Introduction
Breast cancer is a serious disease threatening the
health of women. In 2010, the WHO statistics showed that breast
cancer alone is expected to account for 28% (207,090) of all new
cancer cases among women and is the third leading cause of
cancer-related deaths (1). The
occurrence of breast cancer involves multiple processes, including
enhanced tumor cell proliferation and reduced apoptosis (2), increase in tumor cell migration and
invasion capacity (3), formation of
vascular mimicry by tumor cells (4)
and evasion of immunity of an organism (5). It is known that tumor invasion and
metastasis of breast cancer is the main reason for mortality.
Therefore, studying the molecular mechanisms of breast cancer cells
in the process of invasion and metastasis has meaningful
significance for the pathogenesis of breast cancer and its early
diagnosis and treatment. Tumor cell migration and invasion is a
complex process involving many molecules. In recent years, many
scholars have been committed to exploring the mechanisms of breast
cancer recurrence, invasion and metastasis. They demonstrated that
various molecules involved in cell adhesion, such as integrin,
E-cadherin (6) and ICAM-1 (7) are associated with breast cancer
invasion and migration. Multiple subtype expression of CD44 in
tumors is markedly increased and is positively correlated with
tumor capsule invasion and epithelial-to-mesenchymal transition
(EMT) (8). Yet, the specific
molecular mechanisms involved in breast cancer metastasis remain
unknown (9). Recently, it has been
suggested that miRNAs regulate the development of breast cancer as
a novel gene regulation mechanism.
MicroRNAs (miRNAs) are non-coding single-stranded
RNAs with a length of ~18–22 nucleotides, and play an important
role in regulating gene expression (10). miRNAs cause target mRNA degradation
or translation inhibition through the negative regulation of its
target gene mRNAs, acting as tumor promoters or suppressors
(11). For example, miR-23a was
found to play a role in tumor promotion by downregulating PPP2R5E
thereby inhibiting gastric cancer cell apoptosis (12). miR-429 was demonstrated to have
cancer-suppressive function by targeting the PAK6 signaling pathway
and by inhibiting the migration and invasion of colon cancer cells
(13). Research shows that miRNAs
can also regulate the malignant process in breast cancer. In human
breast cancers, miRNAs can promote the occurrence and development
of breast cancer. For example, miR-217 can promote breast cancer
cell invasive ability and drug resistance (14). miRNA-24-3p can promote the growth of
breast cancer cells and inhibit apoptosis (15). More and more studies suggest that
miR-340 also plays an important role in the malignant behavior of
tumor cells. miR-340 was found to be able to inhibit the cancer
stem cell-like function of glioma tumor cells through targeting
tissue plasminogen activator (16).
In non-small cell lung cancer, miR-34 inhibited tumor cell
proliferation and induced apoptosis by targeting P27 multiple
negative regulatory factors (17).
However, research concerning the regulation of miR-340 in breast
cancer is rare. Thus, in the present study, we investigated the
effect of miR-340 on cell migration and invasion. As the classical
pathway of miRNA regulation is through target genes, we predicted
and verified myosin X (MYO10) as a candidate target gene of
miR-340. Existing research also confirmed that MYO10 can
participate in pseudopodium formation, blood vessel formation and
other important cell migration and invasion processes (18,19).
It is important for us to detect tumor cell migration and invasion
in breast cancer. If the expression level of miR-340 in breast
cancer cells can be effectively regulated, we may find a new method
for the treatment of breast cancer metastasis and may also
facilitate the early diagnosis of human breast cancer.
In the present study, we confirmed that miR-340 was
consistently expressed at a low level in multiple breast cancer
cell lines, and overexpression was able to inhibit breast cancer
cell migration and invasion. Meanwhile, we predicted and verified
that overexpression of miR-340 can regulate the expression of MYO,
and overexpression of MYO10 reversed the inhibition of migration
and invasion by miR-340. Furthermore, in vivo experiments in
mice showed that pulmonary metastasis of breast cancer cells was
significantly decreased by miR-340 which further verified our
conclusion. In brief, our study demonstrated that miR-340 inhibits
the migration and invasion of breast cancer cells by targeting
MYO10, thereby inhibiting the metastasis of breast cancer.
Materials and methods
Cell lines and cell culture
Human breast cancer cell lines MCF-7, MCF-10A,
MDA-MB-231, MDA-MB-468 and SKBR3 were purchased from the American
Type Culture Collection (ATCC, Manassas, VA, USA) and preserved in
our laboratory. Cell culture medium for MCF-7 and MCF-10A cells
contained 10% FBS and 1% double resistant RPMI-1640 medium. Cell
culture medium for the MDA-MB-231 and MDA-MB-468 cells contained
10% FBS and 1% double resistant Leibovitz L-15. Cell culture mediun
for SKBR3 cells contained 10% FBS and 1% double resistant
Dulbecco's modified eagle's medium (DMEM). All the above cells were
cultured in an incubator with 5% CO2 at 37°C.
RNA extraction and real-time PCR
The process of extracting the total RNA in cells and
tissues was carried out according to the instructions of the
mirvana miRNA isolation kit (Ambion, Austin, TX, USA). The
extraction concentration was measured by spectrophotometer NanoDrop
(NanoDrop, Wilmington, De, USA). Finally, it was standby preserved
at −80°C. As for RT-qPCR, we first used reverse transcriptase M-MLV
and nucleic acid enzyme inhibitor RiboLock (Applied Biosystems,
Foster City, CA, USA) to transcribe RNA into cDNA in reverse. Next,
SYBR Green (GenePharma, Shanghai, China) was used to conduct the
real-time quantitative PCR reaction with IQ-5 (Bio-Rad
Laboratories, Hercules, CA, USA). The PCR procedure consisted of 4
min at 95°C firstly, and 94°C for 30 sec, 50°C for another 30 sec,
72°C for 30 sec by cycles. 2−ΔΔCt method was used as the
quantification approach. Primer information is as follows: miR-340
forward, 5′-GCGGTTATAAAGCAATGAGA-3′ and reverse primer,
5′-GTGCGTGTCGTGGAGTCG-3′; U6 forward,
5′-GCTTCGGCAGCACATATACTAAAAT-3′ and reverse primer,
5′-CGCTTCACGAATTTGCGTGTCAT-3′; MYO10 forward,
5′-AAGTGGGGCAGGTAAAACCG-3′ and reverse primer,
5′-GCTCGTTCAACACAGGATGTC-3′; GAPDH forward,
5′-TGCACCACCAACTGCTTAGC-3′ and reverse primer,
5′-GGCATGGACTGTGGTCATGAG-3′.
Plasmid, ASO-miRNA and siRNA
transfection
The SKBR3 and MCF-7 cells were plated in 6-well
plates at a density of 1×106 cells/well and kept
overnight. Then, we transferred the miR-340 mimic or ASO-miR-340,
to pCMVP/MYO10 or si-MYO10 (all from GenePharma) using the liposome
method. Lipofectamine 2000 was purchased from Invitrogen Life
Technologies (Carlsbad, CA, USA); miR-340 mimic,
5′-UUAUAAAGCAAUGAGACUGAUU-3′ (sense) and
5′-UCAGUCUCAUUGCUUUAUAAUU-3′ (antisense); ASO-miR-340:
5′-UUCUCCGAACGUGUCACGUTT-3′ (sense) and 5′-ACGUGACACGUUCGUAGAATT-3′
(antisense); MYO10-siRNA, 5′-GCCCAACUACAGAAAUGGUTT-3′ (sense) and
5′-ACCAUUUCUGUAGUUGGGCTT-3′ (antisense). Six hours after
transfection, the cells were cultured in normal culture medium, and
48 h later, further assessement was conducted.
Protein extraction and western
blotting
The transfected cells were lysed with RIPA buffer.
The proteins (50 µg) were then subjected to 10% SDS-PAGE
electrophoresis. Subsequently, the proteins were transferred to the
membranes. The membranes were incubated with 5% blocking buffer and
then incubated with antibody at 4°C overnight, and added secondary
antibodies (1:1,000; Abcam, Cambridge, MA, SA) for 2 h. The protein
expression was accessed by exposing film. To quantitate band
intensities, labWorks™ gel imaging and analysis system was adopted
for photographing and analyzing the luminance value of each group
of target bands.
Luciferase reporter assay
Approximately 3×104 cells were plated in
a 12-well plate evenly. Approximately 24 h later, breast cancer
cells were transfected with 200 ng miR-340 and 50 ng
pGL3-MYO10-3-UTR. Thirty-six hours after transfection, the cells
were split, and their fluorescence activity was measured with a
dual luciferase system (Promega, Madison, WI, USA).
In vitro scratch test
In vitro scratch test was used to analyze the
migration ability of the cells. The cells were transfected in a
6-well plate. After the cell fusion, the surface of the cell layer
was scratched with a sterile plastic gun. The cells were then
cultured with double-nutrient medium. Forty-eight hours later, we
observed scratch healing on the cell layer surface under a
microscope.
Transwell invasion experiment
The aperture of the bottom membrane of the Transwell
chambers or wells (Corning Inc., Corning, NY, USA) was 8 µm.
The chambers were coated with Matrigel (Sigma-Aldrich, St. Louis,
MO, USA), and were used for detecting the cell invasive ability.
The underlayer was filled with 600 µl l15/RPMI-1640/DMEM
nutrient solution which contained 10% FBS. The volume of the upper
layer of the Transwell was 200 µl inoculated with a certain
amount of breast cancer cells, 5×105 MDAMB-231 or
1×106 MCF-7. The cells were cultured in an incubator at
37°C with 5% CO2 for 36 or 48 h, and then the well was
removed and fixed in liquid, which consisted of methanol and
glacial acetic acid at a ratio of 3:1, for 30 min. Then the wells
were washed with PBS, stained with 0.1% crystal violet and finally
mounted. Five wells were randomly selected, and cells were observed
and numbers counted under a microscope.
Immunohistochemical (IHC) analysis
We detected the tissue expression levels of
candidate target gene MYO10 with IHC testing technology. The TMA
slice was dewaxed. Endogenous peroxidase activity was halted with
3% hydrogen peroxide for 10 min. The section was infiltrated in
0.01 M citric acid buffer (pH 6.0) and then heated in a microwave
for 10 min to carry out antigen recovery. Then it was incubated
with the primary antibody anti-MYO10 (1:100) for 1 h at room
temperature, and the secondary antibody was added after washing
with PBS, and the incubation was continued. Finally it was
incubated with dimethylbenzidine and counterstained with
hematoxylin. Using a microscope, we observed the staining intensity
and the percentage of stained cells with respect to the background.
A positive signal was indicated when over 10% of the tumor cells
were stained.
Animal experiment
All of the animal experiments conformed to the
requirements of the ethics committee. MCF-7-transfected cells
(1×106) were injected into 6- to 8-week-old nude mice by
tail intravenous injection. The mice were sacrificed 5–7 weeks
later. Under a microscope, we observed and counted the metastatic
pulmonary nodules. IHC staining was also carried out and
assessed.
Statistical analysis
Statistical software SPSS22 was used. Data are
expressed as mean ± standard deviation. A t-test was used for
analyzing the data. P<0.05 or P<0.01 were considered to
indicate a statistically significant difference.
Results
miR-340 is expressed at a low level and
directly targets MYO10 in breast cancer cell lines
In order to explore the role of miR-340 in breast
cancer cells, RT-qPCR technology was applied to analyze miR-340
expression levels in different breast cancer cell lines. We took
the expression level of miR-340 in MCF10A cells as a standard 1,
and found that miR-340 showed low expression in multiple breast
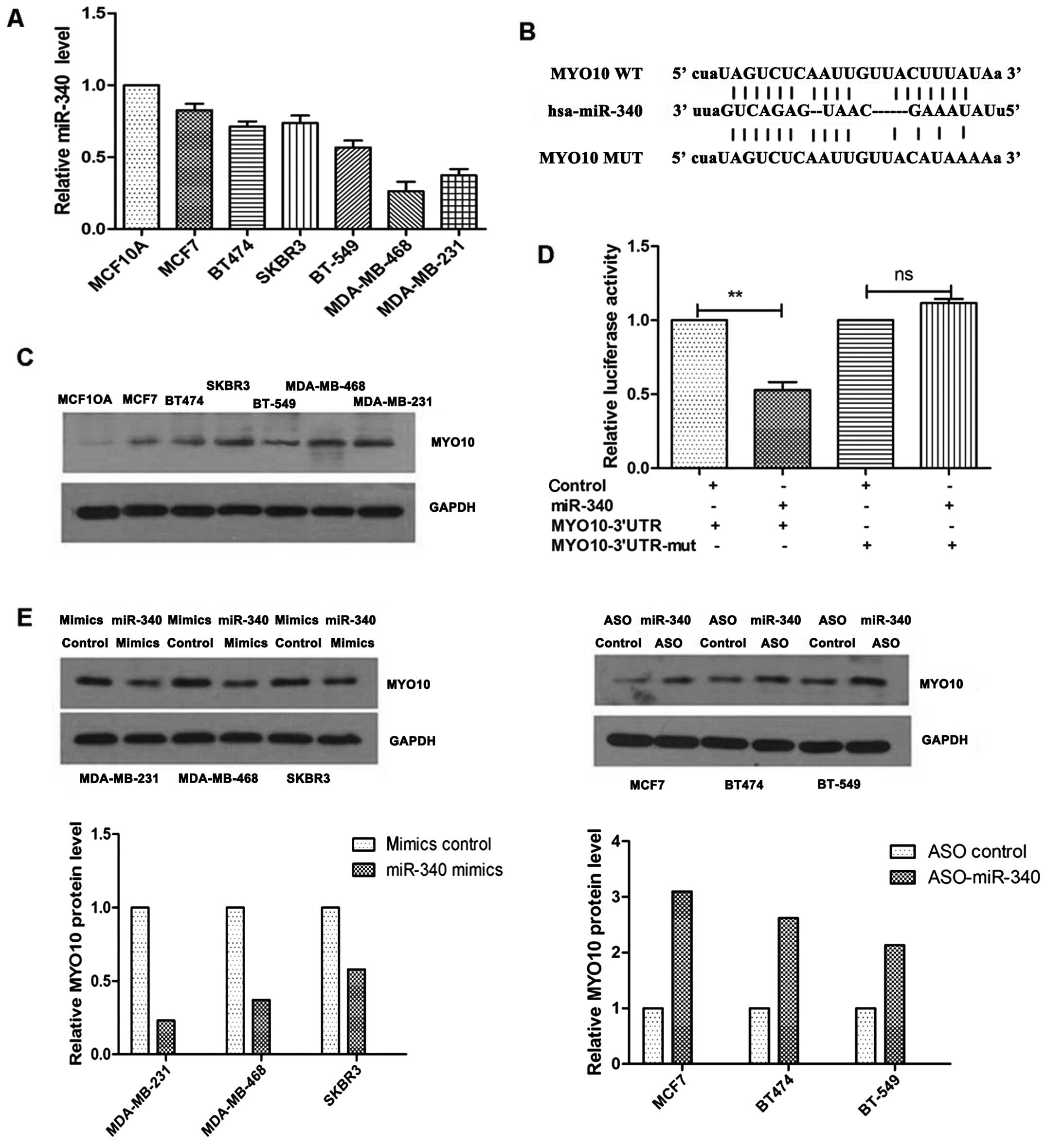
cancer cell lines, compared to the MCF10A cells (Fig. 1A). As the function of miRNA is
realized by regulating its corresponding target gene, we searched
TargetScan database for the target gene of miR-340. Among several
candidate target genes of miR-340, we chose MYO10 as the object of
further study. The reason was that previous reports indicated that
MYO10 could promote the development of breast cancer, and we
predicted that there are three potential targets for miR-340 in the
3′UTR region in MYO10 (Fig. 1B).
Meanwhile, we assessed the MYO10 expression level in 8 different
breast cancer cell lines using western blot analysis, and found
that the expression level was higher in the breast cancer cell
lines than that in the MCF10A cells (Fig. 1C). In order to verify that MYO10 is
the direct target gene of miR-340, we constructed MYO10-3′UTR,
which contained miR-340 binding sites, into the fluorescent
reporter vector, and observed the target given situation by
detecting luciferase activity. The result suggested that the
fluorescence activity of miR-340 decreased due to overexpression,
while, the fluorescence activity of the mutant UTR was not
significantly changed after the mutation (Fig. 1D). This showed that there was a
direct and inverse relationship between miR-340 and MYO10.
Overexpression of miR-340 in the breast cancer MDA-MB-231,
MDA-MB-468 or SKBR3 cells led to a decrease in the MYO10 protein
level at varying degrees. Moreover, the expression of MYO10 was
significantly increased after blocking miR-340 expression (Fig. 1e). Thus, we confirmed that miR-340
is expressed at a low level in breast cancer cells and MYO10 is a
direct target of miR-340, which negatively regulates its
expression.
Overexpression of MYO10 reverses the
inhibition of breast cancer cell migration and invasion mediated by
miR-340
To further explore the regulation of malignant
behavior of breast cancer cells by MYO10 and miR-340, we studied
breast cancer cell migration and invasion. We used miR-340 mimics
and pCMVP/MYO10 to overexpress miR-340 and MYO10 in the MCF-7
cells. First we measured the protein expression level of MYO10 by
western blotting when miR-340 and MYO10 were stained respectively
or conjointly, and the miR-340 mimics and pCMVP/MYO10 plasmids were
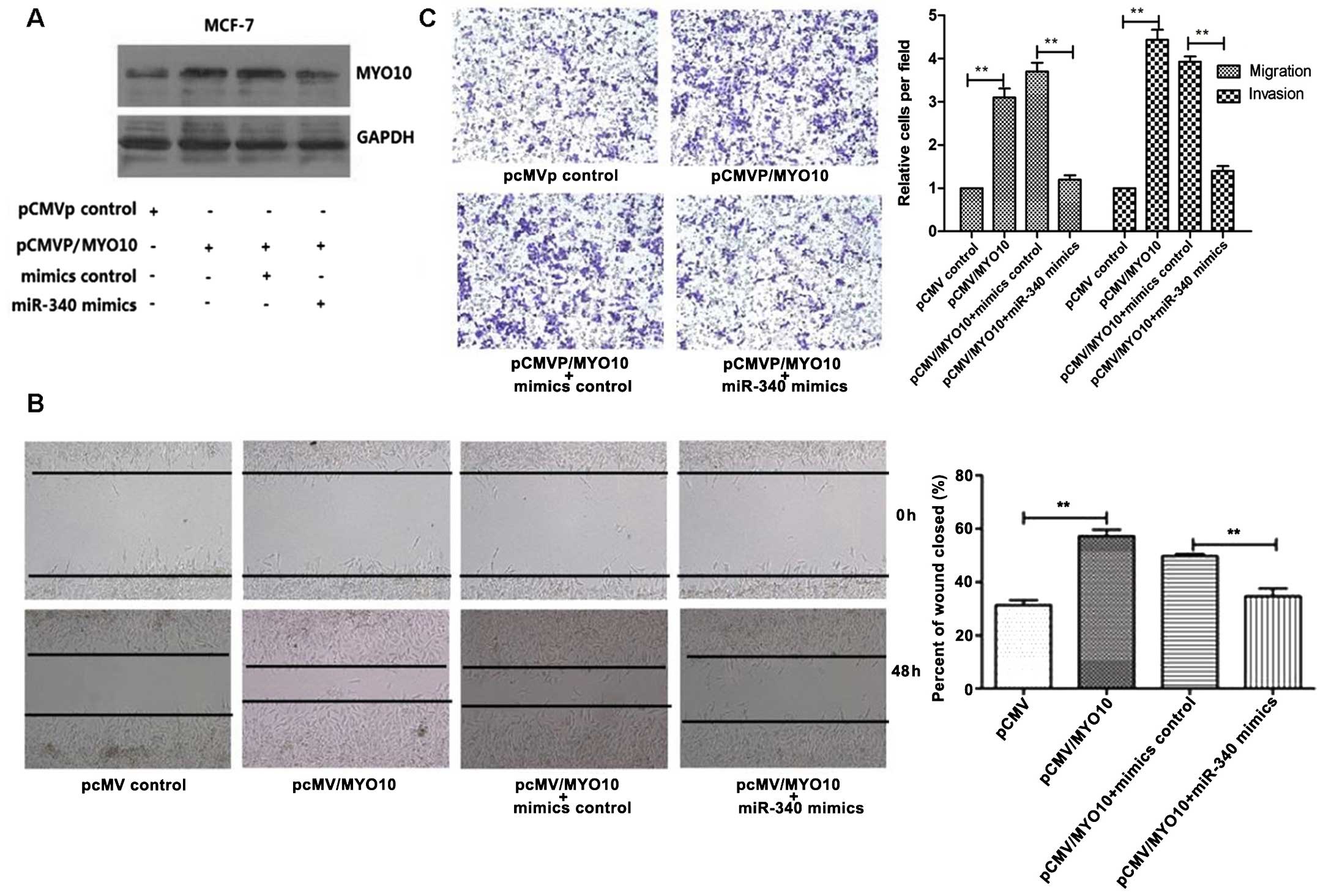
verified to be effective (Fig. 2A).
Then we assessed the effects of miR-340 and MYO10 on cell migration
through in vitro scratch test. As shown in Fig. 2B, when MYO10 was overexpressed
alone, cell migration ability was enhanced. When miR-340 and MYO10
were overexpressed together, miR-340 induced cell migration; MYO10
reversed the condition partly. The same result appeared in
Transwell invasion and migration experiment. When overexpressing
MYO10 alone, the ability of cell invasion and migration increased
about 4.5 times. When miR-340 and MYO10 were overexpressed
conjointly, miR-340 induced cell migration; MYO10 saved the
condition partly. The reduction in cell migration and invasion due
to miR-340 may be saved by MYO10 (Fig.
2C).
Knockdown of MYO10 weakens the
enhancement of cell migration and invasion induced by silencing
miR-340
In order to further verify whether MYO10 has a
necessary influence on the change in breast cancer cell migration
and invasion mediated by miR-340, on the basis of previous
experiments, we assessed the function of knockdown of MYO10 and
silencing of miR-340. First, we tested MYO10 expression with
western blotting, and verified that siRNA MYO10 could effectively
knock down the expression of MYO10. MYO10 expression obviously
increased after ASO-miR-340 transfection illustrating the
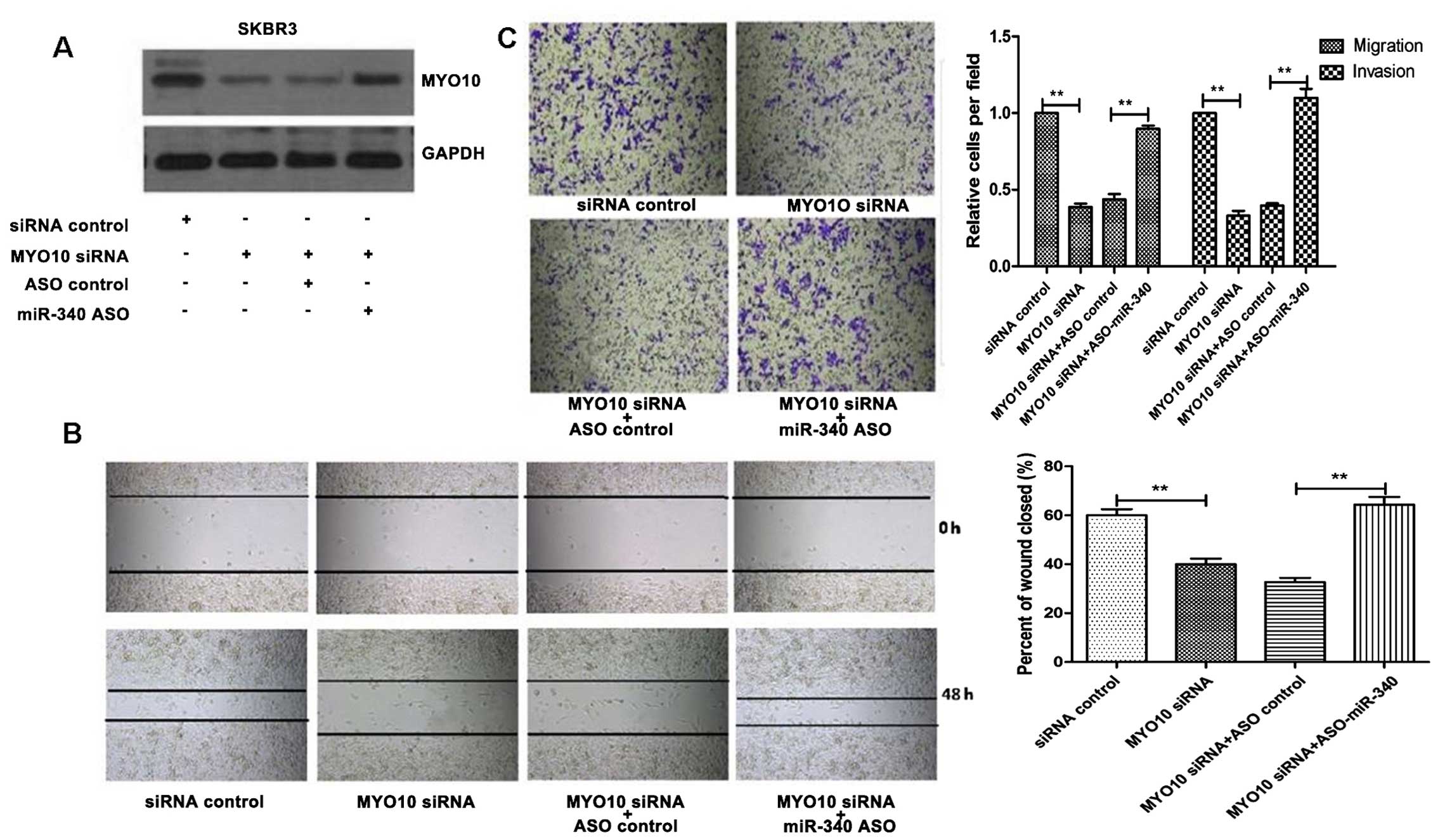
effectiveness of the two knockdowns (Fig. 3A). Following knockdown of MYO10
alone, cell migration decreased. When cells were transfected wiht
MYO10 siRNA and ASO-miR-340 jointly, ASO-miR-340-induced
enhancement of cell migration was inhibited by MYO10 siRNA
(Fig. 3B). We found the same
results in the Transwell invasion and migration experiments.
Following knockdown of MYO10 alone, cell migration and invasion
ability decreased by ~35%. Following treatment of MYO10 siRNA and
ASO-miR-340 together, cell migration enhancement induced by
ASO-miR-340 was inhibited by MYO10 siRNA (Fig. 3C).
Expression level of miR-340 and MYO10 in
high metastatic and low metastatic breast cancer specimens
Based on the cytological experiments mentioned above
on the expression levels of MYO10 and miR-340 and on their
functions, we attempted to validate our conclusions from the levels
in tissue specimens. Before the detection of miR-340 expression in
the breast cancer specimens, pathological classification was
carried out according to the number of cancer cell lymph node
metastasis. Those with lymph node metastasis <1 were considered
as the no metastasis group, those with lymph node metastasis from 1
to 3 were considered as the low metastasis group, and those with
lymph node metastasis >3 were regarded as the high metastasis
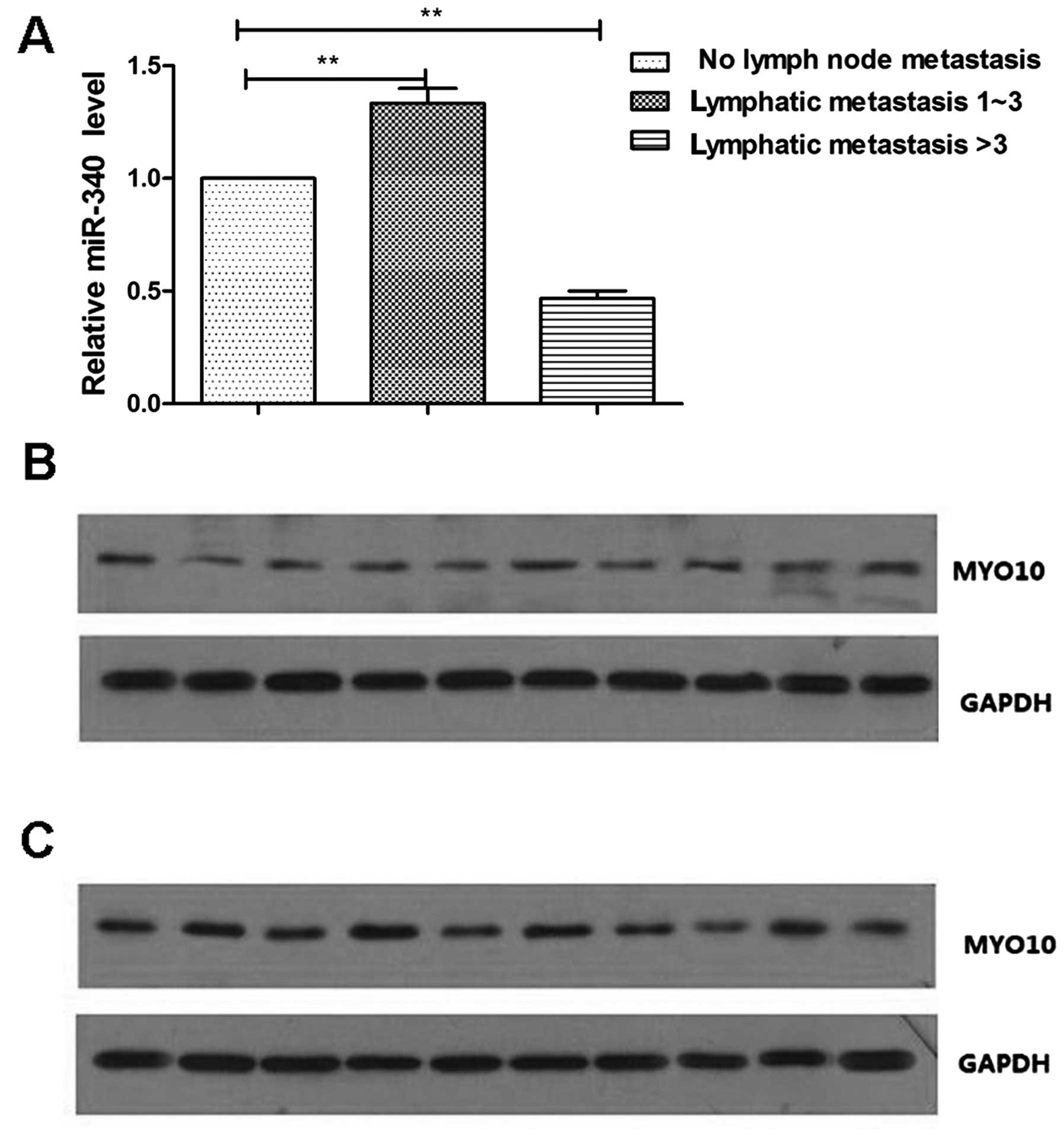
group. The result revealed that, when compared to the no metastasis
group, the expression level of miR-340 in the high metastasis group
was decreased by 60%, while the expression level of miR-340 in the
low metastasis group increased by ~1.2 times (Fig. 4A). These results confirm our
previous conclusion that miR-340 inhibits tumor cell migration and
invasion. We detected the expression levels of MYO10 in 10 high
metastatic and low metastatic carcinomas using western blotting. We
found that in high metastatic carcinomas, the expression level of
MYO10 was increased (Fig. 4B),
while the MYO10 expression level was decreased in the low
metastatic carcinomas (Fig. 4C).
Thus, miR-340 inhibits tumor cell migration and invasion by
negative regulation of MYO10 expression.
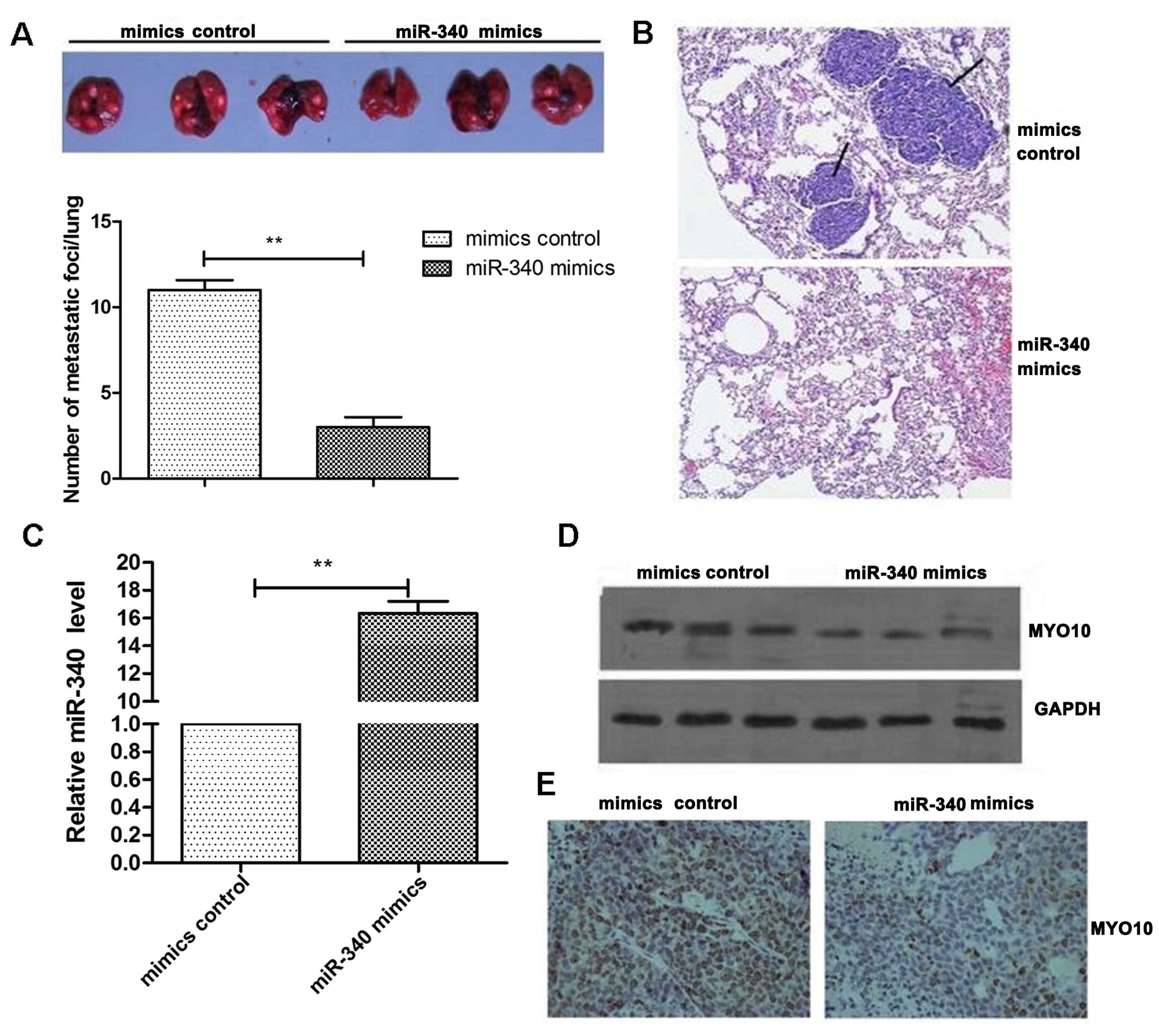
miR-340 inhibits lung metastasis of
breast cancer in mice and is negatively correlated with MYO10
To further clarify the correlation between miR-340
and the MYO10, we performed animal experiments in mice by tail vein
injection. We injected MCF-7 cells overexpressing miR-340 into mice
as described earlier. Metastatic lung nodules were counted after
the mice were sacrificed. It was found that overexpression of
miR-340 significantly decreased the lung metastasis of breast
cancer in the mice. Compared to the control group, lung metastasis
decreased by 70% (Fig. 5A). We also
confirmed that miR-340 effectively inhibited the lung metastasis of
breast cancer using IHC staining for carcinoembryonic antigen (CEA)
(Fig. 5B). We obtained a small
portion of the tumor tissues to extract RNA and detect expression
levels of miR-340. The results confirmed that overexpression of
miR-340 was effective (Fig. 5C).
Western blotting also demonstrated that the expression of miR-340
negatively regulated MYO10 expression in the tissues (Fig. 5D). Likewise, using
immunohistochemical staining techniques we also found that the
expression of MYO10 in the miR-340 overexpression group was
reduced, compared to the control group (Fig. 5E). All of the above confirmed that
miR-340 and MYO10 expression were negatively correlated in the
tissues.
Discussion
Tumor cell metastasis is a critical stage which
threatens the life of cancer patients and involves a complex
cascade of events. Progressing from in situ is the first step,
which includes change in tumor cell adhesion, cell migration,
invasion of the basement membrane, enzymatic hydrolysis substrate,
angiogenesis and survival in the circulatory system (20). Next, tumor cells settle over a long
distance and grow and form a metastatic tumor. In this series of
events, tumor cell migration and invasion of surrounding tissue is
a crucial early step. A large number of recent studies indicate
that miRNAs play an important role in the migration and invasion of
tumor cells (21). For instance,
miR-20a/b can inhibit the invasion of prostate cancer cells
(22) and miR-140-5p can inhibit
the migration of hepatocellular carcinoma cells. Many studies have
also reported that in the course of breast cancer development, many
miRNAs, which include miR-205, miR-206 and miR-146, can promote or
inhibit the migration and invasion of breast cancer cells (24,25).
In the present study, we assessed a number of different breast
cancer cell lines and detected low expression of miR-340. A number
of studies previously confirmed that miR-340 can play a regulatory
role in different types of tumors. For example, in osteosarcoma
miR-340 can target ROCK1 and inhibit cell growth and metastasis
(26). miR-340 expression in bone
marrow was associated with liver metastasis of colon cancer
(27). Yet, few reports have
reported the relationship between miR-340 and breast cancer cell
migration and invasion. Our research focused on the effects of
miR-340 on the migration and invasion of breast cancer cells. And
our final conclusion is that miR-340 can inhibit the migration and
invasion of breast cancer cells.
It is known that miRNAs effect post-transcriptional
expression levels by targeting target genes. Thus, we used
bioinformatic prediction method to identify the potential target
gene of miR-340 as MYO10. The MYO family has 12 members in
vertebrates. Their structure is characterized by the actin
dependent domain in N-terminal and variable C-terminal, which
contains a number of cytoskeleton, cytoplasmic membrane, signaling
molecules and target anchor points of other factors (28). MYO10 role is also crucial in
biological function. At the University of North Carolina, the
researchers in the Cell and Molecular Physiology Laboratory used
scanning electron microscopy to observe the large number of
filopodia on the back of Hela cells. The results suggested that
MYO10 is the necessary molecular motor for the formation of
filopodia (29). Cox et al
(30) found that there is a close
connection between MYO10 and cell phagocytosis, and MYO10 tail
truncated structure could inhibit the phagocytosis of alveolar
macrophages. It was confirmed that the effect of MYO10 is essential
in the process of cell adhesion and migration (31). It was also found by many other
studies that MYO10 plays a major role in cell endocytosis (32). Yet, reports on the regulation of
MYO10 on invasion and gene expression in breast cancer are rare.
Our research confirmed that MYO10 plays a promoting role in the
migration and invasion of breast cancer cells. Further research
found that MYO10 promotes the migration and invasion of breast
cancer cells and restores miR-340-mediated inhibition of breast
cancer cell migration and invasion. The findings are consistent
with previous studies. Thereby it was also confirmed by functional
verification that MYO10 is the direct target gene of miR-340.
Further research will be carried out to ascertain through which
specific molecular mechanisms or signaling pathways is this
functional regulation achieved.
Our experiment demonstrated for the first time that
in breast cancer cells, miR-340 inhibited the migration and
invasion of cancer cells through targeting MYO10. This finding has
important significance in tumor metastasis as it may become an
important method for the inhibition of tumor metastasis. MYO10 also
provides another effective target for the treatment of breast
cancer. Therefore, MYO10 is expected to become a new means of
diagnosis and treatment of breast cancer metastasis to weaken the
migration and invasion of tumor cells through mediating the
expression level of miR-340 in breast cancer cells.
Acknowledgments
The present study was supported by the Breast
Surgery Key Disciplines Fund of Jiaxing (no. 04-F-15) and Pathology
of Jiaxing (no. GJJX-010-001).
References
|
1
|
Jemal A, Siegel R, Xu J and Ward E: Cancer
statistics, 2010. CA Cancer J Clin. 60:277–300. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yang M, Yuan F, Li P, Chen Z, Chen A, Li S
and Hu C: Interferon regulatory factor 4 binding protein is a novel
p53 target gene and suppresses cisplatin-induced apoptosis of
breast cancer cells. Mol Cancer. 13:11–54. 2012.
|
|
3
|
Noh EM, Park YJ, Kim JM, Kim MS, Kim HR,
Song HK, Hong OY, So HS, Yang SH, Kim JS, et al: Fisetin regulates
TPA-induced breast cell invasion by suppressing matrix
metal-loproteinase-9 activation via the PKC/ROS/MAPK pathways. Eur
J Pharmacol. 764:79–86. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cui YF, Liu AH, An DZ, Sun RB, Shi Y, Shi
YX, Shi M, Zhang Q, Wang LL, Feng Q, et al: Claudin-4 is required
for vasculogenic mimicry formation in human breast cancer cells.
Oncotarget. 6:11087–11097. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
McCracken MN, Cha AC and Weissman IL:
Molecular pathways: Activating T cells after cancer cell
phagocytosis from blockade of CD47 'don't eat me' signals. Clin
Cancer Res. 21:3597–3601. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Frixen UH, Behrens J, Sachs M, Eberle G,
Voss B, Warda A, Löchner D and Birchmeier W: E-cadherin-mediated
cell-cell adhesion prevents invasiveness of human carcinoma cells.
J Cell Biol. 113:173–185. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kohm AP and Miller SD: Role of ICAM-1 and
P-selectin expression in the development and effector function of
CD4+ CD25+ regulatory T cells. J Autoimmun.
21:261–271. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Preca BT, Bajdak K, Mock K, Sundararajan
V, Pfannstiel J, Maurer J, Wellner U, Hopt UT, Brummer T, Brabletz
S, et al: A self-enforcing CD44s/ZeB1 feedback loop maintains eMT
and stemness properties in cancer cells. Int J Cancer. Jun
16–2015.Epub ahead of print. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Weigelt B, Peterse JL and van 't veer LJ:
Breast cancer metastasis: Markers and models. Nat Rev Cancer.
5:591–602. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bartel DP: MicroRNAs: Target recognition
and regulatory functions. Cell. 136:215–233. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen K and Rajewsky N: The evolution of
gene regulation by transcription factors and microRNAs. Nat Rev
Genet. 8:93–103. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu X, Liu Q, Fan Y, Wang S, Liu X, Zhu L,
Liu M and Tang H: Downregulation of PPP2R5E expression by miR-23a
suppresses apoptosis to facilitate the growth of gastric cancer
cells. FEBS Lett. 588:3160–3169. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tian X, Wei Z, Wang J, Liu P, Qin Y and
Zhong M: MicroRNA-429 inhibits the migration and invasion of colon
cancer cells by targeting PAK6/cofilin signaling. Oncol Rep.
34:707–714. 2015.PubMed/NCBI
|
|
14
|
Zhang AX, Lu FQ, Yang YP, Ren XY, Li ZF
and Zhang W: MicroRNA-217 overexpression induces drug resistance
and invasion of breast cancer cells by targeting PTEN signaling.
Cell Biol Int. Jun 24–2015.Epub ahead of print. View Article : Google Scholar
|
|
15
|
Lu K, Wang J, Song Y, Zhao S, Liu H, Tang
D, Pan B, Zhao H and Zhang Q: miRNA-24-3p promotes cell
proliferation and inhibits apoptosis in human breast cancer by
targeting p27 Kip1. Oncol Rep. 34:995–1002. 2015.PubMed/NCBI
|
|
16
|
Yamashita D, Kondo T, Ohue S, Takahashi H,
Ishikawa M, Matoba R, Suehiro S, Kohno S, Harada H, Tanaka J, et
al: miR340 suppresses the stem-like cell function of
glioma-initiating cells by targeting tissue plasminogen activator.
Cancer Res. 75:1123–1133. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fernandez S, Risolino M, Mandia N, Talotta
F, Soini Y, Incoronato M, Condorelli G, Banfi S and Verde P:
miR-340 inhibits tumor cell proliferation and induces apoptosis by
targeting multiple negative regulators of p27 in non-small cell
lung cancer. Oncogene. 34:3240–3250. 2015. View Article : Google Scholar
|
|
18
|
Schoumacher M, Goldman RD, Louvard D and
Vignjevic DM: Actin, microtubules, and vimentin intermediate
filaments cooperate for elongation of invadopodia. J Cell Biol.
189:541–556. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gerhardt H, Golding M, Fruttiger M,
Ruhrberg C, Lundkvist A, Abramsson A, Jeltsch M, Mitchell C,
Alitalo K, Shima D, et al: VEGF guides angiogenic sprouting
utilizing endothelial tip cell filopodia. J Cell Biol.
161:1163–1177. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Klein CA: Cancer. The metastasis cascade.
Science. 321:1785–1787. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nicoloso MS, Spizzo R, Shimizu M, Rossi S
and Calin GA: MicroRNAs - the micro steering wheel of tumour
metastases. Nat Rev Cancer. 9:293–302. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yang H, Fang F, Chang R and Yang L:
MicroRNA-140-5p suppresses tumor growth and metastasis by targeting
transforming growth factor β receptor 1 and fibroblast growth
factor 9 in hepatocellular carcinoma. Hepatology. 58:205–217. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kato M, Goto Y, Matsushita R, Kurozumi A,
Fukumoto I, Nishikawa R, Sakamoto S, Enokida H, Nakagawa M,
Ichikawa T, et al: MicroRNA-26a/b directly regulate La-related
protein 1 and inhibit cancer cell invasion in prostate cancer. Int
J Oncol. 47:710–718. 2015.PubMed/NCBI
|
|
24
|
Wu H, Zhu S and Mo YY: Suppression of cell
growth and invasion by miR-205 in breast cancer. Cell Res.
19:439–448. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hurst DR, Edmonds MD, Scott GK, Benz CC,
Vaidya KS and Welch DR: Breast cancer metastasis suppressor 1
up-regulates miR-146, which suppresses breast cancer metastasis.
Cancer Res. 69:1279–1283. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhou X, Wei M and Wang W: MicroRNA-340
suppresses osteosarcoma tumor growth and metastasis by directly
targeting ROCK1. Biochem Biophys Res Commun. 437:653–658. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Takeyama H, Yamamoto H, Yamashita S, Wu X,
Takahashi H, Nishimura J, Haraguchi N, Miyake Y, Suzuki R, Murata
K, et al: Decreased miR-340 expression in bone marrow is associated
with liver metastasis of colorectal cancer. Mol Cancer Ther.
13:976–985. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Berg JS, Powell BC and Cheney RE: A
millennial myosin census. Mol Biol Cell. 12:780–794. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bohil AB, Robertson BW and Cheney RE:
Myosin-X is a molecular motor that functions in filopodia
formation. Proc Natl Acad Sci USA. 103:12411–12416. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cox D, Berg JS, Cammer M, Chinegwundoh JO,
Dale BM, Cheney RE and Greenberg S: Myosin X is a downstream
effector of PI(3)K during phagocytosis. Nat Cell Biol. 4:469–477.
2002.PubMed/NCBI
|
|
31
|
Yonezawa S, Yoshizaki N, Sano M, Hanai A,
Masaki S, Takizawa T, Kageyama T and Moriyama A: Possible
involvement of myosin-X in intercellular adhesion: Importance of
serial pleckstrin homology regions for intracellular localization.
Dev Growth Differ. 45:175–185. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tacon D, Knight PJ and Peckham M: Imaging
myosin 10 in cells. Biochem Soc Trans. 32:689–693. 2004. View Article : Google Scholar : PubMed/NCBI
|