Introduction
Acute myeloid leukemia (AML) is the most common form
of acute leukemia that occurs more frequently in adults, but
relatively rare in children; and AML incidence increases with age
(1). AML is characterized by the
rapid growth of abnormal white blood cells that accumulates in the
bone marrow, which inhibits production and differentiation of
normal blood cells (1,2). Genetically, AML is defined as a clonal
disorder caused by malignant transformation of bone marrow-derived
cells, self-renewing stem cells, or progenitors that demonstrate a
decreased rate of self-destruction and aberrant differentiation
(3). Pediatric AML comprises of up
to 20% of all childhood leukemia (4). Although overall AML prognosis has been
improved in the last decade, further development and identification
of prognostic markers or novel targets for AML treatment could
improve the survival rate of AML patients, especially pediatric AML
patients.
Long non-coding RNA (lncRNA) regulates gene
transcription and protein expressions genetically and
epigenetically, and altered expressions result in cancer
development. Human genomic data has shown that ~75% of the human
genome is transcribed into RNA, and only a few above 1% codes
protein expressions; indicating that a large portion of the genome
is dedicated as regulators (5,6). Many
lncRNAs ranging from 0.2 to 100 kilobases (kb) in length are
transcribed from the genome. Among these newly discovered RNA
elements, lncRNAs have been identified to have functional roles in
a diverse range of cellular functions such as development,
differentiation, cell fate, as well as disease pathogenesis
(7–11). Expression analyses of cancer versus
normal cells have revealed aberrant non-coding RNA (ncRNA)
expression in various human cancers. For example, an altered PCGEM1
expression was associated with increased proliferation and colony
formation in prostate cancer cells (12). MALAT1, also known as NEAT2, was
originally identified as an abundantly expressed ncRNA during
metastasis of early-stage non-small cell lung cancer; and its
overexpression is a prognostic marker for poor patient survival
rate (13,14). MALAT1 was also found to be highly
expressed in hepatocellular carcinoma (15–17).
The oncofetal H19 gene was the first imprinted ncRNA to be
identified, and loss of imprinting (LOI) at chromosome
11p15.5H19/IGF2 locus leads to an imbalanced expression of H19 and
IGF2. H19 dysregulation has been implicated in a variety of human
cancers such as colorectal (18),
HCC (19), breast (20), and bladder cancers (21,22).
Various lncRNAs were reported to be implicated in
malignant hematopoiesis associated with blood cell neoplasms such
as leukemia (23,24). H19 ncRNA was highly expressed in
Bcr-Abl-transformed cell lines and primary cells derived from
patients in a Bcr-Abl kinase-dependent manner (23). lncRNA MONC and MIR100HG were highly
expressed in acute mega-karyoblastic leukemia blasts (24). Thus, lncRNAs may be useful as
diagnostic and prognostic markers in leukemia. In the present
study, we profiled differential expression of lncRNAs in pediatric
AML to better understand AML pathogenesis and identify biomarkers
and novel therapeutic targets.
Materials and methods
Patients and samples
Bone marrow specimens were obtained from 22
pediatric AML patients at the Department of Hematology and
Oncology, Children's Hospital of Soochow University, between 2011
and 2014. This study was approved by the Ethics Committee of the
Children's Hospital of Soochow University (#SUEC2011-021 and
#SUEC2014-037), and written informed consent was obtained from all
parents or guardians. AML diagnosis was made in accordance with the
revised French-American-British (FAB) classification (25). The main clinical and laboratory
features of this cohort of patients are summarized in Table I. Additionally, bone marrow samples
from 20 donors without leukemia were used as controls. Bone marrow
mononuclear cells (BMNCs) were isolated using Ficoll solution
within 2 h after bone marrow samples were harvested, and subjected
for isolation of total cellular RNA.
 | Table IThe main clinical and laboratory
features of the pediatric AML samples. |
Table I
The main clinical and laboratory
features of the pediatric AML samples.
| Gender | Age (years) | Diagnosis | AML typing | Chromosome
analysis | Fusion gene | Mutation |
|---|
| 1 | F | 11 | AML | M5 | 46,
XX,t(9;11)(P22;q23) [9]/46,XX[3] | MLL/AF9 | |
| 2 | F | 5 | AML | M5 | ns | ns | |
| 3 | M | 7 | AML | M2a | 46, XY | AML/ETO | |
| 4 | M | 5 | AML | M2a | 46, XY | (−) | CEBPA TAD1 |
| 5 | F | 6 | AML | M4 | 46, XX | dupMLL,
FLT-TKD | |
| 6 | M | 6 | AML | M2 | 45,
X,-Y,t(8;21)(q22;q22) [7]/46,XY[5] | AML1/ETO | |
| 7 | F | 12 | AML | AML | 46, XX | AML/ETO | |
| 8 | M | 2 | AML | M5 | 46, XY | 46, XY | |
| 9 | F | 10 | AML | M2 | 46,
XX,t(8;21)(q22;q22) | AML/ETO | C-Kit |
| 10 | M | 5 | AML | M4 | 46, XY | (−) | |
| 11 | F | 2 | AML | M5 | ns | ns | |
| 12 | F | 13 | AML | M3 | 46, XX,
t(15;17)(q22;q21) | PML/RARA | |
| 13 | M | 12 | AML | M2a | ns | ns | |
| 14 | F | 2 | AML | M4 | 46, XX | CBF/MYH11 | |
| 15 | M | 4 | AML | M2 | 46, XY | AML/ETO | |
| 16 | F | 12 | AML | M2 | 45, X,
-X,t(8:21)(q22;q22) [6]/46,XX[2] | AML/ETO | C-Kit |
| 17 | M | 6 | AML | M3 |
46,XY,t(15:7)(q22:q210 [9]/46,XY[3] | PML/RARA | |
| 18 | M | 8 | AML | M2a | 46, XY | (−) | |
| 19 | F | 1 | AML | M5b | 46, XX,
t(6;11)(q27;q23) | MLL/AF6 | |
| 20 | M | 10 | AML | M4 | 47, XY,+22,
inv(16)(p13q22) | CBF/MYH11 | |
| 21 | M | 9 | AML | M2a | 46, XY | (−) | |
| 22 | M | 7 | AML | M3 | 46, XY | PML/RARA | |
Profiling of lncRNA expression using
Arraystar Human LncRNA Array V3.0
Arraystar Human LncRNA Array V3.0 was used to
profile expression of lncRNAs, which was performed by KangChen
Bio-tech (Shanghai, China) according to a previous study (26). Briefly, RNA samples from BMNCs were
further purified to remove rRNA, and transcribed into fluorescent
cRNA as probes to hybridize to the Human LncRNA Array V3.0 (8660 K;
Arraystar). The array contains 30,586 lncRNAs and 26,109 coding
transcripts, which were collected from the most authoritative
databases (such as RefSeq, UCSC, Knowngenes, and Ensembl) and
related literature. Array data were then analyzed by
MultiExperiment Viewer software for upregulation or downregulation
of lncRNA expression in AML samples compared to control samples
with a cut-off point of 2-fold for upregulation and a cut-off point
of 0.5-fold for downregulation.
Gene Ontology and Kyoto Encyclopedia of
Genes and Genomes pathway analyses
Gene Ontology (GO) functionally analyzes
differentially expressed genes with GO categories (http://david.abcc.ncifcrf.gov/summary.jsp). Pathway
analysis of differentially expressed genes was performed based on
the latest Kyoto Encyclopedia of Genes and Genomes (KEGG) database
(http://www.genome.jp/kegg).
Differentially expressed genes and gene product enrichment with
particular attention to GO biological processes and molecular
functions were grouped into gene pathways using a p-value ≤0.05, as
shown below.
Construction of the lncRNA-mRNA
co-expression network
The construction of the lncRNA-mRNA co-expression
network included three steps: i), lncRNA screening: lncRNAs that
were upregulated or downregulated with a fold-change >3.0 and a
p-value <0.05 were first selected to enhance data reliability.
Sequences of lncRNAs that have not been recorded in ENCODE were
removed. ii), lncRNA-miRNA interactions were predicted by miRcode
(http://www.mircode.org/). iii), mRNAs targeted by
miRNAs with experimental support were obtained from TarBase
(http://www.microrna.gr/tarbase).
Network construction procedures included the
following: i), preprocessing of data: if one coding gene has
different transcripts, the median value was taken to represent the
value of this gene expression without special treatment of lncRNA
expression values; ii), data were screened and subset of data were
removed according to the lists of differential lncRNA and mRNA
expressions obtained from GO and KEGG pathway analyses; iii),
Pearson's correlation coefficient was calculated, and the R value
was used to calculate the correlation coefficient between lncRNA
and coding genes; and iv), Pearson's correlation coefficient was
used for screening, wherein, RNAs with a Pearson's correlation
coefficient ≥0.98 were considered significant. The lncRNA-mRNA
co-expression network was then constructed by Cytoscape software
(The Cytoscape Consortium, San Diego, CA, USA).
RNA isolation and qRT-PCR
Total cellular RNA was isolated from bone marrow
samples using a TRIzol reagent (Invitrogen, Carlsbad, CA, USA) and
stored at −80°C until use. RNA concentration was determined using a
spectrophotometer (NanoDrop 2000) and purity was assessed by
agarose gel electrophoresis. RNA samples were then reversely
transcribed into cDNA using 4 µg of RNA samples in a
10-µl volume and SuperScript II reverse transcriptase
(Invitrogen) according to manufacturer's protocols. For qPCR, we
first designed PCR primers according to the database of Real-time
primers (Center for Medical Genetics, http://medgen.ugent.be/CMGG/) or using the online
program, Primer 3 (www.fokker.wi.mit.edu/primer3/input.htm). Primer
selection parameters were set to primer size: 20–26 nts; primer
melting temperature: 60–64°C; GC clamp: 1; and product size range:
generally 120–240 bp, which went down to 100 bp if no appropriate
primers could be identified. Primers were synthesized by
Invitrogen. The qPCR amplification was set in a 20-µl
reaction volume containing 1 µl of cDNA, 0.2 mM of each
primer, and 10 µl of SyBR Green Mix (Roche, Indianapolis,
IN, USA); and was performed in a LightCycler 480 (Roche) using
universal thermal cycling parameters (an initial 95°C for 10 min
and 45 cycles of 15 sec at 95°C, 15 sec at 60°C, and 60 sec at
72°C. After that, the melting curve for 10 sec at 95°C and 60 sec
at 65°C). For gene expression levels, we used the comparative Ct
method. First, gene expression levels for each sample were
normalized to the expression level of the housekeeping gene
encoding glyceraldehyde 3-phosphate dehydrogenase (GAPDH) within a
given sample (−ΔCt); and the relative expression of each gene was
calculated with 106 × log2 (−ΔCt). The difference
between pediatric AML samples compared to control samples was used
to determine 106 × log2 (−ΔCt).
Statistical analysis
SPSS v11.5 (SPSS Inc., Chicago, IL, USA) was used
for all statistical analyses. Differentially expressed lncRNAs in
AML samples were statistically compared with normal controls, and a
cut-off point of 2-fold for upregulation and 0.5-fold for
downregulation of lncRNA expressions were used in AML samples. For
gene expression, we performed a Student's t-test; and a p-value
≤0.05 was considered statistically significant.
Results
Differentially expressed lncRNAs and
mRNAs in pediatric AML
The Arraystar Human LncRNA 8×60k V3.01 micro-array
was used to profile differentially expressed lncRNAs and mRNAs in
pediatric AML versus normal controls. A total of 2,335 differently
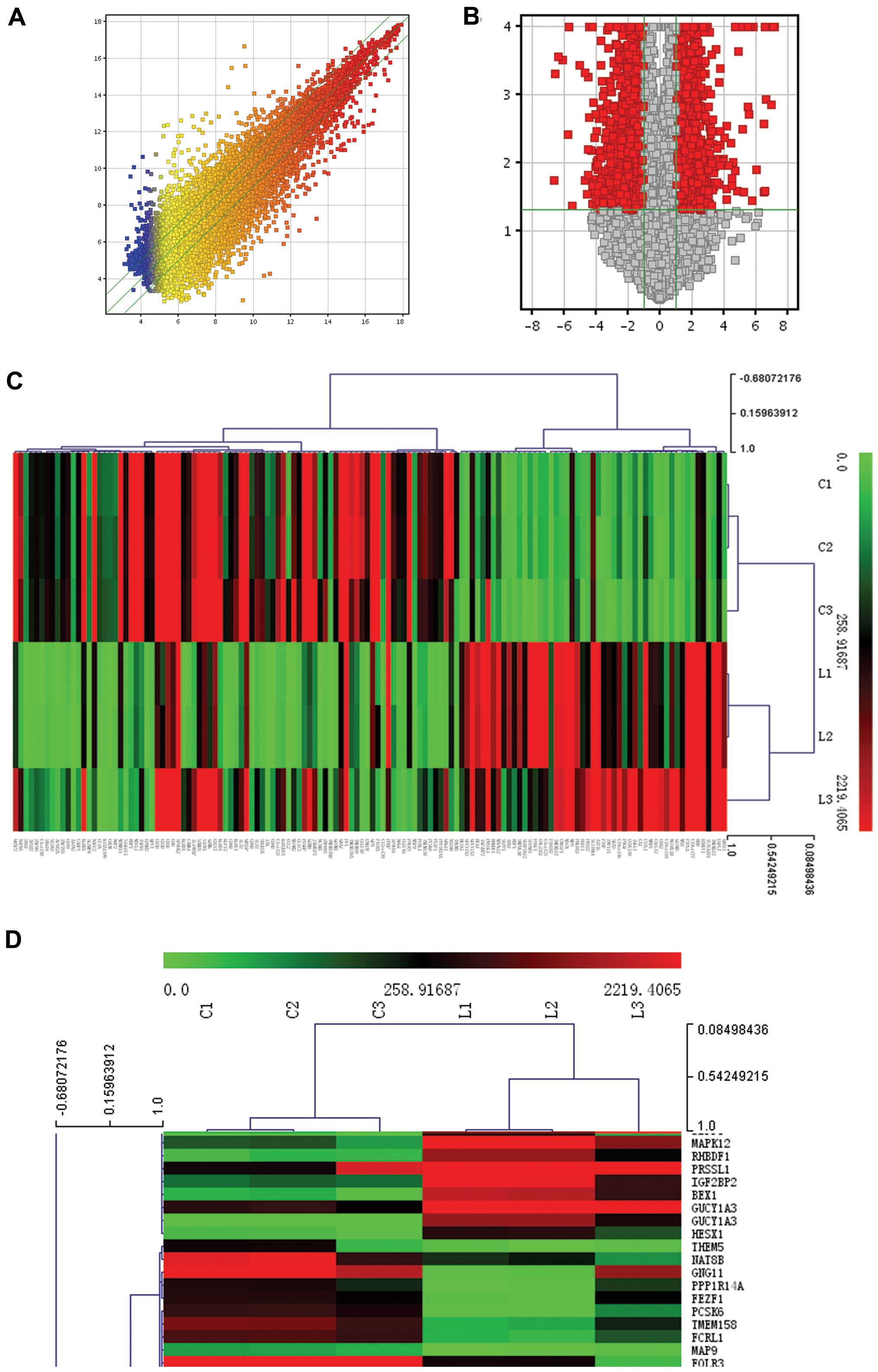
expressed mRNAs were found in pediatric AML (Fig. 1A and B). Among them, 51 mRNAs were
significantly upregulated and 85 mRNAs were significantly
downregulated for >10-fold in pediatric AML compared to normal
controls. Clustering analysis was used to visualize the
relationships between the mRNA expression patterns present in the
samples (fold-changes ≥10; Fig. 1C and
D).
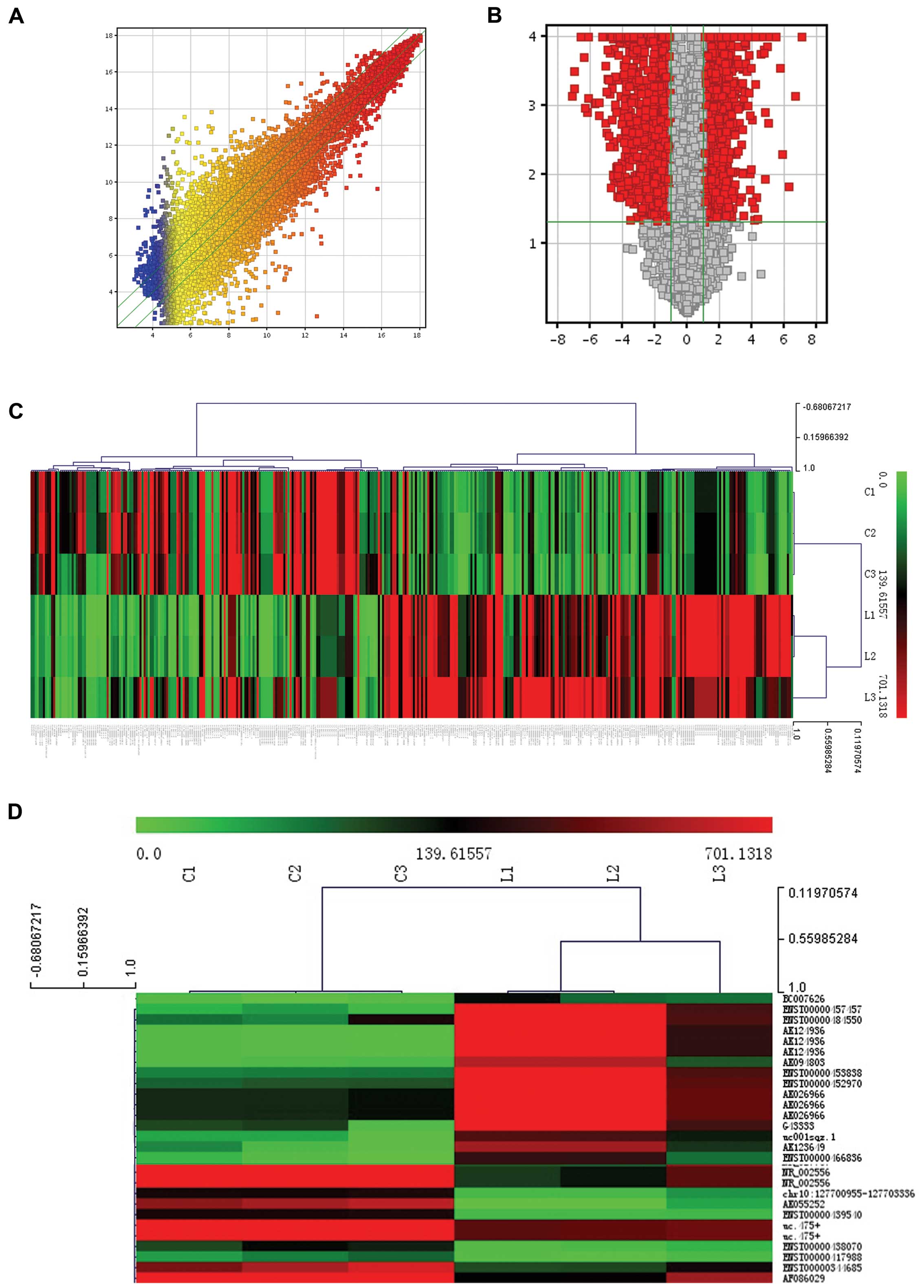
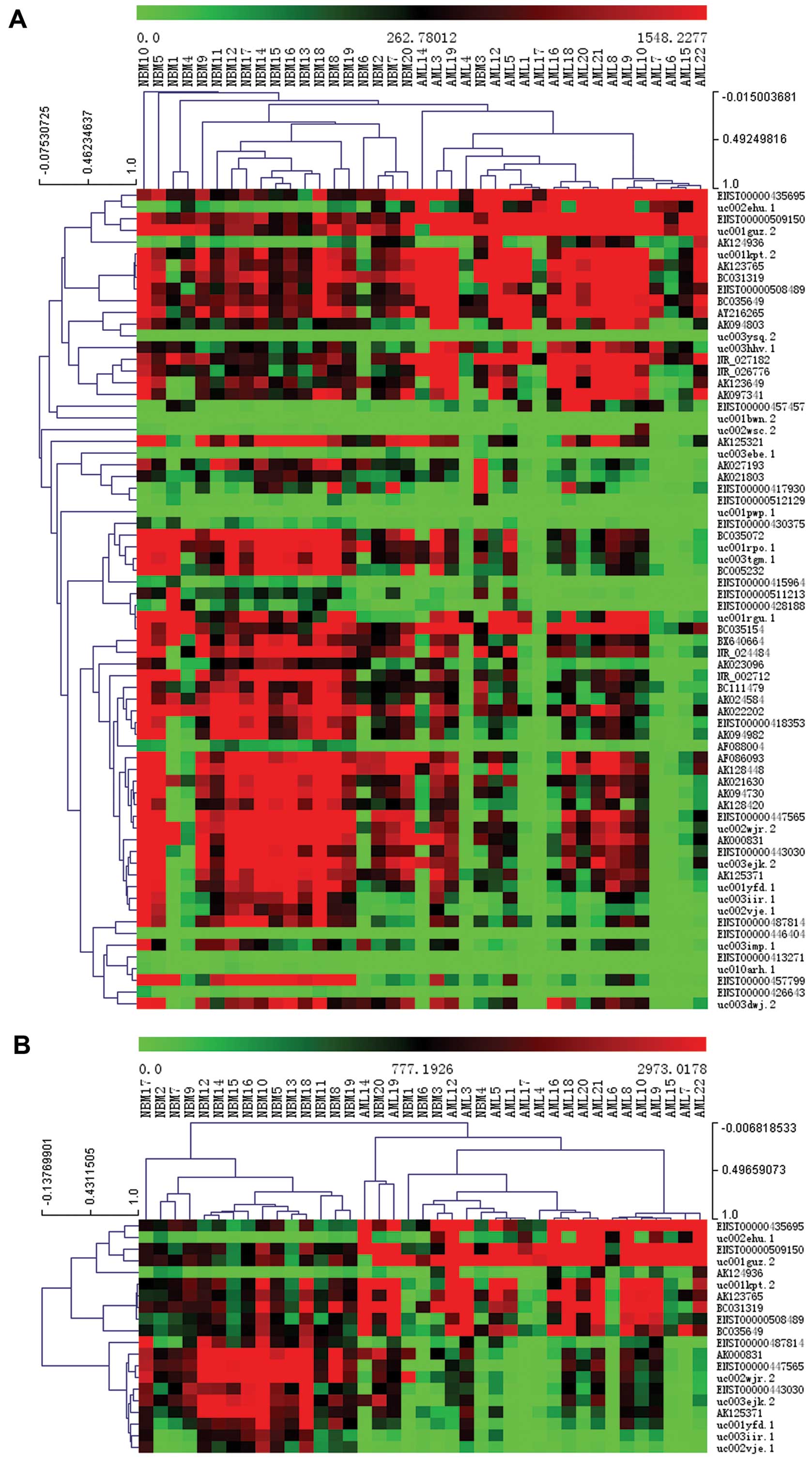
Moreover, a total of 2,413 differentially expressed
lncRNA were identified in pediatric AML (Fig. 2A and B). Hierarchical clustering
analysis of these differentially expressed lncRNAs with >10-fold
difference is shown in Fig. 2C and
D and Tables II and III.
 | Table IIlncRNA upregulated >10-fold in the
pediatric acute myeloid leukemia compared with normal control. |
Table II
lncRNA upregulated >10-fold in the
pediatric acute myeloid leukemia compared with normal control.
| Seqname | Gene symbol | Associated gene
name | Source | [C](raw) | [L](raw) | FC (abs) | P-value |
|---|
| 1 | AK124936 | lincRNA-FAM47E | SCARB2 | lincRNA | 14.110494 | 1536.7562 | 59.8816 | 0.0072902 |
| 2 | uc003ysq.2 | CCDC26 | | UCSC_knowngene | 34.0223 | 3260.5908 | 42.260292 | 0.0027339 |
| 3 | uc002ehu.1 | PNAS-108 | | UCSC_knowngene | 20.46965 | 1096.482 | 39.467697 | 0.000351 |
| 4 | BX952962 |
lincRNA-OBFC2A-2 | | lincRNA | 17.117323 | 788.3796 | 35.642254 | 3.22E-06 |
| 5 |
ENST00000435695 | AC002454.1 | CDK6 | Ensembl | 127.27789 | 9728.407 | 34.028286 | 0.004122 |
| 6 | NR_024259 | LOC728606 | | RefSeq_NR | 24.271034 | 1178.9098 | 33.306793 | 0.000145 |
| 7 |
ENST00000417463 | KB-1592A4.14 | | Ensembl | 32.294987 | 4614.054 | 30.944315 | 0.0318946 |
| 8 | BC035649 | | | misc_RNA | 24.781305 | 1576.078 | 29.303568 | 0.0128913 |
| 9 | AA635039 |
lincRNA-TARBP1-2 | | lincRNA | 22.896029 | 899.75323 | 28.649506 | 0.0000286 |
| 10 | BC022435 | | C9orf41 | misc_RNA | 35.91673 | 3247.7634 | 27.618906 | 0.0135179 |
| 11 |
ENST00000457457 | AC016735.1 | | Ensembl | 37.48748 | 1587.2334 | 26.119883 | 0.013067 |
| 12 | AK127507 | | | misc_RNA | 16.645311 | 605.7293 | 21.048286 | 0.0024883 |
| 13 | uc011mle.1 | DM004476 | | UCSC_knowngene | 26.679949 | 918.6805 | 21.00233 | 0.000982 |
| 14 | uc002ufj.3 | KLHL23 |
PHOSPHO2-KLHL23 | UCSC_knowngene | 133.91594 | 3545.8738 | 19.016815 | 0.0000236 |
| 15 |
ENST00000509500 | U85056.1 | | Ensembl | 24.437654 | 1016.5418 | 18.854773 | 0.0098472 |
| 16 |
ENST00000458624 | AC007009.1 | ICA1 | Ensembl | 35.76904 | 913.7956 | 18.405573 | 0.0051383 |
| 17 | uc001guz.2 | EF413001 | | UCSC_knowngene | 100.33683 | 2223.7722 | 17.687159 | 0.000444 |
| 18 | uc010wia.1 | AK027091 | | UCSC_knowngene | 443.38882 | 9681.8 | 15.688867 | 0.0075263 |
| 19 | AY216265 | | ETS2 | NRED | 547.44006 | 11685.214 | 15.45025 | 0.0000294 |
| 20 | AK000053 | | CLCC1 | RNAdb | 62.11948 | 1246.1871 | 14.599653 | 0.00033 |
| 21 | uc002nrb.1 | AK131472 | | UCSC_knowngene | 33.197628 | 684.31696 | 13.896548 | 0.0102436 |
| 22 | AK123638 | | COPG2 | misc_RNA | 41.249954 | 1012.7471 | 13.175612 | 0.0063138 |
| 23 | uc001sqo.2 | CR602022 | | UCSC_knowngene | 204.72939 | 3436.1982 | 13.079311 | 0.0012676 |
| 24 | AK094803 | | | misc_RNA | 20.941412 | 405.46933 | 12.166367 | 0.0237685 |
| 25 |
ENST00000453838 | KRT18P28 | | Ensembl | 69.50002 | 1220.4552 | 11.720998 | 0.0187785 |
| 26 | BC035154 | | | misc_RNA | 143.23755 | 2030.2422 | 11.628016 | 0.000541 |
| 27 | AK097063 | | ZNF850 | misc_RNA | 13.838933 | 242.19637 | 11.516952 | 0.0014993 |
| 28 | AK123765 | | | misc_RNA | 61.605896 | 854.0999 | 11.402978 | 0.000336 |
| 29 | uc002uwa.2 | AOX2 | | UCSC_knowngene | 33.613018 | 1187.9089 | 10.597581 | 0.0464274 |
| 30 | AK123649 | | | misc_RNA | 31.297625 | 381.76712 | 10.589622 | 0.0417903 |
| 31 | AK123319 | | NETO1 | misc_RNA | 38.455334 | 891.8227 | 10.473429 | 0.0190694 |
| 32 | uc002ndq.1 | CR605427 | HSH2D | UCSC_knowngene | 23.040321 | 295.48752 | 10.46813 | 0.000156 |
| 33 |
ENST00000452408 | KRT18P1 | | Ensembl | 19.09376 | 262.50006 | 10.44417 | 0.0088213 |
| 34 | NR_001458 | MIR155HG | | RefSeq_NR | 1211.0641 | 16439.678 | 10.089632 | 0.0018226 |
 | Table IIIlncRNA downregulated >10-fold in
the pediatric acute myeloid leukemia compared with normal
control. |
Table III
lncRNA downregulated >10-fold in
the pediatric acute myeloid leukemia compared with normal
control.
| Seqname | Gene symbol | Associated gene
name | Source | [C](raw) | [L](raw) | FC (abs) | P-value |
|---|
| 1 | NR_002712 | CXCR2P1 | | RefSeq_NR | 6307.8667 | 94.28683 | 95.082214 | 0.0001815 |
| 2 |
ENST00000340794 | MAGEA13P | Ensembl | 655.6455 | 33.92397 | 54.03028 | 0.0099015 | |
| 3 | AK055252 | | C15orf38-AP3S2 | misc_RNA | 522.1257 | 18.751987 | 53.95123 | 0.0019818 |
| 4 | CR592555 | | DHFR | RNAdb | 52353.11 | 2405.9045 | 28.411339 | 0.0017334 |
| 5 | NR_024420 | LOC389634 | RefSeq_NR | 2986.3733 | 443.83182 | 27.338875 | 0.0243679 | |
| 6 |
ENST00000511213 | RP11-362F19.1 | Ensembl | 390.45132 | 20.542099 | 26.359552 | 0.0014037 | |
| 7 | NR_003191 | GGTA1 | | RefSeq_NR | 5235.906 | 306.32895 | 23.206347 | 6.774E-05 |
| 8 | BC035072 | | | misc_RNA | 264.2821 | 13.62112 | 22.975542 | 0.0019559 |
| 9 | uc003iir.1 | AK124399 | | UCSC_knowngene | 584.21124 | 38.04964 | 20.08043 | 0.000707 |
| 10 | uc003yse.1 | LOC727677 | POU5F1B | UCSC_knowngene | 210.80397 | 12.041702 | 19.793795 | 0.0070152 |
| 11 | AK128410 | | | misc_RNA | 9238.451 | 1925.9315 | 15.957043 | 0.0250141 |
| 12 | M18204 | TEA ncRNAs | lncRNAdb | 3398.365 | 756.7373 | 14.61877 | 0.0316519 | |
| 13 | AL832164 | | RORA | RNAdb | 10414.999 | 2412.2832 | 13.646528 | 0.0276052 |
| 14 | AK125371 | | | misc_RNA | 121.759 | 16.427212 | 13.455201 | 0.0147761 |
| 15 |
ENST00000417930 | AC092580.4 | Ensembl | 1074.5836 | 187.77055 | 13.370509 | 0.0223116 | |
| 16 |
ENST00000406090 | AC009494.3 | Ensembl | 111.52717 | 17.403128 | 13.112155 | 0.0121482 | |
| 17 | AK095282 | | | misc_RNA | 581.82513 | 140.42796 | 12.77245 | 0.0465396 |
| 18 | NR_027767 | TNIK | TNIK | RefSeq_NR | 1542.7079 | 210.12633 | 12.649625 | 0.0047896 |
| 19 | uc001wby.2 | TCRA | | UCSC_knowngene | 229.08073 | 36.00486 | 12.01228 | 0.011543 |
| 20 |
ENST00000425002 | AC079325.6 | Ensembl | 150.33525 | 17.702103 | 11.682941 | 0.0063381 | |
| 21 |
chr1:22577463-22595288+ | lincRNA-ZBTB40 | lincRNA | 591.5555 | 134.24306 | 11.504897 | 0.0407799 | |
| 22 | AK092053 | | | misc_RNA | 590.2487 | 103.68743 | 11.401361 | 0.0197635 |
| 23 |
chr8:128772715-128786539- | lincRNA-MYC-6 | lincRNA | 298.0089 | 32.098663 | 11.27972 | 0.000242 | |
| 24 |
ENST00000447519 | AP001189.4 | LRRC32 | Ensembl | 575.1246 | 64.24173 | 10.889111 | 0.0001103 |
| 25 | uc001rpo.1 | AX747844 | | UCSC_knowngene | 3992.0068 | 857.19037 | 10.817992 | 0.017896 |
| 26 |
ENST00000419624 | AC005392.13 | Ensembl | 207.65968 | 24.788124 | 10.610993 | 0.0003014 | |
| 27 |
ENST00000464405 | TRBC1 | | Ensembl | 469.0503 | 109.8695 | 10.379145 | 0.0380851 |
| 28 | AK023096 | | PCSK5 | NRED | 1533.9199 | 419.10873 | 10.367031 | 0.0380955 |
| 29 | AK097103 | | | misc_RNA | 2026.3512 | 261.61304 | 10.004671 | 0.0020644 |
Altered lncRNA-related gene pathways in
pediatric AML
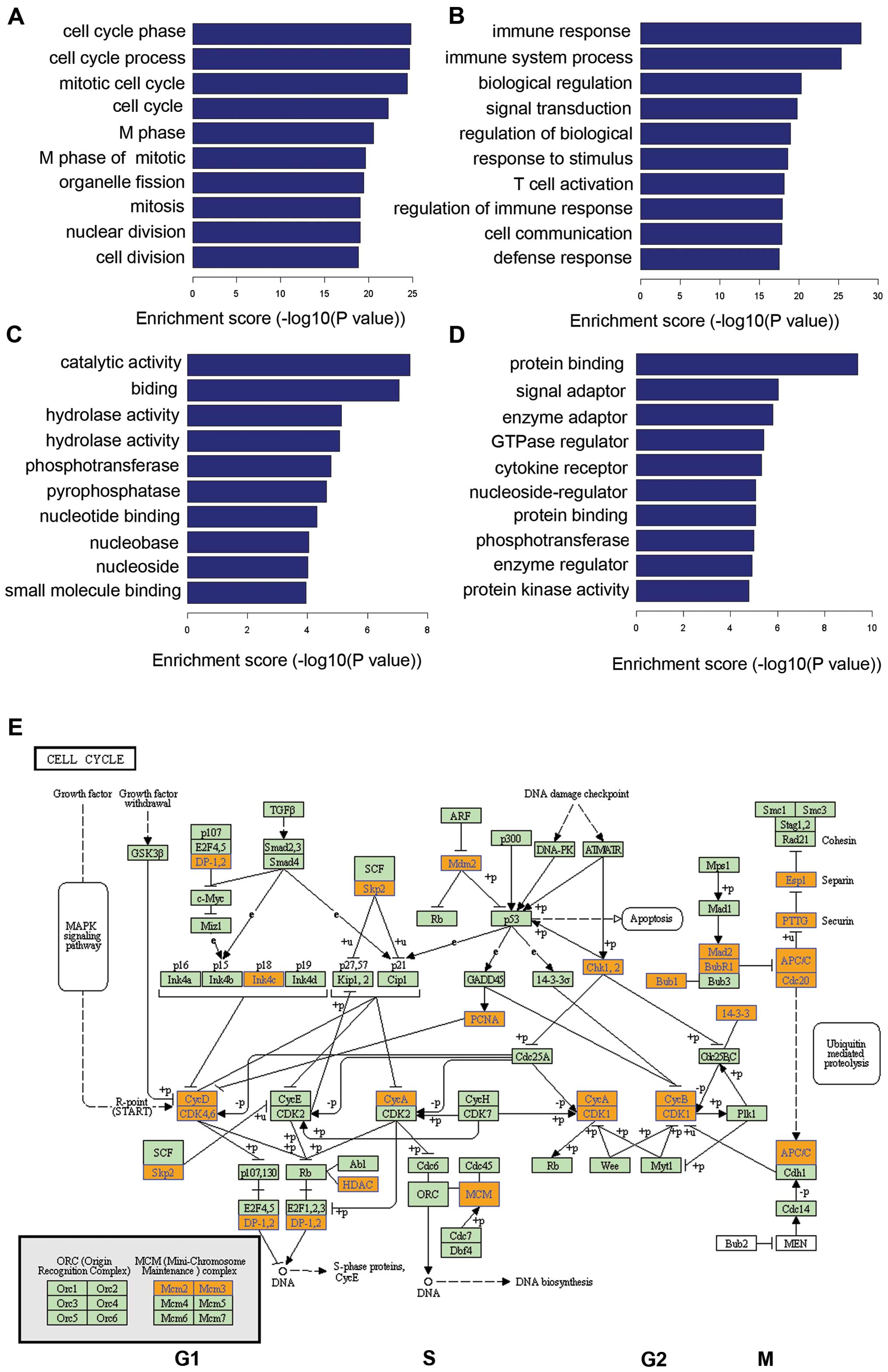
Ontological pathway enrichment analysis was
performed on differentially expressed lncRNA and mRNA, and a
biological process enrichment analysis was performed using the
DAVID tool to gain insights into their functions (GEO accession no.
GSE64975). The most enriched GO targeted by upregulated and
downregulated transcripts were involved in cell cycle regulation,
immune response, and immune system process (Fig. 3A and B). The most significant
molecular function (MF) enrichment scores are shown in Fig. 3C and D. KEGG annotations of the most
enriched pathways are shown in Fig.
3E, and KEGG pathway annotations of the most enriched pathways
were cell cycle regulation with a p-value
<7.22764E−10. Cell cycle pathway proteins included
ANAPC11, ANAPC13, ANAPC7, BUB1,
BUB1B, CCNA2, CCNB1 and CCNB2.
lncRNA/mRNA co-expression network in
pediatric AML
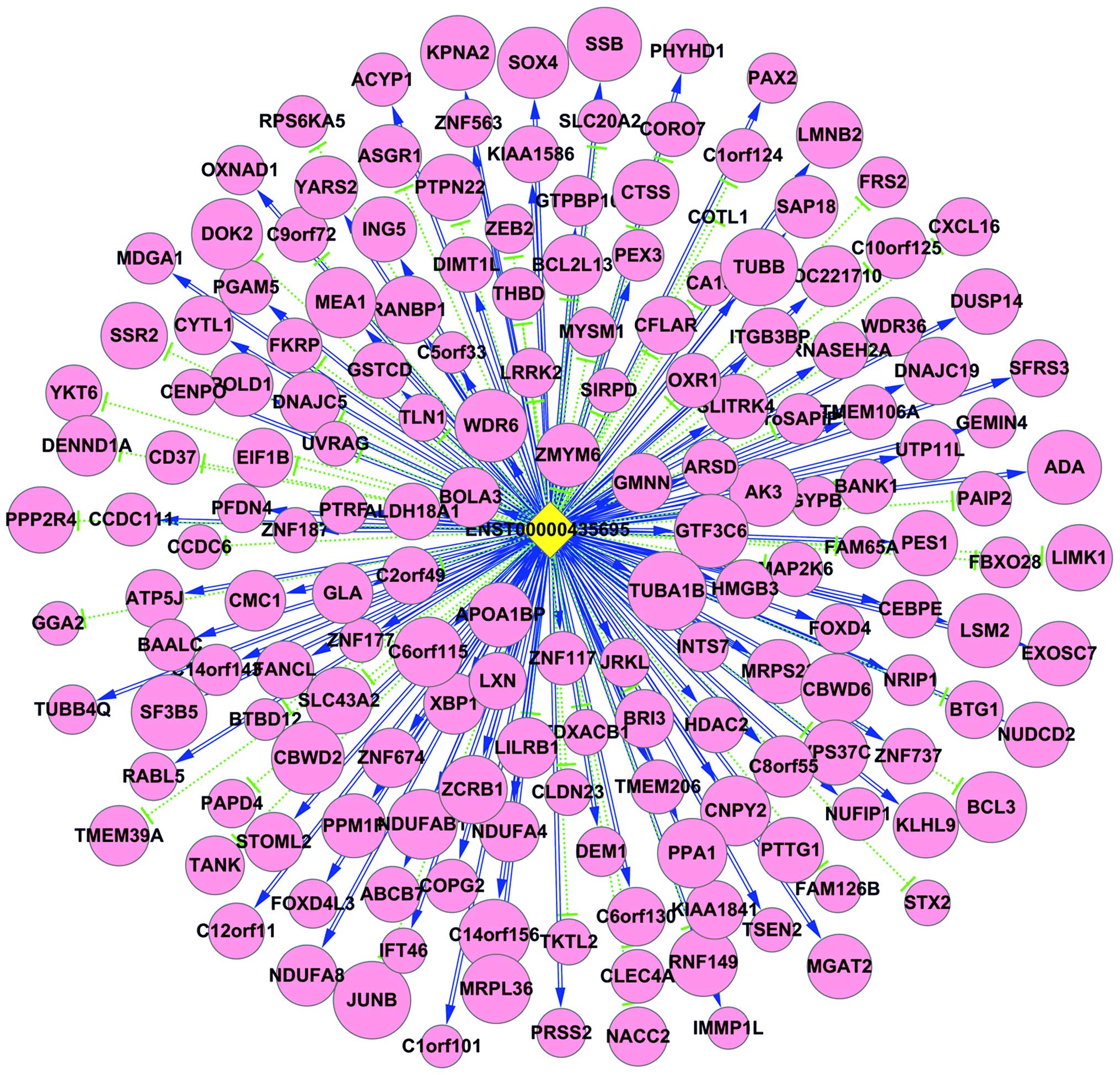
Next, we constructed a coding and non-coding gene
expression network based on the correlation analysis between
differentially expressed lncRNAs and mRNAs. Pearson's correlation
coefficient analysis was performed using a coefficient no less than
0.98 to construct the network (Fig.
4). The expression network indicated that one mRNA or lncRNA
may correlate with one to tens of lncRNAs. The co-expression
network may suggest the inter-regulation of lncRNAs and mRNAs
during AML development.
Confirmation of dysregulated lncRNAs in
pediatric AML versus normal control samples
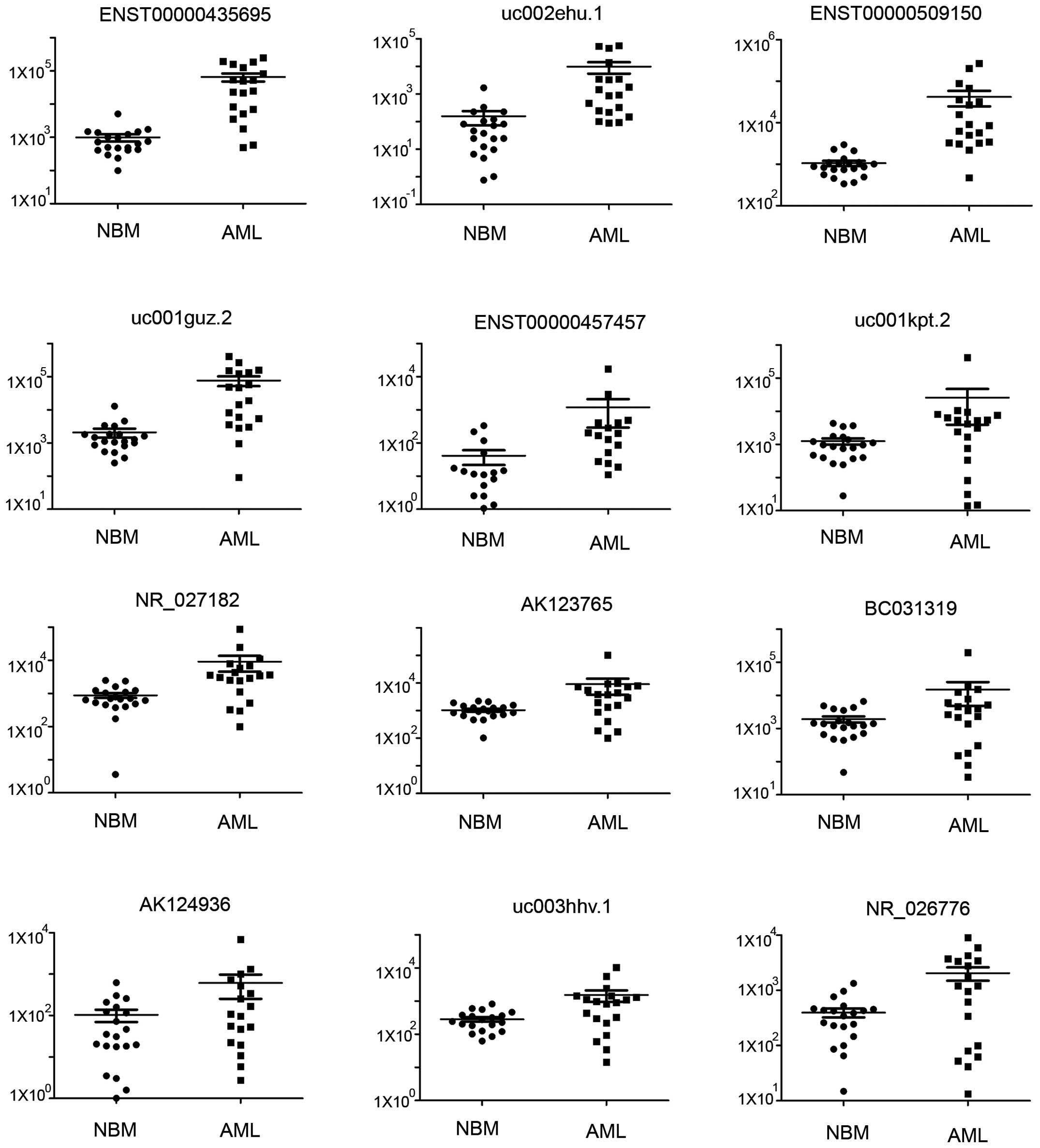
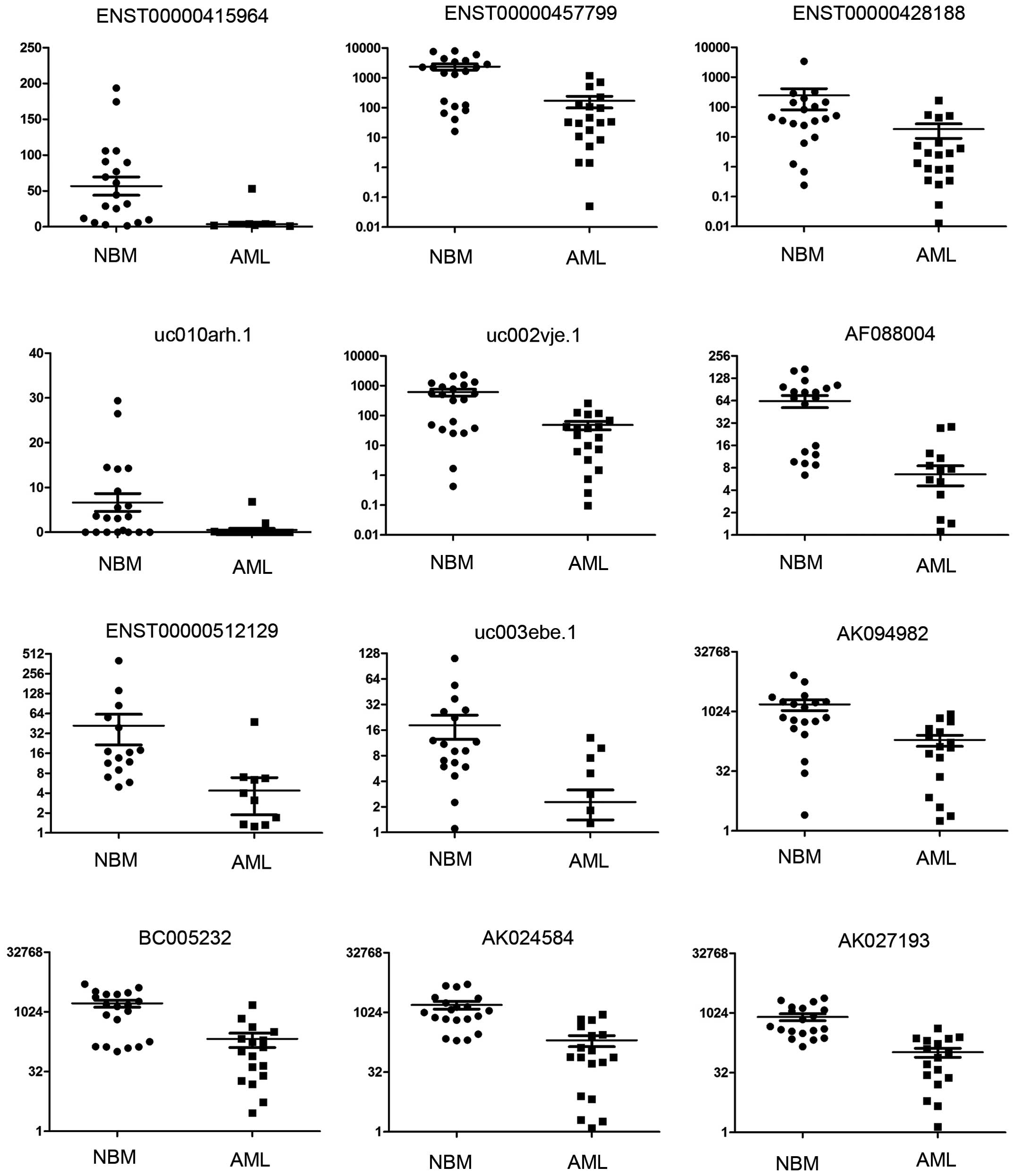
To confirm the microarray data, we selected 97
dysregulated lncRNAs from the micro-array analysis of 22 pediatric
AML samples and 20 control samples using qRT-PCR. Our data revealed
that the lncRNA expression profile in pediatric AML was
significantly different from normal controls (Fig. 5A). A total of 20 genes were
successfully clustered using lncRNA samples (Fig. 5B). A total of 24 lncRNAs were
confirmed to be dysregulated in pediatric AML (Table IV), and detailed expressions of
each upregulated and downregulated lncRNA in pediatric AML are
shown in Figs. 6 and 7. The most upregulated lncRNA in pediatric
AML is ENST00000435695 and the most downregulated lncRNA in
pediatric AML is ENST00000415964.
 | Table IVlncRNAs most dysregulated in the
pediatric acute myeloid leukemia compared with normal control. |
Table IV
lncRNAs most dysregulated in the
pediatric acute myeloid leukemia compared with normal control.
| Seqname | N | AML | FC | P-value |
|---|
| 1 |
ENST00000435695 | 991.3334 | 65836.24647 | 66.41181 | 0.001962 |
| 2 | uc002ehu.1 | 157.1266 | 9872.330406 | 62.83042 | 0.040465 |
| 3 |
ENST00000509150 | 1056.795 | 41764.2482 | 39.51973 | 0.026976 |
| 4 | uc001guz.2 | 2078.731 | 76903.56747 | 36.99544 | 0.00817 |
| 5 |
ENST00000457457 | 41.08492 | 1197.166609 | 29.13883 | 0.021802 |
| 6 | uc001kpt.2 | 1255.126 | 25614.56252 | 20.40796 | 0.027621 |
| 7 | NR_027182 | 878.2717 | 9092.321414 | 10.35252 | 0.008923 |
| 8 | AK123765 | 1042.844 | 9066.866062 | 8.694369 | 0.014631 |
| 9 | BC031319 | 1911.99 | 15009.11256 | 7.849995 | 0.021456 |
| 10 | AK124936 | 104.2268 | 612.3396304 | 5.87507 | 0.017449 |
| 11 | uc003hhv.1 | 283.4595 | 1530.759093 | 5.400274 | 0.043802 |
| 12 | NR_026776 | 394.2164 | 2050.126185 | 5.20051 | 0.008692 |
| 13 | AK027193 | 816.4939 | 105.3354471 | 0.129009 | 0.000302 |
| 14 | AK024584 | 1589.484 | 203.7112857 | 0.128162 | 0.000796 |
| 15 | BC005232 | 1683.387 | 213.9596056 | 0.127101 | 0.000347 |
| 16 | AK094982 | 1550.97 | 196.5648444 | 0.126737 | 0.009100 |
| 17 | uc003ebe.1 | 18.21419 | 2.279349604 | 0.125141 | 0.013112 |
| 18 |
ENST00000512129 | 41.8703 | 4.359070151 | 0.104109 | 0.008417 |
| 19 | AF088004 | 63.27258 | 6.537745743 | 0.103327 | 0.000117 |
| 20 | uc002vje.1 | 610.3662 | 48.49298993 | 0.079449 | 0.001854 |
| 21 | uc010arh.1 | 6.662799 | 0.504782 | 0.075761 | 0.006096 |
| 22 |
ENST00000428188 | 247.9221 | 18.21257952 | 0.073461 | 0.001850 |
| 23 |
ENST00000457799 | 2389.248 | 169.9657438 | 0.071138 | 0.000823 |
| 24 |
ENST00000415964 | 56.81532 | 3.721491039 | 0.065502 | 0.000550 |
Discussion
Our present study profiled differentially expressed
lncRNAs and mRNAs in pediatric AMLs. We demonstrated for the first
time the expression profiles of human lncRNAs in pediatric AML by
microarray; and identified a collection of aberrantly expressed
lncRNAs in pediatric AML compared to normal controls. It is likely
that these dysregulated lncRNAs play a key or partial role in the
development and/or progression of pediatric AML. Previous
genome-wide profiling studies revealed that many transcribed
non-coding ultra conserved regions exhibit distinct profiles in
various human cancers. For example, a genome-wide RNA sequencing
(RNAseq) analysis evaluated the differential expressions of lncRNAs
in a cohort of 102 prostate cancer versus benign samples (27); and identified 121 unannotated
transcripts that could accurately discriminate benign, localized
and metastatic samples. Other lncRNA expression profile studies
were conducted in >100 paired esophageal squamous cell carcinoma
and normal samples (28), 5 pairs
of liver cancer and normal tissues (29), and 6 pairs of renal clear cell
carcinoma and corresponding normal tissues (26); which revealed large numbers of
lncRNAs that were significantly dysregulated in cancer tissues.
Recent studies have started to reveal the importance of lncRNAs in
leukemia tumorigenesis. For example, lncRNA H19 was highly
expressed in Bcr-Abl-transformed cell lines and primary cells
derived from patients in a Bcr-Abl kinase-dependent manner
(23). lncRNA MONC and MIR100HG
were highly expressed in acute megakaryoblastic leukemia blasts
(24). However, it remains to be
determined how these lncRNAs participate and contribute to leukemia
development or progression. In this study, we found that some of
these differentially expressed lncRNAs regulated cell cycle
progression and immunosystem functions. Future study is required to
investigate whether manipulating lncRNA expressions could control
AML progression and be used as a therapeutic target to control
AML.
Our present study profiled and identified a group of
dysregulated lncRNAs and mRNAs in bone marrow samples obtained from
pediatric AML patients versus normal controls. We also verified 97
lncRNAs in >20 AML and normal control samples; and found 24
dysregulated lncRNAs in pediatric AML. These lncRNAs could regulate
cell cycle progression and immunoresponses that could be associated
with AML development or progression. Further study is required to
determine whether these lncRNAs may serve as new therapeutic
targets and diagnostic markers for pediatric AML.
Acknowledgments
The present study was supported in part by grants
from the National '12th Five-Year' Major Science and Technology
fund (#2011ZX09302-007-01), the National Natural Science Foundation
of China (nos. 81100371, 81370627, 81300423 and 81272143), the Key
Medical Subjects of Jiangsu Province (#XK201120), the Key
Laboratory of Suzhou (#SZS201108 and #SZS201307), the Suzhou
Science and Technology Development Planning 2013 Projects
(#SyS201352), the Suzhou City youth Science and Technology Projects
(#KJXW2012021), and the Technological Special Project for
'Significant New Drugs Creation' (#2012ZX09103301-040). The funders
had no role in study design, data collection and analysis, decision
to publish, or preparation of the manuscript.
References
|
1
|
Appelbaum FR, Baer MR, Carabasi MH, Coutre
SE, Erba HP, Estey E, Glenn MJ, Kraut EH, Maslak P, Millenson M, et
al National Comprehensive Cancer Network: NCCN practice guidelines
for acute myelogenous leukemia. Oncology (Williston Park).
14:53–61. 2000.
|
|
2
|
Sanz GF, Sanz MA, Vallespí T, Cañizo MC,
Torrabadella M, García S, Irriguible D and San Miguel JF: Two
regression models and a scoring system for predicting survival and
planning treatment in myelodysplastic syndromes: A multivariate
analysis of prognostic factors in 370 patients. Blood. 74:395–408.
1989.PubMed/NCBI
|
|
3
|
Hope KJ, Jin L and Dick JE: Human acute
myeloid leukemia stem cells. Arch Med Res. 34:507–514. 2003.
View Article : Google Scholar
|
|
4
|
Hasle H, Niemeyer CM, Chessells JM,
Baumann I, Bennett JM, Kerndrup G and Head DR: A pediatric approach
to the WHO classification of myelodysplastic and myeloproliferative
diseases. Leukemia. 17:277–282. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Song X, Wang X, Arai S and Kurokawa R:
Promoter-associated noncoding RNA from the CCND1 promoter. Methods
Mol Biol. 809:609–622. 2012. View Article : Google Scholar
|
|
6
|
Zhang X, Lian Z, Padden C, Gerstein MB,
Rozowsky J, Snyder M, Gingeras TR, Kapranov P, Weissman SM and
Newburger PE: A myelopoiesis-associated regulatory intergenic
noncoding RNA transcript within the human HOXA cluster. Blood.
113:2526–2534. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rinn JL: lncRNAs: Linking RNA to
chromatin. Cold Spring Harb Perspect Biol. 6(6)2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rinn JL and Chang HY: Genome regulation by
long noncoding RNAs. Annu Rev Biochem. 81:145–166. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen Z, Jia S, Li D, Cai J, Tu J, Geng B,
Guan Y, Cui Q and Yang J: Silencing of long noncoding RNA AK139328
attenuates ischemia/reperfusion injury in mouse livers. PLoS One.
8:e808172013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yin Z, Guan D, Fan Q, Su J, Zheng W, Ma W
and Ke C: lncRNA expression signatures in response to enterovirus
71 infection. Biochem Biophys Res Commun. 430:629–633. 2013.
View Article : Google Scholar
|
|
11
|
Kung JT, Colognori D and Lee JT: Long
noncoding RNAs: Past, present, and future. Genetics. 193:651–669.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fu X, Ravindranath L, Tran N, Petrovics G
and Srivastava S: Regulation of apoptosis by a prostate-specific
and prostate cancer-associated noncoding gene, PCGEM1. DNA Cell
Biol. 25:135–141. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gutschner T, Hämmerle M and Diederichs S:
MALAT1 - a paradigm for long noncoding RNA function in cancer. J
Mol Med Berl. 91:791–801. 2013. View Article : Google Scholar
|
|
14
|
Schmidt LH, Spieker T, Koschmieder S,
Schäffers S, Humberg J, Jungen D, Bulk E, Hascher A, Wittmer D,
Marra A, et al: The long noncoding MALAT-1 RNA indicates a poor
prognosis in non-small cell lung cancer and induces migration and
tumor growth. J Thorac Oncol. 6:1984–1992. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li G, Zhang H, Wan X, Yang X, Zhu C, Wang
A, He L, Miao R, Chen S and Zhao H: Long noncoding RNA plays a key
role in metastasis and prognosis of hepatocellular carcinoma.
Biomed Res Int. 2014(780521)2014.
|
|
16
|
He Y, Meng XM, Huang C, Wu BM, Zhang L, Lv
XW and Li J: Long noncoding RNAs: Novel insights into hepatocelluar
carcinoma. Cancer Lett. 344:20–27. 2014. View Article : Google Scholar
|
|
17
|
Lai MC, Yang Z, Zhou L, Zhu QQ, Xie HY,
Zhang F, Wu LM, Chen LM and Zheng SS: Long non-coding RNA MALAT-1
overexpression predicts tumor recurrence of hepatocellular
carcinoma after liver transplantation. Med Oncol. 29:1810–1816.
2012. View Article : Google Scholar
|
|
18
|
Tsang WP, Ng EK, Ng SS, Jin H, Yu J, Sung
JJ and Kwok TT: Oncofetal H19-derived miR-675 regulates tumor
suppressor RB in human colorectal cancer. Carcinogenesis.
31:350–358. 2010. View Article : Google Scholar
|
|
19
|
Ariel I, Miao HQ, Ji XR, Schneider T, Roll
D, de Groot N, Hochberg A and Ayesh S: Imprinted H19 oncofetal RNA
is a candidate tumour marker for hepatocellular carcinoma. Mol
Pathol. 51:21–25. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lottin S, Vercoutter-Edouart AS,
Adriaenssens E, Czeszak X, Lemoine J, Roudbaraki M, Coll J,
Hondermarck H, Dugimont T and Curgy JJ: Thioredoxin
post-transcriptional regulation by H19 provides a new function to
mRNA-like non-coding RNA. Oncogene. 21:1625–1631. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Luo M, Li Z, Wang W, Zeng Y, Liu Z and Qiu
J: Upregulated H19 contributes to bladder cancer cell proliferation
by regulating ID2 expression. FEBS J. 280:1709–1716. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Luo M, Li Z, Wang W, Zeng Y, Liu Z and Qiu
J: Long non-coding RNA H19 increases bladder cancer metastasis by
associating with EZH2 and inhibiting E-cadherin expression. Cancer
Lett. 333:213–221. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Guo G, Kang Q, Chen Q, Chen Z, Wang J, Tan
L and Chen JL: High expression of long non-coding RNA H19 is
required for efficient tumorigenesis induced by Bcr-Abl oncogene.
FEBS Lett. 588:1780–1786. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Emmrich S, Streltsov A, Schmidt F,
Thangapandi VR, Reinhardt D and Klusmann JH: LincRNAs MONC and
MIR100HG act as oncogenes in acute megakaryoblastic leukemia. Mol
Cancer. 13(171)2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bennett JM, Catovsky D, Daniel MT,
Flandrin G, Galton DA, Gralnick HR and Sultan C: Proposals for the
classification of the acute leukaemias. French-American-British
(FAB) co-operative group. Br J Haematol. 33:451–458. 1976.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yu G, Yao W, Wang J, Ma X, Xiao W, Li H,
Xia D, yang y, Deng K, Xiao H, et al: LncRNAs expression signatures
of renal clear cell carcinoma revealed by microarray. PLoS One.
7:e423772012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Prensner JR and Chinnaiyan AM: The
emergence of lncRNAs in cancer biology. Cancer Discov. 1:391–407.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li J, Chen Z, Tian L, Zhou C, He MY, Gao
Y, Wang S, Zhou F, Shi S, Feng X, et al: LncRNA profile study
reveals a three-lncRNA signature associated with the survival of
patients with oesophageal squamous cell carcinoma. Gut.
63:1700–1710. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yang F, Zhang L, Huo XS, Yuan JH, Xu D,
Yuan SX, Zhu N, Zhou WP, Yang GS, Wang YZ, et al: Long noncoding
RNA high expression in hepatocellular carcinoma facilitates tumor
growth through enhancer of zeste homolog 2 in humans. Hepatology.
54:1679–1689. 2011. View Article : Google Scholar : PubMed/NCBI
|