Introduction
Lung cancer is the leading cause of cancer-related
deaths among both men and women, with an estimated 228,190 cases of
lung cancer in the United States and 159,480 deaths from the
disease in 2013 (1). The majority
of patients are diagnosed at an advanced stage when curative
treatment options are limited (2).
The overall five-year survival rate is only 16% (3), and the prognosis has remained
unchanged for the last three decades.
Digitoxin (Dig) is an FDA approved drug for the
treatment of cardiac disease. The therapeutic plasma levels of
digitoxin is considered to be in the ranges of 13–33 nM (4,5) and up
to 46 nM (6). Dig at micromolar
concentrations inhibits the Na/K-ATPase, but it is believed that at
nanomolar concentrations, it activates the
Na+/K+-ATPase signalosome to transmit
intracellular signals, leading to anticancer effects (7). Paclitaxel (PX) remains a first-line
treatment for advanced NSCLC in the United States (8). PX at therapeutic concentrations acts
as a microtubule stabilizer, inducing cell cycle arrest in the
G2/M phase; its dose-limiting toxicities are neutropenia
and peripheral neuropathy (9).
Resistance to PX is associated with expression of multidrug
resistance efflux pumps and tumor hypoxia (10). In patients, relevant plasma
concentrations of PX are between 80–280 nM (11), but peak concentration after
intravenous infusion can reach 10 µM (12). In the case of hydroxyurea (HU),
plasma levels of 1 mM can be achieved and maintained in patients
(13). These concentrations are
high enough to inhibit in vitro the proliferation of lung
cancer cells, but as single drugs neither PX nor HU have been
successful.
Lung cancers display intratumoral heterogeneity
(14,15). It is known that complex crosstalk
exists between cancer cells and the stromal microenvironment via
the secretion of a variety of growth factors (3). Other environmental factors such as
hypoxia, blood flow, pH have profound effects on this interaction
and contribute to the intratumoral heterogeneity of lung cancer.
Different tumor microenvironments are characterized by different
cell populations with varying rates of proliferation and varying
degrees of selective pressures such as oxygen, acidity, and tumor
growth factors (16). Therefore, in
addition to testing anticancer drugs growing under routine culture
conditions [media supplemented with fetal bovine serum (FBS)]
studying the effect of these compounds in serum starved cells that
in part mimic the behaviour of low proliferating cells, such as
cancer stem-like cells, may offer additional information on the
chemosensitivity of cancers in general. In this study we
characterized the anticancer activity of clinically relevant
concentrations of Dig and its synthetic analog MonoD on H460 lung
cancer cells growing under different culture conditions. We also
evaluated the effect of these cardiac glycosides (CGs) in
combination with clinically relevant concentrations of paclitaxel
and hydroxyurea.
Materials and methods
Drugs
Dig and MonoD (β-D-digitoxose) were stored as stock
solution (10 mM) in DMSO in glass containers. Dig was obtained from
Sigma-Aldrich (St. Louis, MO, USA). MonoD was synthesized using a
methodology previously described (17). Final dilutions were freshly prepared
in culture media before use. All control treatments were
supplemented with the highest concentration (~0.001%) of DMSO used
in drug treatment. HU, PX and colchicine were purchased from
Sigma-Aldrich. HU and colchicine were prepared as stock solution
(500 and 10 mM, respectively) in distilled sterile water and stored
in aliquots at −20°C. PX was prepared as stock solution of 1 mM in
DMSO and stored in aliquots at −20°C.
Cell culture
The human lung epithelial cancer cell line NCI-H460
was obtained from American Type Culture Collection (Manassas, VA,
USA). This cell line is considered highly resistant to chemotherapy
(1). NCI-H460 cells were cultured
in complete media (CM, RPMI-1640 supplemented with 5% FbS, 2 mM
L-glutamine, 100 U/ml penicillin, and 100 mg/ml streptomycin)
(18). All cells were cultured in a
5% CO2 environment at 37°C.
Short-term antiproliferative effect of
Dig and MonoD (MTT assay)
Cells (~2,000 cells/well) were plated in 96-well
cell-culture microplates (Costar, USA) and incubated overnight in
cell culture medium to allow them to adhere. Cells were then
exposed to the appropriate concentration of drug or vehicle for
24–72 h. Depending on the culture conditions, drugs were added in
either CM or serum-free media (SFM, same as CM but without FbS).
Cell viability was evaluated by the MTT (Sigma-Aldrich) assay. The
absorbance of solubilized formazan was read at 570 nm using Gen 5
2.0 All-In-One microplate reader (bio-Tek Instruments Inc.). In all
cases, the highest concentration of DMSO was used in the control
and this concentration was maintained at or below 0.001% (v/v).
This DMSO concentration did not show any significant
anti-proliferative effect on the cell line in a short-term
assay.
Colony-forming assay
Colony-forming assay was performed according to
Rafehi et al (19). Briefly,
200 cells/well were plated in 6-well plates and allowed to adhere
overnight. Cells were then treated with drugs in CM at the
indicated concentration or with vehicle alone for 72 h. After drug
exposure cells were incubated with complete media for 7–10 days
(media was changed every 72 h). Then cells were fixed with 3.7%
formaldehyde for 15 min, stained with 0.01% crystal violet and
photographed. Colonies were counted using ImageJ software (ImageJ
v.1.48, http://imagej.nih.gov/ij/).
Statistic analysis
The drug concentrations inhibiting cell growth by
50% (IC50) were determined by interpolation from the
dose-response curves using a sigmoidal logistic 3 parameters
equation. Curve fitting was performed with SigmaPlot (v.11.0)
Software. Each point represents the mean ± standard error (Se) of
triplicate or quadruplicate wells (see figures for details).
Comparison between groups has been done by ANOVA.
Combination index assay
The combination index (CI) was calculated to
investigate the combined effect of Dig or MonoD and PX. The CI was
calculated using the following formula (6): CI = [IC50 (CG +
PX)/IC50 (CG alone)] + [IC50 (PX +
CG)/IC50 (PX alone)]. Where CG = Dig or MonoD and PX =
paclitaxel. CI >1 was defined as an antagonistic effect, CI =1
as an additive ef fect, and CI <1 as a synergistic effect
(20).
Results
Serum potentiates the antiproliferative
effect of Dig but not of MonoD
Cells were seeded at 2,000 cells/well and allowed to
adhere overnight, and treated with increasing doses of Dig or MonoD
(0, 1, 5, 10, 25, 50, 100 or 200 nM) in CM for 24, 48 or 72 h. Cell
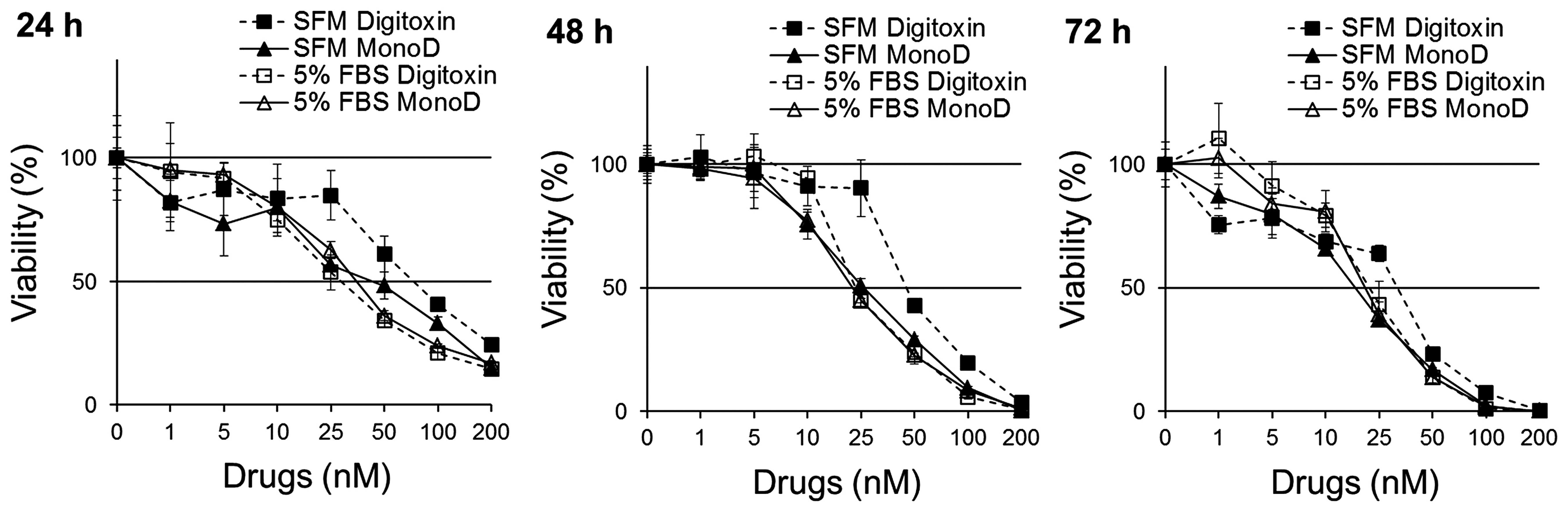
viability was determined using MTT assay. Fig. 1 shows that cells incubated with Dig
in SFM were less sensitive (IC50 at 24 h, 91.3±11.4) to
this drug when compared to cells incubated in CM (IC50
at 24 h, 29.4±2.1). The effect was maximal after 24 h and observed
for up to 72 h. In contrast, cells treated with MonoD showed
similar sensitivity to this drugs in the absence (IC50
at 24 h, 29.4±2.1) or in the presence (IC50 at 24 h,
37.3±4.5) of 5% FbS. Table I shows
the IC50 for 24, 48 and 72 h and clearly indicates that
the potency of Dig but not MonoD are potentiated when incubated in
CM.
 | Table IIC50 for digitoxin and
MonoD at 24, 48 or 72 h. |
Table I
IC50 for digitoxin and
MonoD at 24, 48 or 72 h.
| H460 cells | IC50
(mean ± Se)
|
|---|
24 h
| 48 h
| 72 h
|
|---|
| SFM | 5% FbS | SFM | 5% FbS | SFM | 5% FbS |
|---|
| Digitoxin | 91.3±11.4 | 29.4±2.1 | 49.2±2.6 | 24.7±1.3 | 36.4±3.1 | 19.4±1.7 |
| MonoD | 49.1±11.2 | 37.3±4.5 | 24.9±1.2 | 22.3±0.9 | 17.6±0.9 | 19.6±1.1 |
Short-term serum starvation attenuates
the antiproliferative effects of both Dig and MonoD
Cells seeded at 2,000 cells/well were allowed to
adhere overnight and later starved for 24 h in SFM. This procedure
is widely used to synchronize and reversibly arrest cells at the
G0/G1 transition of the cell cycle (21,22).
After starvation, cells were treated with Dig or MonoD (0, 1, 5,
10, 25, 50, 100 or 200 nM) in either SFM or CM for 24, 48 or 72 h.
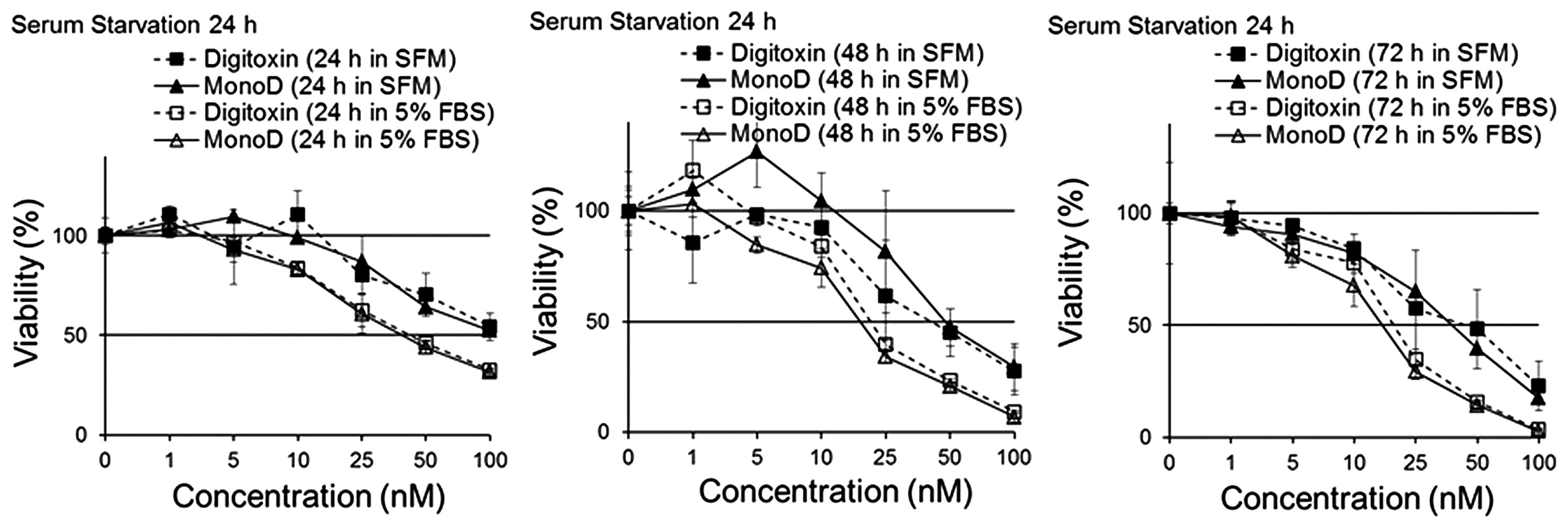
Cell viability was determined by the MTT assay. Fig. 2 shows that serum starvation for 24 h
attenuated the effect of both Dig and MonoD, suggesting that slow
proliferating cells are less sensitive to both drugs. Table II shows the IC50 for 24,
48 and 72 h following 24 h of serum starvation.
 | Table IIIC50 for digitoxin and
MonoD at 24, 48 or 72 h after 24 h serum starvation. |
Table II
IC50 for digitoxin and
MonoD at 24, 48 or 72 h after 24 h serum starvation.
| H460 cells | IC50
(mean ± Se)
|
|---|
24 h
| 48 h
| 72 h
|
|---|
| SFM | 5% FbS | SFM | 5% FbS | SFM | 5% FbS |
|---|
| Digitoxin | 100.41±28.3 | 41.90±3.0 | 47.32±9.4 | 27.98±7.8 | 43.78±12.7 | 18.63±1.0 |
| MonoD | 88.78±13.4 | 37.99±3.9 | 46.27±7.8 | 17.56±1.7 | 39.87±6.2 | 15.03±1.1 |
Hydroxyurea pretreatment does not affect
the antiproliferative effect of Dig and MonoD
The differential effect of serum on the
antiproliferative activity of Dig and MonoD and the effect of serum
starvation on their activity prompted us to investigate whether CGs
can be more effective in combination with drugs that act at
specific phases of the cell cycle. We first evaluated the effect of
Dig and MonoD in combination with varying concentrations of HU. Dig
and MonoD at 20 nM were chosen since this concentration is close to
their IC50. Cells were treated with HU (0, 0.1, 0.25,
0.5, 0.75, 1 or 2 mM) in CM for 72 h in the presence or absence of
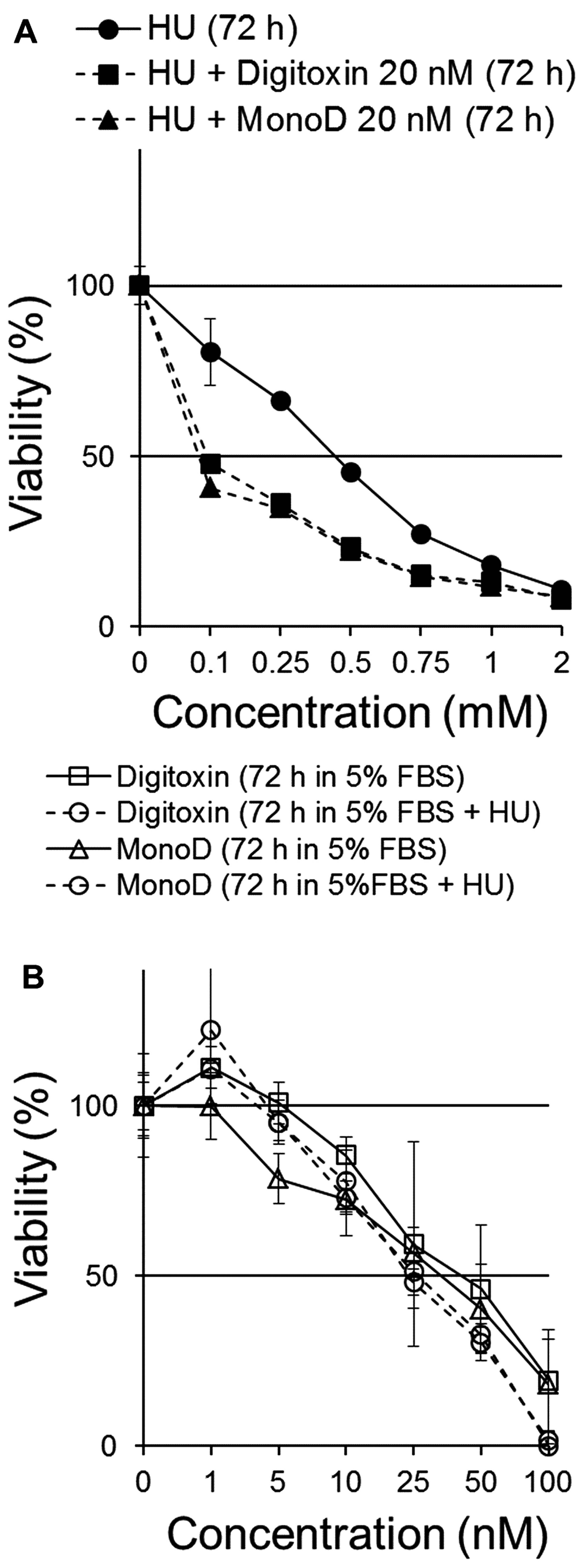
20 nM Dig or MonoD. Fig. 3A shows
that both Dig and MonoD slightly increase the antiproliferative
effect of HU when both drugs were added simultaneously. The
IC50 for HU alone, HU + Dig and HU + MonoD 20 nM were
0,56±0.02, 0.28±0.02 and 0.27±0.01 mM, respectively.
We next investigated the effect of pretreatment with
HU (2 mM) on the antiproliferative effect of Dig and MonoD. It is
well established that HU-treated cells accumulate in early S phase
due to a dose-dependent inhibiting effect of HU on DNA synthesis
(23). Upon release from the block,
cells synchronously progress through S, G2 and M phases
of the cell cycle (24). Cells
seeded at 2,000 cells/well were allowed to adhere overnight and
later incubated for 24 h in CM in the presence of 2 mM HU, which
inhibited proliferation by ~80–90% (Fig. 3A). The arrested/surviving cells were
treated with Dig or MonoD (0, 1, 5, 10, 25, 50, 100 or 200 nM) in
CM for 72 h. Cell viability was determined by the MTT assay.
Parallel cultures with HU-untreated cells were used for comparison.
Fig. 3B shows that Dig and MonoD
treatment for 72 h decreased the proliferation of HU-pretreated
cells with a similar potency compared to HU-untreated cells.
Overall, the data show that pretreatment with HU does not
significantly affect the antiproliferative effect of Dig and MonoD,
but suggests that CGs can still induce cytotoxicity in cells that
survived HU treatment, thereby positing a potential role for CGs
when added after HU.
Long-term serum starvation attenuates the
antiproliferative effect of paclitaxel, colchicine, hydroxyurea and
digitoxin but not MonoD
It is known that lung cancer cells can grow in
serum-free media for prolonged period (25); however, the chemosensitivity of
cells growing under this condition is poorly characterized. We
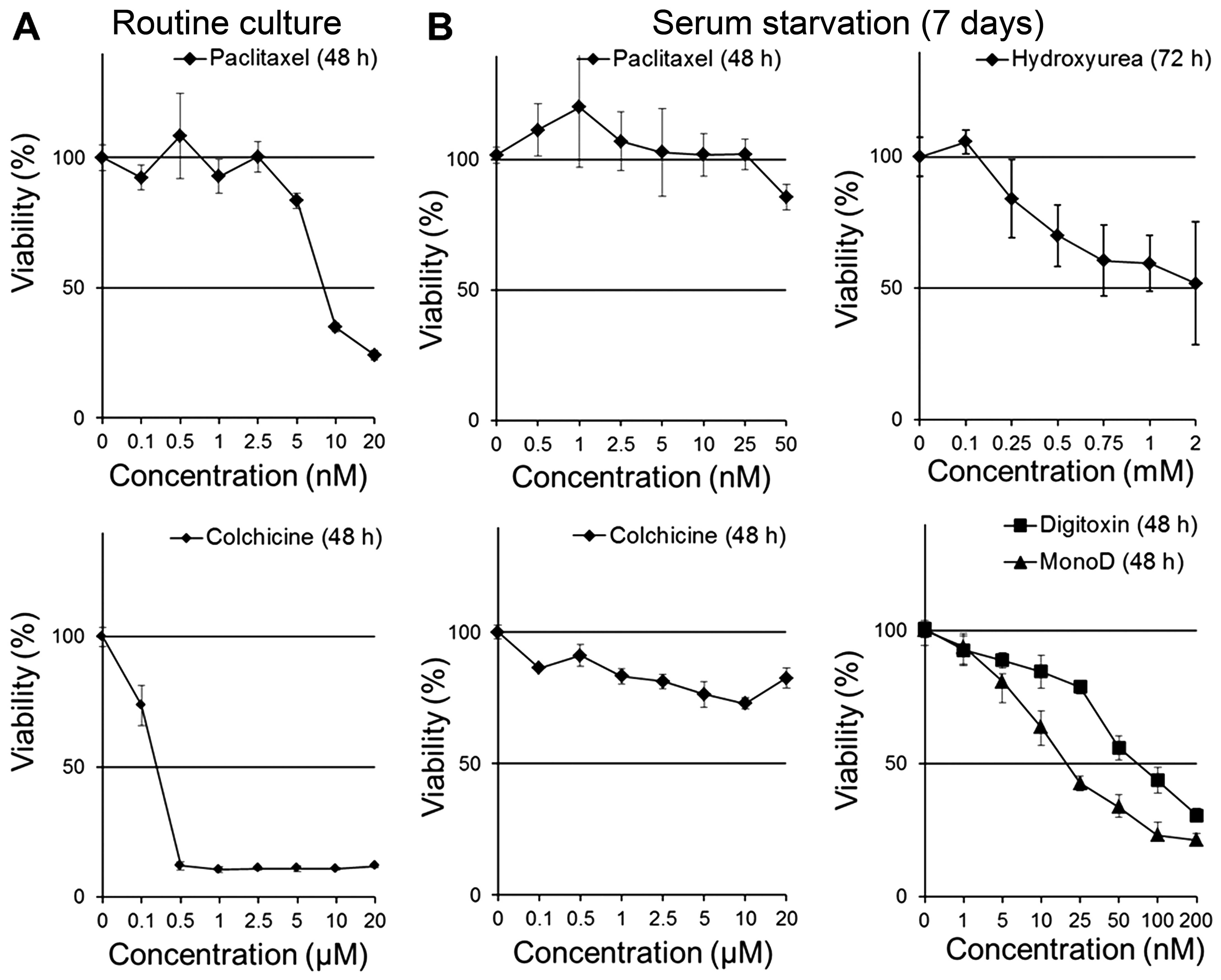
first evaluated the antiproliferative effects of PX and colchicine
on H460 cells in CM. Fig. 4A shows
that H460 cells were highly sensitive to PX and colchicine
(IC50, 8.9 nM and 1.8 µM, respectively). Next we
evaluated the effect of PX, colchicine, HU, Dig and MonoD on H460
cells serum-starved for prolonged periods: cells seeded at 2,000
cells/well were allowed to adhere overnight and later starved for
7–8 days in SFM. It is important to note that H460 cells grew in
serum-free conditions at reduced proliferation rates (data not
shown). After starvation, cells were treated with Dig or MonoD (0,
1, 5, 10, 25, 50, 100 or 200 nM) in SFM for 24, 48 or 72 h. Cell
viability was determined by the MTT assay. As shown in Fig. 4, following prolonged serum
starvation, H460 cells became insensitive to PX and colchicine
(Fig. 4B), less sensitive to HU and
Dig but remain highly sensitive to MonoD (compare activity with
Figs. 1Figure 2–3). Table
III shows the IC50 for paclitaxel, and colchicine in
cells growing under routine culture conditions and for digitoxin
and MonoD in cells growing under prolonged serum starvation.
Table IV shows the IC50
of paclitaxel, paclitaxel + digitoxin and paclitaxel + MonoD.
 | Table IIIIC50 for paclitaxel,
colchicine (routine culture), digi-toxin and MonoD (prolonged serum
starvation). |
Table III
IC50 for paclitaxel,
colchicine (routine culture), digi-toxin and MonoD (prolonged serum
starvation).
| H460 cells | IC50
(mean ± SD) 48 h | IC50
(mean ± SD) 72 h |
|---|
| Paclitaxel
(nM) | 8.93±0.7 | ND |
| Colchicine
(µM) | 0.1815±0.02 | ND |
| Dig (nM) | 82.32±7.26 | 88.66±8.12 |
| MonoD (nM) | 21.09±2.61 | 19.15±1.52 |
 | Table IVIC50 for paclitaxel,
paclitaxel + digitoxin and paclitaxel + MonoD. |
Table IV
IC50 for paclitaxel,
paclitaxel + digitoxin and paclitaxel + MonoD.
| H460 cells | IC50
(mean ± SD) 48 h | IC50
(mean ± SD) 72 h |
|---|
| Paclitaxel | 11.82±1.20 | 10.22±0.55 |
| Paclitaxel +
digitoxin (20 nM) | 3.02±0.42 | 2.71±0.33 |
| Paclitaxel + MonoD
(20 nM) | 3.39±0.48 | 2.04±0.37 |
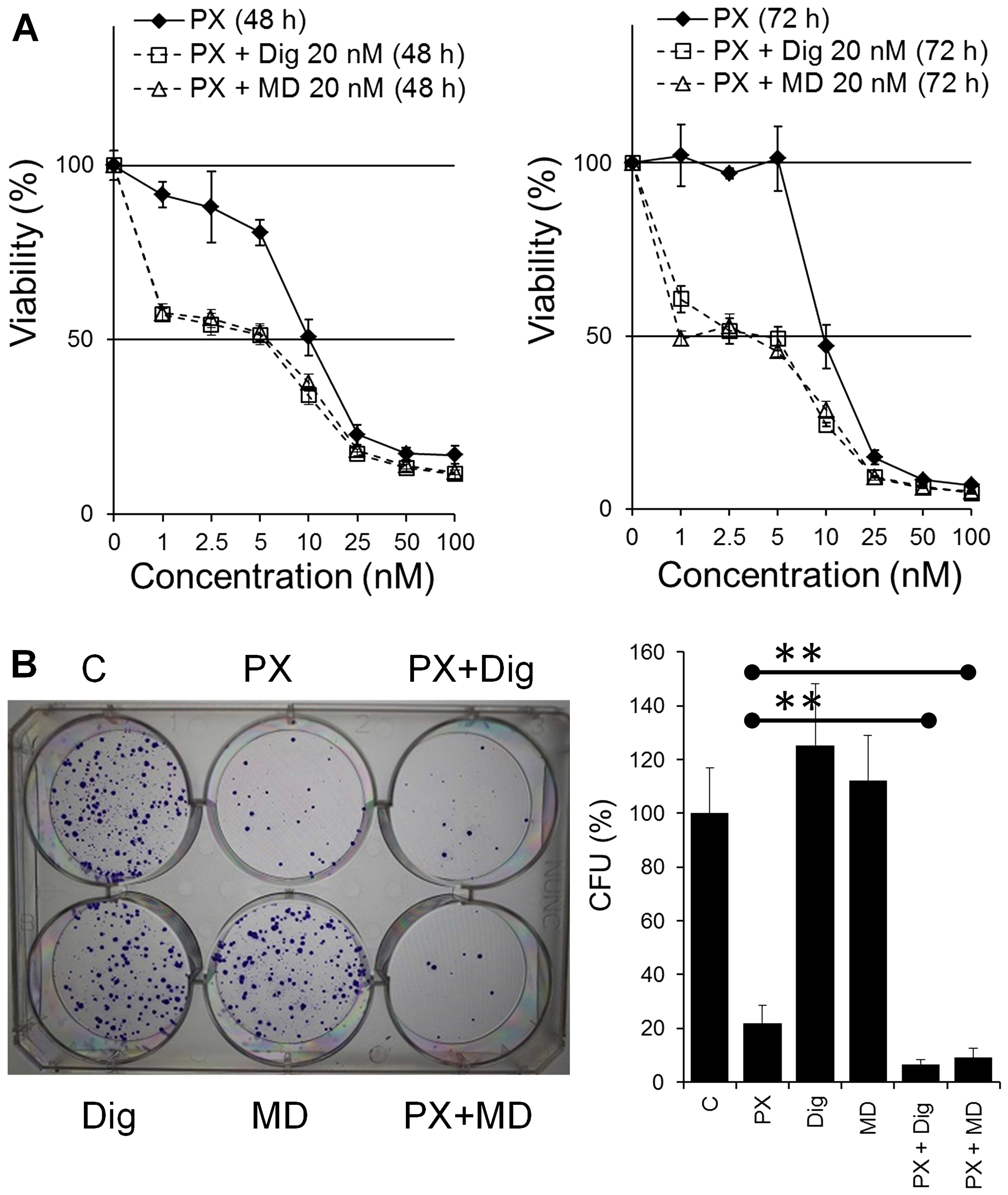
Dig and MonoD synergize with paclitaxel
on H460 cancer cells
PX exerts cytotoxic effects against H460 cells with
IC50 ranging from 5 nM (20) to 25 nM (26). However, 24 h treatment with PX can
induce a cell cycle arrest at the G2/M transition and
this effect was observed to be maximum at a concentration that is
~10 times higher that its IC50 (27).
Cells seeded at 2,000 cells/well were allowed to
adhere overnight and treated with PX (0, 0.5, 1, 2.5, 5, 10, 25 or
50 nM) in the absence (vehicle alone, DMSO) or presence of Dig or
MonoD (20 nM) in CM for 48 or 72 h. Cell viability was determined
by the MTT assay. The co-treatment showed increased
antiproliferative activity compared to PX alone (Fig. 5) and the effect was found to be
synergistic. Synergism was confirmed by the CI that was calculated
using the IC50 from dose responses curves for Dig alone
or MonoD alone (Fig. 1), PX alone
and PX co-treated with either Dig or MonoD (Fig. 5). The CI values were 0.404 and 0.403
for PX + Dig and PX + MonoD, respectively. The enhanced effect due
to the co-treatment was also observed by the colony-forming assay.
H460 cells were plated in 6-well plates at 200 cells/well and
allowed to adhere overnight. Cells were then treated with drugs
alone (PX 10 nM, Dig 20 nM or MonoD 20 nM) or combined (PX 10 nM +
Dig 20 nM or PX 10 nM + MonoD 20 nM) for 72 h. Cells treated with
DMSO alone were included as control and equivalent DMSO
concentrations were included in all treatment (~0.001%). Following
drug treatment, cells were incubated in complete media for 10 days
(media was changed every 3 days) and colonies were stained and
quantified as described in the Materials and methods section.
Fig. 5B shows that co-treatment
with either PX 10 nM + Dig 20 nM or PX 10 nM + MonoD 20 nM has
enhanced antiproliferative activity when compared to drugs
alone.
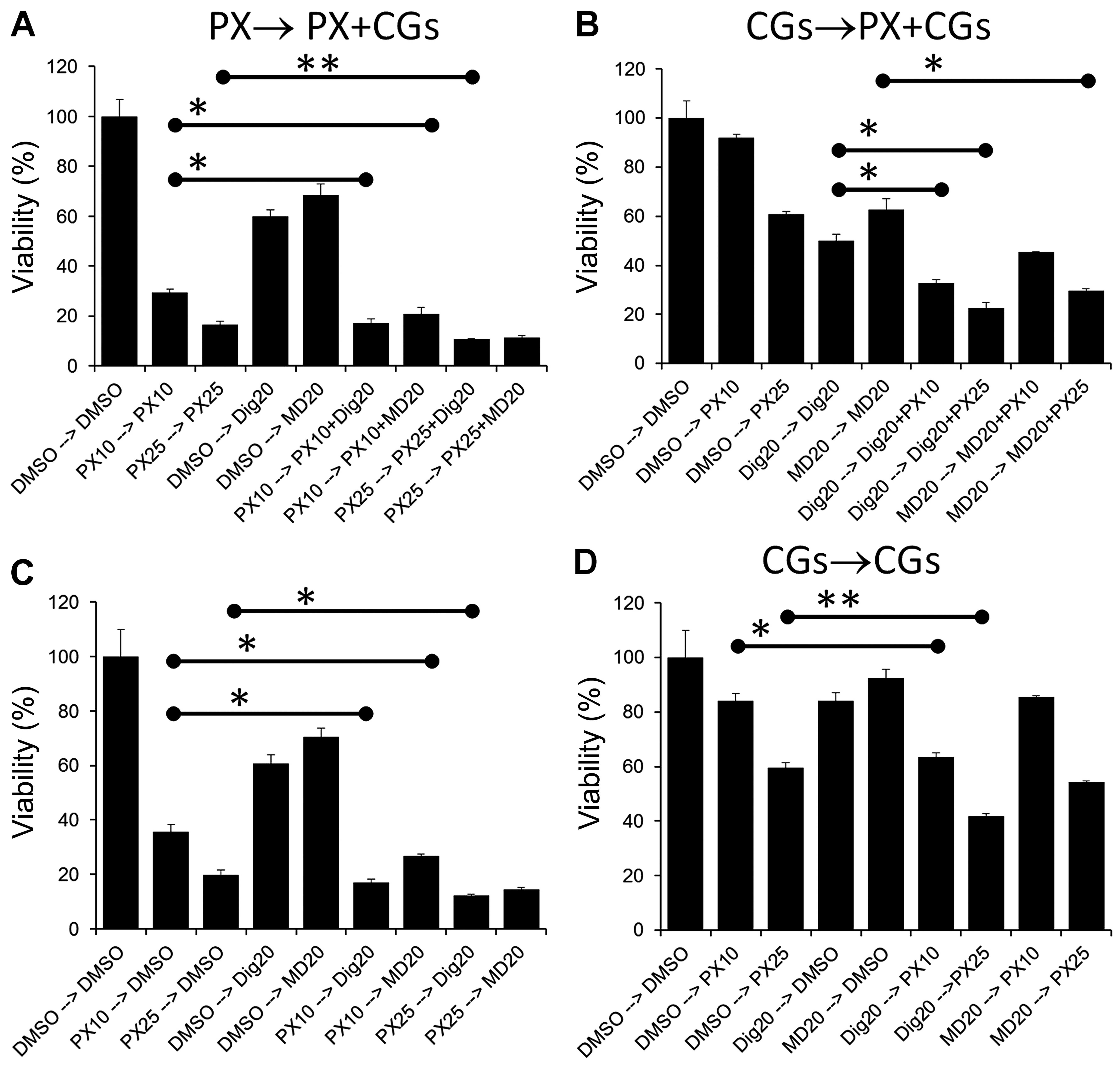
Effect of sequential treatment of lung
cancer cells in single or multi-drug modalities has alternative
effects
PX combination treat ments by other groups have
demonstrated that sequential regimens can have additive,
synergistic or antagonistic effects on cancer cell lines depending
on the order in which the drugs are administered (20,28–30).
Two types of sequential treatments were performed. In the first
protocol (single drug → co-treatment) cells were treated with a
single drug (PX, Dig or MonoD) for 24 h, followed by PX + Dig or PX
+ MonoD (PX → PX + Dig, PX → PX + MD, respectively) for 48 h. Cell
viability was assessed using the MTT assay. These regimens were
compared to single drugs alone incubated for the same period of
time. To eliminate any potential effect of media consumption and/or
drug inactivation during the first 24 h, all media and drugs were
freshly prepared and added at 24 h. The same procedure with the
reverse sequence was also performed (Dig → PX + Dig) (Fig. 6A and B). In the second protocol
(single drug → single drug), cells were treated with a single drug
(PX, Dig or MonoD) for 24 h followed by Dig or MonoD (PX → Dig, PX
→ MD, respectively) for 48 h. The same procedure with the reverse
sequence was also performed (Dig → PX) (Fig. 6C and D). Overall the results
indicated that enhanced antiproliferative activity was obtained
when PX was added first.
Discussion
Combination chemotherapy with drugs that show
enhanced antitumor efficacy is considered a promising approach to
improve clinical success by decreasing single drug doses and
minimizing or slowing drug resistance development (31). Drugs with different mechanisms of
action, relative non-cross-resistance, and partially
non-overlapping toxicities are considered good candidates (29).
In this study we first demonstrated that Dig and
MonoD have, at clinically relevant concentrations, potent
antiproliferative activity against the human H460 lung cancer cell
line and that there is a serum-dependent effect on the
antiproliferative activity of Dig but not MonoD (Fig. 1).
Short periods of serum deprivation (24 h prior to
the addition of drugs), a procedure known to increase the
percentage of cells in the G0/G1 phase of the
cell cycle, slightly decreased the effectiveness of both CGs
(Fig. 2). The effect was more
evident when cells were incubated with drugs in SFM for 24 h. After
72 h of drug exposure, there was no significant difference in the
potency of Dig and MonoD when compared to cells not subjected to
starvation for 24 h. It is important to mention that the
IC50 of digitoxin for H460 cells subjected to short
periods of starvation was still within the therapeutic range
(<46 nM) (Fig. 2). When Dig (20
nM) or MonoD (20 nM) were used alone for 72 h in complete media, it
led to decreased cell viability by ~50% (Fig. 5A) in perfect agreement with their
respective IC50 previously shown in Fig. 1. However, when both CGs were used
alone at the same concentration (each at 20 nM) in the
colony-forming assay they showed no effect compared to DMSO alone.
This paradoxical effect is not due to experimental variations and
may be explained by assuming that Dig and MonoD at 20 nM have a
reversible effect, which was confirmed subsequently (Fig. 6).
Both Dig and MonoD potentiated the effect of HU. HU
at 0.75–1 mM in combination with Dig 20 nM or MonoD 20 nM decreased
the viability of H460 cells by >80% (Fig. 3A). These are clinically relevant
concentrations since a mean serum level of HU >1 mM can be
achieved and maintained in patients (13). Pretreatment with HU 2 mM, a
concentration that slow down and partially synchronize cells in S
phase inhibited the proliferation of H460 cells by ~80% within 24 h
(Fig. 3A). Cells that survived 2 mM
HU for 24 h were sensitive to Dig or MonoD (Fig. 3B).
When grown in routine culture media supplemented
with 5% FbS, H460 cells were very sensitive to HU (Fig. 3A), PX and colchicine (Fig. 4A). Prolonged serum deprivation (7
days) markedly decreased the antiproliferative activity of HU, PX
and colchicine and decreased the effectiveness of Dig, but had no
effect on MonoD (Fig. 4B). In fact,
the antiproliferative effect of MonoD was high when the drugs were
added in complete media (IC50 ~20 nM at 72 h) (Fig. 1), low when cells were incubated in
SFM for up to 96 h (IC50 ~40 nM at 72 h) (Fig. 3), and again high when cells were
incubated in SFM for 7 days (IC50 ~20 nM at 72 h)
(Fig. 4). The data suggest that
MonoD exerts its antiproliferative action in a manner that is
distinct from Dig, and that MonoD may potentially be a more
suitable anti-neoplastic agent for the treatment of cancers that
are not in the dividing phase and thus able to resist the effect of
traditional chemotherapy including PX and HU.
The ability of CGs, especially MonoD, to kill cancer
cells under different culture conditions compared to other drugs
such as PX or colchicine is important since CGs may be less
sensitive to intratumoral heterogeneity typically found in cancer
tumors (16,32). In this context, MonoD by its ability
to kill cancer cells in the presence or absence of serum (a rich
source of growth factors) with similar potency (Figs. 1, 3
and 4) offers an additional
advantage.
Co-treatment of cancer cells with Dig + PX has been
explored in vitro in a few types of cancer. Studies in the
MDA-MB-453 breast cancer cell line showed a synergistic
antiproliferative effect with Dig concentrations as low as 13 nM
(6). On the other hand, in the
human prostate cancer cells (PC-3), Dig reversed both
G2/M arrest and induction of apoptosis by PX (33). Based on these studies, it is clear
that the effects of PX-based combinations are cell
type-dependent.
We explored the antiproliferative effects of PX +
CGs combinations on H460 cells. We found that both Dig or MonoD in
combination with PX have a synergistic effect (Fig. 5). In the present study we chose a
fixed concentration of Dig (20 nM) or MonoD (20 nM) since these
value are clinically relevant and close to their IC50 in
serum containing media (Fig.
1).
PX in combination with other drugs showed
antagonistic, additive or synergistic effect in a
schedule-dependent manner (28–30).
For this reason, we investigated sequential treatments (Fig. 6). Several important observations
should be highlighted: i) maximal antiproliferative effect was
obtained when PX (25 nM) was added as a first drug for 24 h
followed by 20 nM of either Dig or MonoD (Fig. 6A). However, this effect was only
slightly higher when compared to PX followed by only one CG (20 nM
Dig or MonoD) (Fig. 6C). ii) The
maximum antiproliferative effect obtained with PX 25→20 nM of
either Dig or MonoD was only slightly higher when compared to PX 25
nM alone for 72 h (Fig. 6A). iii)
The effect of 24 h treatment with the CGs were almost completely
reversible, when cells treated for 24 h with either 20 nM Dig or
MonoD were incubated for 48 h in drug free media (DMSO alone), the
proliferative activity was similar to control values (Fig. 6D). In contrast, the effects of
either 10 or 25 nM PX were irreversible: cells treated for 24 h
with PX followed by 48 h in drug-free media (DMSO alone)
demonstrated proliferative activity that was only slightly higher
to cells treated with equivalent PX concentrations for 72 h
(compare 10 and 20 nM PX alone in Fig.
6C vs. D). The irreversible effect of PX could be due to the
intracellular accumulation of this drug that can reach
concentration levels up to 9 µM, and is likely retained in
tumor tissue for a substantial period of time (11). The intracellular uptake of PX may
explain the increased antiproliferative activity of PX-based
combinations when PX is added first.
Overall, the aforementioned observations are
important since sequential treatment with drugs (PX → Dig or MonoD)
will be likely less toxic for several reasons: i) the exposure time
to PX, due to its irreversible effect can be shortened and, ii) the
doses of both drugs can be reduced, limiting potential adverse
effects. Despite the fact that the antiproliferative effects of Dig
and MonoD as single drugs were partially reversible (Figs. 5 and 6), both drugs were able to significantly
increase the antiproliferative effect of PX in the colony-forming
assay (Fig. 5), and demonstrate
that the combination of PX + CGs have long-term antiproliferative
effects. Therefore PX-CGs, and especially PX in combination with
MonoD have the potential to target wider subpopulations of cancer
cells.
Due to intratumor heterogeneity, the poor
antiproliferative activity of PX on serum starved cells as well as
the reversible effect of Dig and MonoD may limit the in vivo
efficacy of the drug combinations described above. Furthermore,
lung cancer stem cells that are able to grow in serum-free media
(with few additives such as FGF and eGF) are known to be resistant
to PX (34). To circumvent these
limitations, other PX analogs with enhanced antiproliferative
activity to serum starved cells and cancer stem cells, and Dig
analogs similar to MonoD but with irreversible effects can be
screened for testing new combinations with higher efficacy toward
cancer cells that are resistant to traditional chemotherapeutic
regimens.
In conclusion, we reported that both Dig and MonoD
have potent antiproliferative activity against the chemoresistant
NSCLC NCI-H460 cell line. Our studies have demonstrated that both
CGs potentiated the antiproliferative effects of HU and PX, two
anticancer drugs with different mechanism of actions. We also
showed that sequential administration of PX followed by either Dig
or MonoD resulted in the most signifi-cant cytotoxic effects. The
latter has implications for rational translation of
chemotherapeutic regimens for the treatment of lung cancer.
Finally, by testing the efficacy of anticancer drugs in cells
growing under different culture conditions (short and prolonged
serum starvation) it was possible to identify that MonoD, contrary
to PX, colchicine or HU, has potent antipro-liferative activity
against cells growing under prolonged serum starvation conditions.
These studies offer a new strategy to screen and develop drugs and
combinations of drugs that have higher probabilities of success in
clinical trials.
Abbreviations:
|
PX
|
paclitaxel
|
|
Dig
|
digitoxin
|
|
HU
|
hydroxyurea
|
Acknowledgments
This study was supported by grants CA173069 from the
National Cancer Institute (NIH/NCI) to A.I. and HL112630 to
N.A.
References
|
1
|
Coughlin SS, Matthews-Juarez P, Juarez PD,
Melton CE and King M: Opportunities to address lung cancer
disparities among African Americans. Cancer Med. 3:1467–1476. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Detterbeck FC, Mazzone PJ, Naidich DP and
Bach PB: Screening for lung cancer: Diagnosis and management of
lung cancer, 3rd ed: American College of Chest Physicians
evidence-based clinical practice guidelines. Chest. 143(Suppl 5):
e78S–e92S. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nana-Sinkam SP and Powell CA: Molecular
biology of lung cancer: Diagnosis and management of lung cancer,
3rd ed: American College of Chest Physicians evidence-based
clinical practice guidelines. Chest. 143(Suppl 5): e30S–e39S. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
López-Lázaro M, Pastor N, Azrak SS, Ayuso
MJ, Austin CA and Cortés F: Digitoxin inhibits the growth of cancer
cell lines at concentrations commonly found in cardiac patients. J
Nat Prod. 68:1642–1645. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kometiani P, Liu L and Askari A:
Digitalis-induced signaling by Na+/K+-ATPase
in human breast cancer cells. Mol Pharmacol. 67:929–936. 2005.
View Article : Google Scholar
|
|
6
|
Einbond LS, Wu HA, Su T, Chang T,
Panjikaran M, Wang X and Goldsberry S: Digitoxin activates EGR1 and
synergizes with paclitaxel on human breast cancer cells. J
Carcinog. 9:102010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Elbaz HA, Stueckle TA, Tse W, Rojanasakul
Y and Dinu CZ: Digitoxin and its analogs as novel cancer
therapeutics. Exp Hematol Oncol. 1:42012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Socinski MA: Update on taxanes in the
first-line treatment of advanced non-small-cell lung cancer. Curr
Oncol. 21:e691–e703. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lück HJ and Roché H: Weekly paclitaxel: An
effective and well-tolerated treatment in patients with advanced
breast cancer. Crit Rev Oncol Hematol. 44(Suppl): S15–S30. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Das V, Štěpánková J, Hajdúch M and Miller
JH: Role of tumor hypoxia in acquisition of resistance to
microtubule-stabilizing drugs. Biochim Biophys Acta. 1855:172–182.
2015.PubMed/NCBI
|
|
11
|
Zasadil LM, Andersen KA, Yeum D, Rocque
GB, Wilke LG, Tevaarwerk AJ, Raines RT and Burkard ME: Cytotoxicity
of paclitaxel in breast cancer is due to chromosome missegregation
on multipolar spindles. Sci Transl Med. 6:229ra432014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wiernik PH, Schwartz EL, Strauman JJ,
Dutcher JP, Lipton RB and Paietta E: Phase I clinical and
pharmacokinetic study of taxol. Cancer Res. 47:2486–2493.
1987.PubMed/NCBI
|
|
13
|
Veale D, Cantwell BM, Kerr N, Upfold A and
Harris AL: Phase 1 study of high-dose hydroxyurea in lung cancer.
Cancer Chemother Pharmacol. 21:53–56. 1988.PubMed/NCBI
|
|
14
|
Kang SR, Song HC, Byun BH, Oh JR, Kim HS,
Hong SP, Kwon SY, Chong A, Kim J, Cho SG, et al: Intratumoral
metabolic heterogeneity for prediction of disease progression after
concurrent chemoradiotherapy in patients with inoperable stage III
non-small-cell lung cancer. Nucl Med Mol Imaging. 48:16–25. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Neelakantan D, Drasin DJ and Ford HL:
Intratumoral heterogeneity: Clonal cooperation in
epithelial-to-mesenchymal transition and metastasis. Cell Adh Migr.
9:1–12. 2015. View Article : Google Scholar
|
|
16
|
Cheng X and Chen H: Tumor heterogeneity
and resistance to EGFR-targeted therapy in advanced nonsmall cell
lung cancer: Challenges and perspectives. Onco Targets Ther.
7:1689–1704. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Iyer AK, Zhou M, Azad N, Elbaz H, Wang L,
Rogalsky DK, Rojanasakul Y, O'Doherty GA and Langenhan JM: A direct
comparison of the anticancer activities of digitoxin
MeON-Neoglycosides and O-Glycosides: Oligosaccharide chain
length-dependent induction of caspase-9-mediated apoptosis. ACS Med
Chem Lett. 1:326–330. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Medan D, Luanpitpong S, Azad N, Wang L,
Jiang BH, Davis ME, Barnett JB, Guo L and Rojanasakul Y:
Multifunctional role of Bcl-2 in malignant transformation and
tumorigenesis of Cr(VI)-transformed lung cells. PLoS One.
7:e370452012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rafehi H, Orlowski C, Georgiadis GT,
Ververis K, El-Osta A and Karagiannis TC: Clonogenic assay:
Adherent cells. J Vis Exp. 49:25732011.PubMed/NCBI
|
|
20
|
Park S, Kim JH, Hwang YI, Jung KS, Jang YS
and Jang SH: Schedule-dependent effect of
epigallocatechin-3-gallate (EGCG) with paclitaxel on H460 cells.
Tuberc Respir Dis (Seoul). 76:114–119. 2014. View Article : Google Scholar
|
|
21
|
Kues WA, Anger M, Carnwath JW, Paul D,
Motlik J and Niemann H: Cell cycle synchronization of porcine fetal
fibroblasts:Effects of serum deprivation and reversible cell cycle
inhibitors. Biol Reprod. 62:412–419. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Khammanit R, Chantakru S, Kitiyanant Y and
Saikhun J: Effect of serum starvation and chemical inhibitors on
cell cycle synchronization of canine dermal fibroblasts.
Theriogenology. 70:27–34. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Maurer-Schultze B, Siebert M and Bassukas
ID: An in vivo study on the synchronizing effect of hydroxyurea.
Exp Cell Res. 174:230–243. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Darzynkiewicz Z, Halicka HD, Zhao H and
Podhorecka M: Cell synchronization by inhibitors of DNA replication
induces replication stress and DNA damage response: Analysis by
flow cytometry. Methods Mol Biol. 761:85–96. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Brower M, Carney DN, Oie HK, Gazdar AF and
Minna JD: Growth of cell lines and clinical specimens of human
non-small cell lung cancer in a serum-free defined medium. Cancer
Res. 46:798–806. 1986.PubMed/NCBI
|
|
26
|
Soriano AF, Helfrich B, Chan DC, Heasley
LE, Bunn PAJ Jr and Chou TC: Synergistic effects of new
chemopreventive agents and conventional cytotoxic agents against
human lung cancer cell lines. Cancer Res. 59:6178–6184. 1999.
|
|
27
|
Kroep JR, Giaccone G, Tolis C, Voorn DA,
Loves WJ, Groeningen CJ, Pinedo HM and Peters GJ: Sequence
dependent effect of paclitaxel on gemcitabine metabolism in
relation to cell cycle and cytotoxicity in non-small-cell lung
cancer cell lines. Br J Cancer. 83:1069–1076. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zoli W, Ricotti L, Barzanti F, Dal Susino
M, Frassineti GL, Milandri C, Casadei Giunchi D and Amadori D:
Scheduledependent interaction of doxorubicin, paclitaxel and
gemcitabine in human breast cancer cell lines. Int J Cancer.
80:413–416. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Oliveras-Ferraros C, Vazquez-Martin A,
Colomer R, De Llorens R, Brunet J and Menendez JA:
Sequence-dependent synergism and antagonism between paclitaxel and
gemcitabine in breast cancer cells: The importance of scheduling.
Int J Oncol. 32:113–120. 2008.
|
|
30
|
Cheng H, An SJ, Zhang XC, Dong S, Zhang
YF, Chen ZH, Chen HJ, Guo AL, Lin QX and Wu YL: In vitro
sequencedependent synergism between paclitaxel and gefitinib in
human lung cancer cell lines. Cancer Chemother Pharmacol.
67:637–646. 2011. View Article : Google Scholar
|
|
31
|
Baldan F, Mio C, Allegri L, Puppin C,
Russo D, Filetti S and Damante G: Synergy between HDAC and PARP
inhibitors on proliferation of a human anaplastic thyroid
cancer-derived cell line. Int J Endocrinol. 2015:9783712015.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gerlinger M, Rowan AJ, Horswell S, Larkin
J, Endesfelder D, Gronroos E, Martinez P, Matthews N, Stewart A,
Tarpey P, et al: Intratumor heterogeneity and branched evolution
revealed by multiregion sequencing. N Engl J Med. 366:883–892.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Huang DM, Guh JH, Huang YT, Chueh SC, Wang
HP and Teng CM: Cardiac glycosides induce resistance to
tubulindependent anticancer drugs in androgen-independent human
prostate cancer. J Biomed Sci. 9:443–452. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Larzabal L, El-Nikhely N, Redrado M,
Seeger W, Savai R and Calvo A: Differential effects of drugs
targeting cancer stem cell (CSC) and non-CSC populations on lung
primary tumors and metastasis. PLoS One. 8:e797982013. View Article : Google Scholar : PubMed/NCBI
|