Introduction
Ovarian cancer is a significant cause of mortality
in women around the world. Although ovarian cancer treatment has
advanced in recent years, long-term survival remains stable
(1,2). Evidence shows that the targeting of
epigenetics including acetylation and deacetylation of the core
nucleosome histones show great promise for improving the treatment
of cancers (3–5).
Recent studies show that epigenetic disorder in
cancer plays an important role in regulating cancer development
(6–9). It is regarded as the most powerful
regulator to mediate other transcription regulatory pathways which
are related to transcription factors. It has been proven that the
aberrant HDAC activity is linked to the development of cancers.
Inhibition of HDACs leads to the restoration of transcriptionally
silenced pathways or the repression of aberrantly expressed genes
(10–16). Increasing evidence indicates that
the causes of cancer are related not only to a variety of signaling
pathways, but also to epigenetic modification, which allows
adaptation to environmental alteration such as ECM remodeling,
hypoxia stress and nutrient deprivation (17–19).
HDAC4 is a class II histone deacetylase, which
modulates gene expression by undergoing nuclei-cytoplasmic shutting
via its phosphorylation state. Nuclear HDAC4 represses
transcription procedure when tethered to a promoter. Class II HDACs
contain an N-terminal regulatory domain that is subject to
phosphorylation. Nuclei-cytoplasmic shuttling of HDAC4 is
controlled by its phosphorylation state. PP1α/PP2A is the critical
regulator of HDAC4 function (20).
Dephosphorylation of HDAC4 by PP1α/PP2A stabilizes and promotes its
nuclear accumulation, whereas phosphorylated HDAC4 is located in
the cytoplasm and is prone to degradation (20–23).
Previous studies showed that fibrillar collagen
matrices enhanced the proliferation and invasion of epithelial
ovarian cancer cells via activation of PTEN/Akt signaling (17). Therefore, we postulate that HDAC4 is
associated with poor prognosis of ovarian cancer. We also
investigated whether fibrillar collagen matrices influence the
aberrant HDAC4 in epithelial ovarian cancer. Herein, we demonstrate
that nuclear HDAC4 is a key regulator promoting the progressive
epithelial ovarian cancer on fibrillar collagen matrices via
co-localized PP1α, in which HDAC4 represses the mRNA/protein of
p21.
Materials and methods
Epithelial ovarian cancer cell lines, OVCAR3 and
SKOV3, were purchased from the American Type Culture Collection.
Three-Dimensional fibrillar collagen (0.25 mg/ml final
concentration) was as previously described (17). The antibodies of HDAC4, lamin-A,
Sp1, p21 and Acetyl-histone-H3 were obtained from Cell Signaling
Technology (Danvers, MA, USA). Both GAPDH and PP1α were from Santa
Cruz Biotechnology, Inc. (Santa Cruz, CA, USA).
Cell and culture
All patients fulfilled the criteria for diagnosis of
epithelial ovarian cancer with grades and stages as established by
the International Federation of Gynecology and Obstetrics (FIGO)
2014. One hundred and two patients with epithelial ovarian cancer
were confirmed by immunohistochemistry and/or western blotting.
Less than 25% of nuclear HDAC4 expression was the lower HDCA4
patient group, and 25 to 100% the higher HDAC4 patient group
according to evaluation of 200 cancer cells. Tumor tissues were
collected from surgeries performed at Affiliated Nanjing Maternity
and Child Health Care Hospital at Nanjing Medical University. Cells
were cultured as previous described (17). We began our experiments by studying
the proliferation of epithelial ovarian cancer cells which were
treated with HDAC4 inhibitor, Trichostatin A (TSA). We observed the
proliferation of cells with TSA treatment. To investigate the role
of fibrillar collagen in regulating HDAC4 function, we examined
HDAC4 location in the epithelial ovarian cancer cell line OVCAR3 in
cells seeded with or without fibrillar collagen for 2 h.
Immunohistochemistry (IHC)
We studied HDAC4 expression in epithelial ovarian
cancer tissue specimens with stage I/II (n=36) or stage III/IV
(n=66) by immunohistochemistry and western blot analysis (17). Immunohistochemistry studies were
performed on formalin-fixed sections of tissue specimens. Sections
were pretreated with trypsin (10 mg per 50 ml in Tris buffer, pH
8.1) for 10 min at 37°C, followed by anti-HDAC4 antibody (1:1000
dilution in PBS) for 30 min. Slides were washed in PBS and
incubated sequentially for 15 min with peroxidase-conjugated swine
anti-mouse immunoglobulin G (1:50 dilution). Staining was performed
using a Dako auto-stainer. Localization of reaction products was
performed using diaminobenzidene reaction.
Immunofluorescence assay (IFA)
PP1α location was examined by subcellular
fractionation assay and immunofluorescence in epithelial ovarian
cancer cells with or without fibrillar collagen. HDAC4 localization
was analyzed also by immunofluorescence assay in epithelial ovarian
cancer cells with or without the PP1α inhibitor calyculin A.
Immunofluorescence was performed as previously described (17).
Immunoprecipitation assay (IP)
We examined that HDAC4 interacted with Sp1 in
nuclear fraction of OVCAR3 cells using immunoprecipitation assay by
anti-HDAC4 antibody.
Western blot analysis
We examined HDAC4 expression by western blotting in
OVCAR3 cells seeded with or without fibrillar collagen for 2 h and
with overexpression of PP1α in adenoviral vector. In addition,
protein of p21 was examined by western blotting in OVCAR3 cells
performing gain/loss-of-function of HDAC4 by either overexpression
of HDCA4 or knock-down of HDAC4 with shRNA. The cells were
harvested in lysis buffer. The samples were centrifuged for 20 min
at 13,000 × g. The protein concentration of the supernatant was
determined by BCA assay. Sodium dodecylsulfate-poly-acrylamide gel
electrophoresis(SDS-PAGE) was carried out loading equal amount of
proteins/lane. Gels were transferred to cellulose nitrate membranes
and blocked with 5% non-fat milk in Tris-buffered saline with
Tween-20 (TBST) buffer for 1 h. Then, the membranes were incubated
with primary antibodies at a 1:5,000 dilution in 5% non-fat milk
overnight at 4°C, and washed five times with TBST for a total of 30
min. The membranes were then incubated which the secondary
antibodies conjugated with horseradish peroxidase at a 1:5,000
dilution for 1 h at room temperature and then washed five times
with TBST. The blots were visualized with Amersham ECL Plus Western
Blotting Detection Reagents according to the manufacturer's
instructions (17).
Cell migration assay
Cell migration was analyzed by Electric
Cell-substrate Impedance Sensing (ECIS; Applied BioPhysics Troy,
NY, USA), which is an impedance based method to study cell
activities in tissue culture in real-time. We modified the protocol
described by the company: 25,000 cells/cm2 was seeded in
ECIS arrays (8-well), and grown to about 100% confluency in
approximately 24 h. The cells were then killed in a small active
electrode. The additional fibrillar collagen was polymerized in
each well at 0.25 mg/ml for 90 min. The migration was assessed by
continuing impendent measurements for 20 h.
Quantitative qPCR
mRNA of p21 was examined by qPCR. cDNA was reverse
transcribed using TaqMan reverse transcriptase kit (Roche) and qPCR
was performed using the Roche Light-Cycler with SYBR Green dye
(Roche) (24). The following human
primers were used: p21 FW: 5′-GAG GCCGGGATGAGTTGGGAGGAG, RV:
5′-CAGCCGG CGTTTGGAGTGGTAGAA; p53 FW: 5′-CCCCTCCT GGCCCCTGTCATCTTC,
RV: 5′-GCAGCGCCTCAC AACCTCCGTCAT; GAPDH FW: 5′-AATCCCATCACC
ATCTTCCA, RV: 5′-TGGACTCCACGACGTACTCA.
Statistics
Data are expressed as mean ± SD. The data in each
experiment were performed with unpaired Student's t-tests using
SPSS 13.0 software. The experiments were done a minimum of three
times. P<0.05 was considered to indicate a statistically
significant difference.
Results
HDAC inhibitor attenuates the
proliferation of epithelial ovarian cancer cells
An epigenetic disorder is one of the main factors
leading to poor prognosis of cancer (18–20).
Previous work indicated that fibrillar collagen matrices enhanced
the proliferation and invasion in epithelial ovarian cancer cells
(17). To directly examine the role
of HDACs in contribution to modulation of epithelial ovarian cancer
cells, we began our experiments by studying the proliferation of
epithelial ovarian cancer cells in cells treated with the HDAC
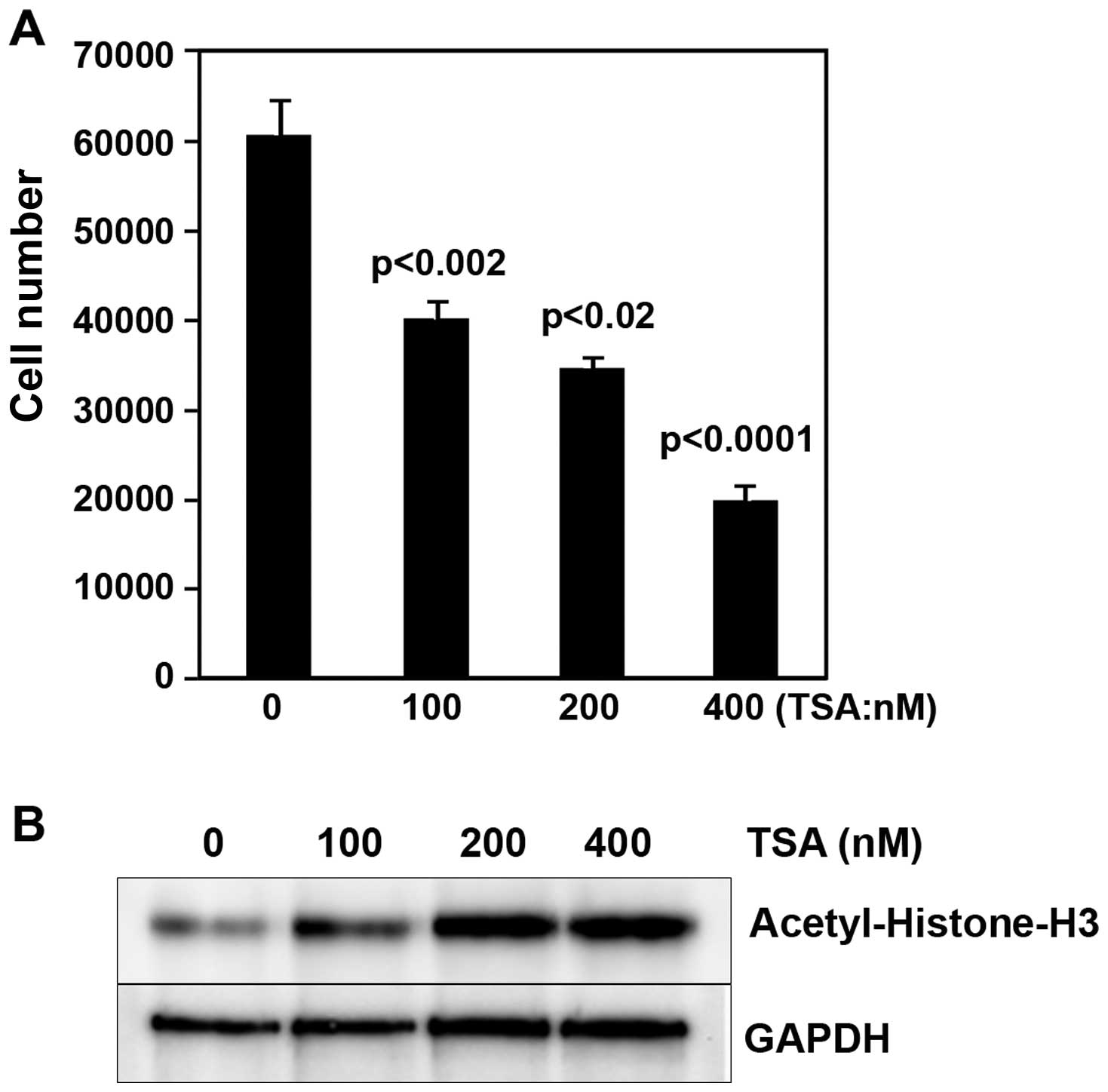
inhibitor, Trichostatin A (TSA), for 48 h in a dose-dependent
manner. We observed that i) the proliferation of cells with TSA
treatment was markedly decreased (Fig.
1A); ii) acetyl-histone-H3 expression in cells with TSA was
increased (Fig. 1B). This is one of
the reasons that HDAC4 promotes the proliferation of epithelial
ovarian cancer cells. These data indicated that the epigenetic
changes of HDACs might play a pivotal role in regulating epithelial
ovarian cancer.
Higher HDAC4 expression was related to
high stage of the epithelial ovarian cancer
HDACs are aberrant in many cancers including
epithelial ovarian cancer (25–29).
We postulated that aberrant HDAC4 expression was associated with
fibrillar collagen matrices which were remodeled in the cancer
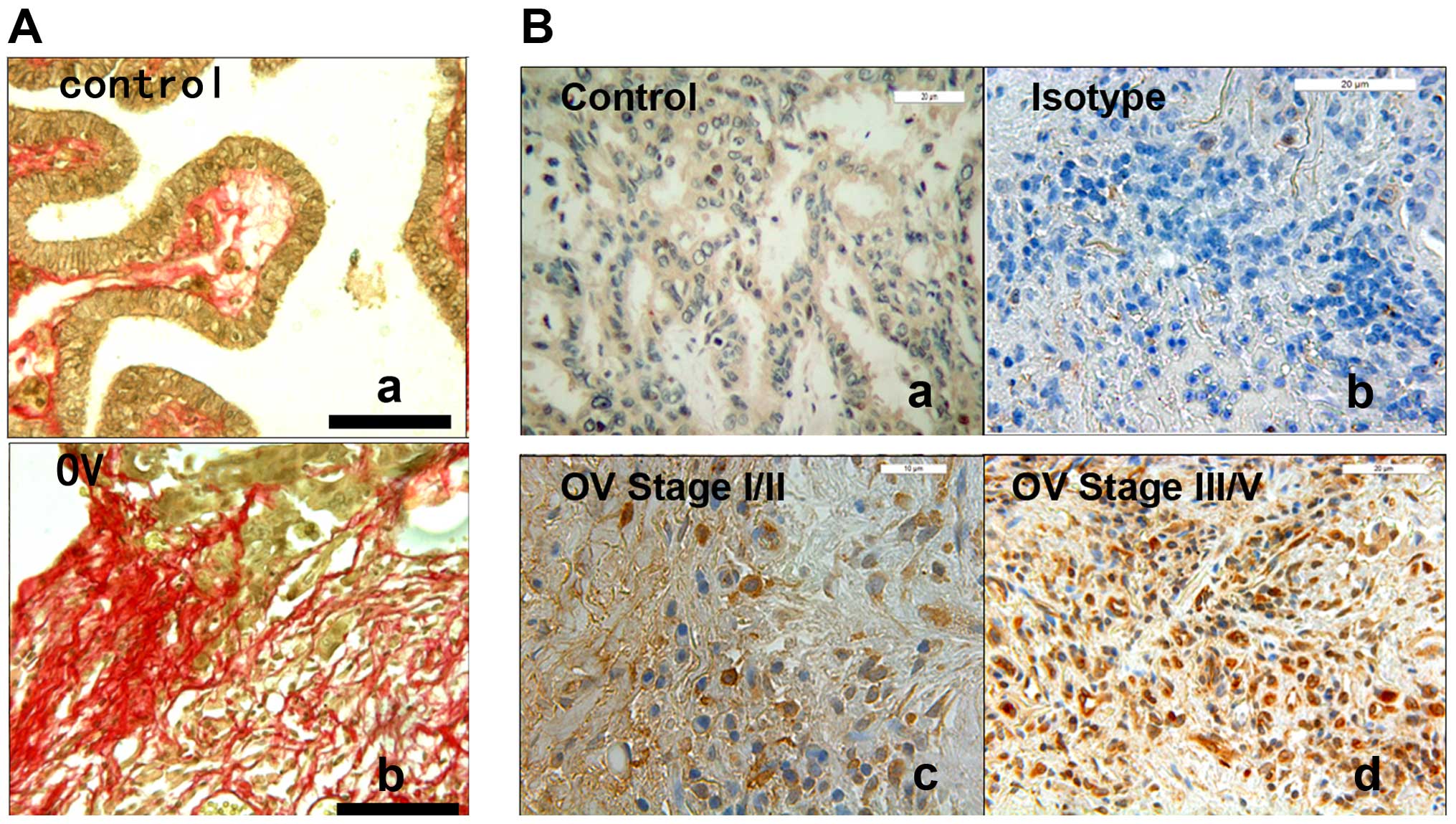
microenvironment. To support this idea, we examined that synthesis
and deposition of fibrillar collagen that was increased in
epithelial ovarian cancer tissues specimens in comparison with
controls by picrosirius red stain (Fig.
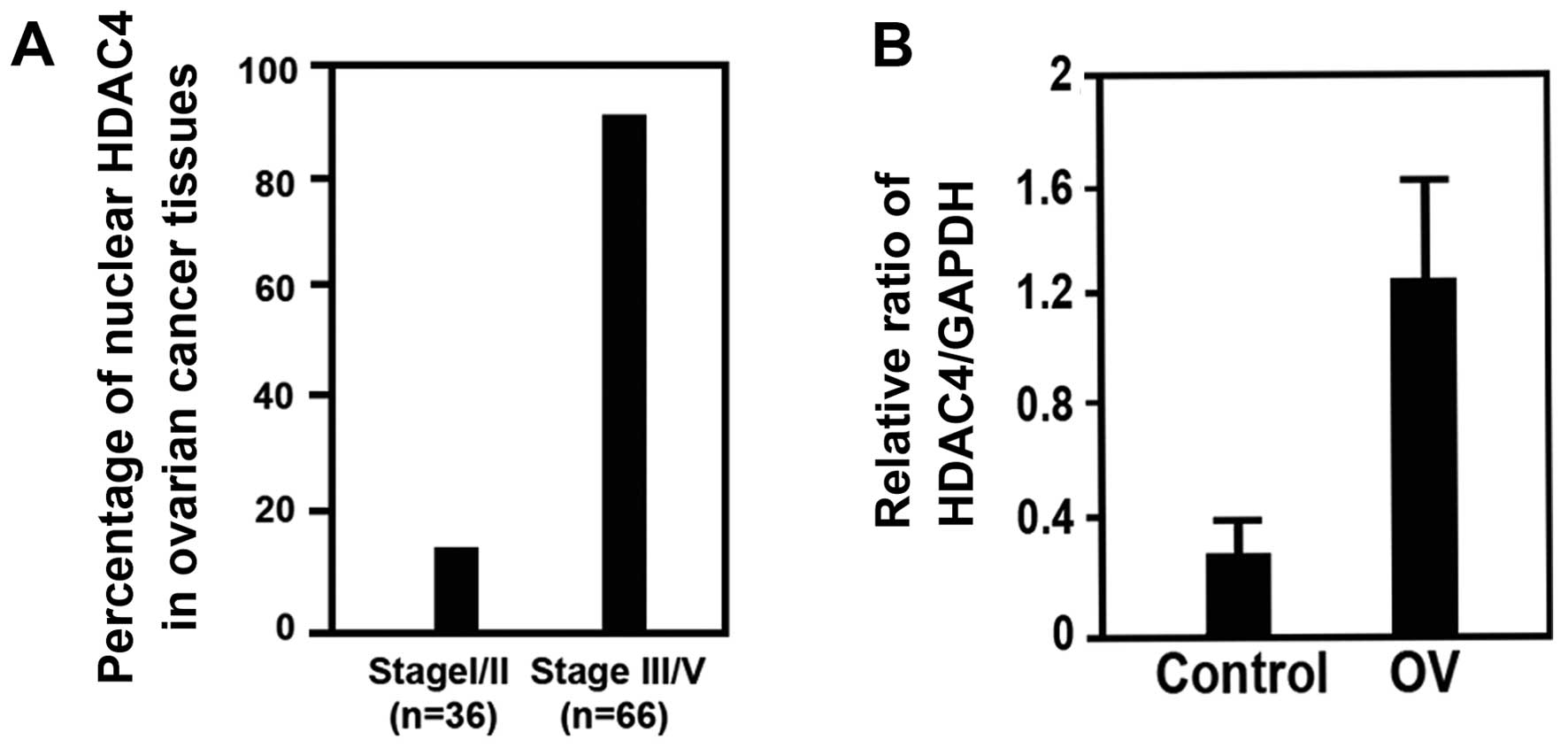
2A). To explore in vivo the relevance of our findings,
we studied HDAC4 expression in epithelial ovarian cancer tissue
specimens with stage I/II (n=36) or stage III/IV (n=66) by
immunohistochemistry (IHC). We found that 87.8% of epithelial
ovarian cancer tissues with stage III/IV had higher HDAC4
expression, compared to 13.8% of that with stage I/II (Figs. 2B and 4A). Finally, we found that HDAC4
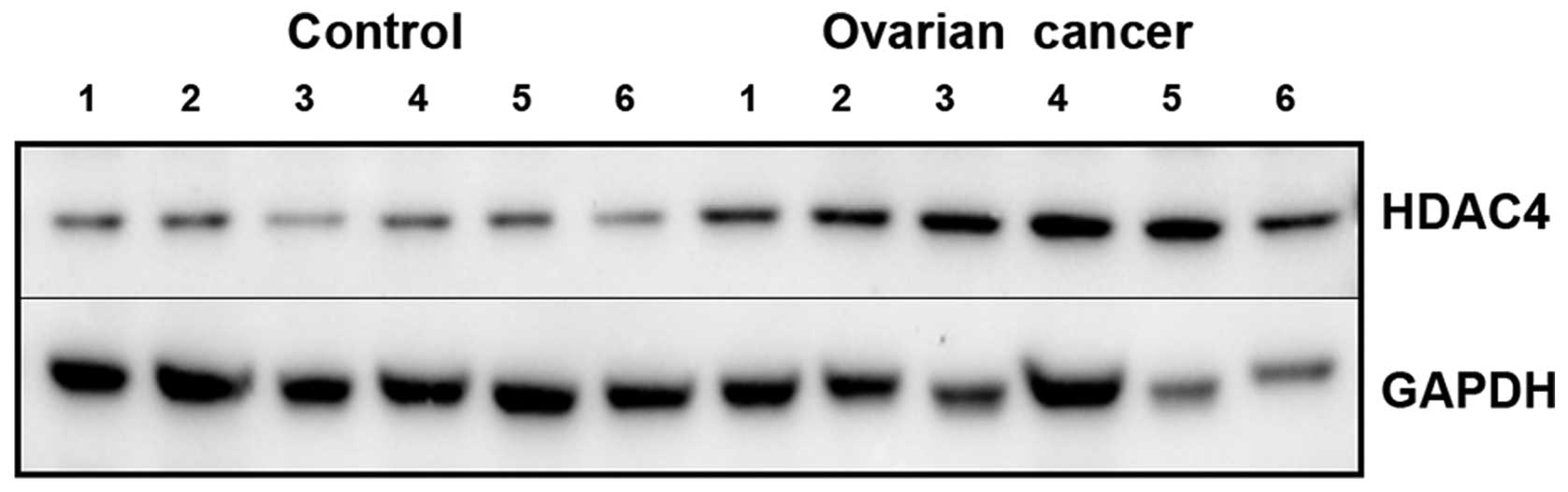
expression was increased in epithelial ovarian cancer tissues with
stage III/IV (n=24), compared to control (n=18) by western blotting
(Figs. 4B and 5). This shows that the expression rate of
HDAC4 in epithelial ovarian cancer is related to the stage of the
epithelial ovarian cancer, that is, later stage of epithelial
ovarian cancer had higher expression rate of HDAC4. These data
supported the concept of higher HDAC4 expression in epithelial
ovarian cancer might be a biomarker, which was relevant to poor
prognosis of epithelial ovarian cancer.
We also found that nuclear HDAC4 fibrillar
collagen-induced was regulated via colocalization of PP1α. Previous
data showed that fibrillar collagen matrices were abnormally
deposited in epithelial ovarian cancer tissues (Fig. 2A), leading to enhanced proliferation
and invasion of cancer cells (17).
To further investigate the role of fibrillar collagen in regulating
HDAC4 function, we examined HDAC4 location in the epithelial
ovarian cancer cell line OVCAR3 in cells seeded with or without
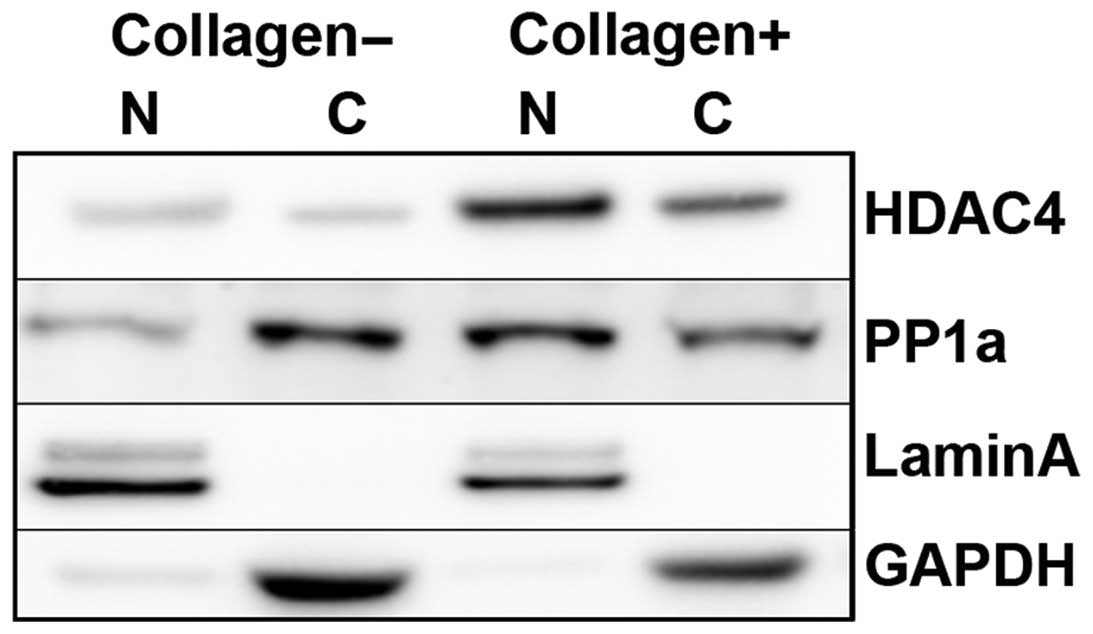
fibrillar collagen for 2 h. We found that HDAC4 expression was
increased in response to fibrillar collagen matrices. Of note, we
found that HDAC4 expression was relatively elevated in nuclear
fraction in response to fibrillar collagen (Fig. 6). HDAC4, which plays a vital role in
up- and down-regulation of the transcription factors, is one of the
most frequent epigenetic changes.
Paroni and colleagues (20–22)
reported that PP1/PP2A regulates HDAC4 cellular fraction via
phosphorylation of HDAC4. To explore whether the HDAC4 was retained
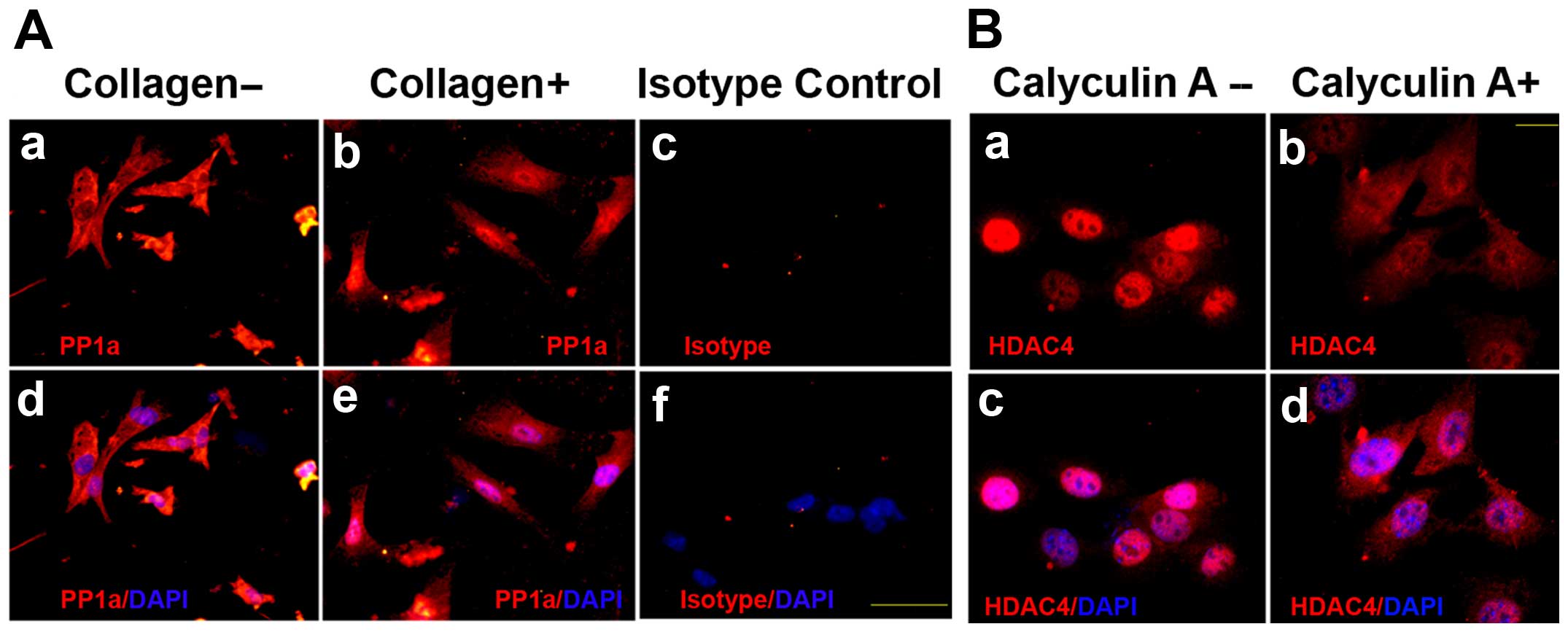
in the nucleus by regulation of PP1α, PP1α location was examined by
subcellular fractionation assay and immunofluorescence in
epithelial ovarian cancer cells in response to fibrillar collagen.
The results showed that PP1α expression was also retained and
colocalized with HDAC4 in the nucleus on fibrillar collagen
(Figs. 6 and 3A). To ascertain whether HDAC4 mainly
remained in the nucleus via regulation of PP1α on fibrillar
collagen, OVCAR3 cells were seeded on fibrillar collagen matrices
for 2 h with or without PP1α pretreatment with 5 nM calyculin A.
The images by immunofluorescence showed that HDAC4 expression in
nuclear fraction was decreased in cells with calyculin A, compared
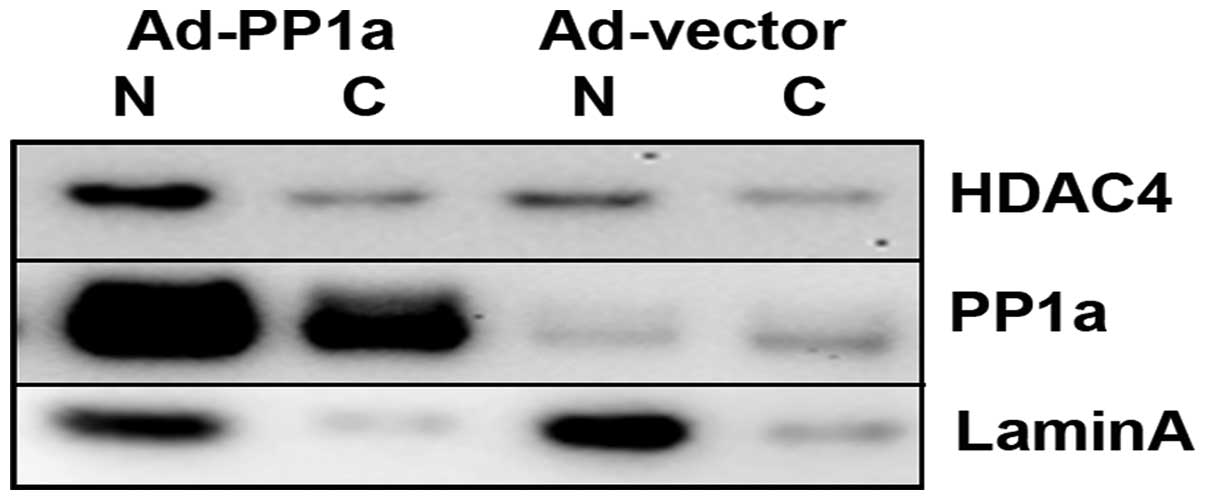
to that without calyculin A on fibrillar collagen (Fig. 3B). We confirmed the HDAC4 in the
nucleus was increased through regulation of PP1α by overexpression
of PP1α in adenoviral vector in OVCAR3 cells. The results of
western blotting showed that HDAC4 expression was increased in the
nucleus fraction (Fig. 7).
HDAC4 promotes the proliferation via
repression of p21 in epithelial ovarian cancer
Sp1 is involved in the regulation of many tumors.
Prior studies showed that HDAC4 mediated p21 through either
p53-dependent or p53-independent pathway (30,31).
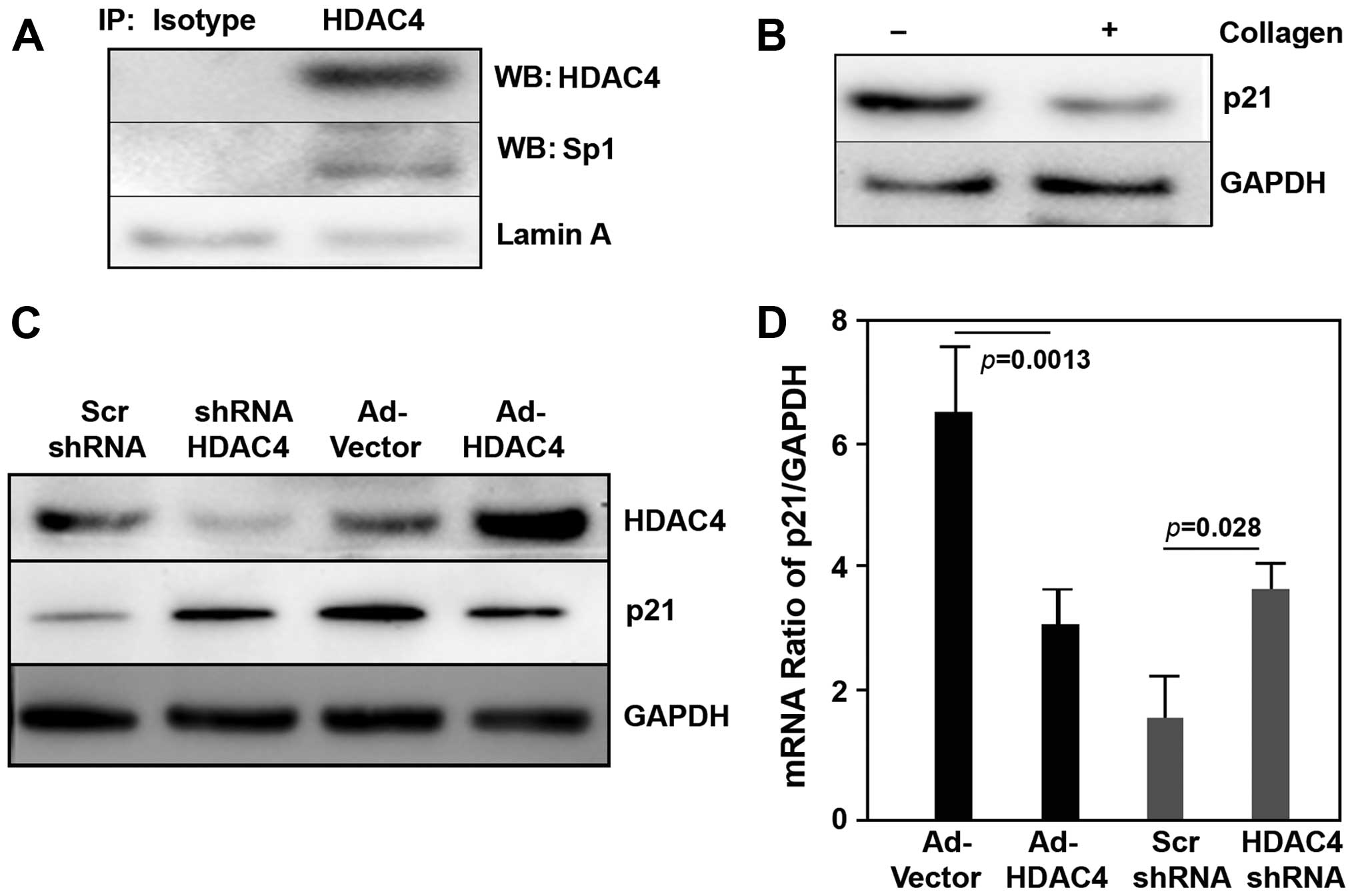
We investigated the HDAC4 interaction with Sp1 in the nuclear
fraction of OVCAR3 cells using immunoprecipitation assay with
anti-HDAC4 antibody. We found that HDAC4 bound to Sp1 in epithelial
ovarian cancer cells (Fig. 8A). we
also examined that p21 expression was suppressed when OVCAR3 cells
were seeded on fibrillar collagen (Fig.
8B), whereas p53 did not change.
To directly test whether HDAC4/Sp1 complex regulated
the downstream p21 signal, both protein and mRNA of p21 were
examined by western blotting and qPCR when OVCAR3 cells were
performed via gain/loss-of-function of HDAC4 by either
overexpression of HDCA4 or knock-down of HDAC4 with shRNA. The
results showed that overexpression of HDCA4 markedly decreased
protein and mRNA level of p21 (Fig. 8C
and D). In contrast, knock-down of HDAC4 significantly
increased protein and mRNA of p21 (Fig.
8C and D). While we did not find that the gain/loss of HDAC4
regulated protein and mRNA of p53 in these experiments. These data
supported a model of increased nuclear HDAC4/Sp1 complex, which in
turn repressed p21, promoting the aggressive epithelial ovarian
cancer in response to fibrillar collagen matrices.
To ascertain whether the nuclear HDAC4 operated the
proliferation and migration of epithelial ovarian cancer cells
through its effect on p21 signal on fibrillar collagen matrices, we
knocked down HDAC4 by shRNA in epithelial ovarian cancer cells. We
performed analysis of colony formation in matrigel and migration by
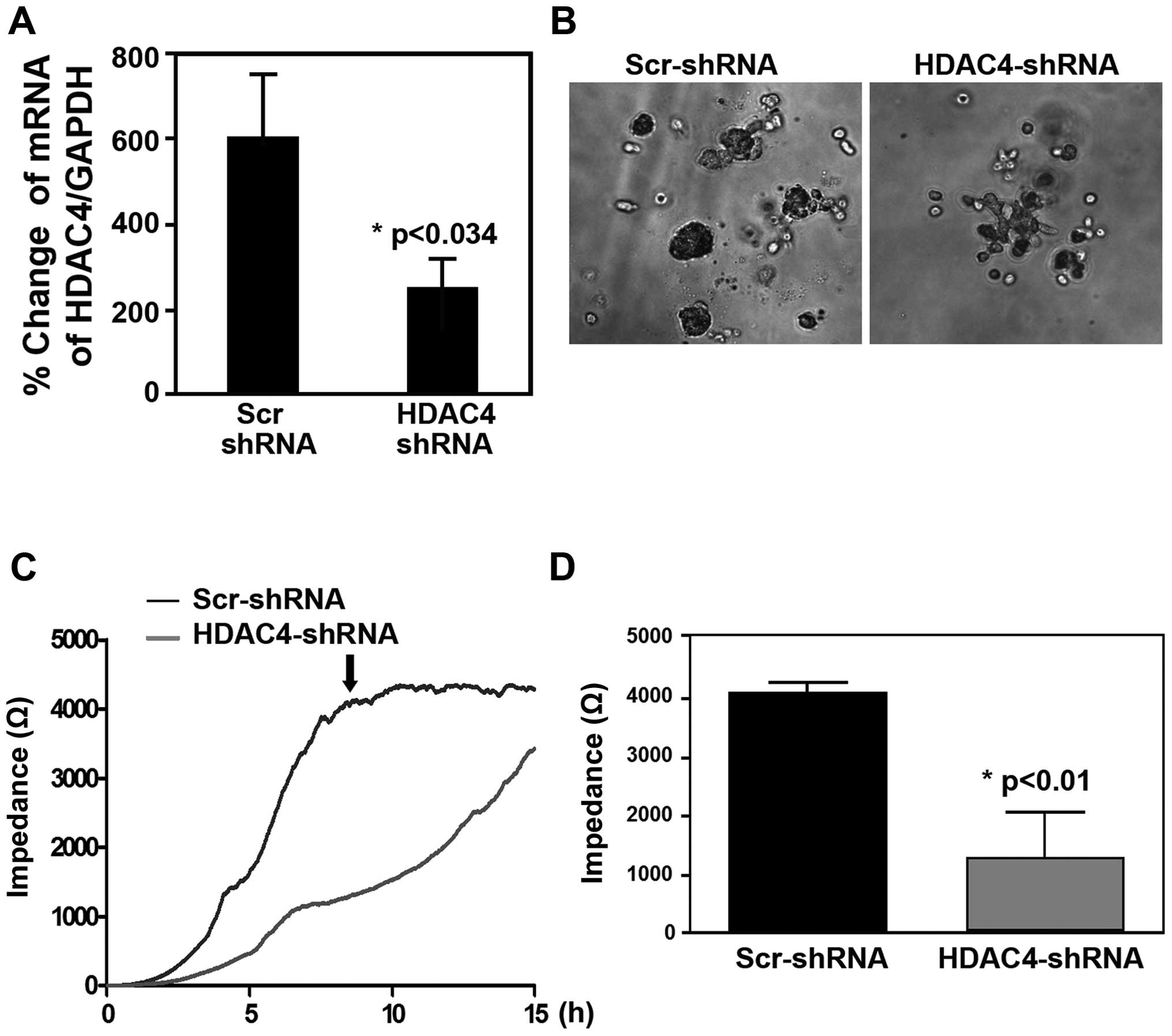
ECIS. The knock-down results showed that i) ~60% of HDAC4 by shRNA
inhibited the proliferation and colony formation (Fig. 9A and B); ii) knock-down of HDAC4
suppressed the migration of epithelial ovarian cancer cells
(Fig. 9C). Together, these data
indicated that increased nuclear HDAC4/Sp1 complex played a
critical role in leading to enhancement of progressive epithelial
ovarian cancer cells via repressing p21.
Discussion
Recent evidence of epigenetic disorders has led to
the discovery of a potential approach to screen and treat
epithelial ovarian cancer (2,3,5,6).
Previous studies revealed that fibrillar collagen matrices promoted
tumor progression (17,32,33).
Our initial data indicate that the epithelial ovarian cancer cells
with stage III/IV have higher HDAC4 expression than those with
stage I/II. However, the mechanism of aberrant regulation of HDAC4
by fibrillar collagen in epithelial ovarian cancer remained
unclear. In this study, we demonstrated that an aberrant nuclear
HDAC4/Sp1 complex via co-localization of PP1α increases the
aggressive epithelial ovarian cancer cells through repressing p21
genes when epithelial ovarian cancer cells interact with fibrillar
collagen matrices. These data indicate an important role of
HDAC4/Sp1/PP1α/p21 axis, which is a hallmark feature of epithelial
ovarian cancer, in regulation of cancer cell behavior,
Studies in cancer indicate that cross-linked type I
collagen matrices affect tumor progression. It is consistent with
our finding that fibrillar collagen enhances proliferation and
invasion of epithelial ovarian cancer. Of note, we demonstrate that
increase of nuclear HDAC4 and PP1α co-localization is in response
to fibrillar collagen. Changes in the pathologic ECM
microenvironment can reprogram cells through epigenetic modifying
mechanisms, which include alteration of histone modifications via
HDACs activity. Deacetylation of histones by HDACs results in
chromatin compaction, which represses gene transcription. HDAC4
shutting between cytoplasm and nucleus is controlled by its
phosphorylation state. PP1 is a critical regulator of HDAC4
functions (21,22). As HDAC4 is phosphorylated by PP1,
HDAC4 is exported and kept in the cytoplasm where it is susceptible
to degradation by the proteasome. Tissue specimens in vivo
showed that ~88% of patients with epithelial ovarian cancer with
stage III/IV have higher nuclear expression, compared to ~14% of
that with stage I/II.
In vitro, we discover that HDAC4 aberrantly
remains in the nucleus of epithelial ovarian cancer cell via
colocalization with PP1α on fibrillar collagen. We further
confirmed that increase of HDAC4 in the nucleus of epithelial
ovarian cancer cells is regulated by fibrillar collagen or
overexpression of PP1α. In contrast, we showed that PP1α inhibitor
leads to nuclear HDAC4 reduction. Although HDAC4 undergoes nuclear
import or retentive nucleus when HDAC4 is dephosphorylated by PP1α
we cannot eliminate PP2A regulation. The PP2A function is regulated
by expression, localization and holoenzyme composition (20). PP2A location might be a reason why
PP2A plays a dual role in up or down-regulating signals in the
cells.
It has been reported that microenvironmental changes
play a role in regulating cell behavior, which eventually causes
epigenetic disorders in cancer cells (34,35).
Additionally, we found that both protein and mRNA of p21 are
suppressed when epithelial ovarian cancer cells are seeded on
fibrillar collagen. It is related to the increase of nuclear HDCA4,
which represses p21 transcription and then leads to decreasing p21
protein. In this case, p53 does not changed significantly in
epithelial ovarian cancer cells in response to fibrillar collagen
(data not shown).
Upon nuclear HDAC4 accumulation, HDAC4 forms a
complex with a variant of transcription factors, which repress
their function and alter gene transcription (31). Prior studies indicated that HDCA4
might complex with Sp1, or Mef (36), which bound to transcription factors.
Kang et al (31) showed that
HADC4/Sp1 complex repressed p21 expression in cancer cells via Sp1
binding the promoter of p21. To verify this, we performed a series
of experiments. We examined HDAC4 binding to Sp1 epithelial ovarian
cancer cells by immunoprecipitation. Then, we demonstrated that
protein and mRNA of p21 were negatively affected by know-down or
overexpression of HDAC4. Our data indicate that the increase of
HDCA4 collagen-matrices-induced in nucleus suppresses the
mRNA/protein expression of p21, leading to promoting the
proliferation of epithelial ovarian cancer cells. Noteworthy,
change of HDAC does not affect mRNA/protein of p53, which is
usually considered as one of the regulators of p21. Finally, we
demonstrated that loss-of-function of HDAC4 by shRNA can inhibit
the colony formation and migration of epithelial ovarian cancer
cells. These data are consistent with the previous findings
(30). Despite this progress, we
have not constructed the relationship between HDAC4 and survival;
this work will be done in the future.
In summary, our data demonstrate that the
accumulation of HDAC4 which was induced by fibrillar collagen
matrices in the nucleus via co-localization of PP1α, leads to
repressing the mRNA/protein of p21, and in turn promotes the
proliferation and migration of epithelial ovarian cancer cells.
References
|
1
|
Engel J, Eckel R, Schubert-Fritschle G,
Kerr J, Kuhn W, Diebold J, Kimmig R, Rehbock J and Hölzel D:
Moderate progress for ovarian cancer in the last 20 years:
Prolongation of survival, but no improvement in the cure rate. Eur
J Cancer. 38:2435–2445. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
van der Burg ME: Advanced ovarian cancer.
Curr Treat Options Oncol. 2:109–118. 2001. View Article : Google Scholar
|
|
3
|
Watanabe T: Investigational histone
deacetylase inhibitors for non-Hodgkin lymphomas. Expert Opin
Investig Drugs. 19:1113–1127. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Horwitz SM: The emerging role of histone
deacetylase inhibitors in treating T-cell lymphomas. Curr Hematol
Malig Rep. 6:67–72. 2011. View Article : Google Scholar
|
|
5
|
Modesitt SC and Parsons SJ: In vitro and
in vivo histone deacetylase inhibitor therapy with vorinostat and
paclitaxel in ovarian cancer models: Does timing matter? Gynecol
Oncol. 119:351–357. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Egger G, Liang G, Aparicio A and Jones PA:
Epigenetics in human disease and prospects for epigenetic therapy.
Nature. 429:457–463. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jones PA and Baylin SB: The fundamental
role of epigenetic events in cancer. Nat Rev Genet. 3:415–428.
2002.PubMed/NCBI
|
|
8
|
Jones PA and Baylin SB: The epigenomics of
cancer. Cell. 128:683–692. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Baylin SB and Ohm JE: Epigenetic gene
silencing in cancer -a mechanism for early oncogenic pathway
addiction? Nat Rev Cancer. 6:107–116. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Takai N, Desmond JC, Kumagai T, Gui D,
Said JW, Whittaker S, Miyakawa I and Koeffler HP: Histone
deacetylase inhibitors have a profound antigrowth activity in
endometrial cancer cells. Clin Cancer Res. 10:1141–1149. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Takai N, Kawamata N, Gui D, Said JW,
Miyakawa I and Koeffler HP: Human ovarian carcinoma cells: Histone
deacetylase inhibitors exhibit antiproliferative activity and
potently induce apoptosis. Cancer. 101:2760–2770. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Timmermann S, Lehrmann H, Polesskaya A and
Harel-Bellan A: Histone acetylation and disease. Cell Mol Life Sci.
58:728–736. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Murdoch WJ: Ovarian surface epithelium,
ovulation and carcinogenesis. Biol Rev Camb Philos Soc. 71:529–543.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Agarwal R and Kaye SB: Ovarian cancer:
Strategies for overcoming resistance to chemotherapy. Nat Rev
Cancer. 3:502–516. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hayashi A, Horiuchi A, Kikuchi N, Hayashi
T, Fuseya C, Suzuki A, Konishi I and Shiozawa T: Type-specific
roles of histone deacetylase (HDAC) overexpression in ovarian
carcinoma: HDAC1 enhances cell proliferation and HDAC3 stimulates
cell migration with downregulation of E-cadherin. Int J Cancer.
127:1332–1346. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Weichert W, Denkert C, Noske A,
Darb-Esfahani S, Dietel M, Kalloger SE, Huntsman DG and Köbel M:
Expression of class I histone deacetylases indicates poor prognosis
in endometrioid subtypes of ovarian and endometrial carcinomas.
Neoplasia. 10:1021–1027. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shen Y, Shen R, Ge L, Zhu Q and Li F:
Fibrillar type I collagen matrices enhance metastasis/invasion of
ovarian epithelial cancer via β1 integrin and PTEN signals. Int J
Gynecol Cancer. 22:1316–1324. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Isaacs JT, Antony L, Dalrymple SL, Brennen
WN, Gerber S, Hammers H, Wissing M, Kachhap S, Luo J, Xing L, et
al: Tasquinimod is an allosteric modulator of HDAC4 survival
signaling within the compromised cancer microenvironment. Cancer
Res. 73:1386–1899. 2013. View Article : Google Scholar :
|
|
19
|
Geng H, Harvey CT, Pittsenbarger J, Liu Q,
Beer TM, Xue C and Qian DZ: HDAC4 protein regulates HIF1α protein
lysine acetylation and cancer cell response to hypoxia. J Biol
Chem. 286:38095–38102. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Paroni G, Cernotta N, Dello Russo C,
Gallinari P, Pallaoro M, Foti C, Talamo F, Orsatti L, Steinkühler C
and Brancolini C: PP2A regulates HDAC4 nuclear import. Biol Cell.
19:655–667. 2008.
|
|
21
|
Perry RLS, Yang C, Soora N, Salma J,
Marback M, Naghibi L, Ilyas H, Chan J, Gordon JW and McDermott JC:
Direct interaction between myocyte enhancer factor 2 (MEF2) and
protein phosphatase 1α represses MEF2-dependent gene expression.
Mol Cell Biol. 29:3355–3366. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu Y, Randall W and Schneider MF:
Activity-dependent and -independent nuclear fluxes of HDAC4
mediated by different kinases in adult skeletal muscle. J Chem
Biol. 168:887–897. 2005.
|
|
23
|
Li J, Chen J, Ricupero CL, Hart RP,
Schwartz MS, Kusnecov A and Herrup K: Nuclear accumulation of HDAC4
in ATM deficiency promotes neurodegeneration in ataxia
telangiectasia. Nat Med. 18:783–790. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
O'Mahony J and Hill C: A real time PCR
assay for the detection and quantitation of Mycobacterium avium
subsp. paratuberculosis using SYBR Green and the Light Cycler. J
Microbiol Methods. 51:283–293. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Krusche CA, Vloet AJ, Classen-Linke I, von
Rango U, Beier HM and Alfer J: Class I histone deacetylase
expression in the human cyclic endometrium and endometrial
adenocarcinomas. Hum Reprod. 22:2956–2966. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Weichert W, Röske A, Gekeler V, Beckers T,
Ebert MP, Pross M, Dietel M, Denkert C and Röcken C: Association of
patterns of class I histone deacetylase expression with patient
prognosis in gastric cancer: A retrospective analysis. Lancet
Oncol. 9:139–148. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ozdağ H, Teschendorff AE, Ahmed AA, Hyland
SJ, Blenkiron C, Bobrow L, Veerakumarasivam A, Burtt G,
Subkhankulova T, Arends MJ, et al: Differential expression of
selected histone modifier genes in human solid cancers. BMC
Genomics. 7:902006. View Article : Google Scholar
|
|
28
|
Ahn MY, Kang DO, Na YJ, Yoon S, Choi WS,
Kang KW, Chung HY, Jung JH, Min do S and Kim HS: Histone
deacetylase inhibitor, apicidin, inhibits human ovarian cancer cell
migration via class II histone deacetylase 4 silencing. Cancer
Lett. 28(325): 189–199. 2012. View Article : Google Scholar
|
|
29
|
Stronach EA, Alfraidi A, Rama N, Datler C,
Studd JB, Agarwal R, Guney TG, Gourley C, Hennessy BT, Mills GB, et
al: HDAC4-regulated STAT1 activation mediates platinum resistance
in ovarian cancer. Cancer Res. 71:4412–4422. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mottet D, Pirotte S, Lamour V, Hagedorn M,
Javerzat S, Bikfalvi A, Bellahcène A, Verdin E and Castronovo V:
HDAC4 represses p21(WAF1/Cip1) expression in human cancer cells
through a Sp1-dependent, p53-independent mechanism. Oncogene.
28:243–256. 2009. View Article : Google Scholar
|
|
31
|
Kang ZH, Wang CY, Zhang WL, Zhang JT, Yuan
CH, Zhao PW, Lin YY, Hong S, Li CY and Wang L: Histone deacetylase
HDAC4 promotes gastric cancer SGC-7901 cells progression via p21
repression. PLoS One. 9:e988942014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Fenner J, Stacer AC, Winterroth F, Johnson
TD, Luker KE and Luker GD: Macroscopic stiffness of breast tumors
predicts metastasis. Sci Rep. 4:55122014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kaemmerer E, Melchels FP, Holzapfel BM,
Meckel T, Hutmacher DW and Loessner D: Gelatine
methacrylamide-based hydrogels: an alternative three-dimensional
cancer cell culture system. Acta Biomater. 10:2551–2562. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Curry JM, Sprandio J, Cognetti D,
Luginbuhl A, Bar-ad V, Pribitkin E and Tuluc M: Tumor
microenvironment in head and neck squamous cell carcinoma. Semin
Oncol. 41:217–234. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Cohen AL, Piccolo SR, Cheng L, Soldi R,
Han B, Johnson WE and Bild AH: Genomic pathway analysis reveals
that EZH2 and HDAC4 represent mutually exclusive epigenetic
pathways across human cancers. BMC Med Genomics. 6:352013.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Liu F, Pore N, Kim M, Voong KR, Dowling M,
Maity A and Kao GD: Regulation of histone deacetylase 4 expression
by the SP family of transcription factors. Mol Biol Cell.
17:585–597. 2006. View Article : Google Scholar :
|