Introduction
Radiation-induced lung injury (RILI), in the form of
pneumonitis and fibrosis, is fairly common in patients receiving
thoracic radiation therapy. Unfortunately, the prognosis of
patients with RILI is poor with a median survival time of less than
3 years (1,2). Currently, extensive studies have been
conducted to investigate the relationship between cytokines and the
pathogenesis of RILI (3,4). For example, overproduction of several
cytokines appears to be related to the development of acute and
late pulmonary toxicities after RILI, including pro-inflammatory
cytokines such as interleukin-1α (IL-1α), IL-1β, interferon
(IFN)-γ, IL-6, tumor necrosis factor α (TNF-α), and pro-fibrogenic
cytokines such as transforming growth factor β1 (TGF-β1) and TGF-α
(5–9).
Recently, accumulative evidence suggests that
mesenchymal stem cells (MSCs), as gene therapy delivery vehicles,
are involved in the repair of lung tissue damage by differentiating
into functional cells and facilitating lung tissue regeneration by
means of the generation of cytokines (10,11).
For example, hepatocyte growth factor gene-modified MSCs
contributed to the attenuation of RILI and inhibition of lung
fibrosis (12). In addition,
Asmussen et al (13)
reported that human MSCs contributed to the improvement of
oxygenation and attenuation of pulmonary oedema, especially in a
high-dose group. Moreover, Devaney et al (14) revealed that a high dose of hMSCs was
most effective in reducing E. coli-induced lung injury
compared with a low-dose group. In the present study, we
hypothesized that the therapeutic potential of bone marrow
(BM)-derived MSCs on RILI may function in a dose-dependent manner.
To this end, three different doses of hBM-MSCs were administrated
in a mouse model of RILI to evaluate the therapeutic effects of
different doses of hBM-MSCs in vivo. Our results revealed
that low-dose hBM-MSCs contributed to functional recovery in mice
with RILI.
Materials and methods
Cell culture
hBM-MSCs, kindly provided by the Cancer Center of
the First Bethune Hospital of Jilin University (Changchun, China),
were seeded into a flask with basal MSC medium supplemented with 5%
fetal bovine serum (FBS), and 1% mesenchymal stem cell growth
supplement (MSCGS) and 1% penicillin/streptomycin (Biowit
Technologies, Shenzhen, China). Subsequently, the cells were
cultured at 37°C with 5% CO2 in a humidified atmosphere.
Passaging was conducted every 2–3 days, and cells of passage 5 (P5)
were used in the present study.
Animals
Mice (weighing 20±3 g) of the same genetic
background were purchased from HFK Bioscience Co., Ltd. (Beijing,
China). All animals were maintained under specific pathogen-free
conditions, and had free access to water and a standard rodent diet
provided by the Animal Center of Jilin University (Changchun,
China). The study protocols were approved by the Animal Care and
Use Committee of the Chinese Academy of Medical Sciences (Beijing,
China).
Induction of RILI
To establish the RILI model, the whole lung was
exposed to irradiation with a dose rate of 1,500 mGy/min using a
low pass energy X-RAD 320 X-ray system (Precision X-ray; North
Branford, CT, USA). The total dose was 18 Gy. The voltage was set
at 300 kV, and the current was 11.79 mA.
Experimental design
A total of 100 mice were randomly divided into: i) a
control group (n=25), which was subject to lung irradiation
followed by injection of phosphate-buffered solution (PBS) via the
tail vein; ii) a low-dose hBM-MSC group which was subject to lung
irradiation followed by injection of 1×103 hBM-MSCs/g in
PBS through the tail vein; iii) a medium-dose hBM-MSC group which
was subject to lung irradiation followed by 5×103
hBM-MSCs/g through the tail vein; and iv) a high-dose hBM-MSC group
which was subject to lung irradiation followed by 1×104
hBM-MSCs/g through the tail vein. The delivery of hBM-MSCs was
performed within 24 h after radiation.
Three mice randomly selected in each group were
sacrificed under euthanasia on day 3, 7, 14, 28 and 84 after
irradiation. Eye venous blood was collected to determine the
cytokine levels using enzyme-linked immunosorbent assay
(ELISA).
ELISA
Cytokine levels including HGF, IL-10 and TGF-β1, and
Col were measured using standard ELISA kits purchased from R&D
Systems (Minneapolis, MN, USA), eBioscience (San Diego, CA, USA)
and USCN (Wuhan, China), according to the manufacturer's
instructions.
Flow cytometry
Flow cytometry was carried out to determine the
expression of cell-surface markers of the MSCs. Cells (P5) were
fixed for 30 min in ice-cold 1% paraformaldehyde. Afterwards, the
cells were stained at room temperature in the dark with the
following primary mouse anti-human antibodies purchased from BD
Biosciences (Franklin Lakes, NJ, USA): PE-conjugated CD11b,
FITC-conjugated CD19, FITC-conjugated CD34, PE-conjugated CD45,
PE-conjugated CD73, PE-conjugated CD90, PE-conjugated CD105 and
PE-conjugated HLA-DR. FITC- or PE-conjugated mouse IgG1 served as
the isotype control.
Tri-lineage differentiation
Cells (P5) were induced to differentiate into
adipocytes, osteoblasts and chondrocytes using the
StemPro® Adipogenesis differentiation kit,
StemPro® Osteogenesis differentiation kit, and
StemPro® Chondrogenesis differentiation kit (Life
Technologies, Carlsbad, CA, USA) according to the manufacturer's
instructions. The medium was replaced every 2–3 days. Approximately
21 days later, cell identification was performed by staining with
Red Oil O, Alizarin Red and Aniline Blue, respectively.
Histological analysis
The left lungs were fixed using 10% neutral formalin
for 8–10 h, followed by embedding with paraffin. Then the sections
(4-µm) were subjected to hematoxylin and eosin (H&E)
staining, Masson's trichrome staining and immunohistochemical
staining, respectively. The images were evaluated by two qualified
staff blinded to the details of this study using an Olympus BX51
microscope (Olympus, Tokyo, Japan).
Real-time PCR analysis
Total RNA was extracted from lung tissues using
TRIzol (Invitrogen Inc., Carlsbad, CA, USA). The cDNA synthesis of
TNF-α was performed using 1 µg RNA using M-MLV reverse
transcriptase (Takara, Shiga, Japan). Real-time PCR amplification
was carried out using SYBR on a Life 7500 Fast system (Life
Technologies) with the following primers: TNF-α,
5′-ATCCgCgACgTggAACTg-3′ and 5′-ACCgCCTggAgTTCTggAA-3′; and
β-actin, 5′-TGAGC TGCGTTTTACACCCT-3′ and 5′-AGGGTGAGGGACTTCC
TGTAA-3′. PCR reactions were performed using a total of 20
µl containing 10 µl 2X SYBR Premix, 0.6 µl of
each specific primer to a final concentration of 300 nM, and 1
µl cDNA template. PCR was performed under the following
conditions: degeneration at 95°C for 15 min, followed by 40 cycles
of denaturation at 95°C for 10 sec, annealing at 65°C for 30 sec
and extension at 72°C for 30 sec. The mRNA level was normalized by
β-actin. The amplification results were calculated as
2−ΔΔCt according to a previous description (15).
Immunofluorescent staining
Immunofluorescent staining was performed to evaluate
the tri-lineage differentiation capacity of hBM-MSCs in vivo
by analyzing the expression of SPC and PECAM. In this section, 10
immunodeficiency mice were randomly divided into a non-radiation +
hBM-MSC group, subject to injection of 1×104 hBM-MSCs/g
in PBS through the tail vein; and a lung irradiation + hBM-MSC
group, subject to lung irradiation followed by injection of
1×104 hBM-MSCs/g in PBS through the tail vein. The
animals were sacrificed 56 days after injection, and the left lung
was collected. Subsequently, the tissues were dewaxed in xylene and
rehydrated with a gradient series of ethanol. Thereafter, the
sections were incubated with the primary antibody against β2
microglobulin, SPC and PECAM at 4°C overnight, followed by
incubation with the secondary antibodies for 2 h at room
temperature. The images were observed using PerkinElmer UltraView
Vox confocal microscopy (PerkinElmer, Waltham, MA, USA).
Statistical analysis
Data are presented as the mean ± standard deviation.
Statistical evaluation was performed using analysis of variance
with a Tukey's post hoc test. P<0.05 was indicative of a
significant difference.
Results
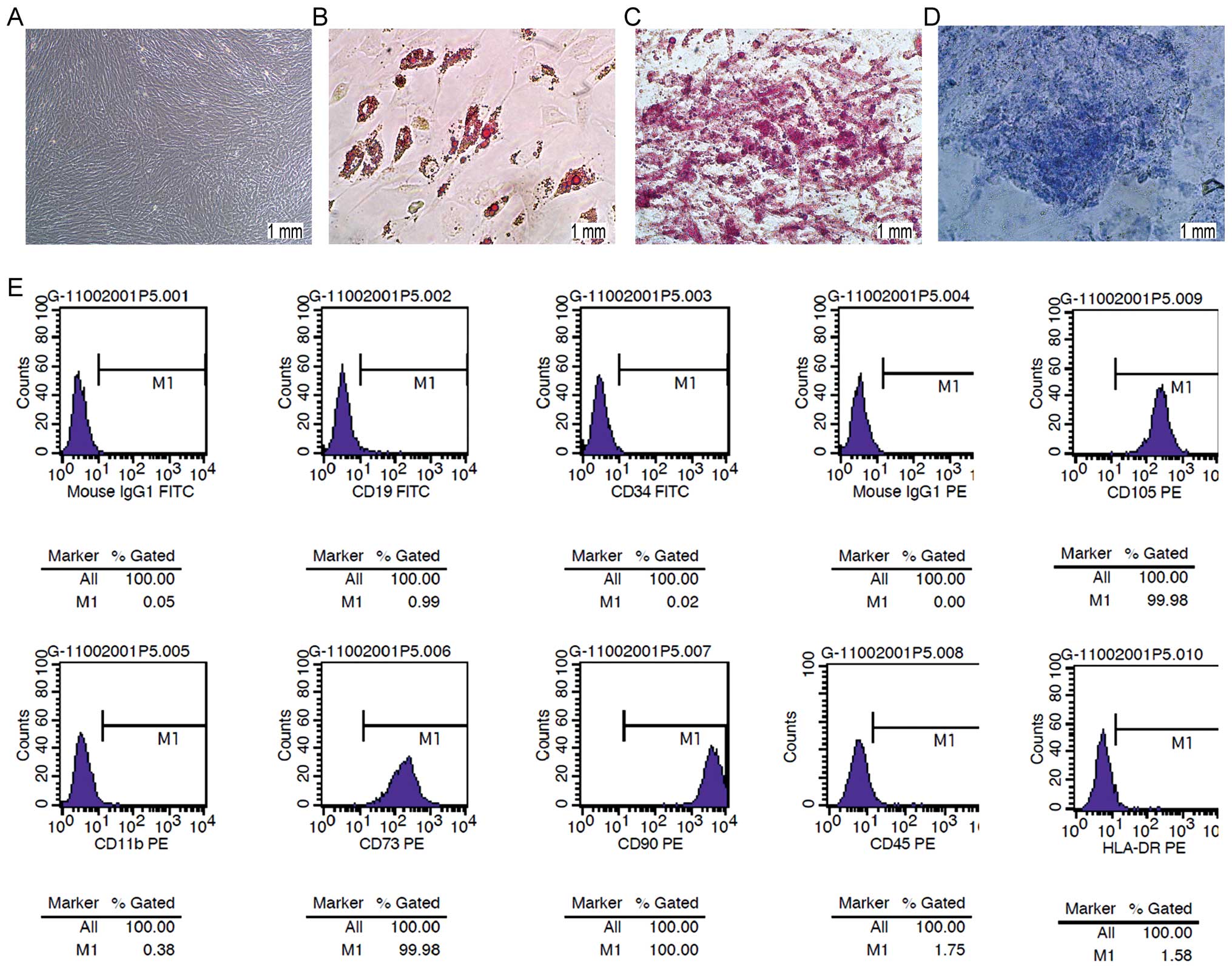
Morphology and features of hBM-MSCs
Under in vitro conditions, hBM-MSCs are
characterized by their spindle-like shape and clear cellular
boundaries (Fig. 1A). In addition,
hBM-MSCs show the capability of differentiating into adipocytes,
osteoblasts and chondrocytes after culturing in defined medium
(Fig. 1B–D). Cell-surface marker
analysis showed that the hBM-MSCs represented a population of cells
with expression of CD73, CD90 and CD105, and absence of hemapoietic
markers such as CD11b, CD19, CD34 and CD45 (Fig. 1E).
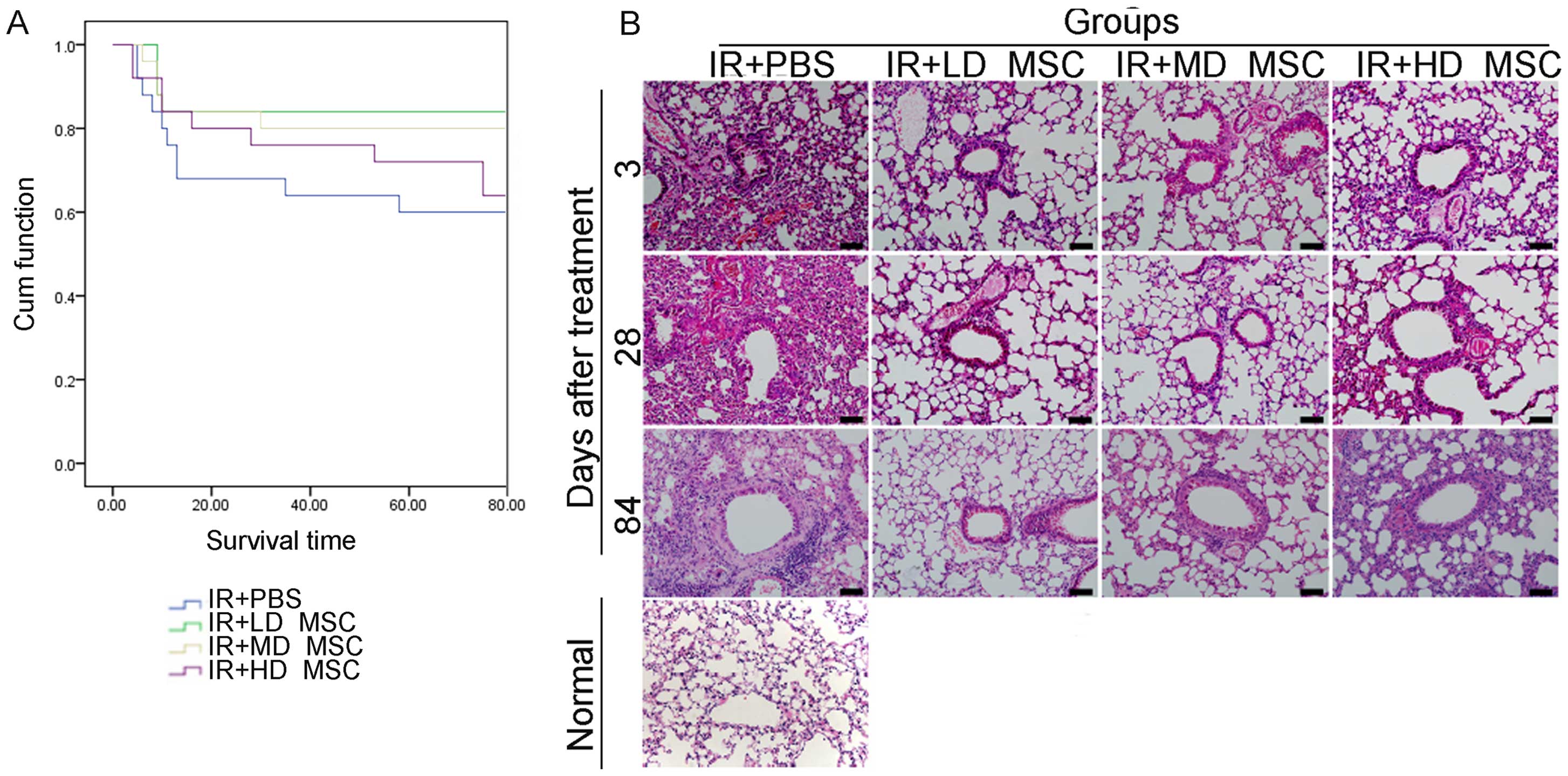
Low-dose hBM-MSCs improve the survival
rate and histopathological features in irradiated mice
The survival rate of animals in the hBM-MSC
treatment groups was higher than the rate in the irradiation alone
group. The survival rate of the mice in the low-dose MSC group was
higher than that of mice in the high-dose group (Fig. 2A). For the histopathological
results, RILI-associated features were initially observed on day 3
and were characterized by degradation of capillaries within
alveolar septa and extravasation of erythrocytes into alveolar
spaces (Fig. 2B). On day 28,
alveolar structures with pathological lesions were observed in the
lung with infiltration of inflammatory cells in the interstitial
part and abnormal stromal hyperplasia. On day 84, infiltration of
inflammatory cells and severe interstitial hyperplasia were
observed. Compared with the control group, reduction in airspace
inflammation and alveolar hemorrhage was observed in the groups
treated with hBM-MSCs. In addition, the thickness of the
interalveolar septa was modestly increased in the control group.
Whereas, a slight reduction was noted in the thickness of the
interalveolar septa in the high-dose group, while a significant
reduction was noted in that of the low-dose group. These features
may be responsible for the differences in the survival rate of the
mice in each group.
hBM-MSCs decrease the collagen deposition
in radiation lung injury
Masson staining on day 28 showed alveolar,
bronchial, and vascular collagen deposition, which was increased on
day 84 (Fig. 3A). Treatment of
hBM-MSCs contributed to a decrease in collagen deposition in the
radiation lung injury, especially in the low-dose group.
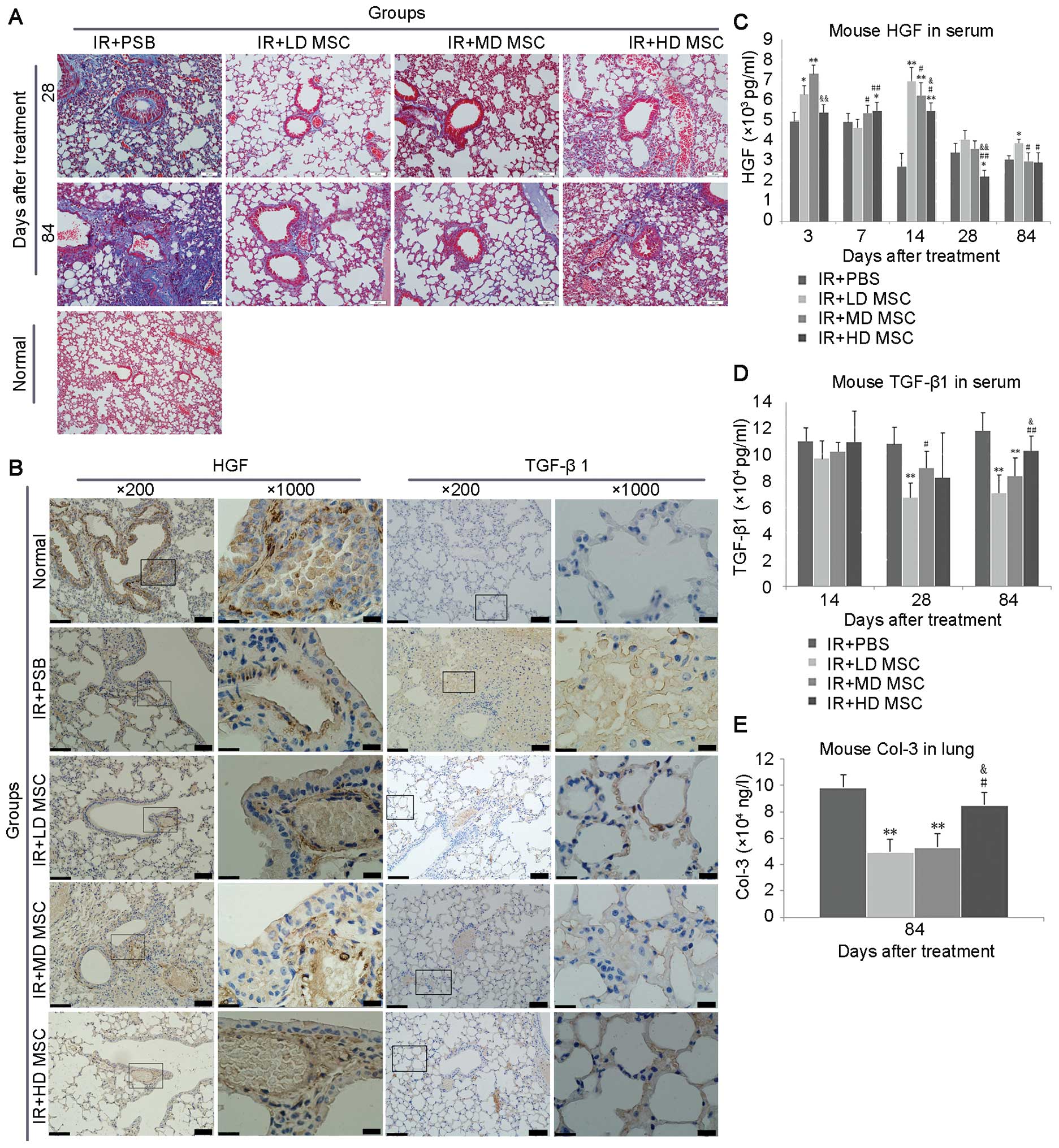 | Figure 3Expression of fibrosis-related
markers in each group. (A) Collagen deposition was markedly induced
in mice following administration of hBM-MSCs, especially in the
low-dose group, compared with the irradiation alone group. (B)
overexpression of HGF was observed in normal mice, while its
expression was significantly decreased in the irradiation alone
group. However, after interference of hBM-MSCs, the expression of
HGF was increased compared with the irradiation alone group.
hBM-MSCs inhibited the expression of TGF-β1 compared with the
irradiation group, indicating its role in the inhibition of
fibrosis in vivo. (C–E) Expression levels of hepatocyte
growth factor (HGF), transforming growth factor (TGF)-β1 in serum
and Col-3 in lung tissues after treatment were measured by ELISA.
*P<0.05 vs. the radiation group;
**P<0.01 vs. the radiation group;
#P<0.05 vs. the low-dose MSC group.
##P<0.01 vs. the low-dose MSC group.
&P<0.05 vs. middle-dose MSC group;
&&P<0.01 vs. middle-dose MSC group. IR+PBS,
irradiation alone group; IR+LD MSC, irradiation + low-dose MSC
group; IR+MD MSC, irradiation + middle-dose MSC group; IR+HD MSC,
irradiation + high-dose MSC group. Scale bar, 20 µm. |
The expression of HGF and TGF-β1 in the stroma was
aberrant after radiotherapy (Fig.
3B). After radiation, HGF expression was downregulated in blood
vessels and bronchus. TGF-β1 was not expressed in normal lung
tissues. Marked upregulation of TGF-β1 expression was noted in the
radiation group; however, its expression was significantly
decreased in the hBM-MSC groups compared with the radiation alone
group.
HGF and TGF-β1 protein concentrations in peripheral
blood were measured by ELISA (Fig.
3C and D). Serum TGF-β1 was reduced on day 28 and 84 after
hBM-MSC interference. Compared with the non-treatment group, the
serum HGF was increased after hBM-MSC interference on day 3, 7, 14,
28 and 84, respectively (P<0.05). Regarding Colla3 protein, the
expression of Col was downregulated in the lung tissue on day 84
after hBM-MSC interference compared with the control group
(Fig. 3E). Among the hBM-MSC
groups, the expression of Col was significantly reduced in the
low-dose group compared with the middle- and high-dose groups,
respectively.
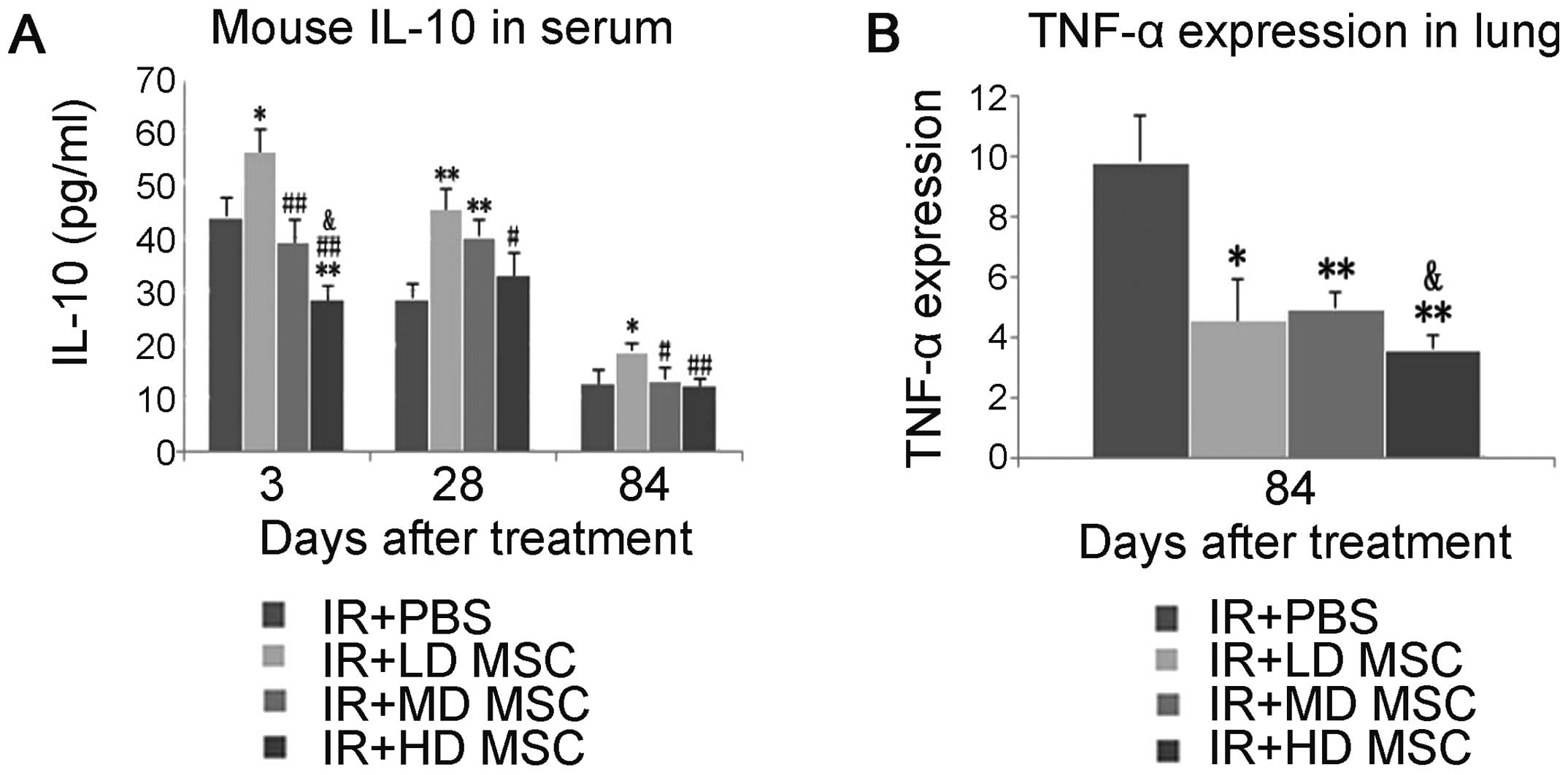
hBM-MSC therapy attenuates the secretion
and expression of pro-inflammatory cytokines and improves the
expression of anti-inflammatory cytokines
To evaluate the anti-inflammatory activity of
hBM-MSCs, IL-10 was measured by ELISA in peripheral blood on day 3,
28 and 84 after radiation (Fig.
4A). The results indicated that hBM-MSCs significantly
increased the expression of IL-10.
Real-time RT-PCR was used to detect the local
inflammatory reaction of lung tissue on day 28 after radiation.
Compared with the radiation group, hBM-MSCs reduced the expression
of TNF-α mRNA (Fig. 4B).
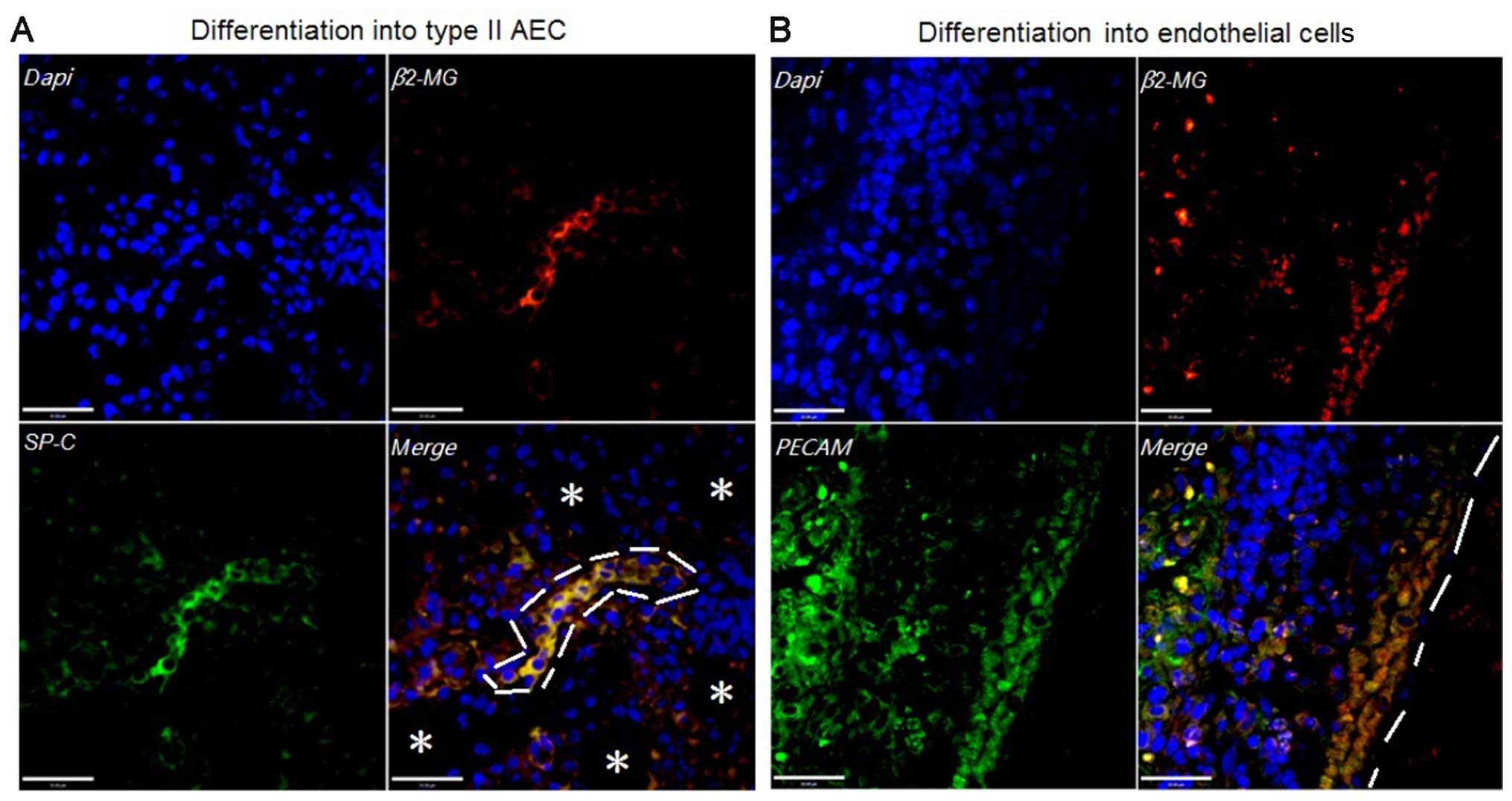
hBM-MSCs differentiate into functional
cells in the presence of lung injury
In order to prove the differentiation potential of
hBM-MSCs, 10 immunodeficiency mice were divided into a
non-radiation plus hBM-MSC (1×104 hBM-MSCs/g) group, and
a lung irradiation plus hBM-MSC (1×104 hBM-MSCs/g)
group, respectively. The epithelial cell markers, SP-C, and hBM-MSC
marker, β2 microglobulin (β2-MG), were co-expressed in the lung
irradiation plus hBM-MSC group (Fig.
5A). In addition, endothelial cell markers, PECAM and β2-MG,
were co-expressed in the lung irradiation plus hBM-MSC group
(Fig. 5B). In contrast, such
markers were not expressed in the non-radiation plus hBM-MSC group.
Taken together, the differentiation capacity of hBM-MSCs into
functional cells only occured in the presence of lung damage.
Discussion
In the present study, we hypothesized that the
therapeutic potential of BM-MSCs on RILI may occur in a
dose-dependent manner. Our results revealed that BM-MSCs could
attenuate RILI in mice compared with a control group. The survival
rate of the mice in the low-dose MSC group was higher than that of
mice in the high-dose group.
Previous studies have investigated the protective
effects of clinical grade hMSCs under in vivo conditions
using a low dose and a high dose, respectively. These results
revealed that different doses of MSCs could exert protective
effects in vivo compared with the control groups.
Interestingly, the low-dose group was proven to be the more
effective in improving the functional properties compared with the
high-dose groups (13,16–19).
In addition, a higher incidence of adverse events may occur in the
high-dose group. For example, Li et al (20) showed that the high-dose
(1.0×106 and 5.0×105) groups induced lethal
portal vein embolization (PVE) in mice with liver disease. On the
contrary, no PVE and related death was observed in the low-dose
(2.5×105) group. Similarly, a low dose of MSCs showed
greater safety and better therapeutic effects for RILI compared
with the high-dose group. Compared with the control group, the hMSC
treatment groups experienced alleviation of RILI, especially in the
low-dose group.
To investigate the potential mechanism of the
therapeutic effects of MSCs involved in RILI, we firstly assessed
the expression of fibrosis-related factors TGF-β1, HGF and Col.
TGF-β1, a major mediator involved in pro-inflammatory responses and
fibrotic tissue remodeling, has been considered to play a crucial
role in RILI (21). Meanwhile,
inflammation may promote the expression of TGF-β1, forming a
vicious circle in RILI. In the present study, the expression of
TGF-β1 in the treatment groups was markedly downregulated compared
with the irradiation group on day 28 and 84 after hBM-MSC
interference, indicating that HBM-MSCs play important roles in the
inhibition of fibrosis. HGF, associated with mitogenic, morphogenic
and anti-apoptotic activities of BM-MSCs, was found to enhance the
regeneration of the lung and to have an inhibitory effect on
fibrosis (22). Wang et al
(12) reported that Ad-HGF-modified
MSCs improved histopathological and biochemical markers of RILI by
attenuating the expression of inflammatory factors and fibrosis
factors (e.g., TGF-β) and inhibiting fibrotic progression. In
addition, compared with the non-transfected MSC group,
Ad-HGF-modified MSCs contributed to the improvement of RILI by
enhancing the release of endogenous HGF. Moreover, in a previous
study, Kim et al (23)
reported that MSC/HGF interference resulted in a significant
reduction in liver fibrosis associated with elevated HGF levels and
decreased TGF-β1 after MSC/HGF therapy. In the present study, a
significant difference was noted in the expression of HGF after
hBM-MSC interference compared with the control group on day 3, 14
and 28, especially the low-dose hBM-MSC group. Based on these
results, we concluded that HGF plays an important role in the
inhibition of fibrosis in RILI.
Next, we assessed the effects of BM-MSCs on
inflammation-related factors such as TNF-α and IL-10. Previous
studies revealed that murine MSCs could respond to pulmonary
injury, reduce pro-inflammatory cytokines (IL-6 and TNF-α),
collagen deposition and increase the expression levels of IL-10 and
PGE2 (10,24–28).
In addition, Lee et al showed that MSCs could restore
alveolar fluid clearance, reduce inflammation, and exert
antimicrobial activity partly through secretion of keratinocyte
growth factors (29). In our study,
hBM-MSCs appeared to be mediated by a shift from a pro-inflammatory
response to RILI, and contributed to the alleviation of radioactive
lung injury. Compared with the control group, downregulation of
TNF-α and upregulation of IL-10 were observed in the BM-MSC group,
especially the low-dose hBM-MSC group.
BMSCs have been reported to have the capacity to
differentiate into various cell types, including endothelial cells,
epithelial cells (30), adipocytes
and osteocytes (31–33). In the present study, infrequent
BM-MSC engraftment resulted in differentiation of BM-MSCs into
epithelial cells and endothelial cells as revealed by double
immunofluorescence staining. Furthermore, few MSCs were observed
within the injured sites compared to tremendous loss of functional
cells in the impaired tissues. Thus, we speculate that the tissue
regeneration may not largely depend on direct differentiation of
MSCs into functional cells. Considering the fact that therapeutic
effects were observed even though heterogenic MSCs were rapidly
cleared by the host after transplantation, we conclude that BM-MSCs
facilitated tissue repair mainly though paracrine or autocrine
actions.
In conclusion, a low dose of hBM-MSCs is superior to
a high dose of hBM-MSCs with excellent safety and differentiation
capacity in mice with RILI. We speculate that hBM-MSCs attenuate
lung injury mainly via paracrine mechanisms, including upregulation
of HGF and IL-10, as well as downregulation of TNF-α, TGF-β1 and
Col-3. Thus, low-dose hMSCs may be a promising therapeutic approach
for the management of RILI.
Acknowledgments
This study was supported by grants from the National
Natural Science Foundation of China (grant nos. 81272999 and
81372929).
Abbreviations:
|
hBM-MSCs
|
human bone marrow-derived mesenchymal
stem cells
|
|
HGF
|
hepatocyte growth factor
|
|
IL-1α
|
interleukin-1α
|
|
IL-1β
|
interleukin-1β
|
|
IFN-γ
|
interferon-γ
|
|
IL-10
|
interleukin-10
|
|
PBS
|
phosphate-buffered solution
|
|
PVE
|
portal vein embolization
|
|
PECAM
|
platelet endothelial cell adhesion
molecule
|
|
RILI
|
radiation-induced lung injury
|
|
SPC
|
surfactant protein C
|
|
TNF-α
|
tumor necrosis factor α
|
|
TGF-β1
|
transforming growth factor β1
|
|
β2-MG
|
β2 microglobulin
|
References
|
1
|
Mehta V: Radiation pneumonitis and
pulmonary fibrosis in non-small-cell lung cancer: Pulmonary
function, prediction, and prevention. Int J Radiat Oncol Biol Phys.
63:5–24. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Xie L, Zhou J, Zhang S, Chen Q, Lai R,
Ding W, Song C, Meng X and Wu J: Integrating microRNA and mRNA
expression profiles in response to radiation-induced injury in rat
lung. Radiat Oncol. 9(111)2014. View Article : Google Scholar
|
|
3
|
Rube CE, Uthe D, Schmid KW, Richter KD,
Wessel J, Schuck A, Willich N and Rube C: Dose-dependent induction
of transforming growth factor beta (TGF-beta) in the lung tissue of
fibrosis-prone mice after thoracic irradiation. Int J Radiat Oncol
Biol Phys. 47:1033–1042. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Arpin D, Perol D, Blay JY, Falchero L,
Claude L, Vuillermoz-Blas S, Martel-Lafay I, Ginestet C, Alberti L,
Nosov D, et al: Early variations of circulating interleukin-6 and
interleukin-10 levels during thoracic radiotherapy are predictive
for radiation pneumonitis. J Clin Oncol. 23:8748–8756. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chiang CS, Liu WC, Jung SM, Chen FH, Wu
CR, McBride WH, Lee CC and Hong JH: Compartmental responses after
thoracic irradiation of mice: Strain differences. Int J Radiat
oncolBiol Phys. 62:862–871. 2005. View Article : Google Scholar
|
|
6
|
Rübe CE, Rodemann HP and Rübe C: The
relevance of cytokines in the radiation-induced lung reaction.
Experimental basis and clinical significance. Strahlenther Onkol.
180:541–549. 2004.In German.
|
|
7
|
Dhainaut JF, Charpentier J and Chiche JD:
Transforming growth factor-beta: A mediator of cell regulation in
acute respiratory distress syndrome. Crit Care Med. 31(Suppl 4):
S258–S264. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kim JY, Kim YS, Kim YK, Park HJ, Kim SJ,
Kang JH, Wang YP, Jang HS, Lee SN and Yoon SC: The TGF-β1 dynamics
during radiation therapy and its correlation to symptomatic
radiation pneumonitis in lung cancer patients. Radiat Oncol.
4(59)2009. View Article : Google Scholar
|
|
9
|
Chung EJ, Hudak K, Horton JA, White A,
Scroggins BT, Vaswani S and Citrin D: Transforming growth factor
alpha is a critical mediator of radiation lung injury. Radiat Res.
182:350–362. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Németh K, Leelahavanichkul A, Yuen PS,
Mayer B, Parmelee A, Doi K, Robey PG, Leelahavanichkul K, Koller
BH, Brown JM, et al: Bone marrow stromal cells attenuate sepsis via
prostaglandin E(2)-dependent reprogramming of host macrophages to
increase their interleukin-10 production. Nat Med. 15:42–49. 2009.
View Article : Google Scholar
|
|
11
|
Shi Y, Hu G, Su J, Li W, Chen Q, Shou P,
Xu C, Chen X, Huang Y, Zhu Z, et al: Mesenchymal stem cells: A new
strategy for immunosuppression and tissue repair. Cell Res.
20:510–518. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang H, Yang YF, Zhao L, Xiao FJ, Zhang
QW, Wen ML, Wu CT, Peng RY and Wang LS: Hepatocyte growth factor
gene-modified mesenchymal stem cells reduce radiation-induced lung
injury. Hum Gene Ther. 24:343–353. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Asmussen S, Ito H, Traber DL, Lee JW, Cox
RA, Hawkins HK, McAuley DF, McKenna DH, Traber LD, Zhuo H, et al:
Human mesenchymal stem cells reduce the severity of acute lung
injury in a sheep model of bacterial pneumonia. Thorax. 69:819–825.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Devaney J, Horie S, Masterson C, Elliman
S, Barry F, O'Brien T, Curley GF, O'Toole D and Laffey JG: Human
mesenchymal stromal cells decrease the severity of acute lung
injury induced by E. coli in the Rat. Thorax. 70:625–635. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rao X, huang X, Zhou Z and Lin X: An
improvement of the 2ˆ(-delta delta CT) method for quantitative
real-time polymerase chain reaction data analysis. Biostat
Bioinforma Biomath. 3:71–85. 2013.PubMed/NCBI
|
|
16
|
Saether EE, Chamberlain CS, Leiferman EM,
Kondratko-Mittnacht JR, Li WJ, Brickson SL and Vanderby R: Enhanced
medial collateral ligament healing using mesenchymal stem cells:
Dosage effects on cellular response and cytokine profile. Stem Cell
Rev. 10:86–96. 2014. View Article : Google Scholar :
|
|
17
|
Yavagal DR, Lin B, Raval AP, Garza PS,
Dong C, Zhao W, Rangel EB, Mcniece I, Rundek T, Sacco RL, et al:
Efficacy and dose-dependent safety of intra-arterial delivery of
mesenchymal stem cells in a rodent stroke model. PLoS One.
9:e937352014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fukuda Y, Horie N, Satoh K, Yamaguchi S,
Morofuji Y, Hiu T, Izumo T, Hayashi K, Nishida N and Nagata I:
Intra-arterial transplantation of low-dose stem cells provides
functional recovery without adverse effects after stroke. Cell Mol
Neurobiol. 35:399–406. 2015. View Article : Google Scholar
|
|
19
|
Kean TJ, Lin P, Caplan AI and Dennis JE:
MSCs: Delivery routes and engraftment, cell-targeting strategies,
and immune modulation. Stem Cells Int. 2013(732742)2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li Z, Hu X, Mao J, Liu X, Zhang L, Liu J,
Li D and Shan H: Optimization of mesenchymal stem cells (MSCs)
delivery dose and route in mice with acute liver injury by
bioluminescence imaging. Mol Imaging Biol. 17:185–194. 2015.
View Article : Google Scholar
|
|
21
|
Song YS, Lee HJ, Doo SH, Lee SJ, Lim I,
Chang KT and Kim SU: Mesenchymal stem cells overexpressing
hepatocyte growth factor (HGF) inhibit collagen deposit and improve
bladder function in rat model of bladder outlet obstruction. Cell
Transplant. 21:1641–1650. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang K, Yang S, Zhu Y, Mo A, Zhang D and
Liu L: Protection against acute radiation-induced lung injury: A
novel role for the anti-angiogenic agent Endostar. Mol Med Rep.
6:309–315. 2012.PubMed/NCBI
|
|
23
|
Kim MD, Kim SS, Cha HY, Jang SH, Chang DY,
Kim W, Suh-Kim H and Lee JH: Therapeutic effect of hepatocyte
growth factor-secreting mesenchymal stem cells in a rat model of
liver fibrosis. Exp Mol Med. 46:e1102014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ortiz LA, Gambelli F, McBride C, Gaupp D,
Baddoo M, Kaminski N and Phinney DG: Mesenchymal stem cell
engraftment in lung is enhanced in response to bleomycin exposure
and ameliorates its fibrotic effects. Proc Natl Acad Sci USA.
100:8407–8411. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ooi YY, Dheen ST and Tay SS: Paracrine
effects of mesenchymal stem cells-conditioned medium on microglial
cytokines expression and nitric oxide production.
Neuroimmunomodulation. 22:233–242. 2015. View Article : Google Scholar
|
|
26
|
Cho KS, Park MK, Kang SA, Park HY, Hong
SL, Park HK, Yu HS and Roh HJ: Adipose-derived stem cells
ameliorate allergic airway inflammation by inducing regulatory T
cells in a mouse model of asthma. Mediators Inflamm.
2014(436476)2014. View Article : Google Scholar
|
|
27
|
Curley GF, Hayes M, Ansari B, Shaw G, Ryan
A, Barry F, O'Brien T, O'Toole D and Laffey JG: Mesenchymal stem
cells enhance recovery and repair following ventilator-induced lung
injury in the rat. Thorax. 67:496–501. 2012. View Article : Google Scholar
|
|
28
|
Zhao MM, Cui JZ, Cui Y, Li R, Tian YX,
Song SX, Zhang J and Gao JL: Therapeutic effect of exogenous bone
marrow derived mesenchymal stem cell transplantation on silicosis
via paracrine mechanisms in rats. Mol Med Rep. 8:741–746.
2013.PubMed/NCBI
|
|
29
|
Lee JW, Krasnodembskaya A, McKenna DH,
Song Y, Abbott J and Matthay MA: Therapeutic effects of human
mesenchymal stem cells in ex vivo human lungs injured with live
bacteria. Am J Respir Crit Care Med. 187:751–760. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kotton DN, Ma By, Cardoso WV, Sanderson
EA, Summer RS, Williams MC and Fine A: Bone marrow-derived cells as
progenitors of lung alveolar epithelium. Development.
128:5181–5188. 2001.PubMed/NCBI
|
|
31
|
Prockop DJ: Marrow stromal cells as stem
cells for nonhematopoietic tissues. Science. 276:71–74. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ye J and Gimble JM: Regulation of stem
cell differentiation in adipose tissue by chronic inflammation.
Clin Exp Pharmacol Physiol. 38:872–878. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
François S, Bensidhoum M, Mouiseddine M,
Mazurier C, Allenet B, Semont A, Frick J, Saché A, Bouchet S,
Thierry D, et al: Local irradiation not only induces homing of
human mesenchymal stem cells at exposed sites but promotes their
widespread engraftment to multiple organs: A study of their
quantitative distribution after irradiation damage. Stem Cells.
24:1020–1029. 2006. View Article : Google Scholar
|