Introduction
Melittin which constitutes 40–60% of dry whole
honeybee venom is the most abundant component of whole bee venom
(1). This amphipathic peptide of 26
residues contains a hydrophobic stretch of 19 amino acids followed
by a cluster of 4 positively charged residues at the C terminus
(2). Although it is a small
peptide, melittin exhibits a variety of effects such as
anti-inflammatory, anti-arthritic and anti-virus effects and
pain-relieving effects in various cell types (1,3,4). It
also induces cell cycle arrest, growth inhibition and apoptosis in
various tumor cells. Its potential use as an agent to treat
hepatocellular carcinoma, breast, ovarian and prostate cancer has
been tested in vivo or in vitro, with positive
outcomes (5–8). Due to the non-specific toxicity,
melittin can impair not only cancer cells but also normal tissue.
Therefore, many approaches, such as, preparing nanoparticle or
recombinant immunotoxin were developed in recent years to make
melittin exhibit specific toxicity towards target cells (9–11).
The urokinase-type plasminogen activator (uPA) which
is an extracellular serine protease plays a central role in tissue
remodelling events occurring in normal physiology and in
pathophysiology, including cancer invasion and metastasis. uPA
consists of an epidermal growth factor-like domain (EGF-domain;
residues 5–46) and a kringle domain (residues 50–131), which
together comprise the amino-terminal fragment (ATF), followed by an
interdomain linker (residues 132–147), and the catalytic domain
(residues 148–411) (12). Its
binding to the cell surface receptor (the urokinase-type
plasminogen activator receptor, uPAR) via ATF renders it more
accessible to plasminogen on the cell surface (13). Active uPA cleaves inactive
plasminogen to generate active plasmin, a broad-specific serine
protease, which can degrade a variety of extracellular matrix (ECM)
proteins.
uPAR is predominantly expressed on inflammatory
cells (14) and many types of
cancer, including gastric, colon, breast and ovarian cancers, and
cholangiocarcinoma in areas of cancer invasion and metastasis and
low levels of uPAR are associated with a better survival (15–18).
Moreover, prevention of uPA from binding to uPAR decreases invasion
(12). uPA and uPAR are involved in
ECM degradation, cancer invasion and metastasis by regulating the
plasminogen/plasmin system (19).
Thus, uPA-uPAR systems could be an ideal candidate for targeted
cancer therapy.
A number of small molecule uPA inhibitors have been
developed (20), however, most of
these inhibitors lack sufficiently documented specificity (21). Moreover, ATF of uPA was studied as
antagonist inhibitor by competing with uPA for binding to tumor
cell surfaces (22). It efficiently
inhibited the proliferation, migration and invasiveness of cancer
cells in vitro and inhibited tumor invasion in vitro
(13).
In the present study, we attempted to prepare a type
of fusion protein, which could take advantage of anticancer effects
of mellitin and could make use of the specific binding of ATF to
upregulated uPAR on tumor surfaces as targeted part of fusion
protein at the same time, and apply it to effective targeted
treatment. Thereby, recombinant ATF-mellitin (rATF-mellitin) which
contains amino acid sequences of ATF and melittin was expressed in
P. pastoris and its anticancer effects were detected in
vitro after purification.
Materials and methods
Strains, vectors and reagents
T4 DNA ligase, Taq DNA polymerase, plasmid
preparation kit, DNA and protein markers, and restrictive enzymes
were purchased from Takara Co., Ltd. (Otsu, Japan). The P.
pastoris X-33, E. coli XL blue, pPICZαC vector and
zeocin antibiotic were obtained from Invitrogen (Carlsbad, CA,
USA), and all primers were synthesized by Sangon Biotechnology Co.,
Ltd. (Shanghai, China). pPICZαC vector without cleavage Ste13 was
reconstructed and kept by our laboratory. The murine anti-human
urokinase monoclonal antibody was obtained from American
Diagnostica Inc. (USA). PCR purification kit was purchased from
Sangon Biotechnology Co., Ltd. SP Sepharose XL and
SourceTM30 RPC reversed phase hydrophobic chromatography
were purchased from Pharmacia (Sweden). HBL100 human breast
epithelial cell line and SKOV3 human ovarian cancer cell line were
obtained from the type culture collection of the Chinese Academy of
Sciences (Shanghai, China).
Yeast culture media
P. pastoris was grown in yeast extract
peptone dextrose (YPD) (2% peptone, 1% yeast extract and 2%
dextrose) or buffered minimal glycerol-complex medium (BMGY) (0.1 M
potassium phosphate, 2% peptone, 1% yeast extract, 1.34% YNB and 1%
glycerol). Buffered minimal methanol-complex medium (BMMY) was used
for protein induction (0.1 M potassium phosphate, 2% peptone, 1%
yeast extract, 1.34% YNB and 0.5% methanol). YPD-zeocin plates (1%
yeast extract, 2% peptone, 2% dextrose, 2% agar and 0.1 g/l zeocin)
were used for selecting positive transformants.
Construction of recombinant vector
pPICZαC-ATF
The DNA that encodes 155–156 amino acids of uPA was
SacII site (ccgcgg) which could be used to connect ATF with
the DNA sequence of melittin. Thus, we attempted to acquire DNA
that encodes uPA 1-156 amino acids that includes DNA sequence
encoding ATF and interdomain linker. Total RNA was extracted from
SKOV3 cells and was used as the template for reverse transcription
(RT) reaction. RT reaction was carried out with the following
parameters: 42°C 60 min for RT, and then 94°C 5 min to inactivate
AMV reverse transcriptase. Then the obtained cDNA was used as the
template for further PCR to amplify the DNA of human uPA. The
upstream primer was: 5′-GTT CCA TCG AAC TGT GAC TG-3′ and the
downstream primer was, 5′-GGT TCT CGA TGG TGG TGA AT-3′; the
temperature of annealing was 50°C and the polymerase was Takara Ex
Taq. The amplified DNA fragment was detected by electrophoresis on
1.0% agarose gel. The amplified human uPA DNA fragment was inserted
into cloning vector pMD18T and the recombinant plasmid which
contained correct sequence of uPA was used as template for another
PCR. The primers were: 5′-AAT CTC GAG AAG AGA TCT AAC GAG CAC CAA
GTT-3′ (upstream primer), which contained the XhoI site and
the Kex2 site, and 5′-AAT GAA TTC TCA AAT CTT AAA CCG CGG
GCC TCA-3′ (downstream primer), which contained the EcoRI
site. The temperature of annealing was 60°C and the polymerase was
Takara Ex Taq. The amplified DNA fragment was detected by
electrophoresis on 1.0% agarose gel. The amplified DNA fragment
that contains uPA 1-156 amino acids was digested with XhoI
and EcoRI, and were then inserted into the corresponding
sites of the expression vector pPICZαC. The recombinant plasmid was
transformed into the competent cells of Escherichia coli
XL-Blue and the recombinant colonies were selected by zeocin (25
μg/ ml) resistance. Both the nucleotide sequences of the
inserted DNA and flanking sequence were verified by sequencing with
GenomeLab DTCS-Quick Start kit and CEQ 2000 DNA analysis system
(Beckman, USA).
Construction of expression vector
pPICZαC-ATF-melittin
The DNA that encodes 1–26 amino acids of melittin
was synthesized according to its native amino acid sequences by
Sangon Biotechnology Co., Ltd., and was inserted into plasmid
pBluescript II (pBs). For the sake of insertion into pPICZαC-ATF
vector, the cohensive end of SacII was added to the 5′ end,
whereas, termination codon and the cohensive end of EcoRI
were added to the 3′ end. In order to improve the yield of fusion
protein, synonymous codons were replaced by yeast biased codons.
The full length DNA sequence which we designed to insert into
pPICZαC-ATF was as follows: 5′-GG TTC AAG ATT GGT GCT GTT TTG AAG
GTT TTG ACT ACT GGT TTG CCA GCT TTG ATT TCT TGG ATT AAG AGA AAG AGA
CAA CAA TGA G-3′; complementary strand, 5′-AA TTC TCA TTG TTG TCT
CTT TCT CTT AAT CCA AGA AAT CAA AGC TGG CAA ACC AGT AGT CAA AAC CTT
CAA AAC AGC ACC AAT CTT GAA CCG C-3′. Annealing was performed as
follows: 6 μl 0.5 mmol NaCl and 25 μmol single strand
DNA were mixed. Then the compound was boiled for 3 min at 80°C and
cooled down to room temperature gradually. After being annealed,
the complementary strands contained cohesive end of SacII at
the 5′ end, whereas, termination codon and the cohensive end of
EcoRI were added at the 3′ end.
The recombinant plasmid pPICZαC-ATF was digested
with SacII and EcoRI, and then the annealed DNA
sequence that encodes melittin was inserted into the corresponding
sites of pPICZαC-ATF vector. Then the recombinant plasmid was
transformed into competent cells of Escherichia coli XL-Blue
and the recombinant colonies were selected by zeocin (25
μg/ml) resistance. The positive clones were incubated in LB
liquid medium for 12 h, respectively, and the plasmids were
extracted by plasmid preparation kit. Both the nucleotide sequences
of the inserted DNA and flanking sequence were verified by
sequencing with GenomeLab DTCS-Quick Start kit and CEQ 2000 DNA
analysis system.
Transformation of P. pastoris and selection
of high-level expression colonies. Plasmid DNA pPICZαC-ATF-melittin
was linearized with SacI and introduced into competent cells
of P. pastoris X-33 strain by electroporation using a
MicroPulser (Bio-Rad, USA) according to the pPICZa vector manual.
After the electroporation, 1 M ice-cold sorbitol was added
immediately, and the cuvette contents were incubated at 30°C for 60
min. The mixture was spread on yeast extract peptone dextrose (YPD)
agar plates containing zeocin and cultured at 30°C for 2 days.
Antibiotic zeocin was used in the concentration of 0.1 g/l. The
blank plasmid of pPICZαC was also transformed as a negative
control.
After the transformants with zeocin resistance
appeared, some transformants were randomly picked from the plates
and initially cultured in a 50-ml conical tube containing 10 ml
BMGY medium at 28°C with shaking at 225 rpm for 24 h. The culture
media (0.5 ml) was sampled, respectively, centrifuged at 4°C,
10,000 rpm for 5 min, and the cell pellets were used for genomic
DNA analysis. The yeast genomic DNA was extracted and PCR
identification was carried out to select P. pastoris X-33
strain which was the transformed recombinant plasmid. The primers
were: 5-GAC TGG TTC CAA TTG ACA AGC-3′ (upstream primer) and 5-ATC
GAT CTC ACA GTG TTG ACC-3′ (downstream primer). The temperature of
annealing was 52°C and the polymerase was Takara Ex Taq.
The rest of the culture media were then centrifuged
and cell pellets were resuspended in 10 ml BMMY medium to induce
expression for 7 days. Methanol was added every 24 h to a final
concentration of 0.5% (v/v) for inducing the expression of the
target protein. The culture media (0.5 ml) were sampled per day and
centrifuged at 4°C, 10,000 rpm for 5 min. The supernatant was used
for recombinant protein detection and selection of high-level
expression colonies.
SDS-PAGE and western blot assays
SDS-PAGE analysis was performed using a 12% gel. For
western blot analysis, proteins in the gel were transferred to
polyvinylidene fluoride membrane. The membrane was blocked with 5%
non-fat milk powder which was dissolved in Tris-buffered saline
Tween-20 (TBST) for 1 h and then incubated with the murine
anti-human urokinase monoclonal antibody for 12 h at 4°C. After
being washed by TBST, the membrane was incubated with the goat
anti-mouse IgG conjugated to HRP (Abclonal, USA), diluted to
1:2,000. The bound antibody was detected using EasyBlot ECL kit
(Beyotime, China).
Optimized expression of rATF-melittin in
P. pastoris
In order to achieve high level expression of
rATF-melittin, different culture parameters including pH value and
induction time were evaluated in the expression procedure. The pH
values were adjusted to 3.5–7.5 with 0.5 intervals. High-level
expression cell colony was cultured with the above processes as and
the pH values were adjusted every day with disodium hydrogen
phosphate and citric acid. At the desired time points, 0.5 ml cell
aliquots were withdrawn, and were then replaced with equal amount
of fresh medium. The supernatant samples were used for
enzyme-linked immunosorbent assay (ELISA).
ELISA
Individual wells of ELISA plate (Costar, USA) were
coated with 50 μl supernatant sample of rATF-melittin which
had been diluted with 50 μl coating buffer (15 mM
Na2CO3, 35 mM NaHCO3, pH 9.6)
overnight at 4°C. The plates were blocked with 5% (w/v) non-fat
milk powder in TBST and were incubated for 2 h at room temperature.
The murine anti-human urokinase monoclonal antibody (American
Diagnostica Inc.) was used at 1:1,000, incubated for 1 h at 37°C.
Following several washes with TBST, the plates were again incubated
with goat anti-mouse IgG conjugated to HRP (Abclonal) (1:2,000
dilution with blocking buffer) for 1 h. The color reaction was
developed by addition of the substrate solution
orthophenylenediamine (OPD) and incubated for 5 min at room
temperature in the dark. Then 50 μl stop solution (2 M
H2SO4) was added to each well. The absorbance
values at 490 nm were read in ELx800 microplate reader (Bio-Tek,
USA) within 2 h.
Large-scale expression of
rATF-melittin
The highest-level expression transformant was
cultured in a 5-l shake flask containing 2 l BMGY medium at 28°C
until the culture reached OD 600=6.0. The shake flask culture was
used to inoculate an 80-l NBS BioFlo 5000 fermentor (New Brunswick
Scientific, USA) containing 40 l of fermentation basal salts medium
FM 21 supplemented with PTM1 trace salts (1.1 ml of stock
solution/l) and biotin (0.4 ml of the stock solution/l). The
dissolved oxygen level (DO) was set at 30% and the stirring rate
was 700 rpm. The pH of the medium was maintained 6.5 by automatic
addition of 5M NH4OH or 1 M phosphoric acid. Antifoam
(5%) was delivered as required and temperature was maintained at
28°C. Growth was divided into three phases designated as glycerol,
glycerol-fed and methanol-fed batch phases. After complete
consumption of glycerol in the medium, a glycerol fed-batch phase
was initiated by addition of 50% glycerol [containing 1.2% (v/v) of
PTM1 trace salts] at a rate of 400 ml/h. The glycerol feed was
carried out until the cell wet weight reached 180 g/l. The third
phase was initiated by addition of methanol which contained 1.2%
(v/v) of PTM1 trace salts after glycerol was exhausted. Methanol
was initially added at 144 ml/h for 4 h to allow the culture adapt
to growth with methanol, then methanol was gradually increased to
440 ml/h. Sampling of the culture medium was performed at the end
of each phase and every 3 h to analyze cells wet weight, optical
density, and to measure rATF-melittin expression based on SDS-PAGE
analysis (ELISA analysis).
Purification of rATF-melittin
The supernatant was collected by centrifugation at
15,000 rpm for 10 min and was clarified with a 0.45-μm
cellulose membrane. After being diluted four times with 20 mM
NaAc-HAc (pH 4.0) buffer, the pH of the fermentation broth was
adjusted to 4.0 with 1 M acetate acid. A cation exchange
chromatographic column (20 ml; SP Sepharose XL, Sweden) was
equilibrated with 20 mM NaAc-HAc (pH 4.0) buffer. The supernatant
was loaded onto the cation exchange chromatographic column at the
rate of 0.5 ml/min. Then the column was extensively washed with the
same buffer at the rate of 1 ml/min. The bound protein was eluted
with a linear gradient of 0.1–1.0 M NaCl while the flow rate was
maintained at the rate of 1 ml/min. Protein elution was monitored
by measuring the absorbance at 280 nm and identified by SDS-PAGE
analysis. Column effluent containing rATF-melittin was collected
and loaded onto a reverse phase column (2.0×15 cm; Source 30;
Sweden) which was equilibrated with 0.1% trifluoroacetic acid (TFA)
for further purification. rATF-melittin was eluted using 50%
methanol that containing 0.1% TFA at the rate of 1 ml/min and
monitored by measuring the UV absorbance at 280 nm. Column effluent
containing rATF-melittin was concentrated by vacuum distillation
and freeze drying to remove methanol. The finally purified
rATF-melittin was stored at −80°C for further studies.
N-terminal amino acid sequence
analysis
To determine the N-terminal sequence, the purified
rATF-melittin was electrophoresed on 12% SDS-PAGE gel and
electroblotted on a PVDF membrane. After being blotted, the PVDF
membrane was stained with Coomassie brilliant blue R250, and the
rATF-melittin band was cut out and determined by automated Edman
degradation performed on a model PPSQ-21A protein sequencer
(Shimadzu, Japan).
Cytotoxicity assay of rATF-melittin
The cytotoxicity of rATF-melittin on normal cells
was monitored with using Cell Counting Kit-8 (CCK-8) assay.
Briefly, human epithelial cells HBL100 were maintained in RPMI-1640
with 10% fetal bovine serum (FBS; HyClone, USA) and 100 U/ml of
penicillin/ streptomycin, at 37°C in humidified atmosphere
containing 5% CO2. HBL100 cells were seeded into 96-well
plates containing complete medium at a density of 1×104
cells/well and incubated for 24 h followed by different doses of
rATF-melittin. HBL100 cells were treated with RPMI-1640 (as
control) and 7.5, 15, 30, 60 and 120 μg/ml rATF-melittin for
24 h, respectively. Then the medium was replaced with 200 μl
of fresh culture medium and 20 μl CCK-8 solution was added
to each well. After being incubated for 2 h, the absorbance was
detected at 490 nm using a microplate reader (Bio-Rad Instruments,
USA).
Inhibition effects of rATF-melittin on
proliferation of ovarian cancer cells
SKOV3 cells were maintained in H-DMEM with 10% FBS
and 100 U/ml of penicillin/streptomycin, at 37°C in humidified
atmosphere containing 5% CO2. To test the inhibition
effects of rATF-melittin on proliferation of ovarian cancer cells,
SKOV3 cells were seeded into 96-well plates at a density of
1×104 cells/well and were incubated for 24 h followed by
different doses of rATF-melittin. SKOV3 cells were treated with
H-DMEM (as control) and 7.5, 15, 30, 60 and 120 μg/ml
rATF-melittin for 24 h, respectively. CCK-8 assay was performed as
above.
Results
Construction and transformation of
pPICZαC-ATF-melittin
Results of DNA sequence analysis of the recombinant
expression vector pPICZαC-ATF-melittin (data not shown)
demonstrated that the DNA sequences encoding human uPA amino acids
1-156 and melittin were correctly inserted into pPICZαC vector and
the amino acid sequence of ATF-melittin encoded was identical with
that logged in GenBank.
After being cultured at 30°C for 2 days, dozens of
transformants with zeocin resistance appeared on YPD agar plates
which contained 0.1 g/l zeocin. The PCR analysis of genomic DNA
showed that the DNA sequence encoding human uPA amino acids 1-156
and melittin was indeed integrated into over 90% of clones which
transformed with recombinant expression vector
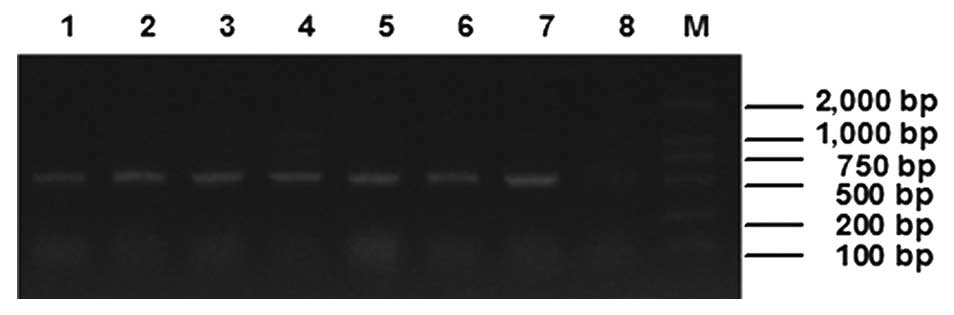
pPICZαC-ATF-melittin. As the result showed (Fig. 1), there were ~500 bp amplification
bands for the samples that were transformed with
pPICZαC-ATF-melittin, however, the control sample which was
transformed with pPICZαC blank plasmid was negative.
Expression and detection of rATF-melittin
in P. pastoris
After induction with methanol for rATF-melittin
expression, the transformant which presented the highest expression
level of rATF-melittin could be used for further experiments.
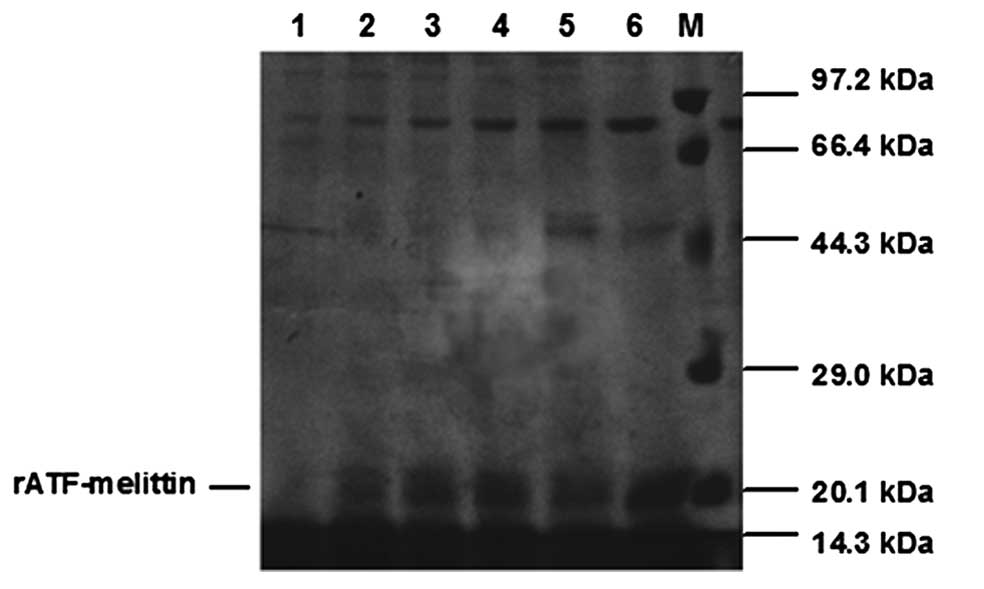
SDS-PAGE analysis of rATF-melittin culture medium indicated
rATF-melittin expressed after the inducing of methanol, however,
the recombinant protein expression in the transformant containing
blank plasmid of pPICZαC was negative. Based on the amino acids
sequence, the calculated molecular weight of rATF-melittin was 20.9
kDa, which was consistent with the result of SDS-PAGE measurement
(Fig. 2).
Optimized expression of rATF-melittin in
P. pastoris
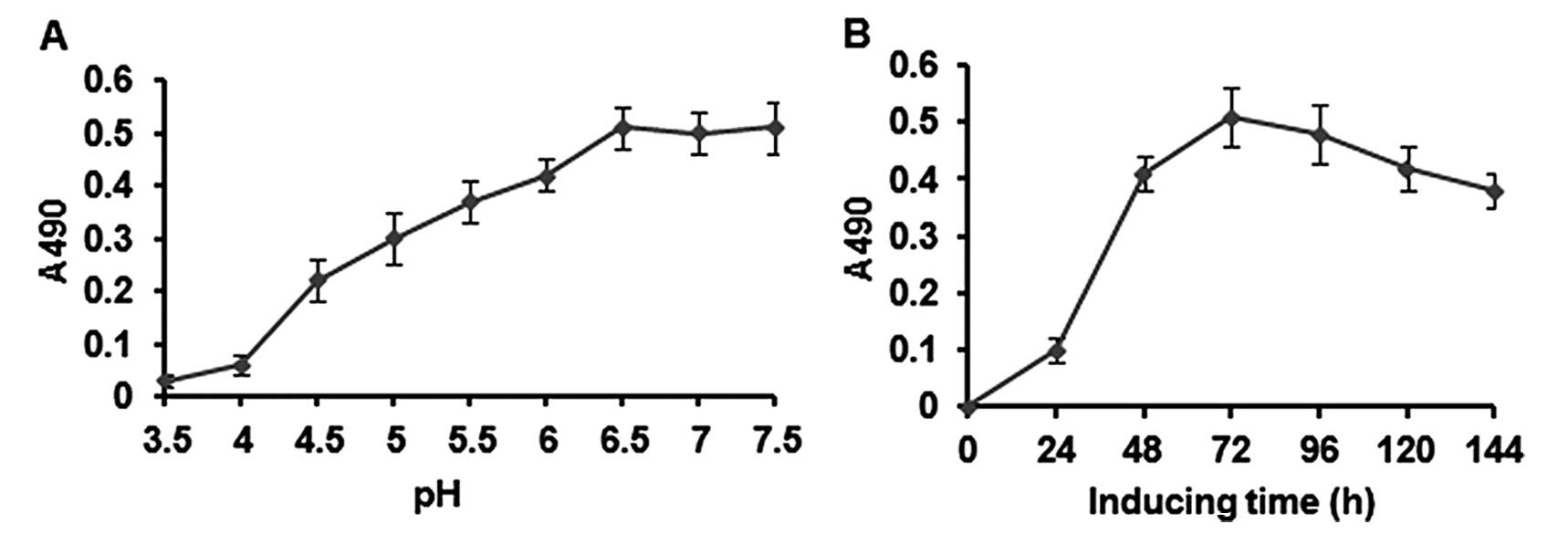
After a series of experiments, the optimal
expression conditions of rATF-melittin were obtained as follows:
the optimal pH was 6.5 (Fig. 3A)
and the optimal induction time-point was about day 3 for the strain
(Fig. 3B) at 28°C and with methanol
daily addition concentration of 0.5% (v/v) in 50-ml tube. Under
these conditions, the transformant of P. pastoris that
presented the highest expression level was chosen for scaled-up
protein production.
Large-scale expression and purification
of rATF-melittin
The transformant which presented the highest-level
expression was cultured to inoculate an 80-l fermentor under the
optimal fermentation conditions. Glycerol batch phase lasted ~24 h,
and then 50% glycerol was added until the wet weight of culture
cells reached 180 g/l. When glycerol was exhausted, OD value
abruptly increased. After being induced with methanol, samples were
withdrawn every 3 h for SDS-PAGE analysis. The result indicated
that during biomass generation phase, the carbon source (glycerol)
only met the growth requirement of P. pastoris and there was
no recombinant protein expression. Expression of rATF-melittin was
initiated when methanol became the unique carbon source. After
being induced for 18 h, the transformant presented the highest
expression level. The yield was not visibly changed between 18 and
24 h (Fig. 4).
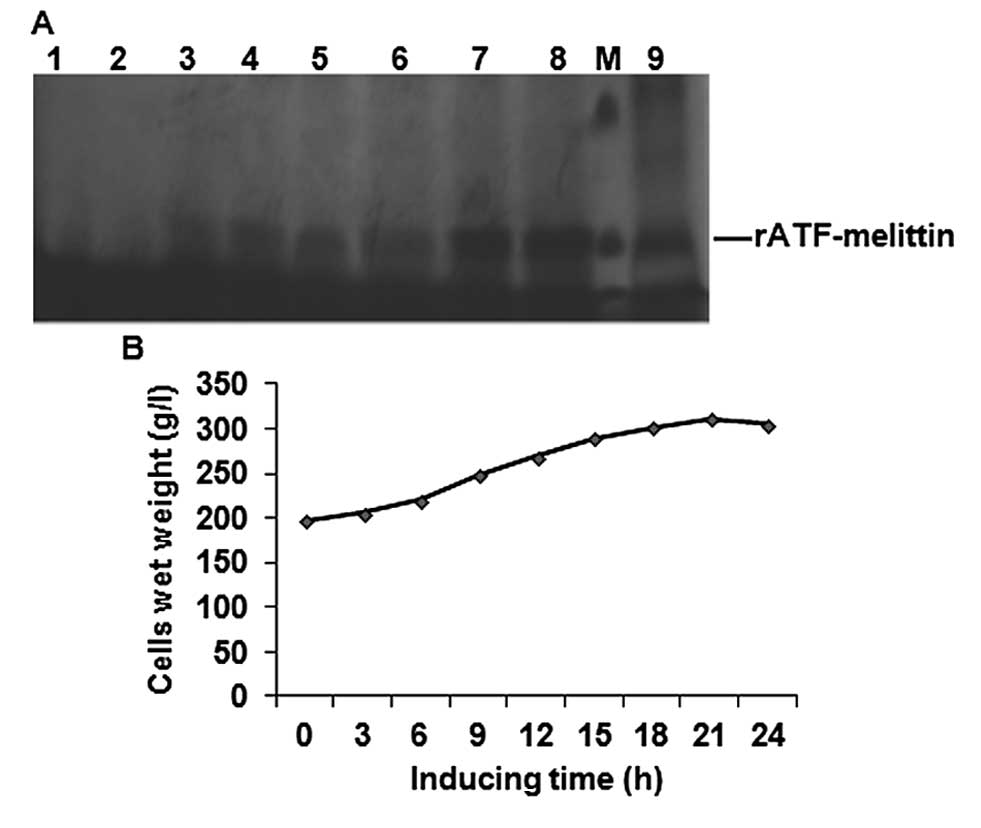 | Figure 4Large-scale expression of
rATF-melittin. (A) SDS-PAGE analysis of supematant at different
fermentation stage in 40 l fermentation broth. Lanes 1-9, samples
collected at different time after methanol inducing: 0, 3, 6, 9,
12, 15, 18, 2 and 24 h, respectively. Lane M, protein molecular
weight marker (from up to down: 29 and 20.1 kDa). (B) Profile of
P. pastoris growth at different times after methanol
induction. |
Purification of rATF-melittin and
characterization of purified rATF-melittin
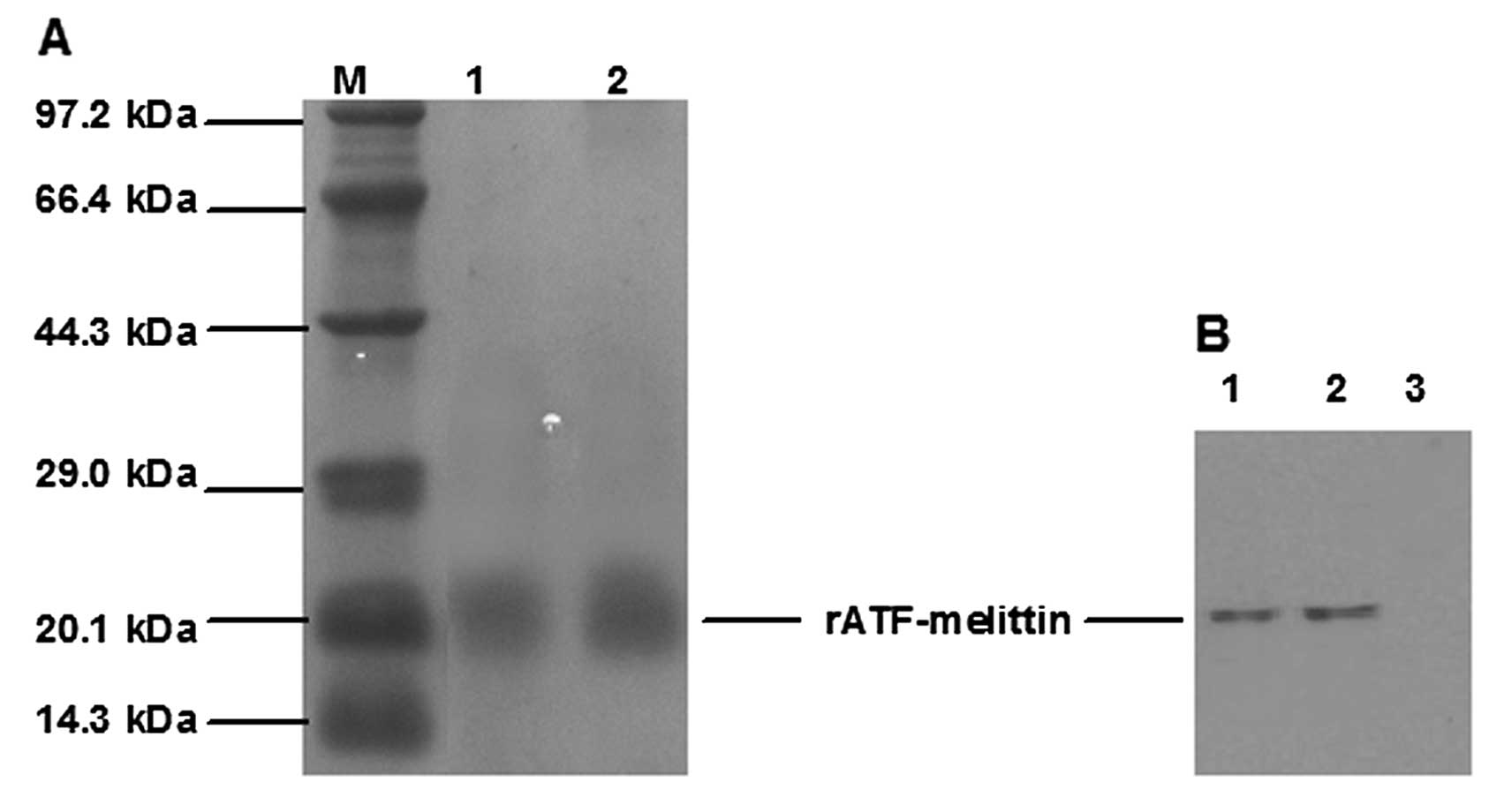
The rATF-melittin supernatant was purified with a
cation exchange chromatography and a reverse phase chromatography.
Using an AKTA Explorer 100 chromatography system, we optimized the
purification parameters. The optimal concentration of NaCl for
elution was 0.5 M and 50% methanol (containing 0.1% TFA) can elute
the bound rATF-melittin from the reverse phase chromatographic
column (Fig. 5A). After being
purified, 5.19 g rATF-melittin was obtained from 40 l fermentation
broth. The concise purification protocol of rATF-melittin is
presented in Table I.
 | Table IPurification process of
rATF-melittin. |
Table I
Purification process of
rATF-melittin.
| Purification
steps | Total protein
(g) | rATF-melittin
(g) | Recovery (%) | Purity (%) |
|---|
| Supenatant | 12.48 | 7.74 | | 62 |
| SP Sepharose
XL | 7.41 | 6.07 | 78.4 | 81.9 |
| Source™ 30 RPC | 5.45 | 5.19 | 67.2 | 95.2 |
The primary purified recombinant protein was
identified by western blot analysis. The results demonstrated that
the recombinant protein could bind with murine anti-human urokinase
monoclonal antibody. No band was observed in lane 3, which was the
supernatant before adding methanol (Fig. 5B).
N-terminal sequencing of rATF-melittin yielded the
first 14 amino acids as S N E L H Q V P S N C D C L. The N-terminal
sequence of rATF-melittin was identical to that of human uPA,
confirming the successful expression and purification of
rATF-melittin.
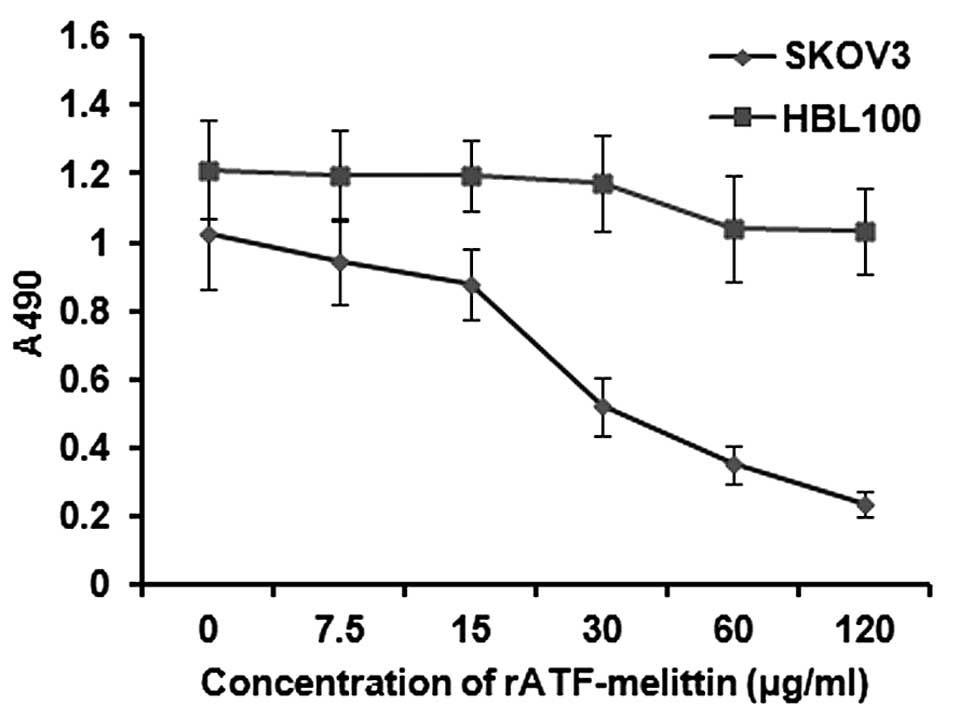
Anticancer effects of rATF-melittin
After being treated with rATF-melittin at different
doses, some SKOV3 cells showed membrane blebbing, ballooning and
chromatin condensation. However, the cells grew well with adherence
and the cells were fusiform or diamond shaped in the control group
CCK-8 assay was used to detect the quantity of cells. As a result,
rATF-melittin treatment caused a dose-dependent inhibition of
growth on SKOV3 at 24 h. The inhibition rate is ~80% at the dose of
120 μg/ml. However, the result (Fig. 6) showed that rATF-melittin did not
have much influence on the proliferation of normal cells (HBL100).
The reason is that uPAR is highly expressed in ovarian cancer cells
SKOV3, but it is undetectable in normal cells such as HBL100. In
other words, the anticancer effect of rATF-melittin can be exerted
when uPA combines with its receptor-uPAR followed by the release of
melittin. Thus, the fusion protein expressed showed no obvious
toxicity on normal tissues and can be applied to ovarian cancer
targeted therapy.
Discussion
Ovarian cancer is the fourth leading cause of cancer
mortality among women in Western societies (23). Patients with ovarian cancer have
less than 50% chance of 5-year survival and a majority of patients
die of disease recurrence or metastasis (24). In case of relapse, therapeutic
options are limited, particularly if the relapse occurs within 6
months after completion of primary treatment (25). Consequently, it is clear that new
treatments are necessary for ovarian cancer.
Melittin is well known to possess cytolytic activity
with wide-spectrum lytic properties. Thus, we chose melittin as the
cytotoxic agent to treat cancer cells. However, it is not only
cytotoxic to tumors, but also vital to normal cells. It is crucial
to control melittin to exhibit specific toxicity towards target
cells and to reduce its toxicity towards normal tissue. Melittin
had low toxicity when coupled with target peptides (26). Thus, tumor-targeted toxins may be
helpful for developing novel anticancer therapeutics.
It has been shown that uPAR is predominantly
expressed on many types of cancer, including gastric, colon, breast
and ovarian cancers, and cholangiocarcinoma. In the present study,
uPA was used as the cell-targeting domain to identify and bond with
ovarian cancer cells. uPA was connected with melittin to construct
the tumor-targeted toxins-rATF-mellitin. uPA was able to identify
cancer cells and led melittin to bond with them, and then perform
its lytic properties on the one hand, and on the other
rATF-mellitin played a role as antagonist inhibitor by competing
with native uPA by binding to tumor cell surfaces.
The present study focuses on expression of the
targeted toxin rATF-mellitin, which can be used in ovarian cancer
target therapy. In the present study, pPICZαC-ATF-melittin
eukaryotic expression vector was successfully constructed. After
transformed into P. pastoris and induced by methanol,
rATF-mellitin was detected by SDS-PAGE and western blot analysis.
After induction with methanol, the expression level of
rATF-mellitin was 312 mg/l in 80-l fermentor which contains 40 l
fermentation broth. rATF-mellitin was purified to >95% purity
using SP Sepharose ion exchange chromatography and source™ 30 RPC
with 67.2% recovery. Cell proliferation assay showed that
rATF-melittin inhibited growth of SKOV3 cells and had no
cytotoxicity effect on normal cells. For the first time, we
established a stable and effective rATF-mellitin P. pastoris
expression system to obtain a high level of expression of secreted
rATF-mellitin, which was purified by a highly efficient and easy to
handle purification procedure.
Abbreviations:
|
ATF
|
amino-terminal fragment
|
|
BMGY
|
buffered minimal glycerol-complex
medium
|
|
BMMY
|
buffered minimal methanol-complex
medium
|
|
CCK-8
|
Cell Counting Kit-8
|
|
DO
|
dissolved oxygen level
|
|
ECM
|
extracellular matrix
|
|
EGF-domain
|
epidermal growth factor-like
domain
|
|
ELISA
|
enzyme-linked immunosorbent assay
|
|
FBS
|
fetal bovine serum
|
|
GPI
|
glycosylphosphatidylinositol
|
|
rATF-mellitin
|
recombinant amino-terminal
fragment-melittin
|
|
RT
|
reverse transcription
|
|
OPD
|
orthophenylenediamine
|
|
pBs
|
plasmid bluescript II
|
|
PBS
|
phosphate-buffered saline
|
|
RT
|
reverse transcription
|
|
TBST
|
Tris-buffered saline Tween-20
|
|
TFA
|
trifluoroacetic acid
|
|
uPA
|
urokinase plasminogen activator
|
|
uPAR
|
urokinase plasminogen activator
receptor
|
|
YPD
|
yeast extract peptone dextrose
|
Acknowledgments
The present study was supported by grants from the
National Natural Science Foundation of China (81272875, 30973187,
81401221 and 81302242), the Ministry of Education for Young Teacher
Foundation of China (20110061120084), the Jilin Science and
Technology Funds (20140520047JH, 20150204007YY, 20130102094JC and
20140204022YY), and the Basic Scientific Research of Jilin
University Funds and Young Scholars Program of Norman Bethune
Health Science Center of Jilin University (20142116).
References
|
1
|
Raghuraman H and Chattopadhyay A:
Melittin: A membrane-active peptide with diverse functions. Biosci
Rep. 27:189–223. 2007. View Article : Google Scholar
|
|
2
|
Sommer A, Fries A, Cornelsen I, Speck N,
Koch-Nolte F, Gimpl G, Andrä J, Bhakdi S and Reiss K: Melittin
modulates keratinocyte function through P2 receptor-dependent ADAM
activation. J Biol Chem. 287:23678–23689. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Son DJ, Lee JW, Lee YH, Song HS, Lee CK
and Hong JT: Therapeutic application of anti-arthritis,
pain-releasing, and anti-cancer effects of bee venom and its
constituent compounds. Pharmacol Ther. 115:246–270. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang C, Chen T, Zhang N, Yang M, Li B, Lü
X, Cao X and Ling C: Melittin, a major component of bee venom,
sensitizes human hepatocellular carcinoma cells to tumor necrosis
factor-related apoptosis-inducing ligand (TRAIL)-induced apoptosis
by activating CaMKII-TAK1-JNK/p38 and inhibiting IkappaBalpha
kinase-NFkappaB. J Biol Chem. 284:3804–3813. 2009. View Article : Google Scholar
|
|
5
|
Jo M, Park MH, Kollipara PS, An BJ, Song
HS, Han SB, Kim JH, Song MJ and Hong JT: Anti-cancer effect of bee
venom toxin and melittin in ovarian cancer cells through induction
of death receptors and inhibition of JAK2/STAT3 pathway. Toxicol
Appl Pharmacol. 258:72–81. 2012. View Article : Google Scholar
|
|
6
|
Liu S, Yu M, He Y, Xiao L, Wang F, Song C,
Sun S, Ling C and Xu Z: Melittin prevents liver cancer cell
metastasis through inhibition of the Rac1-dependent pathway.
Hepatology. 47:1964–1973. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Russell PJ, Hewish D, Carter T,
Sterling-Levis K, Ow K, Hattarki M, Doughty L, Guthrie R, Shapira
D, Molloy PL, et al: Cytotoxic properties of immunoconjugates
containing melittin-like peptide 101 against prostate cancer: In
vitro and in vivo studies. Cancer Immunol Immunother. 53:411–421.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Soman NR, Baldwin SL, Hu G, Marsh JN,
Lanza GM, Heuser JE, Arbeit JM, Wickline SA and Schlesinger PH:
Molecularly targeted nanocarriers deliver the cytolytic peptide
melittin specifically to tumor cells in mice, reducing tumor
growth. J Clin Invest. 119:2830–2842. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Moghadam BY, Hou WC, Corredor C,
Westerhoff P and Posner JD: Role of nanoparticle surface
functionality in the disruption of model cell membranes. Langmuir.
28:16318–16326. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Funayama JC, Pucca MB, Roncolato EC,
Bertolini TB, Campos LB and Barbosa JE: Production of human
antibody fragments binding to melittin and phospholipase A2 in
Africanised bee venom: Minimising venom toxicity. Basic Clin
Pharmacol Toxicol. 110:290–297. 2012. View Article : Google Scholar
|
|
11
|
Holle L, Song W, Holle E, Wei Y, Li J,
Wagner TE and Yu X: In vitro- and in vivo-targeted tumor lysis by
an MMP2 cleavable melittin-LAP fusion protein. Int J Oncol.
35:829–835. 2009.PubMed/NCBI
|
|
12
|
Botkjaer KA, Deryugina EI, Dupont DM,
Gårdsvoll H, Bekes EM, Thuesen CK, Chen Z, Ploug M, Quigley JP and
Andreasen PA: Targeting tumor cell invasion and dissemination in
vivo by an aptamer that inhibits urokinase-type plasminogen
activator through a novel multifunctional mechanism. Mol Cancer
Res. 10:1532–1543. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li J, Lin Y, Zhuang H and Hua ZC:
Expression, purification, and biological characterization of the
amino-terminal fragment of urokinase in Pichia pastoris. J
Microbiol Biotechnol. 23:1197–1205. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yang L, Sajja HK, Cao Z, Qian W, Bender L,
Marcus AI, Lipowska M, Wood WC and Wang YA: uPAR-targeted optical
imaging contrasts as theranostic agents for tumor margin detection.
Theranostics. 4:106–118. 2013. View Article : Google Scholar
|
|
15
|
Ding Y, Zhang H, Zhong M, Zhou Z, Zhuang
Z, Yin H, Wang X and Zhu Z: Clinical significance of the uPA system
in gastric cancer with peritoneal metastasis. Eur J Med Res.
18:282013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Thummarati P, Wijitburaphat S, Prasopthum
A, Menakongka A, Sripa B, Tohtong R and Suthiphongchai T: High
level of urokinase plasminogen activator contributes to
cholangiocarcinoma invasion and metastasis. World J Gastroenterol.
18:244–250. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Foekens JA, Peters HA, Look MP, Portengen
H, Schmitt M, Kramer MD, Brünner N, Jänicke F, Meijer-van Gelder
ME, Henzen-Logmans SC, et al: The urokinase system of plasminogen
activation and prognosis in 2780 breast cancer patients. Cancer
Res. 60:636–643. 2000.PubMed/NCBI
|
|
18
|
Kim TD, Song KS, Li G, Choi H, Park HD,
Lim K, Hwang BD and Yoon WH: Activity and expression of
urokinase-type plasminogen activator and matrix metalloproteinases
in human colorectal cancer. BMC Cancer. 6:2112006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Laufs S, Schumacher J and Allgayer H:
Urokinase-receptor (u-PAR): an essential player in multiple games
of cancer: a review on its role in tumor progression, invasion,
metastasis, proliferation/dormancy, clinical outcome and minimal
residual disease. Cell Cycle. 5:1760–1771. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Rockway TW, Nienaber V and Giranda VL:
Inhibitors of the protease domain of urokinase-type plasminogen
activator. Curr Pharm Des. 8:2541–2558. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Carriero MV and Stoppelli MP: The
urokinase-type plasminogen activator and the generation of
inhibitors of urokinase activity and signaling. Curr Pharm Des.
17:1944–1961. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li H, Soria C, Griscelli F, Opolon P,
Soria J, Yeh P, Legrand C, Vannier JP, Belin D, Perricaudet M, et
al: Amino-terminal fragment of urokinase inhibits tumor cell
invasion in vitro and in vivo: Respective contribution of the
urokinase plasminogen activator receptor-dependent or-independent
pathway. Hum Gene Ther. 16:1157–1167. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Longuespée R, Boyon C, Desmons A, Vinatier
D, Leblanc E, Farré I, Wisztorski M, Ly K, D'Anjou F, Day R, et al:
Ovarian cancer molecular pathology. Cancer Metastasis Rev.
31:713–732. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Su D, Katsaros D, Xu S, Xu H, Gao Y,
Biglia N, Feng J, Ying L, Zhang P, Benedetto C, et al:
ADP-ribosylation factor-like 4C (ARL4C), a novel ovarian cancer
metastasis suppressor, identified by integrated genomics. Am J
Transl Res. 7:242–256. 2015.PubMed/NCBI
|
|
25
|
Coosemans A, Baert T and Vergote I: A view
on dendritic cell immunotherapy in ovarian cancer: How far have we
come? Facts Views Vis Obgyn. 7:73–78. 2015.PubMed/NCBI
|
|
26
|
Sun D, Sun M, Zhu W, Wang Z, Li Y and Ma
J: The anti-cancer potency and mechanism of a novel tumor-activated
fused toxin, DLM. Toxins. 7:423–438. 2015. View Article : Google Scholar : PubMed/NCBI
|