Introduction
Prostate cancer is the most commonly diagnosed
cancer and the second leading cause of cancer-related deaths among
males (1). Although commonly used
treatments (surgery, radiation therapy and chemotherapy) can reduce
tumor burden and prolong survival time, prostate cancer is not
curative in about a half of patients. Together with the high
recurrence rate, there is a strong demand to develop more effective
strategies for prostate cancer therapy (2–4). Gene
therapy provides a promising treatment strategy for cancer therapy,
among which suicide gene therapy is considered as one of the most
effective ones.
β-glucuronidase (βG) is a type of acid hydrolase
present in the microsomes and lysosomes of many human tissues,
which can convert nontoxic glucuronide prodrugs with glycoside
bonds into toxic glucuronic acid (5). The most commonly used prodrug is
N-[4-doxorubicin-N-carbonyl (oxymethyl) phenyl]
O-β-glucuronyl carbamate (DOX-GA3), which can be converted
into toxic DOX by βG (6,7). After successful conversion, not only
the cells that express βG but the neigh-boring βG-non-expressing
cells are effectively killed, showing a strong bystander effect,
which is a typical phenomenon in suicide gene therapy (8,9). βG
together with its glucuronide prodrug has been considered as a new
suicide gene therapy system (5,10–12).
In addition, as it is a human protein, βG can avoid the
immunogenicity of other suicide genes such as thymidine kinase (TK)
and cytosine deaminase (CD), thus showing greater potential for
clinical application.
For successful application of suicide gene therapy,
specific high expression of the suicide gene in cancer cells is of
vital importance for the specific killing of cancer cells. In some
larger tumors, such as pancreatic carcinoma and ovarian carcinoma,
βG is overexpressed and high levels of βG are present in the
necrotic area, so a directly applied glucuronide prodrug can be
converted into a toxic drug in these tumors and specifically kill
the cancer cells (7,13,14).
But actually, most other tumors may not have increased βG
expression or do not have necrosis, thus monotherapy by using a
glucuronide prodrug of βG is inapplicable. Under such conditions,
targeted delivery or high expression levels of a suicide gene is
the key factor for successful therapy.
For the targeted killing of cancer cells, selective
expression of βG is of vital importance. The prostate-specific
antigen (PSA) is known to be highly specific in prostate tissues,
and has been used for targeted expression of therapeutic genes in
prostate cancer. However, the relatively low transcription
efficiency has limited its further application (15,16).
In the present study, a GAL4-regulated bicistronic adenoviral
vector (Ad/PSAP-GV16-βG) was constructed to express βG under the
control of the PSA promoter. In such a vector, the PSA promoter
controls the expression of GAL4/VP16, which can transactivate the
βG expression controlled by the GAL4/TATA promoter. This expression
system was used to evaluate whether this βG/DOX-GA3 suicide gene
system may have application prospect for the therapy of prostate
cancer.
Materials and methods
The protocol of the present study was approved by
the Ethics Committee of Jinling hospital, in compliance with the
Helsinki Declaration.
Cell lines
LNCaP, which is a prostate cancer cell line that
expresses PSA, and DU145, which is a prostate cancer cell line from
brain metastasis that does not express PSA, were cultured in
RPMI-1640 media supplemented with 10% fetal bovine serum. Cells
were maintained at 37°C in a humidified incubator with 5%
CO2.
Genomic DNA extraction, cDNA isolation
and PCR amplification
The primers used to amplify the predicted PSA
promoter were forward, 5′-tttagatcttagaggatctgtggaccacaa-3′ and
reverse, 5′-tttaagcttggtgacacagctctccgggtg-3′. PCR was performed
using Ex Taq HS (Takara, Shiga, Japan) with the following
program: 95°C for 2 min, 30 cycles of 95°C for 30 sec, 55°C for 30
sec and 72°C for 30 sec, and a final step of 7 min at 72°C.
Total RNA was harvested from cultured HepG2 cells
using the RNeasy Mini kit (Qiagen, Hilden, Germany). Total RNA (1
µg) was reverse-transcribed into 20 µl cDNA using the
PrimeScript PCR kit (Takara). The primers used to amplify the βG
transcript were forward, 5′-tttgagctcatggcccgggggtcggcggttgcc-3′
and reverse, 5′-aaacctaggagttcatttgcccgacaaaaggttt-3′. PCR was
performed with the following program: 95°C for 2 min, 30 cycles of
95°C for 30 sec, 55°C for 30 sec and 72°C for 30 sec, and a final
step of 7 min at 72°C. PCR products were analyzed using agarose gel
electrophoresis and cloned into the pcDNA3.1 (Takara) vector for
sequencing to ensure that the sequence was correct.
Plasmid construction and preparation of
the bicistronic adenoviruses
The DNA fragments encoding the human PSA promoter
(PSAP) and βG were amplified and subcloned. The pshuttle-GT-TRAIL
and the pshuttle-hTERT-GV16-TRAIL plasmid were kindly provided by
Dr B. Fang (17). The new
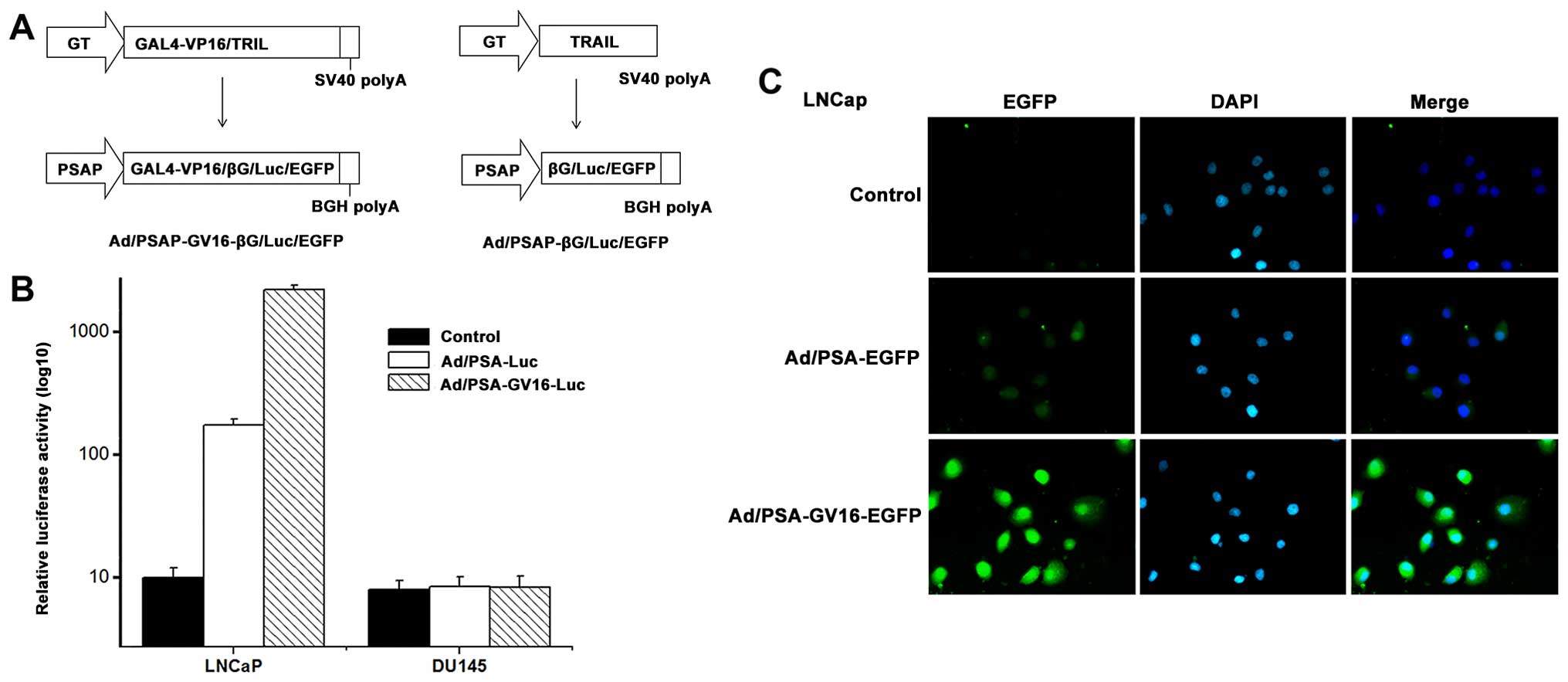
bicistronic shuttle vector pshuttle-PSAP-GV16-βG (Fig. 1) was constructed as follows. i) The
TRAIL gene in the shuttle plasmid pshuttle-hTERT-GV16-TRAIL was
replaced by the βG gene to obtain the pshuttle-hTERT-GV16-βG
plasmid and ii) the hTERT promoter in pshuttle-hTERT-GV16-βG was
replaced by PSAP to obtain the pshuttle-PSAP-GV16-βG plasmid. The
shuttle vector pshuttle-PSAP-βG was constructed by replacing the GT
promoter and the TRAIL gene in pshuttle-GT-TRAIL with PSAP and the
βG gene.
Adenoviral vectors were constructed as described
previously (12). Briefly, the
shuttle plasmids were cotransfected into 293 cells along with a
35-kb ClaI fragment from adenovirus type 5. Then,
recombinant vector Ad/PSAP-βG and Ad/PSAP-GV16-βG were generated by
homologous recombination and plaque-purified. The expression
cassette sequence was then confirmed by DNA sequencing. The
adenoviral vectors Ad/PSAP-GV16-Luc, Ad/PSAP-GV16-EGFP, Ad/PSAP-Luc
and Ad/PSAP-EGFP were also constructed in a similar manner. Virus
titers were determined by optical absorbance at A260 nm
(one A260 nm unit=1012 particles/ml) and by
plaque assay. Titers determined by A260 (i.e., viral
particles) were used in all of the experiments. Particle: plaque
ratios normally fell between 30:1 and 100:1. All of the viral
preparations were free of contamination by E11 adenovirus and
endotoxin.
Western blot analysis
Protein extracts were prepared from cultured cells
or tumor tissue after they were homogenized in lysis buffer (50 mM
NaCl, 50 mM EDTA, 1% Triton X-100) containing protease inhibitor
cocktail (Roche, Indianapolis, IN, USA). Cell lysates (30
µg) were separated on 12% sodium dodecyl sulfate
polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto
polyvinylidene difluoride membranes (Amersham Pharmacia Biotech,
Uppsala, Sweden) according to standard protocol. The membranes were
then blocked in phosphate-buffered saline (PBS) containing 5%
bovine serum albumin (BSA) and 0.05% Tween-20 for 2 h at room
temperature, before being incubated with the appropriate primary
antibody [anti-human βG (1:1,000; Molecular Probes Life
Technologies, Carlsbad, CA, USA) or anti-β-actin (1:2,500; Santa
Cruz Biotechnology, Santa Cruz, CA, USA)] overnight at 4°C. The
membranes were washed with PBS containing 0.05% Tween and then
incubated with the appropriate secondary antibody (1:10,000) for 1
h at room temperature. The bands were visualized using MultiImage™
Light Cabinet Filter Positions (Alpha Innotech, San Leandro, CA,
USA).
MTT assay
For the
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay, the LNCaP and DU145 cells were plated in 96-well plates at a
density of 1,000 cells/well before infection with the control
adenovirus, Ad/PSAP-βG or Ad/PSAP-GV16-βG. At 48 h after the
infection, different concentrations of DOX-GA3 or 10 µg/ml
DOX-GA3 was added and incubated for the indicated times. Aliquots
of 20 µl of 5 mg/ml MTT (Sigma, St Louis, MO, USA) in PBS
were added to each well, and the cells were incubated for another 4
h followed by the addition of 150 µl dimethyl sufoxide
(DMSO). The A570 nm values were assayed in a Sunrise
microplate reader (Tecan, Groedig, Austria). The mean and standard
deviations of three parallel samples were calculated.
Tumor growth model
BALB/c nude mice (4 to 6-weeks old, body weight
20–30 g) were purchased from National Rodent Laboratory Animal
Resources, Shanghai Branch (Shanghai, China) and maintained under
specific pathogen-free condition. The experiment was approved by
the Animal Use and Care Committee of Jinling Hospital, School of
Medicine, Nanjing University. The mice were inoculated
subcutaneously (s.c.) with 2×106 human PSA-producing
prostate cancer LNCaP cells. Tumors were allowed to grow until they
reached a diameter of 0.3–0.5 cm (day 0). The mice were then
randomly divided into different treatment groups. Six mice were
included in each group.
In vivo treatment with the recombinant
bicistronic adenovirus
To detect the selective expression of luciferase or
EGFP in vivo, 1×108 PFU of the control
adenovirus, Ad/PSA-Luc or Ad/PSAP-EGFP were injected into the mice
bearing the LNCaP tumors by tail vein. Mice were sacrificed 48 h
after the infection and tissues were lysed for luciferase activity
detection or snap-frozen for cryosectioning for the observation of
EGFP by fluorescence microscopy.
To detect the antitumor effect of targeted βG
expression plus prodrug DOX-GA3 in vivo, 1×108
PFU of the control adenovirus, Ad/PSAP-GV16-βG or Ad/PSAP-βG was
administered by an intravenous injection into the lateral tail, and
500 mg/kg DOX-GA3 was simultaneously injected. The maximum
tolerated dose (MTD) of DOX-GA3 (500 mg/kg weekly ×2) was
determined according to a previous report (6). The treatment was performed twice, once
a week. The tumor sizes were determined by measuring two diameters
perpendicular to each other with a caliper every 3 days. The tumor
volume (V) was estimated using the equation V = 2/3ab2,
where a is the longest diameter and b is the shortest diameter. The
survival times of the nude mice were recorded. Once the animals
died, the tumor tissues were removed immediately for additional
analysis.
Histological analysis
The formalin-fixed mouse tissues were embedded in
paraffin. Serial 5-µm sections were obtained with a Leica
microtome. The sections were then dewaxed, hydrated and incubated
in methanol-H2O2 for 20 min to remove
endogenous peroxidase. The slides of the tumor tissues were
incubated sequentially with rabbit monoclonal antibody to human βG,
horseradish peroxidase-conjugated goat anti-rabbit IgG. The
sections were counterstained with hematoxylin and evaluated
independently in a blinded manner.
Statistical analysis
Statistical analysis was performed using the SPSS
11.5 software package for Windows (SPSS, Chicago, IL, USA).
Statistical significance was assessed by comparing mean ± SD values
with the Student's t-test for independent groups. Tumor volumes
were analyzed by the analysis of covariance (ANCOVA) method
followed by LSD as post-hoc, with comparisons between treatment
groups made by covariance test with the beginning differences
occurring by grouping eliminated. P<0.05 was considered to
indicate a statistically significant difference.
Results
Targeted gene expression is amplified
using the PSA promoter-controlled GAL4-regulated bicistronic
adenovirus
To construct the recombinant adenovirus, the human
PSA promoter gene was amplified from the PSA-producing prostate
cancer cell line LNCaP, and the human βG gene was amplified from
the human hepatocarcinoma cell line HepG2. New bicistronic shuttle
plasmids (pshuttle-PSAP-GV16-βG, pshuttle-PSAP-GV16-luc and
pshuttle-PSAP-GV16-EGFP) were constructed by replacing the hTERT
promoter and TRAIL gene in pshuttle-hTERT-GV16-TRAIL (15) with the PSA promoter and the βG,
luciferase or EGFP gene, respectively. Recombinant vectors
(Ad/PSAP-GV16-βG, Ad/PSAP-GV16-luc and Ad/PSAP-GV16-EGFP) were
generated by homologous recombination. By using these adenoviruses,
the expression of βG, luciferase or EGFP was controlled by the PSA
promoter and the GAL4-regulatory system (Fig. 1A). The shuttle plasmids in which βG,
luciferase or EGFP expression was controlled directly by the PSA
promoter were also constructed (pshuttle-PSAP-βG, pshuttle-PSAP-luc
and pshuttle-PSAP-EGFP) and recombinant vectors (Ad/PSAP-βG,
Ad/PSAP-Luc and Ad/PSAP-EGFP) were generated by homologous
recombination.
To evaluate the effectiveness of the augmented
expression system, PSA-producing LNCaP cells and PSA-non-producing
DU145 cells were infected with the control adenovirus,
Ad/PSAP-GV16-Luc or Ad/PSAP-Luc at a multiplicity of infection
(MOI) of 1,000 viral particles/cell. The cells were lysed and
luciferase activities were determined 48 h after the infection. In
the PSA-producing LNCaP cells, there was a relatively low
luciferase level after infection with Ad/PSAP-Luc, while the
luciferase activity increased 80- to 100-fold in the
Ad/PSAP-GV16-Luc-infected cell lysates (P=0.001). In contrast,
luciferase activity was scarcely detected in the PSA-non-producing
DU145 cells after infection with either Ad/PSAP-Luc or
Ad/PSAP-GV16-Luc (Fig. 1B).
Furthermore, when the LNCaP and DU145 cells were
infected with the control adenovirus, Ad/PSAP-GV16-EGFP or
Ad/PSAP-EGFP for 48 h, the expression of EGFP was only observed in
the PSA-producing LNCaP cells, with Ad/PSAP-GV16-EGFP-infected
cells showing significantly stronger EGFP expression than the
Ad/PSAP-EGFP-infected cells (Fig.
1C). EGFP expression was hardly observed in the
PSA-non-producing DU145 cells either infected with Ad/PSAP-EGFP or
Ad/PSAP-GV16-EGFP (data not shown).
Amplified βG expression significantly
increases the sensitivity to DOX-GA3 and the bystander effect in
LNCaP cells
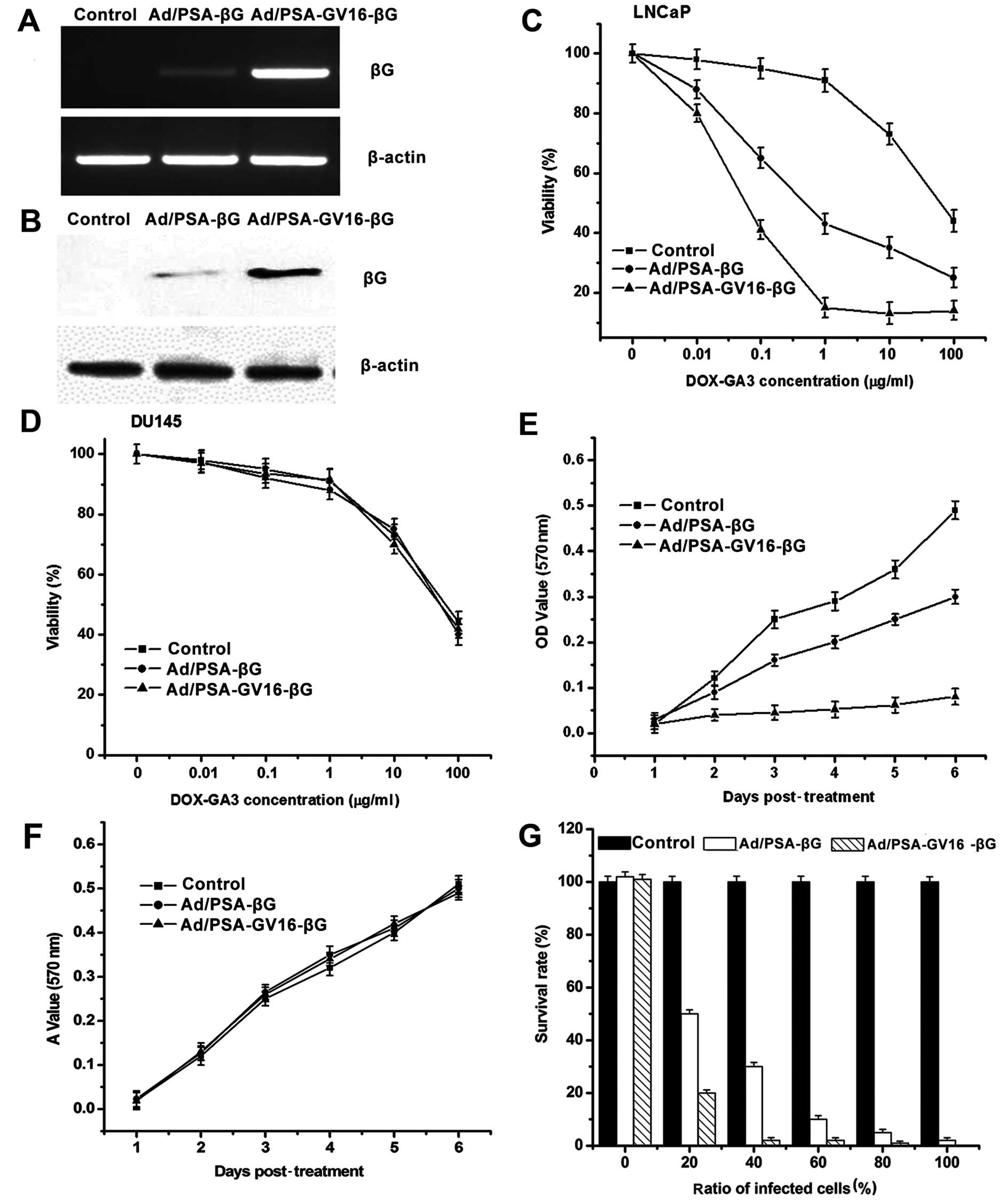
Expression of βG was detected 48 h after the LNCaP
and DU145 cells were infected with the control adenovirus,
Ad/PSAP-βG or Ad/PSAP-GV16-βG at an MOI of 1,000 viral
particles/cell. The results of RT-PCR and western blot analysis
showed that the βG expression was only detected in the LNCaP cells,
and βG expression was significantly amplified in the
Ad/PSAP-GV16-βG-infected cells compared with the
Ad/PSAP-βG-infected cells (Fig. 2A and
B).
The cytotoxicity of DOX-GA3 on the infected LNCaP
and DU145 cells was evaluated by MTT assay. When different amounts
of DOX-GA3 were added into the supernatant of the cells which were
infected for 48 h, both Ad/PSAP-βG- and Ad/PSAP-GV16-βG-infected
LNCaP cells showed an apparent dose-dependent relationship between
DOX-GA3 concentration and growth inhibition (Fig. 2C). DOX-GA3 cytotoxity on the
Ad/PSAP-GV16-βG-infected cells was much more potent than on the
Ad/PSAP-βG-infected group, indicating an increased sensitivity to
DOX-GA3 (P=0.01). In the DU145 cells, the DOX-GA3 cytotoxity was
comparable whether they were infected with the control adenovirus
or the βG-expressing adenoviruses (Fig.
2D).
To detect the time-dependent cytotoxity of DOX-GA3
on LNCaP and DU145 cells after they were infected with Ad/PSAP-βG
or Ad/PSAP-GV16-βG, 10 µg/ml DOX-GA3 was added into the
supernatant of the infected cells for different times before MTT
assay. As shown in Fig. 2E, obvious
cytotoxity was observed in both the Ad/PSAP-βG- and
Ad/PSAP-GV16-βG-infected LNCaP cells, and the cytotoxity was more
significant in the Ad/PSAP-GV16-βG-infected cells (P= 0.027),
indicating the increased sensitivity to DOX-GA3 in these cells. On
the other hand, DU145 cells did not show increased sensitivity to
DOX-GA3 whether they were infected with Ad/PSAP-βG or
Ad/PSAP-GV16-βG (Fig. 2F).
Next, to evaluate the effect of the amplified βG
expression on the bystander effect, different percentages of
infected LNCaP cells were mixed with uninfected cells before
DOX-GA3 (10 µg/ml) was applied and incubated for 72 h. In
the Ad/PSAP-GV16-βG-infected group, the cells were almost
completely killed after the DOX-GA3 incubation with only ~40%
infected cells. Even when only 20% of the Ad/PSA-GV16-βG-infected
cells were applied, ~80% of total cells were killed, showing a
stronger bystander effect than that noted in the Ad/PSA-βG-infected
cells (P=0.001) (Fig. 2G).
Amplified and selective target gene
expression in vivo using the GAL4-regulated bicistronic
adenovirus
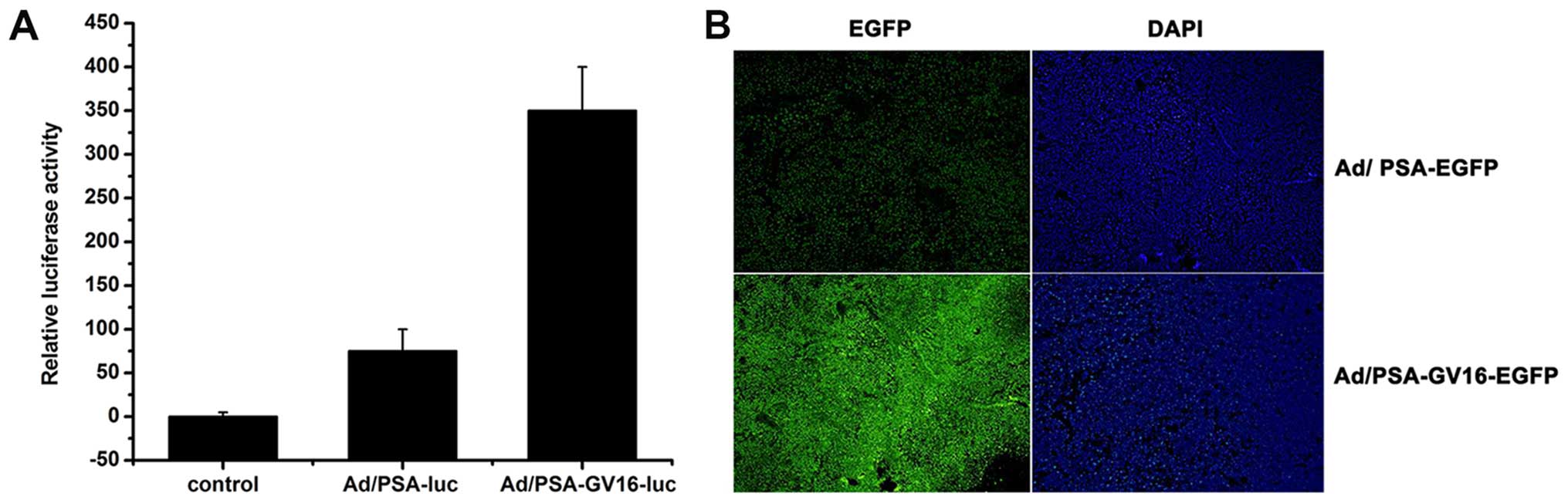
To evaluate whether selective and amplified
expression of target genes can be achieved in vivo, nude
mouse models with PSA-producing LNCaP xenografts were established.
When tumors reached a diameter of ~0.3–0.5 cm, control adenovirus,
Ad/PSAP-GV16-Luc or Ad/PSAP-Luc were injected into the tail vein,
mice were sacrificed 48 h later, and luciferase activities were
determined in the tumors and different tissues. Results showed that
the luciferase activity was only detected in tumor lysates of both
the Ad/PSAP-Luc- and Ad/PSAP-GV16-Luc-infected mice, and the
luciferase activity was significantly higher in the
Ad/PSAP-GV16-Luc-infected mice than that in the
Ad/PSAP-Luc-infected mice (P=0.001) (Fig. 3A).
To directly observe the selective and amplified gene
expression, mice with LNCaP xenografts were infected with the
control adenovirus, Ad/PSAP-GV16-EGFP or Ad/PSAP-EGFP for 48 h
before tumors and different tissues were snap-frozen and
cryosectioned for direct fluorescence observation. EGFP expression
was only observed in the tumors and significantly stronger
fluorescence was observed in the Ad/PSAP-GV16-EGFP-infected mice
than that in the Ad/PSAP-EGFP-infected mice (Fig. 3B).
Targeted and amplified βG expression
combined with DOX-GA3 administration effectively suppresses the
growth of PSA-producing tumors
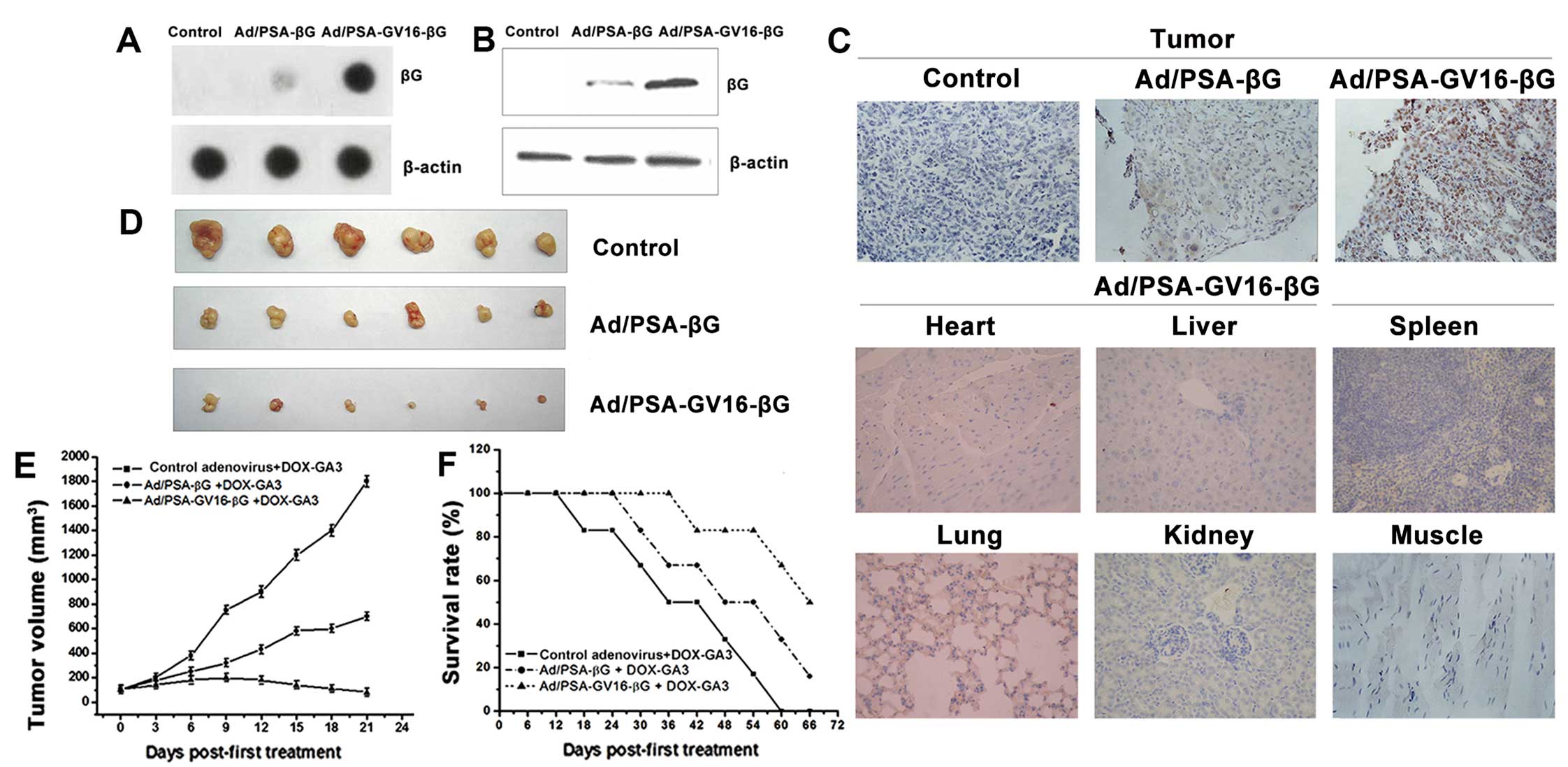
To confirm the targeted and amplified βG expression
in vivo, the control adenovirus, Ad/PSAP-βG or
Ad/PSAP-GV16-βG were intravenously injected into nude mice with
LNCaP xenografts. The mice were sacrificed 48 h after the
injection, and tumors were lysed to detected βG expression by dot
blot and western blot analysis. βG expression was detected in the
Ad/PSAP-βG- and Ad/PSAP-GV16-βG-infected groups, with the
Ad/PSAP-GV16-βG-infected mice showing significantly higher βG
expression than the Ad/PSAP-βG-infected mice (Fig. 4A and B). Results of the
immunohistochemical staining confirmed the increased expression of
βG, and there was no detectable βG expression in the heart, liver,
spleen, lung, kidney, or muscle, indicating the selective βG
expression in PSA-producing tumor tissue (Fig. 4C).
To evaluate the therapeutic effect of this strategy,
500 mg/kg DOX-GA3 was simultaneously injected with the control
adenovirus, Ad/PSAP-βG or Ad/PSAP-GV16-βG into the established nude
mouse model. After twice weekly sequential intravenous injections
of adenovirus and DOX-GA3, tumor volume was monitored every 3 days.
Treatment with Ad/PSAP-GV16-βG and 500 mg/kg DOX-GA3 resulted in
more effective tumor growth inhibition than the Ad/PSAP-βG and
DOX-GA3-treated group (P<0.05) (Fig.
4D and E). Survival time of the treated mice was also
evaluated. In mice treated with Ad/PSAP-βG or Ad/PSAP-GV16-βG and
DOX-GA3, the survival time was prolonged, especially in the
Ad/PSAP-GV16-βG-infected group (Fig.
4F), and the difference was significant (P<0.05).
Discussion
The aim of the present study was to investigate
whether a GAL4-regulated bicistronic adenovirus could achieve
selective and amplified βG expression in PSA-producing prostate
cancer cells by using the PSA promoter. If successful, this suicide
gene system would have potential for application in the treatment
of prostate cancer. Our results showed that by using the system,
selective and amplified expression of both reporter genes and βG
were achieved in the PSA-producing LNCaP cells but not in the
PSA-non-producing DU145 cells. This selective and amplified βG
expression combined with the prodrug DOX-GA3 was shown to have
strong cytotoxicity and a strong bystander effect in the
PSA-producing LNCaP cells, but not in the PSA-non-producing DU145
cells.
Because of the unique bystander effect, suicide gene
therapy is considered to be one of the most effective strategies
for cancer therapy (18–22). Compared with other organs, the
prostate is more suitable for suicide gene therapy for the
following reasons. i) It is easy to access by transurethral or
transrectal route; ii) several proteins are known to be expressed
in a tissue-specific manner, including prostate-specific antigen
(PSA), human glandular kallikrein 2 (hKLK2) and prostate-specific
membrane antigen (PSMA), showing great potential for
tissue-specific targeted expression of toxic gene by using
tissue-specific promoter and (iii) both normal and malignant cells
in the prostate have been shown to have abundant connexin proteins.
The connexin proteins assemble to form tiny water-filled channels
to execute gap junction functions, which play a significant role in
the bystander effect (23,24).
There are two main approaches to achieve high and
specific expression of a suicide gene for specific killing. One is
antibody-directed enzyme prodrug therapy (ADEPT), in which a
suicide gene protein is fused with a tumor-specific antibody which
can specifically direct the suicide gene protein to the tumor
(6,11,25–27).
Another promising approach is gene-directed enzyme prodrug therapy
(GDEPT) in which the expression of a suicide gene is controlled by
a tumor-specific promoter which can mediate its expression
specifically in tumors (5,28–30).
Compared with the ADEPT strategy, GDEPT seems to be more attractive
since it can avoid the instability and the fast renal clearance of
the antibody-suicide gene fusion protein. However, although
specific suicide gene expression can be achieved using
tumor-specific promoters such as the carcinoembryonic antigen (CEA)
promoter, PSA promoter and prostate-specific membrane antigen
(PSMA) promoter (31–35), the relatively weak transcription
activity of these promoters often results in poor expression of
suicide genes which severely attenuates the therapeutic effect. To
overcome this problem, several augmented expression systems have
been designed to improve the targeted expression of therapeutic
genes, among which the GAL4-VP16 regulation system seemed to be
most attractive (15,36–38).
In this system, the target gene expression is controlled by a
synthesized GAL4/TATA (GT) promoter, which can be transactivated by
GAL4-VP16, whose expression is controlled by a tissue-specific
promoter. The GAL4-VP16 regulatory system is also attractive for
the following reasons. i) GAL4/TATA (GT) promoter has extremely low
basal activity in most organs and ii) the GT promoter is highly
inducible when co-delivered with the transactivating protein
GAL4-VP16 at a low dose.
In the present study, we first evaluated the
effectiveness of the GAL4 regulatory system by using luciferase and
EGFP as reporter genes. By using the PSA promoter-controlled and
GAL4-VP16-regulated bicistronic adenovirus, we showed that
expression of the reporter gene could be augmented 80- to 100-fold,
while the specificity was well maintained. Amplification of βG
expression was also achieved by using this expression system, and
because of the selective and amplified βG expression in
PSA-producing LNCaP cells, their sensitivity to DOX-GA3 was
significantly improved, and a stronger bystander effect was also
observed in these cells. Our in vivo results showed that the
specific and augmented βG expression together with the application
of its prodrug DOX-GA3 resulted in significantly stronger
suppression on tumor growth in nude mice with PSA-producing LNCaP
xenografts.
βG is an acid hydrolase present in many human
tissues. It can convert the nontoxic glucuronide prodrug with
glycoside bonds into toxic glucuronic acid. Normally the endogenous
βG expression is very low, but under certain conditions, for
example, in several types of tumor tissue, the expression of βG may
increase. To test whether the relatively low βG expression may
result in obvious toxicity after the application of its prodrug
DOX-GA3, we detected the ALT and AST levels in the
adenovirus-infected and DOX-GA3-treated mice, and the results did
not show an obvious increase in ALT and AST levels (data not
shown). Our immunohistochemical staining of different tissues also
did not show any obvious changes. Potential toxicity of this system
still needs to be evaluated before clinical use.
In conclusion, this is the first trial that
demonstrates that selective and amplified suicide gene expression
can be achieved by using a GAL4-regulated bicistronic adenovirus
controlled by the PSA promoter, and that the selective and
amplified βG expression can strongly inhibit the growth of prostate
cancer growth. The combination of the GAL4 system and the PSA
promoter resulted in high gene expression efficiency while
maintaining good specificity. These results support the possibility
of the application of the PSA promoter-controlled βG suicide gene
system for prostate cancer gene therapy.
Acknowledgments
The present study was supported by grants from the
Nature Science Foundation of China (project nos. 81372741,
81172146, 81372771, 81171924 and 30300413).
References
|
1
|
MacRae EJ, Giannoudis A, Ryan R, Brown NJ,
Hamdy FC, Maitland N and Lewis CE: Gene therapy for prostate
cancer: Current strategies and new cell-based approaches. Prostate.
66:470–494. 2006. View Article : Google Scholar
|
|
2
|
Trojan L, Kiknavelidze K, Knoll T, Alken P
and Michel MS: Prostate cancer therapy: Standard management, new
options and experimental approaches. Anticancer Res. 25:551–561.
2005.PubMed/NCBI
|
|
3
|
Ward JF and Moul JW: Biochemical
recurrence after definitive prostate cancer therapy. Part I:
Defining and localizing biochemical recurrence of prostate cancer.
Curr Opin Urol. 15:181–186. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ward JF and Moul JW: Biochemical
recurrence after definitive prostate cancer therapy. Part II:
Treatment strategies for biochemical recurrence of prostate cancer.
Curr Opin Urol. 15:187–195. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
de Graaf M, Boven E, Scheeren HW, Haisma
HJ and Pinedo HM: Beta-glucuronidase-mediated drug release. Curr
Pharm Des. 8:1391–1403. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Houba PH, Boven E, van der Meulen-Muileman
IH, Leenders RG, Scheeren JW, Pinedo HM and Haisma HJ: Pronounced
antitumor efficacy of doxorubicin when given as the prodrug DOX-GA3
in combination with a monoclonal antibody beta-glucuronidase
conjugate. Int J Cancer. 91:550–554. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Houba PH, Boven E, van der Meulen-Muileman
IH, Leenders RG, Scheeren JW, Pinedo HM and Haisma HJ: A novel
doxorubicin-glucuronide prodrug DOX-GA3 for tumour-selective
chemotherapy: Distribution and efficacy in experimental human
ovarian cancer. Br J Cancer. 84:550–557. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
van Dillen IJ, Mulder NH, Vaalburg W, de
Vries EF and Hospers GA: Influence of the bystander effect on
HSV-tk/GCV gene therapy. A review. Curr Gene Ther. 2:307–322. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mesnil M and Yamasaki H: Bystander effect
in herpes simplex virus-thymidine kinase/ganciclovir cancer gene
therapy: Role of gap-junctional intercellular communication. Cancer
Res. 60:3989–3999. 2000.PubMed/NCBI
|
|
10
|
Fonseca MJ, Storm G, Hennink WE, Gerritsen
WR and Haisma J: Cationic polymeric gene delivery of
beta-glucuronidase for doxorubicin prodrug therapy. J Gene Med.
1:407–414. 1999. View Article : Google Scholar
|
|
11
|
de Graaf M, Boven E, Oosterhoff D, van der
Meulen-Muileman IH, Huls GA, Gerritsen WR, Haisma HJ and Pinedo HM:
A fully human anti-Ep-CAM scFv-beta-glucuronidase fusion protein
for selective chemotherapy with a glucuronide prodrug. Br J Cancer.
86:811–818. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wei G, Loktionova NA, Pegg AE and Moschel
RC: Beta-glucuronidase-cleavable prodrugs of
O6-benzylguanine and
O6-benzyl-2′-deoxyguanosine. J Med Chem. 48:256–261.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Houba PH, Boven E, Erkelens CA, Leenders
RG, Scheeren JW, Pinedo HM and Haisma HJ: The efficacy of the
anthracycline prodrug daunorubicin-GA3 in human ovarian cancer
xenografts. Br J Cancer. 78:1600–1606. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sperker B, Werner U, Mürdter TE, Tekkaya
C, Fritz P, Wacke R, Adam U, Gerken M, Drewelow B and Kroemer HK:
Expression and function of beta-glucuronidase in pancreatic cancer:
Potential role in drug targeting. Naunyn Schmiedebergs Arch
Pharmacol. 362:110–115. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Park HS, Cheon J, Cho HY, Ko YH, Bae JH,
Moon DG and Kim JJ: In vivo characterization of a prostate-specific
antigen promoter-based suicide gene therapy for the treatment of
benign prostatic hyperplasia. Gene Ther. 10:1129–1134. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yu D, Chen D, Chiu C, Razmazma B, Chow YH
and Pang S: Prostate-specific targeting using PSA promoter-based
lentiviral vectors. Cancer Gene Ther. 8:628–635. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lin T, Gu J, Zhang L, Huang X, Stephens
LC, Curley SA and Fang B: Targeted expression of green fluorescent
protein/tumor necrosis factor-related apoptosis-inducing ligand
fusion protein from human telomerase reverse transcriptase promoter
elicits antitumor activity without toxic effects on primary human
hepatocytes. Cancer Res. 62:3620–3625. 2002.PubMed/NCBI
|
|
18
|
Nasu Y, Kusaka N, Saika T, Tsushima T and
Kumon H: Suicide gene therapy for urogenital cancer: Current
outcome and prospects. Mol Urol. 4:67–71. 2000. View Article : Google Scholar
|
|
19
|
Yazawa K, Fisher WE and Brunicardi FC:
Current progress in suicide gene therapy for cancer. World J Surg.
26:783–789. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Poulsen TT, Pedersen N and Poulsen HS:
Replacement and suicide gene therapy for targeted treatment of lung
cancer. Clin Lung Cancer. 6:227–236. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nicholas TW, Read SB, Burrows FJ and Kruse
CA: Suicide gene therapy with herpes simplex virus thymidine kinase
and ganciclovir is enhanced with connexins to improve gap junctions
and bystander effects. Histol histopathol. 18:495–507.
2003.PubMed/NCBI
|
|
22
|
Fillat C, Carrió M, Cascante A and Sangro
B: Suicide gene therapy mediated by the herpes simplex virus
thymidine kinase gene/ganciclovir system: Fifteen years of
application. Curr Gene Ther. 3:13–26. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Satoh T, Irie A, Egawa S and Baba S: In
situ gene therapy for prostate cancer. Curr Gene Ther. 5:111–119.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shalev M, Kadmon D, Teh BS, Butler EB,
Aguilar-Cordova E, Thompson TC, Herman JR, Adler HL, Scardino PT
and Miles BJ: Suicide gene therapy toxicity after multiple and
repeat injections in patients with localized prostate cancer. J
Urol. 163:1747–1750. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Biela BH, Khawli LA, Hu P and Epstein AL:
Chimeric TNT-3/human beta-glucuronidase fusion proteins for
antibody-directed enzyme prodrug therapy (ADEPT). Cancer Biother
Radiopharm. 18:339–353. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Haisma HJ, Sernee MF, Hooijberg E,
Brakenhoff RH, vd Meulen-Muileman IH, Pinedo HM and Boven E:
Construction and characterization of a fusion protein of
single-chain anti-CD20 antibody and human beta-glucuronidase for
antibody-directed enzyme prodrug therapy. Blood. 92:184–190.
1998.PubMed/NCBI
|
|
27
|
Kaliberov SA, Chiz S, Kaliberova LN,
Krendelchtchikova V, Della Manna D, Zhou T and Buchsbaum DJ:
Combination of cytosine deaminase suicide gene expression with DR5
antibody treatment increases cancer cell cytotoxicity. Cancer Gene
Ther. 13:203–214. 2006. View Article : Google Scholar
|
|
28
|
Djeha HA, Todryk SM, Pelech S, Wrighton
CJ, Irvine AS, Mountain A and Lipinski KS: Antitumor immune
responses mediated by adenoviral GDEPT using nitroreductase/CB1954
is enhanced by high-level coexpression of heat shock protein 70.
Cancer Gene Ther. 12:560–571. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Miki K, Xu M, Gupta A, Ba Y, Tan Y,
Al-Refaie W, Bouvet M, Makuuchi M, Moossa AR and Hoffman RM:
Methioninase cancer gene therapy with selenomethionine as suicide
prodrug substrate. Cancer Res. 61:6805–6810. 2001.PubMed/NCBI
|
|
30
|
Denny WA: Prodrugs for gene-directed
enzyme-prodrug therapy (suicide gene therapy). J Biomed Biotechnol.
2003:48–70. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Dabrowska A, Szary J, Kowalczuk M, Szala S
and Ugorski M: CEA-negative glioblastoma and melanoma cells are
sensitive to cytosine deaminase/5-fluorocytosine therapy directed
by the carcinoembryonic antigen promoter. Acta Biochim Pol.
51:723–732. 2004.PubMed/NCBI
|
|
32
|
Ikegami S, Tadakuma T, Suzuki S, Yoshimura
I, Asano T and Hayakawa M: Development of gene therapy using
prostate-specific membrane antigen promoter/enhancer with Cre
Recombinase/LoxP system for prostate cancer cells under androgen
ablation condition. Jpn J Cancer Res. 93:1154–1163. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Peng W, Chen J, Huang YH and Sawicki JA:
Tightly-regulated suicide gene expression kills PSA-expressing
prostate tumor cells. Gene Ther. 12:1573–1580. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Qiao J, Doubrovin M, Sauter BV, Huang Y,
Guo ZS, Balatoni J, Akhurst T, Blasberg RG, Tjuvajev JG, Chen SH,
et al: Tumor-specific transcriptional targeting of suicide gene
therapy. Gene Ther. 9:168–175. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ueda K, Iwahashi M, Nakamori M, Nakamura
M, Matsuura I, Yamaue H and Tanimura H: Carcinoembryonic
antigen-specific suicide gene therapy of cytosine
deaminase/5-fluorocytosine enhanced by the cre/loxP system in the
orthotopic gastric carcinoma model. Cancer Res. 61:6158–6162.
2001.PubMed/NCBI
|
|
36
|
Koch PE, Guo ZS, Kagawa S, Gu J, Roth JA
and Fang B: Augmenting transgene expression from carcinoembryonic
antigen (CEA) promoter via a GAL4 gene regulatory system. Mol Ther.
3:278–283. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ray S, Paulmurugan R, Hildebrandt I, Iyer
M, Wu L, Carey M and Gambhir SS: Novel bidirectional vector
strategy for amplification of therapeutic and reporter gene
expression. Hum Gene Ther. 15:681–690. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Iyer M, Salazar FB, Lewis X, Zhang L, Wu
L, Carey M and Gambhir SS: Non-invasive imaging of a transgenic
mouse model using a prostate-specific two-step transcriptional
amplification strategy. Transgenic Res. 14:47–55. 2005. View Article : Google Scholar : PubMed/NCBI
|