Introduction
Liver cancer is one of the most common malignant
cancers (1). It is ranked 3rd in
deaths from various malignant cancers (1). Liver cancer mostly occurs in the
conditions of hepatic disease and liver cirrhosis, and most
patients have weak liver function (2). Therefore, patients often cannot
tolerate an operation or endure traditional chemotherapeutics in
strong enough doses. Also, liver cancer is insensitive to
traditional chemotherapeutics (3).
Hence, chemotherapy often has no satisfactory results for the
improvement of prognosis for patients. Therefore, identification of
natural chemotherapeutics without harmful effects has become one of
the strategies to improve therapeutic effect of liver cancer
(4).
The combination between NF-κB and sequence κB on DNA
may adjust the transcriptional activation of multiple genes, which
are closely related with the inhibition of transcriptional
activation, vasculogenesis, tumor metastasis and apoptosis, which
are the key link of promoting tumor growth and resistance (5). The inhibition of NF-κB activity may
increase the sensitivity of cancer cells for chemotherapeutics and
radiotherapy (6). The expression
and mutation of cancer suppressor gene p53 is closely related with
tumorigenesis, development and apoptosis of multiple tumors
(7). However, the expression of
both p53 and NF-κB is adjusted by Akt (8).
As an isoprene flavonoid existing in hops,
xanthohumol has multiple biological activities. It plays a
significant role in prevention and cure of diabetes and atherosis.
Also, it has antioxidant, and antiviral functions, inhibiting
cancer cell growth in breast, colon, ovarian and prostatic cancer
(9,10). However, the molecular mechanism of
such function is not yet clear. In the present study, we elucidated
anticancer effect of xanthohumol inducing growth inhibition and
apoptosis of human liver cancer through the NF-κB/p53-apoptosis
signaling pathway.
Materials and methods
Chemicals and materials
Dulbecco's modifid Eagle's medium (DMEM) and fetal
bovine serum (FBS) were acquired from Gibco Technologies (Carlsbad,
CA, USA). Penicillin and streptomycin were acquired from Life
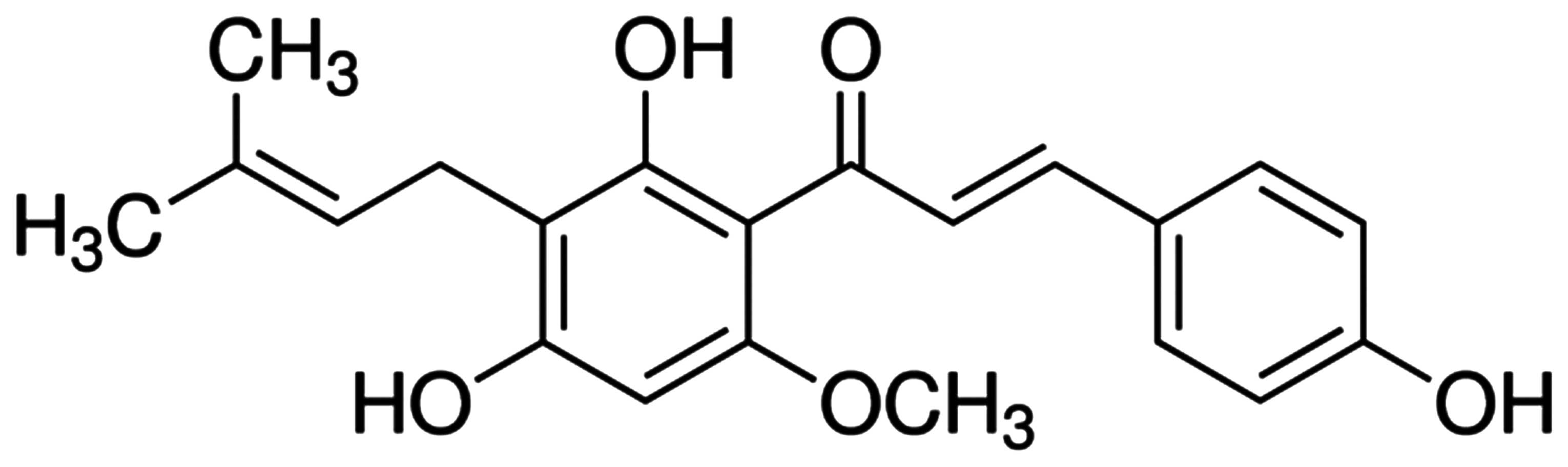
Technologies. Xanthohumol and
3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2-H-tetrazolium bromide
(MTT) were acquired from Sigma-Aldrich (St. Louis, MO, USA). The
chemical structure of xanthohumol is shown in Fig. 1. FITC Annexin V apoptosis detection
kit was acquired from BD Biosciences (San Jose, CA, USA). Caspase-3
activity kit was acquired from GeneTex, Inc. (Irvine, CA, USA).
Cell culture
Human liver cancer HepG2 cells were maintained in
DMEM medium (Gibco) supplemented with 10% (v/v) FBS and 2%
penicillin/streptomycin (Life Technologies) in 5% CO2
incubator at 37°C in a humidified atmosphere.
Cell viability measurement
The effect of xanthohumol on cell viability was
measured using the MTT assay. HepG2 cells were seeded in 96-well
culture plates (5,000 cells/well) and treated with concentrations
of (0–200 µM) xanthohumol for 1–3 days. After treatment,
cells were incubated with 200 µl MTT (1 mg/ml) for 4 h,
followed by removal of the supernatant and dissolution of 20
µl DMSO. The absorbance of the resulting solution was
recorded using a microplate reader (Perkin Elmer Inc., Waltham, MA,
USA) at 570 nm.
Apoptosis detection by Annexin V-FITC/PI
staining
HepG2 cells were seeded in 6-well culture plates
(2.5×105 cells/well) and treated with 10, 20, 30 and 40
µM of xanthohumol for 1 day (24 h). The cells were
resuspended in 1X binding buffer according to the manufacturer's
instruction. Then, HepG2 cells were stained with 5 µl V-FITC
and 5 µl propidium iodide (PI) for 15 min on ice. At the end
of the staining process, 10,000 cells were acquired for each
replicate using Accuri C6 flow cytometer.
Caspase-3 activity
HepG2 cells were seeded at a density of
1×106 cells/culture dish. At the end of the incubation
period, the cells were centrifuged at 3,000 rpm for 5 min and the
supernatant was removed. Then, the cells were resuspended in 0.5 ml
wash buffer and centrifuged at 2,000 rpm for 10 min. An equal
amount of total protein was incubated with Ac-IETD-pNA for
caspase-3 assay for 4–6 h. Fluorescence intensity was measured at
485 nm (excitation wavelength) and 535 nm (mission wavelength).
Western blot analysis
HepG2 cells were seeded at a density of
1×106 cells/culture dish. After xanthohumol treatment,
cells were washed with cold PBS and lysed with cold RIPA buffer
containing protease inhibitors. Protein concentrations were
measured using BSA (Bio-Rad Laboratories, Hercules, CA, USA). Next,
40 µg protein was separated with 10–12% sodium dodecyl
sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and
transferred to 0.2 µm nitrocellulose membrane blocking with
1% BSA in PBS-T. Each membrane was incubated with a specific
primary antibody NF-κB (1:1,000), p53, PARP, XIAP, AIF, Bax,
cytochrome c and β-actin at 4°C overnight. After three
washes in 1% BSA in PBS-T, each membrane was incubated with the
appropriate secondary antibody at room temperature for 2 h and
visualized using an ECL Advanced Western blot detection kit (Thermo
Fisher Scientif, Waltham, MA, USA).
Statistical analysis
Data are expressed as means ± standard deviation
using sample triplicates. Comparisons between control and treated
groups were conducted using one-way ANOVA with post hoc Tukey's
test. Results of P<0.05 were considered to be significant.
Results
Anticancer effect of xanthohumol induces
growth inhibition of human liver cancer
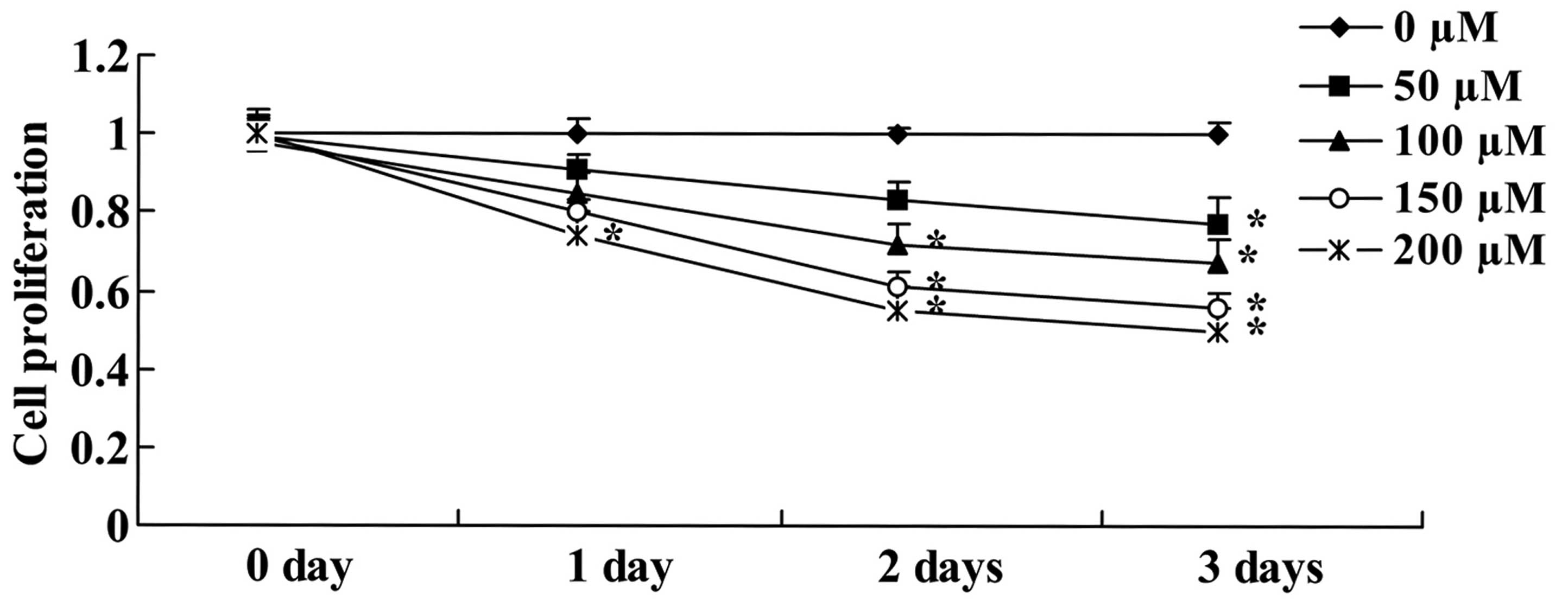
HepG2 cells were subjected to the MTT assay to
evaluate the anticancer effect of xanthohumol treatment by
measuring cell proliferation. Xanthohumol reduced cell
proliferation of HepG2 cells in a concentration- and time-dependent
manner (Fig. 2). As shown in
Fig. 2, this change was markedly
observed after exposure to 200 µM of xanthohumol for 1 day,
100–200 µM of xanthohumol markedly reduced cell
proliferation of HepG2 cells in 2 or 3 day treatments (Fig. 2). At 50 µM xanthohumol
significantly inhibited cell proliferation of HepG2 cells up to 3
days (Fig. 2).
Anticancer effect of xanthohumol induces
apoptosis of human liver cancer
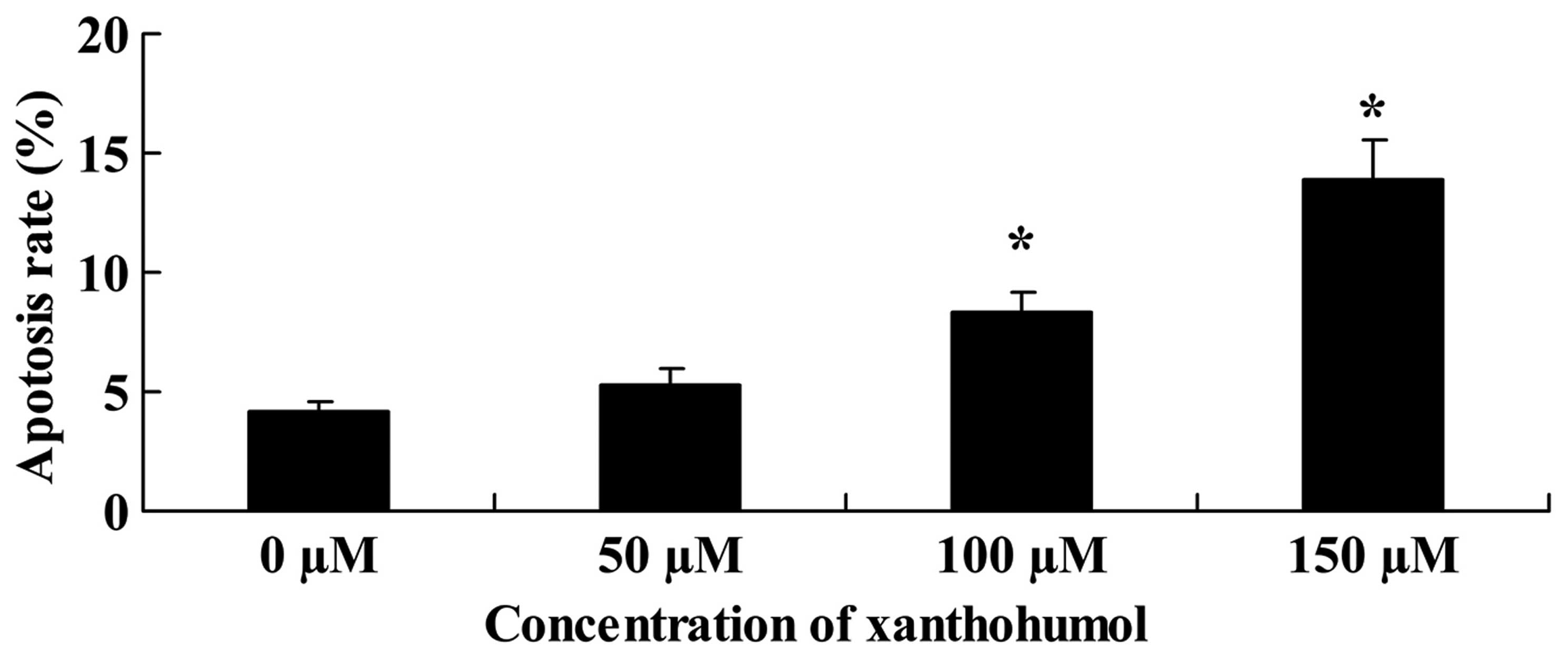
The cell apoptosis was determined by analysis using
PI staining. As shown in Fig. 2,
apoptosis of HepG2 cells was treated with 0–150 µM of
xanthohumol. The group treated with 100–150 µM of
xanthohumol showed a significant increase in apoptosis rate in
comparison with the 0 µM xanthohumol group (Fig. 3).
Anticancer effect of xanthohumol induces
caspase-3 activity of human liver cancer
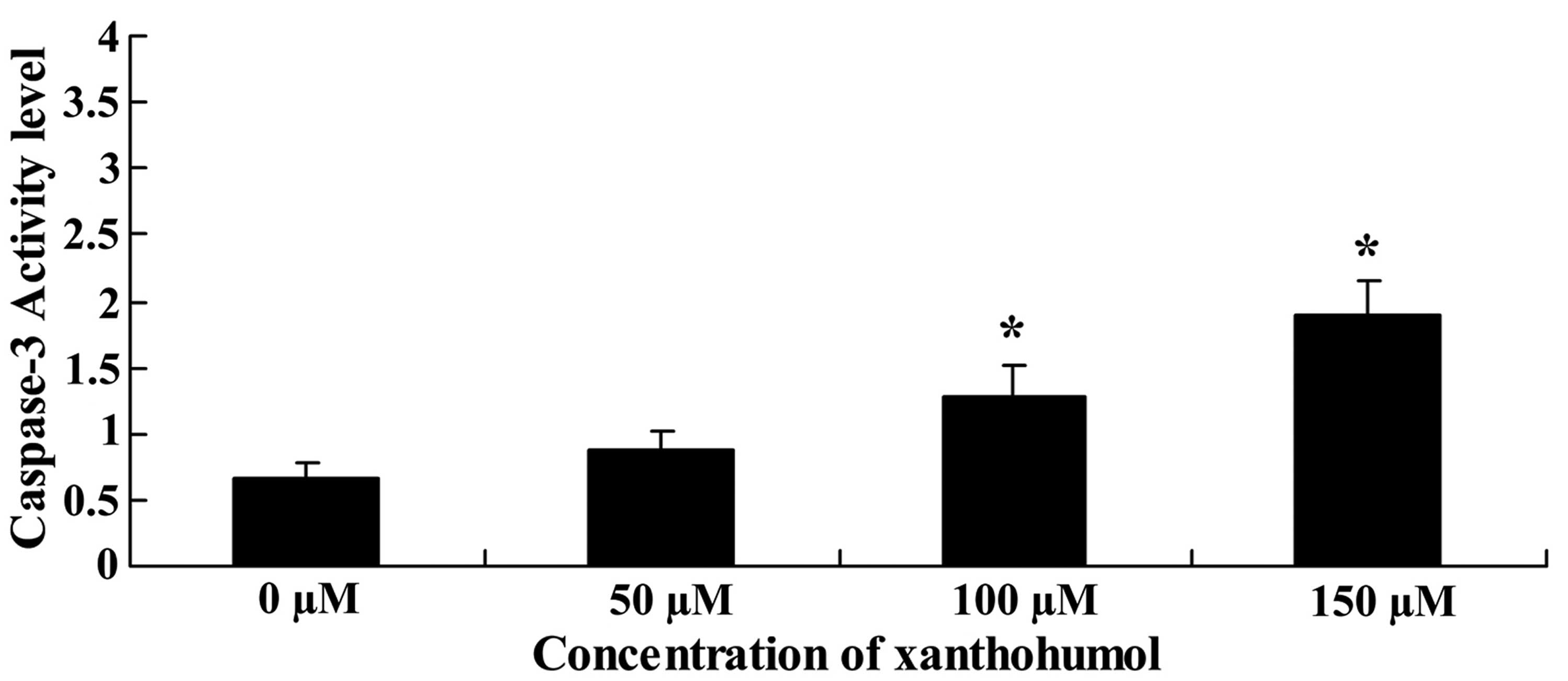
To determine whether xanthohumol induced apoptosis
in HepG2 cells, caspase-3 activity was conducted. Fig. 4 shows caspase-3 activity was
significantly increased by treatment with xanthohumol.
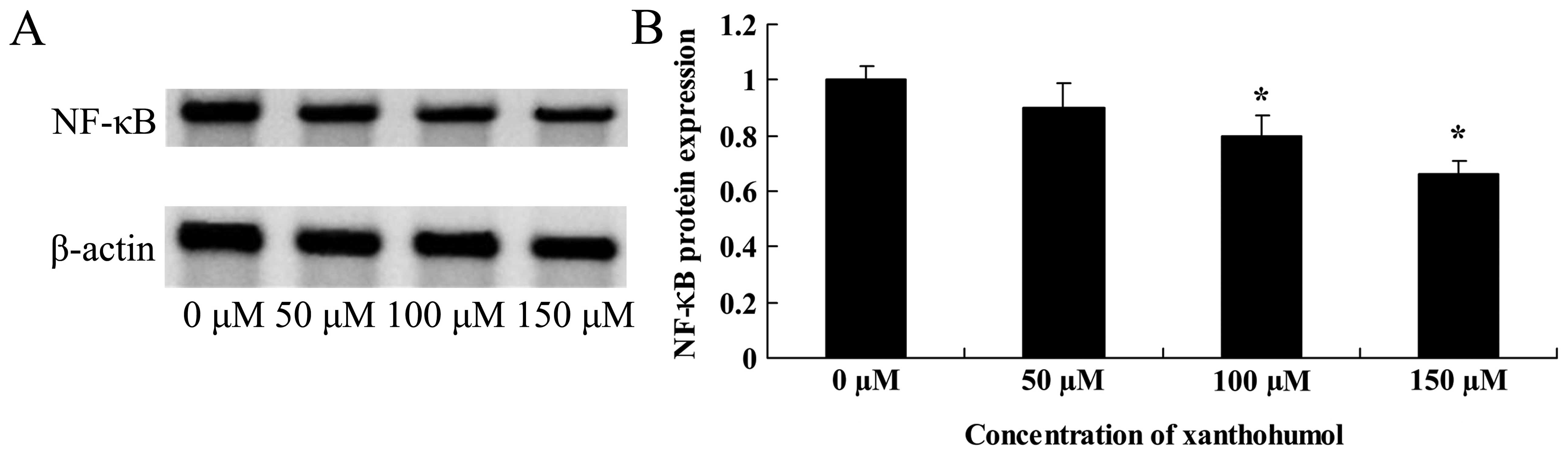
Anticancer effect of xanthohumol inhibits
NF-κB signaling of human liver cancer
Furthermore, we explored whether xanthohumol
inhibits NF-κB signaling in HepG2 cells, NF-κB protein expression
was executed using western blot analysis. Dose response curves
shown in Fig. 5 indicate that NF-κB
protein expression was significantly inhibited with 100 or 150
µM of xanthohumol.
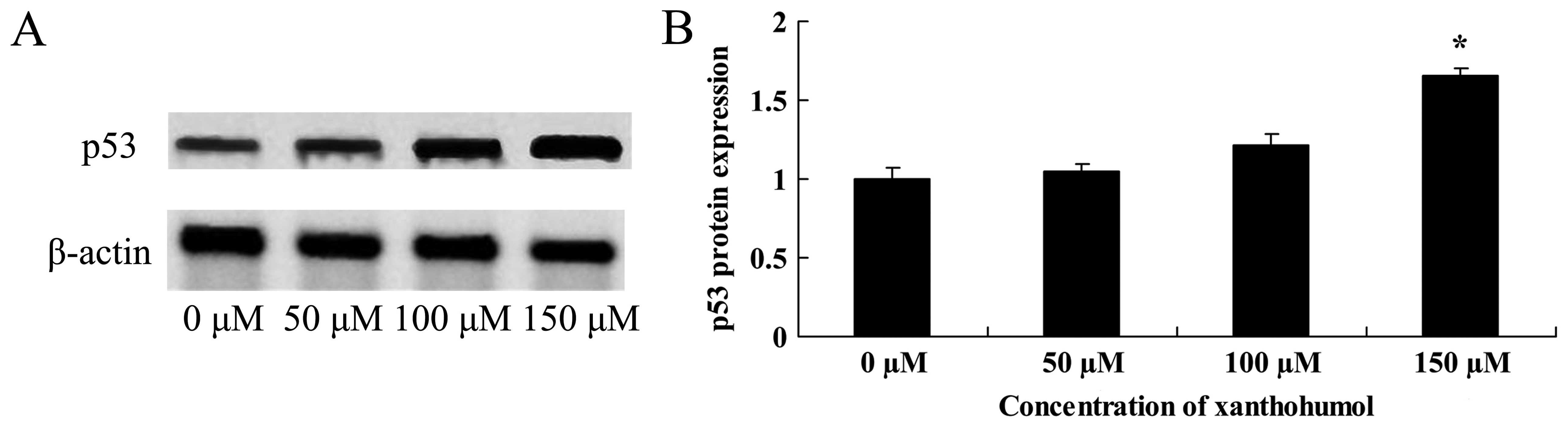
Anticancer effect of xanthohumol induces
p53 signaling of human liver cancer
The anticancer effect of xanthohumol induces p53
signaling of human liver cancer, western blot analysis was executed
for p53 protein expression. As shown in Fig. 6, after treatment with xanthohumol,
promotion of p53 protein expression was significantly observed in
HepG2 cells exposed with 100 or 150 µM of xanthohumol.
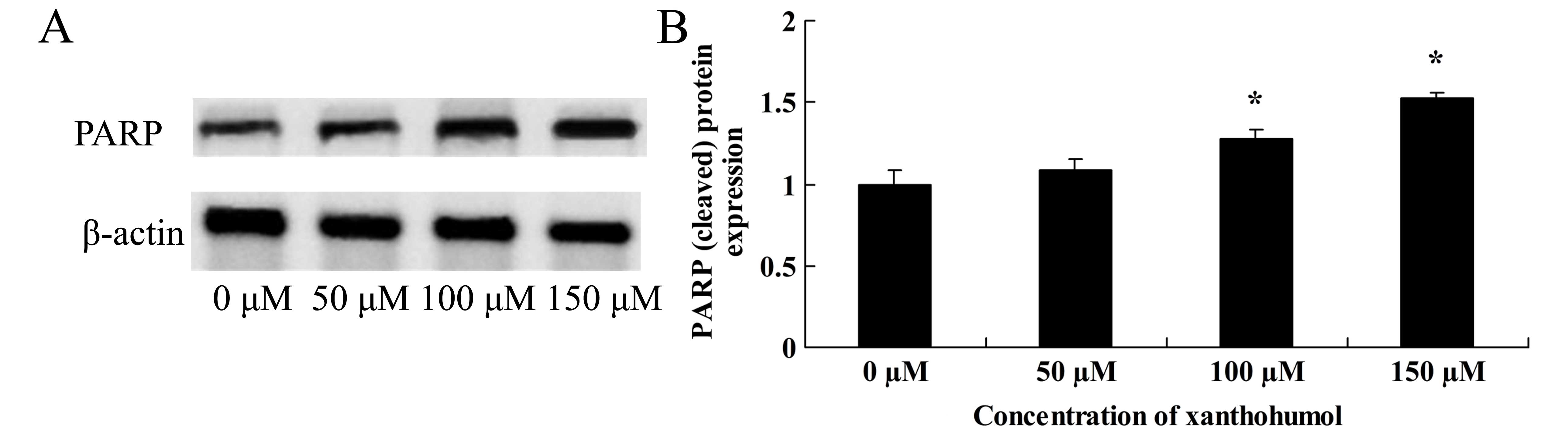
Anticancer effect of xanthohumol induces
PARP signaling of human liver cancer
We identified the anticancer effect of xanthohumol
on PARP signaling of human liver cancer. When HepG2 cells treated
with xanthohumol, PARP protein expression was significantly
increased in HepG2 cells (Fig.
7).
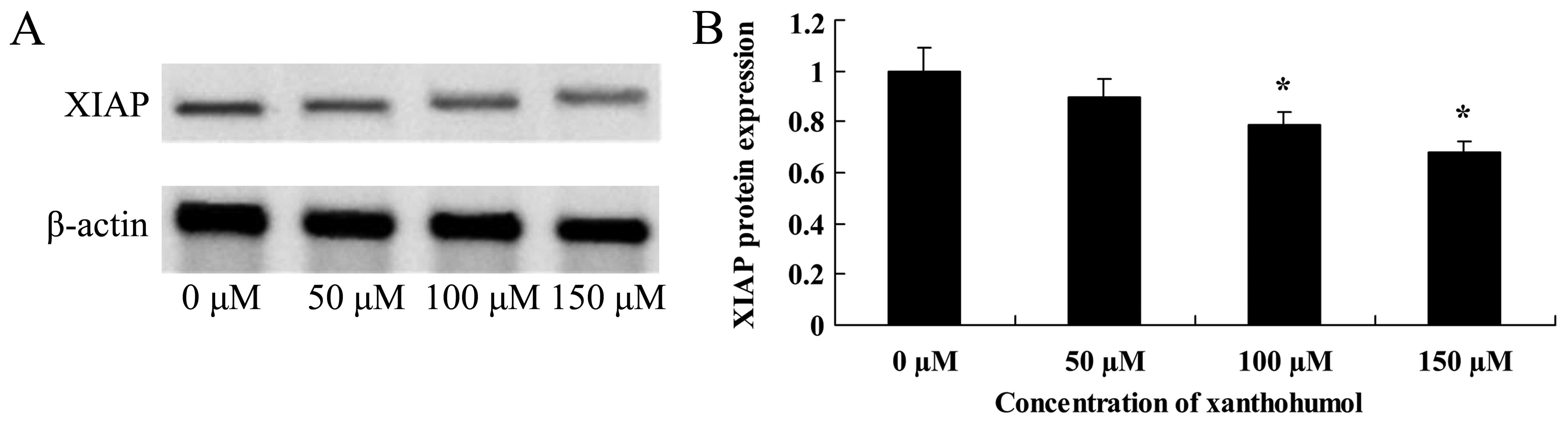
Anticancer effect of xanthohumol inhibits
XIAP signaling of human liver cancer
Furthermore, the anticancer effect of xanthohumol
inhibiting XIAP signaling of human liver cancer was determined. As
shown in Fig. 8, following
xanthohumol treatment, the XIAP proteins expression decreased in
HepG2 cells.
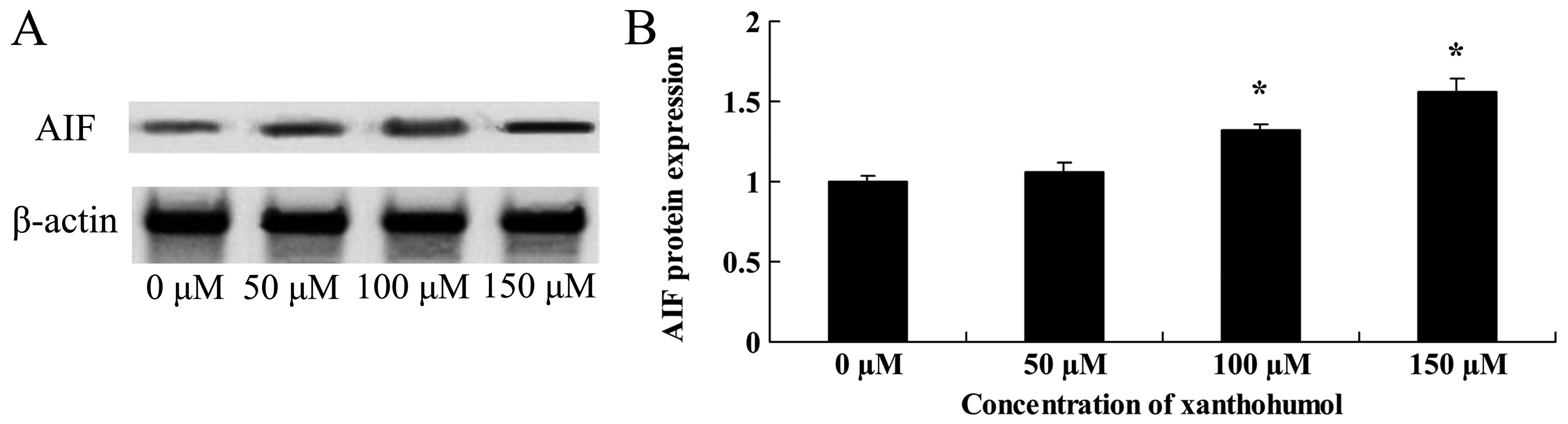
Anticancer effect of xanthohumol inhibits
AIF signaling of human liver cancer
Increased AIF signaling is an important
characteristic of cell apoptosis. In addition, a remarkable
increase in the abundance of AIF protein expression was also
detected after xanthohumol treatment (Fig. 9).
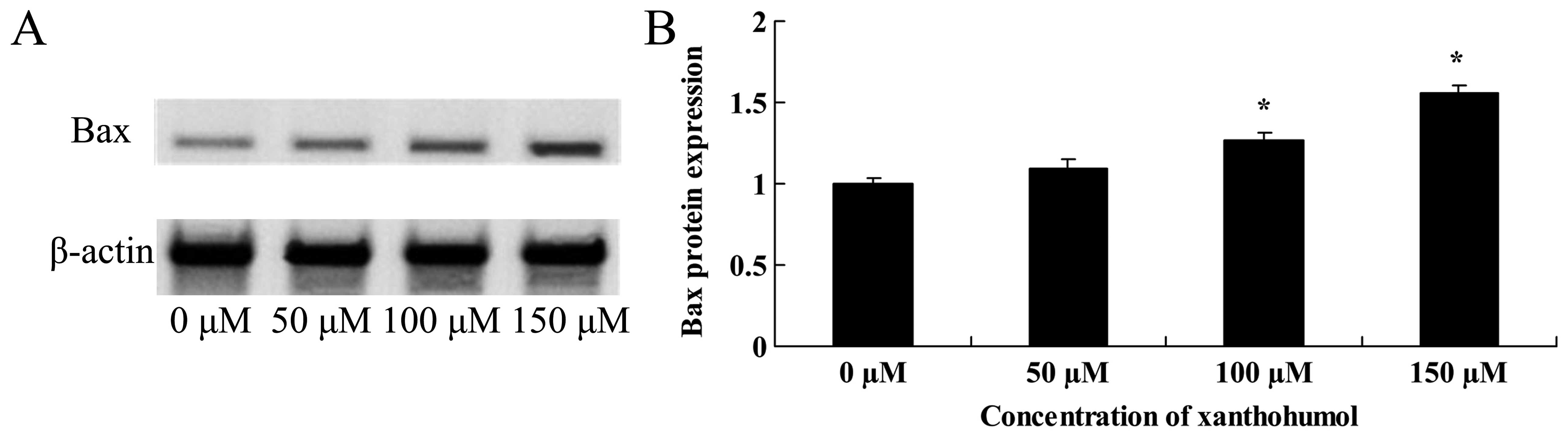
Anticancer effect of xanthohumol induces
Bax signaling of human liver cancer
To investigate whether Bax signaling is involved in
xanthohumol-induced apoptosis, we measured Bax protein expression
in HepG2 cells by western blot analysis. As shown in Fig. 10, HepG2 cells treated with 20
µM of xanthohumol showed a time-dependent promotion of Bax
signaling.
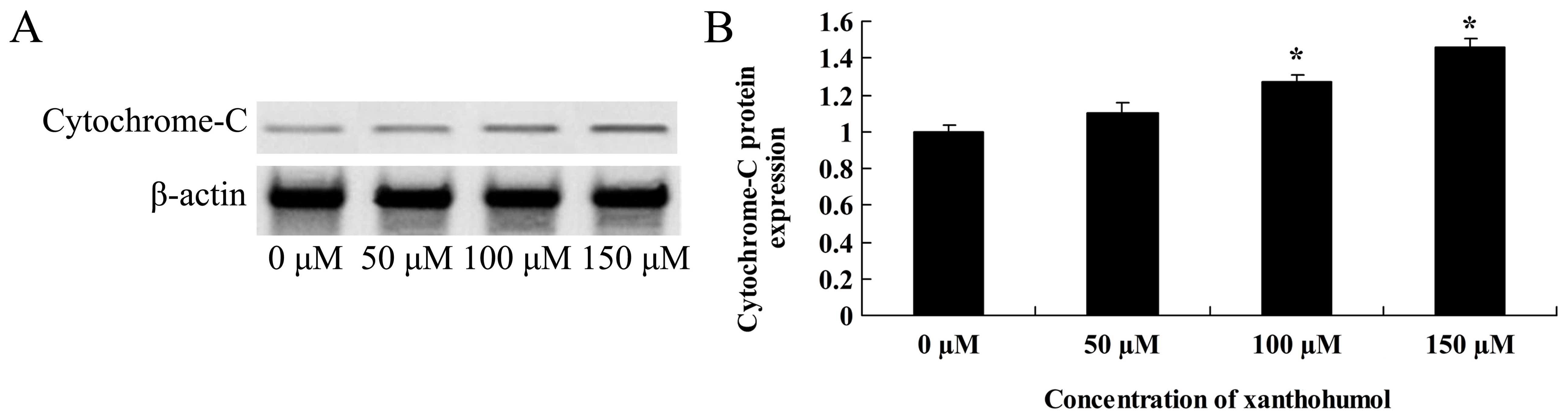
Anticancer effect of xanthohumol induces
cytochrome c signaling of human liver cancer
Activation of cytochrome c signaling is
involved in the anticancer effect of xanthohumol in HepG2 cells. As
expected, the xanthohumol treatment (100–150 µM) resulted in
a significant decrease in cytochrome c signaling of HepG2
cells (Fig. 11).
Discussion
Apoptosis, which is an intrinsic function of cells,
is the reverse of cell proliferation (3). Cell death that occurs via triggering
the suicide program of cells is called apoptosis. The essence of
the so-called apoptosis program is a set of gene programs in cells
responsible for perception, adjustment and execution of the cell
death signal (11). Apoptosis is an
initiative death, which is very common and has important
physiological and pathological significance. Under physiological
status, the death of body cells is a very common phenomenon, which
happens continually. Nevertheless, the essence of such physiologic
death is apoptosis, which is a significant method to adjust the
balance between growth and death by the body. It keeps the
homeostasis of body cell population together with cell
proliferation. The normal physiological process of body cannot do
without apoptosis. In hodiernal study, the anticancer effect of
xanthohumol induces growth inhibition, enhance apoptosis and
advance caspase-3 activity of HepG2 cells. Yong et al
(12) indicated that xanthohumol
induces growth inhibition and apoptosis of human cervical cancer
cells, breast (10) and prostate
cancer (9). These results confirmed
that xanthohumol can suppress cell growth and induce apoptosis of
human liver cancer.
NF-κB may adjust the transcriptional activation of
multiple genes, which is closely related with the inhibiting effect
of cell proliferation, vasculogenesis, tumor metastasis and
apoptosis and also is a key link of promoting tumor growth and
resistance (13). The inhibition of
NF-κB activity may increase the sensitivity of cancer cells against
chemotherapeutics and radiotherapy (14). Research has shown that by the
inhibition of NF-κB excitation, the expression of p53 significantly
increases by downstream factors of upregulated p53 (15). The loss of gene structural stability
is a key factor of multiple tumorigenesis, in which the cancer
suppressor gene p53 is the stress reaction gene of cells,
responding to DNA damage arising from various factors (16). About half of human tumors show loss
of p53 function. The structure and function of p53 as well as the
gene activity network focusing on p53 have been summarized
(16). Also, the correlation
between p53 and HBV and their effect in primary
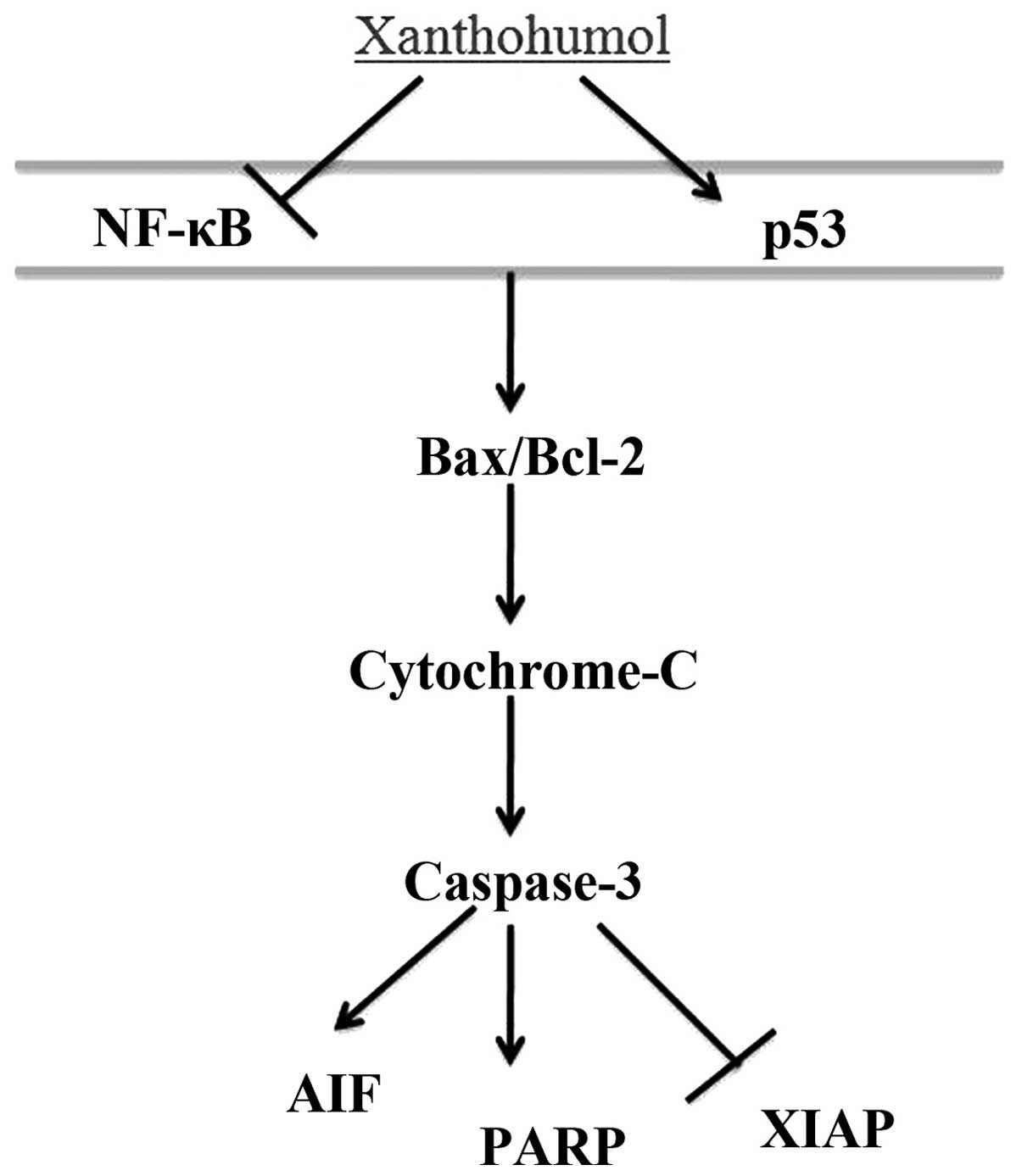
hepatocarcinogenesis have been emphasized (17). In the present study, the anticancer
effect of xanthohumol inhibited the NF-κB signaling and induced p53
signaling of human liver cancer as demonstrated in Fig. 12. Dell'Eva et al (18) reported xanthohumol, an AKT/NF-κB
inhibitor, treatment in leukemia and endothelial cells.
PARP can keep the structural integrity of chromosome
and participate in DNA replication and transcription (19). It plays a role in maintaining a
stable genome and apoptosis course. PARP is activated when DNA is
broken and damaged as a molecular receptor of DNA damage (20). It distinguishes and combines with
the DNA breakages, activating and catalyzing the poly ADP
ribosylation of receptor protein and participating in DNA repair
(21). PARP combines with histone
H1, which influences the normal structure of nucleosome, allowing
the chromosome to form an open and loose structure, which assists
in DNA repair. PARP is activated after DNA damage, identifying and
combining the DNA breakages, thereby protecting bare DNA terminal
from the catabolic reaction by nuclease (20). In the present study, anticancer
effect of xanthohumol induced PARP signaling of human liver cancer
(Fig. 12). Drenzek et al
(22) suggested that xanthohumol
decreases cell growth and induces epithelial ovarian cancer via
cleaved caspase-3 and cleaved PARP.
The XIAP regulation and control of cell
proliferation and migration are reported in the literature. The
conclusion has proved that such a course is achieved by a signal
path, which may allow the activation of NF-κB transcription factor,
thereby promoting the expression of genes for cell proliferation
and migration (23). Research has
shown that XIAP is the critical regulatory protein between the
apoptosis pathway and cell cycle pathway of XIAP; XIAP may promote
the hepatoma carcinoma cell to enter the G1 cycle by adjusting the
expression of cdk4, cdk6 and cyclin DI of G1 phase protein of
hepatoma carcinoma cells, thus reducing cells entering apoptosis
pathway and promoting cell proliferation (24,25).
In the present study, the anticancer effect of xanthohumol inhibits
XIAP signaling of human liver cancer (Fig. 12). Taken together, Yong et
al (12) indicated that
xanthohumol induces growth inhibition and apoptosis through
increasing of cleaved PARP, p53 and AIF, and decreasing of Bcl-2
and XIAP pathways in CaSki human cervical cancer cells.
Tumorigenesis and tumor progression are related with
an inbalance between cell proliferation and apoptosis. NCTD not
only inhibits the tumor cell proliferation, but also induces cell
apoptosis (26). AIF is a
flavoprotein with relative molecular weight of 57 kD encoded by
nuclear gene, located in the intermembrane zone of mitochondria
with double-layer coating. AIF may enter the karyon from
mitochondria by transposition, independently splitting DNA into DNA
fragments with ~60 kb, directly causing the chromatin condensation
and DNA breakage (27). Bcl family
may adjust and control the release of AIF by opening and closing
the PT pore, but it cannot influence its activity (28). Apoptosis of AIF is independent of
the activity of caspase, self-oxidase and reductase. Furthermore,
research shows that AIF antibody may inhibit the release of
cytochrome c and the grade chain reaction of caspase, but
such inhibition cannot influence the release of AIF. Thus, it can
be seen that AIF has an apoptosis-promoting effect in the upstream
of the apoptotic pathway of cytochrome c or caspase
(29). In the present study, the
anticancer effect of xanthohumol inhibited AIF signaling, induced
Bax and cytochrome c signaling of human liver cancer. Festa
et al (30) suggested that
the anticancer effect of xanthohumol induces apoptosis through
activation of caspase-3, caspase-9, PARP cleavage, Bcl-2 and
cytochrome c in human malignant glioblastoma. Yong et
al (12) indicated that
xanthohumol induces growth inhibition and apoptosis through
increasing cleaved PARP, p53 and AIF, and decreasing of Bcl-2 and
XIAP pathways in CaSki human cervical cancer cells.
The present study showed that xanthohumol induced
growth inhibition, apoptosis and caspase-dependent cell death, by
the NF-κB/p53-apoptosis signaling pathway, as well as upregulation
of Bax/Bcl-2-cytochrome c-caspase-3-PARP and AIF,
suppression of XIAP signaling pathway in HepG2 cells. Our findings
provide confirmation of the anticancer effect of xanthohumol on
human liver cancer.
References
|
1
|
Bárcena C, Stefanovic M, Tutusaus A,
Martinez-Nieto GA, Martinez L, García-Ruiz C, de Mingo A,
Caballeria J, Fernandez-Checa JC, Marí M, et al: Angiogenin
secretion from hepatoma cells activates hepatic stellate cells to
amplify a self-sustained cycle promoting liver cancer. Sci Rep.
5:79162015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lin CL, Chien RN, Yeh C, Hsu CW, Chang ML,
Chen YC and Yeh CT: Significant renoprotective effect of
telbivudine during preemptive antiviral therapy in advanced liver
cancer patients receiving cisplatin-based chemotherapy: A
case-control study. Scand J Gastroenterol. 49:1456–1464. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Iwazawa J, Ohue S, Hashimoto N, Muramoto O
and Mitani T: Clinical utility and limitations of tumor-feeder
detection software for liver cancer embolization. Eur J Radiol.
82:1665–1671. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Padhy AK and Dondi M: A report on the
implementation aspects of the International Atomic Energy Agency's
first doctoral coordinated research project, 'Management of liver
cancer using radionuclide methods with special emphasis on
trans-arterial radio-conjugate therapy and internal dosimetry'.
Semin Nucl Med. 38:S5–S12. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nagel D, Vincendeau M, Eitelhuber AC and
Krappmann D: Mechanisms and consequences of constitutive NF-κB
activation in B-cell lymphoid malignancies. Oncogene. 33:5655–5665.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wan S, Pestka S, Jubin RG, Lyu YL, Tsai YC
and Liu LF: Chemotherapeutics and radiation stimulate MHC class I
expression through elevated interferon-beta signaling in breast
cancer cells. PLoS One. 7:e325422012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chaturvedi MM, Sung B, Yadav VR, Kannappan
R and Aggarwal BB: NF-κB addiction and its role in cancer: 'one
size does not fit all'. Oncogene. 30:1615–1630. 2011. View Article : Google Scholar :
|
|
8
|
Athar M, Back JH, Kopelovich L, Bickers DR
and Kim AL: Multiple molecular targets of resveratrol:
Anti-carcinogenic mechanisms. Arch Biochem Biophys. 486:95–102.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Venè R, Benelli R, Minghelli S, Astigiano
S, Tosetti F and Ferrari N: Xanthohumol impairs human prostate
cancer cell growth and invasion and diminishes the incidence and
progression of advanced tumors in TRAMP mice. Mol Med.
18:1292–1302. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yoshimaru T, Komatsu M, Tashiro E, Imoto
M, Osada H, Miyoshi Y, Honda J, Sasa M and Katagiri T: Xanthohumol
suppresses oestrogen-signalling in breast cancer through the
inhibition of BIG3-PHB2 interactions. Sci Rep. 4:73552014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chie WC, Blazeby JM, Hsiao CF, Chiu HC,
Poon RT, Mikoshiba N, Al-Kadhimi G, Heaton N, Calara J, Collins P,
et al: EORTC Quality of Life Group: International cross-cultural
field validation of an European Organization for Research and
Treatment of Cancer questionnaire module for patients with primary
liver cancer, the European Organization for Research and Treatment
of Cancer quality-of-life questionnaire HCC18. Hepatology.
55:1122–1129. 2012. View Article : Google Scholar
|
|
12
|
Yong WK and Abd Malek SN: Xanthohumol
induces growth inhibition and apoptosis in ca ski human cervical
cancer cells. Evid Based Complement Alternat Med. 2015:9213062015.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dey A, Tergaonkar V and Lane DP:
Double-edged swords as cancer therapeutics: Simultaneously
targeting p53 and NF-kappaB pathways. Nat Rev Drug Discov.
7:1031–1040. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chariot A: The NF-kappaB-independent
functions of IKK subunits in immunity and cancer. Trends Cell Biol.
19:404–413. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Johnson RF and Perkins ND: Nuclear
factor-κB, p53, and mitochondria: Regulation of cellular metabolism
and the Warburg effect. Trends Biochem Sci. 37:317–324. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
He XX, Zhang YN, Yan JW, Yan JJ, Wu Q and
Song YH: CP-31398 inhibits the growth of p53-mutated liver cancer
cells in vitro and in vivo. Tumour Biol. Aug 7–2015.Epub ahead of
print.
|
|
17
|
Zhang X, Zhang H and Ye L: Effects of
hepatitis B virus X protein on the development of liver cancer. J
Lab Clin Med. 147:58–66. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dell'Eva R, Ambrosini C, Vannini N,
Piaggio G, Albini A and Ferrari N: AKT/NF-kappaB inhibitor
xanthohumol targets cell growth and angiogenesis in hematologic
malignancies. Cancer. 110:2007–2011. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kulkarni A, Oza J, Yao M, Sohail H,
Ginjala V, Tomas-Loba A, Horejsi Z, Tan AR, Boulton SJ and Ganesan
S: Tripartite Motif-containing 33 (TRIM33) protein functions in the
poly(ADP-ribose) polymerase (PARP)-dependent DNA damage response
through interaction with Amplified in Liver Cancer 1 (ALC1)
protein. J Biol Chem. 288:32357–32369. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Booth L, Cruickshanks N, Ridder T, Dai Y,
Grant S and Dent P: PARP and CHK inhibitors interact to cause DNA
damage and cell death in mammary carcinoma cells. Cancer Biol Ther.
14:458–465. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ciccarone F, Klinger FG, Catizone A,
Calabrese R, Zampieri M, Bacalini MG, De Felici M and Caiafa P:
Poly(ADP-ribosyl)ation acts in the DNA demethylation of mouse
primordial germ cells also with DNA damage-independent roles. PLoS
One. 7:e469272012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Drenzek JG, Seiler NL, Jaskula-Sztul R,
Rausch MM and Rose SL: Xanthohumol decreases Notch1 expression and
cell growth by cell cycle arrest and induction of apoptosis in
epithelial ovarian cancer cell lines. Gynecol Oncol. 122:396–401.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hehlgans S, Petraki C, Reichert S, Cordes
N, Rödel C and Rödel F: Double targeting of Survivin and XIAP
radiosensitizes 3D grown human colorectal tumor cells and decreases
migration. Radiother Oncol. 108:32–39. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Srivastava AK, Singh PK, Singh D, Dalela
D, Rath SK, Goel MM and Bhatt ML: Evaluation of urinary XIAP as a
diagnostic biomarker of carcinoma of urinary bladder. Tumour Biol.
35:8243–8248. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lee FA, Zee BC, Cheung FY, Kwong P, Chiang
CL, Leung KC, Siu SW, Lee C, Lai M, Kwok C, et al: Randomized phase
II study of the X-linked inhibitor of apoptosis (XIAP) antisense
AEG35156 in combination with sorafenib in patients with advanced
hepatocellular carcinoma (HCC). Am J Clin Oncol. Jun 23–2014.Epub
ahead of print.
|
|
26
|
Lewis EM, Wilkinson AS, Davis NY, Horita
DA and Wilkinson JC: Nondegradative ubiquitination of apoptosis
inducing factor (AIF) by X-linked inhibitor of apoptosis at a
residue critical for AIF-mediated chromatin degradation.
Biochemistry. 50:11084–11096. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mendivil-Perez M, Velez-Pardo C and
Jimenez-Del-Rio M: TPEN induces apoptosis independently of zinc
chelator activity in a model of acute lymphoblastic leukemia and ex
vivo acute leukemia cells through oxidative stress and mitochondria
caspase-3- and AIF-dependent pathways. Oxid Med Cell Longev.
2012:3132752012. View Article : Google Scholar
|
|
28
|
Park SY, Kim HY, Lee JH, Yoon KH, Chang MS
and Park SK: The age-dependent induction of apoptosis-inducing
factor (AIF) in the human semitendinosus skeletal muscle. Cell Mol
Biol Lett. 15:1–12. 2010. View Article : Google Scholar
|
|
29
|
Doti N, Reuther C, Scognamiglio PL, Dolga
AM, Plesnila N, Ruvo M and Culmsee C: Inhibition of the AIF/CypA
complex protects against intrinsic death pathways induced by
oxidative stress. Cell Death Dis. 5:e9932014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Festa M, Capasso A, D'Acunto CW, Masullo
M, Rossi AG, Pizza C and Piacente S: Xanthohumol induces apoptosis
in human malignant glioblastoma cells by increasing reactive oxygen
species and activating MAPK pathways. J Nat Prod. 74:2505–2513.
2011. View Article : Google Scholar : PubMed/NCBI
|