Introduction
Prostate cancer (PCa) is the most common newly
diagnosed cancer in the United States in 2015 and remains the
second leading cause of cancer-related mortality, trailing only
lung cancer (1). Metastasis, a
highly complex, multistep biological process that involves several
important events and molecular factors, is a major cause of death
in patients with PCa (2). Although
various oncogenes and tumor-suppressor genes have been reported in
a previous research to play important roles in the process of
metastasis, the molecular mechanisms of prostate carcinogenesis
remain unclear (3). Therefore, a
better understanding of the underlying molecular mechanisms of
metastasis is essential for the development of novel and effective
therapies against PCa.
miRNAs are a large class of endogenous small
non-coding RNAs that are ~22 nucleotides long. miRNAs regulate gene
expression by binding to the 3′ untranslated regions (UTRs) of
target mRNAs in a partially complementary manner, which leads to
mRNA degradation or suppression of translation (5).
An increasing number of studies have shown that
miRNAs may not only play a significant role in PCa progression
(6,7) such as proliferation (7), the cell cycle (8), apoptosis (9), invasion and metastasis (10–12)
but also function as tumor suppressors or oncogenes depending on
the genes that they regulate (13).
Previous studies have shown that the proliferation,
invasion and metastasis of PCa involve various genomic alterations.
Dubovenko et al (14)
reported a contribution effect of the RAS-RAF-mitogen-activated
protein kinase (MAPK) signaling pathway in PCa development. Qiu
et al (15) found that the
low expression of cyclin-dependent kinases (CDKs) (CDK2, CDK4,
CDK6) downregulated by sodium butyrate play an important role in
the G1 arrest of PCa cells. Meanwhile, the alterative expression of
cell cycle regulatory proteins such as cyclin was found to be
related to PCa cell growth as well (16).
Previous studies have found that miR-340 plays
critical roles in the biological processes of cancers. Li et
al (17) demonstrated that
miR-340 inhibits glioblastoma cell proliferation by directly
targeting CDK6, cyclin D1 and cyclin D2. Furthermore, Fernandez
et al found that miR-340 was identified as a novel tumor
suppressor in non-small cell lung cancer (18), and also inhibits the tumorigenic
phenotype of melanomas by regulating the RAS-RAF-mitogen-activated
protein kinase (MAPK) signaling pathway (19). However, the role and underlying
mechanisms of miR-340 in PCa remains unclear. In the present study,
for the first time, we showed that miR-340 acts as a tumor
suppressor in PCa based on the finding that miR-340 inhibited PCa
cell proliferation, migration and invasion by targeting the MDM2
proto-oncogene and by inhibiting MDM2-dependent p53 inhibition. Our
findings suggest the potential efficacy of a therapy involving the
overexpression of miRNA-340 to treat human PCa.
Materials and methods
Tissue specimens and cell lines
PCa tissue specimens and corresponding non-tumor
samples were collected from 50 PCa patients who underwent radical
prostatectomy at the Third Xiangya Hospital of Central South
University from 2010 to 2013. Written informed consent was obtained
from all of the patients, and this study was conducted in
accordance with the ethical standards set forth by the
Institutional Committee, approved by the IRB of Third Xiangya
Hospital, Central South University, no. 2015-S147. Human PCa cell
lines (DU145 and PC-3) and normal prostate epithelial cells
(RWPE-1) were purchased from the Cell Bank at the Chinese Academy
of Sciences. Human benign prostate hyperplasia (BPH-1) cells were
obtained from the American Type Culture Collection (ATCC; Manassas,
VA, USA). DU145, PC-3 and BPH-1 cells were grown in Dulbecco's
modified Eagle's medium (DMEM), F-12 and RPMI-1640 medium
(Invitrogen, Carlsbad, CA, USA), respectively, and RWPE-1 cells
were maintained in K-SFM containing 0.05 mg/ml bovine pituitary
extract (BPE) and 5 ng/ml epidermal growth factor (EGF). All media
were supplemented with 10% fetal bovine serum and a 1%
antibiotic/antimycotic solution (Sigma, St. Louis, MO, USA). All
cell lines were incubated at 37°C in the presence of 5%
CO2.
Plasmids and cell transfection
The miR-340 mimic/inhibitor and the scrambled
control were purchased from GenePharma (Shanghai, China). The
pcDNA3.1-MDM2 plasmid was constructed by YRBIO (Changsha, China).
The cells were seeded in 6- or 96-well plates at 30% confluency 24
h prior to transfection, and the cells were then transfected with
the miR-340 mimic/inhibitor or the controls using Lipofectamine
2000 reagent (Invitrogen) according to the manufacturer's
protocol.
Quantitative real-time PCR
miRNA and total RNA were extracted from the cells or
tissues using the miRNeasy Mini kit (Qiagen, Valencia, CA, USA) and
TRIzol reagent (Invitrogen), respectively. Reverse transcription
was performed using a miScript II RT kit (Qiagen), and qRT-PCR was
performed on a CFX96 real-time thermocycler (BioRad, Hercules, CA,
USA) using SYBR Premix Ex Taq kits (Takara, Dalian, China). All the
primer sets are listed in Table I.
Each assay was performed in triplicate. U6 and GAPDH were used as
endogenous controls, and relative expression was calculated using
the 2−ΔΔCT method.
 | Table IPrimer sequences. |
Table I
Primer sequences.
| Name | Primer | Sequence
(5′−3′) |
|---|
| miRNA-340 | Reverse
transcription primer: |
GTCGTATCCAGTGCGTGTCGTGGAGTCGGCAATTGCACTGGATACGACAATCAG |
| Forward
primer: |
GCGGTTATAAAGCAATGAGA |
| Reverse
primer: |
GTGCGTGTCGTGGAGTCG |
| U6 snRNA | Reverse
transcription prime: |
AAAATATGGAACGCTTCACGAATTTG |
| Forward
primer: |
CTCGCTTCGGCAGCACATATACT |
| Reverse
primer: |
ACGCTTCACGAATTTGCGTGTC |
| GAPDH | Forward
primer: |
CCAGGTGGTCTCCTCTGA |
| Reverse
primer: |
GCTGTAGCCAAATCGTTGT |
| MDM2 | Forward
primer: |
GCAGGGCTTATTCCTTTTCTTTA |
| Reverse
primer: |
CATTGAACCTTGTGTGATTTGTC |
| p21 | Forward
primer: |
TCCTCTAGCTGTGGGGGTGA |
| Reverse
primer: |
GAAGGTCGCTGGACGATTTG |
| BCL-2 | Forward
primer: |
CGCCCTGTGGATGACTGAGTA |
| Reverse
primer: |
CCTCAGCCCAGACTCACATCA |
Cell proliferation assay
A total of 104 cells/well were seeded in
96-well plates in triplicate. At 24, 48 and 72 h after
transfection, cell proliferation was assessed using the MTT reagent
(Roche, Indianapolis, IN, USA), and the absorbance was measured for
each time-point at a wavelength of 450 nm using a 96-well plate
reader.
In vitro cell migration and invasion
assays
Cells were resuspended in serum-free medium before
assessing cell migration and invasion capacity. A total of
5×104 cells were seeded into the top chamber of
Transwell inserts (Corning, Tewksbury, MA, USA) for the migration
assay, and 1×105 cells were plated in the upper chamber
precoated with Matrigel (BD Biosciences) for the invasion assay.
Medium containing 10% FBS was added to the bottom chamber, and the
plates were incubated for 24 h. After incubation, the inserts
containing cells adherent to the lower membrane were fixed and
stained with 20% methanol and 0.2% crystal violet, respectively.
The stained cells were imaged and counted.
Flow cytometric analysis for
apoptosis
Cells were collected after transfection with the
miR-340 mimic, inhibitor or control, and the percentage of
apoptotic cells was measured by flow cytometry using an Annexin
V-FITC/PI double-staining kit and a FACSCalibur flow cytometer
(Beckman Instruments, Fullerton, CA, USA). Five independent assays
were performed.
Western blot analysis
Cells were harvested, and protein was extracted
using RIPA buffer. The bicinchoninic acid method was used to
determine protein concentration. A total of 25 µg of protein
was separated by SDS-PAGE and transferred to PVDF membranes
(Millipore, Billerica, MA, USA) to be probed with the following
antibodies: mouse anti-MDM2 (1:1,000), mouse anti-p21 (1:800) and
mouse anti-BCL2 (1:500) (Abcam, Cambridge, UK). HRP-conjugated goat
anti-mouse antibody (1:1,000; Cell Signaling Technology, Danvers,
MA, USA) was used as the secondary antibody. Signals were detected
using ECL Western Blotting Substrate (Thermo Fisher Scientific,
USA) and were quantified using Image J software.
Generation of stable
miR-340-overexpressing PCa cells
miR-340 overexpression or control lentiviral vectors
were purchased from YRBIO and were used to infect the PCa cells
(DU145 and PC-3) for 72 h. Cells were selected with 2 µg/ml
puromycin at ~2 weeks.
Dual-luciferase reporter assay
Luciferase reporter vectors containing the seed
sequence for miR-340 corresponding to the wild-type (WT) 3′UTR of
MDM2 and a mutated version of the 3′UTR of MDM2 containing
mutations within the core binding site for miR-340 were
constructed. HEK-293 cells were seeded into 96-well plates and were
co-transfected with WT 3′UTR MDM2 or mutant 3′UTR MDM2 and miR-340
or a control scrambled sequence using Lipofectamine 2000. Firefly
and Renilla luciferase activities were determined using the
Dual-Luciferase reporter system (Promega, Madison, WI, USA) 48 h
after transfection. The experiments were performed independently in
triplicate, and the data are presented as the mean ratio of
Renilla activity/firefly luciferase activity.
Tumorigenesis assays
A cell suspension of 2×106 cells/mouse
was injected subcutaneously into the flanks of 4- to 6-week-old
female nude mice (n=5/group). The tumor volume was calculated twice
a week using microcalipers, and the study was terminated 30 days
after implantation. The tumor tissues were snap frozen in liquid
nitrogen and stored at −80°C for subsequent analysis.
Statistical analysis
All statistical analyses were performed with SPSS
13.0 (SPSS Inc., Chicago, IL, USA). The data values were presented
as the mean ± SEM. Mean value differences between two groups were
analyzed by the two-tailed Student's t-test, and the means of three
groups were compared with one-way ANOVA. P-value <0.05 was
considered to indicate a statistically significant difference.
Results
Expression of miR-340 is significantly
downregulated in PCa cells and tissues
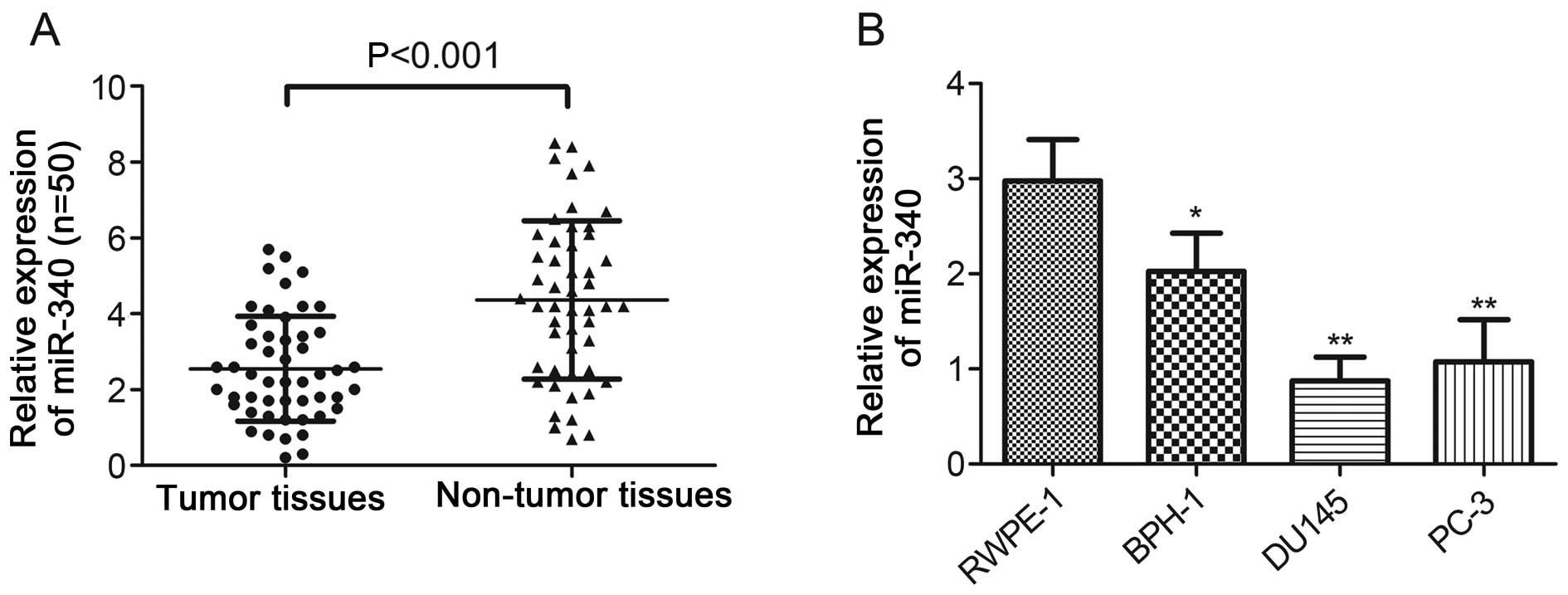
To evaluate the role of miR-340 in PCa progression,
50 pairs of tumor and adjacent non-tumor tissues obtained from PCa
patients were analyzed. The real-time PCR results indicated that
miR-340 expression was significantly downregulated in the PCa
tissues when compared with that noted in the matched normal tissues
(P<0.001, Fig. 1A). Furthermore,
the miR-340 levels were substantially downregulated both in the PCa
cell lines (P<0.01) and BPH-1 cells (P<0.05) compared with
the level in the normal prostate epithelial cells (RWPE-1),
respectively (Fig. 1B). The
downregulation of miR-340 both in PCa cells and tissues indicates
that miR-340 may play an important role in PCa.
miR-340 suppresses PCa cell
proliferation, migration and invasion as well as induces
apoptosis
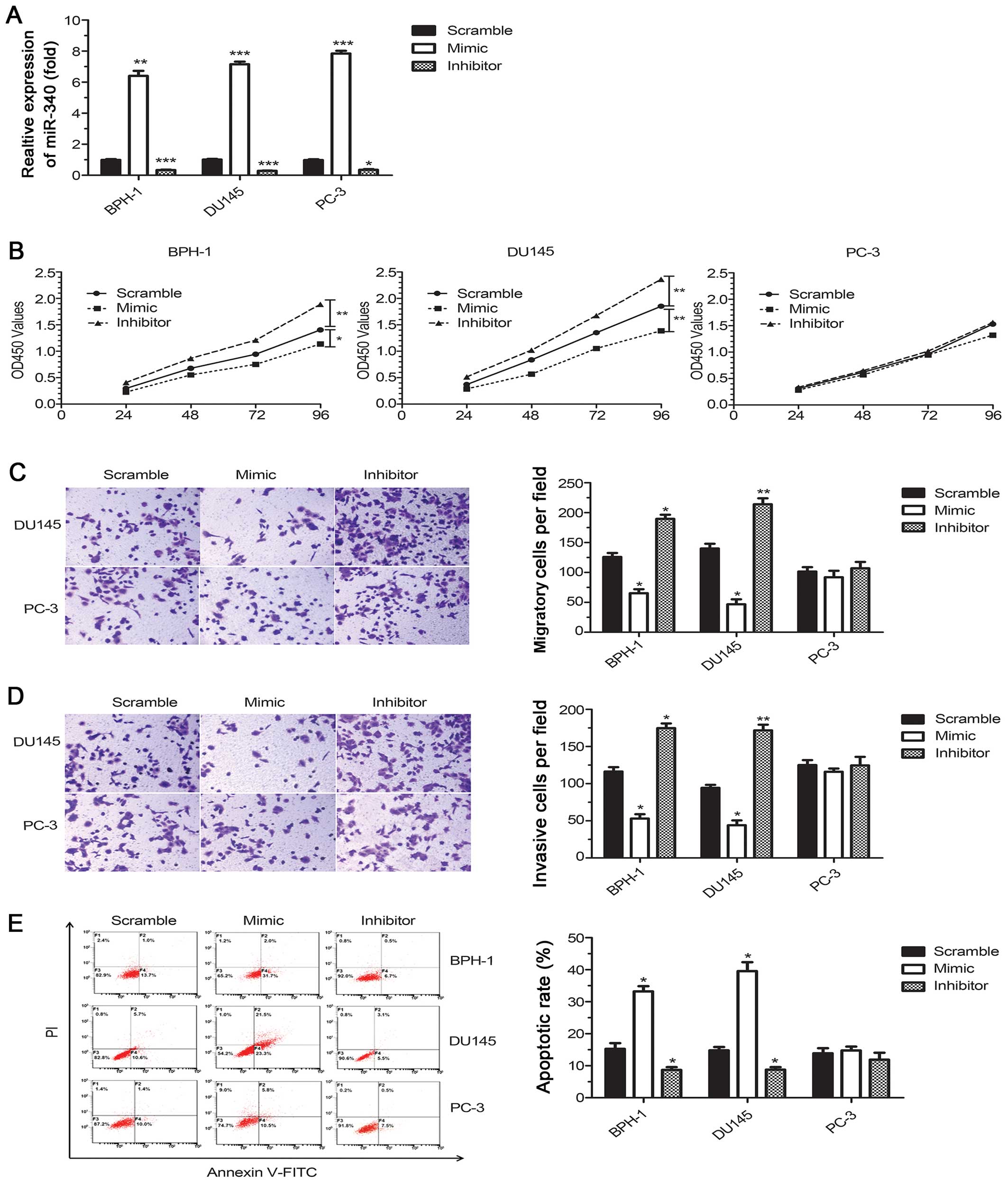
To investigate the function of miR-340 in PCa
tumorigenesis, two PCa cell lines (DU145 and PC-3) and BPH-1 cells
were transfected with a commercially available miR-340 mimic or
inhibitor. The levels of miR-340 were significantly increased upon
transfection with the miR-340 mimic and were decreased upon
transfection with the miR-340 inhibitor (P<0.05, Fig. 2A). Upregulation of miR-340 resulted
in a significant decrease in cell proliferation in the two p53 WT
cell lines (DU145 and BPH-1) but not in the PC-3 cells, which lack
WT p53. Consistently, downregulation of miR-340 increased cell
proliferation in the DU145 and BPH-1 cells (P<0.05) but had no
effect on the PC-3 cells (P>0.05, Fig. 2B). Furthermore, the overexpression
of miR-340 reduced cell migration and invasion in the cell lines
expressing WT p53 (P<0.05, Fig. 2C
and D). Flow cytometric results showed a sharp increase in the
percentage of apoptotic cells for miR-340 mimic recipients and a
decrease in apoptosis in the miR-340 inhibitor recipients compared
with the scrambled control (P<0.05, Fig. 2E). Notably, these effects were
absent in the PC-3 cells, which lack WT p53, indicating that
miR-340 may interact with the p53 pathway.
miR-340 directly targets MDM2 to regulate
the MDM2-p53 pathway
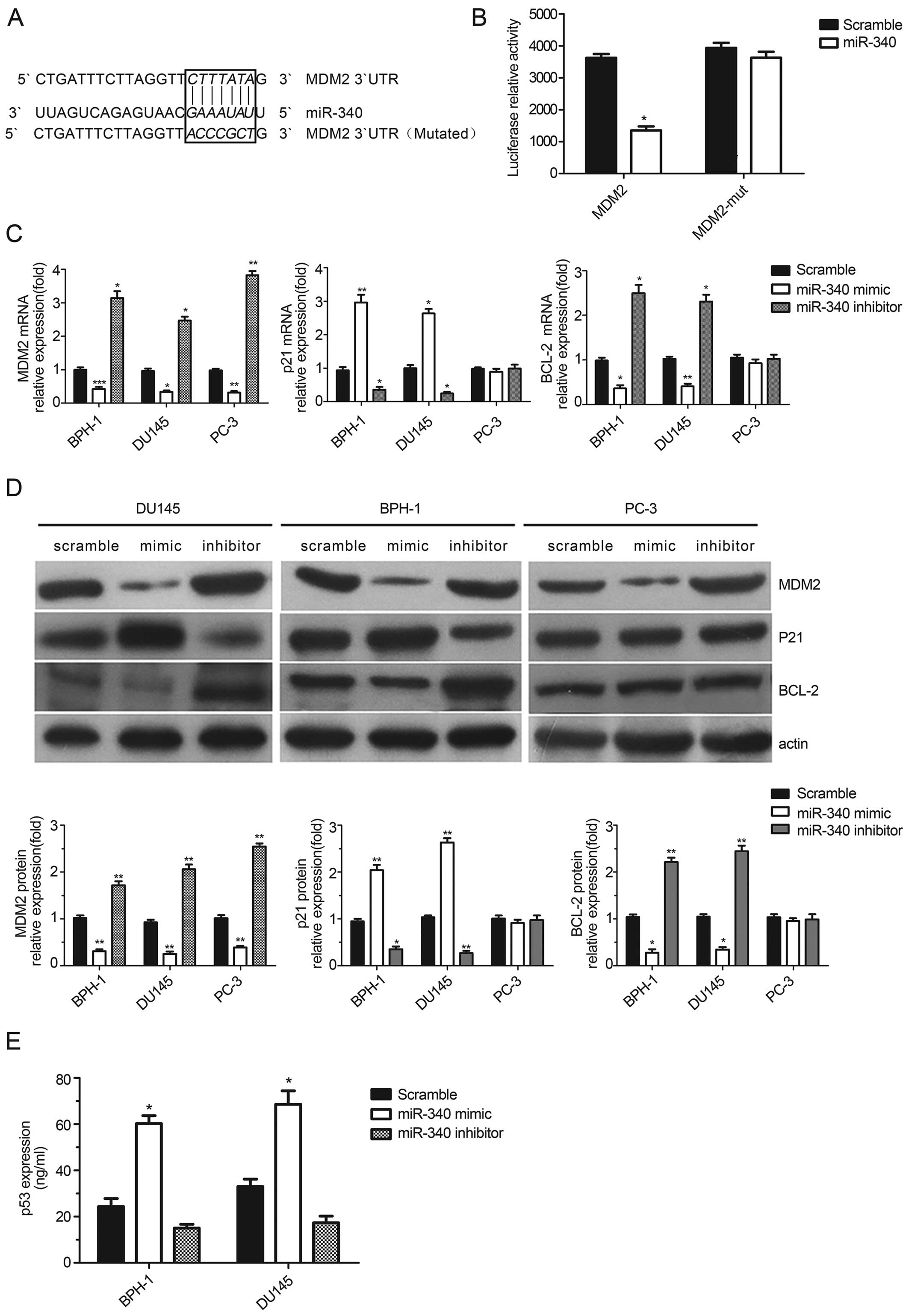
To determine the mechanism by which miR-340 affects
the functions of cancer cells and interacts with p53, we predicted
miR-340 targets by TargetScan and focused on target genes encoding
proteins involved in the p53 pathway. Mouse double minute 2 (MDM2),
an important regulator of the p53 tumor suppressor, was found to be
a novel target candidate of miR-340 as the 4293–4299 position of
the MDM2 3′UTR was complementary to the seed sequence of miR-340.
To validate this potential direct interaction, we constructed
luciferase reporter vectors containing the WT and mutant (Mut)
3′UTR of MDM2 (Fig. 3A).
Dual-luciferase reporter assays showed a statistically significant
decrease (63% reduction) in the luciferase activity of 3′UTR MDM2
(P<0.05), but no changes were found in the luciferase activity
of the mutant (P>0.05, Fig. 3B).
In addition, all cell lines transfected with the miR-340 mimics
showed a decrease both in MDM2 mRNA and protein levels as measured
by real-time PCR and western blot analyses, respectively
(P<0.05, Fig. 3C and D). These
finding showed that miR-340 may directly target the 3′UTR of MDM2
and have an effect on the regulation of its expression.
To confirm the effect of miR-340 on p53 expression,
we measured p53 levels in cell lysates by ELISA. A significant
increase in p53 expression was detected after miR-340 upregulation
in the BPH-1 and DU145 cells, which harbor WT p53 (P<0.05,
Fig. 3E). To determine whether the
antitumor activity of miR-340 is dependent on p53, we analyzed the
mRNA and protein levels of p21WAF1/CIP1, a
cyclin-dependent kinase inhibitor that functions as a p53-dependent
cell cycle checkpoint. Our analysis revealed a statistically
significant increase in p21 levels in cells treated with the
miR-340 mimics and a decrease in p21 expression in cells treated
with the miR-340 inhibitors (P<0.05). Furthermore, the
expression of the anti-apoptotic gene, BCL-2, exhibited the
opposite trend in the WT p53 cell lines (Fig. 3C and D). Consistent with a
p53-dependence, the mRNA and protein levels of p21 and BCL-2
remained unchanged in the PC-3 p53-null cells despite clear changes
in MDM2 expression levels (Fig. 3C and
D). These results suggest that miR-340 may play a
tumor-suppressive role in the presence of functional p53 protein by
targeting MDM2.
MDM2 reverses miR-340-induced inhibition
of growth, migration and invasion in PCa cells
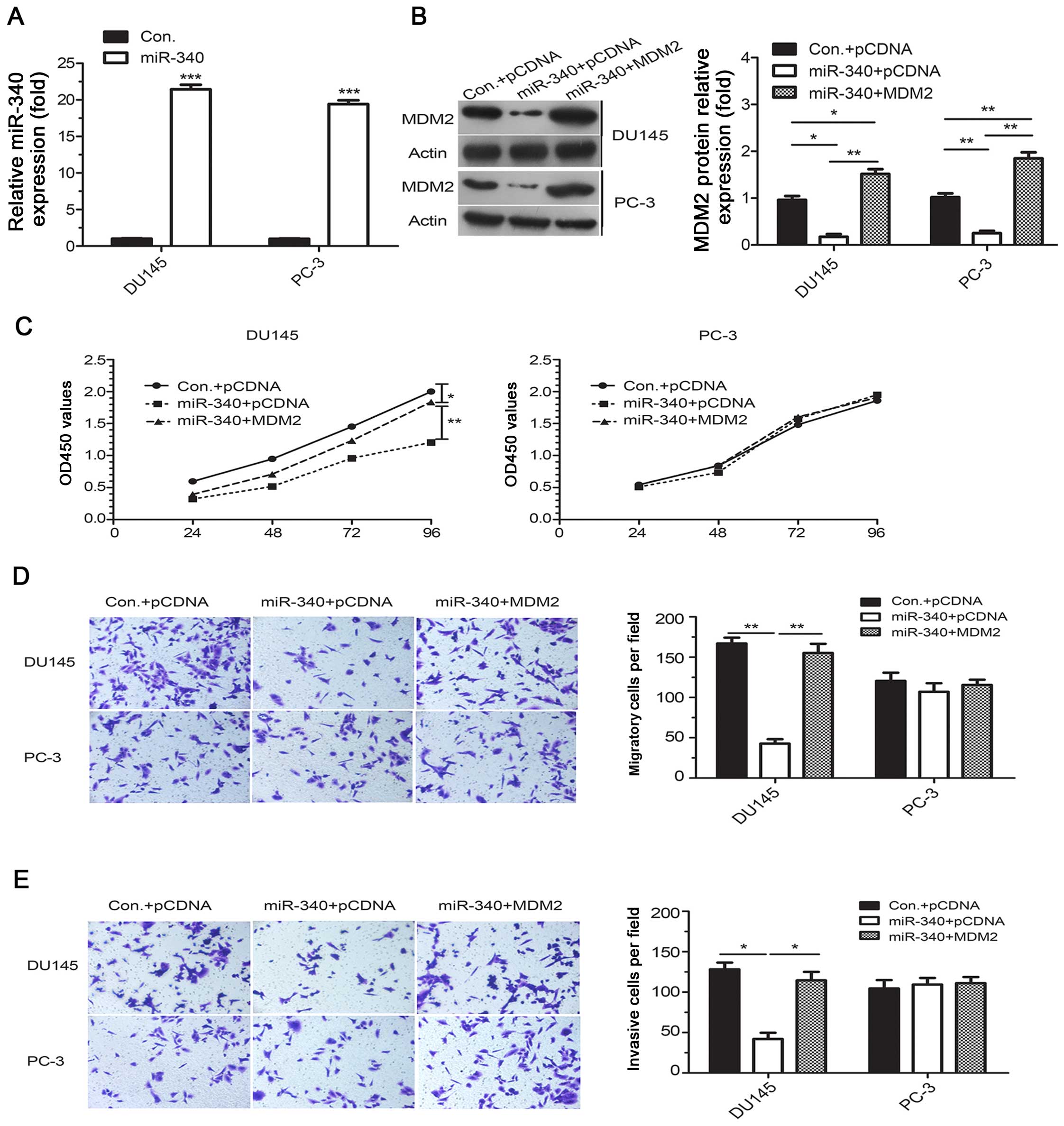
Stable miR-340-overexpressing PCa cells were
generated using lentiviral vectors, and all the stable cells
expressed significantly increased levels of miR-340 and
significantly decreased levels of MDM2 protein compared to their
corresponding controls (P<0.05, Fig.
4A and B). Moreover, a series of in vitro assays
revealed an inhibition of cell growth and a marked decrease in
migration and invasion in these stable miR-340-overexpressing
cells. Furthermore, rescue experiments showed that re-expression of
MDM2 by transfection with the pcDNA3.1-MDM2 plasmid mitigated the
inhibition of proliferation, migration and invasion in the
miR-340-overexpressing DU145 cells (Fig. 4C–E). As expected, these effects were
abolished in the miR-340-over-expressing PC-3 cells lacking WT p53,
which was consistent with the data obtained from the transient
transfection experiments in the PC-3 cells. Overexpression of MDM2
alleviated the effects induced by miR-340 in the WT p53 cells but
not in the p53-null cells, which suggests that functional p53
protein is essential for the tumor suppressive activity of
miR-340.
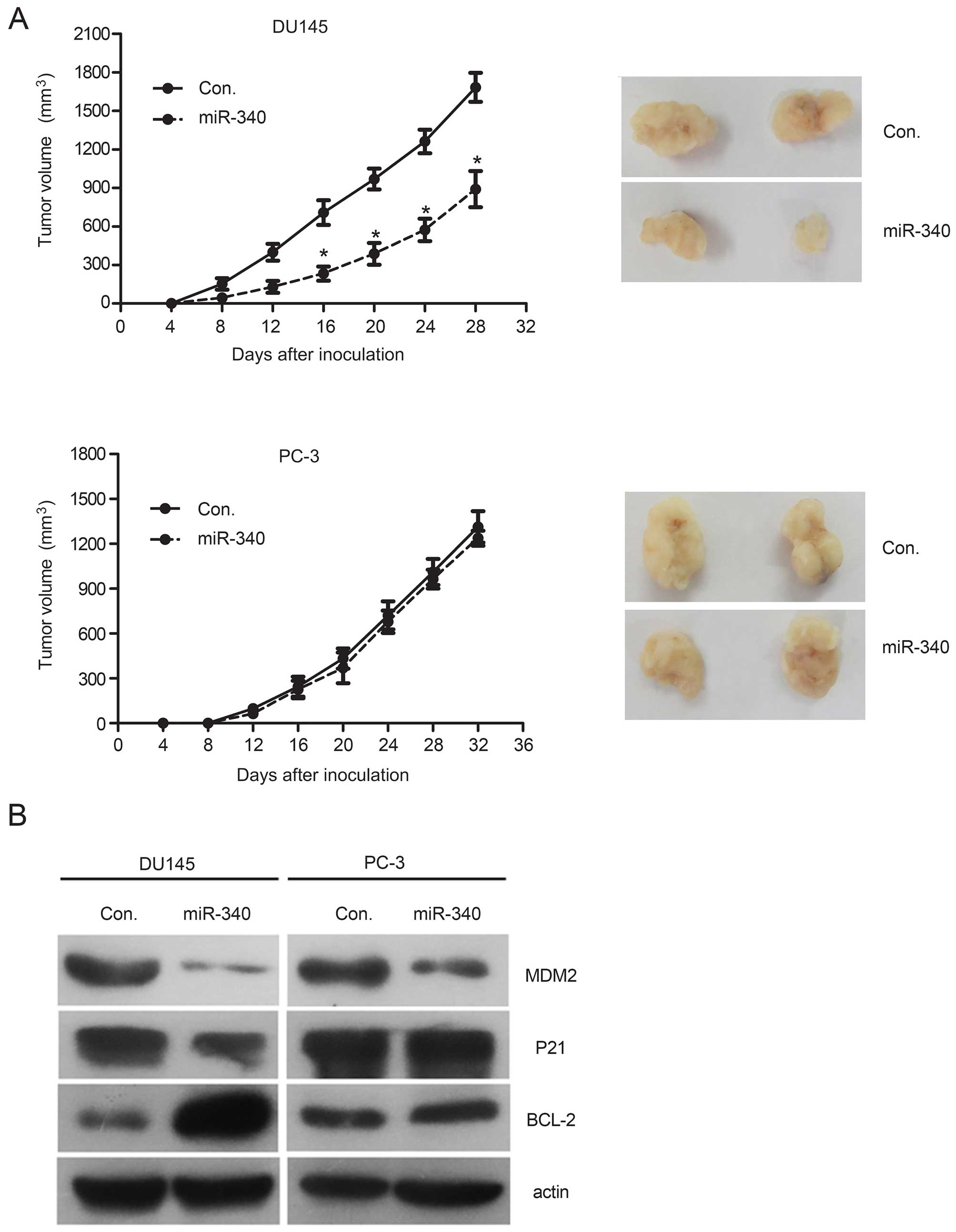
miR-340 inhibits xenograft tumor
growth
To evaluate the tumor-suppressive effects of miR-340
in vivo, we subcutaneously injected stable
miR-340-overexpressing PCa cells into nude mice. A significant
suppressive effect on the growth of miR-340-overexpressing tumors
was observed in the DU145 cells (WT p53), but these effects were
not observed in the PC-3 cells lacking WT p53 protein (Fig. 5A). Although the protein level of
MDM2 in the tumor tissues was reduced in all miR-340-overexpressing
cells, upregulation of p21 protein and downregulation of BCL-2
protein were observed in the WT p53 cells but not in the p53-null
cells compared with the control (P>0.05, Fig. 5B), which was similar to the in
vitro results. These results support the tumor-suppressive role
of miR-340 and underscore the importance of the MDM2-p53 pathway in
the miR-340-induced tumor suppressive effects using an in
vivo xenograft model.
Discussion
miRNAs are involved in many critical biological
processes such as tumorigenesis and cancer metastasis. Growing
evidence indicates that miRNAs can act as ideal molecular targets
for cancer diagnosis, prognosis and therapy in many types of
cancers (20–22). Various miRNAs have been used to
detect cancer and to develop miRNA-based therapeutic strategies in
PCa (23–25). To explore different strategies for
cancer therapeutics, more studies are needed to identify novel and
valuable miRNAs and further, to investigate the role and molecular
mechanisms of these miRNAs in PCa tumorigenesis.
Previous findings have confirmed that miR-340
suppresses tumor cell migration and invasion in non-small cell lung
cancer (17), melanomas (18), breast cancer (26) and osteosarcomas (27). In the present study, we found that
miR-340 is a potential novel tumor suppressor associated with PCa
which was partly consistent with previous studies. Although there
is evidence that miR-340 is downregulated in several types of
cancers, the role of miR-340 and the underlying mechanisms of
action in the tumorigenesis of PCa have not yet been reported. Our
results demonstrated for the first time that miR-340 is
significantly downregulated in PCa cell lines and tissues.
Overexpression of miR-340 decreased cell proliferation, migration
and invasion. It promoted apoptosis in vitro, and inhibited
tumor growth in vivo as well. Our findings indicate that
miR-340 may be associated with PCa tumorigenesis, which could be
considered as a potential biomarker for PCa. The inhibitory effect
of miR-340 overexpression on cell migration and invasion was only
observed in p53 WT cells such as DU145 and BPH-1 cells, but not in
PC-3 p53-null cells which suggests that miR-340 may function in a
p53-dependent manner. Using in silico algorithms and
functional analyses, we identified MDM2, a p53 E3 ubiquitin ligase
(28) and principal negative
regulator of the expression and function of p53 (29,30),
as a miR-340 target. A core complementary sequence of miR-340 was
identified in the 3′UTR of MDM2 as miR-340 overexpression
suppressed luciferase activity and downregulated MDM2 mRNA and
protein levels in cells. These results indicated that miR-340
functions as a tumor suppressor, in part, by targeting the MDM2
oncogene in PCa.
MDM2 plays an important role in the progression of
several cancers (31,32), and MDM2 expression is positively
correlated with tumor volume and the proliferative index of PCa
cells (33). Additionally, MDM2 has
been suggested as an intriguing therapeutic target for PCa therapy,
and MDM2 inhibitors, such as second-generation antisense oligos,
may have a broad spectrum of antitumor activity in human cancers
(34). MDM2 promotes the
degradation of p53 by ubiquitination, functioning in a negative
feedback regulatory loop of p53, which regulates cell growth and
apoptosis. In our study, the relationship between miR-340 and p53
was determined, and the role of miR-340 in the regulation of
migration and invasion was dependent on the p53 status, as
restoration of functional MDM2 reversed the effects induced by
miR-340 expression in WT p53 cells but not in p53-null cells.
Similar data were obtained in vivo, whereby significant
inhibition of tumor xenograft growth was detected with miR-340
stably transfected DU145 cells but not with miR-340 stably
transfected p53-null PC-3 cells. These data were consistent with
previous studies showing that miR-660 and miR-18b also hinder cell
proliferation and metastasis only in the presence of WT p53
(32,35).
Furthermore, our in vivo and in vitro
WT p53 miR-340 overexpression models showed the upregulation of
p21WAF1/CIP and the downregulation of MDM2 and BCL-2,
which were accompanied by substantial arrest of cell proliferation
and induction of apoptosis in PCa cells. Although small-molecule
antagonists of the p53-MDM2 interaction such as nutlins (36,37) or
MDM2 inhibitors (4,38), have been developed in the past few
years, the development of novel molecular targeting methods for
MDM2 is a remaining need for cancer therapy. Our data suggest that
the restoration of p53 activity by targeting MDM2 using miR-340 may
be an effective approach in PCa therapy.
However, there were some limitations to our study.
The correlation between miR-340 and MDM2 levels in PCa tissues
should be examined, and additional cell types should be tested.
Moreover, miR-340 likely targets other genes and participates in
other signaling pathways in addition to the MDM2 pathway for the
regulation of cell growth in PCa.
In conclusion, our findings showed that miR-340 may
be a novel tumor-suppressive miRNA and has a regulatory effect on
the MDM2-p53 pathway, which could be considered as a basis for the
development of miRNA-targeted therapies for prostate and other
cancers.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Valastyan S and Weinberg RA: Tumor
metastasis: Molecular insights and evolving paradigms. Cell.
147:275–292. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Xu L, Wang Z, Li XF, He X, Guan LL, Tuo
JL, Wang Y, Luo Y, Zhong HL, Qiu SP, et al: Screening and
identification of significant genes related to tumor metastasis and
PSMA in prostate cancer using microarray analysis. Oncol Rep.
30:1920–1928. 2013.PubMed/NCBI
|
|
4
|
Baek D, Villén J, Shin C, Camargo FD, Gygi
SP and Bartel DP: The impact of microRNAs on protein output.
Nature. 455:64–71. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang YL, Wu S, Jiang B, Yin FF, Zheng SS
and Hou SC: Role of microRNAs in prostate cancer pathogenesis. Clin
Genitourin Cancer. 13:261–270. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bonci D, Coppola V, Musumeci M, Addario A,
Giuffrida R, Memeo L, D'Urso L, Pagliuca A, Biffoni M, Labbaye C,
et al: The miR-15a-miR-16-1 cluster controls prostate cancer by
targeting multiple oncogenic activities. Nat Med. 14:1271–1277.
2008. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wu Z, He B, He J and Mao X: Upregulation
of miR-153 promotes cell proliferation via downregulation of the
PTEN tumor suppressor gene in human prostate cancer. Prostate.
73:596–604. 2013. View Article : Google Scholar
|
|
8
|
Lewis H, Lance R, Troyer D, Beydoun H,
Hadley M, Orians J, Benzine T, Madric K, Semmes OJ, Drake R, et al:
miR-888 is an expressed prostatic secretions-derived microRNA that
promotes prostate cell growth and migration. Cell Cycle.
13:227–239. 2014. View
Article : Google Scholar :
|
|
9
|
Verdoodt B, Neid M, Vogt M, Kuhn V,
Liffers ST, Palisaar RJ, Noldus J, Tannapfel A and
Mirmohammadsadegh A: Micro-RNA-205, a novel regulator of the
anti-apoptotic protein Bcl2, is downregulated in prostate cancer.
Int J Oncol. 43:307–314. 2013.PubMed/NCBI
|
|
10
|
Ren D, Wang M, Guo W, Huang S, Wang Z,
Zhao X, Du H, Song L and Peng X: Double-negative feedback loop
between ZEB2 and miR-145 regulates epithelial-mesenchymal
transition and stem cell properties in prostate cancer cells. Cell
Tissue Res. 358:763–778. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Josson S, Gururajan M, Sung SY, Hu P, Shao
C, Zhau HE, Liu C, Lichterman J, Duan P, Li Q, et al: Stromal
fibroblast-derived miR-409 promotes epithelial-to-mesenchymal
transition and prostate tumorigenesis. Oncogene. 34:2690–2699.
2015. View Article : Google Scholar
|
|
12
|
Rajendiran S, Parwani AV, Hare RJ,
Dasgupta S, Roby RK and Vishwanatha JK: MicroRNA-940 suppresses
prostate cancer migration and invasion by regulating MIEN1. Mol
Cancer. 13:2502014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sun Q, Zhao X, Liu X, Wang Y, Huang J,
Jiang B, Chen Q and Yu J: miR-146a functions as a tumor suppressor
in prostate cancer by targeting Rac1. Prostate. 74:1613–1621. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dubovenko A, Serebryiskaya T, Nikolsky Y,
Nikolskaya T, Perlina A, JeBailey L, Bureeva S, Katta S, Srivastava
S, Dobi A, et al: Reconstitution of the ERG gene expression network
reveals new biomarkers and therapeutic targets in ERG positive
prostate tumors. J Cancer. 6:490–501. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Qiu J, Gao Z and Shima H: Growth of human
prostate cancer cells is significantly suppressed in vitro with
sodium butyrate through apoptosis. Oncol Rep. 27:160–167. 2012.
|
|
16
|
Shi C, Yu L, Yang F, Yan J and Zeng H: A
novel organoselenium compound induces cell cycle arrest and
apoptosis in prostate cancer cell lines. Biochem Biophys Res
Commun. 309:578–583. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li X, Gong X, Chen J, Zhang J, Sun J and
Guo M: miR-340 inhibits glioblastoma cell proliferation by
suppressing CDK6, cyclin-D1 and cyclin-D2. Biochem Biophys Res
Commun. 460:670–677. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fernandez S, Risolino M, Mandia N, Talotta
F, Soini Y, Incoronato M, Condorelli G, Banfi S and Verde P:
miR-340 inhibits tumor cell proliferation and induces apoptosis by
targeting multiple negative regulators of p27 in non-small cell
lung cancer. Oncogene. 34:3240–3250. 2015. View Article : Google Scholar
|
|
19
|
Poenitzsch Strong AM, Setaluri V and
Spiegelman VS: MicroRNA-340 as a modulator of RAS-RAF-MAPK
signaling in melanoma. Arch Biochem Biophys. 563:118–24. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yu X, Luo L, Wu Y, Yu X, Liu Y, Yu X, Zhao
X, Zhang X, Cui L, Ye G, et al: Gastric juice miR-129 as a
potential biomarker for screening gastric cancer. Med Oncol.
30:3652013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang Y, Jia LS, Yuan W, Wu Z, Wang HB, Xu
T, Sun JC, Cheng KF and Shi JG: Low miR-34a and miR-192 are
associated with unfavorable prognosis in patients suffering from
osteosarcoma. Am J Transl Res. 7:111–119. 2015.PubMed/NCBI
|
|
22
|
Ma L, Reinhardt F, Pan E, Soutschek J,
Bhat B, Marcusson EG, Teruya-Feldstein J, Bell GW and Weinberg RA:
Therapeutic silencing of miR-10b inhibits metastasis in a mouse
mammary tumor model. Nat Biotechnol. 28:341–347. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gordanpour A, Nam RK, Sugar L and Seth A:
MicroRNAs in prostate cancer: From biomarkers to molecularly-based
therapeutics. Prostate Cancer Prostatic Dis. 15:314–319. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bhatnagar N, Li X, Padi SK, Zhang Q, Tang
MS and Guo B: Downregulation of miR-205 and miR-31 confers
resistance to chemotherapy-induced apoptosis in prostate cancer
cells. Cell Death Dis. 1:e1052010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li X, Wan X, Chen H, Yang S, Liu Y, Mo W,
Meng D, Du W, Huang Y, Wu H, et al: Identification of miR-133b and
RB1CC1 as independent predictors for biochemical recurrence and
potential therapeutic targets for prostate cancer. Clin Cancer Res.
20:2312–2325. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wu ZS, Wu Q, Wang CQ, Wang XN, Huang J,
Zhao JJ, Mao SS, Zhang GH, Xu XC and Zhang N: miR-340 inhibition of
breast cancer cell migration and invasion through targeting of
oncoprotein c-Met. Cancer. 117:2842–2852. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhou X, Wei M and Wang W: MicroRNA-340
suppresses osteosarcoma tumor growth and metastasis by directly
targeting ROCK1. Biochem Biophys Res Commun. 437:653–658. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Honda R, Tanaka H and Yasuda H:
Oncoprotein MDM2 is a ubiquitin ligase E3 for tumor suppressor p53.
FEBS Lett. 420:25–27. 1997. View Article : Google Scholar
|
|
29
|
Montes de Oca Luna R, Wagner DS and Lozano
G: Rescue of early embryonic lethality in mdm2-deficient mice by
deletion of p53. Nature. 378:203–206. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chen J, Wu X, Lin J and Levine AJ: mdm-2
inhibits the G1 arrest and apoptosis functions of the p53 tumor
suppressor protein. Mol Cell Biol. 16:2445–2452. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Rayburn E, Zhang R, He J and Wang H: MDM2
and human malignancies: Expression, clinical pathology, prognostic
markers, and implications for chemotherapy. Curr Cancer Drug
Targets. 5:27–41. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Fortunato O, Boeri M, Moro M, Verri C,
Mensah M, Conte D, Caleca L, Roz L, Pastorino U and Sozzi G:
mir-660 is downregulated in lung cancer patients and its
replacement inhibits lung tumorigenesis by targeting MDM2-p53
interaction. Cell Death Dis. 5:e15642014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Leite KR, Franco MF, Srougi M, Nesrallah
LJ, Nesrallah A, Bevilacqua RG, Darini E, Carvalho CM, Meirelles
MI, Santana I, et al: Abnormal expression of MDM2 in prostate
carcinoma. Mod Pathol. 14:428–436. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhang Z, Li M, Wang H, Agrawal S and Zhang
R: Antisense therapy targeting MDM2 oncogene in prostate cancer:
Effects on proliferation, apoptosis, multiple gene expression, and
chemotherapy. Proc Natl Acad Sci USA. 100:11636–11641. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Dar AA, Majid S, Rittsteuer C, de Semir D,
Bezrookove V, Tong S, Nosrati M, Sagebiel R, Miller JR III and
Kashani-Sabet M: The role of miR-18b in MDM2-p53 pathway signaling
and melanoma progression. J Natl Cancer Inst. 105:433–442. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Tovar C, Rosinski J, Filipovic Z, Higgins
B, Kolinsky K, Hilton H, Zhao X, Vu BT, Qing W, Packman K, et al:
Small-molecule MDM2 antagonists reveal aberrant p53 signaling in
cancer: Implications for therapy. Proc Natl Acad Sci USA.
103:1888–1893. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Vassilev LT, Vu BT, Graves B, Carvajal D,
Podlaski F, Filipovic Z, Kong N, Kammlott U, Lukacs C, Klein C, et
al: In vivo activation of the p53 pathway by small-molecule
antagonists of MDM2. Science. 303:844–848. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Allen JG, Bourbeau MP, Wohlhieter GE,
Bartberger MD, Michelsen K, Hungate R, Gadwood RC, Gaston RD, Evans
B, Mann LW, et al: Discovery and optimization of
chromenotriazolopyrimidines as potent inhibitors of the mouse
double minute 2-tumor protein 53 protein-protein interaction. J Med
Chem. 52:7044–7053. 2009. View Article : Google Scholar : PubMed/NCBI
|