Introduction
Esophageal cancer (EC) is one of the most common
malignant diseases worldwide, particularly in China, where a high
incidence of EC is noted. Esophageal squamous cell carcinoma (ESCC)
is the predominant histological type of esophageal carcinoma in
China (1). Several treatment
approaches, including surgery, radiotherapy and chemotherapy are
used in the clinic. However, the overall 5-year survival rate
remains dismal (2). Therapeutic
resistance at a late stage of ESCC is one of the significant
reasons for the low survival rate (3).
Recent research indicates that tumorigenic signaling
pathways are involved in the processes of ESCC, including tumor
cell growth, cell cycling, apoptosis, angiogenesis and invasion
(2,4). Hence, the key factors of signaling
pathways can be effective therapeutic targets for the treatment of
ESCC. Unlike breast cancer and non-small cell lung cancer (5-8), there
is no reported data concerning the targeted therapy of ESCC.
Therefore, there is needed to develop an effective strategy to
investigate the mechanisms of esophageal carcinogenesis and explore
therapeutic strategies for specific individuals to improve the
prognosis of esophageal patients.
Animal models are appropriate tools to resolve both
basic and clinical EC research problems. In recent years,
patient-derived xenografts (PDXs) have been used to evaluate the
effectiveness of targeted treatments for different types of tumors,
such as breast and non-small cell lung cancer. The response of PDXs
to chemotherapy resembles the patient response in different
clinical treatment trials (9,10).
Hence, PDX models provide a platform with which to study genetic
and biological alterations as well as specific large-scale
anticancer therapies for cancer patients (11).
In the present study, we established and
characterized ESCC PDXs by transplanting 26 ESCC patient tumor
specimens into severe combined immunodeficiency (SCID) mice. The
ʻsuccess-rateʼ of the PDX models was 53.8%. Tumor growth in the
first, second and third passage was observed. The pathology and the
CK5/6, p63 and p40 expression levels in the patient samples of the
first and third passages were detected and compared with each other
by hematoxylin and eosin (H&E), and immunochemical staining. In
addition, tumor genetic signaling pathway kinases, including mTOR
and p-mTOR (Ser2481), Stat1 and p-Stat1 (Tyr701), Stat3 and p-Stat3
(Tyr705), Akt1 and p-Akt (Ser473), Erk and p-Erk (Thr202/Tyr204)
were detected in five PDX models by western blotting. These PDX
models provided a platform to further understand the molecular
mechanisms of ESCC and screen new specialized therapeutic regimens
for ESCC patients in preclinical research.
Materials and methods
Patient tissue procurement
All 26 ESCC patients underwent surgical operations
at the First Affiliated Hospital of Zhengzhou University
(Zhengzhou, China), and ESCC tissues were obtained intraoperatively
from October 2013 to October 2014. All patients had not received
chemotherapy and radiotherapy prior to surgery. Written informed
consent was provided by all patents for using the tissue samples.
All research protocols were approved by the Research Ethics
Committee of Zhengzhou University. Tissue histology was confirmed
by two pathologists. Fresh harvested ESCC specimens were obtained
from the edge of whole tumor tissues to maintain high active and
low necrotic part of the tissues. All tissues were transported to
the laboratory in transport media [fetal bovine serum (FBS)-free,
RPMI-1640 with penicillin and streptomycin].
Animals
Female CB17/SCID mice (6-8 weeks of age) with an
average body weight of 18-20 g (Vital River, Beijing, China) were
used in the study. Mice (4-5) were kept in a pathogen-free environment
in light controlled rooms (12-h cycles) and were provided with food
and water ad libitum.
Patient tumor xenografts
Tissue samples were placed in a Petri plate
containing PBS with penicillin and streptomycin. The tissues were
divided into three parts. One portion was implanted into SCID mice,
the second portion was fixed in 10% formalin and the third portion
was used for protein extraction. The mice were anesthetized using
0.3 ml of 0.4% (w/v) pentobarbital sodium for every 20 g of body
weight. Then, they were subcutaneously implanted with tissues of
different weight and size (0.10-0.12 g and ~3 mm). Animals were
monitored periodically for their weight and tumor growth. Second
passage was performed at a tumor size of ~1,500 mm3. The
tumor specimen was cut into three parts and followed the same
protocol as previously described. Successful tumor grafts were
recognized as those with rapid tumor growth in vivo with
more than three passages, and these were used for experimental
studies.
Tumor growth measurements
The tumor xenograft size was measured with a Vernier
caliper two times every week. The tumor volume was calculated using
the formula: V = LD × (SD)2/2, where V is the tumor
volume, LD is the longest tumor diameter and SD is the shortest
tumor diameter. Tumor growth curves were plotted as tumor
volume.
Histological and immunohistochemical
analysis
The second part of the tumor was embedded in
paraffin for histopathologic examination and immunohistochemical
analysis. All of the slides were stained with Harris hematoxylin
after dewaxing the 5-µm sections with dimethylbenzene and
were evaluated by two pathologists. Tissue sections were stained
with antibodies against CK5/6, p63, p40 (Abcam, UK) and were
incubated overnight at 4°C. HRP-IgG secondary antibody was used at
37°C for 15 min. Then diaminobenzidine (DAB) was used for
detection. All slides were observed and measured by an Olympus
microscope (Japan).
Western blotting
The PDX tissues were ground in liquid nitrogen and
were lysed by tissue lysate. Next, tissue protein was extracted by
centrifugation at 12,000 rpm for 15 min at 4°C. The protein (50
µg) was separated on 10% SDS-polyacrylamide gel, and then
transferred to a PVDF membrane at 90 V for 2 h. The membrane was
blocked with 5% non-fat milk for 60 min. Then the membrane was
incubated with mTOR, p-mTOR (Ser2481), Stat1, p-Stat1 (Tyr701),
Stat3, p-Stat3 (Tyr705), Akt1, p-Akt (Ser473), Erk1/2 and p-Erk1/2
(Thr202/Tyr204) antibodies (Cell Signaling Technology, USA)
overnight at 4°C (all antibody were used at 1:1,000, Stat3 antibody
was from mouse and other antibodies were all from rabbit). HRP-IgG
secondary antibody was incubated at room temperature for 2 h.
Protein bands were visualized by an enhanced chemiluminescence
detection kit (ECL; Pierce). The PVDF membranes were scanned by
ImageQuant LAS 4000 and analyzed by ImageJ software v2.1.4.7.
Statistical analysis
All statistical analyses were performed using SPSS
statistical software 17.0. Experimental values were reported as
mean ± standard error of the mean. One-way analysis of variance was
used for statistical analysis. P<0.05 was considered to indicate
a statistically significant result.
Results
Clinical characteristics of the
patients
In the present study, 26 ESCC subjects underwent
surgical resection. The subjects consisted of 17 men and 9 women
ranging from 46-82 years of age (62.6±7.4 years). The subjects did
not have any apparent distant metastases, and none had been
previously treated. In regards to differentiation, 2 samples were
established from 3 well differentiated samples, 4 were from 6
well-moderate differentiated samples, 7 were from 10 moderately
differentiated samples, only 1 sample was established from 5
moderate-poorly differentiated samples while no sample was
established from 2 poorly differentiated samples. Among these
patients, 17 patients were stage II, 3 patients were stage III, 2
patients were stage II-III, 3 patients were stage I. One patient
was stage I-II. According to lymph node metastasis, 10 samples had
node metastasis and 6 samples were established, 16 samples had no
node metastasis and 8 samples were established. Clinical data of
all the patients are shown in Table
I.
 | Table IClinical characteristics of the
studied patients. |
Table I
Clinical characteristics of the
studied patients.
| No. | Age (years) | Gender | Tumor
differentiation | TNM staging | Lymph node
metastasis | Engrafted mode |
|---|
| EG1 | 63 | Male | Poor | T4N0M0 III | No | No |
| EG2 | 64 | Male | Well-Moderate | T2N0M0 IIa | No | Yes |
| EG3 | 74 | Female | Well-Moderate | T2N0M0 IIa | No | Yes |
| EG4 | 60 | Female | Moderate-poor | T3N0M0 IIa | No | No |
| EG5 | 61 | Male | Moderate | T2N0M0 IIa | No | Yes |
| EG6 | 65 | Female | Moderate-poor | TIN0M0 I | No | No |
| EG7 | 60 | Female | Moderate | T3N2M0 III | Yes | No |
| EG8 | 63 | Male | Moderate | T2N0M0 IIa | Yes | Yes |
| EG9 | 66 | Male | Well-Moderate | T2N1M0 IIb | Yes | Yes |
| EG10 | 62 | Female | Well-Moderate | T2N0M0 IIa | No | No |
| EG11 | 65 | Male | Poor | T3N0M0 IIa | No | Yes |
| EG12 | 65 | Female | Well-Moderate | T2N0M0 IIa | No | No |
| EG13 | 63 | Male | Moderate | T3N0M0 IIa | No | Yes |
| EG14 | 59 | Male | Moderate | T2N0M0 IIa | No | Yes |
| EG15 | 60 | Male | Moderate | T2N1M0 IIb | Yes | No |
| EG16 | 58 | Female | Moderate-poor | T3N1M0 III | Yes | No |
| EG17 | 82 | Male | Moderate | T3N0M0 IIa | No | No |
| EG18 | 52 | Male | Well-Moderate | T3N1M0 I-II | Yes | Yes |
| EG19 | 70 | Male | Moderate | T1N1M0 II | Yes | Yes |
| EG20 | 46 | Female | Moderate | T2N0M0 II | No | Yes |
| EG21 | 68 | Female | Moderate-poor | T3N1M0 II–III | Yes | Yes |
| EG22 | 60 | Male | Well | T2N0M0 II | No | No |
| EG23 | 61 | Male | Moderate-poor | T3N1M0 II–III | Yes | No |
| EG24 | 47 | Male | Well | T3N1M0 I | Yes | Yes |
| EG25 | 69 | Male | Well | T2N0M0 I | No | Yes |
| EG26 | 64 | Male | Moderate | T3N0M0 II | No | Yes |
Growth of ESCC xenografts in SCID
mice
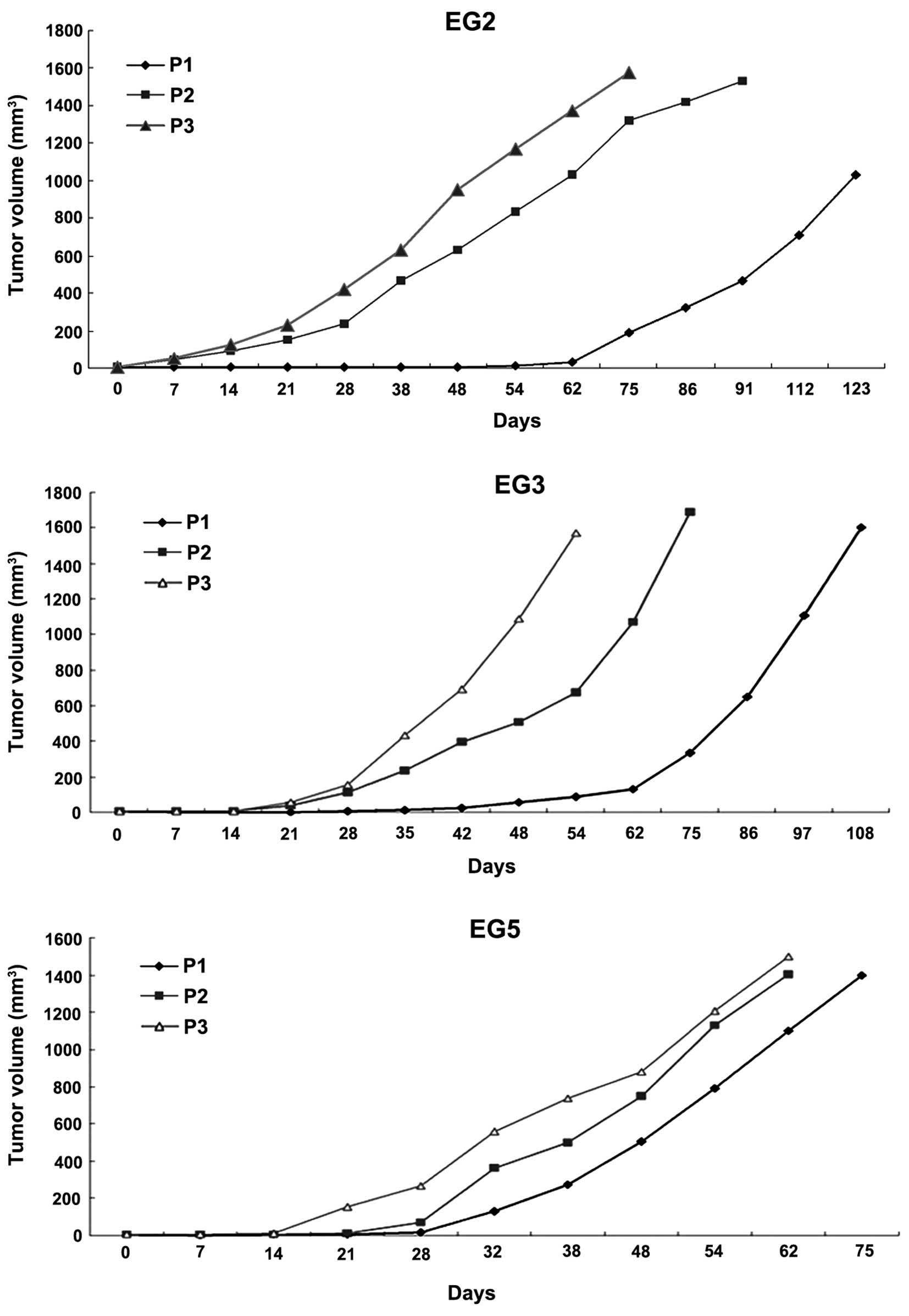
Fourteen transplantable xenografts were established,
and the success rate was 53.8%. EG2, EG3 and EG5 were chosen to
assess the pathological characteristics. The growth curves of
passage three xenografts (first, second and third passage) were
plotted as tumor volume over time (Fig.
1). The growth time of the first passage was 123, 108 and 83
days respectively, the second passage was 90, 74 and 81 days, and
third passage was 62, 52 and 72 days. Every group period was
shorter in the third passage than the first and second passage. In
addition, the growth was stable after the third passage.
Comparison of the histology and
immmunohistochemistry between the xenografts and the patient
tumors
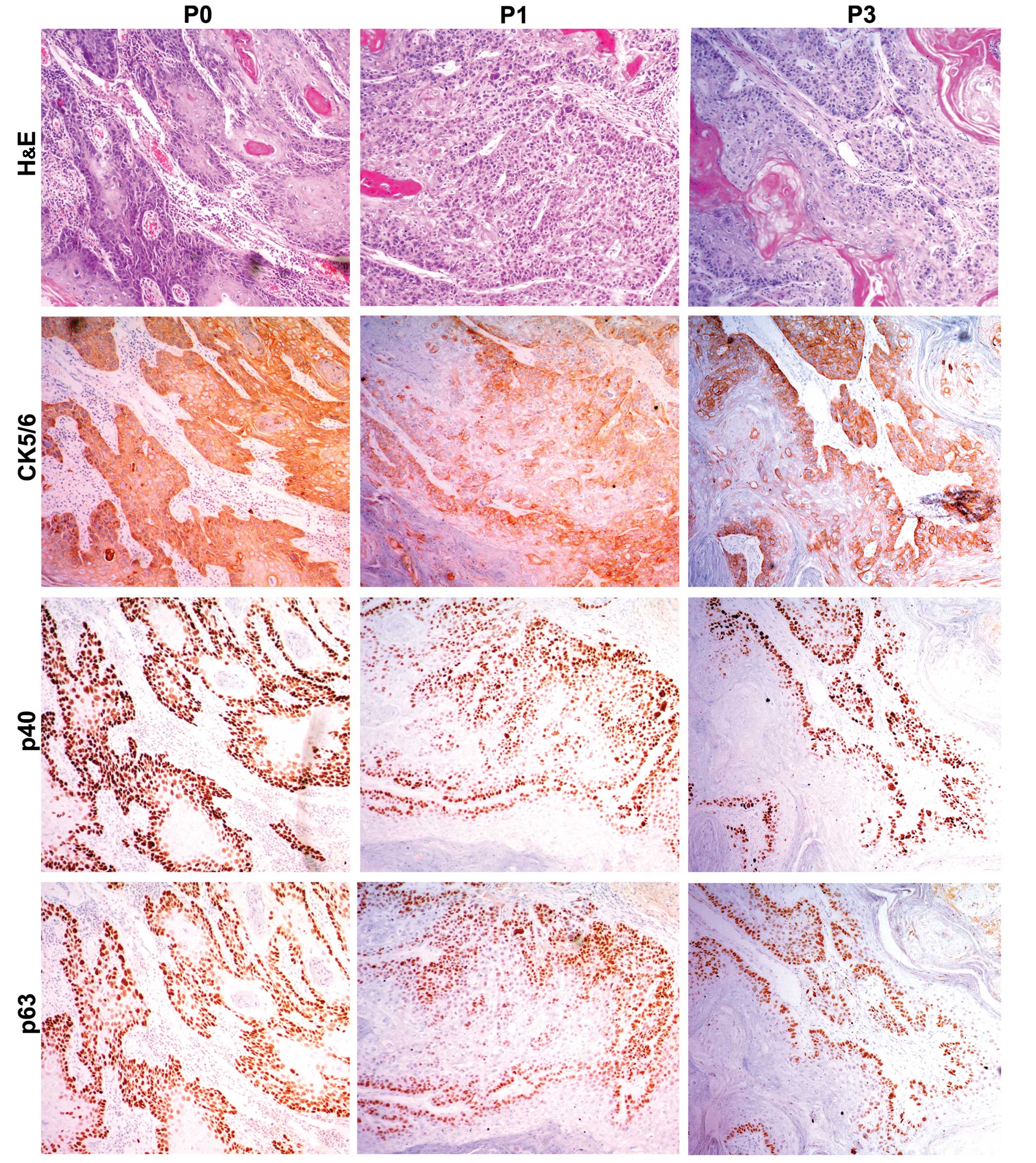
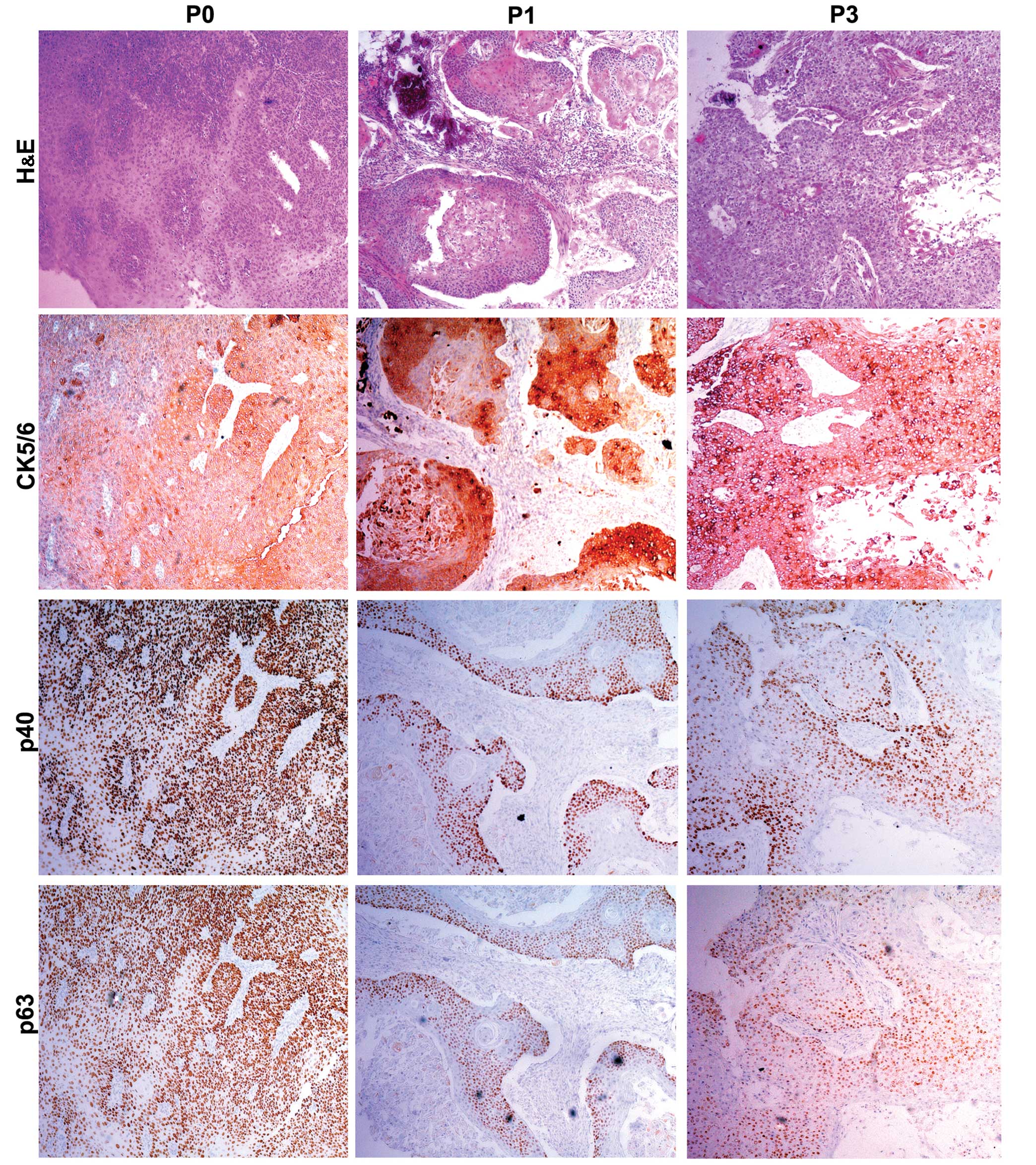
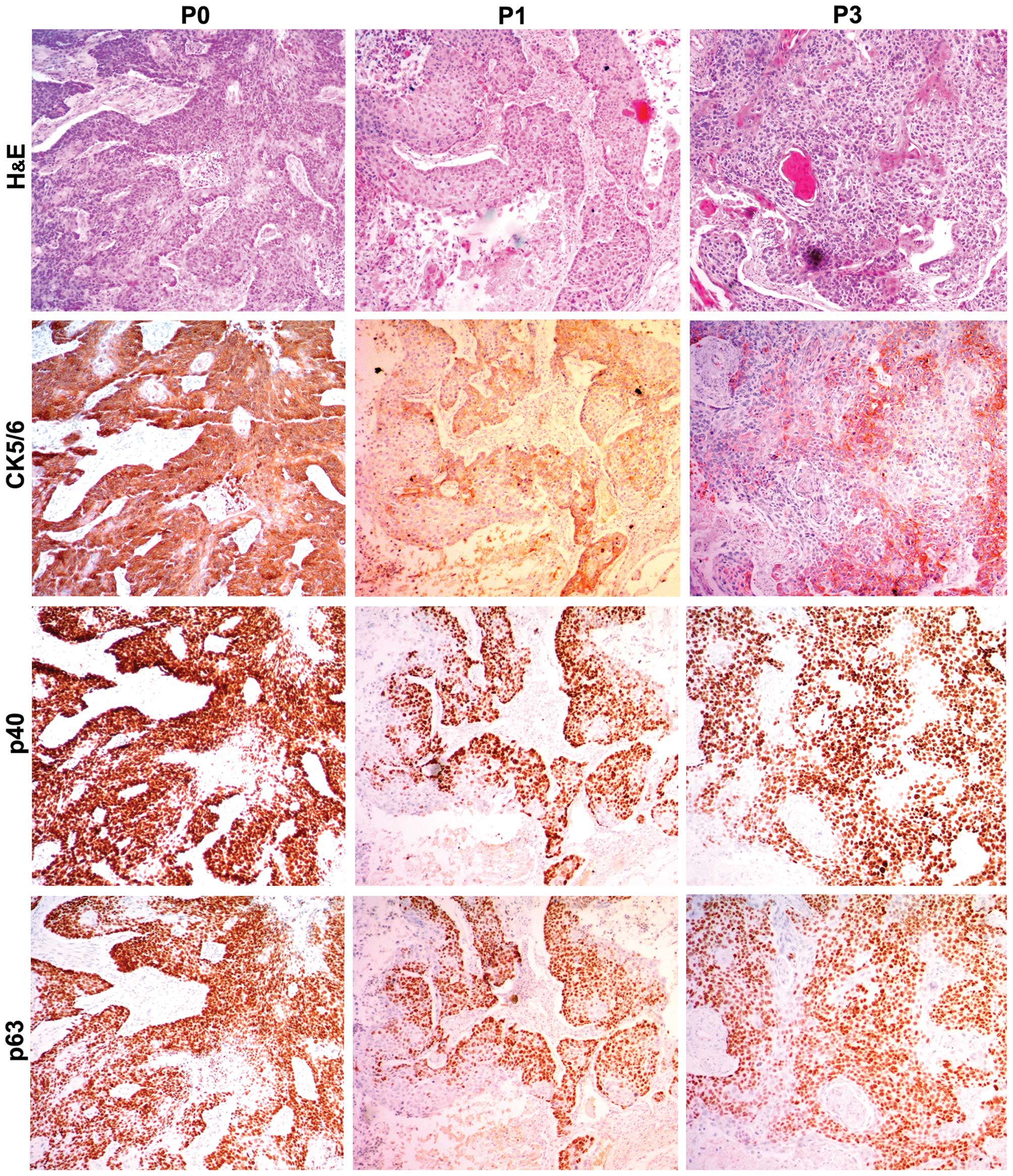
In order to further evaluate the PDX xenografts, we
compared the histology of the original patient tumors with the
first and third passage xenografts for samples EG2, EG3 and EG5. We
found that the pathological characteristics of the third passage
xenografts were in accordance with the original patient samples.
The expression of CK5/6, p40 and p63 was positive in the three
passages by immunohistochemical staining (Figs. 2Figure 3–4).
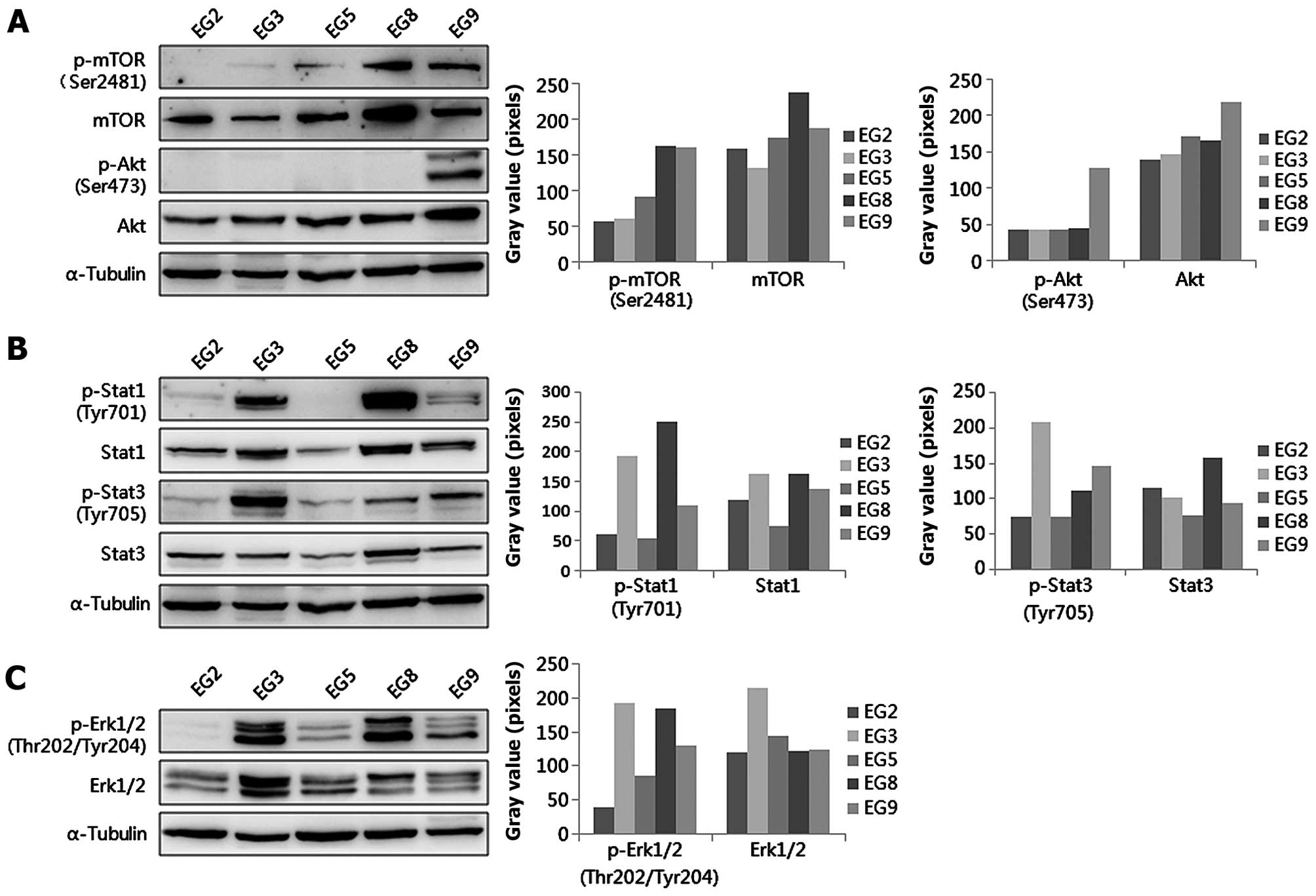
Activated signal transduction pathways in
the established esophageal tumor xenografts
The levels of phosphorylated and total proteins of
AKT-mTOR, Stat3 and Erk 1/2 were detected by western blotting. The
level of p-mTOR (Ser2481) was higher in EG8 and EG9 than in EG2,
EG3 and EG5, however, p-Akt (Ser473) was higher in EG9 than in EG2,
EG3, EG5 and EG8 (Fig. 5A). The
level of p-Stat3 (Tyr705) was highest in EG3, however, the level of
p-Stat1 (Tyr701) was higher in EG3 and EG8 (Fig. 5B). Moreover, p-ERK1/2
(Thr202/Tyr204) was highest in EG3 (Fig. 5C). The difference in activated
signal transduction pathways in these PDXs had statistical
significance (P=0.000).
Discussion
Esophageal cancer (EC) is a serious malignancy with
a high rate of mortality and poor prognosis. While many other types
of cancer are expected to decrease in incidence over the next 10
years by 2025, the prevalence of EC is expected to increase by 140%
(12). ESCC is the most common
histological type of EC, with a high incidence in China (13). Current clinical treatment strategies
for ESCC include surgery, chemotherapy and radiotherapy. Recently,
neoadjuvant chemoradiation followed by surgery has been used in the
treatment of EC in the clinic (14). Unfortunately, the 5-year survival
rate has not improved. Therefore, it is necessary to develop
effective therapeutic strategies for ESCC patients.
Animal models are the most useful tool for
preclinical evaluation of novel therapeutic strategies in cancer.
PDX models can reproduce tumor development, proliferation and
metastasis (11) and can predict
phase II clinical trial performance (15). Therefore, PDX models play an
important role in developing personalized treatment for EC patients
(14). In the present study, we
established 26 ESCC PDXs subcutaneously. In addition, the success
ʻtake-rateʼ of the xenografts was 53.8% and it was higher compared
to other EC PDX models for which the success rate was 38.5%
(16). We further evaluated the
established PDX models represented as EG2, EG3 and EG5. The three
established ESCC xenografts showed decreased degree of tumor growth
curves from first passage to third passage (Fig. 1). In the three PDX models, the first
passage reached 1,500 mm3 on day 133, 106 and 75 for
EG2, EG3 and EG8, respectively. Next, we performed histology and
growth curve analyses of the established xenografts. Expression of
CK5/6, p63 and p40 was assessed by immunohistochemsistry.
Cytokeratin 5/6 is useful for differentiating epithelioid
mesotheliomas from pulmonary carcinomas (17). P63 protein plays important roles in
the carcinogenesis of ESCC through the Akt signaling pathway
(18). An investigation into the
combined expression of p63 may be helpful in early diagnosis and in
evaluating the prognosis of ESCC (19). In our study, histological analysis
and immunohistochemical analysis were used for assessing the
characteristics of the PDX tumors. Expression of CK5/6, p63, p40 in
patient samples was positive in the patient tissue and in the first
and third passages. Our results indicated that the ESCC PDX models
maintained the histological characteristics and the differentiation
status of the original patient tumor. In a word, we successfully
established ESCC PDX models from patient minimal surgical tissue.
The established ESCC PDX models provide a superior in vivo
preclinical platform to explore novel cancer therapeutics and
analysis of drug activity, including the discovery of predictive
biomarkers, the study of ESCC cancer stem-cell biology and
stromal-tumor interactions in the clinic.
The drugs used for ESCC patient treatment are toxic
to cells and have many side-effects. More effective and innovative
treatment strategies are urgently needed (3). However, the molecular tumorigenesis
mechanisms of ESCC have not been fully elucidated. Many signaling
pathways, for example Akt/mTOR, Erk1/2, Stat1 and Stat3 are
involved in the processes of cell growth, cell cycling, apoptosis
and invasion in ESCC (20-24). In the present study, we examined the
expression of Akt and p-Akt (Ser473), mTOR and p-mTOR in five PDX
models. Our results found that the level of p-Akt (Ser473) was
positive only in EG9. Our results showed that all groups expressed
total mTOR, however, the level of p-mTOR (Ser2481) was higher in
EG8 and EG9 than in EG2 and EG5. Thus, we chose the activated
Akt/mTOR pathway from our established PDX models to further
research the role of the Akt/mTOR pathway in ESCC. The
extracellular signal-regulated kinase (Erk1/2) signaling pathway is
important for the regulation of cell growth by controlling
transcription (25,26). In addition, p-Erk1/2 (Thr202/Tyr204)
is higher in poorly differentiated tissues than in well and
moderately differentiated tissues in ESCC (25,27).
Our results indicated that Erk1/2 was activated in EG2 compared to
the other patients. In addition, its levels were different in these
five patient models.
Signal transducer and activator of transcription-3
(Stat3) plays a role in esophageal carcinogenesis by promoting cell
proliferation, motility and suppression of apoptosis (28). In addition, high p-Stat3 (Tyr705) is
correlated with poor prognosis in ESCC (29). However, Stat1 has opposing
biological effects compared with Stat3. The loss of Stat1
expression significantly correlates with a worse clinical outcome
in ESCC (30). Recent research
found that Stat1 downregulates Stat3 and p-Stat3 (Tyr705), and the
relative proportions of Stat1:Stat3 heterodimers decide the cell
fate in ESCC (31). In our study,
we found that p-Stat1 and p-Stat3 were overexpressed in EG3, in
contrast, there was absence of both in EG2 and EG5. In contrast,
p-Stat1 (Tyr701) was lost and p-Stat3 (Tyr705) was activated in
EG9. This indicated that the relationship between Stat3 and Stat1
is complicated. Investigation of the role of Stat1 and Stat3 and
the relationship between both in ESCC are warranted using these
xenografts.
In conclusion, we successfully established ESCC
patient tumor-derived xenografts. These xenografts maintained the
patient's pathological characteristics. They had different growth
cycles and tumor texture. Notably, different signaling pathways
were activated in ESCC, which reflected the biological
heterogeneity observed in the patient population. Furthermore,
these PDX models provide an important platform for understanding
the molecular mechanisms of ESCC, for elucidating critical
signaling pathways involved in esophageal tumorigenesis and
progression. These models can also be used to identify potential
individual therapeutic targets, investigate the biological activity
and toxicity profiles of preclinical drugs and provide a more
relevant system to test clinically directed hypotheses in ESCC.
Acknowledgments
The present study was supported by the National
Sciences Foundation of China (nos. 81372269, U1304813 and
81472324), the Science Foundation of the Henan Province of China
(nos. 13A310553 and 15B310013), the Young Teacher Special Fund of
Zhengzhou University (no. 1421328055) and the Young Teacher
Training of Zhengzhou University. We would like to express our
thanks to Dr Fred Bogott for critically reading the manuscript and
offering good suggestions.
References
|
1
|
Zhao P, Dai M, Chen W and Li N: Cancer
trends in China. Jpn J Clin Oncol. 40:281–285. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Adachi Y, Ohashi H, Imsumran A, Yamamoto
H, Matsunaga Y, Taniguchi H, Nosho K, Suzuki H, Sasaki Y, Arimura
Y, et al: The effect of IGF-I receptor blockade for human
esophageal squamous cell carcinoma and adenocarcinoma. Tumour Biol.
35:973–985. 2014. View Article : Google Scholar
|
|
3
|
Belkhiri A and El-Rifai W: Advances in
targeted therapies and new promising targets in esophageal cancer.
Oncotarget. 6:1348–1358. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Honjo S, Ajani JA, Scott AW, Chen Q,
Skinner HD, Stroehlein J, Johnson RL and Song S: Metformin
sensitizes chemotherapy by targeting cancer stem cells and the mTOR
pathway in esophageal cancer. Int J Oncol. 45:567–574.
2014.PubMed/NCBI
|
|
5
|
Harb WA: Management of patients with
hormone receptor-positive breast cancer with visceral disease:
Challenges and treatment options. Cancer Manag Res. 7:37–46. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Geuna E, Milani A, Martinello R, Aversa C,
Valabrega G, Scaltriti M and Montemurro F: Buparlisib, an oral
pan-PI3K inhibitor for the treatment of breast cancer. Expert Opin
Investig Drugs. 24:421–431. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Inoue A, Saijo Y, Maemondo M, Gomi K,
Tokue Y, Kimura Y, Ebina M, Kikuchi T, Moriya T and Nukiwa T:
Severe acute interstitial pneumonia and gefitinib. Lancet.
361:137–139. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Baselga J, Rischin D, Ranson M, Calvert H,
Raymond E, Kieback DG, Kaye SB, Gianni L, Harris A, Bjork T, et al:
Phase I safety, pharmacokinetic, and pharmacodynamic trial of
ZD1839, a selective oral epidermal growth factor receptor tyrosine
kinase inhibitor, in patients with five selected solid tumor types.
J Clin Oncol. 20:4292–4302. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Marangoni E, Vincent-Salomon A, Auger N,
Degeorges A, Assayag F, de Cremoux P, de Plater L, Guyader C, De
Pinieux G, Judde JG, et al: A new model of patient tumor-derived
breast cancer xenografts for preclinical assays. Clin Cancer Res.
13:3989–3998. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Moro M, Bertolini G, Tortoreto M,
Pastorino U, Sozzi G and Roz L: Patient-derived xenografts of non
small cell lung cancer: Resurgence of an old model for
investigation of modern concepts of tailored therapy and cancer
stem cells. J Biomed Biotechnol. 2012:5685672012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Siolas D and Hannon GJ: Patient-derived
tumor xenografts: Transforming clinical samples into mouse models.
Cancer Res. 73:5315–5319. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tian H, Hou L, Xiong YM, Huang JX, She YJ,
Bi XB and Song XR: miR-218 suppresses tumor growth and enhances the
chemosensitivity of esophageal squamous cell carcinoma to
cisplatin. Oncol Rep. 33:981–989. 2015.
|
|
13
|
Napier KJ, Scheerer M and Misra S:
Esophageal cancer: A Review of epidemiology, pathogenesis, staging
workup and treatment modalities. World J Gastrointest Oncol.
6:112–120. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Uemura N and Kondo T: Current status of
predictive biomarkers for neoadjuvant therapy in esophageal cancer.
World J Gastrointest Pathophysiol. 5:322–334. 2014.PubMed/NCBI
|
|
15
|
Morton CL and Houghton PJ: Establishment
of human tumor xenografts in immunodeficient mice. Nat Protoc.
2:247–250. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dong SW, Zhang H, Wang BL, Sun P, Wang YG
and Zhang P: Effect of the downregulation of SMYD3 expression by
RNAi on RIZ1 expression and proliferation of esophageal squamous
cell carcinoma. Oncol Rep. 32:1064–1070. 2014.PubMed/NCBI
|
|
17
|
Blobel GA, Moll R, Franke WW, Kayser KW
and Gould VE: The intermediate filament cytoskeleton of malignant
mesotheliomas and its diagnostic significance. Am J Pathol.
121:235–247. 1985.PubMed/NCBI
|
|
18
|
Ye S, Lee KB, Park MH, Lee JS and Kim SM:
p63 regulates growth of esophageal squamous carcinoma cells via the
Akt signaling pathway. Int J Oncol. 44:2153–2159. 2014.PubMed/NCBI
|
|
19
|
Thépot A, Hautefeuille A, Cros MP,
Abedi-Ardekani B, Pétré A, Damour O, Krutovskikh V and Hainaut P:
Intraepithelial p63-dependent expression of distinct components of
cell adhesion complexes in normal esophageal mucosa and squamous
cell carcinoma. Int J Cancer. 127:2051–2062. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lin DC, Hao JJ, Nagata Y, Xu L, Shang L,
Meng X, Sato Y, Okuno Y, Varela AM, Ding LW, et al: Genomic and
molecular characterization of esophageal squamous cell carcinoma.
Nat Genet. 46:467–473. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Song Y, Li L, Ou Y, Gao Z, Li E, Li X,
Zhang W, Wang J, Xu L, Zhou Y, et al: Identification of genomic
alterations in oesophageal squamous cell cancer. Nature. 509:91–95.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li B, Li J, Xu WW, Guan XY, Qin YR, Zhang
LY, Law S, Tsao SW and Cheung AL: Suppression of esophageal tumor
growth and chemoresistance by directly targeting the PI3K/AKT
pathway. Oncotarget. 5:11576–11587. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hou G, Yang S, Zhou Y, Wang C, Zhao W and
Lu Z: Targeted inhibition of mTOR signaling improves sensitivity of
esophageal squamous cell carcinoma cells to cisplatin. J Immunol
Res. 2014:8457632014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Huang Y, Xi Q, Chen Y, Wang J, Peng P, Xia
S and Yu S: A dual mTORC1 and mTORC2 inhibitor shows antitumor
activity in esophageal squamous cell carcinoma cells and sensitizes
them to cisplatin. Anticancer Drugs. 24:889–898. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ciccarelli A and Giustetto M: Role of ERK
signaling in activity-dependent modifications of histone proteins.
Neuropharmacology. 80:34–44. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bhalla S, Evens AM, Dai B, Prachand S,
Gordon LI and Gartenhaus RB: The novel anti-MEK small molecule
AZD6244 induces BIM-dependent and AKT-independent apoptosis in
diffuse large B-cell lymphoma. Blood. 118:1052–1061. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang J, Zhi H, Zhou C, Ding F, Luo A,
Zhang X, Sun Y, Wang X, Wu M and Liu Z: Up-regulation of
fibronectin in oesophageal squamous cell carcinoma is associated
with activation of the Erk pathway. J Pathol. 207:402–409. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sakamoto C: STAT1 and STAT3 might be
regulated differently in esophageal squamous cell carcinoma. J
Gastroenterol. 37:575–577. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang Z, Zhu S, Shen M, Liu J, Wang M, Li
C, Wang Y, Deng A and Mei Q: STAT3 is involved in esophageal
carcinogenesis through regulation of Oct-1. Carcinogenesis.
34:678–688. 2013. View Article : Google Scholar
|
|
30
|
Qing Y and Stark GR: Alternative
activation of STAT1 and STAT3 in response to interferon-gamma. J
Biol Chem. 279:41679–41685. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Regis G, Pensa S, Boselli D, Novelli F and
Poli V: Ups and downs: the STAT1:STAT3 seesaw of Interferon and
gp130 receptor signalling. Semin Cell Dev Biol. 19:351–359. 2008.
View Article : Google Scholar : PubMed/NCBI
|