Introduction
In Western countries, prostate cancer has surpassed
lung cancer as the most prevalent cancer and has become the second
leading cause of cancer-related deaths among men (1). Distant metastasis is the primary cause
of death for the majority of prostate cancer patients. Early-stage
prostate cancer depends upon androgens. However, 70–80% of patients
with metastatic disease respond initially to androgen-deprivation
therapy (ADT), yet the tumors may become hormone-refractory and
lethal due to metastatic spread several years after initial
treatment with anti-androgens (2).
Although microtubule-targeting agents, such as docetaxel, improve
the overall survival of patients with distant disease by 2–3
months, tumor resistance eventually occurs (3). In addition, several targeted therapies
involved in clinical trials have shown little effect on prolonging
survival. Therefore, new agents for the treatment of
androgen-resistant prostate cancer are needed.
Metastasis is a complex process whereby tumor cells
penetrate the basement membranes of blood vessels, survive in the
blood stream until extravasating to secondary sites, and form
metastases (4). This process
requires epithelial-to-mesenchymal transition (EMT). During EMT,
tumor cells lose epithelial polarity and adopt a spindle-shaped
morphology and migratory fibroblastoid phenotype. These transitions
endow cancer cells with enhanced invasive and metastatic potential
(5). EMT involves multiple
signaling pathways, and is regulated by a set of transcription
factors, including Snail, Slug and Twist, which lead to loss of
cell-cell adhesion molecules such as E-cadherin and gain of
mesenchymal proteins such as vimentin (6,7).
Importantly, these transcription factors as key regulators of EMT
have been confirmed to be critical to metastasis and the invasive
ability of cancer cells in prostate cancer progression (8,9).
Furthermore, loss of E-cadherin expression was found in high-grade
prostate cancer and is associated with a reduction in survival
(10,11). Therefore, clarifying the initial
molecular mechanisms regulating the EMT phenotype allows the
development of novel therapeutic strategies for the prevention and
treatment of prostate cancer.
Recent findings have suggested that a wide variety
of molecules promote EMT, such as chemokines (12). Chemokines are small cytokine-like
secreted proteins with selective chemoattractant properties and
have emerged as important molecular regulators in cancer biology.
Growing evidence has demonstrated that various chemokines promote
tumor growth and metastasis via mediating EMT (13). Fractalkine also known as chemokine
(C-X3-C motif) ligand 1 (CX3CL1) is the only described member of
the CX3C family. Recent studies have confirmed that CX3XL1 is
highly expressed in various cancers, and is involved in tumor
spread and organ-specific metastases (14–16).
In prostate cancer, the CX3CL1/CX3CR1 axis activates the PI3K/AKT
survival pathway and plays a crucial role in skeletal metastasis
(17). Our previous study showed
that hypoxia exposure upregulated CX3CR1 expression via HIF and the
NF-κB pathway in androgen-independent prostate cancer cells
(18). Although there has been
increasing interest in the role of CX3CL1/CX3CR1 during tumor
metastasis, little is known concerning the detailed mechanisms
involved.
Hypoxia, a well-recognized microenvironmental factor
in prostate cancer development, is closely related with cancer
relapse, metastases and resistance to chemotherapy (19). Futhermore, hypoxia has been
implicated in the promotion of the EMT process by activating a
multitude of molecular signaling pathways that drive EMT (20). The present study focused on whether
CX3CL1/CX3CR regulate EMT and promote tumor migration and invasion
in androgen-independent prostate cancer cells under hypoxic
condition, and explored the potential molecular mechanisms
involved.
Materials and methods
Reagents and antibodies
Recombinant human fractalkine (CX3CL1) and TGF-α
were purchased from PeproTech (Rocky Hill, NJ, USA). EGFR inhibitor
AG1478 was obtained from Cell Signaling Technology (Beverly, MA,
USA).
Primary antibodies against E-cadherin (cat. #3195),
vimentin (cat. #5741) and slug (cat. #9585) were purchased from
Cell Signaling Technology (Danvers, MA, USA). Antibodies for EGFR
(cat.# sc-03), phosphor-EGFR (cat.# sc-101668, Tyr1173) and β-actin
(cat.# sc-47778) were purchased from Santa Cruz Biotechnology
(Santa Cruz, CA, USA). TGF-α neutralizing antibody (cat.# sc-9043)
was from Calbiochem (La Jolla, CA, USA).
Cell culture and hypoxia treatment
Human prostate cancer cell lines DU145 and PC-3 were
purchased from the American Type Culture Collection (ATCC;
Manassas, VA, USA). Cells were cultrued in RPMI-1640 medium
containing 10% fetal bovine serum (FBS; Gibco-BRL, Grand Island,
NY, USA) and 1X penicillin/streptomycin (Invitrogen, Carlsbad, CA,
USA) at 37°C in a humidified atmosphere (5% CO2/95%
air). For hypoxia treatment, the cells were incubated in a hypoxic
chamber (Thermo Scientific) maintained at 1% O2, 5%
CO2 and 94% N2 at 37°C for 24–48 h.
Matrigel invasion and migration
assays
The cell invasion assay was performed using
Matrigel-coated 24-well Transwell inserts containing polycarbonate
filters with 8-µm pores (BD Biosciences) according to a
previously published protocol. Briefly, 5×104 cells in
200 µl of serum-free RPMI-1640 medium were seeded onto the
upper chambers, whereas basal serum-free medium or medium with
recombinant human CX3CL1 (200 ng/ml), TGF-α (50 µg/ml) or
Ab-TGF-α (10 µg/ml) was added into the bottom chamber. After
the assay chambers were incubated for 24 or 48 h under hypoxic
conditions, the non-invading cells on the upper surface of the
membrane were carefully removed with a cotton swab, and the filter
membrane was fixed with cool methanol for 15 min, subsequently
stained with 0.1% crystal violet for 30 min. Invading cells on the
lower surface of the membrane were examined and counted under a
microscope (Olympus IX51; Olympus, Japan) at a magnification of
×200. Five random fields were numerically averaged and counted for
each assay. Cell migration assay was performed with a similar
procedure without Matrigel coating. All experiments were performed
in triplicate and repeated three times.
ELISA assay
An equal number of cells were plated and cultured
for the indicated times in RPMI-1640 medium supplemented with 10%
FBS. The supernatant was collected and TGF-α levels were determined
using a commercial human TGF-α ELISA kit (Oncogene, Boston, MA,
USA) according to the manufacturer's instructions.
Western blot analysis
Western blot analysis was carried out as described
previously (18). Briefly, cells
were harvested, washed, and lysed in ice cold lysis buffer
containing a mixture of protease inhibitors and phosphatase
inhibitor. After centrifuged at 12,000 × g for 30 min, the
supernatant was collected and the protein concentration in the
extracts was determined using BCA reagent (Beyotime Institute of
Biotechnology, Nanjing, China) according to the manufacturer's
protocols. Equal amounts of proteins (40 µg) were loaded,
fractionated by 12% SDS-PAGE and transferred onto nitrocellulose
membranes. After being blocked with 5% non-fat milk in TBST for 2 h
at room temperature, the membranes were incubated with primary
antibodies for E-cadherin (1:1,000 rabbit monoclonal), vimentin
(1:1,000 rabbit monoclonal), Slug (1:1,000 rabbit monoclonal), EGFR
(1:500 rabbit monoclonal), p-EGFR (1:300 rabbit monoclonal) and
β-actin (1:1,000 rabbit monoclonal) at 4°C overnight. The bound
primary antibody was detected by incubating with appropriate
horseradish peroxidase-conjugated secondary antibodies at a
dilution of 1:2,000 in TBST for 2 h. The expression levels of
β-actin were monitored as an internal control for the
semi-quantitative PCR. Immunoreactive bands were visualized using
the western blot analysis Super ECL Plus detection reagents
(Applygen Technologies Inc., Beijing, China) and analyzed by
Quantity One software.
RNA interference
For the small interfering RNA (siRNA) treatment,
oligonucleotides corresponding to nucleotide sequences of Slug and
ADAM17 were synthesized commercially by Invitrogen
(Life-Technologies). The siRNA sequences were as follows: Slug
siRNA 1, 5′-AUGAGUUGUAACCAG GUCAGCUUCC-3′; Slug siRNA 2,
5′-AUACAUGACAUAUUUCCCUCCCUGG-3′; Slug siRNA 3,
5′-UUUCUUUGCUGUCAACACGAUUCUG-3′; and ADAM17 siRNA,
5′-CAGAAUCGUGUUGACAGCAAAGAAA-3′. Cells were transfected at 40–60%
confluency with siRNA specific for Slug, ADAM17 or a scrambled
sequence at the concentration of 100 nM using Lipofectamine 2000
transfection reagent (Invitrogen) according to the manufacturer's
recommendations. The transfected cells were collected at 48 or 72 h
and efficiency of protein knockdown was assessed by western blot
analysis.
Statistical analysis
Each experiment was replicated in triplicate. All
data are presented as mean ± SD. Statistical analyses were
performed using Statistics Package for Social Science (SPSS 20.0
for Windows; IBM, Armonk, NY, USA). One-way ANOVA was used for
multiple comparisons. A p-value <0.05 was considered
statistically significant.
Results
CX3CL1 increases the migration and
invasiveness of hypoxic androgen-independent prostate cancer
cells
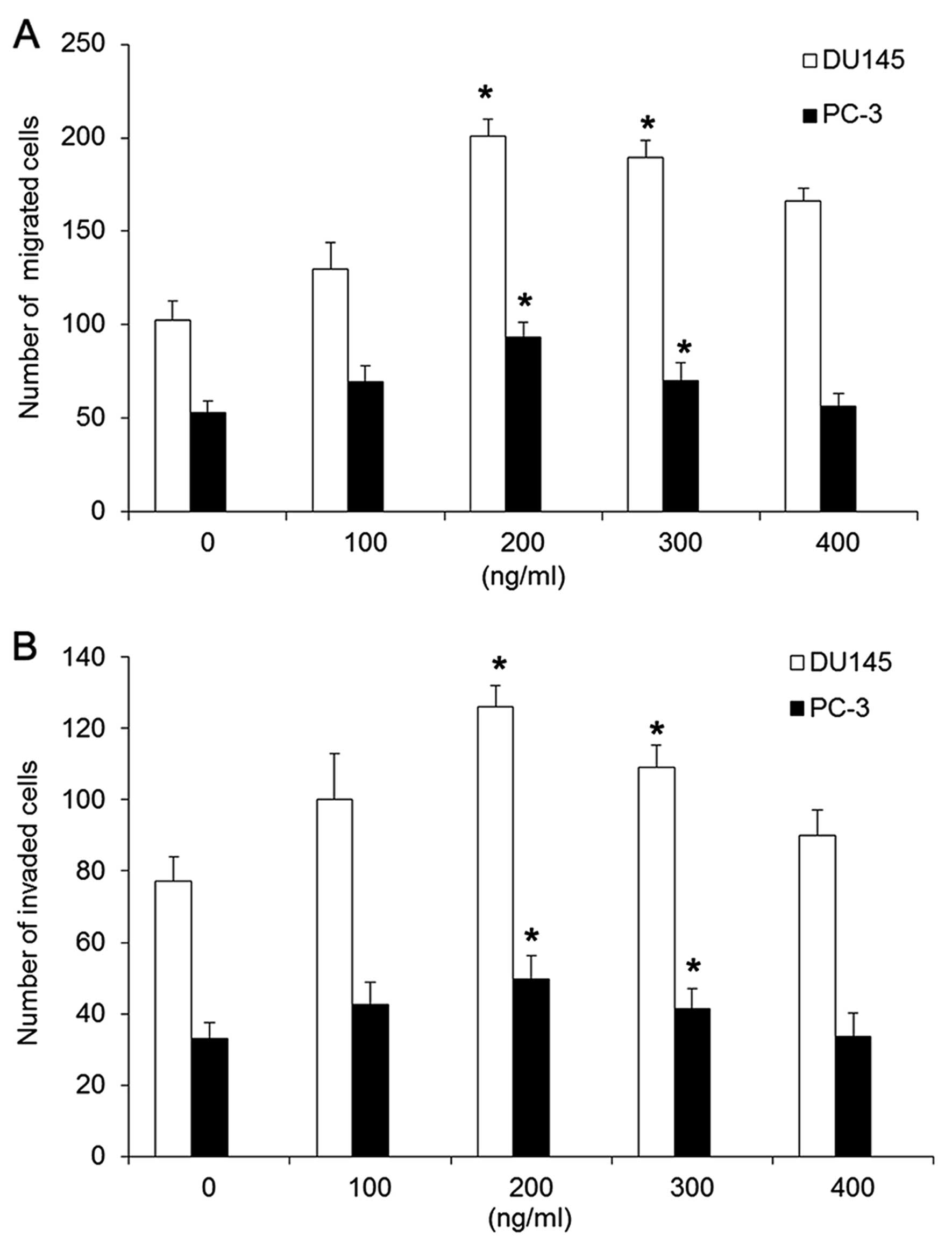
Initially, we examined the effect of CX3CL1 on the
abilities of invasion and migration in androgen-independent
prostate cancer cells. Two types of androgen-independent prostate
cancer cell lines DU145 and PC-3 were treated with CX3CL1 at
various concentrations (0–400 ng/ml) for a period of 48 h under
hypoxic conditions. Transwell assays showed that the ability of
DU145 and PC-3 cells to cross the basement membrane matrix
following treatment with CX3CL1 at a concentration of 200 or 300
ng/ml was markedly increased compared to the untreated cells. DU145
cells exhibited more aggressive and invasive activity than PC-3
cells responding to CX3CL1 stimulation (Fig. 1).
CX3CL1 induces a mesenchymal phenotype in
hypoxic androgen-independent prostate cancer cells
EMT is a major process leading to increased
migration and invasive abilities in cancer cells. To investigate
whether the enhancement of migration and invasive abilities in
androgen-independent prostate cancer cells resulted from the EMT
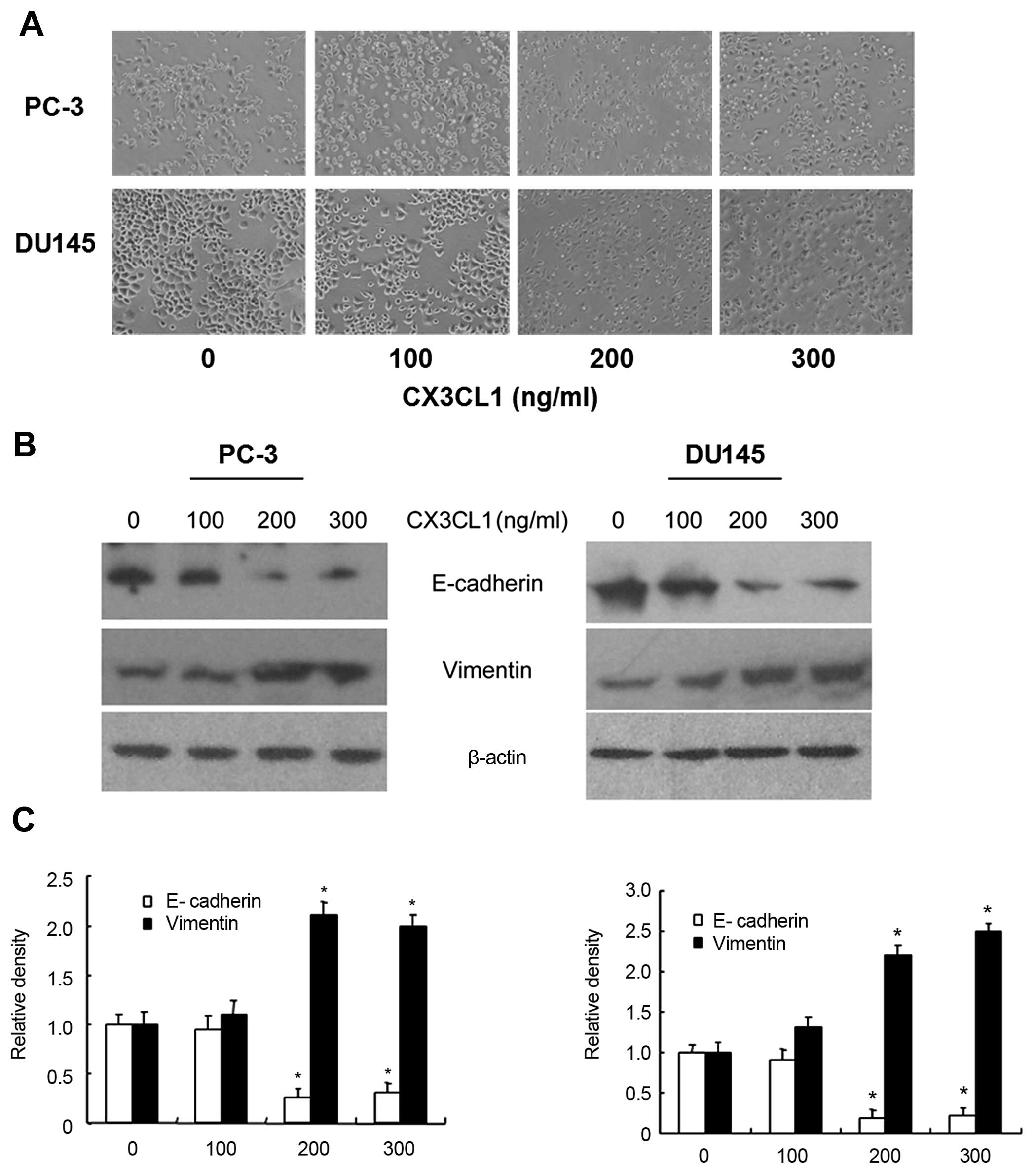
process, we observed morphological changes of DU145 and PC-3 cells
following treatment with CX3CL1 at various concentrations (0–300
ng/ml) for a period of 48 h under hypoxic conditions. As shown in
Fig. 2A, most treated cells not
only exhibited an fibroblastic and elongated morphology but also
showed occasional misorientation and a loose association compared
to the closely contacted monolayer, polygon cobblestone-like cells.
An accompanying alteration in expression of epithelial and
mesenchymal markers is often used to identify cells which are
undergoing EMT. Western blot analysis showed that there was
decreased expression of E-cadherin but elevated expression of
vimentin in the DU145 and PC-3 cells upon CXCL3 stimulation at a
concentration of 200 or 300 ng/ml for 48 h (Fig. 2B and C). Taken together, we provided
strong evidence that CXCL3 induced an EMT-like phenotype in the
androgen-independent prostate cancer cells.
EGFR-dependent Slug pathway is implicated
in CX3CL1-induced EMT
Epidermal growth factor receptor (EGFR), an
erbB-family receptor tyrosine kinase, is constitutively active in a
variety of tumors and overexpressed in ~30% of prostate cancer
cases (21). Experimental and
preclinical evidence showed that EGFR overexpression is correlated
with prostate cancer aggressiveness (22). Thus, to identify whether EGFR
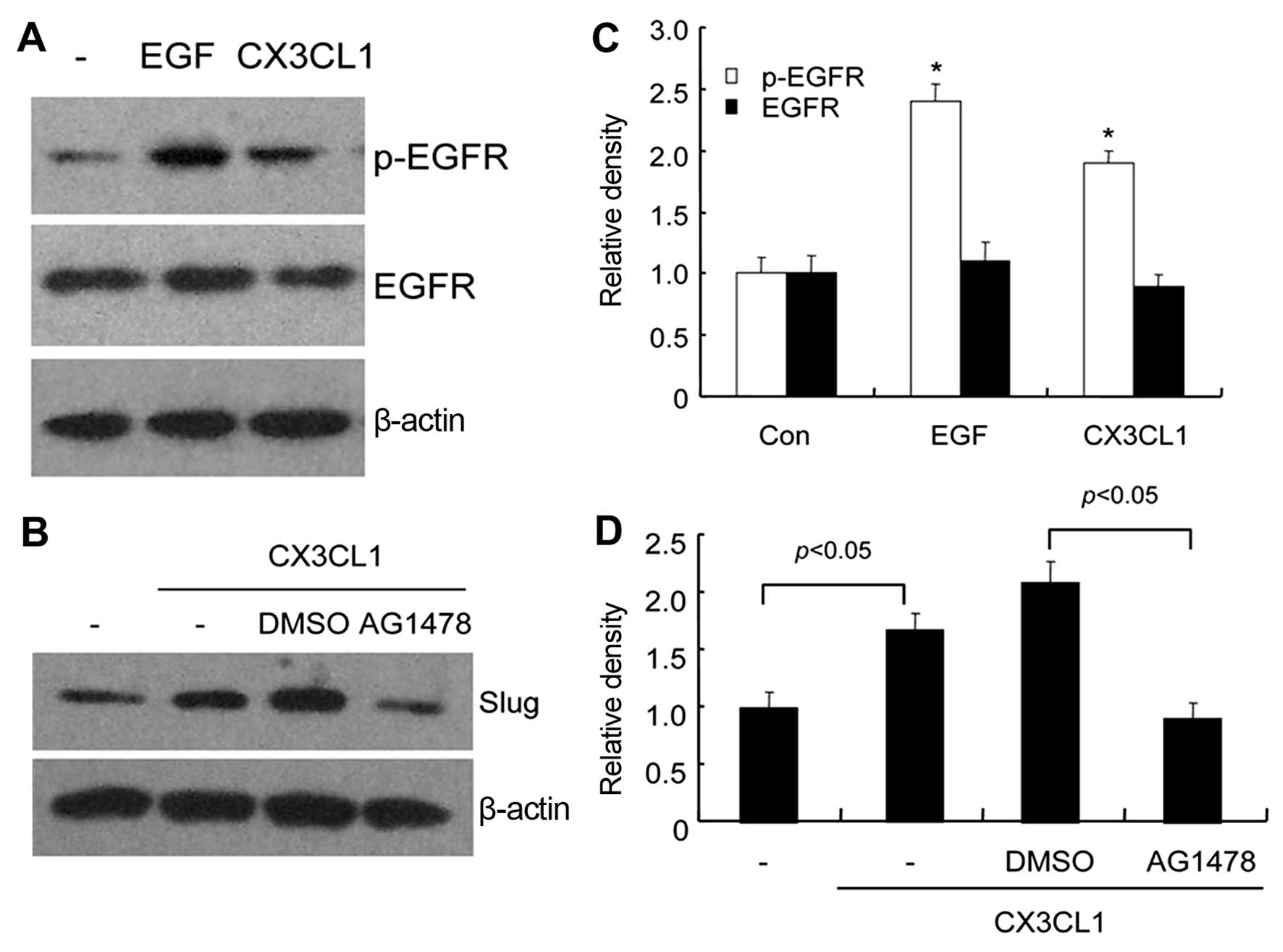
pathway activation exists in CX3CL-induced EMT occurrence, we
determined the expression levels of total EGFR and phospho-EGFR
(p-EGFR) in DU145 cells treated with 200 ng/ml of CX3CL1. Western
blot analysis showed that p-EGFR expression was significantly
elevated in the DU145 cells treated with CX3CL1 compared with the
untreated cells (Fig. 3A and C),
indicating CX3CL1-mediated activation of the EGFR signaling
pathway. Accumulating evidence suggests that the EGFR family and
its downstream mediators, for instance, Slug, serving as a
transcription repressor of E-cadherin, are involved in EMT
(23). Our results showed that
CX3CL1 markedly elevated the protein expression level of Slug in
the DU145 cells, and the EGFR inhibitor AG1478 abrogated this
elevation (Fig. 3B and D),
suggesting that CX3CL1 regulated Slug expression in an
EGFR-dependent manner.
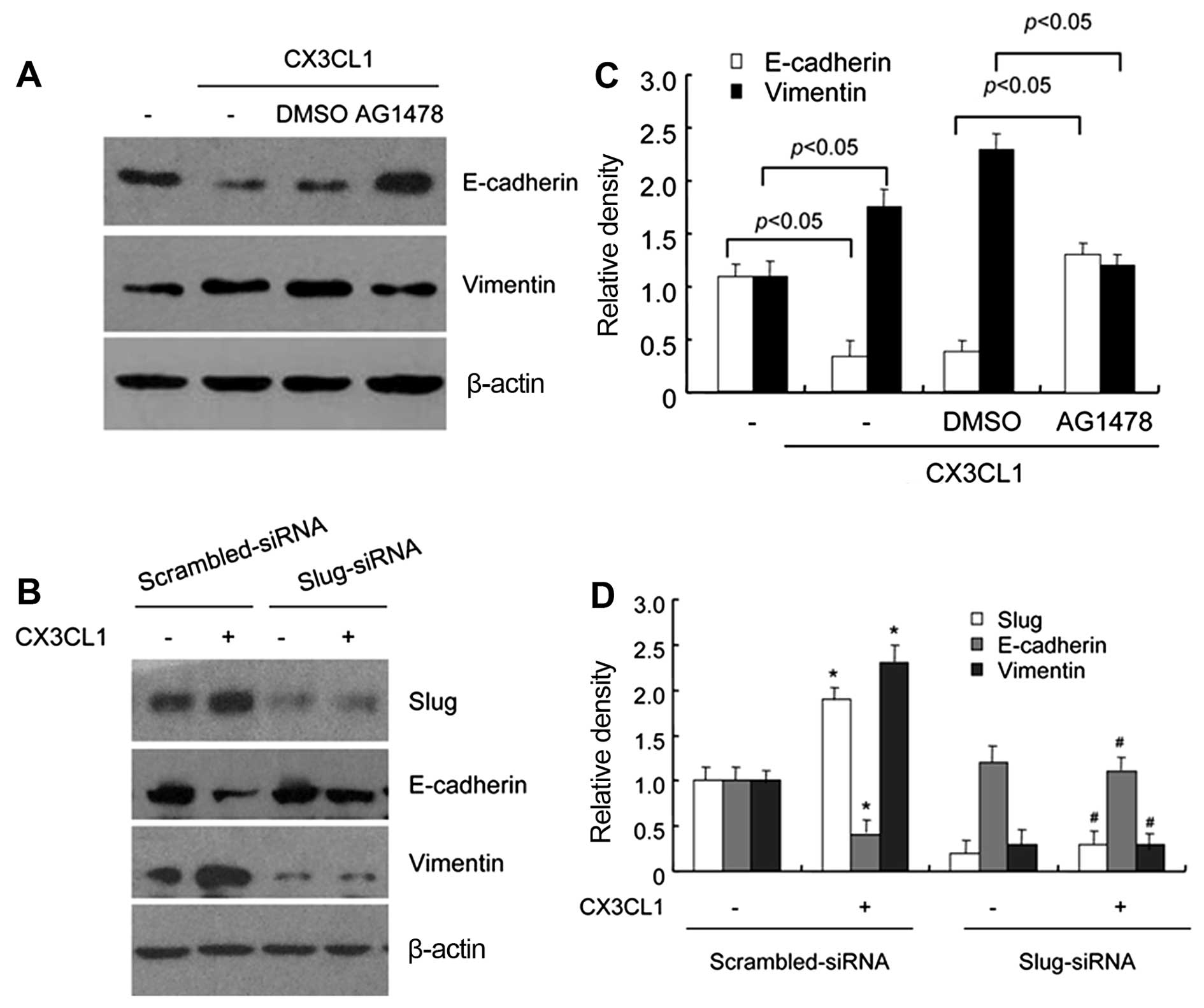
Next, to further confirm the role of the
EGFR-dependent Slug pathway in CX3CL-induced EMT, we examined the
protein expression levels of EMT markers in the DU145 cells
following CX3CL1 treatment alone or combined with AG1478
pretreatment. The western blot results demonstrated that CX3CL1
treatment led to a significant EMT change as indicated by
E-cadherin and vimentin protein levels, whereas AG1478 pretreatment
inhibited CX3CL1-induced downregulation of E-cadherin and
upregulation of vimentin (Fig. 4A and
C). In order to determine whether Slug is associated with the
changes in the protein levels of E-cadherin and vimentin, DU145
cells were transfected with Slug-specific siRNA or non-specific
siRNA, and western blotting was performed after 24 h of the
transfections. As shown in Fig. 4B and
D, Slug protein expression level was greatly reduced in the
Slug-siRNA-transfected cells, compared with scrambled-siRNA
transfected cells. Meanwhile, transfection with Slug-siRNA
attenuated the regulatory effect of CX3CL1 on E-cadherin and
vimentin protein expression, which indicated that Slug was involved
in CX3CL-induced EMT. Taken together, we provided evidence that
CX3CL caused EGFR pathway activation and subsequent Slug
expression, which led to the EMT process.
TACE/TGF-α is responsible for
CX3CL1-induced EGFR activation and EMT in hypoxic DU145 cells
EGFR is activated by binding of its specific
ligands. TGF-α is one of the key ligands for EGFR. Thus, we
hypothesized that CX3CL1 may activate EGFR through increased
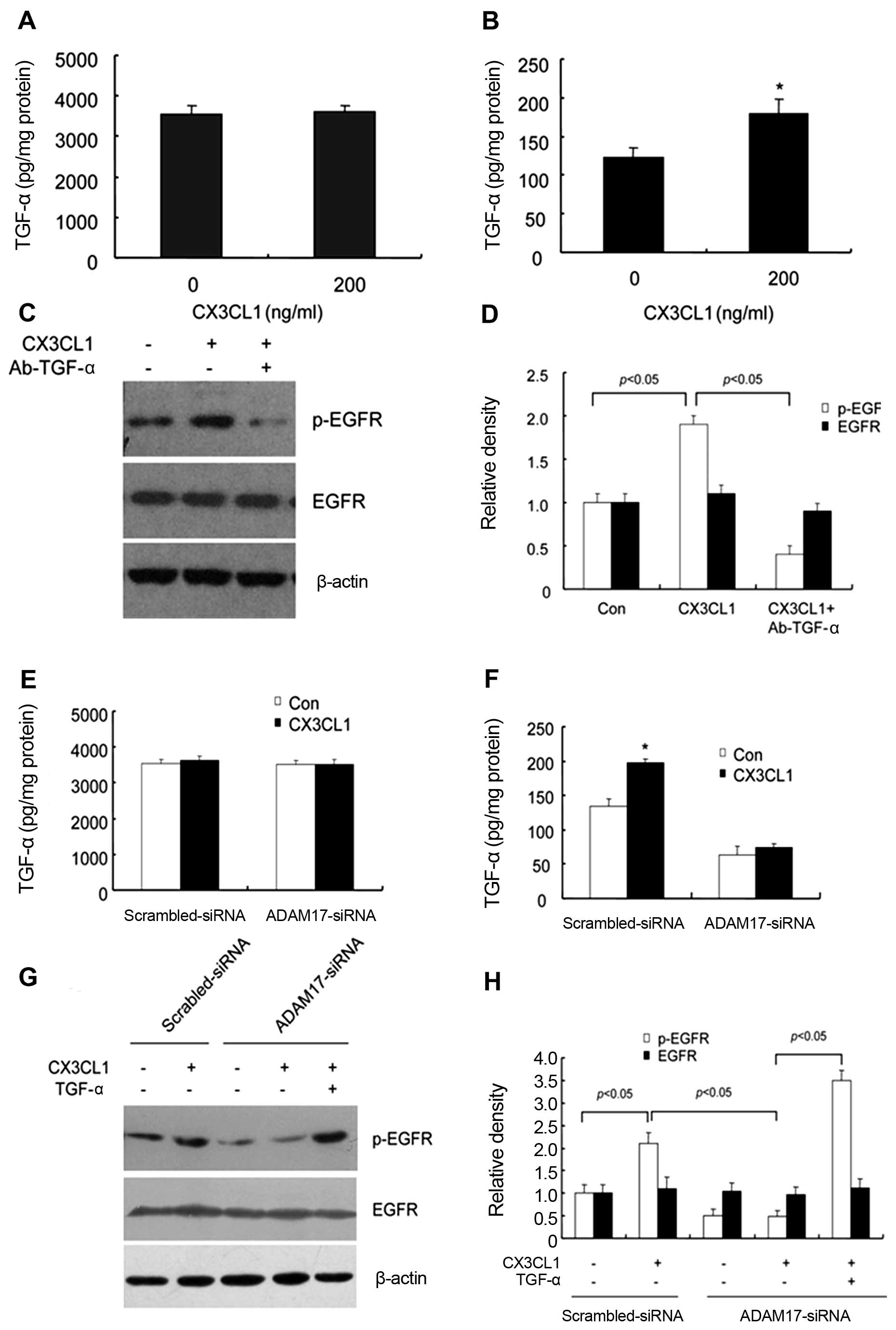
shedding of pro-TGF-α. After CX3CL1 treatment for 20 min, the
concentrations of soluble and total TGF-α in culture medium were
detected by ELISA. Our results demonstrated that the concentration
of soluble TGF-α significantly increased but that of total TGF-α
remained unchanged upon CX3CL1 stimulation (Fig. 5A and B). To further investigate the
role of TGF-α in CX3CL-induced EGFR activation, DU145 cells were
pretreated with a neutralizing antibody of TGF-α (Ab-TGF-α) to
inhibit TGF-α activity. The western blot analysis showed that
CX3CL1-induced EGFR activation was significantly inhibited by
Ab-TGF-α (Fig. 5C and D),
indicating that TGF-α activity is essential to CX3CL1-induced EGFR
activation in DU145 cells.
Tumor necrosis factor-α converting enzyme (TACE or
ADAM17) has been found to be a metalloprotease that cleaves
membrane-bound TGF-α, resulting in release of TGF-α ectodomains and
transactivation of EGFR (24). Our
previous study reported that TACE/ADAM17 activity is essential for
TGF-α maturation in prostate cancer cells. DU145 cells were
transfected with specific siRNA for ADAM17 (verified in our
previous studies) or Scrambled-siRNA, prior to treatment with
CX3CL1. ELISA analysis revealed that the content of soluble TGF-α
in culture supernatants was markedly decreased in the hypoxic DU145
cells pre-transfected with ADAM17-siRNA, but not Scrambled-siRNA
(Fig. 5E and F), which suggest that
downregulation of ADAM17 attenuated CX3CL1-induced increase in
TGF-α activity. These results indicate that ADAM17 is responsible
for CX3CL1-induced TGF-α secretion.
To verify the role of TACE/TGF-α in CX3CL1-induced
EGFR activation, DU145 cells were pre-transfected with ADAM17-siRNA
or Scrambled-siRNA, followed by treatment with CX3CL1 for 24 h.
Western blot analysis showed that CX3CL1-induced p-EGFR expression
was inhibited by ADAM17-siRNA pre-transfection. Moreover, exogenous
supplement of TGF-α compensated the inhibitory effect of ADAM17
downregulation (Fig. 5G and H).
Taken together, these data suggest that TACE/TGF-α was responsible
for CX3CL1-induced EGFR activation in hypoxic DU145 cells.
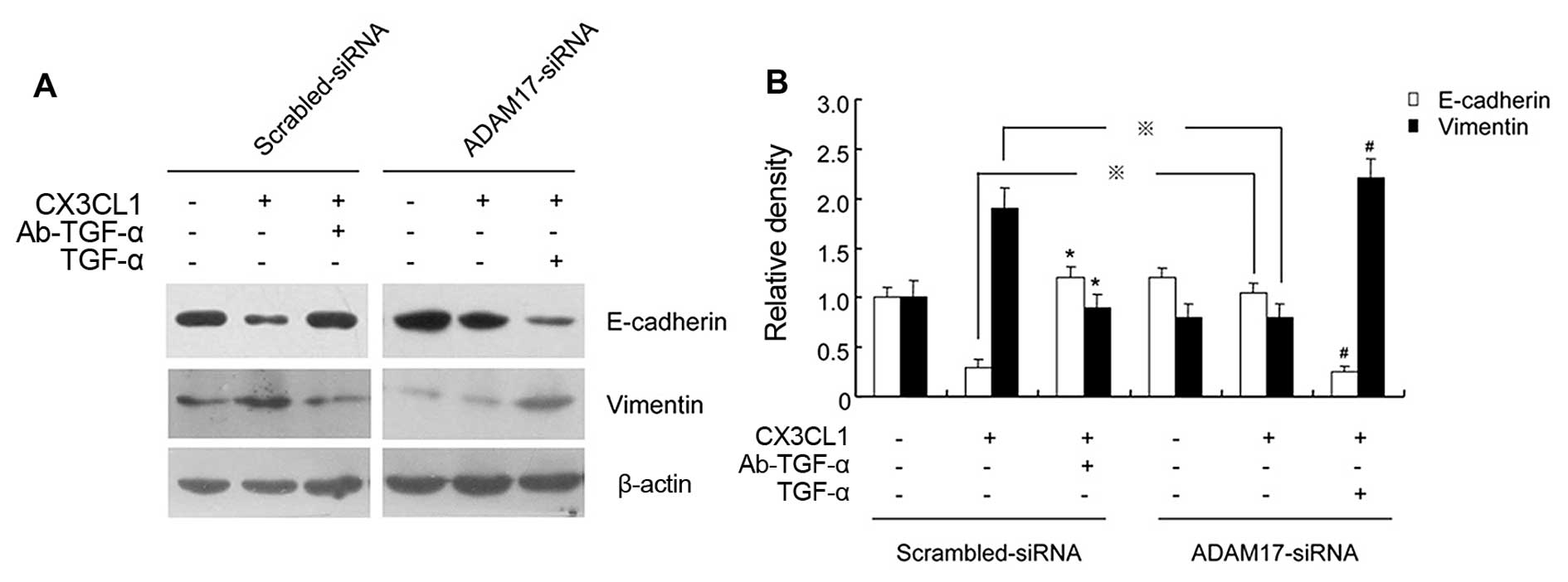
Finally, we investigated the role of TACE/TGF-α in
EMT marker protein expression regulated by CX3CL1. Western blot
results showed that there was decreased expression of E-cadherin
but elevated expression of vimentin in DU145 cells upon CX3CL1
stimulation compared with the control group, whereas either
ADAM17-siRNA pre-transfection or Ab-TGF-α pretreatment abrogated
the regulatory effect of CX3CL1 on EMT marker protein expression.
In addition, exogenous supplement of TGF-α compensated the
inhibitory effect of ADAM17 or TGF-α blockade on expression levels
of EMT markers regulated by CX3CL1 (Fig. 6). These data demonstrated that the
TACE/TGF-α signaling pathway was involved in CX3CL1-induced EMT of
DU145 cells.
CX3CL1-mediated TACE/TGF-α/EGFR signaling
pathway contributes to migration and invasion of DU145 cells
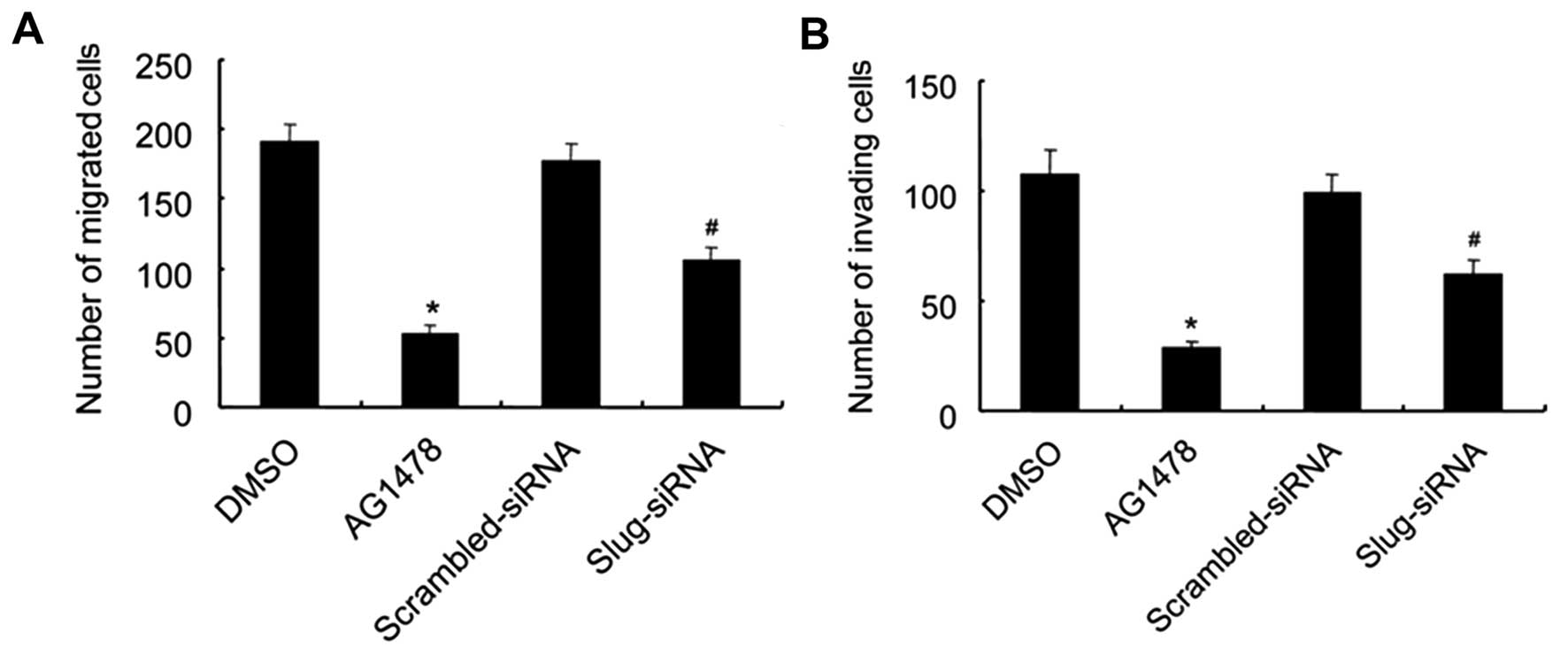
Firstly, to investigate the role of EGFR and Slug in
the enhancement of migration and invasive abilities of DU145 cells
induced by CX3CL1, Matrigel invasion and migration assays were
carried out in DU145 cells after treatment with CX3CL1 alone or
combined with pretreatment with AG1478 or Slug-siRNA transfection.
As shown in Fig. 7, CX3CL1
treatment markedly enhanced the abilities of DU145 cells to cross
the basement membrane matrix. Moreover, inhibition of either EGFR
or Slug significantly repressed the migration and invasive
abilities of the CX3CL1-treated DU145 cells, indicating that the
EGFR-dependent Slug pathway was involved in CX3CL1-induced
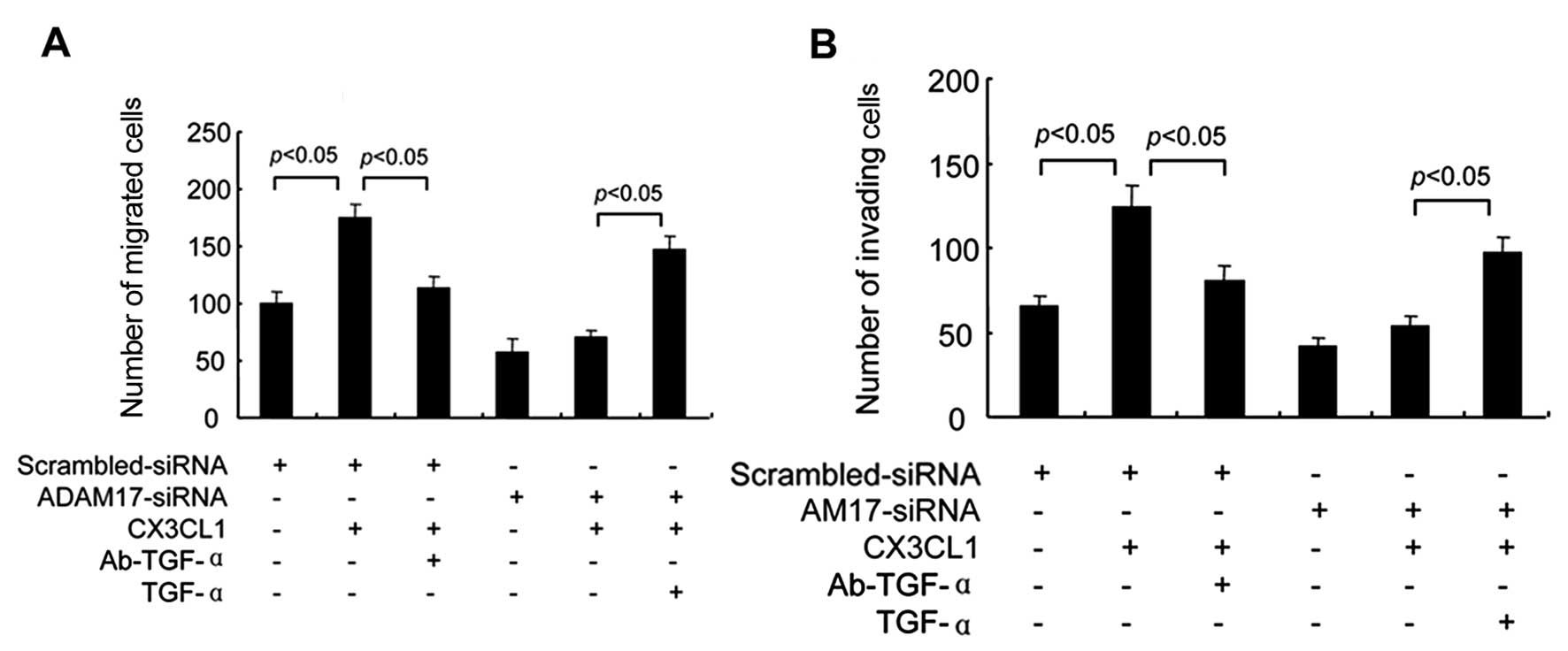
migration and invasion of DU145 cells. Subsequently, we determined
the role of TACE/TGF-α in cell migration and invasion induced by
CX3CL1. Consistent with the alterations of EMT mentioned above,
Matrigel invasion and migration assays demonstrated that CX3CL1
treatment alone increased cell migration and invasion, whereas
either ADAM17-siRNA pre-transfection or Ab-TGF-α pretreatment
abrogated the regulatory effects of CX3CL1 on cell migration and
invasion. Meanwhile, exogenous supplement of TGF-α compensated the
inhibitory effect of ADAM17 down-regulation (Fig. 8). These results suggest that
TACE/GF-α was essential for the regulatory effects of CX3CL1 on
cell migration and invasion.
Discussion
Epithelial-to-mesenchymal transition (EMT), first
described by developmental biologists, is a critical process during
embryonic development and organogenesis (2–6,8,10,12–14,17–25).
Recently, EMT is increasingly considered to play a vital role in
tumor invasion and metastasis, and is closely correlated with tumor
recurrence. Accumulating evidence indicates that EMT could drive
many phenotypic and functional alterations that endow tumor cells
with the ability to mobilize via a complex signaling pathway
(26). Therefore, regulation of EMT
could be an effective strategy to control cancer progression.
CX3CL1, the only member recognized so far that
belongs to the CX3C chemokine subfamily, was reported to
participate in the molecular events that regulate cell adhesion,
migration and survival of human prostate cancer cells (27). In addition, CX3CL1-CX3CR1 binding
was also found to play a crucial role in prostate cancer
progression and skeletal metastasis (17). However, whether CX3CL1 exerts its
function by initiating the EMT process in prostate cancer remains
unknown. In the present study, we found that CX3CL1 induced
migration and invasion of androgen-independent prostate cancer
cells under hypoxic condition in vitro. We hypothesized that
CX3CL1-induced migration and invasion of prostate cancer cells
resulted from EMT initiation. Our results showed that under hypoxic
conditions, notably morphological changes of DU145 and PC-3 cells
were observed after CX3CL1 stimulation for 48 h. Most untreated
cells exhibited classical round with tight junctions, and polygon
cobblestone-like with typical epithelial cell morphology. However,
upon CX3CL1 stimulation, most treated cells became spindle-like
with loose connections instead of epithelial-like with tight
connections, which morphologically indicated that CX3CL1 induced
EMT in DU145 and PC-3 cells. Subsequently, we detected changes in
the expression levels of EMT marker proteins in the DU145 and PC-3
cells to further verify whether these morphological changes
resulted from functional alterations. Our results revealed that
CX3CL1 reduced the expression of epithelial cell marker,
E-cadherin, whereas expression of mesenchymal cell marker,
vimentin, was significantly elevated. These results imply that
CX3CL1 induced an EMT-like phenotype in androgen-independent
prostate cancer cells.
EMT is a complicated pathological process, involving
complex network regulation. A variety of molecules regulate EMT.
The epidermal growth factor receptor (EGFR) family belongs to the
tyrosine kinase receptor family, which is widely involved in many
physiological or pathological processes such as cell proliferation
and differentiation, embryonic development, apoptosis and
metastasis (28,29). Our study provides initial evidence
that CX3CL1 exposure resulted in EGFR transactivation and
subsequent Slug expression, which led to the EMT process. G
protein-coupled receptor (GPCR) is an important cell membrane
receptor, which could be activated by a variety of extracellular
physical and chemical stimuli, such as neurotransmitters,
chemokines, hormones and drugs. These signals play vital roles in
regulating cellular functions (30). Currently, it is generally accepted
that EGFRs act as an important conduit for multiple GPCR-related
stimuli. Evidence suggests that the EGFR and GPCR pathways usually
overlap and interact in cell growth and tissue remodeling (31). CX3CR is the highly selectively
chemokine receptor for CX3CL1, belonging to the GPCR family.
Commonly, CX3CL1 exerts its functions though binding with its
receptor CX3CR1. Thus, we speculated that CX3CL1/CX3CR1 binding
activates EGFR and its downstream signaling pathway.
The matrix metalloprotease (MMP or ADAM) family are
surface membrane-associated proteins responsible for the cleavage
of several membrane proteins such as TGF-α, one of the ligands for
the EGFR (32). This biological
cell process is called ectodomain shedding. TACE/ADAM17, one of the
ADAM family members, is upregulated in several types of cancers and
correlates with tumor aggressiveness. The crosstalk between
TACE/ADAM17 and EGFR regulates cell proliferation, migration and
invasion and survival (30). In the
present study, we evaluated the requirement for TACE-dependent EGFR
ligand shedding for CX3CL-1 induced EGFR transactivation and EMT.
Our results showed that CX3CL1 significantly increased the
concentration of soluble TGF-α in culture medium, suggesting that
CX3CL1 resulted in release of TGF-α ectodomains. In addition,
inhibition of TGF-α or downregulation of ADAM17 depressed
CX3CL1-induced EGFR transactivation. Our data demonstrated that
TACE/TGF-α was responsible for CX3CL1-induced EGFR activation in
hypoxic DU145 cells. Meanwhile, our results showed that the
TACE/TGF-α signaling pathway was also responsible for
CX3CL1-induced EMT-related gene expression, such as E-cadherin and
vimentin. As expected, final Matrigel invasion and migration assays
demonstrated that TACE/TGF-α were essential for the regulatory
effects of CX3CL1 on cell migration and invasion.
In conclusion, CX3CL1/CX3CR1 induces EMT and
migration and invasion of androgen-independent prostate cancer
cells through TACE/TGF-α/EGFR pathway activation. These findings
revealed that CX3CL1 may serve as a new target by which to treat
prostate cancer.
Acknowledgments
The present study was supported by grants from the
National Natural Science Foundation of China (no. 30973010), the
Research Foundation of the Department of Health of Heilongjiang
Province (2012-669) and the Startup Fund of The Affiliated Third
Hospital of Harbin Medical University (JJ2011-12).
References
|
1
|
Siegel R, Ma J, Zou Z and Jemal A: Cancer
statistics, 2014. CA Cancer J Clin. 64:9–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gittes RF: Carcinoma of the prostate. N
Engl J Med. 324:236–245. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tannock IF, de Wit R, Berry WR, Horti J,
Pluzanska A, Chi KN, Oudard S, Théodore C, James ND, Turesson I, et
al: TAX 327 Investigators: Docetaxel plus prednisone or
mitoxantrone plus prednisone for advanced prostate cancer. N Engl J
Med. 351:1502–1512. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sulzmaier FJ and Ramos JW: RSK isoforms in
cancer cell invasion and metastasis. Cancer Res. 73:6099–6105.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Thiery JP, Acloque H, Huang RY and Nieto
MA: Epithelial-mesenchymal transitions in development and disease.
Cell. 139:871–890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liu YN, Yin JJ, Abou-Kheir W, Hynes PG,
Casey OM, Fang L, Yi M, Stephens RM, Seng V, Sheppard-Tillman H, et
al: miR-1 and miR-200 inhibit EMT via Slug-dependent and
tumorigenesis via Slug-independent mechanisms. Oncogene.
32:296–306. 2013. View Article : Google Scholar
|
|
7
|
Hotz B, Arndt M, Dullat S, Bhargava S,
Buhr HJ and Hotz HG: Epithelial to mesenchymal transition:
Expression of the regulators snail, slug, and twist in pancreatic
cancer. Clin Cancer Res. 13:4769–4776. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ding G, Feng C, Jiang H, Ding Q, Zhang L,
Na R, Xu H and Liu J: Combination of rapamycin, CI-1040, and 17-AAG
inhibits metastatic capacity of prostate cancer via Slug
inhibition. PLoS One. 8:e774002013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kwok WK, Ling MT, Lee TW, Lau TC, Zhou C,
Zhang X, Chua CW, Chan KW, Chan FL, Glackin C, et al: Up-regulation
of TWIST in prostate cancer and its implication as a therapeutic
target. Cancer Res. 65:5153–5162. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Whiteland H, Spencer-Harty S, Thomas DH,
Davies C, Morgan C, Kynaston H, Bose P, Fenn N, Lewis PD, Bodger O,
et al: Putative prognostic epithelial-to-mesenchymal transition
biomarkers for aggressive prostate cancer. Exp Mol Pathol.
95:220–226. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Umbas R, Isaacs WB, Bringuier PP,
Schaafsma HE, Karthaus HF, Oosterhof GO, Debruyne FM and Schalken
JA: Decreased E-cadherin expression is associated with poor
prognosis in patients with prostate cancer. Cancer Res.
54:3929–3933. 1994.PubMed/NCBI
|
|
12
|
Bertran E, Crosas-Molist E, Sancho P, Caja
L, Lopez-Luque J, Navarro E, Egea G, Lastra R, Serrano T, Ramos E,
et al: Overactivation of the TGF-β pathway confers a
mesenchymal-like phenotype and CXCR4-dependent migratory properties
to liver tumor cells. Hepatology. 58:2032–2044. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Izumi K, Fang LY, Mizokami A, Namiki M, Li
L, Lin WJ and Chang C: Targeting the androgen receptor with siRNA
promotes prostate cancer metastasis through enhanced macrophage
recruitment via CCL2/CCR2-induced STAT3 activation. EMBO Mol Med.
5:1383–1401. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Blum DL, Koyama T, M'Koma AE, Iturregui
JM, Martinez-Ferrer M, Uwamariya C, Smith JA Jr, Clark PE and
Bhowmick NA: Chemokine markers predict biochemical recurrence of
prostate cancer following prostatectomy. Clin Cancer Res.
14:7790–7797. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xu X, Wang Y, Chen J, Ma H, Shao Z, Chen H
and Jin G: High expression of CX3CL1/CX3CR1 axis predicts a poor
prognosis of pancreatic ductal adenocarcinoma. J Gastrointest Surg.
16:1493–1498. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gaudin F, Nasreddine S, Donnadieu AC,
Emilie D, Combadière C, Prévot S, Machelon V and Balabanian K:
Identification of the chemokine CX3CL1 as a new regulator of
malignant cell proliferation in epithelial ovarian cancer. PLoS
One. 6:e215462011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jamieson WL, Shimizu S, D'Ambrosio JA,
Meucci O and Fatatis A: CX3CR1 is expressed by prostate epithelial
cells and androgens regulate the levels of CX3CL1/fractalkine in
the bone marrow: Potential role in prostate cancer bone tropism.
Cancer Res. 68:1715–1722. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xiao LJ, Chen YY, Lin P, Zou HF, Lin F,
Zhao LN, Li D, Guo L, Tang JB, Zheng XL, et al: Hypoxia increases
CX3CR1 expression via HIF-1 and NF-κB in androgen-independent
prostate cancer cells. Int J Oncol. 41:1827–1836. 2012.PubMed/NCBI
|
|
19
|
Yuan WC, Lee YR, Huang SF, Lin YM, Chen
TY, Chung HC, Tsai CH, Chen HY, Chiang CT, Lai CK, et al: A
cullin3-KLHL20 ubiquitin ligase-dependent pathway targets PML to
potentiate HIF-1 signaling and prostate cancer progression. Cancer
Cell. 20:214–228. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Philip B, Ito K, Moreno-Sánchez R and
Ralph SJ: HIF expression and the role of hypoxic microenvironments
within primary tumours as protective sites driving cancer stem cell
renewal and metastatic progression. Carcinogenesis. 34:1699–1707.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Traish AM and Morgentaler A: Epidermal
growth factor receptor expression escapes androgen regulation in
prostate cancer: A potential molecular switch for tumour growth. Br
J Cancer. 101:1949–1956. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Antonarakis ES, Carducci MA and
Eisenberger MA: Novel targeted therapeutics for metastatic
castration-resistant prostate cancer. Cancer Lett. 291:1–13. 2010.
View Article : Google Scholar
|
|
23
|
Lo HW, Hsu SC, Xia W, Cao X, Shih JY, Wei
Y, Abbruzzese JL, Hortobagyi GN and Hung MC: Epidermal growth
factor receptor cooperates with signal transducer and activator of
transcription 3 to induce epithelial-mesenchymal transition in
cancer cells via up-regulation of TWIST gene expression. Cancer
Res. 67:9066–9076. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Maretzky T, McIlwain DR, Issuree PD, Li X,
Malapeira J, Amin S, Lang PA, Mak TW and Blobel CP: iRhom2 controls
the substrate selectivity of stimulated ADAM17-dependent ectodomain
shedding. Proc Natl Acad Sci USA. 110:11433–11438. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Floor S, van Staveren WC, Larsimont D,
Dumont JE and Maenhaut C: Cancer cells in epithelial-to-mesenchymal
transition and tumor-propagating-cancer stem cells: Distinct,
overlapping or same populations. Oncogene. 30:4609–4621. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tomaskovic-Crook E, Thompson EW and Thiery
JP: Epithelial to mesenchymal transition and breast cancer. Breast
Cancer Res. 11:2132009. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shulby SA, Dolloff NG, Stearns ME, Meucci
O and Fatatis A: CX3CR1-fractalkine expression regulates cellular
mechanisms involved in adhesion, migration, and survival of human
prostate cancer cells. Cancer Res. 64:4693–4698. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
DeHaan AM, Wolters NM, Keller ET and
Ignatoski KM: EGFR ligand switch in late stage prostate cancer
contributes to changes in cell signaling and bone remodeling.
Prostate. 69:528–537. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Shah RB, Ghosh D and Elder JT: Epidermal
growth factor receptor (ErbB1) expression in prostate cancer
progression: Correlation with androgen independence. Prostate.
66:1437–1444. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kenny PA: TACE: A new target in epidermal
growth factor receptor dependent tumors. Differentiation.
75:800–808. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gschwind A, Hart S, Fischer OM and Ullrich
A: TACE cleavage of proamphiregulin regulates GPCR-induced
proliferation and motility of cancer cells. EMBO J. 22:2411–2421.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Harris RC, Chung E and Coffey RJ: EGF
receptor ligands. Exp Cell Res. 284:2–13. 2003. View Article : Google Scholar : PubMed/NCBI
|