Introduction
Gold nanoparticles (Au-NPs) have attracted wide
attention in various biomedical applications and also have
appealing potential to positively impact the health care system
(1–3). Not only can Au-NPs be made as
scaffolds for more and more potent cancer drug delivery but they
can also act as intrinsic antineoplastic agents (4–6).
Arvizo et al (7) have
demonstrated that unmodified Au-NPs could inhibit the proliferation
of cancer cells by abrogating MAPK-signaling.
Although the application of Au-NPs have been studied
in detail, response of biological systems to the nanoparticles
remain to be elucidated. In addition, exploration of the
size-dependent physicochemical properties of the Au-NPs also
resulted in controversial conclusions. Arvizo et al
(8) showed that nanoparticle size
plays a very important role in the therapeutic effect. Small Au-NPs
(diameter <2 nm) could penetrate the cell nucleus and be highly
toxic (9). However, Connor et
al (10) demonstrated that
Au-NPs with a size of 18 nm could also be endocytosed by cells, but
showing no inherently toxicity to human cells. Due to the
contradictory results of the previous investigations, the effect of
Au-NPs on cells requires more study.
The most pivotal pathologic feature of cancer cells
is generally thought as invasion and metastasis (11). The process of cell invasion and
metastasis begins with cell proliferation, then dissociate from the
primary lesions and migrate in the blood or lymph stream leading to
adhesion in a secondary organ (12). Despite increased progression in
surgery, chemotherapy and radiotherapy, recurrence is almost
inescapable in case of an aggressive metastatic spread (13,14).
Previous studies have demonstrated that intercellular adhesion
molecule-1 (ICAM-1) and matrix metalloproteinase 9 (MMP9) play an
important role in cancer cell adhesion, invasion and migration
(15,16). These proteins have been used as
prognostic biomarkers for gastric cancer progression (17).
This study concentrated on effects of Au-NPs on cell
proliferation, invasion and protein expression. Besides, we chose
to investigate these effects on the human gastric cancer cell line
(SGC-7901), which is a high epidemic tumor in China (18). Moreover, to explore the value of
particle size in biomedical application, four different sizes (5,
10, 20 and 40 nm) were chosen for detailed analysis of this system.
Therefore, the goal of the present study was to explore the effect
of Au-NPs on proliferation and invasion in SGC-7901 cells, which
may promote the application of Au-NPs in gastric cancer
therapy.
Materials and methods
Syntheses of Au-NPs
Syntheses of 5-nm and 10-nm Au-NPs were conducted
using a reducing agent (tannic acid) and a stabilizing agent
(citrate) (19). For each
synthesis, two original solutions were prepared: a) 1 ml of 1%
(w/v) HAuCl4 solution added with 79 ml of water; and b)
a mixed solution consisting of 4 ml of 1% (w/v) citrate solution,
0.7 ml or 0.1 ml of 1% tannic acid and water (20 ml in total). The
solutions were heated to 60°C and then solution b was poured to
solution a with constant stirring. The finished Au-NPs were cooled
to room temperature (RT) before use.
Syntheses of 20- and 40-nm Au-NPs were made by the
classic citrate reduction method (20). For each synthesis, 100 ml of 0.01%
HAuCl4 solution was heated to boiling. Citrate solution
(1%) (4.5 or 1.0 ml) were added and then heated to boiling until
the color changed. The solution was cooled to RT for subsequent
experiments.
Transmission electron microscopy (TEM; JEM-2100EX;
JEOL, Tokyo, Japan) was used to determine the morphology of Au-NPs.
Ultraviolet-visible (UV-Vis) spectra was obtained by a
spectrophotometer (300–1100 nm; Shimadzu Corp., Kyoto, Japan).
Cell culture
The human gastric cancer cell line, SGC-7901 cells
(Chinese Academy of Sciences), were cultured in Dulbecco's modified
eagle's medium (DMEM) with fetal bovine serum (FBS; Invitrogen,
Carlsbad, CA, USA) at 37°C with 5% CO2. Cells were
co-cultured with Au-NPs solution (50 μg/ml) for 24 h. Cells
at the logarithmic growth phase were collected by EDTA (Invitrogen)
detachment for other uses.
Uptake and TEM studies
The uptake of Au-NPs by cells was observed by TEM.
Before exposure to the Au-NPs, the SGC-7901 cells with a
concentration of 1×106 cells/dish (100-mm; Corning
Incorporated, Corning, NY, USA) were incubated for 24 h. After
co-incubation with Au-NPs for 24 h, the cells were fixed for TEM
analysis. Firstly, cells were fixed in 1% osmium tetroxide for 2 h,
and then cells were dehydrated with ethanol of increasing
concentration for 15 min each. The cells were then embedded at 80°C
by resin in propylene oxide polymerise. Lastly, the samples in
ultrathin sections were analyzed by TEM.
Cell viability assay
SGC-7901 cells, seeded in 96-well plates (2,000
cells/well) overnight, were treated with different Au-NPs. After 24
h, each well was added with the reagent (Cell Counting kit-8;
Nanjing KeyGen Biotech. Inc., Jiangsu, China) and co-incubated for
1, 2, 3 or 4 h. A microculture plate reader (Bio-Rad laboratories,
Hercules, CA, USA) was used to test the absorbance at 450 nm and
the cell viability was calculated as a percentage of the control.
This assay was conducted in triplicate and the experiment was
repeated thrice.
Invasion assay
For this assay, an insert with the pore size of 8.0
μm, pre-coated with Matrigel (BD Biosciences, San Jose, CA,
USA), was put on the culture plate. After treated with Au-NPs
solution for 24 h, the harvested cells (2.5×105 cells)
were seeded in the upper of the insert with 200 μl DMEM
containing 0.2% bovine serum albumin. Then, 750 μl DMEM
supplemented with 5% FBS was added to the bottom of the well for 24
h in the incubator.
The cells in the upper and bottom of the chamber
were fixed with 4% formaldehyde by replacing the culture medium.
After 15 min, the chambers were washed with PBS and stained with
0.1% crystal violet for 10 min. After washing the chambers five
times with dH2O, the cells left on the top of membrane
were removed by Q-tips. The cells that remained were those that had
invaded through the membrane.
The invasion cells were also quantified using the
QCM™ 24-well Cell Invasion Fluorometric assay (Millipore). This
assay provides an efficient system for quantitative detection of
cell invasion through a basement membrane model. SGC-7901 cells,
treated with or without Au-NPs, were cultured in complete medium
for 24 h. Then, cells were harvested and seeded (2.5×105
cells) in a plate chamber with 250 μl serum-free medium.
DMEM containing 10% FBS was used as chemoattractant to add to the
lower chamber. After 24 h, cells on the upper membrane were
removed. Next, the inserts were put into a new well added with cell
detachment solution at 37°C for half an hour. After removing the
inserts, the detached cells were split and stained with lysis
buffer/dye solution (Millipore) for 15 min. Lastly, relative
fluorescence unit (RFU) values of the mixtures were recorded using
a fluorescence plate reader (Synergy HT) at 480/520 nm.
Quantitative reverse transcription
polymerase chain reaction (qRT-PCR) assays
Total RNA was isolated from SGC-7901 cells in each
group using TRIzol reagent (Invitrogen-Life Technologies). Reverse
transcription into cDNA was conducted with 1 μg of total
RNA, and then qRT-PCR was performed with SYBR-Green Mix (ABI7300
Real-Time PCR system). The primer sequences were as follows:
glyceraldehyde 3-phosphate dehydrogenase (GAPDH), forward,
GGAGCCAAACGGGTCATCATCTC and reverse, GAGGGGCCATCCACAGTCTTCT; MMP9,
forward, GGCTACGTGACCTATGACATCCT and reverse,
TCCTCCCTTTCCTCCAGAACA; ICAM-1, forward, ACACTAGGCCACGCATCTGAT and
reverse, AGCATACCCAATAGGCAGCAA. PCR was performed at 95°C for 30
sec, at 60°C for 30 sec, and at 70°C for 60 sec for 35 cycles. The
relative abundance of mRNA was evaluated by comparative Ct method.
The experiment was conducted three times independently.
Protein quantitative assays
Protein expression of MMP9 and ICAM-1 in the cell
lysate was detected by an MMP/ICAM-1 Panel magnetic bead kit
(Luminex technology; EMD Millipore, Billerica, MA, USA). Firstly,
MMP9/ICAM-1 capture antibodies and detection antibodies were
combined with Luminex beads or biotin, respectively. MILLIPLEX MAP
lysis buffer and cell assay buffer (Millipore) were used to lyse
cells or dilute cells, respectively. The capture antibody beads,
diluted with 25 μl of cell assay buffer, were transferred to
a magnetic plate (96-wells; Millipore). Next, 25 μl of the
diluted cell lysate was added to the magnetic plate. After
incubation for 2 h at RT with shaking, beads were washed twice
using wash buffer. Then, 25 μl of detection antibodies was
added into the magnetic plate. After incubation for 1 h at RT with
shaking, 25 μl of MILLIPLEX MAP streptavidin-phycoerythrin
(Millipore) was supplemented for 30-min incubation at RT with
shaking. Lastly, the signal data was recorded using a Luminex
FLEXMAP 3D™.
Statistical analysis
All statistical analyses in the present study were
carried out with the GraphPad Prism software version 5.0 (GraphPad
Software, Inc., San Diego, CA, USA). The data are reported as mean
± standard deviation (SD). Statistical comparisons were performed
by one-way analysis of variance (ANOVA), and the Dunnett's t-test
was conducted for comparison with the control group. P-value
<0.05 was considered as significant statistical difference.
Results
Synthesis and characterization of
Au-NPs
Au-NPs without any further modification, referred to
as unmodified nanoparticles, were used in the present study. To
explore the size-dependent effect of the nanoparticles, Au-NPs were
synthesized in four sizes (5, 10, 20 and 40 nm) and characterized
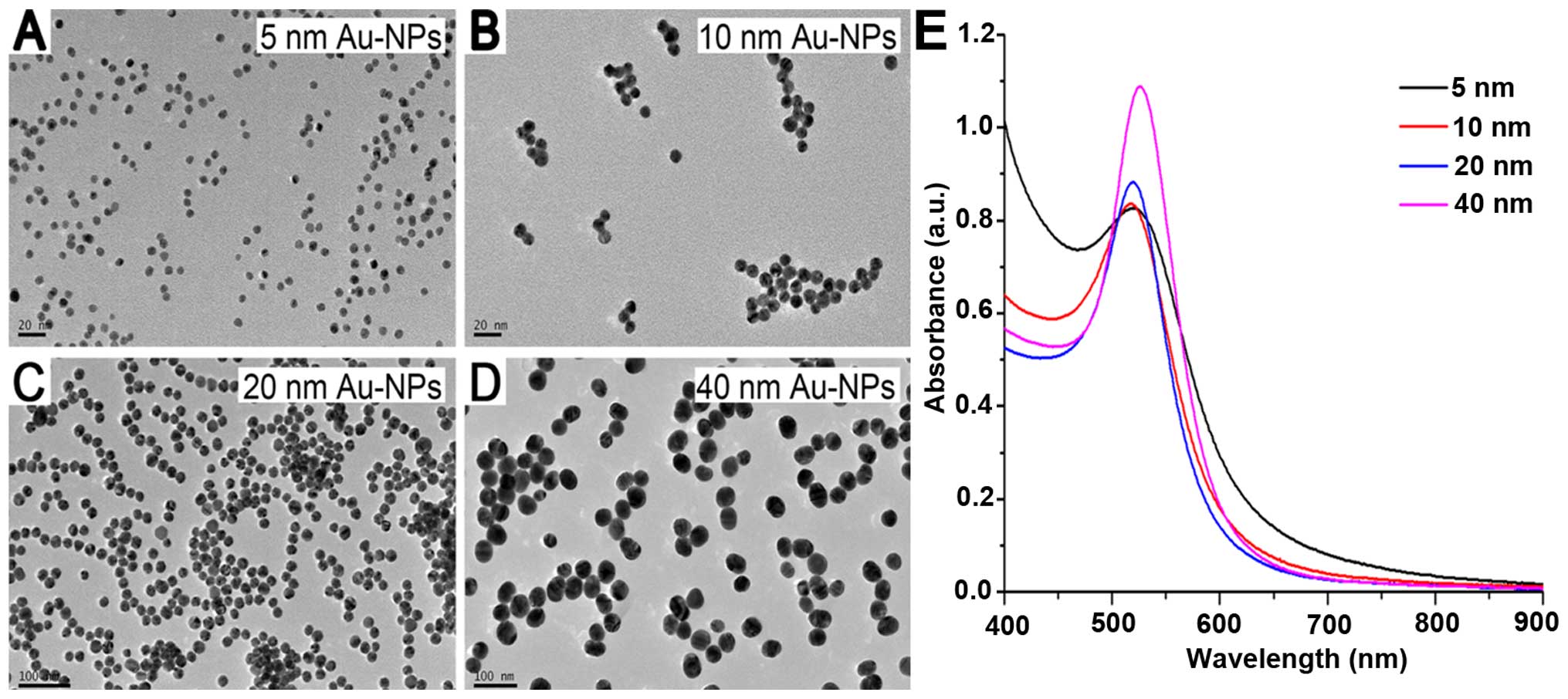
by TEM and UV-Vis spectra (Fig. 1).
The particles exhibited spherical shape and were quite uniform in
size in each group (Fig. 1A–D). All
samples presented a sharp and single absorption band (Fig. 1E).
Internalization of Au-NPs
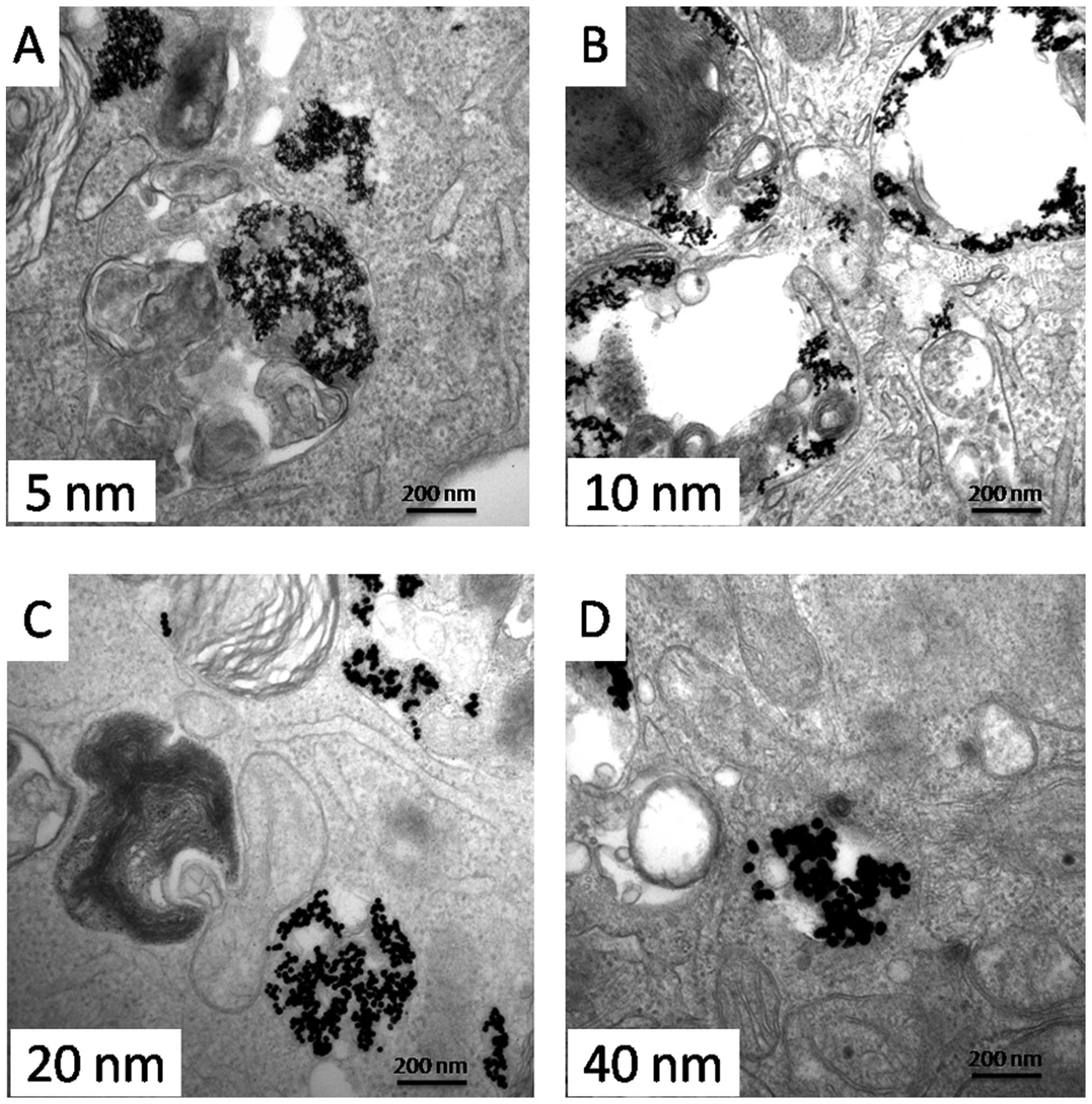
To establish whether Au-NPs entered the cells and
where they located, SGC-7901 cells were cultured in complete medium
containing Au-NPs (5, 10, 20 or 40 nm) for 24 h. Fig. 2 shows the internalization and
distribution of Au-NPs with various sizes in SGC-7901 cells. Most
of the particles appeared in vesicles or perinuclear region within
the cells.
Effect of Au-NPs on proliferation of
SGC-7901 cells
Then, we sought to investigate the effect of these
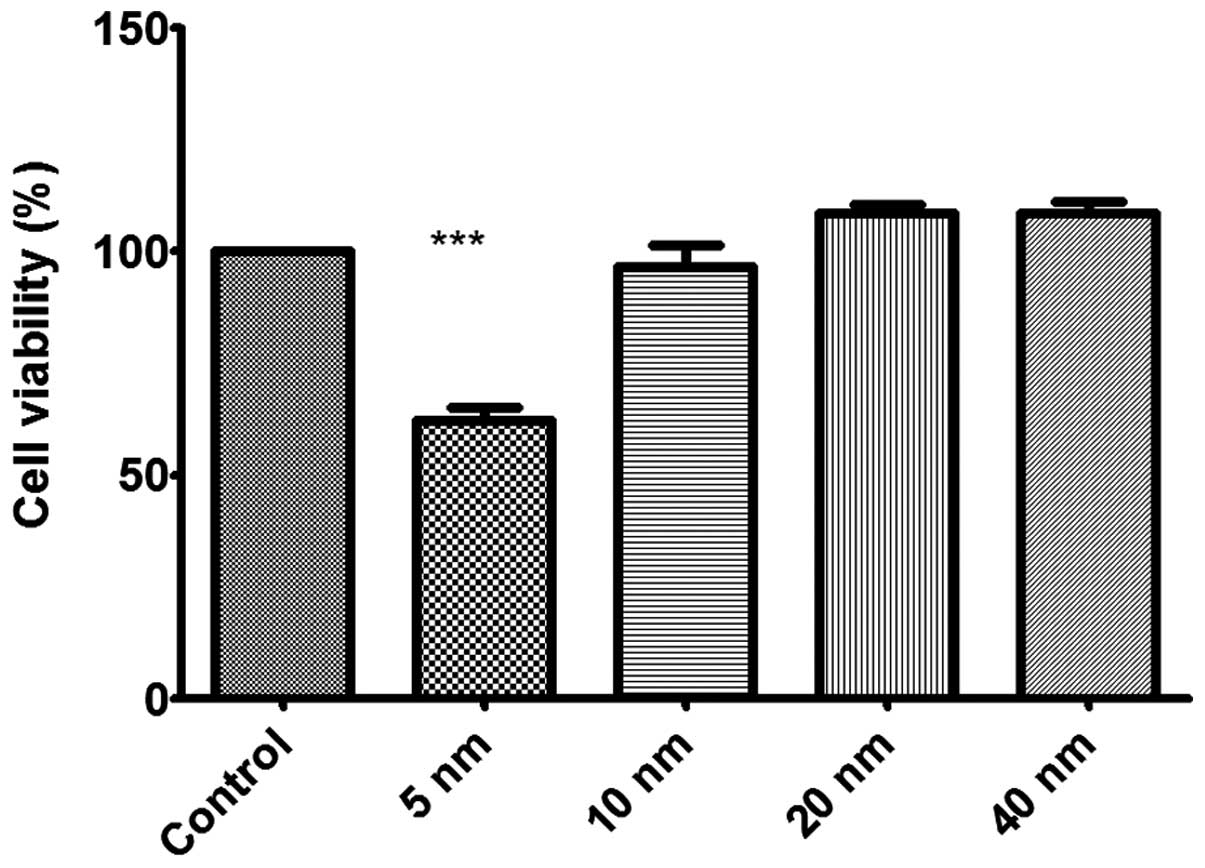
Au-NPs on cell viability in human gastric carcinoma SGC-7901 cells.
The results showed that only 5-nm Au-NPs exhibited obvious
inhibition on the proliferation of SGC-7901 cells (Fig. 3). Au-NPs 10, 20 and 40 nm in size
showed no significant effects on cell viability in SGC-7901
cells.
Invasion assay
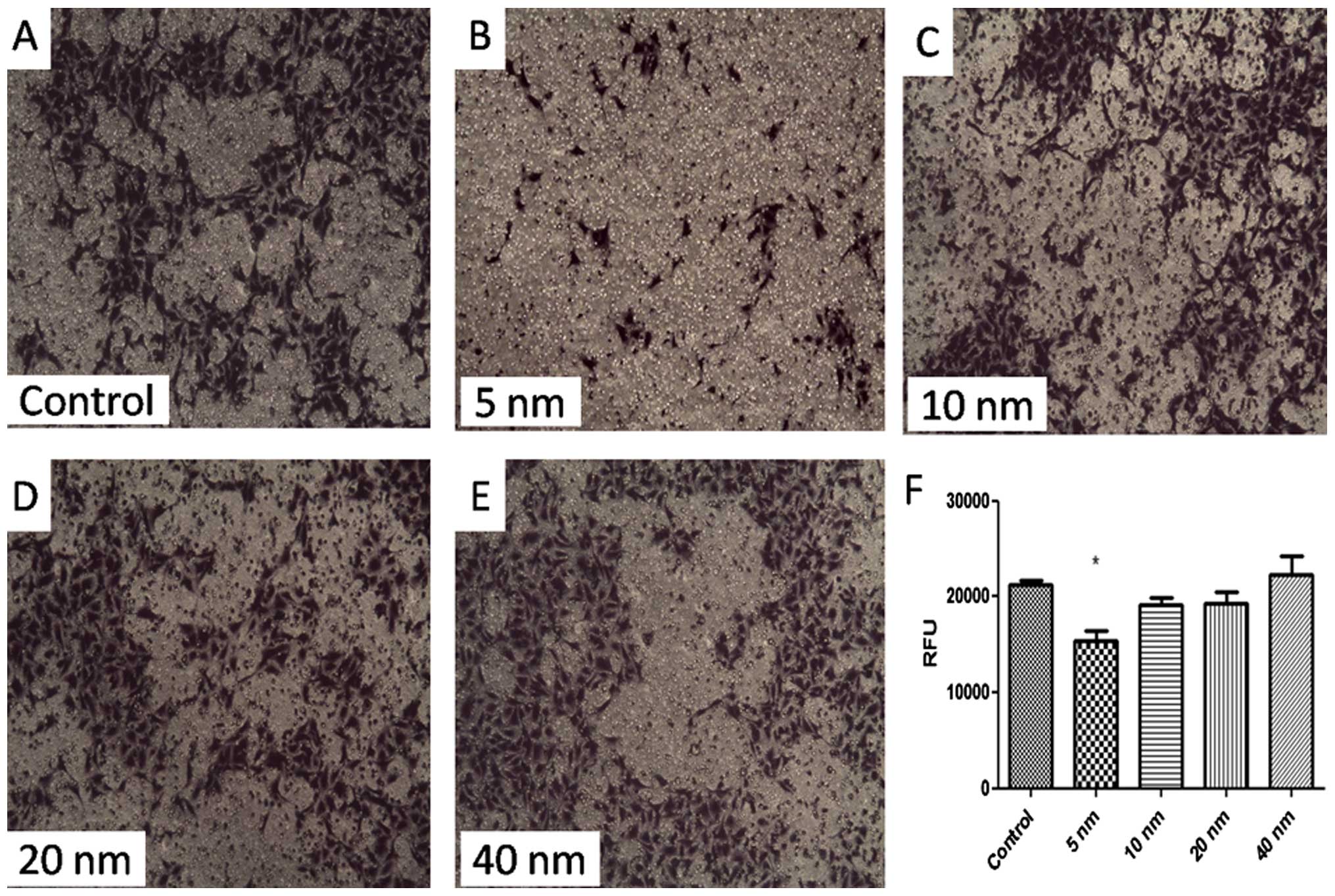
A classic membrane model was built for determining
the invasion ability of SGC-7901 cells. Fig. 4 shows that cell invasion was
suppressed significantly by 5-nm Au-NPs (P<0.05). While 10-, 20-
or 40-nm Au-NPs showed no significant influence on invasion ability
of SGC-7901 cells. These findings indicated that the effects of
Au-NPs on cell invasion might be size-dependent.
Effects of Au-NPs on the expression of
MMP9 and ICAM-1 in SGC-7901 cells
To obtain further understanding of the above
phenomenon, qRT-PCR was carried out to evaluate the mRNA expression
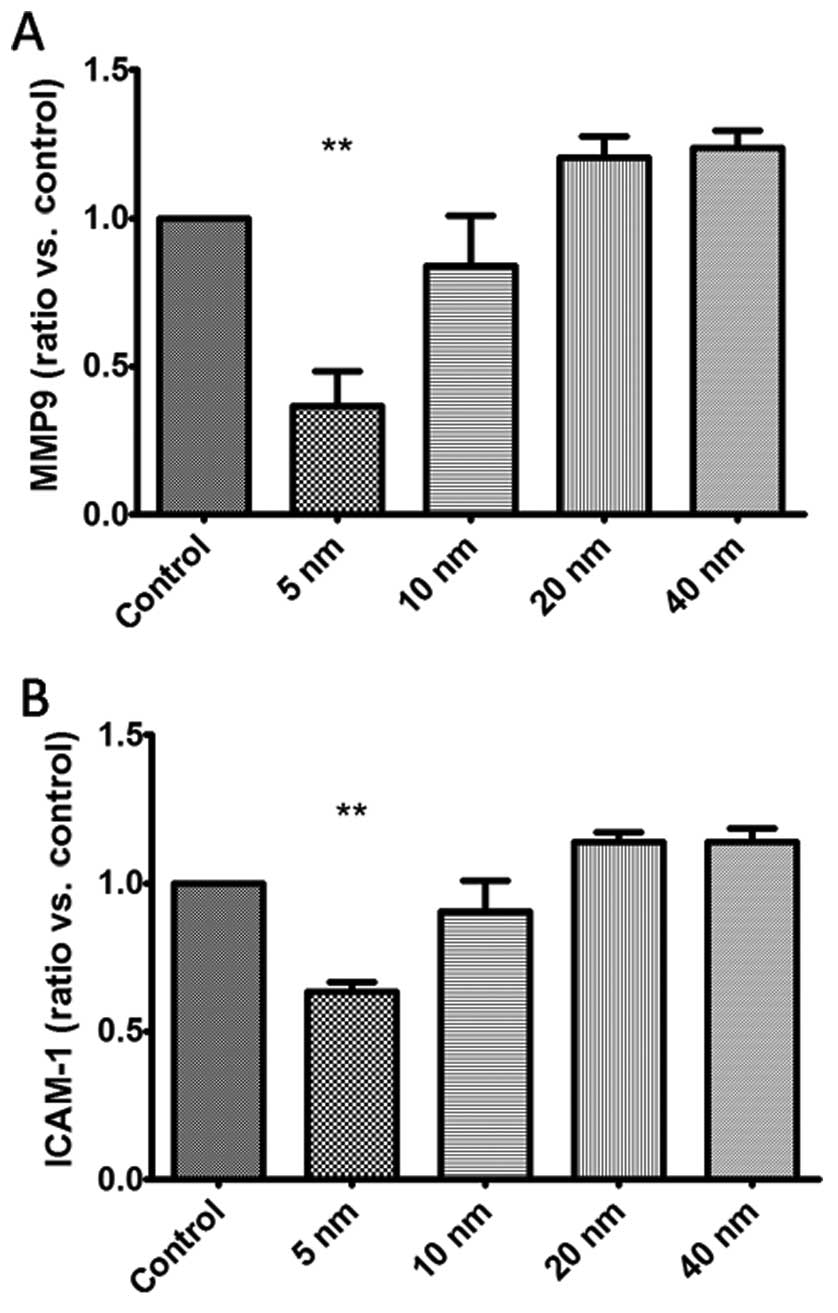
of MMP9 and ICAM-1 after the treatment of Au-NPs. Our findings
demonstrated that 5-nm Au-NPs notably reduced the mRNA expression
of MMP9 and ICAM-1 in SGC-7901 cells (Fig. 5), while no significant difference
were observed in 10-, 20- and 40-nm Au-NPs groups.
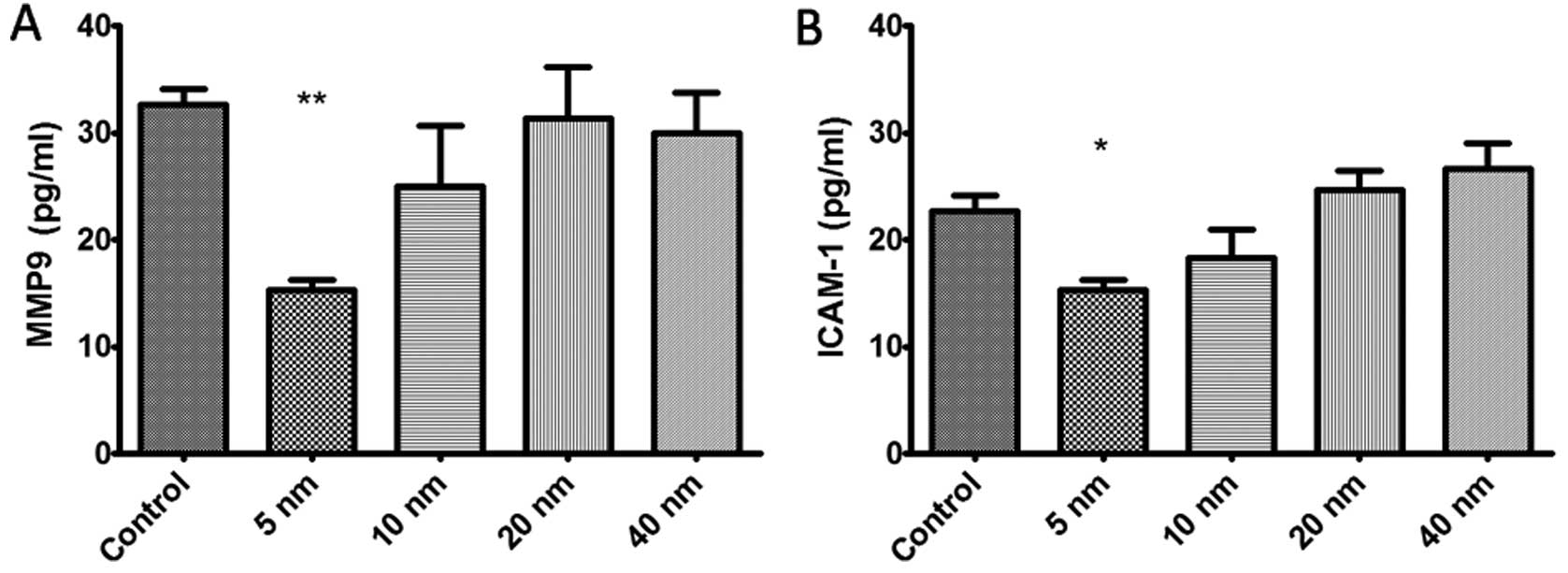
Furthermore, we conducted a Luminex-based experiment
using MMP9/ICAM-1 magnetic beads to quantify the protein expression
of MMP9 and ICAM-1 in SGC-7901 cells. Fig. 6 shows that treatment with 5-nm
Au-NPs obviously decreased the protein expression of MMP9 and
ICAM-1 in SGC-7901 cells (P<0.05), while no obvious change was
found in 10-, 20- and 40-nm Au-NPs groups.
Discussion
Nanotechnology is one of the most active research
fields in modern sciences. Nanotechnology has created a new world
in the development of nanomedicine such as diagnostic and
therapeutic applications. In this filed, Au-NPs become viable for
future biomedical applications because of the low production cost
and ease of synthesizing (21–23).
However, more information is required on the basal biological
interactions before Au-NPs transit from the laboratory to the
clinic. Before Au-NPs are applied to biological systems, it is
vital to demonstrate the biocompatibility of these nanoparticles.
Similar to many previous reports (24,25),
this study also found that Au-NPs were easily endocytosed by
SGC-7901 cells and localized in vesicles and the perinucleus.
We found that smaller Au-NPs (5 nm) inhibited cell
proliferation obviously in SGC-7901 cells. While, no obvious
cytotoxicity was found in 10-, 20- and 40-nm groups, which is in
line with some previous research. Arvizo et al (26) showed that surface size plays a vital
role in the biomedical effect of Au-NPs. While some other studies
came to different conclusions. Connor et al (10) reported that AuNPs in size of 4, 12
and 18 nm had no acute toxicity on K562 leukemia cells, and they
concluded the cytotoxicity came from cetyltrimethyl ammonium
bromide (CTAB) coating of AuNPs. Cui et al (27) even found the opposite results that
AuNPs promoted cell proliferation when AuNPs gathered on the cell
surface instead of within the cells. Furthermore, Patra et
al (28) demonstrated that
AuNPs did not universally target all cell types, which may explain
the controversy among the above studies.
At present, the mechanism on inhibition of cell
proliferation by AuNPs is not exact. Firstly, most research
considered that the generation of reactive oxygen species (ROS) was
the main reason that AuNPs caused cytotoxicity (29,30).
Secondly, AuNPs may cause cell morphological change and
cytoskeleton defects, leading to cell damage and inhibition of
proliferation (28). Besides, AuNPs
could also interfere in the expression level of
proliferation-related genes (31).
In the present study, the inhibited invasion ability
was associated by a remarkable downregulation of MMP9 and ICAM-1
expression. As known from tumor metastasis, tumor cells spread
along the blood vessel or lymph-vessel after invasion through the
ECM. Matrix metalloproteinase, a kind of endopeptidase, can degrade
ECM components, allowing tumor cells to access the blood vessel or
lymph-vessel (32,33). As an important matrix
metalloproteinase, MMP9 is able to degrade type IV collagen, which
is the basic component of the basement membrane. Some studies found
that increased expression of MMP9 in patients with gastric cancer
was correlated with a greater risk of advanced cancers (34,35);
therefore, drugs restraining the expression of the MMPs could
suppress tumor cell metastasis. Also, ICAM-1 is a pivotal adhesion
molecule affecting ECM (36). High
expression of ICAM-1 in human gastric cancer has been reported
(37). Rosette et al
(38) found that downregulation of
ICAM-1 mRNA or protein caused strong inhibition of human breast
cell invasion. In this study, 5-nm Au-NPs effectively suppressed
the expression of MMP9 and ICAM-1 in SGC-7901 cells, which might
explain the weakened effect of the nanoparticles on tumor cell
invasion. The downregulation effects of Au-NPs on MMP9 and ICAM-1
expression in SGC-7901 cells indicated that small nanoparticles
might possess the ability to suppress the invasion of gastric
cancer cells, while further studies in vivo are needed to
confirm the mechanisms.
To the best of our knowledge, this is the first
evidence for the effect of gold nanoparticles on gastric cancer
cell proliferation and invasion in vitro, which would make a
great contribution to the application of Au-NPs to novel therapies
in gastric cancer. Our research suggested that the biomedical
effects of unmodified Au-NPs depended largely on the particle size.
There are opportunities in developing Au-NPs to possess intrinsic
therapeutic potential for clinical application.
In conclusion, the present study provides new
evidence of unmodified Au-NPs of different sizes on the
proliferation and invasion in gastric cancer cells. Particle size
is an essential factor to their biomedical effects. Only 5-nm
Au-NPs inhibited proliferation and invasion in SGC-7901 cell, and
the decreased invasion activity may be attributed to the
downregulation of MMP9 and ICAM-1 expression. This study provides
useful information on the effects of Au-NPs on cell proliferation
and invasion, which could make a great contribution to the
application of Au-NPs to novel therapies in gastric cancer.
Acknowledgments
We thank all the participants in the present
study.
References
|
1
|
Arvizo RR, Bhattacharyya S, Kudgus RA,
Giri K, Bhattacharya R and Mukherjee P: Intrinsic therapeutic
applications of noble metal nanoparticles: Past, present and
future. Chem Soc Rev. 41:2943–2970. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dreaden EC, Alkilany AM, Huang X, Murphy
CJ and El-Sayed MA: The golden age: Gold nanoparticles for
biomedicine. Chem Soc Rev. 41:2740–2779. 2012. View Article : Google Scholar
|
|
3
|
Dykman LA and Khlebtsov NG: Gold
nanoparticles in biology and medicine: Recent advances and
prospects. Acta Naturae. 3:34–55. 2011.PubMed/NCBI
|
|
4
|
Huang X, Jain PK, El-Sayed IH and El-Sayed
MA: Gold nanoparticles: Interesting optical properties and recent
applications in cancer diagnostics and therapy. Nanomedicine
(Lond). 2:681–693. 2007. View Article : Google Scholar
|
|
5
|
Giljohann DA, Seferos DS, Daniel WL,
Massich MD, Patel PC and Mirkin CA: Gold nanoparticles for biology
and medicine. Angew Chem Int Ed Engl. 49:3280–3294. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dreaden EC, Mackey MA, Huang X, Kang B and
El-Sayed MA: Beating cancer in multiple ways using nanogold. Chem
Soc Rev. 40:3391–3404. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Arvizo RR, Saha S, Wang E, Robertson JD,
Bhattacharya R and Mukherjee P: Inhibition of tumor growth and
metastasis by a self-therapeutic nanoparticle. Proc Natl Acad Sci
USA. 110:6700–6705. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Arvizo RR, Rana S, Miranda OR,
Bhattacharya R, Rotello VM and Mukherjee P: Mechanism of
anti-angiogenic property of gold nanoparticles: Role of
nanoparticle size and surface charge. Nanomedicine. 7:580–587.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Alkilany AM and Murphy CJ: Toxicity and
cellular uptake of gold nanoparticles: What we have learned so far?
J Nanopart Res. 12:2313–2333. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Connor EE, Mwamuka J, Gole A, Murphy CJ
and Wyatt MD: Gold nanoparticles are taken up by human cells but do
not cause acute cytotoxicity. Small. 1:325–327. 2005. View Article : Google Scholar
|
|
11
|
Eccles SA, Box C and Court W: Cell
migration/invasion assays and their application in cancer drug
discovery. Biotechnol Annu Rev. 11:391–421. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ju D, Sun D, Xiu L, Meng X, Zhang C and
Wei P: Interleukin-8 is associated with adhesion, migration and
invasion in human gastric cancer SCG-7901 cells. Med Oncol.
29:91–99. 2012. View Article : Google Scholar
|
|
13
|
Busch J, Stephan C, Klutzny A, Hinz S,
Kempkensteffen C, Kilic E, Lein M, Weikert S, Miller K and Magheli
A: Impact of positive surgical margins on oncological outcome
following laparoscopic radical prostatectomy (LRP): long-term
results. World J Urol. 31:395–401. 2013. View Article : Google Scholar
|
|
14
|
Dassen AE, Dikken JL, van de Velde CJ,
Wouters MW, Bosscha K and Lemmens VE: Changes in treatment patterns
and their influence on long-term survival in patients with stages
I-III gastric cancer in The Netherlands. Int J Cancer.
133:1859–1866. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li Y, Tan BB, Zhao Q, Fan LQ, Wang D and
Liu Y: ZNF139 promotes tumor metastasis by increasing migration and
invasion in human gastric cancer cells. Neoplasma. 61:291–298.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Manu KA, Shanmugam MK, Ramachandran L, Li
F, Fong CW, Kumar AP, Tan P and Sethi G: First evidence that
γ-tocotrienol inhibits the growth of human gastric cancer and
chemosensitizes it to capecitabine in a xenograft mouse model
through the modulation of NF-κB pathway. Clin Cancer Res.
18:2220–2229. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen P, Zhao D, Sun Y, Huang L, Zhang S
and Yuan Y: Protein inhibitor of activated STAT-1 is downregulated
in gastric cancer tissue and involved in cell metastasis. Oncol
Rep. 28:2149–2155. 2012.PubMed/NCBI
|
|
18
|
Jiang Z, Guo J, Xiao B, Miao Y, Huang R,
Li D and Zhang Y: Increased expression of miR-421 in human gastric
carcinoma and its clinical association. J Gastroenterol. 45:17–23.
2010. View Article : Google Scholar
|
|
19
|
Slot JW and Geuze HJ: A new method of
preparing gold probes for multiple-labeling cytochemistry. Eur J
Cell Biol. 38:87–93. 1985.PubMed/NCBI
|
|
20
|
Frens G: Controlled nucleation for the
regulation of the particle size in monodisperse gold suspensions.
Nature. 241:20–22. 1973.
|
|
21
|
Khlebtsov N and Dykman L: Biodistribution
and toxicity of engineered gold nanoparticles: A review of in vitro
and in vivo studies. Chem Soc Rev. 40:1647–1671. 2011. View Article : Google Scholar
|
|
22
|
Mesbahi A: A review on gold nanoparticles
radiosensitization effect in radiation therapy of cancer. Rep Pract
Oncol Radiother. 15:176–180. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lévy R, Shaheen U, Cesbron Y and Sée V:
Gold nanoparticles delivery in mammalian live cells: A critical
review. Nano Rev. 1:12010. View Article : Google Scholar
|
|
24
|
Coulter JA, Jain S, Butterworth KT,
Taggart LE, Dickson GR, McMahon SJ, Hyland WB, Muir MF, Trainor C,
Hounsell AR, et al: Cell type-dependent uptake, localization, and
cytotoxicity of 1.9 nm gold nanoparticles. Int J Nanomed.
7:2673–2685. 2012. View Article : Google Scholar
|
|
25
|
Tsai SW, Liaw JW, Kao YC, Huang MY, Lee
CY, Rau LR, Huang CY, Wei KC and Ye TC: Internalized gold
nanoparticles do not affect the osteogenesis and apoptosis of MG63
osteoblast-like cells: A quantitative, in vitro study. PLoS One.
8:e765452013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Arvizo R, Bhattacharya R and Mukherjee P:
Gold nanoparticles: opportunities and challenges in nanomedicine.
Expert Opin Drug Deliv. 7:753–763. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cui W, Li J, Zhang Y, Rong H, Lu W and
Jiang L: Effects of aggregation and the surface properties of gold
nanoparticles on cytotoxicity and cell growth. Nanomedicine.
8:46–53. 2012. View Article : Google Scholar
|
|
28
|
Patra HK, Banerjee S, Chaudhuri U, Lahiri
P and Dasgupta AK: Cell selective response to gold nanoparticles.
Nanomedicine. 3:111–119. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Thakor AS, Paulmurugan R, Kempen P,
Zavaleta C, Sinclair R, Massoud TF and Gambhir SS: oxidative stress
mediates the effects of Raman-active gold nanoparticles in human
cells. Small. 7:126–136. 2011. View Article : Google Scholar
|
|
30
|
Pan Y, Leifert A, Ruau D, Neuss S,
Bornemann J, Schmid G, Brandau W, Simon U and Jahnen-Dechent W:
Gold nanoparticles of diameter 1.4 nm trigger necrosis by oxidative
stress and mitochondrial damage. Small. 5:2067–2076. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yang Y, Qu Y and Lü X: Global gene
expression analysis of the effects of gold nanoparticles on human
dermal fibroblasts. J Biomed Nanotechnol. 6:234–246. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hamano Y, Zeisberg M, Sugimoto H, Lively
JC, Maeshima Y, Yang C, Hynes RO, Werb Z, Sudhakar A and Kalluri R:
Physiological levels of tumstatin, a fragment of collagen IV alpha3
chain, are generated by MMP-9 proteolysis and suppress angiogenesis
via alphaV beta3 integrin. Cancer Cell. 3:589–601. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Egeblad M and Werb Z: New functions for
the matrix metal-loproteinases in cancer progression. Nat Rev
Cancer. 2:161–174. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
34
|
Fanelli MF, Chinen LT, Begnami MD, Costa
WL Jr, Fregnami JH, Soares FA and Montagnini AL: The influence of
transforming growth factor-α, cyclooxygenase-2, matrix
metalloproteinase (MMP)-7, MMP-9 and CXCR4 proteins involved in
epithelial-mesenchymal transition on overall survival of patients
with gastric cancer. Histopathology. 61:153–161. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Mroczko B, Groblewska M, Łukaszewicz-Zajac
M, Bandurski R, Kedra B and Szmitkowski M: Pre-treatment serum and
plasma levels of matrix metalloproteinase 9 (MMP-9) and tissue
inhibitor of matrix metalloproteinases 1 (TIMP-1) in gastric cancer
patients. Clin Chem Lab Med. 47:1133–1139. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Sunami T, Yashiro M and Chung KH: ICAM-1
(intercellular adhesion molecule-1) gene transfection inhibits
lymph node metastasis by human gastric cancer cells. Jpn J Cancer
Res. 91:925–933. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Rosette C, Roth RB, Oeth P, Braun A,
Kammerer S, Ekblom J and Denissenko MF: Role of ICAM1 in invasion
of human breast cancer cells. Carcinogenesis. 26:943–950. 2005.
View Article : Google Scholar : PubMed/NCBI
|