Introduction
Malignant glioma is a devastating neural neoplasm
with no effective treatment. Glioblastoma multiforme (GBM) is one
of the most common and aggressive malignant gliomas in adults and
is associated with an extremely short survival time (<15 months)
(1). Recent findings suggest that
radiotherapy plus concomitant and adjuvant chemotherapy suppress
the proliferation and tumorigenicity of human glioma cells and
represent the standard of care for newly diagnosed GBM patients
(1). However, GBM is difficult to
cure due to the fact that the tumor cells cannot be completely
removed surgically, and that metastatic GBM is rather resistant to
radiotherapy and chemotherapy (1).
This predicament reflects the poor understanding of the exact
molecular mechanisms involved in the onset and pathogenesis of GBM.
An important aspect of the pathogenesis of GBM lies in the
malignant transformation resulting from the accumulation of genetic
alterations and abnormal intracellular signaling pathways and
growth factors (2–5). Aberrant proliferation of glioma cells
is mediated by the combination of growth factors, including
vascular endothelial growth factor, brain-derived neurotrophic
factor, platelet-derived growth factor, hepatocyte growth factor
and transforming growth factor-β (TGF-β) (6,7). These
factors trigger downstream cascades of growth signaling pathways
such as mammalian target of rapamycin (mTOR) (8), ERK1/2 (9), PI3K/AKT (10) and JAK2/STAT3 (11). In addition, when GBM recurs, it
shows progression to a higher histologic grade with metastasis, a
more complex situation (2). Thus, a
further understanding of the mechanisms underlying the
tumorigenesis of GBM is urgently needed.
The sirtuins are a conserved family of proteins
possessing NAD-dependent deacetylase activity. Sirtuins are widely
expressed in different tissues including the brain (12), and there are seven members of the
sirtuin family in mammals (SIRT1-SIRT7) (13,14).
They are involved in promoting longevity, particularly longevity
associated with calorie restriction (14–16).
In addition, they act as cellular sensors to detect intracellular
energy availability and modulate diverse biological functions,
including lipid transport, insulin secretion, inflammation, oxidant
stress and exercise and hypoxia (15–20).
Importantly, they participate in tumorigenic processes such as
epithelial-mesenchymal transition (21). Currently, activators of sirtuins
have attracted much attention by eliciting multiple metabolic
benefits that protect against diet-induced obesity, type 2
diabetes, and non-alcoholic fatty liver disease (14). In addition, several sirtuins have
been reported to have roles in the central nerve system (CNS),
including neuroprotection (22),
neural differentiation (23) and
neurogenesis (24).
SIRT6 is a nuclear histone lysine deacetylase
(25). Similar to other sirtuins,
SIRT6 participates in many intracellular events such as TNF-α
secretion (26) and lipid transport
(27). Interestingly, SIRT6
promotes resistance to DNA damage and suppresses genomic
instability. Knockout of SIRT6 results in abnormalities that
include lymphopenia, fat loss, cyrtosis, metabolic defects and
eventually premature death (28).
This DNA-repair activity of SIRT6 suggests its critical role in
tumorigenesis. Indeed, Sebastian et al demonstrated that
loss of SIRT6 leads to tumor formation, increased glycolysis and
tumor growth (29). Moreover, loss
of SIRT6 induces epigenetic changes that are relevant to
hepatocellular carcinoma development in patients (30). These studies suggest that SIRT6 may
be a tumor suppressor. However, there are conflicting results
concerning this protein. High SIRT6 nuclear staining was found to
be significantly associated with poorer overall survival in breast
cancer (31). TGF-β-mediated
hepatocellular carcinoma tumorigenicity was reported to be promoted
by SIRT6 via suppression of cellular senescence (32). Thus, according to current
information, SIRT6 actually participates in tumor biology, while
the action of SIRT6 may be tumor type-dependent.
The role of SIRT6 in CNS tumors is still largely
unknown. In the present study, we investigated the possible
alteration of expression of SIRT6 in human GBM tissues. Moreover,
we investigated whether SIRT6 can affect GBM cell growth and if so,
to further study the underlying mechanisms.
Materials and methods
Reagents
Antibodies against phospho-JAK2, total-JAK2,
phospho-STAT3 and total-STAT3 were purchased from Cell Signaling
Biotechnology (Danvers, MA, USA). Antibodies against SIRT6, lamin
A/C, apoptosis-inducing factor (AIF) and tubulin were purchased
from Abcam (Cambridge, UK). Cell viability assay (MST-8) was
purchased from Dojindo Molecular Technologies, Inc. (Kumamoto,
Japan). Terminal deoxynucleotidyl transferase dUTP nick end
labeling (TUNEL) kit and LDH leakage assay were purchased from
Promega (Madison, WI, USA). Nuclear and mitochondrial protein
isolation kits were purchased from Pierce (Rockford, IL, USA).
Commercial kits for reactive oxygen species (ROS), malondialdehyde
(MDA) and CuZu/MnSOD activity were purchased from Beyotime
Institute of Biotechnology (Jiangsu, China).
Glioma cell culture
Two human GBM cell lines (U87-MG and T98G) and one
human normal glial cell line (HEB) used in the present study were
purchased from the Cell Bank of the Institute of Biochemistry and
Cell Biology, Shanghai Institutes for Biological Sciences. Cells
were cultured in Dulbecco's modified Eagle's medium (DMEM)
supplemented with 10% fetal bovine serum (FBS) in an incubator with
95% O2 and 5% CO2.
Cell viability assay
Cell viability was evaluated using a non-radioactive
MST-8 assay as described previously (33). U87-MG and T98G cells
(5×103) were transfected with Ad-GFP or Ad-SIRT6 and
then cultured. On the day of measurement, 10 µl of CCK-8
solution was added into the medium at 24, 48 and 72 h for 1 h at
37°C. The absorbance at 450 nm was recorded by a microplate reader,
and the relative cell viability was calculated. Experiments were
performed in duplicate.
Adenovirus construction and
transfection
The adenoviruses were generated with the
RAPAd® CMV Adenoviral Bicistronic Expression System
(Cell Biolabs, San Diego, CA, USA). We cloned the mouse SIRT6 cDNA
and inserted it into pacAd5 CMV-IRES vector and linearized the
vector by PacI. The purified linearized DNAs were
cotransfected into 293 cells using Lipofectamine™ assay
(Invitrogen) (33). The
adenoviruses in the media and 293 cells were harvested 7 days post
transfection. Three freeze/thaw cycles were applied to crush cells
and release the viruses. The viruses were stored at −80°C. For
viral transfection, 20 µl of the virus was added into the
culture medium (2 ml) for 6 h. An adenovirus expressing GFP
(Ad-GFP) was used as a control. Cells were then transfected with
Ad-GFP or Ad-SIRT6 for 6 h.
Lactate dehydrogenase (LDH) assay
Cell injury was determined using the CytoTox-ONE LDH
leakage assay as described previously (22,34).
In brief, the cell culture medium at different time points was
transferred to a black fluorescence plate and incubated for 10 min
with CytoTox-ONE reagent followed by stop solution. Fluorescence
was measured at 560/590 nm.
TUNEL
Fluorescent TUNEL staining in glioma cells was
conducted as previously described (35,36).
At 4 days after transfection, the cells were incubated in TUNEL
reaction solution for 2 h in the dark. The cells were then washed
with phosphate-buffered saline (PBS) for three times. After
washing, the cells were incubated with
4′,6-diamidino-2-phe-nylindole (DAPI) counterstaining solution for
3 min. The stained cells were assessed under a fluorescence
microscope (IX81, Olympus). Apoptotic cells (TUNEL-positive, green)
were counted. At least 10 visual fields were counted to calculate
the proportion of apoptosis.
Oxidant stress levels
ROS, lipid peroxidation and CuZu/Mn-SOD activity
were measured to evaluate the intracellular oxidant stress levels 4
days after transfection. ROS levels were measured as previously
described (37). Lipid peroxidation
and CuZu/MnSOD activity were evaluated using MDA content as
previously described (37).
Quantitative real-time PCR
Real-time PCR analysis was performed on OpticonDNA
engine (MJ Research Inc.) as previously described (38). TRIzol (Invitrogen) was used to
extract total RNA from the human GBM tissues. The samples (100 ng)
were amplified using primers. Amplification primers for SIRT6 were
purchased from GeneChem (Shanghai, China). The primers for β-actin
are as follows: sense, GCACTCTTCCAGCCTTCCTTCC; antisense,
CCGCCAGACAGCACTGTGTT. The mRNA level of housekeeping gene β-actin
was used as a control.
Immunoblotting
Immunoblotting was performed as previously described
(39). Cells were lysed using
Triton X buffer (50 mM Tris, 150 mM NaCl, 1 mM EDTA, 1% Triton X,
pH 7.4) with protease inhibitors and boiled with SDS. Samples were
run on 10% SDS-PAGE gel. Gels were transferred onto PVDF membranes
and processed for immunoblotting with primary antibodies (SIRT6,
1:800; AIF, 1:1,000; lamin A/C, 1:1,000; p-JAK2, 1:500; t-JAK2,
1:500; p-STAT3, 1:500; t-STAT3, 1:500; tubulin, 1:1,000) and by
corresponding HRP-labeled secondary antibodies. For immunoblotting,
images were captured and processed with Odyssay (33).
Patients and tissue samples
A total of 4 patients (WHO grade II) enrolled in the
present study underwent resection for GBM at the Department of
Neurosurgery, Union Hospital, Tongji Medical College, Huazhong
University, China. The tumor tissues and adjacent tissues
(peritumoral) were rinsed in ice-cold PBS. The connective tissue or
vessels were removed. The tissues were stored at −80°C. All of the
tissue samples were re-evaluated according to WHO classifications
(40). None of the patients had
received chemotherapy or radiotherapy prior to surgery. The study
was approved by the Institutional Review Board of Huazhong
University and written informed consent was obtained from the
patients or guardians.
Statistical analysis
Data are expressed as mean ± SEM. Evaluation of the
differences between groups was performed by two-samples Student's
t-test or Mann-Whitney U test (non-normal distribution of values).
P<0.05 was considered to indicate a statistically significant
difference.
Results
Overexpression of SIRT6 inhibits glioma
cell growth
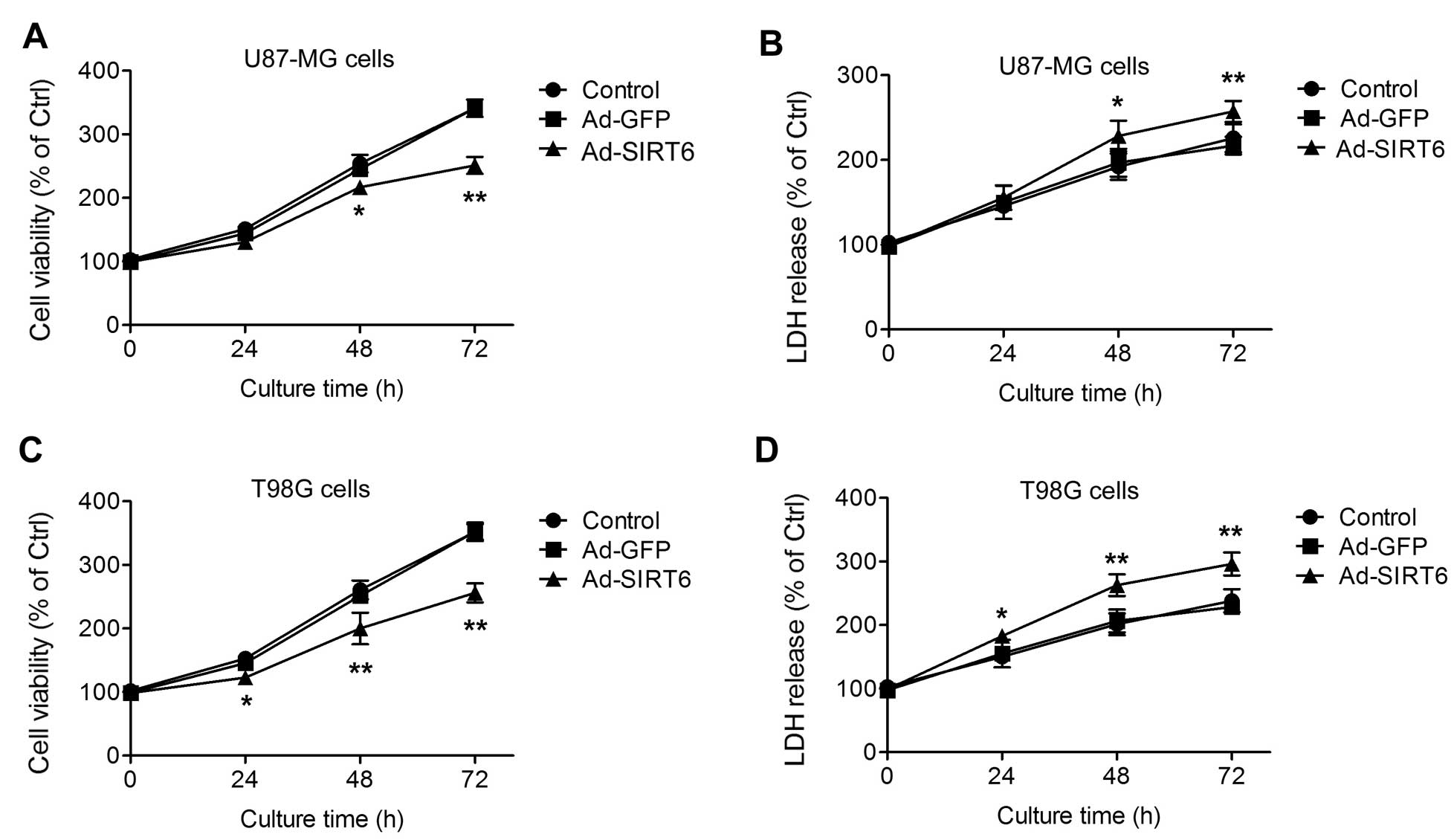
First, the influence of SIRT6 overexpression on GBM
cell growth was assessed in vitro. Two human GBM cell lines
(U87-MG and T98G) were used in the present study. In the U87-MG
cells transfected with Ad-SIRT6, the cell growth was inhibited at
48 and 72 h after seeding (Fig.
1A). Accordingly, the LDH release into the culture medium of
the SIRT6-overexpressing U87-MG cells was increased at 48 and 72 h
(Fig. 1B). The cell viability and
LDH release at the 24-h time-point were not significantly different
among the cells (Fig. 1A and B). In
the T98G cells, similar phenotypes were observed. Overexpression of
SIRT6 inhibited T98G cell growth (Fig.
1C) and increased LDH release (Fig.
1D) at three time-points (24, 48 and 72 h). These results
suggest that overexpression of SIRT6 inhibits glioma cell growth
and induces cell injury.
Overexpression of SIRT6 promotes
apoptosis in glioma cells
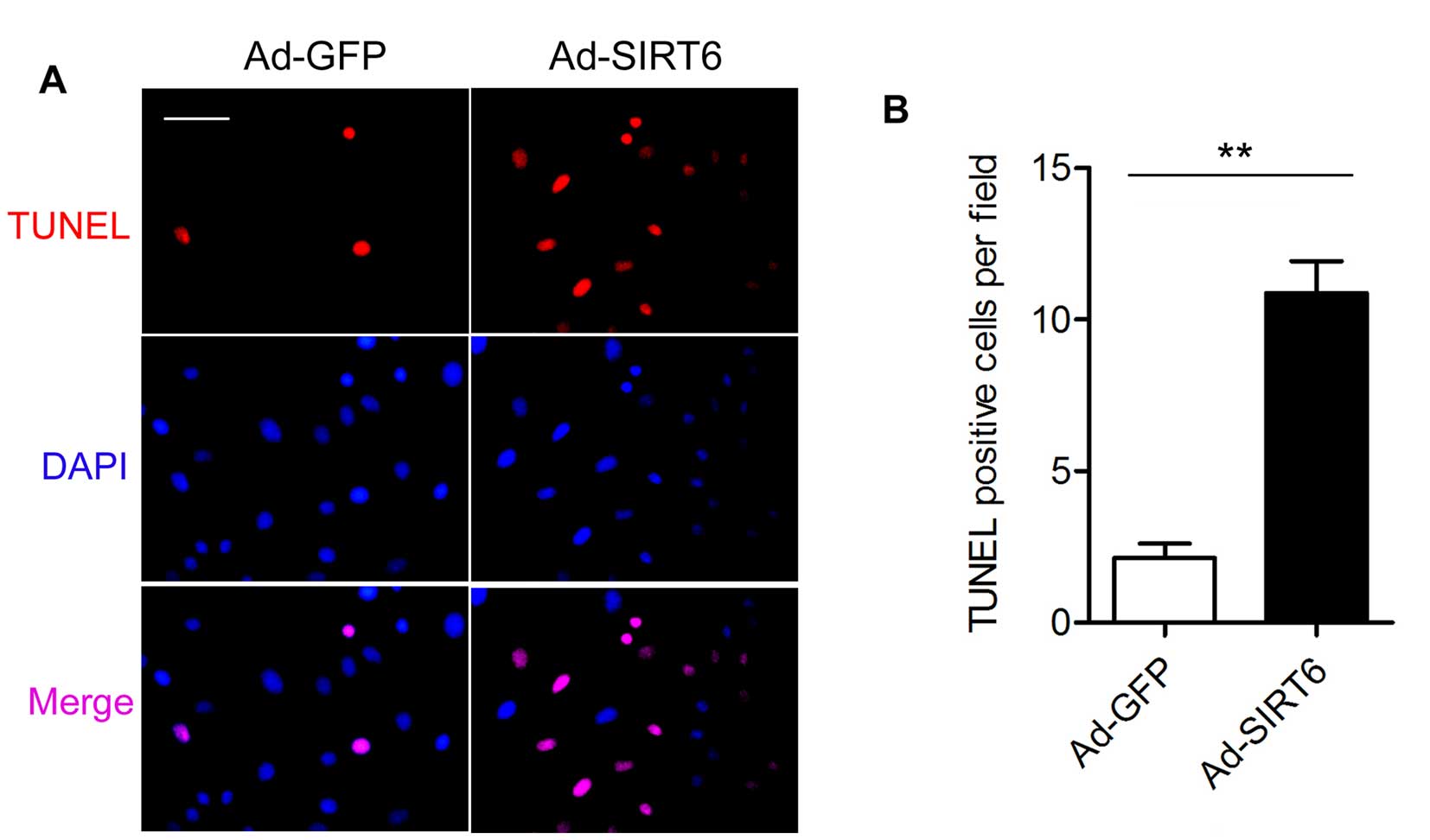
As SIRT6 overexpression was able to inhibit glioma
cell growth in the U87-MG and T98G cells, we investigated the
molecular mechanisms of the tumor-suppressive effects of SIRT6 in
the T98G cells in the following experiments. We performed TUNEL
assay to examine the effect of SIRT6 overexpression on the
apoptosis of the T98G cells. There were fewer TUNEL-positive cells
in the Ad-GFP group than the number in the Ad-SIRT6 group at 4 days
after transfection (Fig. 2).
Overexpression of SIRT6 induces AIF
mitochon- drial-to-nuclear-translocation in the glioma cells
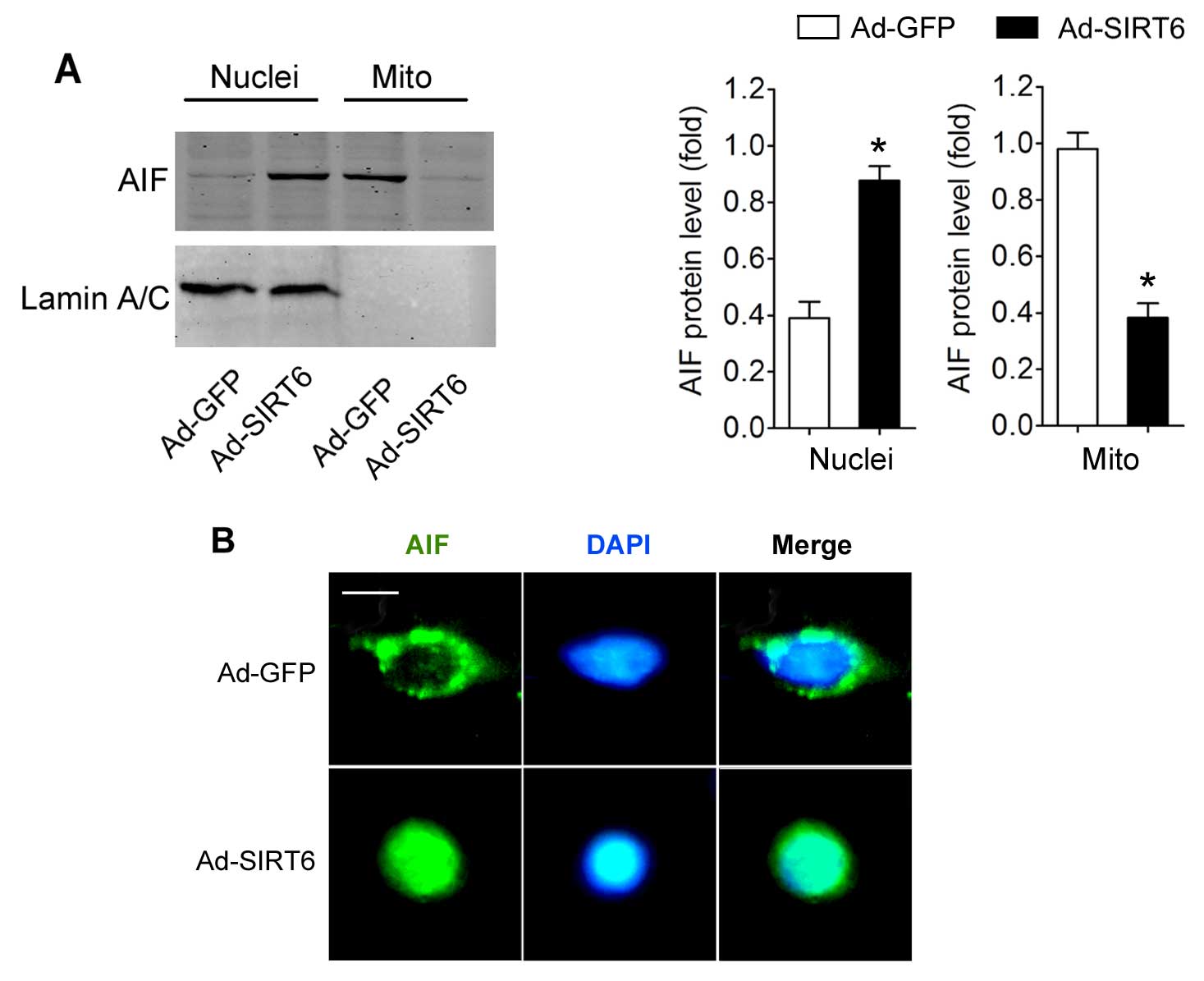
Subsequently, we determined the potential effects of
SIRT6 overexpression on AIF translocation. We found that the
nuclear AIF expression in cells with SIRT6 overexpression was
significantly higher than that in the Ad-GFP-transfected cells.
Moreover, the mitochondrial AIF level in the cells with SIRT6
overexpression was reduced (Fig.
3A). Immunofluorescent staining of AIF clearly demonstrated
that AIF entered the nucleus in the cells with SIRT6
overex-pression (Fig. 3B).
Oxidative stress in glioma cells is
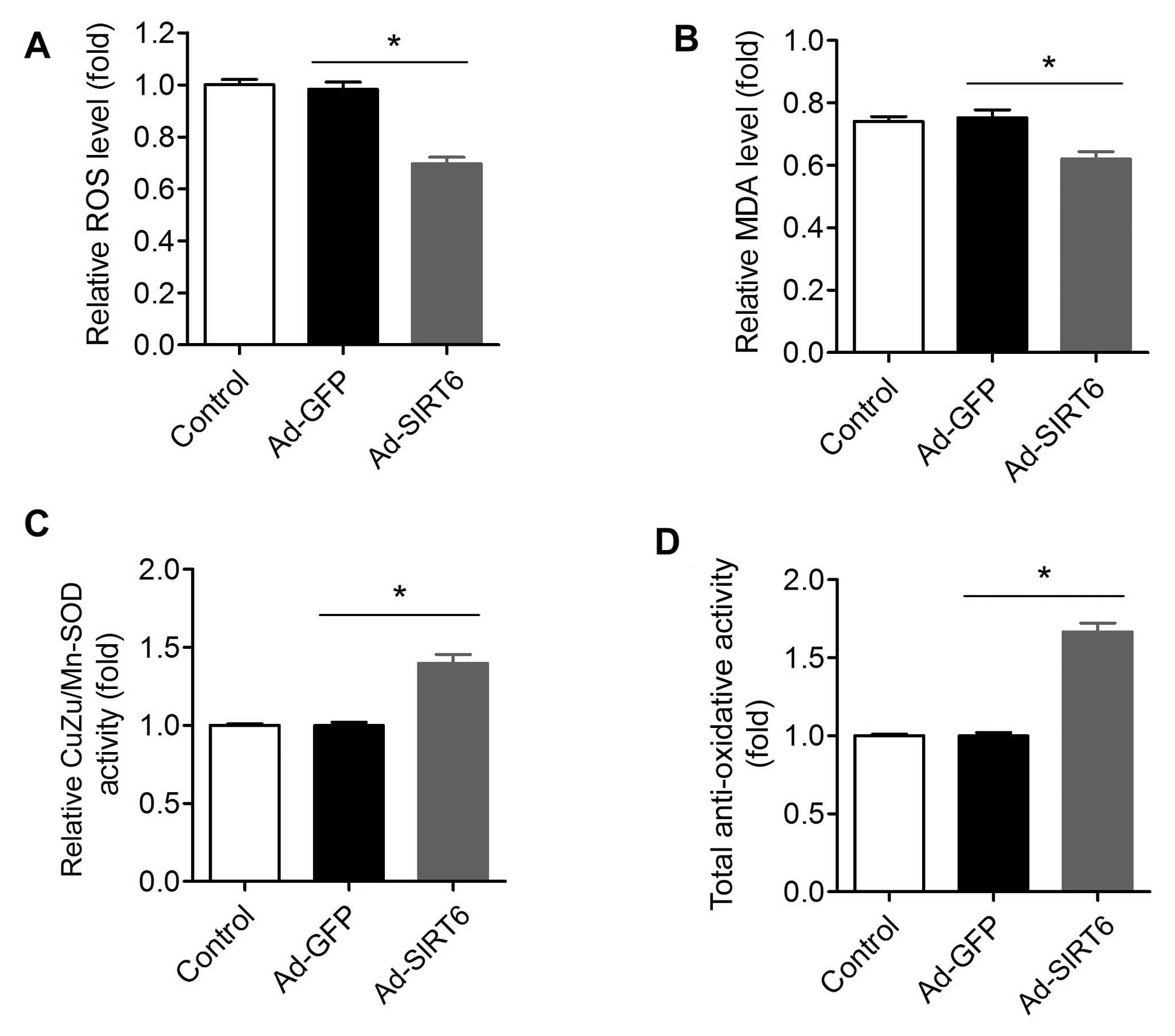
attenuated by SIRT6 overexpression
It is well-known that an abnormal increase in
oxidative stress is an important factor for tumors (41). We compared the oxidant levels in
SIRT6-overexpressing and control tumor cells. The ROS level in
cells with SIRT1 over-expression was significantly lower than this
level in the control cells (Fig.
4A). Moreover, the MDA level, an index for lipid peroxidation,
was also reduced in the Ad-SIRT6-transfected cells (Fig. 4B). Accordingly, the CuZu/Mn-SOD
activity in cells with SIRT1 overexpression was higher than that in
the control cells (Fig. 4C). The
total antioxidant activity in the SIRT6-overexpressed cells was
also higher than that in the control cells (Fig. 4D).
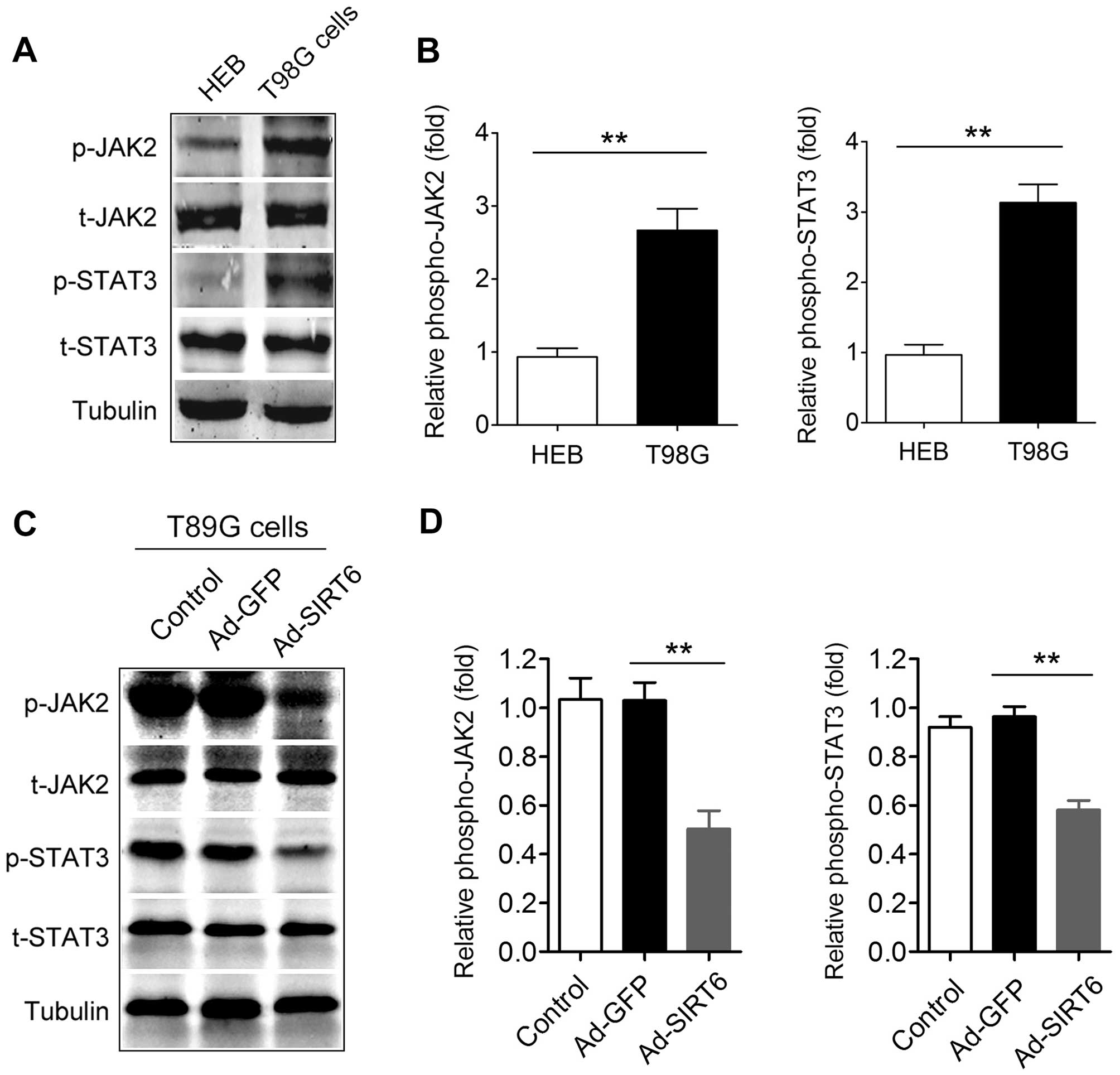
Activation of the JAK2/STAT3 signaling
pathway in glioma cells is suppressed by SIRT6 overexpression
The JAK2/STAT3 signaling pathway is constitutively
activated in most primary malignant gliomas and the extent of
activation is positively correlated with glioma grade (42). Thus, we determined the effect of
SIRT6 overexpression on the JAK2/STAT3 signaling pathway. First, we
confirmed that the JAK2/STAT3 signaling pathway was activated in
the T98G cells compared with that in the HEB cells, a normal glial
cell line (Fig. 5A and B). In
addition, we found that the phosphorylation of JAK2 in the
Ad-SIRT6-transfected cells was significantly reduced compared with
that in the Ad-GFP-transfected cells (Fig. 5C and D). The phosphorylation of
STAT3 in the Ad-SIRT6-transfected cells was also decreased compared
with that in the Ad-GFP-transfected cells (Fig. 5C and D). These data indicate that
overexpression of SIRT6 suppresses the activation of the JAK2/STAT3
signaling pathway in glioma cells.
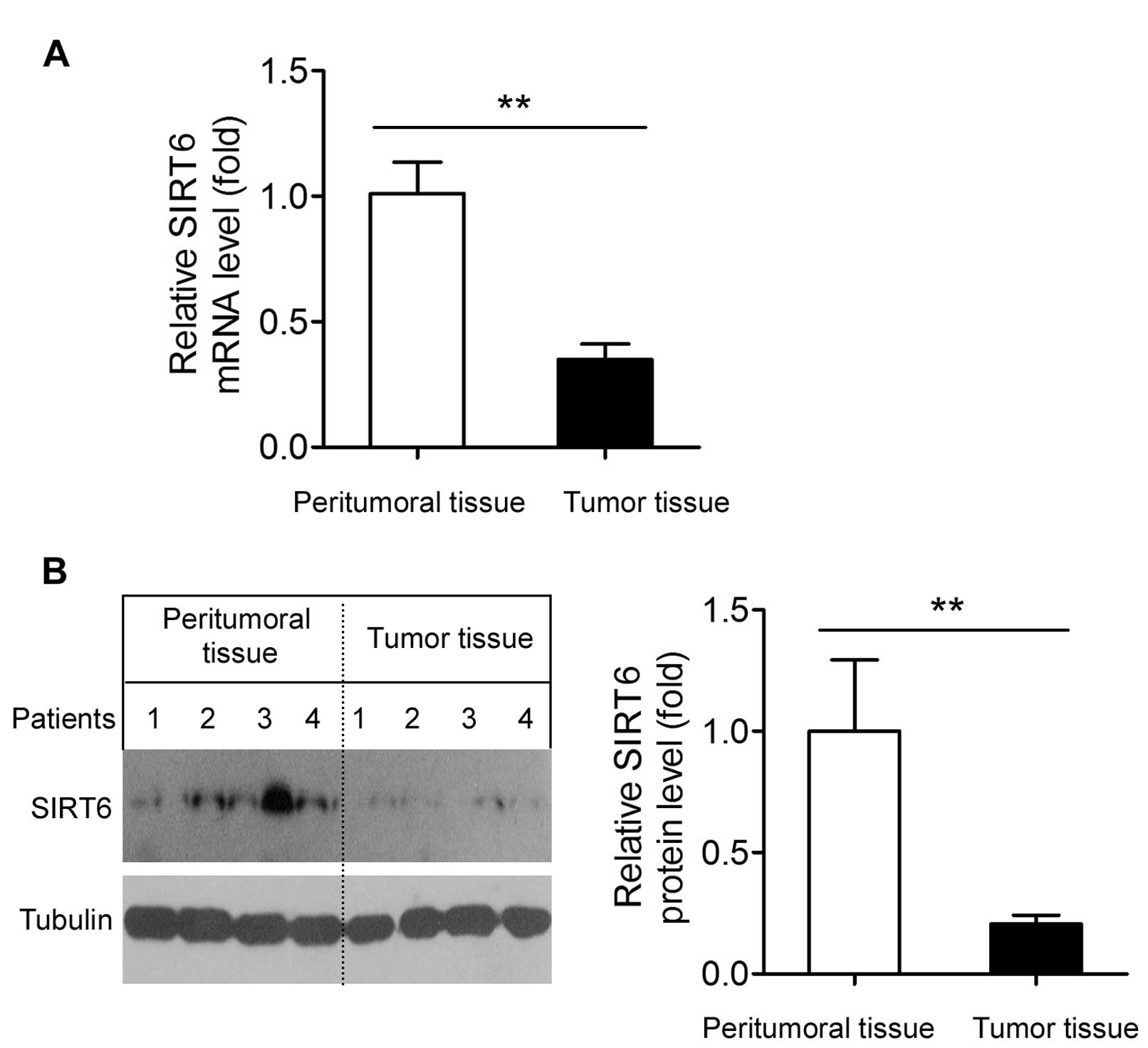
SIRT6 is downregulated in human GBM
tissues
Finally, we compared the SIRT6 mRNA and protein
expression between peritumoral and tumor tissues in human samples.
The SIRT6 mRNA level in the human GBM tissues was significantly
(~65%) lower than that in the peritumoral tissues (Fig. 6A). Accordingly, SIRT6 protein
expression in the GBM tissues was also shown to be significantly
lower than that in the peritumoral tissues (Fig. 6B).
Discussion
In the present study, we showed that the growth of
two GBM cell lines (U87-MG and T98G cells) was inhibited by SIRT6
overexpression. SIRT6 overexpression induced cell injury (LDH
release). Moreover, we showed that SIRT6 overexpression not only
led to apoptosis of the T98G cells, but also increased AIF
mitochondrial-to-nuclear translocation. SIRT6 overexpression also
inhibited the over-activated oxidative stress and JAK2/STAT3
signaling pathway in glioma cells. We also provide evidence that
SIRT6 was markedly downregulated in human GBM tissues. These data
collectively suggest that SIRT6 represents an anti-oncogene in
glioma.
SIRT6 protein is expressed at the highest levels in
the muscle, CNS and heart (43),
and the cerebral SIRT6 expression was found to be reduced upon
ischemic status (44). Notably,
neural-specific SIRT6-knockout mice show attenuated somatic growth
and obesity (45). In a recent
report, Chen et al showed that SIRT6 is downregulated in
human glioma tissues and glioma cell lines (46). They also showed that SIRT6 inhibits
PCBP2 expression by deacetylating H3K9 (46). However, this study did not mention
the type and WHO grade of the glioma tissues. In the present study,
we assessed SIRT6 expression in human WHO grade II GBM tissues. GBM
is the most common adult primary intracranial neoplasm and has a
very poor outcome. In line with the results from Chen et al
(46), we observed significant
downregulation of SIRT6 in the GBM tissues. In addition,
overexpression of SIRT6 suppressed the proliferation and induced
cell injury in the cultured U87-MG and T98G cells. Since glioma
tissue consistently displays a much higher ROS level (47), and SIRT6 overexpression reduces
oxidant stress in glioma cells, we considered that the
downregulation of SIRT6 in GBM tissues may contribute to the
development of GBM by resulting in a pronounced increase in
oxidative stress in GBM tissues to promote its growth.
SIRT6 suppresses genomic instability via binding
chromatin and deacetylating histone (25,28).
Thus, maintenance of genomic integrity is the major function of
SIRT6. Sebastián et al provided initial evidence that SIRT6
may be an anti-oncogene through regulating aerobic glycolysis in
tumor cells (29). Marquardt et
al showed that downregulation of SIRT6 and the dysregulated
genes by loss of SIRT6 displayed oncogenic effects in
hepatocarcinogenesis (30). These
effects of SIRT6 strongly suggest a tumor-suppressing action,
accompanied by modulation of histone deacetylation and glycolysis
metabolism. We speculated that SIRT6 may also play an important
role in cellular apoptosis. Indeed, we found that overexpression of
SIRT6 induced pronounced apoptosis in glioma cells. According to
our knowledge, the present study is the first to show the
pro-apoptotic action of SIRT6 in glioma cells. This result is in
agreement with a study conducted by Van Meter et al
(48). They reported that SIRT6
overexpression induces massive apoptosis in cervical carcinoma,
fibrosarcoma and breast tumor cell lines (48). However, SIRT6 overexpression has no
effect on normal non-transformed cells (48). Intriguingly, they also found that
the cell death induced by SIRT6 overexpression in cancer cells
required the mono-ADP-ribosyltransferase activity of SIRT6
(48).
We observed that SIRT6 overexpression inhibited
oxidative stress, evidenced by the suppressed ROS production and
MDA level, and the increased CuZu/Mn-SOD activity and total
antioxidant activity in the SIRT6-overexpressing glioma cells.
Increased generation of ROS and an altered redox status have long
been noted and considered as features of cancer cells (49). High grade glioma cells commonly
display multiple genetic alterations and high oxidative stress,
suggesting that the excessive redox state might be an important
characteristic of invasive glioma (49). Moreover, the mutation of isocitrate
dehydrogenase 1 (IDH1), a molecular basis of epigenomic changes in
gliomas, is associated with excessive ROS production (50). In the light of these results, we
proposed that the pro-apoptotic activity of SIRT6 in glioma cells
may be attributed to the inhibitory action of SIRT6 on oxidative
stress, at least in part. In addition, we found that SIRT6
overexpression suppressed the activation of the JAK2/STAT3
signaling pathway. The JAK2/STAT3 pathway was demonstrated to be
highly activated in human GBM tissues and GBM-derived brain tumor
stem cell-formed xenografts (51,52).
JAK2/STAT3-targeted therapy slowed the progression of GBM and
suppressed glioma invasion (51,52).
Thus, abnormal JAK2/STAT3 activation is also a feature of GBM. As
SIRT6 overexpression significantly inhibited JAK2/STAT3 activation
in glioma cells, we believe that the inhibitory effect on the
JAK2/STAT3 signaling pathway by SIRT6 may be another molecular
mechanism underlying its tumor-suppressive effect.
Collectively, we demonstrated that SIRT6
overexpression inhibited cell growth, induced apoptosis and
resulted in AIF mitochondrial-to-nuclear translocation in glioma
cell lines. These phenotypes may be achieved by the reduced
oxidative stress and JAK2/STAT3 activation under SIRT6
overexpression. Finally, we found that the expression of SIRT6 was
lost in human GBM tissues. These results suggest that SIRT6 may be
a promising therapeutic target for the treatment of malignant
glioma.
Acknowledgments
The present study was supported by grants from the
National Science Foundation of China (no. 81272778) and the
National Science Foundation of Hubei, China (no. 2013CFB127).
References
|
1
|
Stupp R, Mason WP, van den Bent MJ, Weller
M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn
U, et al European Organisation for Research and Treatment of Cancer
Brain Tumor and Radiotherapy Groups; National Cancer Institute of
Canada Clinical Trials Group: Radiotherapy plus concomitant and
adjuvant temozolomide for glioblastoma. N Engl J Med. 352:987–996.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Alifieris C and Trafalis DT: Glioblastoma
multiforme: Pathogenesis and treatment. Pharmacol Ther. 152:63–82.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mair B, Kubicek S and Nijman SM:
Exploiting epigenetic vulnerabilities for cancer therapeutics.
Trends Pharmacol Sci. 35:136–145. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Huang M, Shen A, Ding J and Geng M:
Molecularly targeted cancer therapy: Some lessons from the past
decade. Trends Pharmacol Sci. 35:41–50. 2014. View Article : Google Scholar
|
|
5
|
Bouteldja N, Andersen LT, Møller N and
Gormsen LC: Using positron emission tomography to study human
ketone body metabolism: A review. Metabolism. 63:1375–1384. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Reardon DA and Wen PY: Glioma in 2014:
Unravelling tumour heterogeneity-implications for therapy. Nat Rev
Clin Oncol. 12:69–70. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nicolaidis S: Biomarkers of glioblastoma
multiforme. Metabolism. 64(Suppl 1): S22–S27. 2015. View Article : Google Scholar
|
|
8
|
Chiarini F, Evangelisti C, McCubrey JA and
Martelli AM: Current treatment strategies for inhibiting mTOR in
cancer. Trends Pharmacol Sci. 36:124–135. 2015. View Article : Google Scholar
|
|
9
|
Ji H, Wang J, Nika H, Hawke D, Keezer S,
Ge Q, Fang B, Fang X, Fang D, Litchfield DW, et al: EGF-induced ERK
activation promotes CK2-mediated disassociation of alpha-catenin
from beta-catenin and transactivation of beta-catenin. Mol Cell.
36:547–559. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Miao H, Li DQ, Mukherjee A, Guo H, Petty
A, Cutter J, Basilion JP, Sedor J, Wu J, Danielpour D, et al: EphA2
mediates ligand-dependent inhibition and ligand-independent
promotion of cell migration and invasion via a reciprocal
regulatory loop with Akt. Cancer Cell. 16:9–20. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kim E, Kim M, Woo DH, Shin Y, Shin J,
Chang N, Oh YT, Kim H, Rheey J, Nakano I, et al: Phosphorylation of
EZH2 activates STAT3 signaling via STAT3 methylation and promotes
tumorigenicity of glioblastoma stem-like cells. Cancer Cell.
23:839–852. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Herskovits AZ and Guarente L: Sirtuin
deacetylases in neurode-generative diseases of aging. Cell Res.
23:746–758. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Haigis MC and Guarente LP: Mammalian
sirtuins - emerging roles in physiology, aging, and calorie
restriction. Genes Dev. 20:2913–2921. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hubbard BP and Sinclair DA: Small molecule
SIRT1 activators for the treatment of aging and age-related
diseases. Trends Pharmacol Sci. 35:146–154. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Polak-Jonkisz D, Rehan L, Laszki-Szcząchor
K and Sobieszczańska M: Novel targets for pharmacological
intervention in age-related diseases. Trends Pharmacol Sci.
35:622–623. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Imai S and Guarente L: NAD+ and
sirtuins in aging and disease. Trends Cell Biol. 24:464–471. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Peng G and Liu Y: Hypoxia-inducible
factors in cancer stem cells and inflammation. Trends Pharmacol
Sci. 36:374–383. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Davis FM, Stewart TA, Thompson EW and
Monteith GR: Targeting EMT in cancer: Opportunities for
pharmacological intervention. Trends Pharmacol Sci. 35:479–488.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yang SJ and Lim Y: Resveratrol ameliorates
hepatic metaflammation and inhibits NLRP3 inflammasome activation.
Metabolism. 63:693–701. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shimada T, Furuta H, Doi A, Ariyasu H,
Kawashima H, Wakasaki H, Nishi M, Sasaki H and Akamizu T: Des-acyl
ghrelin protects microvascular endothelial cells from oxidative
stress-induced apoptosis through sirtuin 1 signaling pathway.
Metabolism. 63:469–474. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen J, Zhang B, Wong N, Lo AW, To KF,
Chan AW, Ng MH, Ho CY, Cheng SH, Lai PB, et al: Sirtuin 1 is
upregulated in a subset of hepatocellular carcinomas where it is
essential for telomere maintenance and tumor cell growth. Cancer
Res. 71:4138–4149. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang P, Xu TY, Guan YF, Tian WW, Viollet
B, Rui YC, Zhai QW, Su DF and Miao CY: Nicotinamide
phosphoribosyltransferase protects against ischemic stroke through
SIRT1-dependent adenosine monophosphate-activated kinase pathway.
Ann Neurol. 69:360–374. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Prozorovski T, Schulze-Topphoff U, Glumm
R, Baumgart J, Schröter F, Ninnemann O, Siegert E, Bendix I,
Brüstle O, Nitsch R, et al: Sirt1 contributes critically to the
redox-dependent fate of neural progenitors. Nat Cell Biol.
10:385–394. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhao Y, Guan YF, Zhou XM, Li GQ, Li ZY,
Zhou CC, Wang P and Miao CY: Regenerative neurogenesis after
ischemic stroke promoted by nicotinamide
phosphoribosyltransferase-nicotinamide adenine dinucleotide
cascade. Stroke. 46:1966–1974. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Michishita E, McCord RA, Berber E, Kioi M,
Padilla-Nash H, Damian M, Cheung P, Kusumoto R, Kawahara TL,
Barrett JC, et al: SIRT6 is a histone H3 lysine 9 deacetylase that
modulates telomeric chromatin. Nature. 452:492–496. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jiang H, Khan S, Wang Y, Charron G, He B,
Sebastian C, Du J, Kim R, Ge E, Mostoslavsky R, et al: SIRT6
regulates TNF-α secretion through hydrolysis of long-chain fatty
acyl lysine. Nature. 496:110–113. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lee J, Hong SW, Park SE, Rhee EJ, Park CY,
Oh KW, Park SW and Lee WY: Exendin-4 regulates lipid metabolism and
fibroblast growth factor 21 in hepatic steatosis. Metabolism.
63:1041–1048. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mostoslavsky R, Chua KF, Lombard DB, Pang
WW, Fischer MR, Gellon L, Liu P, Mostoslavsky G, Franco S, Murphy
MM, et al: Genomic instability and aging-like phenotype in the
absence of mammalian SIRT6. Cell. 124:315–329. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sebastián C, Zwaans BM, Silberman DM,
Gymrek M, Goren A, Zhong L, Ram O, Truelove J, Guimaraes AR, Toiber
D, et al: The histone deacetylase SIRT6 is a tumor suppressor that
controls cancer metabolism. Cell. 151:1185–1199. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Marquardt JU, Fischer K, Baus K, Kashyap
A, Ma S, Krupp M, Linke M, Teufel A, Zechner U, Strand D, et al:
Sirtuin-6-dependent genetic and epigenetic alterations are
associated with poor clinical outcome in hepatocellular carcinoma
patients. Hepatology. 58:1054–1064. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Khongkow M, Olmos Y, Gong C, Gomes AR,
Monteiro LJ, Yagüe E, Cavaco TB, Khongkow P, Man EP, Laohasinnarong
S, et al: SIRT6 modulates paclitaxel and epirubicin resistance and
survival in breast cancer. Carcinogenesis. 34:1476–1486. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Feng XX, Luo J, Liu M, Yan W, Zhou ZZ, Xia
YJ, Tu W, Li PY, Feng ZH and Tian DA: Sirtuin 6 promotes
transforming growth
factor-β1/H2O2/HOCl-mediated enhancement of
hepato-cellular carcinoma cell tumorigenicity by suppressing
cellular senescence. Cancer Sci. 106:559–566. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang P, Xu TY, Guan YF, Su DF, Fan GR and
Miao CY: Perivascular adipose tissue-derived visfatin is a vascular
smooth muscle cell growth factor: Role of nicotinamide
mononucleotide. Cardiovasc Res. 81:370–380. 2009. View Article : Google Scholar
|
|
34
|
Magzoub M and Miranker AD:
Concentration-dependent transitions govern the subcellular
localization of islet amyloid polypeptide. FASEB J. 26:1228–1238.
2012. View Article : Google Scholar :
|
|
35
|
Wang P, Guan YF, Du H, Zhai QW, Su DF and
Miao CY: Induction of autophagy contributes to the neuroprotection
of nicotinamide phosphoribosyltransferase in cerebral ischemia.
Autophagy. 8:77–87. 2012. View Article : Google Scholar
|
|
36
|
Tönjes M, Barbus S, Park YJ, Wang W,
Schlotter M, Lindroth AM, Pleier SV, Bai AH, Karra D, Piro RM, et
al: BCAT1 promotes cell proliferation through amino acid catabolism
in gliomas carrying wild-type IDH1. Nat Med. 19:901–908. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Song J, Ke SF, Zhou CC, Zhang SL, Guan YF,
Xu TY, Sheng CQ, Wang P and Miao CY: Nicotinamide
phosphoribosyltransferase is required for the calorie
restriction-mediated improvements in oxidative stress,
mitochondrial biogenesis, and metabolic adaptation. J Gerontol A
Biol Sci Med Sci. 69:44–57. 2014. View Article : Google Scholar
|
|
38
|
Wang P, Du H, Zhou CC, Song J, Liu X, Cao
X, Mehta JL, Shi Y, Su DF and Miao CY: Intracellular
NAMPT-NAD+-SIRT1 cascade improves post-ischaemic
vascular repair by modulating Notch signalling in endothelial
progenitors. Cardiovasc Res. 104:477–488. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wang P, Xu TY, Wei K, Guan YF, Wang X, Xu
H, Su DF, Pei G and Miao CY: ARRB1/β-arrestin-1 mediates
neuroprotection through coordination of BECN1-dependent autophagy
in cerebral ischemia. Autophagy. 10:1535–1548. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Rousseau A, Mokhtari K and Duyckaerts C:
The 2007 WHO classification of tumors of the central nervous system
- what has changed? Curr Opin Neurol. 21:720–727. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Gorrini C, Harris IS and Mak TW:
Modulation of oxidative stress as an anticancer strategy. Nat Rev
Drug Discov. 12:931–947. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Lo HW, Cao X, Zhu H and Ali-Osman F:
Constitutively activated STAT3 frequently coexpresses with
epidermal growth factor receptor in high-grade gliomas and
targeting STAT3 sensitizes them to Iressa and alkylators. Clin
Cancer Res. 14:6042–6054. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Liszt G, Ford E, Kurtev M and Guarente L:
Mouse Sir2 homolog SIRT6 is a nuclear ADP-ribosyltransferase. J
Biol Chem. 280:21313–21320. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Lee OH, Kim J, Kim JM, Lee H, Kim EH, Bae
SK, Choi Y, Nam HS and Heo JH: Decreased expression of sirtuin 6 is
associated with release of high mobility group box-1 after cerebral
ischemia. Biochem Biophys Res Commun. 438:388–394. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Schwer B, Schumacher B, Lombard DB, Xiao
C, Kurtev MV, Gao J, Schneider JI, Chai H, Bronson RT, Tsai LH, et
al: Neural sirtuin 6 (Sirt6) ablation attenuates somatic growth and
causes obesity. Proc Natl Acad Sci USA. 107:21790–21794. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Chen X, Hao B, Liu Y, Dai D, Han G, Li Y,
Wu X, Zhou X, Yue Z, Wang L, et al: The histone deacetylase SIRT6
suppresses the expression of the RNA-binding protein PCBP2 in
glioma. Biochem Biophys Res Commun. 446:364–369. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Santandreu FM, Brell M, Gene AH, Guevara
R, Oliver J, Couce ME and Roca P: Differences in mitochondrial
function and antioxidant systems between regions of human glioma.
Cell Physiol Biochem. 22:757–768. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Van Meter M, Mao Z, Gorbunova V and
Seluanov A: SIRT6 overexpression induces massive apoptosis in
cancer cells but not in normal cells. Cell Cycle. 10:3153–3158.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Trachootham D, Alexandre J and Huang P:
Targeting cancer cells by ROS-mediated mechanisms: A radical
therapeutic approach? Nat Rev Drug Discov. 8:579–591. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Shi J, Sun B, Shi W, Zuo H, Cui D, Ni L
and Chen J: Decreasing GSH and increasing ROS in chemosensitivity
gliomas with IDH1 mutation. Tumour Biol. 36:655–662. 2015.
View Article : Google Scholar
|
|
51
|
Stechishin OD, Luchman HA, Ruan Y, Blough
MD, Nguyen SA, Kelly JJ, Cairncross JG and Weiss S: On-target
JAK2/STAT3 inhibition slows disease progression in orthotopic
xenografts of human glioblastoma brain tumor stem cells. Neuro
Oncol. 15:198–207. 2013. View Article : Google Scholar :
|
|
52
|
Zheng Q, Han L, Dong Y, Tian J, Huang W,
Liu Z, Jia X, Jiang T, Zhang J, Li X, et al: JAK2/STAT3 targeted
therapy suppresses tumor invasion via disruption of the
EGFRvIII/JAK2/STAT3 axis and associated focal adhesion in
EGFRvIII-expressing glioblastoma. Neuro Oncol. 16:1229–1243. 2014.
View Article : Google Scholar : PubMed/NCBI
|