Introduction
Ovarian cancer is believed to be the third most
common gynaecological malignancy worldwide (1,2). Among
them, epithelial ovarian cancer (EOC) accounts for 90% of all
ovarian cancer, representing the most fatal gynecological
malignancy among women (1,2). EOC is a highly heterogeneous tumor,
consisting of several different histotypes with distinct
characteristics (3). It was
estimated that EOC resulted in 21,980 new cases and 14,270 deaths
in 2014 (2). However, the molecular
mechanism of EOC is far from completely understood. Further
understanding of the molecular pathogenesis and biomarkers of
ovarian cancer would facilitate early detection and reduce
mortality, as well as useful in identifying therapeutic targets,
reducing chemoresistance and prolonging survival at advanced-stage
disease (4).
MicroRNAs (miRNAs) are a group of single-stranded,
non-coding, small RNAs (20–24 nucleotides) (5–7). These
miRNAs play important roles in various biological processes through
regulating gene expression by repressing translation or
facilitating degradation of the target mRNA by partially binding to
the 3′ untranslated region of the target mRNA (8,9).
Accumulating results have shown that approximately one-third of all
mammalian genes were targeted and regulated by miRNAs (10,11).
Moreover, miRNAs are believed to function as important regulators
in the pathogenesis of various types of tumors (12–14).
miRNAs can act as oncomiRs or tumor suppressor miRNAs depending on
the functions of their gene targets in cancer development and
progression (15). In particular,
dysregulation of miRNAs is involved in the development of different
types of ovarian cancer (8,16,17).
Vaksman et al (18)
performed miRNA array analysis and compared ovarian cancer
effusions and primary ovarian cancer, identifying several
differentially expressed miRNAs. Zou et al (19) identified potential microRNAs
associated with drug resistance in ovarian cancer through
web-available microarrays. In our preliminary experiments, we found
that abnormal expression of miR-215 was associated with the
development of ovarian cancer. However, the extract role of miR-215
in the pathogenesis of ovarian cancer is unclear.
The present study was designed to investigate the
role of abnormal expression of miR-215 in the development of EOC
and to elucidate the possible molecular mechanisms. Our results
showed that miR-215 was decreased in EOC tissues and cell lines,
and functioned in EOC cell proliferation, promoted apoptosis and
sensitivity to chemotherapy drugs through inhibition of the
X-chromosome-linked inhibitor of apoptosis (XIAP).
Materials and methods
Chemicals and materials
β-actin and XIAP antibodies were purchased from
Santa Cruz Biotechnology Inc. (Santa Cruz, CA, USA). Paclitaxel was
procured from Sigma. All of the other chemicals used were of the
highest grade available commercially.
Ethics statement, patients and tissue
samples
All patients provided their informed consent, and
the present study was approved by the Ethics Review Committee of
The First Affiliated Hospital of Xi'an Jiaotong University, and
complied with the Declaration of Helsinki. Tissue samples were
collected from 85 subjects who underwent surgery from September
2012 to June 2014 for the treatment of epithelial ovarian cancers
at the Department of Obstetrics and Gynecology, The First
Affiliated Hospital of Xi'an Jiaotong University. All EOC patients
had been histopathologically diagnosed with primary ovarian cancer.
The histological diagnosis was evaluated by two independent
pathologists according to the WHO classification. The International
Federation of Gynecology and Obstetrics (FIGO) staging system was
used to stage cases. The patient features are summarized in
Table I. Samples from 63 cases
involving other ovarian tumors or ovarian cystadenomas were
collected as controls.
 | Table IClinical features of EOC patients. |
Table I
Clinical features of EOC patients.
| Features of
patients | Patients |
|---|
| No. of patients | 85 |
| Age, year (median,
range) | 57, (21–88) |
| Surgery | |
| Primary
debulking | 71 |
| Interval
debulking | 14 |
| FIGO stage (I +
II/III + IV) | 28/57 |
| Histological type
(serous/endometrial/mucinous/clear cell/others) | 41/7/20/5/12 |
| Histological grade
(G1 + G2/G3) | 22/63 |
| Lymph node
metastasis (yes/no) | 66/19 |
| Chemotherapy
(platinum-based/non-platinum-based/none) | 67/2/4 |
| Elevated CA125
levels, U/ml (<5×105/≥5×105) | 16/69 |
| Relapse
(yes/no) | 20/65 |
Cell lines and culture
Human EOC cell lines, OVCAR3, CAOV3, SKOV3 and HEY
cells were purchased from the American Type Culture Collection
(ATCC; Manassas, VA, USA). These cells were cultured in Dulbecco's
modified Eagle's medium (DMEM; Gibco-BRL, Grand Island, NY, USA)
supplemented with 10% fetal bovine serum (FBS; Gibco-BRL), 100 U/ml
penicillin, and 100 µg of streptomycin at 37°C in 5%
CO2. Normal human ovarian surface epithelial (HOSE)
cells were purchased from ScienCell Research Laboratories
(Carlsbad, CA, USA) and maintained in ovarian epithelial cell
medium supplemented with 1X ovarian epithelial cell growth
supplement (ScienCell Research Laboratories). All cell lines were
maintained at 37°C in a humidified incubator containing 5%
CO2.
Cell transfection
The miR-215 mimics, inhibitors and negative miRNA
control, and XIAP overexpression and shRNA lentivirus were
synthesized by Shanghai GenePharma Co., Ltd. (Shanghai, China).
Transient transfection was performed using Lipofectamine 2000
(Invitrogen, Carlsbad, CA, USA) system according to the
manufacturer's protocols. Transfection of XIAP shRNA lentivirus and
stable knockdown of XIAP was conducted according to the
manufacturer's protocols.
RNA isolation and real-time PCR
Total RNA was isolated from EOC tissues or cell
lines using a commercial assay kit (Tiangen Biotech Co., Ltd.,
Beijing, China) according to the manufacturer's instructions. RNA
(1 µg) was reversely transcribed to make cDNA using the
First Strand cDNA synthesis kit (Takara, Tokyo, Japan).
Quantitative real-time polymerase chain reaction (PCR) was used to
precisely quantify miR-215 and XIAP. Real-time PCR was performed
using SYBR-Green reagents (Takara) in a real-time PCR system from
Bio-Rad Biosystems. The 2−ΔΔCT method was used to
evaluate gene expression compared with the endogenous controls
(β-actin or U6 non-coding small nuclear RNA). Amplification was
performed with an initial step at 94°C for 5 min, followed by 40
cycles of denaturation at 94°C for 30 sec, annealing at 63°C for 30
sec and then extension at 72°C for 10 sec.
Protein extraction and western blot
analysis
Total proteins were collected from EOC cell lines
using RIPA lysis buffer according to the manufacturer's protocols.
Cell lysates were washed with cold PBS and incubated on ice in
lysis buffer for 30 min. The lysates were centrifuged at 25,000 × g
for 30 min at 4°C and the protein concentrations in supernatants
were measured using a Bradford protein assay kit (Pierce, Rockford,
IL, USA). Approximately 50 µg of protein was separated using
SDS-PAGE. Then, proteins were transferred to PVDF membrane
(Millipore, Billerica, MA, USA), which was blocked with 5% non-fat
milk. Membranes were incubated overnight at 4°C with primary
antibodies. After washing, the membrane was incubated with a
horseradish peroxidase-conjugated secondary antibody (Pierce) at
37°C for 1 h. Protein bands were visualized with the ECL and
captured using Bio-Rad Imaging Systems (Bio-Rad Laboratories,
Hercules, CA, USA).
Cell viability and proliferation
Cell viability was examined using MTT assay.
Absorbance was measured at 570 nm using a microplate reader
(Bio-Rad Laboratories). Determination of cell proliferation was
conducted using a Cell Counting kit-8 (CCK-8) assay according to
the manufacturer's instructions. Absorbance was detected at 450 nm
using a microplate reader (Bio-Rad Laboratories).
TUNEL assay
TdT-mediated dUTP nick end labeling (TUNEL) staining
was used to detect apoptosis in cells. TUNEL assay was conducted
using a commercial kit (Roche) according to the manufacturer's
protocols. The total number of cells and the number of
TUNEL-positive stained cells were counted in 6 random fields in a
'blinded' manner. Results were expressed as percentage of apoptotic
cells.
Statistical analysis
The data are shown as the means ± SEM, and P<0.05
was considered significant. All experiments were conducted at least
in triplicates. Data analysis was performed using the SPSS 16.0
software (SPSS, Inc., Chicago, IL, USA). One-way analysis of
variance (ANOVA) was used to measure the significance between more
than two groups. The significance of differences between two groups
was analyzed by Student's t-test.
Results
miR-215 is downregulated in EOC tissues
and cell lines
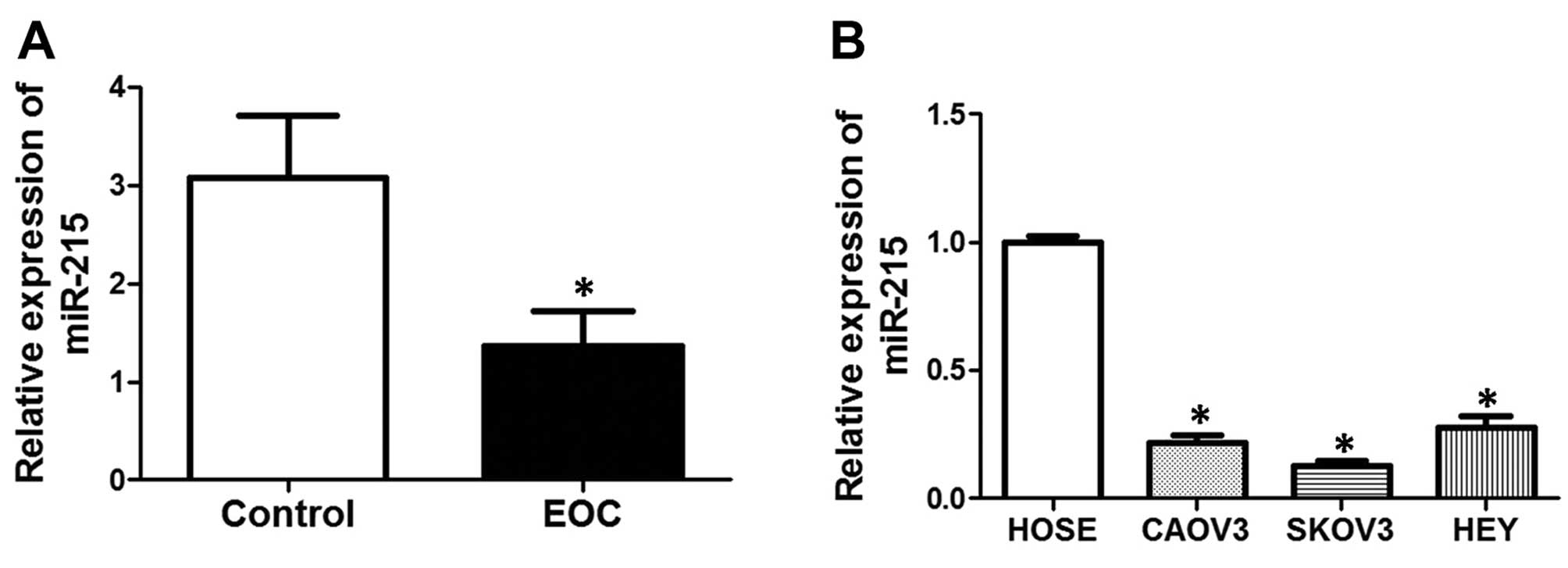
To determine the expression of miR-215 in EOC
tissues and cell lines, we conducted real-time PCR. As shown in
Fig. 1A, mRNA expression of miR-215
was significantly decreased in EOC tissues, compared with controls.
The patient characteristics are shown in Table I. Moreover, we also compared the
expression of miR-215 between normal human ovarian surface
epithelial (HOSE) cells and the human EOC cell lines, CAOV3, SKOV3
and HEY. The results showed that mRNA expression of miR-215 was
notably decreased in EOC cell lines, compared with that in HOSE
cells.
Upregulation of miR-215 inhibited cell
proliferation, promoted apoptosis and increased sensitivity to
chemotherapy drugs
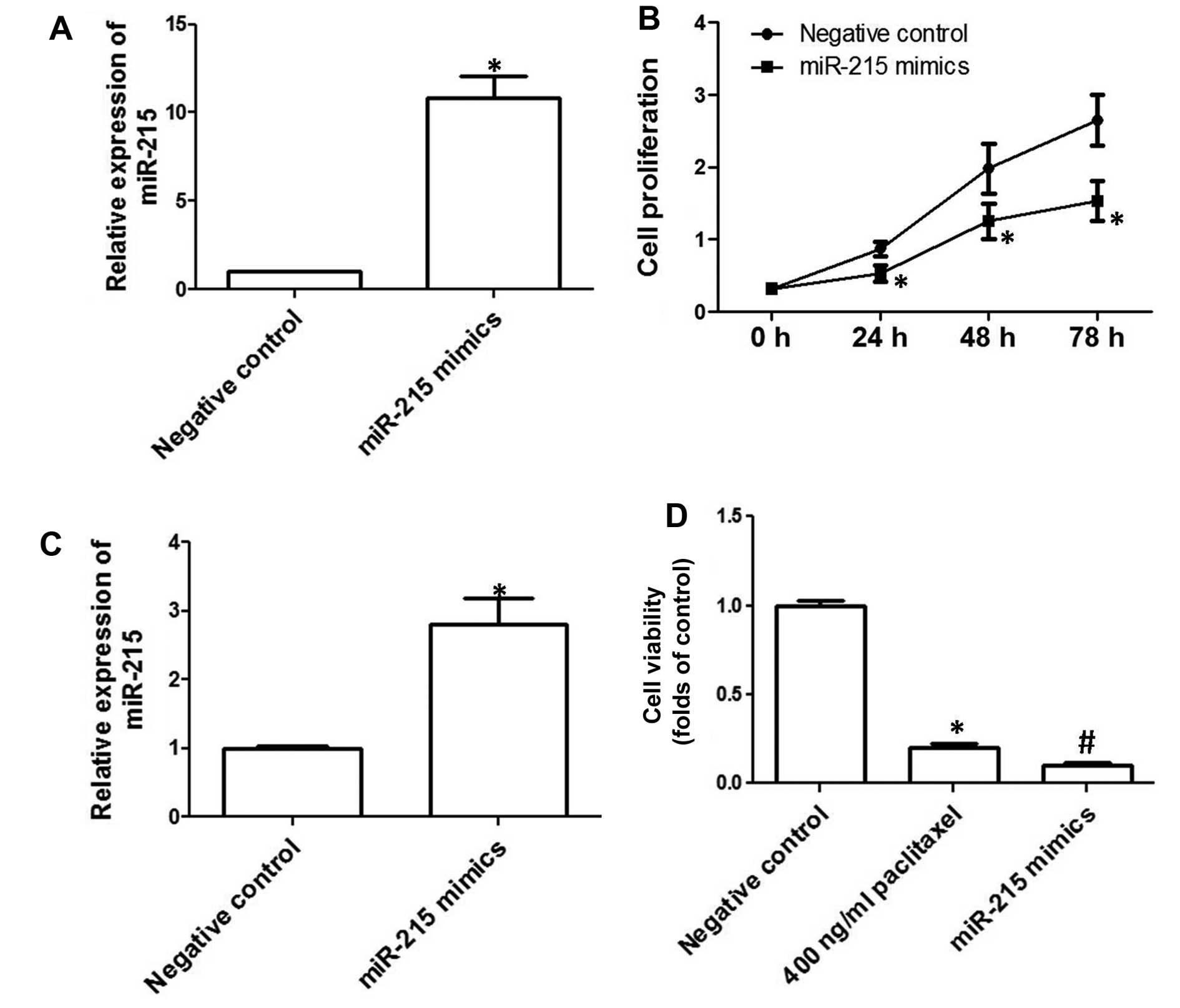
To examine to role of downregulation of miR-215 in
the development of EOC, human EOC cell line SKOV3 was transfected
with miR-215 mimics. As shown in Fig.
2A, miR-215 expression was significantly increased after the
transfection of miR-215 mimics. Cell proliferation of SKOV3 cells
overex-pressing miR-215 was determined and the results showed that
overexpression of miR-215 markedly inhibited cell proliferation in
SKOV3 cells (Fig. 2B). In addition,
we evaluated the effect of miR-215 upregulation on apoptosis. We
showed that overexpression of miR-215 significantly increased
TUNEL-positive cell numbers (Fig.
2C), indicating that miR-215 promoted apoptosis in SKOV3 cells.
Moreover, we examined the effect of overexpression of miR-215 on
the sensitivity to chemotherapy drugs. The results showed that
incubation of 400 ng/ml paclitaxel for 48 h significantly decreased
cell viability (Fig. 2D).
Overexpression of miR-215 markedly promoted paclitaxel-induced
decrease of cell viability (Fig.
2D).
Downregulation of miR-215 promotes cell
proliferation, inhibits apoptosis and decreases sensitivity to
chemotherapy drugs
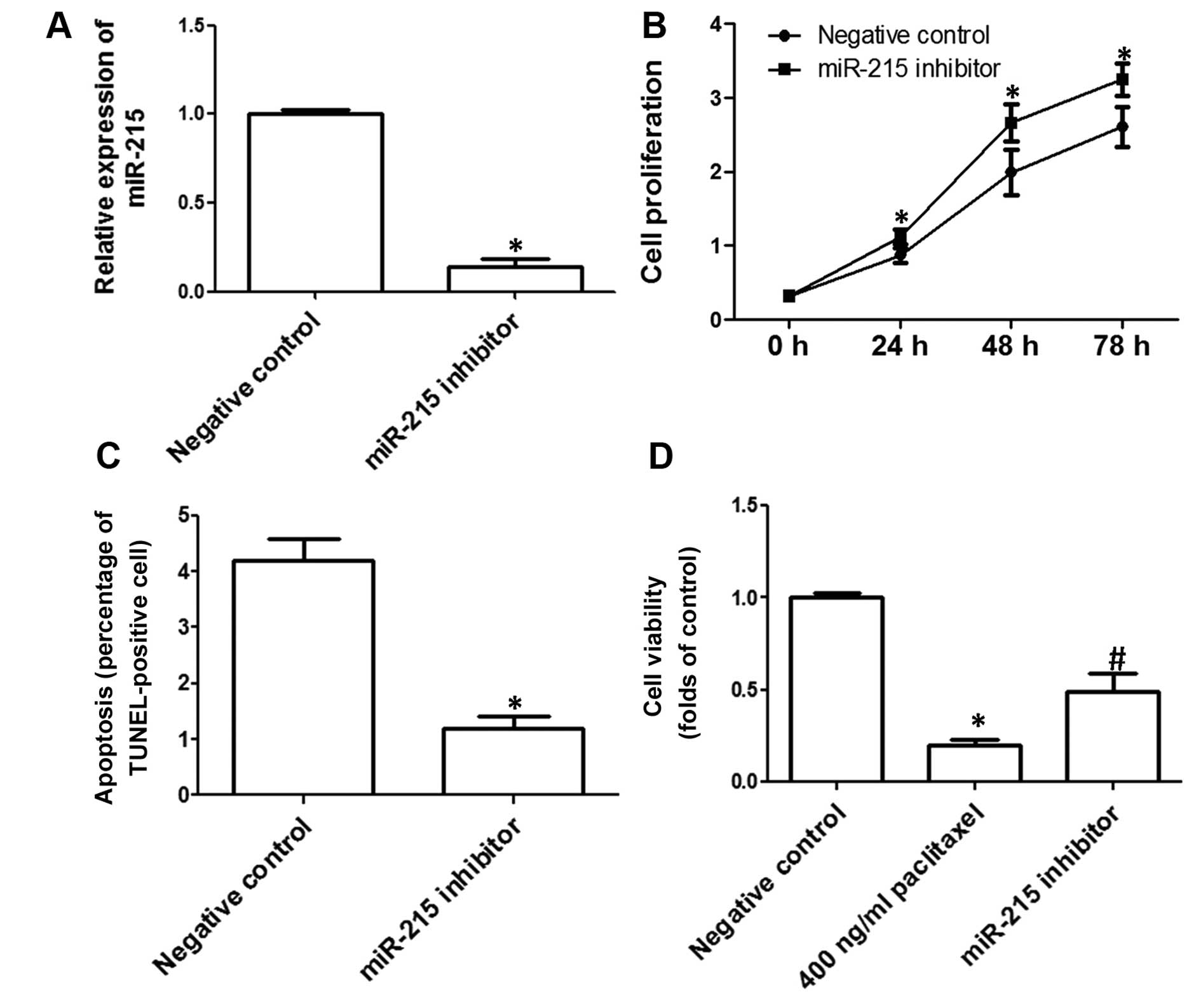
SKOV3 was transfected with miR-215 inhibitors to
investigate the effect of downregulation of miR-215 on cell
proliferation and apoptosis in SKOV3 cells. As shown in Fig. 3A, miR-215 expression was
significantly decreased after the transfection of miR-215
inhibitors. The effect of downregulation of miR-215 on SKOV3 cell
proliferation was detected and the results showed that
downregulation of miR-215 markedly promoted cell proliferation in
SKOV3 cells (Fig. 3B). Moreover, we
evaluated the effect of miR-215 downregulation on apoptosis. We
showed that downregulation of miR-215 significantly decreased
TUNEL-positive cell numbers (Fig.
3C), indicating that downregulation of miR-215 inhibited
apoptosis in SKOV3 cells. We examined the effect of downregulation
of miR-215 on the sensitivity to chemotherapy drugs, and the
inhibition of miR-215 markedly blocked paclitaxel-induced decrease
of cell viability (Fig. 3D).
Regulation of XIAP is involved in
miR-215-induced regulation of cell proliferation, apoptosis and
sensitivity to chemotherapy drugs
Previous results have shown that the
X-chromosome-linked inhibitor of apoptosis (XIAP) could be
regulated by miR-215 (20). In the
present study, we further testing the role of XIAP in
miR-215-exhibiting regulation of cell proliferation, apoptosis and
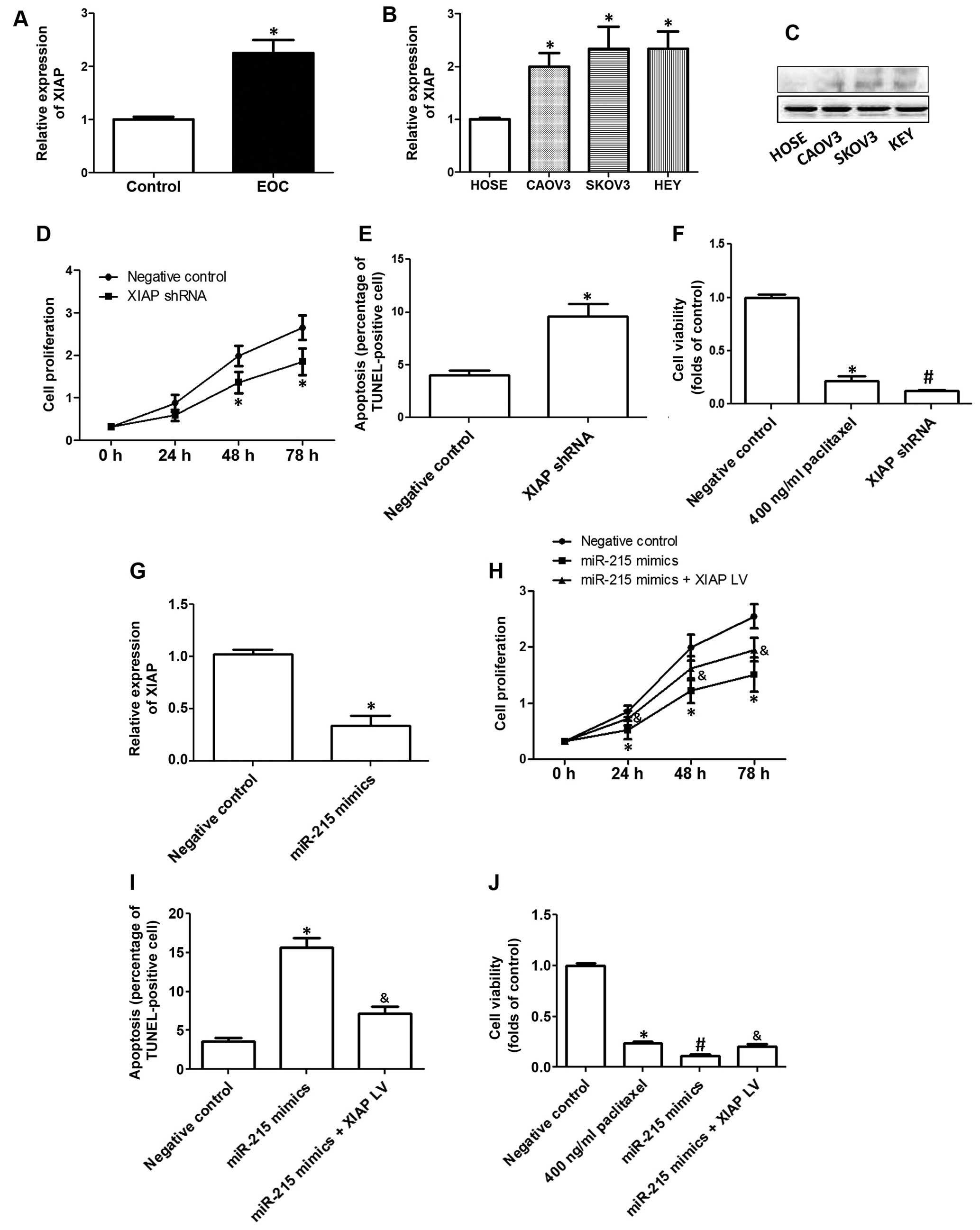
sensitivity to chemotherapy drugs in EOC cells. In Fig. 4A, we show that mRNA expression of
XIAP was significantly increased in EOC tissues, compared with
controls. Moreover, we also compared the expression of XIAP between
HOSE cells and three human EOC cell lines, CAOV3, SKOV3 and HEY
cells. The results showed that mRNA (Fig. 4B) and protein (Fig. 4C) expression of XIAP was notably
increased in EOC cell lines, compared with that in HOSE cells.
In the next step, we evaluated the role of XIAP in
the development of EOC. As shown in Fig. 4D, downregulation of XIAP
significantly inhibited cell proliferation in SKOV3 cells. In
addition, downregulation of XIAP notably increased the percentage
of TUNEL-positive cells in SKOV3 cells (Fig. 4E). Moreover, decrease of XIAP
markedly promoted paclitaxel-induced decrease of cell viability in
OVCAR3 cells (Fig. 4F).
Furthermore, the results showed that overexpression
of miR-215 significantly decreased XIAP expression in EOC cells
(Fig. 4G), indicating that miR-215
was a potential target of miR-215. The decrease of cell viability
induced by miR-215 mimics was markedly blocked by the transfection
of lentivirus overexpressing XIAP (Fig.
4H). Moreover, miR-215 mimic-induced apoptosis was
significantly inhibited by the overexpression of XIAP (Fig. 4I). Overexpression of XIAP also
inhibited miR-215 mimic-promoted sensitivity to chemotherapy drugs,
as reflected by increase of cell viability compared with those
cells treated by paclitaxel plus miR-215 (Fig. 4J).
Discussion
Accumulating literature demonstrates that miRNA
regulation is associated with the pathogenesis of ovarian cancer
(4,16,19,21,22).
However, little is known about the role of miR-215. Our previous
results indicated that miR-215 is abnormally expressed in EOC
samples. In the present study, we investigated the role of miR-215
and the molecular mechanism in the development of EOC.
We found that miR-215 was decreased in EOC tissues
and cell lines. Upregulation of miR-215 inhibited cell
proliferation, promoted apoptosis and increased the sensitivity to
chemotherapy drugs in EOC cells. In contrast, downregulation of
miR-215 increased cell proliferation, inhibited apoptosis, and
decreased the sensitivity to chemotherapy drugs in EOC cells.
Although the literature reporting the relationship between miR-215
and EOC development is limited, there are numerous reports studying
the role of miR-215 in other types of cancer (20,23–26).
Chen et al (23) reported
that miR-215 appeared to exhibit oncogenic properties and promote
the development of gastric cancer (26). Ye et al (20) found that curcumin-activated miR-215
was an important therapeutic target for non-small cell lung cancer.
Zhou et al (24) regarded
the potential of miR-215 as a prognostic predictor for breast
cancer with its high expression in cancer tissues. It was also
found that the expression level of miR-215 was associated with
cervical tumor progression and worse survival rate, suggesting that
it may serve as a potential prognostic marker to identify patients
at higher risk of recurrence (25).
It is suggested that miR-215 plays oncogenic or tumor-suppressive
role in different types of tumors. Our results demonstrate that
miR-215 functions as a tumor suppressor and a chemotherapy
sensitizer of EOC through inhibition of proliferation and promotion
of apoptosis.
We also examined the possible molecular mechanism of
miR-215-exhibiting inhibition of EOC cell proliferation and
promotion of sensitivity to chemotherapy drugs. It was shown that
XIAP was a target of miR-215 in the regulation of lung cancer
(20). Inhibition of XIAP pathways
is involved in the cytotoxicity of EOC cells induced by several
different agents (27–31). The anticancer activity of
20(s)-ginsenoside Rg3 in EOC involved inhibition of XIAP pathways
and downrgulation of inhibitor of apoptosis protein (IAP) family
proteins (27). XIAP expression was
correlated with chemo-resistance of primary chemotherapy and was
identified as a prognostic marker for ovarian clear cell carcinoma
(28). DHA2, a synthesized
derivative of bisbibenzyl, exerted antitumor activity against
ovarian cancer through inhibition of XIAP pathway (29). Bithionol exhibited cytotoxic effects
on various ovarian cancer cell lines, in which inhibition of XIAP
was involved (30). Farrand et
al (31) found that the
diarylheptanoid hirsutenone sensitized chemoresistant ovarian
cancer cells to cisplatin via modulation of XIAP. In the present
study, we also evaluated the possible role of XIAP in
tumor-suppressive effect of miR-215 in EOC. Consistent with
previous results, we found that XIAP acted as an important
oncogenic regulator and desensitized factor for chemotherapy.
Moreover, we found that overexpression of XIAP significantly
inhibited miR-215-exerted decrease of proliferation, increase of
apoptosis and increase of sensitivity to chemotherapy drugs.
Previous results have also shown that XIAP functioned as the
targets of other miRNAs in the regulation of EOC cell proliferation
and chemoresistance (20,32). The data demonstrate that XIAP may be
an important regulator of EOC development and chemotherapy which is
the common downstream target of several tumor-suppressive
miRNAs.
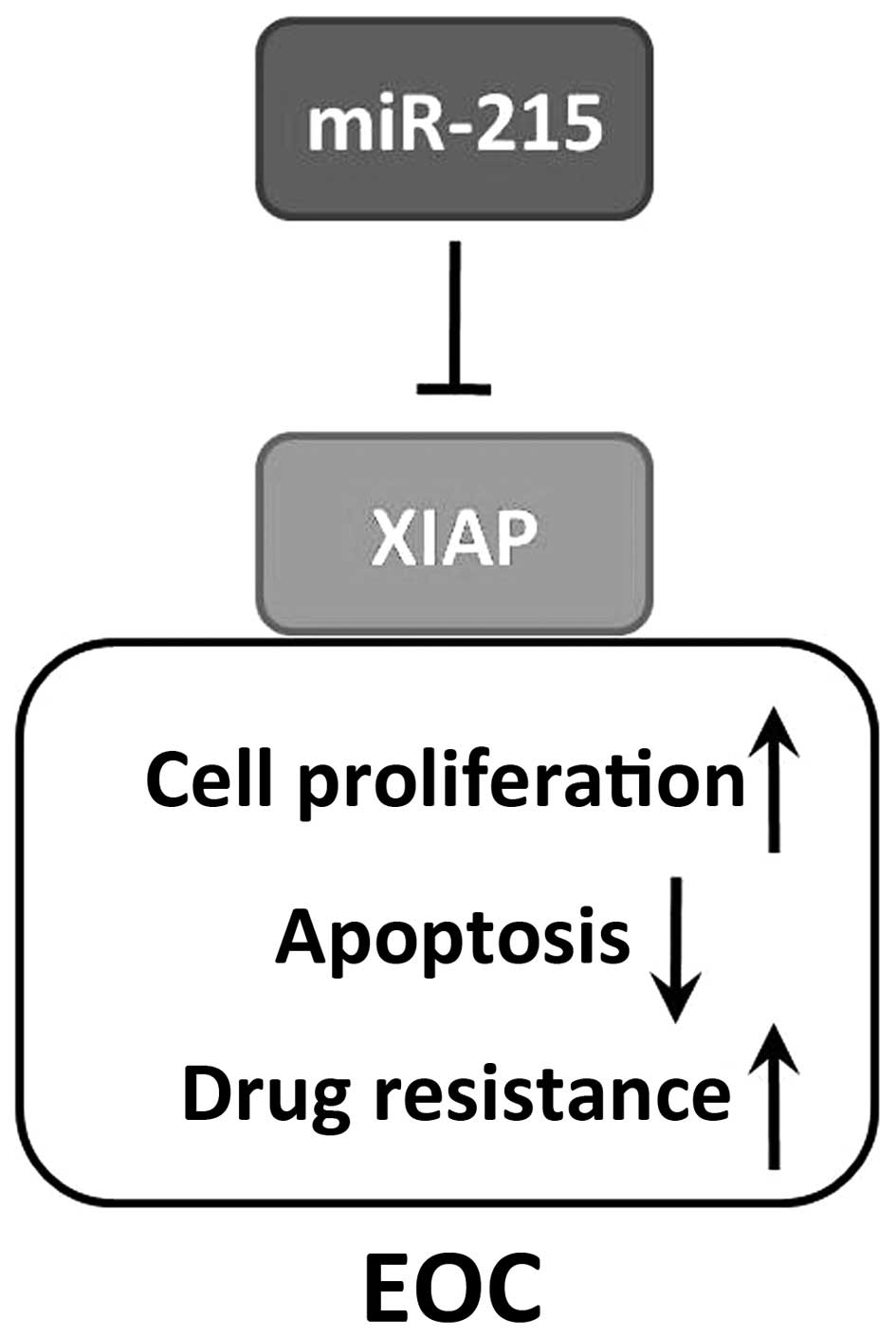
In conclusion, we identified miR-215 as a potential
tumor suppressor in patients with EOC, and it downregulates
expression of the oncogenic regulator XIAP (Fig. 5). Further studies elucidating the
molecular pathways of miR-215/XIAP axis are needed. Overall, the
data demonstrate that miR-215/XIAP pathway may serve as novel
therapeutic targets and prognostic markers in patients with
EOC.
Acknowledgments
The present study was supported by National Natural
Science Funds of China (no. 81402200).
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel R, Ma J, Zou Z and Jemal A: Cancer
statistics, 2014. CA Cancer J Clin. 64:9–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Prat J: Ovarian carcinomas: Five distinct
diseases with different origins, genetic alterations, and
clinicopathological features. Virchows Arch. 460:237–249. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Katz B, Tropé CG, Reich R and Davidson B:
MicroRNAs in ovarian cancer. Hum Pathol. 46:1245–1256. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wen D, Danquah M, Chaudhary AK and Mahato
RI: Small molecules targeting microRNA for cancer therapy: Promises
and obstacles. J Control Release. 219:237–247. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ohtsuka M, Ling H, Doki Y, Mori M and
Calin GA: MicroRNA processing and human cancer. J Clin Med.
4:1651–1667. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li MH, Fu SB and Xiao HS: Genome-wide
analysis of microRNA and mRNA expression signatures in cancer. Acta
Pharmacol Sin. 36:1200–1211. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bartel DP: MicroRNAs: Target recognition
and regulatory functions. Cell. 136:215–233. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen CZ, Li L, Lodish HF and Bartel DP:
MicroRNAs modulate hematopoietic lineage differentiation. Science.
303:83–86. 2004. View Article : Google Scholar
|
|
10
|
Orellana EA and Kasinski AL: MicroRNAs in
Cancer: A historical perspective on the path from discovery to
therapy. Cancers (Basel). 7:1388–1405. 2015. View Article : Google Scholar
|
|
11
|
Garzon R, Marcucci G and Croce CM:
Targeting microRNAs in cancer: Rationale, strategies and
challenges. Nat Rev Drug Discov. 9:775–789. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kishikawa T, Otsuka M, Ohno M, Yoshikawa
T, Takata A and Koike K: Circulating RNAs as new biomarkers for
detecting pancreatic cancer. World J Gastroenterol. 21:8527–8540.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hollis M, Nair K, Vyas A, Chaturvedi LS,
Gambhir S and Vyas D: MicroRNAs potential utility in colon cancer:
Early detection, prognosis, and chemosensitivity. World J
Gastroenterol. 21:8284–8292. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bertoli G, Cava C and Castiglioni I:
MicroRNAs: New biomarkers for diagnosis, prognosis, therapy
prediction and therapeutic tools for breast cancer. Theranostics.
5:1122–1143. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jansson MD and Lund AH: MicroRNA and
cancer. Mol Oncol. 6:590–610. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Braza-Boïls A, Marí-Alexandre J, Gilabert
J, Sánchez-Izquierdo D, España F, Estellés A and Gilabert-Estellés
J: MicroRNA expression profile in endometriosis: Its relation to
angiogenesis and fibrinolytic factors. Hum Reprod. 29:978–988.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Suryawanshi S, Vlad AM, Lin HM,
Mantia-Smaldone G, Laskey R, Lee M, Lin Y, Donnellan N, Klein-Patel
M, Lee T, et al: Plasma microRNAs as novel biomarkers for
endometriosis and endometriosis-associated ovarian cancer. Clin
Cancer Res. 19:1213–1224. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Vaksman O, Stavnes HT, Kaern J, Trope CG,
Davidson B and Reich R: miRNA profiling along tumour progression in
ovarian carcinoma. J Cell Mol Med. 15:1593–1602. 2011. View Article : Google Scholar
|
|
19
|
Zou J, Yin F, Wang Q, Zhang W and Li L:
Analysis of microarray-identified genes and microRNAs associated
with drug resistance in ovarian cancer. Int J Clin Exp Pathol.
8:6847–6858. 2015.PubMed/NCBI
|
|
20
|
Ye M and Zhang J and Zhang J, Miao Q, Yao
L and Zhang J: Curcumin promotes apoptosis by activating the
p53-miR-192-5p/215-XIAP pathway in non-small cell lung cancer.
Cancer Lett. 357:196–205. 2015. View Article : Google Scholar
|
|
21
|
Zhu T, Yuan J, Wang Y, Gong C, Xie Y and
Li H: MiR-661 contributed to cell proliferation of human ovarian
cancer cells by repressing INPP5J expression. Biomed Pharmacother.
75:123–128. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang X, Liu P, Zhang B, Mao H, Shen L and
Ma Y: Inhibitory effects of STAT3 decoy oligodeoxynucleotides on
human epithelial ovarian cancer cell growth in vivo. Int J Mol Med.
32:623–628. 2013.PubMed/NCBI
|
|
23
|
Chen Z, Liu X, Hu Z, Wang Y, Liu M, Liu X,
Li H, Ji R, Guo Q and Zhou Y: Identification and characterization
of tumor suppressor and oncogenic miRNAs in gastric cancer. Oncol
Lett. 10:329–336. 2015.PubMed/NCBI
|
|
24
|
Zhou SW, Su BB, Zhou Y, Feng YQ, Guo Y,
Wang YX, Qi P and Xu S: Aberrant miR-215 expression is associated
with clinical outcome in breast cancer patients. Med Oncol.
31:2592014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liang H, Li Y, Luo RY and Shen FJ:
MicroRNA-215 is a potential prognostic marker for cervical cancer.
J Huazhong Univ Sci Technolog Med Sci. 34:207–212. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Xu YJ and Fan Y: MiR-215/192 participates
in gastric cancer progression. Clin Transl Oncol. 17:34–40. 2015.
View Article : Google Scholar
|
|
27
|
Wang JH, Nao JF, Zhang M and He P:
20(s)-ginsenoside Rg3 promotes apoptosis in human ovarian cancer
HO-8910 cells through PI3K/Akt and XIAP pathways. Tumour Biol.
35:11985–11994. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Miyamoto M, Takano M, Iwaya K, Shinomiya
N, Kato M, Aoyama T, Sasaki N, Goto T, Suzuki A, Hitrata J, et al:
X-chromosome-linked inhibitor of apoptosis as a key factor for
chemoresistance in clear cell carcinoma of the ovary. Br J Cancer.
110:2881–2886. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Pang Y, Si M, Sun B, Niu L, Xu X, Lu T,
Yuan H and Lou H: DHA2, a synthesized derivative of bisbibenzyl,
exerts antitumor activity against ovarian cancer through inhibition
of XIAP and Akt/mTOR pathway. Food Chem Toxicol. 69:163–174. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ayyagari VN and Brard L: Bithionol
inhibits ovarian cancer cell growth in vitro - studies on
mechanism(s) of action. BMC Cancer. 14:612014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Farrand L, Kim JY, Byun S, Im-Aram A, Lee
J, Suh JY, Lee KW, Lee HJ and Tsang BK: The diarylheptanoid
hirsutenone sensitizes chemoresistant ovarian cancer cells to
cisplatin via modulation of apoptosis-inducing factor and X-linked
inhibitor of apoptosis. J Biol Chem. 289:1723–1731. 2014.
View Article : Google Scholar :
|
|
32
|
Pang Y, Mao H, Shen L, Zhao Z, Liu R and
Liu P: MiR-519d represses ovarian cancer cell proliferation and
enhances cisplatin-mediated cytotoxicity in vitro by targeting
XIAP. Onco Targets Ther. 7:587–597. 2014. View Article : Google Scholar : PubMed/NCBI
|