Introduction
Human hepatocellular carcinoma (HCC) is considered
as the third leading cause of cancer-related death worldwide
(1–3). In recent years, although surgical
treatment has greatly improved the survival rate of HCC patients,
its prognosis and survival rate remain poor due to frequent
intra-hepatic and extrahepatic metastases and dissemination
(3,4). Consequently, further investigation of
the underlying molecular mechanisms are vital to identify novel
therapeutic interventions and to improve the prognosis for HCC.
Nanog, a central transcription regulator required
for maintaining the self-renewal capacity and pluripotent state of
embryonic stem cells (ESCs) along with Oct4 and Sox2 (5–10),
exhibits elevated expression in various types of tumor cells
(11,12). Intensive studies indicate that Nanog
also executes parallel functions as in ESCs, participating in
tumorigenesis and promoting the progression of certain types of
tumors, including pancreatic cancer, gastrointestinal tumors,
breast cancer, head and neck squamous cell carcinomas and human HCC
(13–16). In addition, our previous study was
the first to demonstrate that Nanog is involved in the invasion,
chemoresistance, clonogenicity, migration and metastasis of the
cervical carcinoma HeLa cell line (17). Nevertheless, the precise role of
Nanog in human HCC has not been fully explored.
Epithelial-mesenchymal transition (EMT), which was
initially identified as a characteristic of morphogenesis during
embryogenesis, is a transdifferentiation program that reprograms
adherent epithelial cells into more phenotypical mesenchymal cells.
During EMT, epithelial cancer cells exhibit downregulation of the
epithelial marker E-cadherin, but upregulation of mesenchymal
markers N-cadherin and vimentin, promoting the acquisition of
mesenchymal traits that are required for migration and invasion
(2,18,19).
Developmental genetics studies have revealed that the orchestration
of EMT is critical to the development of malignant traits, such as
motility, invasiveness, metastasis, dissemination and
apoptosis-resistance in various tumor cells (20–22).
However, emerging molecular mechanisms and the signaling-network
underlying the regulatory relationship between Nanog and the EMT
pathway in the HCC HepG2 cell line remain unclear.
TALEN is gene editing tools with high efficiency and
specificity and with low genotoxicity in targeted genome
manipulation. TALEN is composed of repeats of DNA-binding domains
and the FokI nuclease domain. TALEN-mediated double-strand breaks
(DSBs) promote endogenous DNA repair mechanisms through error-prone
non-homologous end-joining (NHEJ), by which TALEN successfully
cleave the target genome and induce the mutation of target genes
(23–25). Compared with RNA interference
technology, TALEN offers the advantage of achieving robust
disruption of target gene expression (26).
In the present study, we employed TALEN to induce
diallelic Nanog mutations to disrupt Nanog expression in HepG2
cells. Most significantly, we demonstrated that the disruption of
Nanog expression resulted in reduced proliferation, invasiveness,
migration, enhanced chemosensitivity and reversal of EMT in HepG2
cells. Thereby, Nanog plays an important role in retaining the
malignant phenotype of HepG2 cells.
Materials and methods
Cell lines and culture
The human HCC cell line HepG2 was cultured in
Dulbecco's modified Eagle's medium (DMEM) containing 10% fetal
bovine serum (FBS) (both from Gibco® Life Technologies,
USA) under a humidified condition at 37°C with 5%
CO2.
TALEN plasmid construction and Nanog
targeting
The Nanog-TALEN plasmid was constructed according to
our previous study (17) using a
Fast TALE™ TALEN Assembly kit (SiDan-Sai Biotechnology, China).
Approximately 10 µg of the Nanog-TALEN plasmid (5 µg
each for right and left arm) and control plasmid were mixed with
wild-type HepG2 cells (1×106) in 90 µl of
Opti-MEM (Gibco® Life Technologies), and were
transferred to electroporation cuvettes for electroblotting. After
the cells were transfected with Nanog-TALEN and the control plasmid
under 150 V, the cells were exposed to 3 µg/ml puromycin for
3 days. Then, the medium containing puromycin was replaced with
growth media.
T7 endonuclease 1 (T7E1) and genomic
sequencing
Genomes derived from the Nanog-targeted and control
cells were extracted using a Genomic DNA Extraction Mini kit
(Tiangen, China). The DNA fragment surrounding the Nanog-targeted
site was amplified and then analyzed by T7E1 (View Solid
Biotechnology, China) to evaluate Nanog-mutation efficiency. The
Nanog mutation was confirmed by sequencing.
Cell proliferation assays
The proliferation of targeted and control cells was
monitored using an RTCA SP system, which is a real-time cell-based
assay system (ACEA Biosciences, San Diego, CA, USA). According to
the manufacturer's instructions, 5×103 cells were seeded
in triplicate in an E-Plate VIEW 16 for real-time monitoring of the
proliferative capacities of the cells for 120 h.
Cell migration assays
Scratch assays were used to determine the migratory
ability of HepG2 cells in compliance with our previous description
(17). The cells were maintained in
triplicate into 6-well plates until completely confluent, and then
a scratch was created in the confluent cell monolayers using a
10-µl pipette tip. After the cells were washed 3 times with
phosphate-buffered saline (PBS), the cells was cultured in
serum-free DMEM. The migratory capacity was assessed at 48 h under
a microscope.
Matrigel invasion assays
We employed a Transwell invasion assay (17) to measure the invasive abilities of
the cells in vitro. Cells were trypsinized and resuspended
in DMEM at a density of 2×105 cells/ml. Approximately
200 µl of cell suspension was added in triplicate to the
upper chamber of the polycarbonate membrane filter, which was
embedded with 8-µm pores (Corning, USA), that were
pre-coated with Matrigel. The lower chamber was filled with 500
µl DMEM supplemented with 10% FBS. After the cells were
incubated for 48 h, non-migrating cells on the surface of the
Matrigel in the upper chamber were wiped off by cotton swabs,
whereas the cells migrating to the bottom of the membrane were
fixed in 95% ethanol for 30 min, and then stained with 0.1% crystal
violet. After the stained membrane was washed 3 times with PBS, it
was observed under a microscope and the number of migrating cells
was counted.
Chemosensitivity assays
Chemosensitivity assays were performed to evaluate
the sensitivity of Nanog-targeted and control cells to
chemotherapeutics. Trypsinized cells were washed with PBS and then
seeded in triplicate in an E-Plate VIEW 16 at a density of
1×104 cells/well for real-time monitoring using an RTCA
SP system. After 6 h, cisplatin (40 µg/ml) was added to the
E-Plate VIEW 16 for detection of chemosensitivity at 72 h.
Quantitative PCR
Total RNA from the Nanog-targeted and control cells
was extracted using TRIzol reagent (Invitrogen, USA). The
corresponding cDNA was synthesized using a reverse transcription
kit (Takara, China). SYBR-Green PCR Master Mix was utilized to
amplify the corresponding genes of interest. The PCR cycling
program was as follows: 95°C for 5 min, followed by 32 cycles of
95°C for 30 sec, 58°C for 30 sec, 72°C for 30 sec with a final
10-min incubation at 72°C. The relative levels of gene expression
were analyzed by the 2−ΔΔCt method. The fold-change of
mRNA was normalized to β-actin. Primer sets for corresponding genes
were the same as in our previous study (17).
Western blot analysis
Nanog-targeted and control cells were collected,
washed with PBS; and then lysed on ice using protein lysis buffer
and protein inhibitors. Protein lysates were mixed with SDS-PAGE
protein loading buffer (5:1) and boiled for 5 min, followed by
separation by 12% SDS-PAGE. Then, the proteins of interest were
transferred onto polyvinylidene fluoride (PVDF) membranes
(Millipore, USA). After the membranes were blocked in 5% non-fat
milk in tris-buffered saline with Tween-20 (TBST), they were
incubated overnight at 4°C with primary antibodies, respectively:
anti-Nanog (no. sc-374103), anti-Twist (no. sc-81417) (1:500; Santa
Cruz Biotechnology, USA), anti-Sox2 (no. D6D9), anti-Oct4 (no.
2750) (1:500; Cell Signaling, USA), anti-CD133 (no. bs-4770R),
anti-CDK2 (no. bs-10726R), anti-cyclin E (no bs-0573),
anti-E-cadherin (no bs.1519R), anti-N-cadherin (no. bs-1172R),
anti-vimentin (no. bs-0756R), anti-ABCG2 (no. bs-0662R), anti-MDR1
(no. bs-0563R) (1:400; Bioss, China), anti-cyclin D1 (no. AC853),
anti-cyclin D3 (no AC856), anti-GAPDH (no. AG019) (1:500; Beyotime
Institute of Biotechnology, China). The membranes were then
incubated with a goat anti-rabbit (no. A0208) or anti-mouse (no.
A0216) IgG-conjugated to alkaline phosphatase secondary antibody
for 2 h. Finally, the membranes were washed 3 times with TBST and
imaged using a gel imaging system (Bio-Rad, USA).
Statistical analysis
All data are represented as the mean ± standard
deviation (SD) of 3 repeated experiments. The Student's test was
used to for comparison between two groups. P<0.05 was considered
statistically significant. All data were analyzed with SPSS
statistical software 18.0.
Results
TALEN-mediated diallelic Nanog mutations
in the HepG2 cells
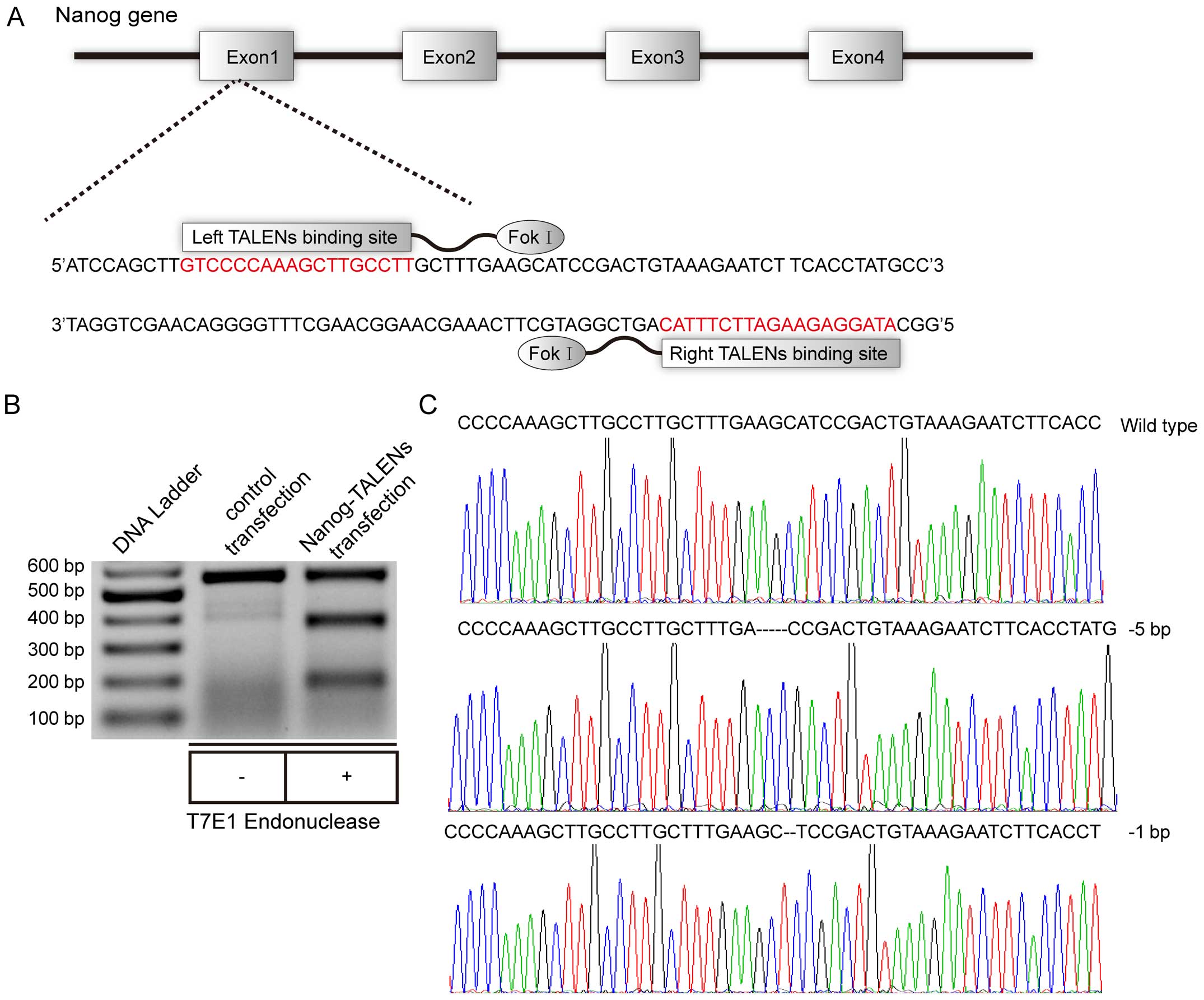
To detect whether TALEN successfully achieved
cleavage of the target site of the Nanog gene (Fig. 1A), genomic DNA from Nanog-targeted
and control cells was extracted and then used to amplify the DNA
fragment containing the Nanog-targeted site. The amplified product
was analyzed by T7E1 to detect the activity and efficiency of
TALEN. Surprisingly, the efficiency of the TALEN-mediated Nanog
gene mutation approached 50% after two transfections (Fig. 1B). Subsequently, we selected out
single cells for further culture from the Nanog-targeted mixture of
cells. After 15 days, single cell-derived subclone genomes were
extracted and analyzed by T7E1. Eventually, we identified subclones
for further genomic sequencing, and found that subclone 3 and 7
exhibited biallelic Nanog mutations. The sequencing results
indicated there were at least 3 Nanog alleles in the HepG2 cells
(Fig. 1C).
Nanog regulates expression of
pluripotency factors to maintain the pluripotency and malignancy of
HepG2 cells
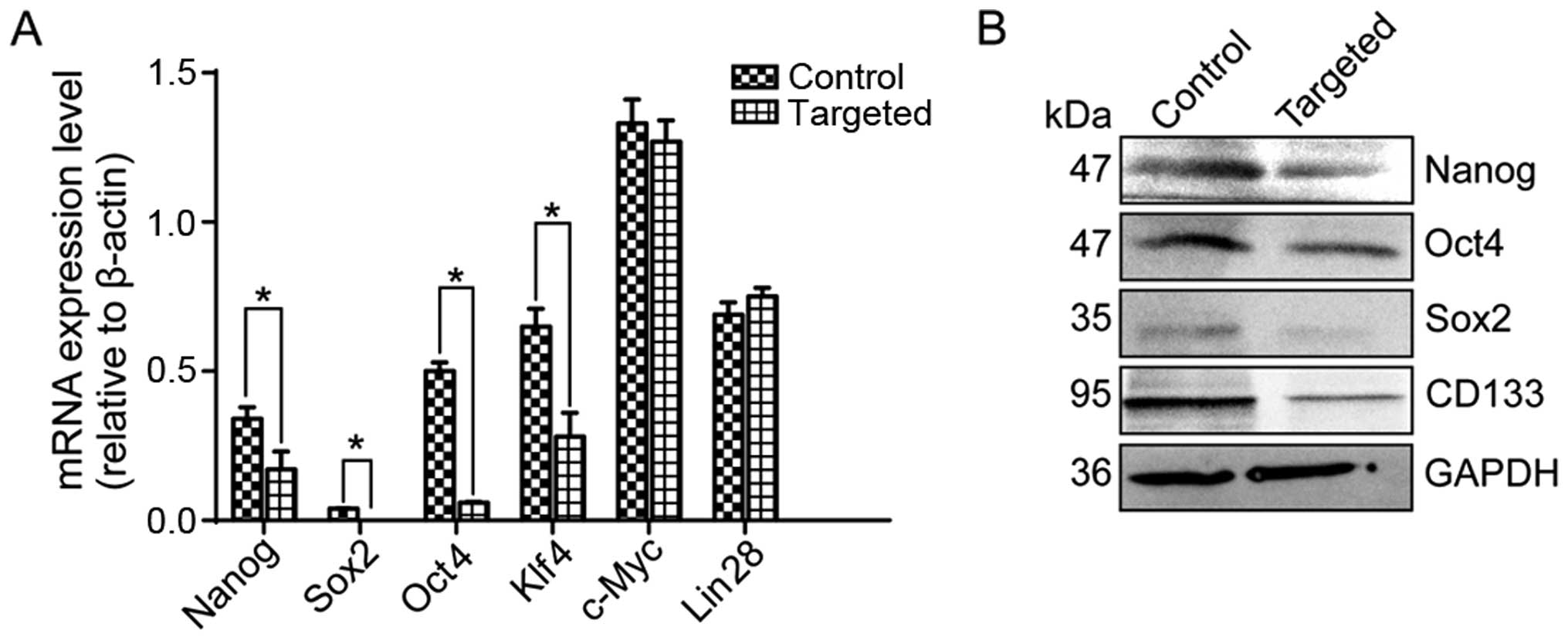
To determine the effects of Nanog disruption on
stemness factors, we detected the expression of the pluripotency
factors Oct4, Sox2, Klf4, c-Myc and Lin28, as well as the
expression of cancer stem cell (CSC) marker CD133 by quantitative
RT-PCR and western blotting. Our results indicated that the
expression of Nanog, Oct4, Sox2, Klf4 and CD133 was markedly
downregulated in the targeted cells, whereas the expression of
c-Myc and Lin28 was not significantly different (Fig. 2A and B).
Disruption of Nanog suppresses the
expression of proliferative markers in the HepG2 cells
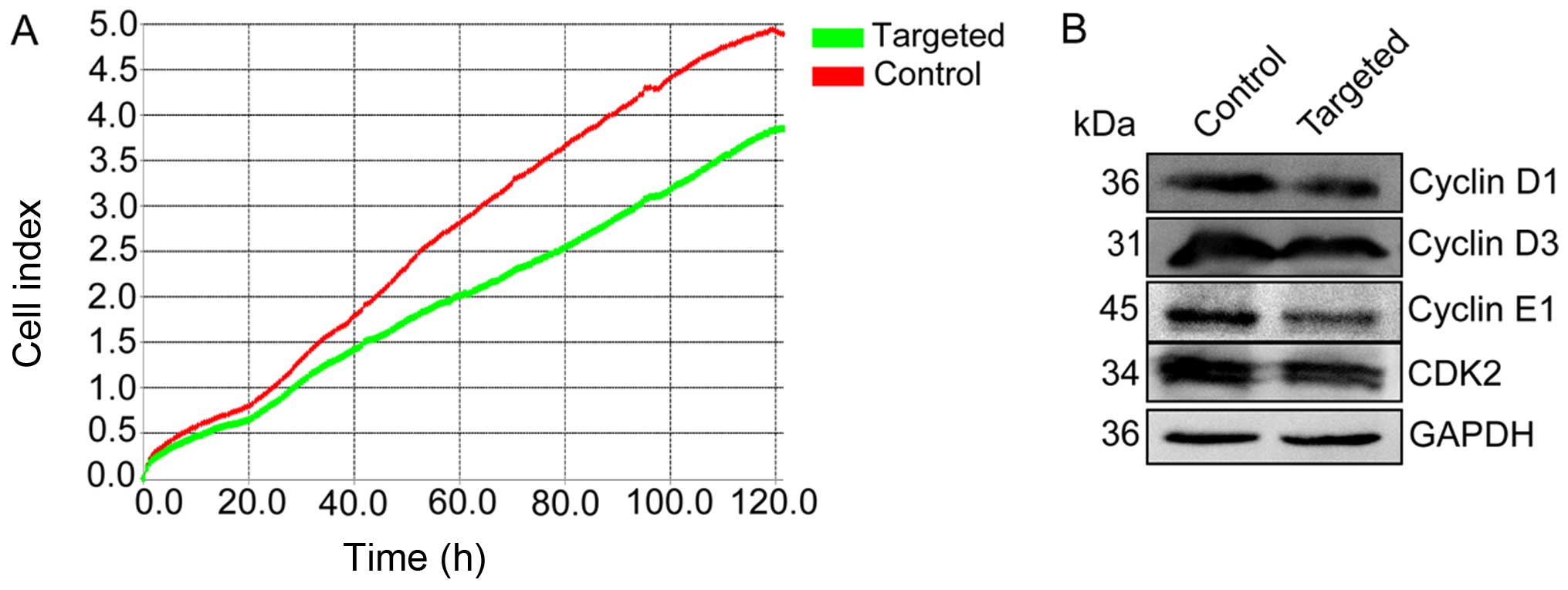
To determine the effect of Nanog disruption on the
apoptosis and proliferation of HepG2 cells, the proliferative
capacity of Nanog-targeted and control cells was measured using an
RTCA SP system, which demonstrated that the proliferative capacity
of Nanog-targeted cells was markedly reduced relative to the
control cells (Fig. 3A). We further
detected cyclin D1/D3/E1 and cyclin-dependent kinase 2 (CDK2)
protein expression by western blotting. In the Nanog-targeted
cells, the expression levels of cyclin D1/D3/E1 and CDK2 protein
were significantly reduced (Fig.
3B).
Disruption of Nanog impairs the migration
and invasion of the HepG2 cells
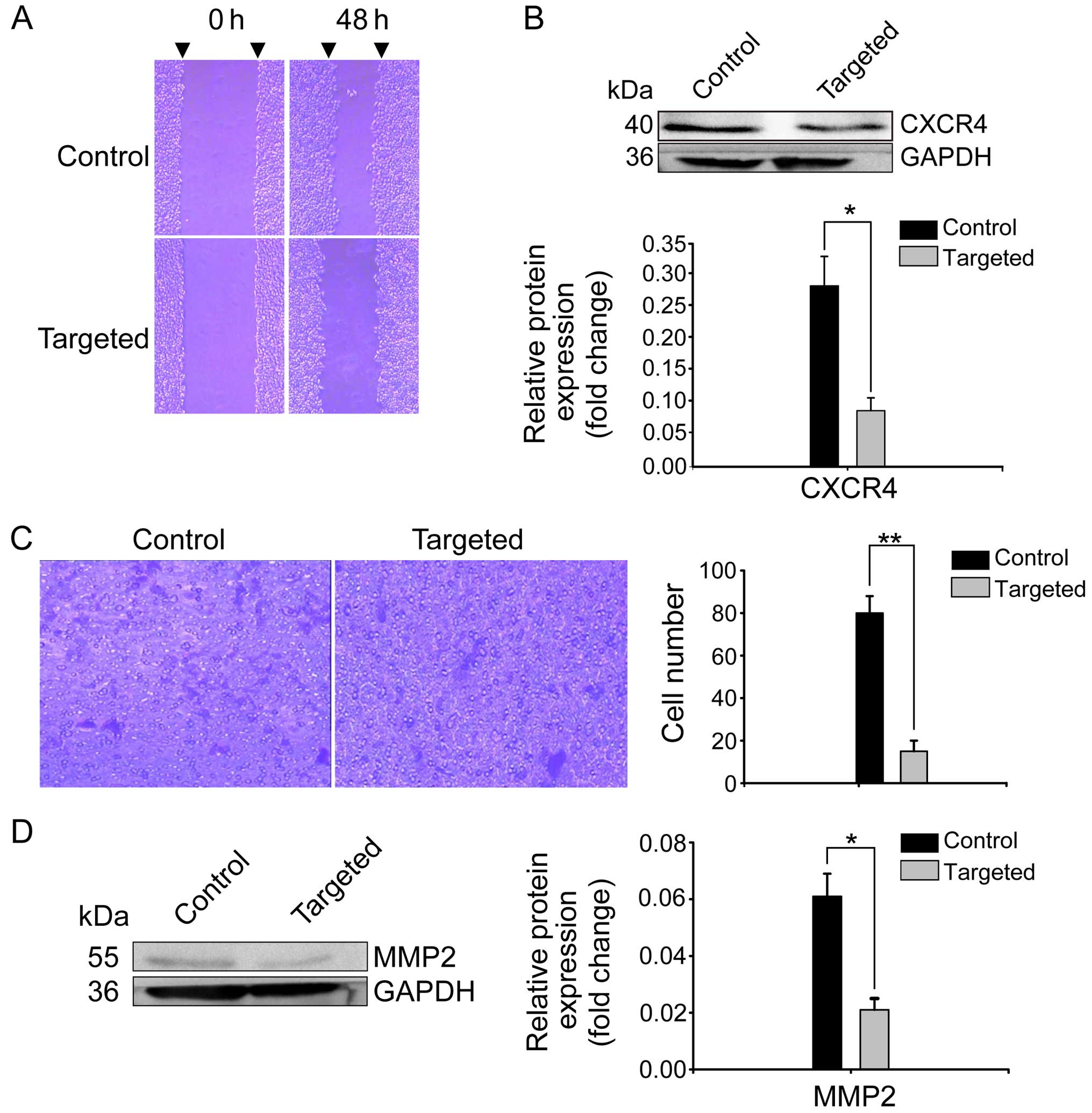
The scratch assays indicated that Nanog disruption
contributed to the reduced migration of HepG2 cells. As depicted in
Fig. 4A, the migration distance of
the targeted cells was significantly less than that of the control
cells. CXCR4 is a migration-related factor that is able to induce
tumor cell migration (14,27,28).
Notably, in the present study, we also found that CXCR4 expression
was significantly decreased in the Nanog-targeted cells (Fig. 4B). Transwell assays indicated that
the number of targeted cells that invaded through the membrane
within 48 h was 15±5, which was less than that of the control cells
(80±8; P<0.01) (Fig. 4C).
Previous studies have reported that matrix metallopeptidase 2
(MMP2) is an invasion-related gene that can regulate the invasive
ability of tumor cells. High expression levels of MMP2 are closely
related to tumor progression, invasion, metastasis and poor
prognosis in various types of cancers (14,29).
In the present study, we also found that MMP2 expression was
noticeably reduced in the targeted cells compared with expression
in the control cells (Fig. 4D;
P<0.05).
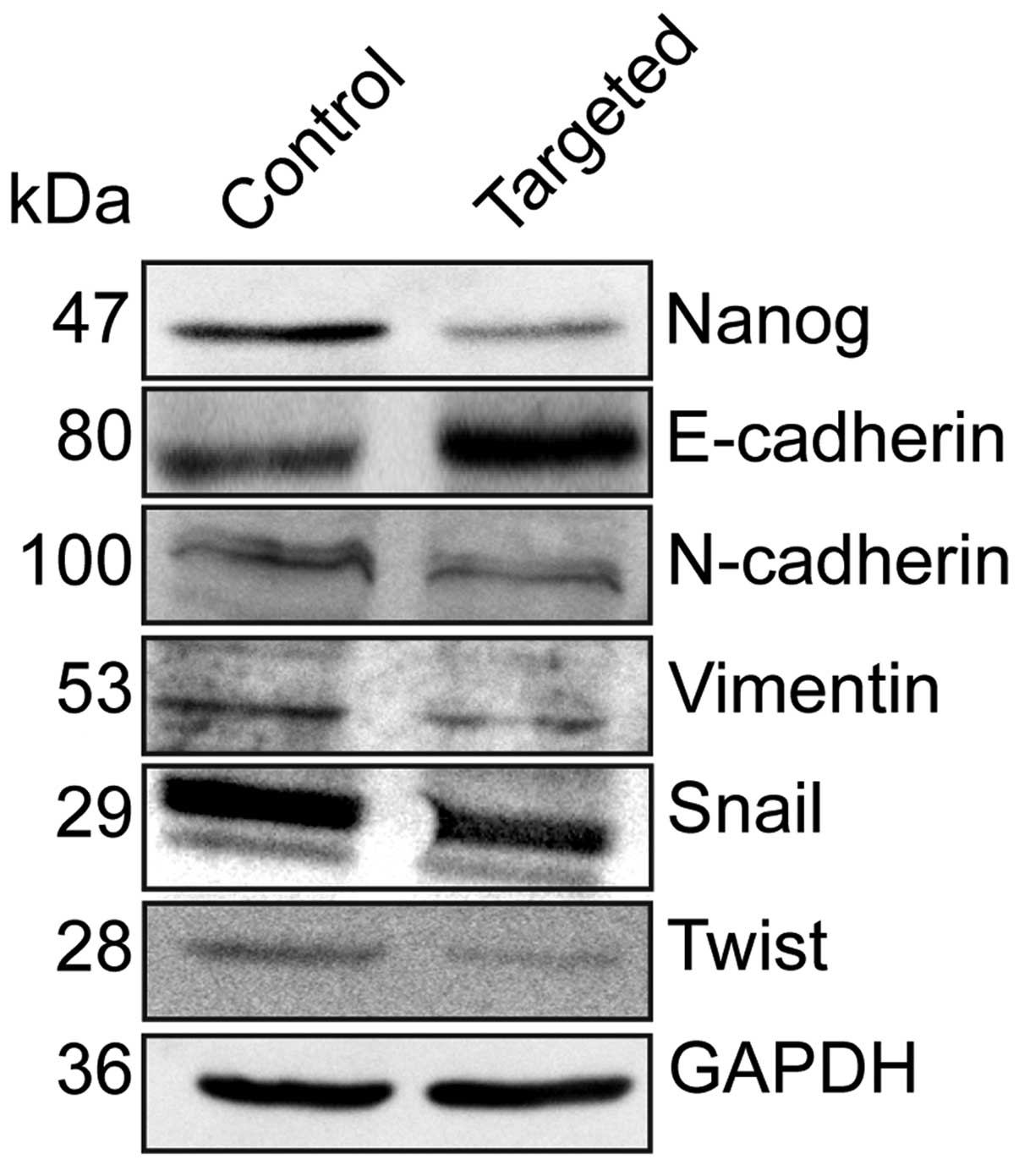
Disruption of Nanog expression decreases
EMT by down-regulating EMT regulators Twist and Snail
To elucidate the effect of the disruption of Nanog
on EMT, we detected the expression of the epithelial marker
E-cadherin, and the mesenchymal markers N-cadherin and vimentin by
western blotting. Relative to the control cells, E-cadherin
expression was elevated, while N-cadherin and vimentin expression
was decreased in the Nanog-targeted cells. We further detected the
expression of the EMT regulators Twist and Snail, and found that
their expression levels were decreased in the Nanog-targeted cells.
(Fig. 5).
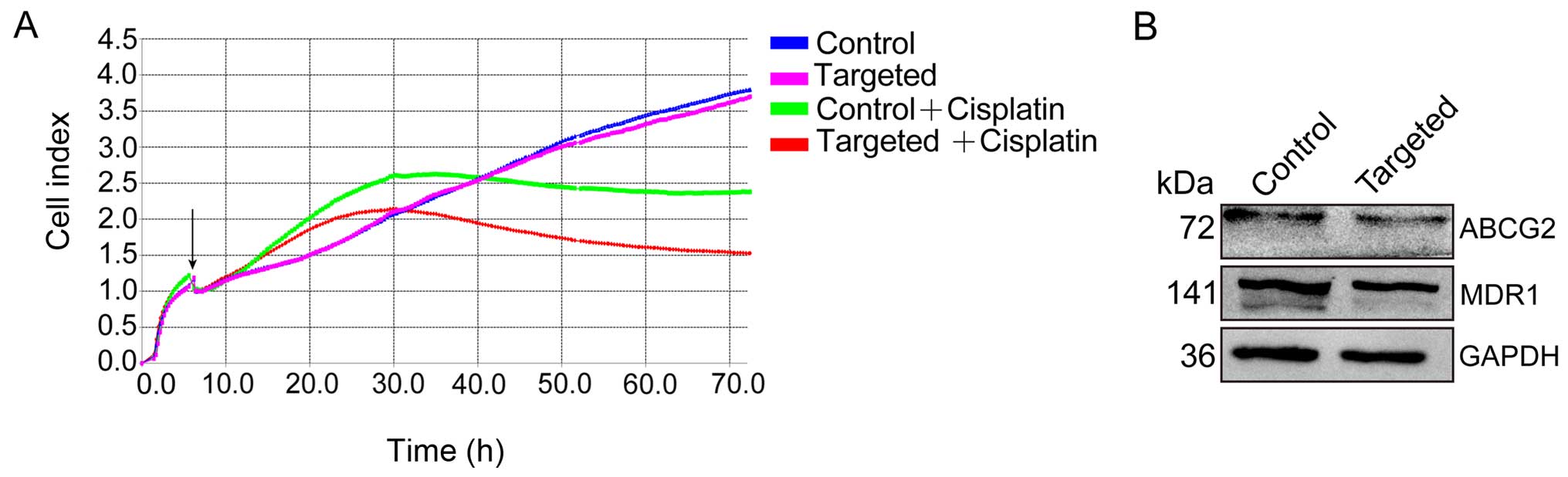
Disruption of Nanog enhances the
chemosensitivity of HepG2 cells by downregulating multidrug
resistance genes
To assess the effect of chemotherapeutics on HepG2
cells, both Nanog-targeted and control cells were exposed to
cisplatin (40 µg/ml), and chemosensitivity was measured
using an RTCA SP system. After 72 h of observation, we noted that
the Nanog-targeted cells were more sensitive to cisplatin than the
control cells (Fig. 6A).
Furthermore, we detected the expression of multi-drug resistant
gene 1 (MDR1) and ATP-binding cassette subfamily G member 2 (ABCG2)
in the Nanog-targeted and control cells, and found that expression
levels of both were significantly downregulated in the
Nanog-targeted cells (Fig. 6B).
Discussion
Increasing evidence suggests that Nanog expression
is elevated in HCC cell lines and in primary tumors, and high Nanog
expression is positively associated with HCC patient prognosis
(1,2,30).
However, currently published investigations have failed to
establish the complete underlying molecular mechanisms of Nanog in
HepG2 cells. In the present study, we aimed to clarify the precise
mechanisms by which Nanog affects the malignant behavior of the HCC
HepG2 cell line.
Previous investigations have verified that Nanog is
able to regulate cell cycle- and apoptosis-related factors to
modulate proliferation and apoptosis in various types of cancers
(12,31). However, whether Nanog exhibits this
function in HepG2 cells remained unclear. In the present study, we
used an RTCA SP system to detect the proliferative ability of
Nanog-targeted and control cells, and demonstrated for the first
time that Nanog can regulate cyclin D1/D3/E and CDK2 to influence
proliferation in HepG2 cells.
To investigate the effect of the disruption of Nanog
on biological behavior, we conducted cell migration, Matrigel
invasion and chemoresistance assays. Our data indicated that the
disruption of Nanog confers an attenuated phenotype with respect to
migration, invasion and chemoresistance. The migration-related gene
CXCR4 and the invasion-related gene MMP2 play critical roles in
migration and invasion in tumor cells, respectively (28,29).
Therefore, we also detected the expression levels of CXCR4 and MMP2
in Nanog-targeted and control cells, demonstrating that the
disruption of Nanog reduced migration and invasion through
downregulation of CXCR4 and MMP2 in the HepG2 cells, respectively.
Therefore, inhibition of CXCR4 and MMP2 hindered HepG2 cell
migration and invasion to a certain degree. The precise mechanisms
by which Nanog regulates CXCR4 and MMP2 require further research,
which may reveal novel therapies and strategies to suppress the
migration and invasion of HCC. Chemoresistance is always a large
barrier for completely eradicating tumors through anticancer drugs.
In the present study, we also detected the sensitivity of
Nanog-targeted and control cells to cisplatin (40 µg/ml)
using an RTCA SP system after 72 h, and demonstrated that the
disruption of Nanog rendered the HepG2 cells more sensitive to
cisplatin (40 µg/ml). We further detected the expression of
ABCG2 and MDR1 in the Nanog-targeted and control cells; and
revealed that the disruption of Nanog expression downregulated
ABCG2 and MDR1 expression to induce chemosensitivity in the HepG2
cells, causing HepG2 cells to have increased sensitivity to
chemotherapeutics. Our results suggest that targeting Nanog, along
with the administration of conventional anticancer drugs, will be
an optimal therapy with which to improve the prognosis and survival
rate of HCC patients.
EMT plays a crucial role in promoting invasion and
metastasis during tumor progression. During EMT, tumor cells reduce
cell-cell adhesion to acquire a mesenchymal-like phenotype and
disseminate into neighboring or distant tissues (32,33).
Therefore, enhanced EMT disrupts E-cadherin-mediated cell-cell
adhesion and converts epithelial-like cells into mesenchymal-like
cells during tumor cell progression. In the present study, we
detected the EMT markers in Nanog-targeted and control cells, and
found that the disruption of Nanog resulted in inhibition of the
EMT process with an elevated E-cadherin level, and with decreased
N-cadherin and vimentin levels in HepG2 cells. Intensive studies
have revealed that transcription factors including Snail and Twist
regulate the EMT process (34–36).
In the present study, we further detected the expression levels of
Snail and Twist in the Nanog-targeted and control cells, and
demonstrating that the expression of these transcription factors
also decreased. These data suggest that Nanog participates in the
regulation of the EMT process to influence the metastasis of HepG2
cells via modulating Snail and Twist. Our data also indicated that
the reversal of Snail and Twist expression may contribute to the
restoration of EMT, which may be an alternative target for the
future gene therapy of HCC patients to block the metastasis and
dissemination of tumor cells.
Abbreviations:
|
TALEN
|
transcription activator-like effector
nucleases
|
|
CSCs
|
cancer stem cells
|
|
HCC
|
hepatocellular carcinoma
|
|
EMT
|
epithelial-mesenchymal transition
|
|
ESCs
|
embryonic stem cells
|
|
CDK2
|
cyclin-dependent kinase 2
|
|
MMP2
|
matrix metallo peptidase 2
|
|
MDR1
|
multi-drug resistant gene 1
|
|
ABCG2
|
ATP-binding cassette subfamily G
member 2
|
|
T7E1
|
T7 endonuclease 1
|
Acknowledgments
The present study was supported by grants from the
Ministry of Science and Technology of China
(2013ZX10001004-002-005), the Natural Science Foundation of Hubei
Province of China (2013CFC033), and the Hubei University of
Medicine Juvenile Scientific and Technological Creativity Team
(2014 CXZ06).
References
|
1
|
Shan J, Shen J, Liu L, Xia F, Xu C, Duan
G, Xu Y, Ma Q, Yang Z, Zhang Q, et al: Nanog regulates self-renewal
of cancer stem cells through the insulin-like growth factor pathway
in human hepatocellular carcinoma. Hepatology. 56:1004–1014. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sun C, Sun L, Jiang K, Gao DM, Kang XN,
Wang C, Zhang S, Huang S, Qin X, Li Y, et al: NANOG promotes liver
cancer cell invasion by inducing epithelial-mesenchymal transition
through NODAL/SMAD3 signaling pathway. Int J Biochem Cell Biol.
45:1099–1108. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hashimoto N, Tsunedomi R, Yoshimura K,
Watanabe Y, Hazama S and Oka M: Cancer stem-like sphere cells
induced from de-differentiated hepatocellular carcinoma-derived
cell lines possess the resistance to anti-cancer drugs. BMC Cancer.
14:7222014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Li X, Luo Q and Song G: Novel therapeutic
strategies for treatment of hepatocellular carcinoma: Targeting
intervention on liver cancer stem cells. Sheng Wu Yi Xue Gong Cheng
Xue Za Zhi. 30:894–898. 2013.In Chinese. PubMed/NCBI
|
|
5
|
Wang J, Levasseur DN and Orkin SH:
Requirement of Nanog dimerization for stem cell self-renewal and
pluripotency. Proc Natl Acad Sci USA. 105:6326–6331. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pan G and Thomson JA: Nanog and
transcriptional networks in embryonic stem cell pluripotency. Cell
Res. 17:42–49. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang J, Rao S, Chu J, Shen X, Levasseur
DN, Theunissen TW and Orkin SH: A protein interaction network for
pluripotency of embryonic stem cells. Nature. 444:364–368. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hyslop L, Stojkovic M, Armstrong L, Walter
T, Stojkovic P, Przyborski S, Herbert M, Murdoch A, Strachan T and
Lako M: Downregulation of NANOG induces differentiation of human
embryonic stem cells to extraembryonic lineages. Stem Cells.
23:1035–1043. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mitsui K, Tokuzawa Y, Itoh H, Segawa K,
Murakami M, Takahashi K, Maruyama M, Maeda M and Yamanaka S: The
homeoprotein Nanog is required for maintenance of pluripotency in
mouse epiblast and ES cells. Cell. 113:631–642. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chambers I, Colby D, Robertson M, Nichols
J, Lee S, Tweedie S and Smith A: Functional expression cloning of
Nanog, a pluripotency sustaining factor in embryonic stem cells.
Cell. 113:643–655. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang ML, Chiou SH and Wu CW: Targeting
cancer stem cells: Emerging role of Nanog transcription factor.
Onco Targets Ther. 6:1207–1220. 2013.PubMed/NCBI
|
|
12
|
Iv Santaliz-Ruiz LE, Xie X, Old M, Teknos
TN and Pan Q: Emerging role of nanog in tumorigenesis and cancer
stem cells. Int J Cancer. 135:2741–2748. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yang L, Zhang X, Zhang M, Zhang J, Sheng
Y, Sun X, Chen Q and Wang LX: Increased Nanog expression promotes
tumor development and cisplatin resistance in human esophageal
cancer cells. Cell Physiol Biochem. 30:943–952. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lu Y, Zhu H, Shan H, Lu J, Chang X, Li X,
Lu J, Fan X, Zhu S, Wang Y, et al: Knockdown of Oct4 and Nanog
expression inhibits the stemness of pancreatic cancer cells. Cancer
Lett. 340:113–123. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang D, Lu P, Zhang H, Luo M, Zhang X, Wei
X, Gao J, Zhao Z and Liu C: Oct-4 and Nanog promote the
epithelial-mesenchymal transition of breast cancer stem cells and
are associated with poor prognosis in breast cancer patients.
Oncotarget. 5:10803–10815. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Huang CE, Yu CC, Hu FW, Chou MY and Tsai
LL: Enhanced chemosensitivity by targeting Nanog in head and neck
squamous cell carcinomas. Int J Mol Sci. 15:14935–14948. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ding Y, Yu AQ, Li CL, Fang J, Zeng Y and
Li DS: TALEN-mediated Nanog disruption results in less
invasiveness, more chemosensitivity and reversal of EMT in Hela
cells. Oncotarget. 5:8393–8401. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chanrion M, Kuperstein I, Barrière C, El
Marjou F, Cohen D, Vignjevic D, Stimmer L, Paul-Gilloteaux P,
Bièche I, Tavares SR, et al: Concomitant Notch activation and p53
deletion trigger epithelial-to-mesenchymal transition and
metastasis in mouse gut. Nat Commun. 5:50052014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rangel MC, Karasawa H, Castro NP, Nagaoka
T, Salomon DS and Bianco C: Role of Cripto-1 during
epithelial-to-mesenchymal transition in development and cancer. Am
J Pathol. 180:2188–2200. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan
A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, et al: The
epithelial-mesenchymal transition generates cells with properties
of stem cells. Cell. 133:704–715. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Polyak K and Weinberg RA: Transitions
between epithelial and mesenchymal states: Acquisition of malignant
and stem cell traits. Nat Rev Cancer. 9:265–273. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Thiery JP and Sleeman JP: Complex networks
orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell
Biol. 7:131–142. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Katsuyama T, Akmammedov A, Seimiya M, Hess
SC, Sievers C and Paro R: An efficient strategy for TALEN-mediated
genome engineering in Drosophila. Nucleic Acids Res. 41:e1632013.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li T, Huang S, Zhao X, Wright DA,
Carpenter S, Spalding MH, Weeks DP and Yang B: Modularly assembled
designer TAL effector nucleases for targeted gene knockout and gene
replacement in eukaryotes. Nucleic Acids Res. 39:6315–6325. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhu H, Lau CH, Goh SL, Liang Q, Chen C, Du
S, Phang RZ, Tay FC, Tan WK, Li Z, et al: Baculoviral transduction
facilitates TALEN-mediated targeted transgene integration and
Cre/LoxP cassette exchange in human-induced pluripotent stem cells.
Nucleic Acids Res. 41:e1802013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jiang D, Zhu W, Wang Y, Sun C, Zhang KQ
and Yang J: Molecular tools for functional genomics in filamentous
fungi: Recent advances and new strategies. Biotechnol Adv.
31:1562–1574. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kucia M, Jankowski K, Reca R, Wysoczynski
M, Bandura L, Allendorf DJ, Zhang J, Ratajczak J and Ratajczak MZ:
CXCR4-SDF-1 signalling, locomotion, chemotaxis and adhesion. J Mol
Histol. 35:233–245. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Müller A, Homey B, Soto H, Ge N, Catron D,
Buchanan ME, McClanahan T, Murphy E, Yuan W, Wagner SN, et al:
Involvement of chemokine receptors in breast cancer metastasis.
Nature. 410:50–56. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Egeblad M and Werb Z: New functions for
the matrix metalloproteinases in cancer progression. Nat Rev
Cancer. 2:161–174. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang XQ, Ng RK, Ming X, Zhang W, Chen L,
Chu AC, Pang R, Lo CM, Tsao SW, Liu X, et al: Epigenetic regulation
of pluripotent genes mediates stem cell features in human
hepatocellular carcinoma and cancer cell lines. PLoS One.
8:e724352013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Han J, Zhang F, Yu M, Zhao P, Ji W, Zhang
H, Wu B, Wang Y and Niu R: RNA interference-mediated silencing of
NANOG reduces cell proliferation and induces G0/G1 cell cycle
arrest in breast cancer cells. Cancer Lett. 321:80–88. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Casas E, Kim J, Bendesky A, Ohno-Machado
L, Wolfe CJ and Yang J: Snail2 is an essential mediator of
Twist1-induced epithelial mesenchymal transition and metastasis.
Cancer Res. 71:245–254. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Acloque H, Adams MS, Fishwick K,
Bronner-Fraser M and Nieto MA: Epithelial-mesenchymal transitions:
The importance of changing cell state in development and disease. J
Clin Invest. 119:1438–1449. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chiou SH, Wang ML, Chou YT, Chen CJ, Hong
CF, Hsieh WJ, Chang HT, Chen YS, Lin TW, Hsu HS, et al:
Coexpression of Oct4 and Nanog enhances malignancy in lung
adenocarcinoma by inducing cancer stem cell-like properties and
epithelial-mesenchymal transdifferentiation. Cancer Res.
70:10433–10444. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Valastyan S and Weinberg RA: Tumor
metastasis: Molecular insights and evolving paradigms. Cell.
147:275–292. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Meng HM, Zheng P, Wang XY, Liu C, Sui HM,
Wu SJ, Zhou J, Ding YQ and Li J: Over-expression of Nanog predicts
tumor progression and poor prognosis in colorectal cancer. Cancer
Biol Ther. 9:295–302. 2010. View Article : Google Scholar
|