Introduction
Breast cancer is the most common malignant cancer
and the leading cause of cancer-related death in women worldwide
(1). The vast majority of breast
cancer-related deaths are due to metastatic diseases (2). Breast cancer metastasis is a complex
and multistep process. Numerous key pathways, such as TGF-β, WNT,
NFκB, PI3K and JAK-STAT signaling pathways, are involved in breast
cancer development and metastasis (3).
Wnt/β-catenin pathway plays an important role in
regulating cell proliferation, fate specification and
differentiation in numerous developmental stages and adult tissue
homeostasis. Wnt/β-catenin pathway is activated when Wnt ligands
bind to a seven-pass transmembrane Frizzled (Fz) receptor and its
co-receptor, low-density lipoprotein receptor-related protein 6
(LRP6) or its close relative LRP5. The activation of Wnt/β-catenin
pathway prevents phosphorylation and degradation of β-catenin, the
main factor of this pathway, by the GSK3β/APC/Axin destruction
complex, and increases the cytosolic and nuclear β-catenin
accumulation. The β-catenin accumulated in the nucleus forms
complexes with T-cell factor/lymphoid enhancing factor (TCF/LEF)
transcription factors and consequently activates target genes
regulating cell proliferation, apoptosis and migration (4,5).
Wnt/β-catenin pathway has been reported to be abnormally activated
in a variety of cancers including breast cancer (6–8).
MicroRNAs (miRNAs) are small endogenous non-coding
RNAs that post-transcriptionally regulate gene expression through
mRNA degradation or translational repression and monitor several
biological processes (9). In
general, individual miRNAs regulate multiple mRNAs and individual
mRNAs can be targeted by multiple miRNAs (9). Several human miRNAs have been shown to
regulate the metastasis of breast cancer cells (10,11).
MicroRNA-148a (miR-148a), as a member of miR-148/152 family, plays
an important role in the growth and development of normal tissues
and is involved in the genesis and development of disease (12). The downregulated expression of
miR-148a has been found in human gastrointestinal (13)and pancreatic cancers (14,15),
and other tumor types (16). Recent
studies have shown that miR-148a is downregulated in breast cancer
cells and tumors (17,18). However, the roles and mechanisms of
miR-148a in breast cancer metastasis remain to be elucidated.
In the present study, we found downregulated
expression of miR-148a in breast cancer tissues and cell lines.
Furthermore, we demonstrated that miR-148a was able to inhibit the
migration and invasion of breast cancer cells by transfecting
miR-148a mimic in MCF-7 and MDA-MB-231 cells. Importantly, our
results showed miR-148a directly inhibited the expression of WNT-1
and inactivated the Wnt/β-catenin pathway in breast cancer cells.
These findings provide new insights into the molecular mechanisms
of breast cancer metastasis and provide a therapeutic strategy for
the treatment of cancer breast.
Materials and methods
Cell lines
Human embryonic kidney cell line 293T, breast cancer
cell lines (MCF-7, MDA-MB-231, SKBR3, T47D, BT549 and MDA-MB-435S),
and mammary epithelial cell (MCF-10A) were all purchased from
American Type Culture Collection (ATCC; Manassas, VA, USA). The
cells were cultured in a humidified atmosphere with 5%
Co2 at 37°C.
Cell transfection
The miR-148a mimic and negative control (NC) mimic
were purchased from RiboBio (Guangzhou, China). MCF-7 and
MDA-MB-231 cells were seeded on 6-well plates
(3×105/well), and cultured overnight. Cells were then
transfected with 15 nM miR-100 mimic or miR-NC using Lipofectamine
2000 according to the manufacturerss instructions (Life
Technologies, USA). After 48 h, the cells were used for western
blotting and qRT-PCR analysis.
RNA isolation and qRT-PCR analysis
Total RNA and miRNAs from breast cancer cells were
isolated using a miRNA isolation kit (BioTeke, China). qRT-PCR for
miR-148a was performed using the TaqMan MicroRNA assay as described
in our previous studies (19). For
mRNA, 100 ng RNA was reverse transcribed to cDNA using M-MLV
reverse transcriptase (Promega, USA), followed by qPCR using SYBR
Premix Ex Taq™ II kit (Takara, Japan) as described in our previous
studies (19). The expression
levels of miR-148a and WNT-1, TCF-4, LEF-1 mRNA were normalized to
that of u6 small nuclear RNA (u6 snRNA) or GAPDH gene. The PCR
amplification primer sequences are shown in Table I. The fold-change for each miRNA and
mRNA relative to the control was calculated using the
2−ΔΔCt method.
 | Table IPrimer sequences used for the qRT-PCR
analysis. |
Table I
Primer sequences used for the qRT-PCR
analysis.
| Application | Oligonudeotides | Sequences
(5′-3′) |
|---|
| miR-148a | F |
GGCAGTCTCAGTGCACTACAG |
| R | GTGCAGGGTCCGAGGT |
| U6 | F |
CTCGCTTCGGCAGCACA |
| R |
AACGCTTCACGAATTTGCGT |
| WNT-1 | F |
TGCACGCACACGCGCGTACTGCAC |
| R |
CAGGATGGCAAGAGGGTTCATG |
| TCF-4 | F |
GCAATGTGGCAACTTGGAC |
| R |
CAGACCAAGCTCCTGATCCT |
| GAPDH | F |
AGCCACATCGCTCAGACAC |
| R |
GCCCAATACGACCAAATCC |
Western blot analysis
Cells were lysed and total proteins were extracted
as previously described (19).
Equal amounts of proteins (30–50 μg) were subjected to 10%
SDS-PADE separation, and then transferred to PVDF membranes.
Membranes were incubated with primary antibodies against human
WNT-1 (1:400; Boster), β-catenin (1:1,000; PeproTech), MMP-7
(1:500; Boster) or GAPDH (1:1,000) followed by incubation with
peroxidase-conjugated secondary antibody (Santa Cruz Biotechnology,
USA). Protein bands were visualized by enhanced chemiluminescence
(ECL; Amersham, Germany). The expression levels of proteins were
quantitatively analyzed with FluorChem V2.0 software (Alpha
Innotech Corp., USA).
Dual luciferase reporter assay
293T cells (1.2×104) in 24-well plates
were co-transfected with 15 nM of miR-148a mimic or miR-NC and 10
ng of luciferase reporter plasmids containing either wild-type or
mutant WNT-1-3′-UTR using Lipofectamine 2000. Forty-eight
hours after transfection, luciferase reporter assays were performed
using the Dual Luciferase Reporter Assay kit (Promega), according
to the manufacturer's protocol.
Transwell migration and invasion
assays
The migration and invasion of cells were analyzed
using 24-well Boyden chambers with 8-μm pore size
polyethylene membranes (Corning, USA). For the invasion assay, the
Transwell membranes were precoated with Matrigel (BD Biosciences,
USA). For both assays, cells were seeded in starvation medium on
the top chamber, and the bottom chamber was filled with 0.5 ml cell
culture medium containing 10% FBS. After 24 h incubation, the cells
that migrated or invaded to the lower chamber were fixed with 4%
paraformaldehyde and stained with crystal violet solution. The
cells were counted under a light microscope (magnification, ×200;
five random fields/well), and were analyzed using ImageJ
software.
Human samples
Human breast cancer and adjacent normal tissues for
qRT-PCR analysis were obtained from 69 breast cancer patients and
for in situ hybridization and immunohistochemistry were
obtained from 55 breast cancer patients, who underwent surgery at
the First Affiliated Hospital of China Medical University between
2011 and 2012. Written informed consent was obtained from all
patients. The study was approved by the Institutional Review Board
of China Medical University Research Ethics Committee. This
research was conducted in accordance with the Declaration of
Helsinki.
Immunohistochemistry
Immunohistochemistry was performed as previously
described (20). Briefly,
4-μm sections obtained from paraffin-embedded tumor tissues
from breast cancer patients were incubated with primary antibody
against WNT-1 (1:200; Boster). Images from each section were
evaluated under a Nikon Eclipse 80i microscope (at a magnification
of ×200; Nikon, Japan). Five random fields without overlaps from
each section were counted. The intensity score was defined as: for
no staining (0), weak (1), moderate
(2) or strong (3) staining. The percentage score was
defined as 0 for <5% staining, 1 for 5–25% staining, 2 for
26–50% staining, 3 for 51–75% staining, and 4 for >75% staining.
The intensity scores were multiplied with the percentage score to
obtain the final scores.
In situ hybridization
In situ hybridization was performed using
Enhanced Sensitive ISH Detection KitII as specified by the
manufacturer (MK1030; Boster, China). Briefly, the slides were
hybridized with 8 μg/ml probe complementary to miR-148a
LNA-modified and DIG-labeled (Shanghai Sangon Biological
Engineering Technology And Service Co., Ltd., China). After
incubation with anti-DIG-HRP Fab fragments conjugated to
horseradish peroxidase, the slides were detected by incubating with
3,3′-diaminobenzidine (DAB) and nuclei were counterstained with
hematoxylin. Quantification of the staining intensity of miR-148a
was performed through image analysis the same manner as
immunohistochemistry.
Statistical analysis
Analyses were performed using SPSS 17.0. A
two-tailed Student's t-test was used to evaluate the statistical
significance of the differences between two groups. One-way
analysis of variance (ANOVA) was used to compare the differences
among three or more groups. The Pearson's rank correlation analysis
was applied to assess the association between the expression of
miR-148a and WNT-1. Probability values <0.05 were considered to
indicate a statistically significant result.
Results
Expression of miR-148a is downregulated
in breast cancer tissues and cell lines
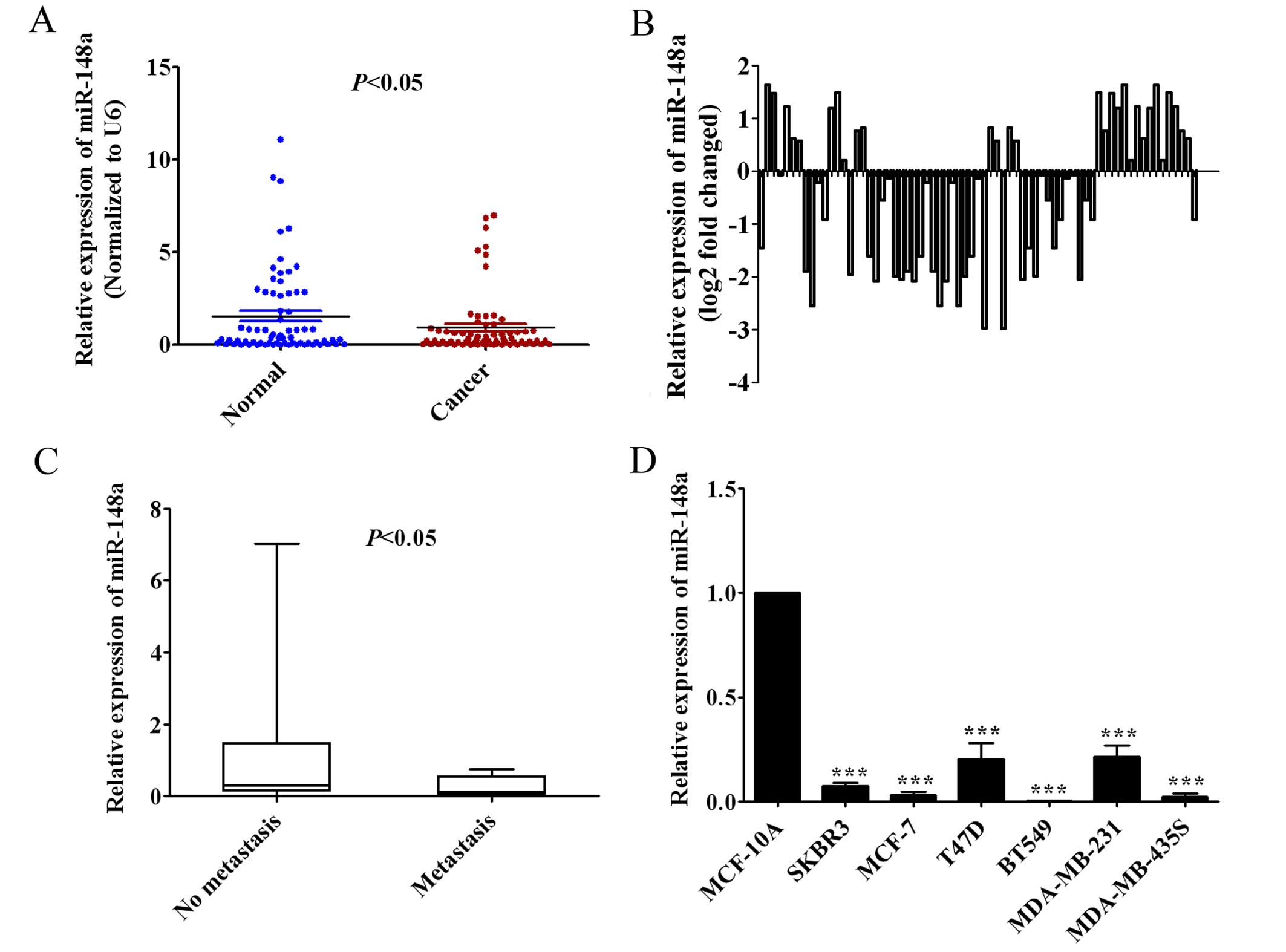
We measured miR-148a expression in 69 pairs of human
breast cancer tissues and adjacent normal breast tissues by qRT-PCR
to observe the clinical relevance of miR-148a in human breast
cancer patients. The findings showed that the expression of
miR-148a in human breast cancer tissues was significantly lower
than in the adjacent normal breast tissues (Fig. 1A; P<0.05). In addition, we found
that miR-148a expression was decreased at least 2-fold compared
with adjacent normal breast tissues in 15.9% (11/69) of human
breast cancer cases (Fig. 1B).
Furthermore, the low expression of miR-148a was shown to be closely
correlated with lymph node metastasis by Mann-Whitney u test
(P<0.05; Fig. 1C). We also found
that the expression of miR-148a was significantly downregulated in
SKBR3, MCF-7, T47D, BT549, MDA-MB-231 and MDA-MB-435S breast cancer
cells compared with human mammary epithelial MCF-10A cells by
qRT-PCR analysis (Fig. 1D).
Overall, these results suggested that the expression of miR-148a
was downregulated in breast cancer tissues and established cell
lines, and the low expression of miR-148a may be relevant to
metastasis of breast cancer.
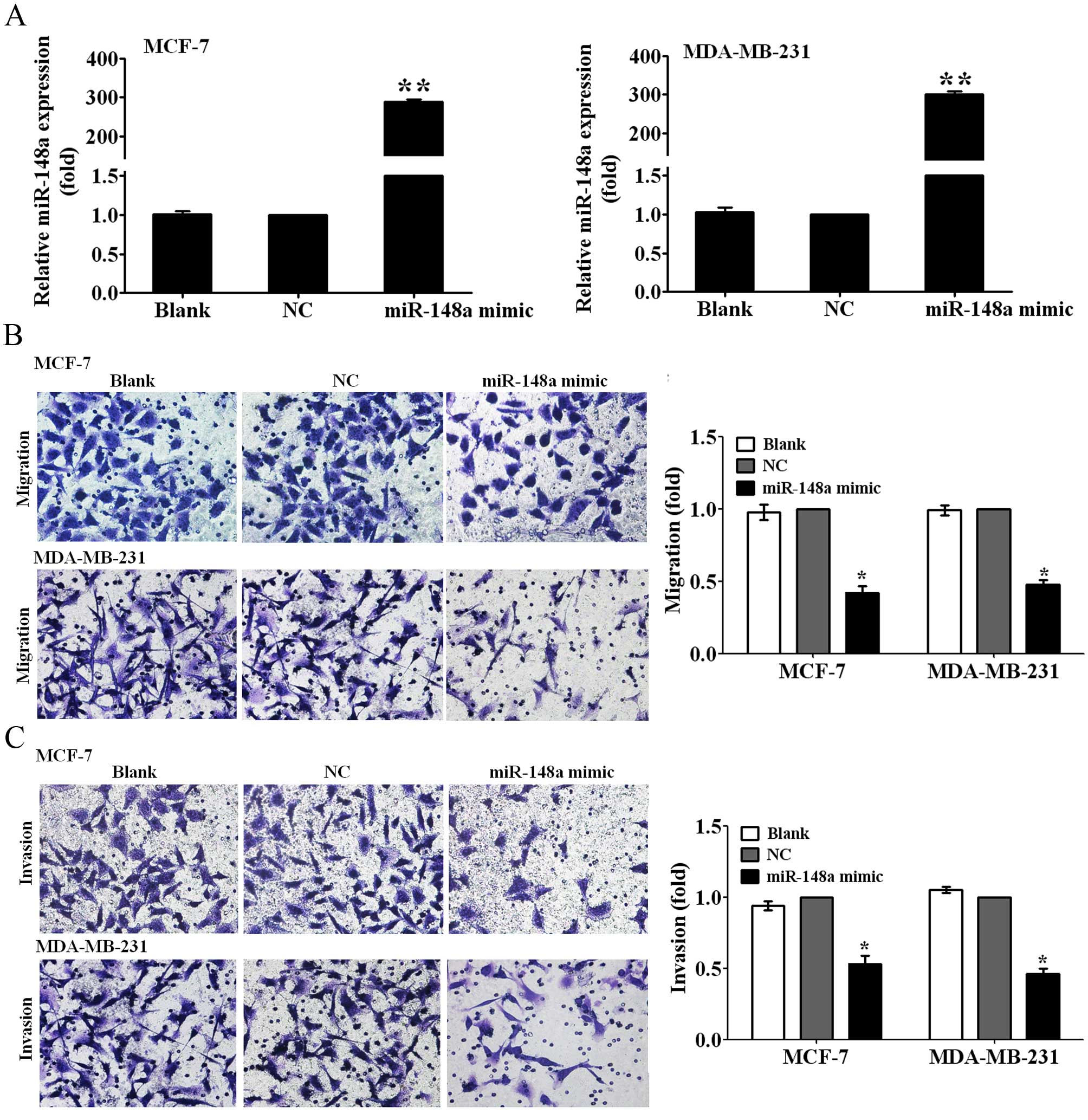
Ectopic miR-148a expression inhibits the
migration and invasion of breast cancer cells
To observe whether miR-148a can inhibit the
migration and invasion of breast cancer cells, we first transfected
MCF-7 and MDA-MB-231 breast cancer cells with miR-148a mimic for 48
h, and then detected the expression levels of miR-148a using
qRT-PCR analysis. It is noteworthy that the expression of miR-148a
was increased by ~280- and 300-fold, respectivly, in MCF-7 and
MDA-MB-231 cells transfected with the miR-148a mimic relative to
those transfected with NC (P<0.01; Fig. 2A). We measured the changes of
migration and invasive abilities of MCF-7 and MDA-MB-231 cells
transfected with the miR-148a mimic by Transwell migration and
invasion assays. The results showed that the overexpression of
miR-148a suppressed the migration ability of MCF-7 and MDA-MB-231
cells to 40 and 45% of the control (P<0.05; Fig. 2B), and decreased the invasion
abilities of MCF-7 and MDA-MB-231 cells to 50 and 45% of the
control (P<0.05; Fig. 2C). The
data suggested that miR-148a inhibited breast cancer cell migration
and invasion.
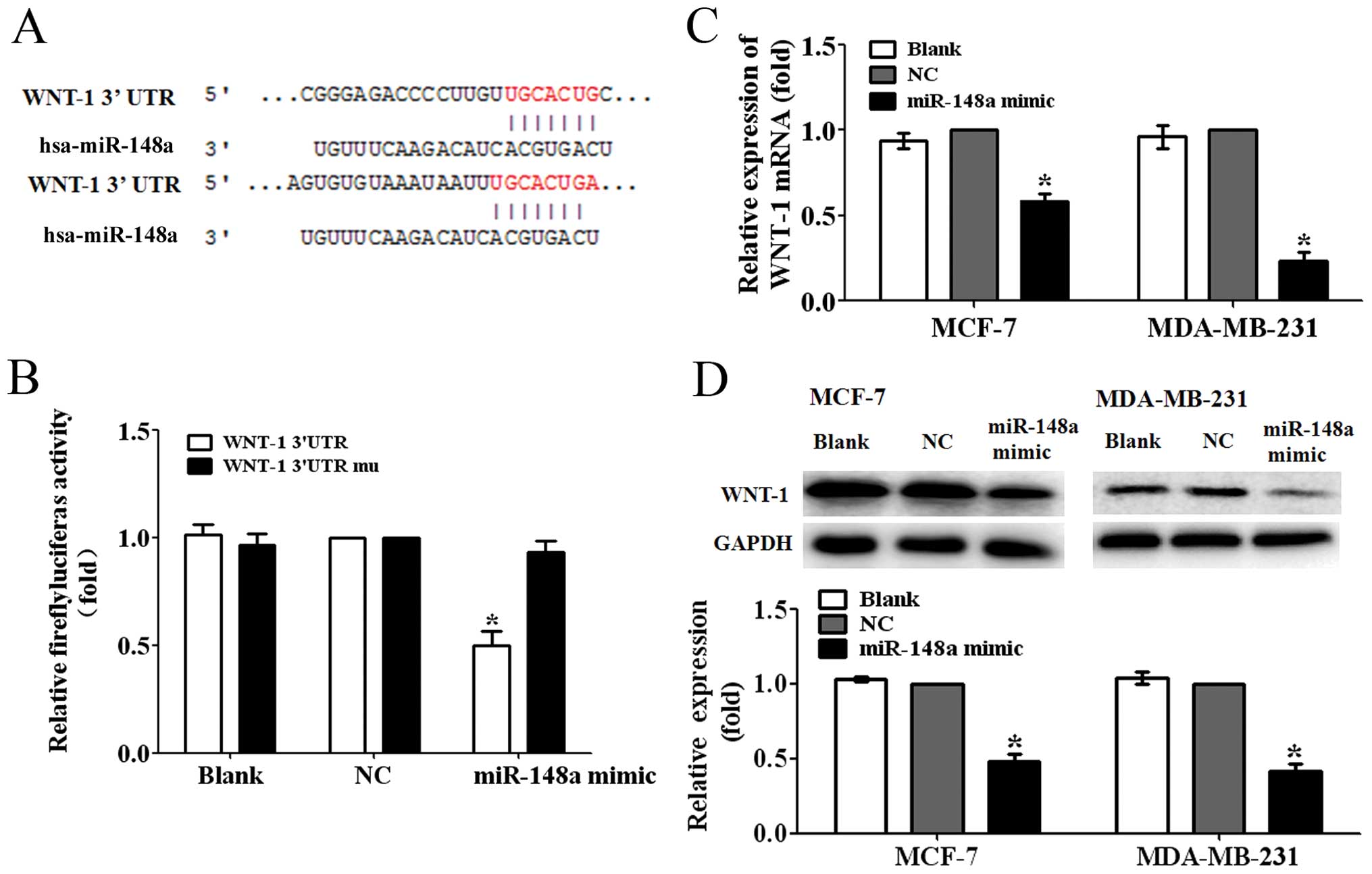
WNT-1 is a direct target of miR-148a
To ascertain the possible mechanisms of miR-148a
suppressing the migration and invasion of breast cancer cells, we
predicted the putative targets of miR-148a by TargetScan. We
focused on the genes related to Wnt/β-catenin signaling pathway
involved in the tumor metastasis. We found that WNT-1, one of the
major ligands of Wnt/β-catenin signaling pathway, was one of the
targets of miR-148a (Fig. 3A). To
further test whether WNT-1 was a direct target of miR-148a, we
constructed a luciferase reporter plasmid containing WNT-1
3′-untranslated region (3′-UTR) harboring a conserved miR-148a
binding site (pGL3-WNT-1-3′UTR) and a plasmid containing
WNT-1-3′-UTR with miR-148a target sequences mutated
(pGL3-WNT-1-3′UTR mu). The pGL3-WNT-1-3′UTR or pGL3-WNT-1-3′UTR mu
was cotransfected with the miR-148a mimic or NC into 293T cells.
The reporter assay showed that miR-148a mimic significantly
decreased the luciferase activity by ~50% in 293T cells
co-transfected with the pGL3-WNT-1-3′UTR. However, the luciferase
activity in the cells co-transfected with the pGL3-WNT-1-3′UTR mu
was not significantly reduced (P<0.05; Fig. 3B). These findings suggested that
WNT-1 was a direct target of miR-148a.
Next, we found that the ectopic miR-148a expression
decreased the WNT-1 mRNA expression levels to ~55 and 25% of the NC
in MCF-7 and MDA-MB-231 cells (P<0.05, Fig. 2C). Furthermore, the protein
expression levels in the MCF-7 and MDA-MB-231 cells transfected
with miR-148a mimic were found suppressed to 50 and 40% of the
control, respectively (P<0.05; Fig.
2D). These data demonstrated that miR-148a was able to inhibit
the expression of WNT-1 in breast cancer cells.
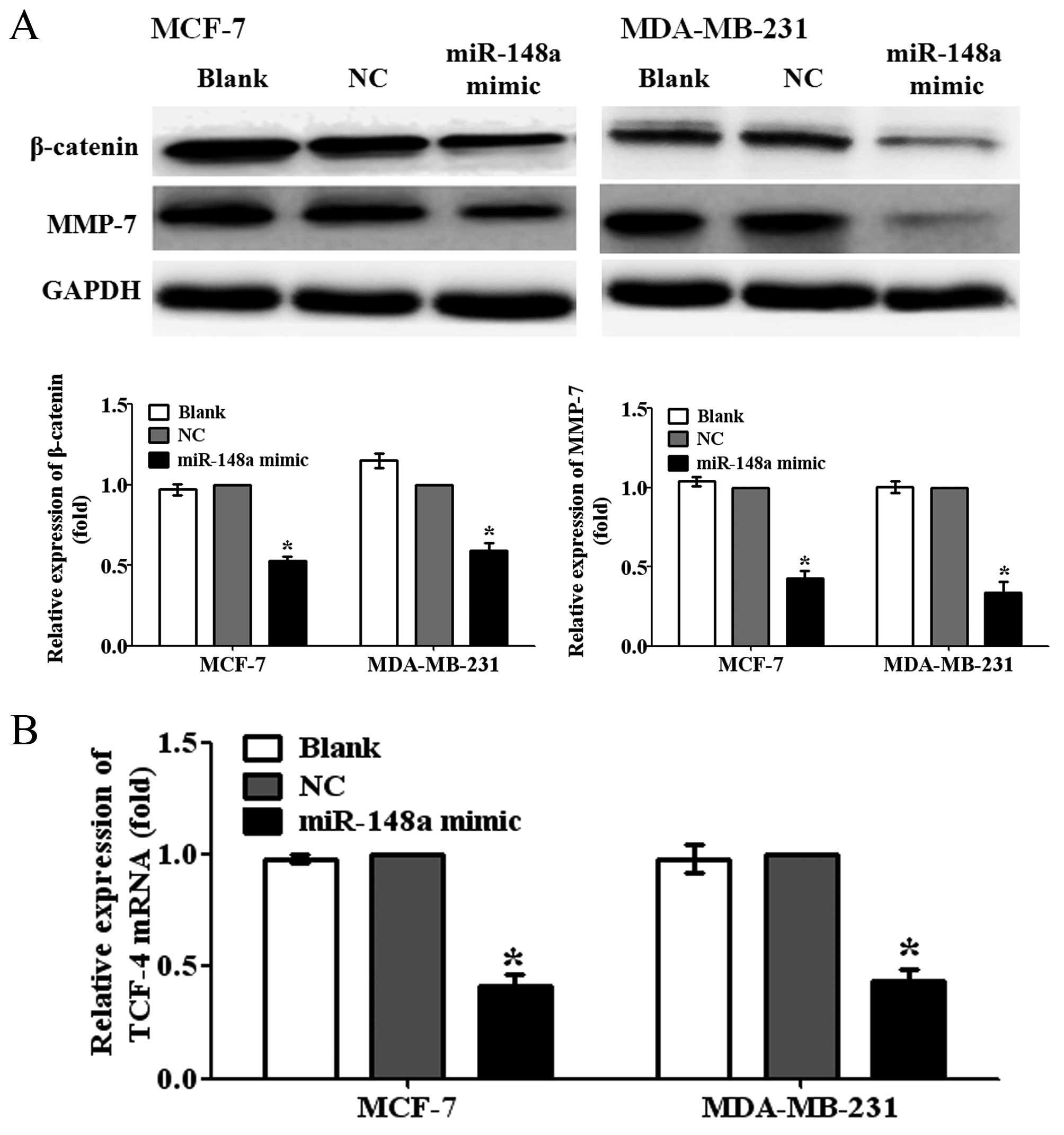
Overexpression of miR-148a inhibits the
activation of Wnt/β-catenin signaling pathway
WNT-1 is an important ligand of Wnt/β-catenin
pathway. To further investigate whether miR-148a can inhibit the
activation of Wnt/β-catenin pathway by targeting WNT-1 in breast
cancer cells, we detected the protein expression levels of
β-catenin, a central component of Wnt/β-catenin pathway, and MMP-7,
a major target gene of Wnt/β-catenin pathway related to metastasis,
in MCF-7 and MDA-MB-231 cells transfected with miR-148a mimic. We
observed that the overexpression of miR-148a significantly reduced
the protein expression levels of β-catenin and MMP-7 in MCF-7 and
MDA-MB-231 cells, compared with NC-transfected cells (Fig 4A; P<0.05). In addition, the
results also showed that the ectopic miR-148a expression obviously
decreased the mRNA expression levels of T cell factor-4 (TCF-4),
one of the important transcription factors of Wnt/β-catenin
pathway, in MCF-7 and MDA-MB-231 cells (Fig. 4B; P<0.05). Taken together, the
findings suggested that miR-148a could suppress the migration and
invasion of breast cancer cells by targeting WNT-1 and inhibiting
the activation of Wnt/β-catenin signaling pathway.
miR-148a expression is negatively
correlated with the expression of WNT-1 in human breast cancer
tissues
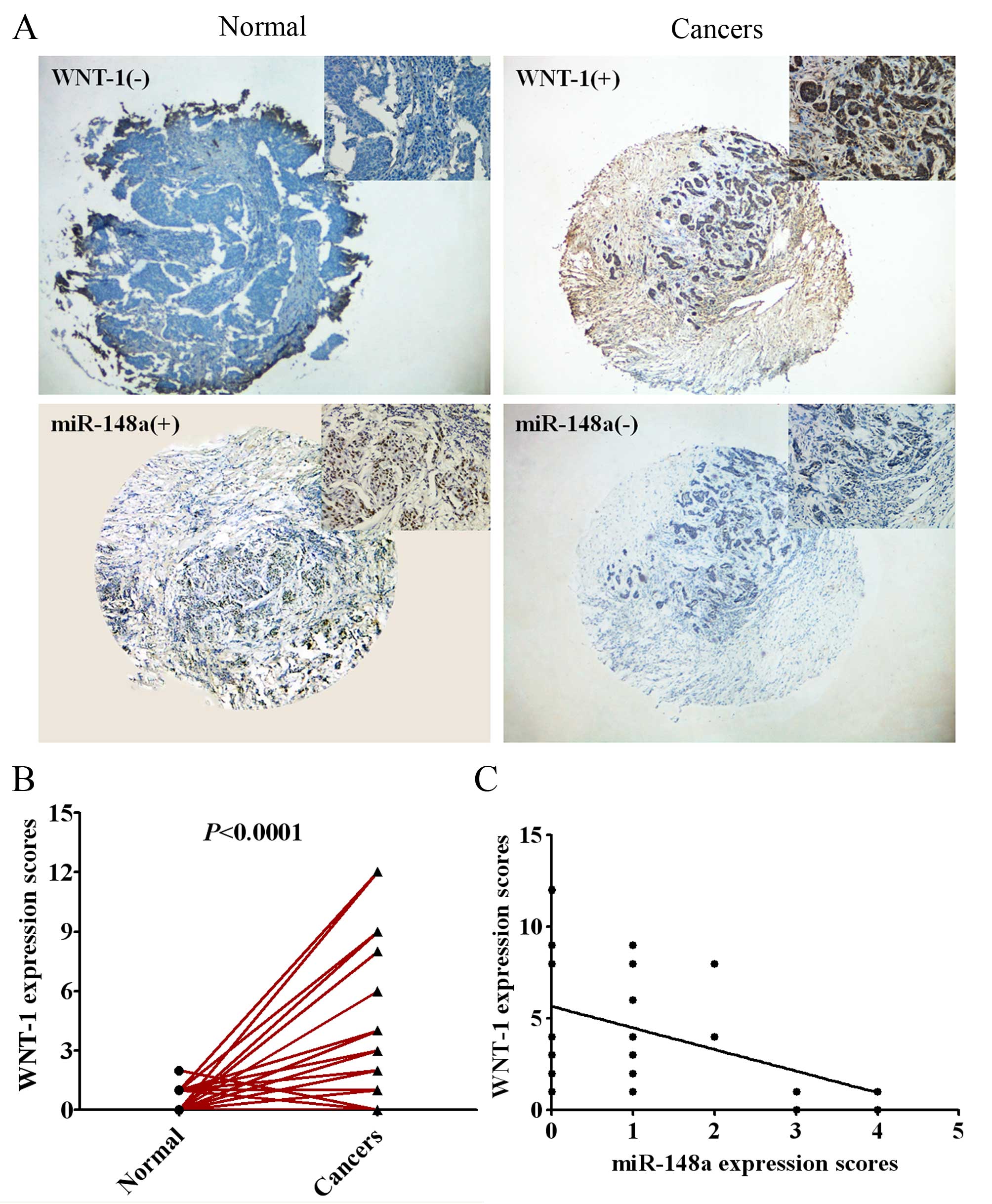
To further evaluate the relevance of the endogenous
expression of miR-148a and WNT-1, we measured the expression of
miR-148a using in situ hybridization and the expression of
WNT-1 protein by immunohistochemistry in 55 pairs of human breast
cancer tissues and adjacent normal tissues with tissue microarray
(TMA). As shown in Fig. 5A and B,
the expression of WNT-1 was significantly higher in human breast
cancer tissues compared with the adjacent normal tissues
(P<0.0001). Pearson rank correlation analysis showed that the
expression of miR-148a was inversely related to the expression of
WNT-1 protein in breast cancer tissues (Fig. 5C; P<0.01).
Discussion
Wnt/β-catenin signaling pathway influences embryonic
development, cell polarity and adhesion, apoptosis and
tumorigenesis (21,22). It is known that Wnt/β-catenin
pathway is upregulated in breast cancer (6) and other types of tumors (8). WNT-1 was the original Wnt identified
as an oncogene in mouse mammary tumors (23). Wong et al reported that there
was a higher positive expression rate in human breast tumors
(24). In our study, we also found
that the WNT-1 was obviously upregulated in human breast cancer
tissues when compared with the adjacent normal tissues.
Wnt/β-catenin pathway has been shown to be involved in the tumor
development and metastasis (5).
Targeting the Wnt/β-catenin pathway would be very important to
inhibit the metastasis of breast cancer.
miRNAs function as regulators of many oncobiological
processes, such as tumorigenesis and metastasis (9). It has been demonstrated that many
miRNAs can target and inhibit the main factors of WNT/β-catenin
pathway and regulate the biological function of cancer cells. Wen
et al reported that miR-126 suppressed papillary thyroid
carcinoma cell proliferation and migration by directly repressing
the expression of LRP6, a major regulator of the Wnt/β-catenin
signaling cascade (25). miR-577
was found to directly target the Wnt/β-catenin pathway components
LRP6 and β-catenin, and inhibit glioblastoma multiforme growth
(26). Subramanian et al
found that miR-29b decreased the transactivation of β-catenin
target genes in human colorectal cancer cells (27).
In the present study, we found that miR-148a could
inhibit the migration and invasion of breast cancer cells by
directly targeting WNT-1 and inhibiting the activation of
Wnt/β-catenin pathway. Furthermore, we also demonstrated that the
expression of miR-148a was inversely related to the expression of
WNT-1 in breast cancer tissues. Similarly, Yan et al also
reported that WNT-1 was a target gene of miR-148a in hepatocellular
carcinoma cells (28). In addition,
Joshi et al found that miR-148a reduced lung tumorigenesis
in vitro and in vivo through the downmodulation of
matrix metalloproteinase 15 (MMP15) and Rho-associated kinase 1
(RoCK1) (29). miR-148a was also
demonstrated as a prognostic oncomiR to target mitogen-inducible
gene 6 (MIG6) and BIM, and regulate EGFR and apoptosis in
glioblastoma (30). Obviously,
miR-148a plays different roles either as an oncomiR or as an
antimiR in the tumor cells of different types by directly targeting
different target genes.
In conclusion, our studies suggest that miR-148a can
inhibit the migration and invasion of breast cancer cells by
directly targeting WNT-1 and downregulating the Wnt/β-catenin
signaling pathway. This will provide a new strategy for treating
metastasis of breast cancer. However, the complex regulatory
network of miR-148a in regulating the migration and invasion of
breast cancer should be further explored.
Acknowledgments
The present study was supported by grants from the
National Natural Science Foundation of China (grant no. 81373427),
the Program for Liaoning Innovative Research Team in university,
LNIRT, China (grant no. LT2014016), the Liaoning Provincial Science
and Technology Program, China (grant no. 2014021085), the Program
for Liaoning Excellent Talents in university, China (grant no.
LJQ2014084), and the S&T Projects in Shenyang, China (grant no.
F14-232-6-05).
References
|
1
|
Donepudi MS, Kondapalli K, Amos SJ and
Venkanteshan P: Breast cancer statistics and markers. J Cancer Res
Ther. 10:506–511. 2014.PubMed/NCBI
|
|
2
|
Gangadhara S, Barrett-Lee P, Nicholson RI
and Hiscox S: Pro-metastatic tumor-stroma interactions in breast
cancer. Future Oncol. 8:1427–1442. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fazilaty H and Mehdipour P: Genetics of
breast cancer bone metastasis: A sequential multistep pattern. Clin
Exp Metastasis. 31:595–612. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Clevers H: Wnt/beta-catenin signaling in
development and disease. Cell. 127:469–480. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
MacDonald BT, Tamai K and He X:
Wnt/beta-catenin signaling: Components, mechanisms, and diseases.
Dev Cell. 17:9–26. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Khramtsov AI, Khramtsova GF, Tretiakova M,
Huo DZ, Olopade OI and Goss KH: Wnt/beta-catenin pathway activation
is enriched in basal-like breast cancers and predicts poor outcome.
Am J Pathol. 176:2911–2920. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Arend RC, Londono-Joshi AI, Straughn JM Jr
and Buchsbaum DJ: The Wnt/beta-catenin pathway in ovarian cancer: A
review. Gynecol Oncol. 131:772–779. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Serafino A, Moroni N, Zonfrillo M,
Andreola F, Mercuri L, Nicotera G, Nunziata J, Ricci R, Antinori A,
Rasi G, et al: WNT-pathway components as predictive markers useful
for diagnosis, prevention and therapy in inflammatory bowel disease
and sporadic colorectal cancer. Oncotarget. 5:978–992. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu P, Tang H, Chen B, He Z, Deng M, Wu M,
Liu X, Yang L, Ye F and Xie X: miR-26a suppresses tumour
proliferation and metastasis by targeting metadherin in triple
negative breast cancer. Cancer Lett. 357:384–392. 2015. View Article : Google Scholar
|
|
11
|
Chan SH, Huang WC, Chang JW, Chang KJ, Kuo
WH, Wang MY, Lin KY, Uen YH, Hou MF, Lin CM, et al: MicroRNA-149
targets GIT1 to suppress integrin signaling and breast cancer
metastasis. Oncogene. 33:4496–4507. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen Y, Song YX and Wang ZN: The
microRNA-148/152 family: Multi-faceted players. Mol Cancer.
12:432013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen Y, Song Y, Wang Z, Yue Z, Xu H, Xing
C and Liu Z: Altered expression of miR-148a and miR-152 in
gastrointestinal cancers and its clinical significance. J
Gastrointest Surg. 14:1170–1179. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang R, Li M, Zang W, Chen X, Wang Y, Li
P, Du Y, Zhao G and Li L: MiR-148a regulates the growth and
apoptosis in pancreatic cancer by targeting CCKBR and Bcl-2. Tumour
Biol. 35:837–844. 2014. View Article : Google Scholar
|
|
15
|
Liffers ST, Munding JB, Vogt M, Kuhlmann
JD, Verdoodt B, Nambiar S, Maghnouj A, Mirmohammadsadegh A, Hahn SA
and Tannapfel A: MicroRNA-148a is down-regulated in human
pancreatic ductal adenocarcinomas and regulates cell survival by
targeting CDC25B. Lab Invest. 91:1472–1479. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Magrelli A, Azzalin G, Salvatore M,
Viganotti M, Tosto F, Colombo T, Devito R, Di Masi A, Antoccia A,
Lorenzetti S, et al: Altered microRNA expression patterns in
hepatoblastoma patients. Transl Oncol. 2:157–163. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xu Q, Jiang Y, Yin Y, Li Q, He J, Jing Y,
Qi YT, Xu Q, Li W, Lu B, et al: A regulatory circuit of
miR-148a/152 and DNMT1 in modulating cell transformation and tumor
angiogenesis through IGF-IR and IRS1. J Mol Cell Biol. 5:3–13.
2013. View Article : Google Scholar :
|
|
18
|
Yu J, Li Q, Xu Q, Liu L and Jiang B:
MiR-148a inhibits angiogenesis by targeting ERBB3. J Biomed Res.
25:170–177. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ma MT, He M, Wang Y, Jiao XY, Zhao L, Bai
XF, Yu ZJ, Wu HZ, Sun ML, Song ZG, et al: MiR-487a resensitizes
mitoxantrone (MX)-resistant breast cancer cells (MCF-7/MX) to MX by
targeting breast cancer resistance protein (BCRP/ABCG2). Cancer
Lett. 339:107–115. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bai X, Song Z, Fu Y, Yu Z, Zhao L, Zhao H,
Yao W, Huang D, Mi X, Wang E, et al: Clinicopathological
significance and prognostic value of DNA methyltransferase 1, 3a,
and 3b expressions in sporadic epithelial ovarian cancer. PLoS One.
7:e400242012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Polakis P: Wnt signaling and cancer. Genes
Dev. 14:1837–1851. 2000.PubMed/NCBI
|
|
22
|
Karim R, Tse G, Putti T, Scolyer R and Lee
S: The significance of the Wnt pathway in the pathology of human
cancers. Pathology. 36:120–128. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Nusse R and Varmus HE: Many tumors induced
by the mouse mammary tumor virus contain a provirus integrated in
the same region of the host genome. Cell. 31:99–109. 1982.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wong SC, Lo SF, Lee KC, Yam JW, Chan JK
and Wendy Hsiao WL: Expression of frizzled-related protein and
Wnt-signalling molecules in invasive human breast tumours. J
Pathol. 196:145–153. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wen Q, Zhao J, Bai L, Wang T, Zhang H and
Ma Q: miR-126 inhibits papillary thyroid carcinoma growth by
targeting LRP6. Oncol Rep. 34:2202–2210. 2015.PubMed/NCBI
|
|
26
|
Zhang W, Shen C, Li C, Yang G, Liu H, Chen
X, Zhu D, Zou H, Zhen Y, Zhang D, et al: miR-577 inhibits
glioblastoma tumor growth via the Wnt signaling pathway. Mol
Carcinog. Mar 12–2015.Epub ahead of print. View Article : Google Scholar
|
|
27
|
Subramanian M, Rao SR, Thacker P,
Chatterjee S and Karunagaran D: MiR-29b downregulates canonical Wnt
signaling by suppressing coactivators of beta-catenin in human
colorectal cancer cells. J Cell Biochem. 115:1974–1984.
2014.PubMed/NCBI
|
|
28
|
Yan H, Dong XG, Zhong XQ, Ye J, Zhou Y,
Yang X, Shen J and Zhang J: Inhibitions of epithelial to
mesenchymal transition and cancer stem cells-like properties are
involved in miR-148a-mediated anti-metastasis of hepatocellular
carcinoma. Mol Carcinog. 53:960–969. 2014.
|
|
29
|
Joshi P, Jeon YJ, Laganà A, Middleton J,
Secchiero P, Garofalo M and Croce CM: MicroRNA-148a reduces
tumorigenesis and increases TRAIL-induced apoptosis in NSCLC. Proc
Natl Acad Sci USA. 112:8650–8655. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kim J, Zhang Y, Skalski M, Hayes J, Kefas
B, Schiff D, Purow B, Parsons S, Lawler S and Abounader R:
microRNA-148a is a prognostic oncomiR that targets MIG6 and BIM to
regulate EGFR and apoptosis in glioblastoma. Cancer Res.
74:1541–1553. 2014. View Article : Google Scholar : PubMed/NCBI
|