Introduction
Osteosarcoma (OS) is one of the most common primary
bone malignancies in childhood and adolescents and it has a high
early metastatic propensity. In the past several decades, the
5-year overall survival rate for OS patients has increased due to
the application of intensive neoadjuvant chemotherapy and surgery.
However, the overall survival rate has plateaued at 60% and the
prognosis remains unsatisfactory for those with pulmonary
metastasis at diagnosis, who have 5-year survival rates of less
than 30% (1). Therefore, new
therapeutic strategies are being sought for this lethal malignancy
and gene therapy appears to be a promising approach. Targeting
specific genes is a proven strategy (2) and gene therapy approaches with some
specific targets, such as tumor necrosis factor α (TNF-α) (3) and endothelin A receptor (ETAR)
(4), have been evaluated as
potential antimetastatic treatments for OS. However, to date no
gene therapy approach has been validated for clinical application
and further investigation is needed to identify new targets.
CD151 is a member of the tetraspanins family, which
is considered to comprise molecular facilitators (5). CD151 is involved in several
pathological activities of tumors, including growth (6), angiogenesis (7) and invasion/metastasis (8). Several lines of evidence indicate that
the primary role of CD151 in tumor progression is to facilitate
metastasis (8–11), and it is suggested that CD151 is
involved in modulating the metastasis of various sarcomas, such as
fibrosarcoma (12), however, the
role and mechanism of CD151 in promoting OS metastasis remain
vague. In our previous study (13),
the results of proteomic and immunohistochemical analyses confirmed
the upregulation of CD151 in OS and suggested CD151 to be a key
regulator of the progression of OS. In addition, protein-protein
interaction network analysis demonstrated a potential relationship
between CD151 and β-catenin, which is the central molecule in the
canonical Wnt (Wnt) signaling pathway and is modulated by glycogen
synthase kinase 3β (GSK-3β) (14).
However the function and mechanism of CD151 in OS metastasis have
not been clarified.
Following the results of our previous study
(13), the anti-metastatic effect
of the downregulation of CD151 was further evaluated using small
interfering RNA (siRNA) in vitro and in vivo, and the
molecular mechanism of CD151 in OS metastasis was investigated
using a highly metastatic human OS cell line.
Materials and methods
Cell line and animals
Two highly metastatic OS cell lines, human 143B and
mouse LM-8 (kindly gifted by Professor Zhengdong Cai, First
Hospital of Shanghai, Shanghai, China) (15), were cultured in Dulbecco's modified
Eagle's medium (DMEM) (Sigma-Aldrich, St. Louis, MO, USA)
supplemented with 10% (v/v) fetal bovine serum (FBS) (Gibco, Grand
Island, NY, USA), and maintained at 37°C in a 5% CO2
incubator (Thermo Scientific, Waltham, MA, USA). Female BALB/c nude
mice, 4–5 weeks old were obtained from Vital River Company,
Beijing, and were fed under specific pathogen free conditions.
Water-soluble vitamin C was added into the drinking water to aid
the healthy growth of the mice. All experimental procedures were
performed in accordance with the guidelines for laboratory animals
established by China Medical University of Animal Care and Use
Committee.
Antibodies
Primary anti-CD151 and matrix metalloproteinase 9
(MMP9) antibodies were purchased from AbD Serotec (Oxford, UK).
Anti-GSK-3β, anti-phosphorylated GSK-3β (pGSK-3β, activated GSK-3β)
and β-catenin antibodies were obtained from Sigma-Aldrich.
HRP-conjugated rabbit anti-mouse IgG secondary antibody was
purchased from Sigma-Aldrich.
Establishment of luciferase-expressing OS
cells
The luciferase-expressing OS cells were prepared for
bioluminescent imaging assay. Stable transfections were carried out
with Lipofectamine® 2000 in 6-well dishes according to
the manufacturer's instructions (Invitrogen Life Technologies,
Carlsbad, CA, USA). After a 48-h transfection, a stable
luciferase-expressing clone was selected by limited dilution to
create a Luc-143B or Luc-LM-8 cell line. Briefly, 1/10, 1/30 and
1/100 of the cells were digested and diluted into 10 ml of
completed medium, and then every dilution of cells were seeded into
96-well plates and grown in medium containing G418 (600
µg/ml). After several days, the single cell clones were
transferred into T25 flasks. After proliferation, the cell lines
with the highest luciferase activity were selected using a
luciferase detection kit (Beyotime) and EnVision imaging
(PerkinElmer).
Transfection of siRNAs
The siRNA sequences targeting CD151, MMP9, GSK-3β
and β-catenin were purchased from Shanghai GenePharma (Shanghai,
China). A non-targeting scrambled sequence, siRNA-MOCK was used as
a control. Transfection of the siRNAs was performed using
Lipofectamine® 2000 (Invitrogen) according to the
manufacturer's instructions. The levels of target gene expression
of the stably transfected clones were validated by quantitative
real-time polymerase chain reaction (data not shown).
Wound healing assay
Wound healing migration assays were performed as
previously described (16).
Subconfluent cells were treated with siRNA-CD151, siRNA-MMP9,
siRNA-MOCK and 0.9% NaCl as the normal control (NC). After 72 h,
the cells were trypsinized, counted and re-plated in 1% (v/v) FBS
complete media in 6-well dishes containing a sterile metal
3-pronged cross (0.75-mm thick) to create a consistent gap in the
monolayer of cells. At 12 h after plating, the cross was removed.
Bright-field images of the field adjacent to the left center arm of
the cross were captured at 0 and 24 h after removing the cross to
assess cell migration across the gap. These assays were carried out
in triplicate. The cell migration rate was calculated as: (width 0
h − width 24 h)/width 24 h × 100.
Matrigel® invasion assay
Matrigel® invasion assays were performed
as previously described (17).
Briefly, subconfluent tumor cells were infected with siRNA-CD151,
siRNA-MMP9, siRNA-MOCK and 0.9% NaCl as NC and assayed at 72 h. The
concentration of the invading cells was determined using
CellTiter-Glo® viability assay (Promega, Madison, WI,
USA) at room temperature and the light absorbance of the medium was
measured by a Bio-Rad 680 microplate reader (Bio-Rad, Hercules, CA,
USA) after 10 min. The assay was performed in triplicate. The cell
invasion rate was calculated as: (value of sample/value of NC) ×
100.
Orthotopic xenograft models
Orthotopic xenograft models were performed as
previously described (16). The
highly metastatic 143B and LM-8 OS cells, transfected with
luciferase were harvested and prepared for injection
(1.5×106 cells/injection) into the left flanks of BALB/c
nude mice. Five mice were used in each group. When the tumor
diameters reached ~0.5 cm, the mice were randomly divided into 3
groups and siRNA-CD151, siRNA-MOCK and 0.9% NaCl as NC,
respectively, were intratumorally injected once every 3 days. At 4
weeks after the implantation of OS cells, animals were sacrificed
and lungs were harvested for evaluation.
Animal histological evaluation
Histological evaluation was performed as previously
described (16). All samples were
assigned a number and the treatment group was blinded. The
harvested lungs were fixed with 10% (w/v) formalin, embedded in
paraffin, serially sectioned and stained with hematoxylin and
eosin. Evaluation of the histologic slides was performed by a
trained pathologist in a blinded manner.
Bioluminescent imaging
BALB/c nude mice transplanted with
luciferase-expressing OS cells underwent non-invasive whole-body
imaging using the bioluminescence imaging system (LB983 NC100;
Berthold) at 4 weeks. The mice were injected with 125 mg/kg
D-luciferin solution intraperitoneally before being anaesthetized
via intraperitoneal injection of pentobarbital (50 mg/kg) for the
procedure. Ten or 12 min after injection, the images were acquired
for 0.5–300 sec. The photon emission from the animals was captured
and quantitated using Indigo software (Indigo Software,
Belgium).
Immunoprecipitation
Proteins were extracted from cells and tumor tissues
with RIPA buffer in the presence of a protease inhibitor, and the
total protein concentration was determined using a BCA protein
assay kit (Thermo Scientific, Rockford, IL, USA). The supernatants
were collected after centrifuging and primary antibodies (5
µg/ml) of the targeted genes pre-conjugated with magnetic
beads (50 µl; Invitrogen) were added to the supernatant and
incubated with gentle agitation, respectively. After washing with
phosphate-buffered saline, proteins bound to the beads were eluted.
The samples were loaded for sodium dodecyl sulfate-polyacrylamide
gel electrophoresis and transferred to polyvinylidene fluoride
membranes. The membranes were blocked and incubated with the
primary antibodies overnight at 4°C. Then, the membranes were
incubated with secondary antibodies at room temperature for 1 h,
and the signal was detected using a Tenon GIS Gel image system
(Shanghai, China).
Statistical analysis
Statistical analysis was performed with SPSS version
16.0 software (SPSS, Inc., Chicago, IL, USA). Data were presented
as the mean ± standard error of the mean (SEM). Student's t-test
and one-way analysis of variance were used for statistical
comparisons among groups. P<0.05 was considered to indicate a
significant difference.
Results
Inhibition of migration and invasion in
vitro
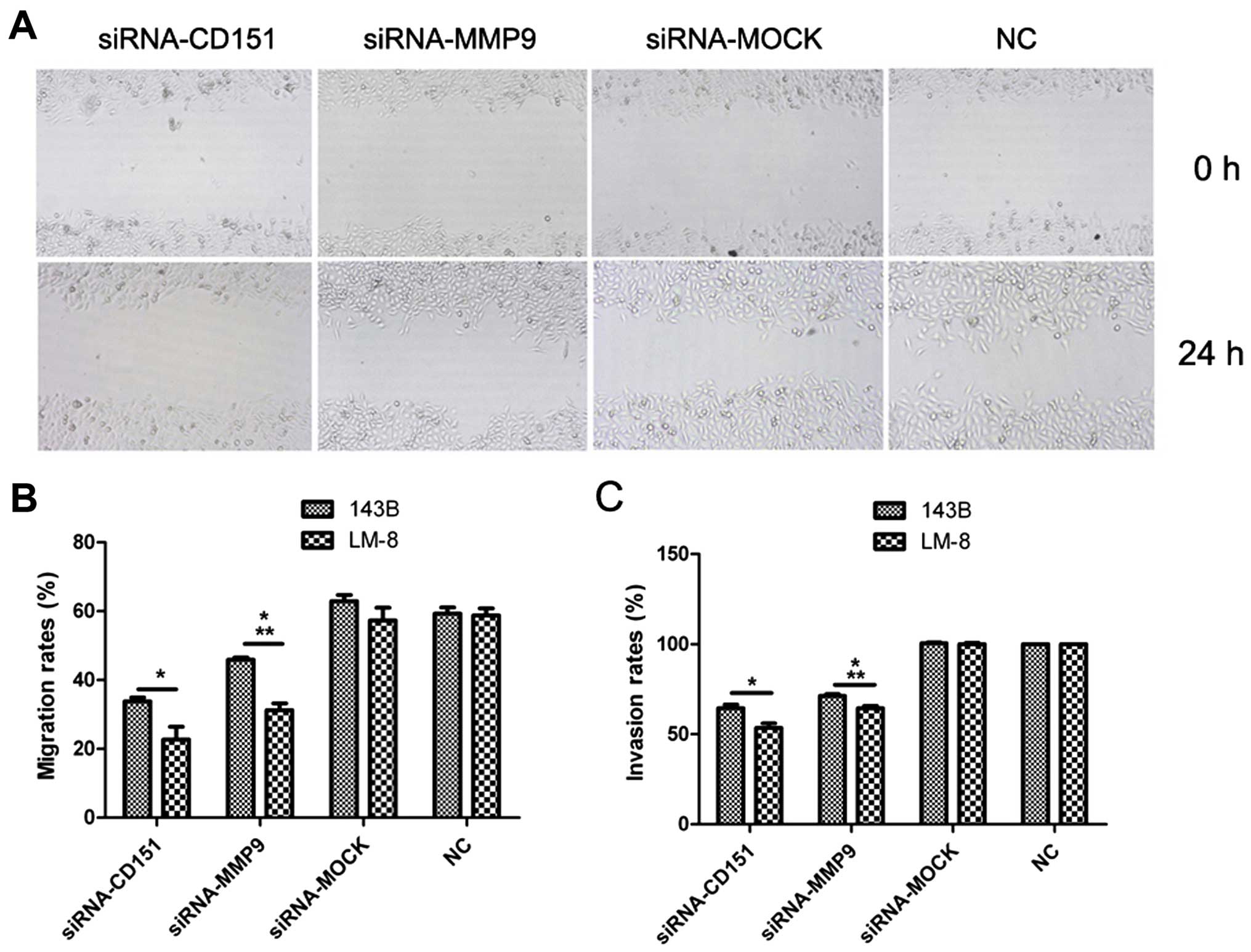
In order to assess the effects of siRNA-CD151 and
siRNA-MMP9 on the migration and invasion of the highly metastatic
OS cells, migration rates were evaluated by wound healing assay and
invasion was evaluated by Matrigel® invasion assay. The
results of the wound healing assay (Fig. 1A and B) showed that the migration
rates were decreased by 45% for the 143B cells and 60% for the LM-8
cells following siRNA-CD151 transfection, and 25% for the 143B
cells and 45% for the LM-8 cells following siRNA-MMP9 transfection,
compared with the rates of the NC group (P<0.01). Inhibition of
the migration rate following siRNA-CD151 transfection was higher
than that following siRNA-MMP9 transfection (P<0.01). According
to the results of the Matrigel invasion assay (Fig. 1C), the invasion rates were decreased
by 36% for 143B cells and 47% for the LM-8 cells following
siRNA-CD151 transfection, and 29% for the 143B cells and 36% for
LM-8 cells following siRNA-MMP9 transfection, compared with those
of the NC group (P<0.01). The invasion rate following
siRNA-CD151 transfection was lower than that following siRNA-MMP9
transfection (P<0.01).
Inhibition of metastasis by siRNA-CD151
in vivo
CD151 was selected as a target for tumor therapy due
to its role in facilitating metastasis in tumors. As described
above, results from wound healing and Matrigel assays demonstrated
that the migration and invasion of the OS cells transfected with
siRNA-CD151 were significantly inhibited, compared with those of
the siRNA-MOCK and NC groups. Inhibition of metastasis by silencing
CD151 in vivo supported the data obtained in vitro.
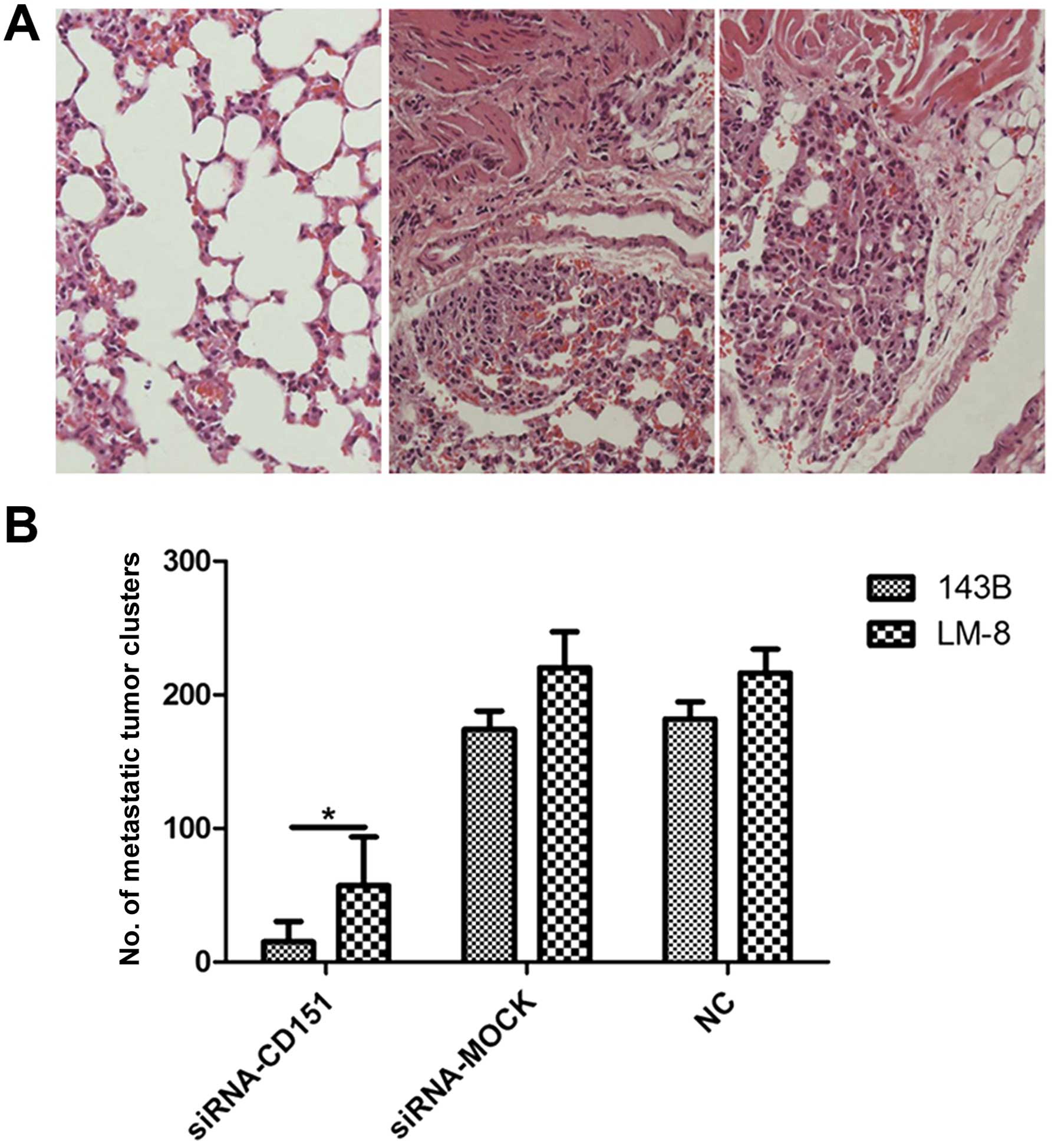
The in vivo results indicated that tumor metastasis rates
and the numbers of pulmonary tumor clusters were significantly
decreased in all siRNA-CD151-treated mice, compared with those of
the controls (Fig. 2). The rate of
pulmonary metastasis was 100% (5/5) in both the siRNA-MOCK and NC
groups, whereas it was 20% (1/5) in the siRNA-CD151 group for 143B
cells and 40% (2/5) for LM-8 cells transplantation. The numbers of
metastatic tumor clusters per mouse were significantly different
between the siRNA-CD151 and the siRNA-MOCK and NC groups,
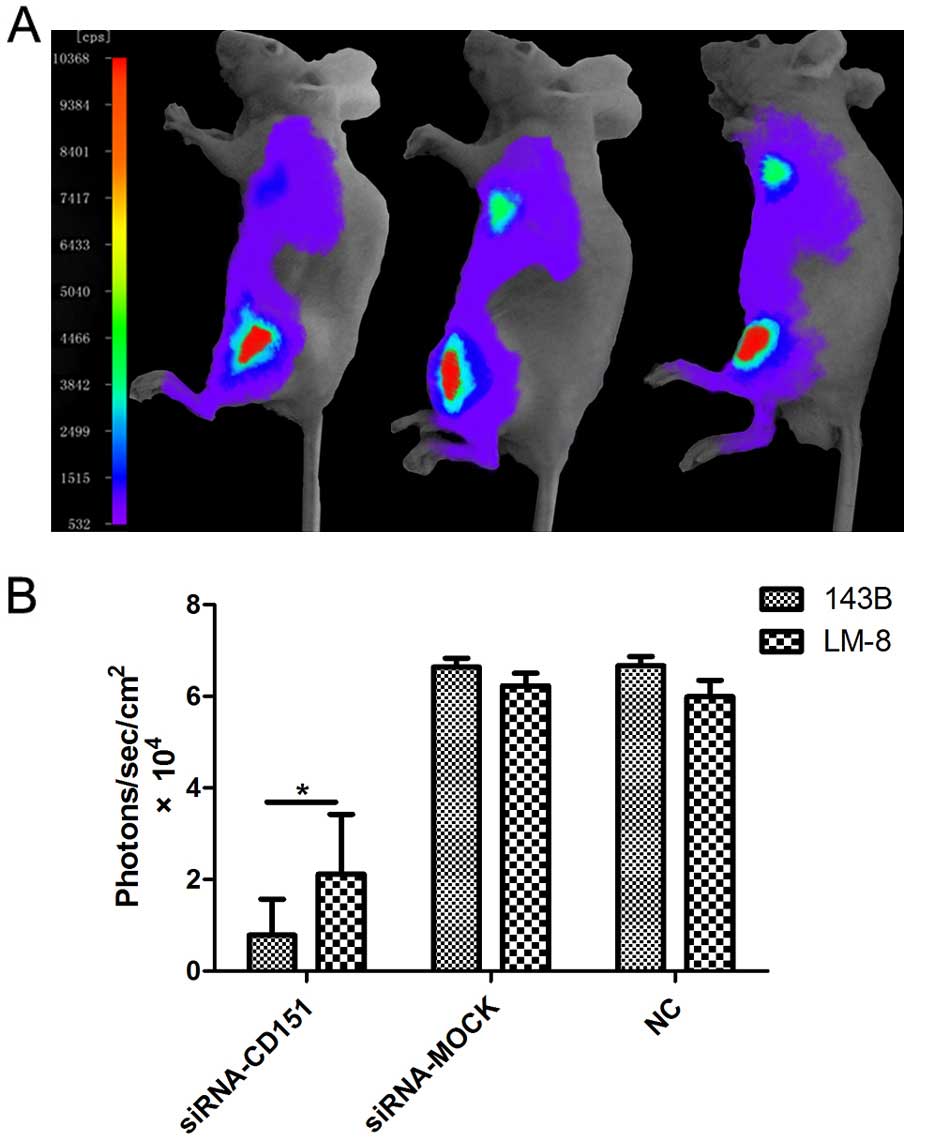
respectively (P<0.01). Moreover, the bioluminescent imaging
assay demonstrated similar results (Fig. 3). After 4 weeks of transplantation,
each of the BALB/c nude mice injected with luciferase-expressing OS
cells demonstrated a strong and similar detectable signal over the
left flanks, whereas the detected signal at the metastatic site of
the lungs demonstrated significant difference between groups.
Signals at the site of lungs in all of the BALB/c nude mice
injected with siRNA-MOCK and NC were similar and moderate,
demonstrating a 100% pulmonary metastatic rate, whereas in the
BALB/c nude mice injected with siRNA-CD151, the pulmonary
metastatic rate was decreased (20% for 143B and 40% for LM-8
cells), which was confirmed with a positive but minimal
bioluminescent signal over the site (Fig. 3A). In addition, statistical analysis
demonstrated that the signals at the lungs in the siRNA-CD151 group
were significantly lower than those in the siRNA-MOCK and NC groups
(Fig. 3B; P<0.01).
Antimetastatic mechanism of CD151
The findings of our previous study (13) suggest that CD151 may interact with
β-catenin, which is the central molecule in the Wnt signaling
pathway and regulate the expression of various genes including MMP9
(18). Moreover, CD151 has been
found to modulate tumor metastasis through regulating MMP9
expression (19). We hypothesized
that CD151 knockdown inhibits OS metastasis through regulation of
MMP9 expression and therefore we scrutinized the molecular
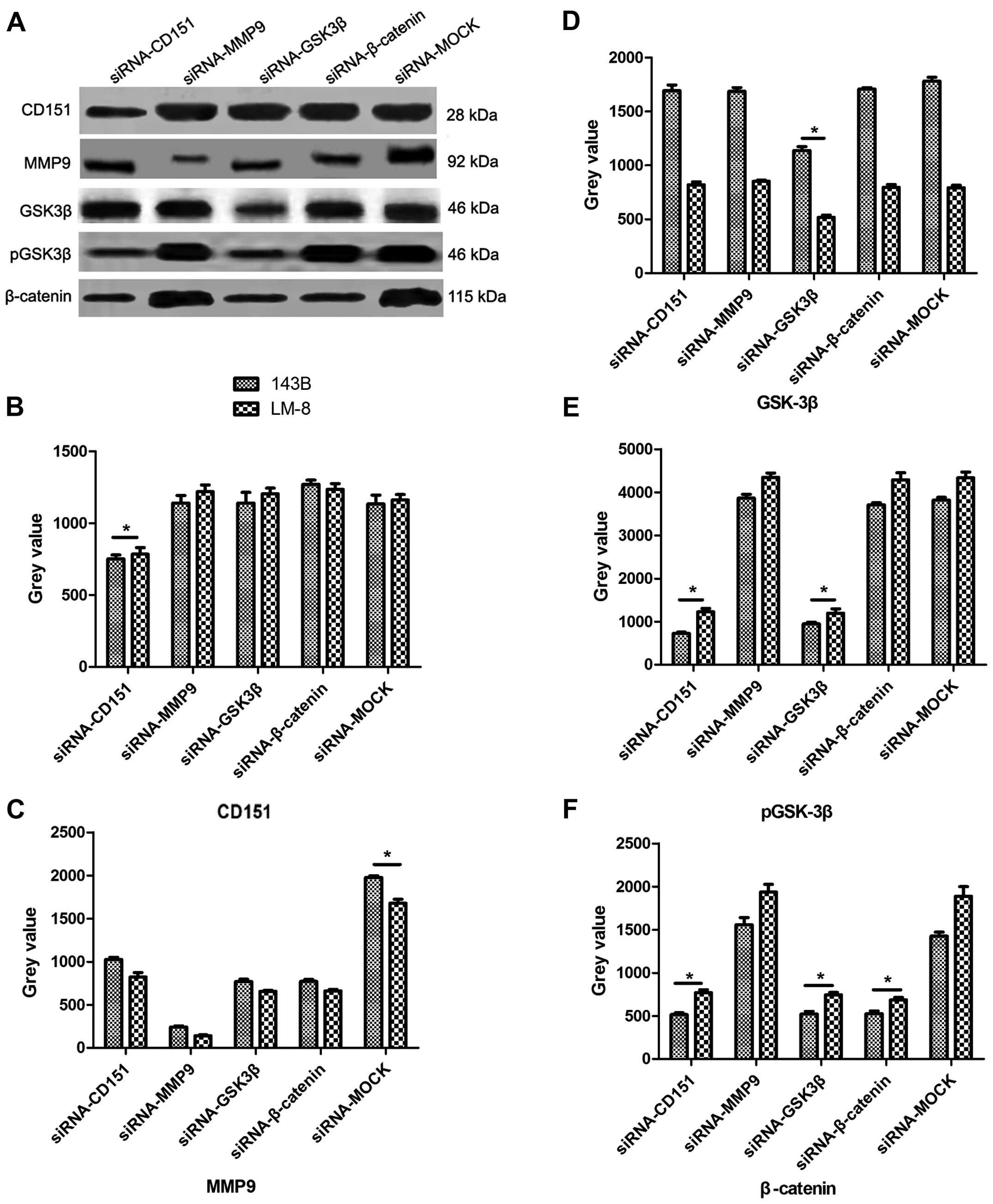
mechanism by IP assay. The protein was extracted from the OS cells
after transfections with targeted siRNAs and evaluated by IP assay
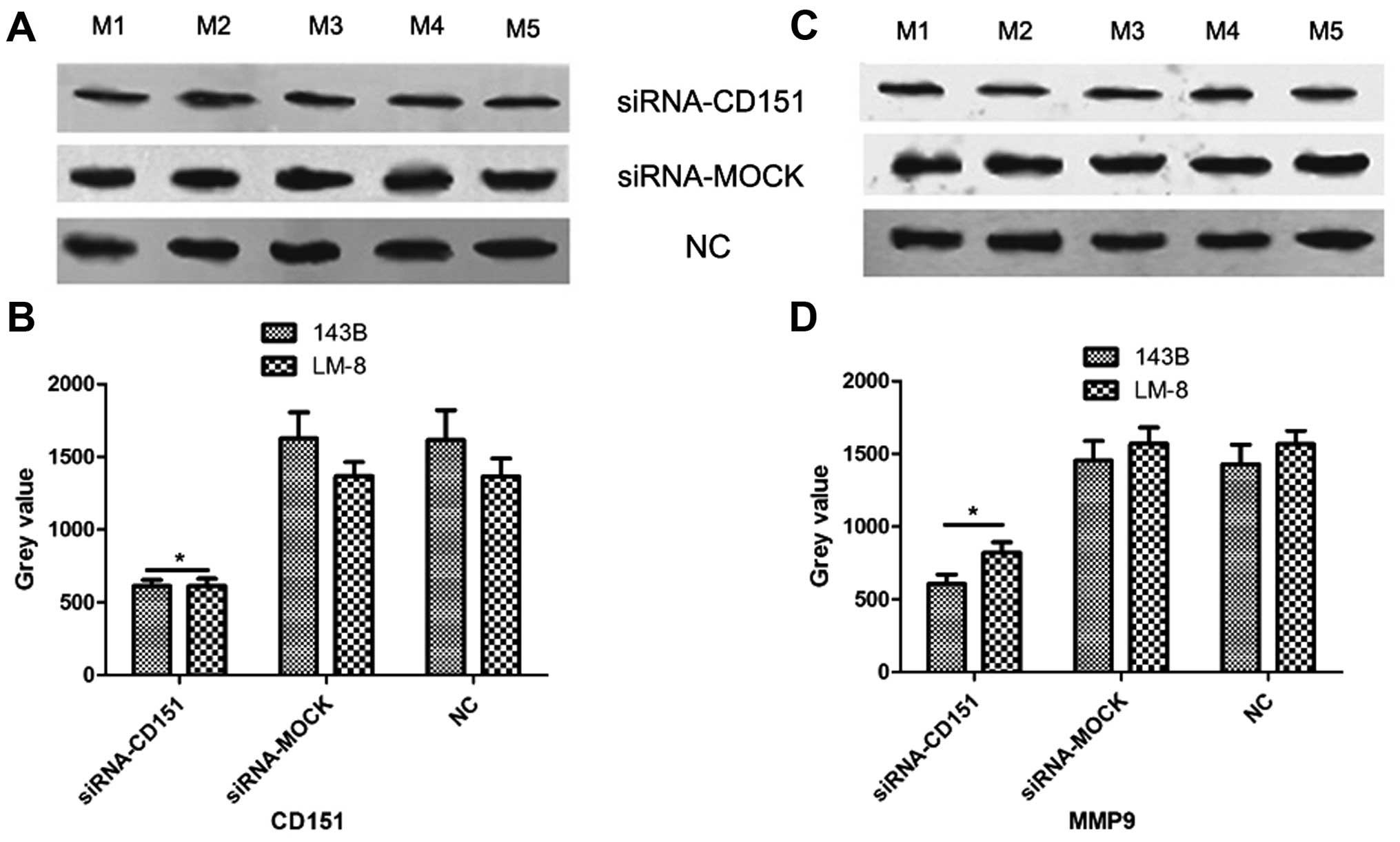
(Fig. 4). After silencing CD151,
the phosphorylation level of GSK-3β (pGSK-3β) and the expression
levels of β-catenin and MMP9 were significantly reduced, compared
with those of the siRNA-MOCK-treated cells. Then, we silenced MMP9
expression and found that except for MMP9, none of the targeted
genes were downregulated by siRNA-MMP9. GSK-3β modulates β-catenin
expression (20), and the results
from the IP assay demonstrated that the expression levels of
GSK-3β, β-catenin, MMP9 and pGSK-3β were significantly reduced by
siRNA-GSK-3β, while CD151 expression remained stable. As MMP9 is a
downstream target gene of the Wnt signaling pathway, all of the
target genes were evaluated after silencing β-catenin, and the
results showed that β-catenin and MMP9 expression levels were
significantly reduced, while CD151, GSK-3β and pGSK-3β expression
remained unchanged. The data obtained from the in vivo
experiments (Fig. 5) consolidated
the results obtained in vitro. In the siRNA-CD151 group
in vivo, the expression levels of CD151 and MMP9 were
significantly reduced compared with those of the siRNA-MOCK and NC
groups.
Discussion
Osteosarcoma (OS) is a primary bone malignancy with
a high early metastatic propensity. Despite some progress in
therapies over the past several decades, the prognosis of patients
with pulmonary metastasis remains unsatisfactory (1). Therefore, it is of great importance to
investigate possible molecular mechanisms and develop
antimetastatic strategies for OS. In the present study, we employed
two highly metastatic OS cell lines, human 143B and mouse LM-8,
along with siRNA to investigate the molecular mechanisms and
antimetastatic effects of CD151 in vitro and in vivo.
Tetraspanin CD151 is considered as a 'facilitator' (5,21) and
has been suggested to have regulatory roles at different stages of
cancer progression, such as tumorigenesis (22), angiogenesis (23) and particularly metastasis (8–11). In
our previous study, by comparing the plasma membrane proteome
between OS and osteoblasts and the expression of potential
OS-related genes between OS tissues and adjacent non-tumorous
tissues, CD151 was validated to be upregulated in OS. In addition,
the expression of CD151 in different cell lines was compared with
the IP assay, presenting similar results (data not shown).
Furthermore, according to bioinformatic analysis, CD151 was
suggested to be a key molecule regulating the progression of OS and
a therapeutic target (13).
However, the exact function(s) of CD151 in promoting OS remains
vague.
As it is of great importance to investigate
antimetastatic strategies for OS, in the present study for the
first time we investigated the role of CD151 in regulating OS
metastasis. Migration through the surrounding extracellular matrix
and invasion of the basement membrane are important steps for
metastasis of tumor cells, and CD151 has been reported to regulate
migration and invasion of tumor cells (24,25).
Wound healing and Matrigel assays were used to investigate the
migration and invasion abilities, respectively, of the OS cells
in vitro. The results showed that after CD151 was knocked
down in vitro using siRNA, the migration and invasion rates
of the OS cells were both decreased, respectively. Furthermore, the
antimetastasis effects of CD151 knockdown in OS in vivo was
evaluated using an orthotopic xenograft model of nude mice. After
intratumoral administration of CD151 siRNA for 4 weeks, the
pulmonary metastatic rate and the mean number of pulmonary
metastatic clusters per mouse were decreased. These values were
significantly greater than those of the siRNA-MOCK and NC groups.
Moreover, the bioluminescent imaging results demonstrated a similar
metastatic inhibition effect with CD151 knockdown. Thus, we
concluded that CD151 knockdown inhibited OS metastasis.
A number of lines of evidence have suggested that
CD151 modulates metastasis through different molecular mechanisms,
including the regulation of integrin-mediated cellular behaviors
(26–28), growth factor-mediated interplay
between tumors and their microenvironments (10), MMP expression and activation
(29,30) and signal transduction (21). The results of our previous study
(13) suggested that β-catenin,
which is a multifunctional molecule and the key effector of the Wnt
signaling pathway (31,32), was mechanistically involved in the
regulation of OS progression by CD151. The Wnt signaling pathway
has been implicated in regulating several stages of OS progression,
including tumorigenesis, tumor growth and metastasis (33,34).
It has been reported that β-catenin accumulation in the cytoplasm
is a common occurrence in OS (35)
and this process is modulated by upstream molecules, including
GSK-3β (32). Our findings showed
that CD151 modulated GSK-3β activation (36). Thus, the relationships among CD151,
β-catenin and GSK-3β were evaluated by siRNA treatments. The
results showed that CD151 knockdown reduced the expression levels
of pGSK-3β and β-catenin, and that GSK-3β knockdown reduced
β-catenin expression. These findings showed that CD151 modulated
β-catenin expression via GSK-3β activation. Moreover, recent
studies demonstrated that CD151 can modulate MMP9 expression
through the FAK/p38/MAPK/JNK/c-Jun and the PI3K/Akt/GSK-3β/Snail
pathways (19,36), which is a downstream target of Wnt
(37) and has been suggested to
modulate OS metastasis (38). Our
in vitro and in vivo results indicated that CD151
knockdown reduced MMP9 expression in OS cells and xenograft model
mice. Since MMP9 was shown to be a target gene of Wnt (37), MMP9 expression was evaluated by
knockdown of GSK-3β and β-catenin, and the results showed that MMP9
expression was increased when GSK-3β or β-catenin expression was
reduced. Therefore, based on our results, we conclude that CD151
knockdown reduces MMP9 expression via GSK-3β/β-catenin signaling in
highly metastatic OS cells.
In conclusion, OS is one of the most common primary
bone malignancies with a high early metastatic propensity and our
findings suggested that in highly metastatic OS cells, CD151
knockdown inhibited their migration, invasion and metastasis
through the GSK-3β/β-catenin/MMP9 signaling pathway. These findings
suggest that CD151 could be considered a potential antimetastatic
target for the treatment of OS, although further investigation
should be made before clinical application.
Abbreviations:
|
OS
|
osteosarcoma
|
|
siRNA
|
small interfering RNA
|
|
MMP9
|
matrix metalloproteinase 9
|
|
GSK-3β
|
glycogen synthase kinase 3
|
|
NC
|
normal control
|
References
|
1
|
Allison DC, Carney SC, Ahlmann ER,
Hendifar A, Chawla S, Fedenko A, Angeles C and Menendez LR: A
meta-analysis of osteosarcoma outcomes in the modern medical era.
Sarcoma. 2012:7048722012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yang J and Zhang W: New molecular insights
into osteosarcoma targeted therapy. Curr Opin Oncol. 25:398–406.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kato H, Wakabayashi H, Naito Y, Kato S,
Nakagawa T, Matsumine A and Sudo A: Anti-tumor necrosis factor
therapy inhibits lung metastasis in an osteosarcoma cell line.
Oncology. 88:139–146. 2015.
|
|
4
|
Li Y, Liao Q, Li K, Zhong D, Weng X and Mi
M: Knockdown of endothelin A receptor expression inhibits
osteosarcoma pulmonary metastasis in an orthotopic xenograft mouse
model. Mol Med Rep. 5:1391–1395. 2012.PubMed/NCBI
|
|
5
|
Zöller M: Tetraspanins: Push and pull in
suppressing and promoting metastasis. Nat Rev Cancer. 9:40–55.
2009. View
Article : Google Scholar
|
|
6
|
Li P, Zeng H, Qin J, Zou Y, Peng D, Zuo H
and Liu Z: Effects of tetraspanin CD151 inhibition on A549 human
lung adenocarcinoma cells. Mol Med Rep. 11:1258–1265. 2015.
|
|
7
|
Peng D, Zuo H, Liu Z, Qin J, Zhou Y, Li P,
Wang D, Zeng H and Zhang XA: The tetraspanin CD151-ARSA mutant
inhibits angiogenesis via the YRSL sequence. Mol Med Rep.
7:836–842. 2013.PubMed/NCBI
|
|
8
|
Copeland BT, Bowman MJ and Ashman LK:
Genetic ablation of the tetraspanin CD151 reduces spontaneous
metastatic spread of prostate cancer in the TRAMP model. Mol Cancer
Res. 11:95–105. 2013. View Article : Google Scholar
|
|
9
|
Zijlstra A, Lewis J, Degryse B, Stuhlmann
H and Quigley JP: The inhibition of tumor cell intravasation and
subsequent metastasis via regulation of in vivo tumor cell motility
by the tetraspanin CD151. Cancer Cell. 13:221–234. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sadej R, Romanska H, Kavanagh D, Baldwin
G, Takahashi T, Kalia N and Berditchevski F: Tetraspanin CD151
regulates transforming growth factor beta signaling: Implication in
tumor metastasis. Cancer Res. 70:6059–6070. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Takeda Y, Li Q, Kazarov AR, Epardaud M,
Elpek K, Turley SJ and Hemler ME: Diminished metastasis in
tetraspanin CD151-knockout mice. Blood. 118:464–472. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sadej R, Grudowska A, Turczyk L, Kordek R
and Romanska HM: CD151 in cancer progression and metastasis: A
complex scenario. Lab Invest. 94:41–51. 2014. View Article : Google Scholar
|
|
13
|
Zhang Z, Zhang L, Hua Y, Jia X, Li J, Hu
S, Peng X, Yang P, Sun M, Ma F, et al: Comparative proteomic
analysis of plasma membrane proteins between human osteosarcoma and
normal osteoblastic cell lines. BMC Cancer. 10:2062010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hoeppner LH, Secreto FJ and Westendorf JJ:
Wnt signaling as a therapeutic target for bone diseases. Expert
Opin Ther Targets. 13:485–496. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Luu HH, Kang Q, Park JK, Si W, Luo Q,
Jiang W, Yin H, Montag AG, Simon MA, Peabody TD, et al: An
orthotopic model of human osteosarcoma growth and spontaneous
pulmonary metastasis. Clin Exp Metastasis. 22:319–329. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Luo X, Sharff KA, Chen J, He TC and Luu
HH: S100A6 expression and function in human osteosarcoma. Clin
Orthop Relat Res. 466:2060–2070. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Su Y, Luo X, He BC, Wang Y, Chen L, Zuo
GW, Liu B, Bi Y, Huang J, Zhu GH, et al: Establishment and
characterization of a new highly metastatic human osteosarcoma cell
line. Clin Exp Metastasis. 26:599–610. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu Z, Rebowe RE, Wang Z, Li Y, Wang Z,
DePaolo JS, Guo J, Qian C and Liu W: KIF3a promotes proliferation
and invasion via Wnt signaling in advanced prostate cancer. Mol
Cancer Res. 12:491–503. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hong IK, Jin YJ, Byun HJ, Jeoung DI, Kim
YM and Lee H: Homophilic interactions of tetraspanin CD151
up-regulate motility and matrix metalloproteinase-9 expression of
human melanoma cells through adhesion-dependent c-Jun activation
signaling pathways. J Biol Chem. 281:24279–24292. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li M, Zhou W, Yuan R, Chen L, Liu T, Huang
D, Hao L, Xie Y and Shao J: ROCK2 promotes HCC proliferation by
CEBPD inhibition through phospho-GSK3β/β-catenin signaling. FEBS
Lett. 589:1018–1025. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hong IK, Jeoung DI, Ha KS, Kim YM and Lee
H: Tetraspanin CD151 stimulates adhesion-dependent activation of
Ras, Rac, and Cdc42 by facilitating molecular association between
β1 integrins and small GTPases. J Biol Chem.
287:32027–32039. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Roselli S, Kahl RG, Copeland BT, Naylor
MJ, Weidenhofer J, Muller WJ and Ashman LK: Deletion of Cd151
reduces mammary tumorigenesis in the MMTV/PyMT mouse model. BMC
Cancer. 14:5092014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Takeda Y, Kazarov AR, Butterfield CE,
Hopkins BD, Benjamin LE, Kaipainen A and Hemler ME: Deletion of
tetraspanin Cd151 results in decreased pathologic angiogenesis in
vivo and in vitro. Blood. 109:1524–1532. 2007. View Article : Google Scholar
|
|
24
|
Palmer TD, Martínez CH, Vasquez C, Hebron
KE, Jones-Paris C, Arnold SA, Chan SM, Chalasani V, Gomez-Lemus JA,
Williams AK, et al: Integrin-free tetraspanin CD151 can inhibit
tumor cell motility upon clustering and is a clinical indicator of
prostate cancer progression. Cancer Res. 74:173–187. 2014.
View Article : Google Scholar :
|
|
25
|
Ranjan A, Bane SM and Kalraiya RD:
Glycosylation of the laminin receptor (α3β1) regulates its
association with tetraspanin CD151: Impact on cell spreading,
motility, degradation and invasion of basement membrane by tumor
cells. Exp Cell Res. 322:249–264. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fei Y, Wang J, Liu W, Zuo H, Qin J, Wang
D, Zeng H and Liu Z: CD151 promotes cancer cell metastasis via
integrins α3β1 and α6β1 in vitro. Mol Med Rep. 6:1226–1230.
2012.PubMed/NCBI
|
|
27
|
Deng X, Li Q, Hoff J, Novak M, Yang H, Jin
H, Erfani SF, Sharma C, Zhou P, Rabinovitz I, et al:
Integrin-associated CD151 drives ErbB2-evoked mammary tumor onset
and metastasis. Neoplasia. 14:678–689. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gustafson-Wagner E and Stipp CS: The
CD9/CD81 tetraspanin complex and tetraspanin CD151 regulate α3β1
integrin-dependent tumor cell behaviors by overlapping but distinct
mechanisms. PLoS One. 8:e618342013. View Article : Google Scholar
|
|
29
|
Shiomi T, Inoki I, Kataoka F, Ohtsuka T,
Hashimoto G, Nemori R and Okada Y: Pericellular activation of
proMMP-7 (promatrilysin-1) through interaction with CD151. Lab
Invest. 85:1489–1506. 2005.PubMed/NCBI
|
|
30
|
Yue S, Mu W and Zöller M: Tspan8 and CD151
promote metastasis by distinct mechanisms. Eur J Cancer.
49:2934–2948. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang Y, Li YP, Paulson C, Shao JZ, Zhang
X, Wu M and Chen W: Wnt and the Wnt signaling pathway in bone
development and disease. Front Biosci. 19:379–407. 2014. View Article : Google Scholar
|
|
32
|
Valenta T, Hausmann G and Basler K: The
many faces and functions of β-catenin. EMBO J. 31:2714–2736. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lin CH, Guo Y, Ghaffar S, McQueen P,
Pourmorady J, Christ A, Rooney K, Ji T, Eskander R, Zi X, et al:
Dkk-3, a secreted wnt antagonist, suppresses tumorigenic potential
and pulmonary metastasis in osteosarcoma. Sarcoma. 2013:1475412013.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ji T, Guo Y, Kim K, McQueen P, Ghaffar S,
Christ A, Lin C, Eskander R, Zi X and Hoang BH: Neuropilin-2
expression is inhibited by secreted Wnt antagonists and its
down-regulation is associated with reduced tumor growth and
metastasis in osteosarcoma. Mol Cancer. 14:862015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Haydon RC, Deyrup A, Ishikawa A, Heck R,
Jiang W, Zhou L, Feng T, King D, Cheng H, Breyer B, et al:
Cytoplasmic and/or nuclear accumulation of the beta-catenin protein
is a frequent event in human osteosarcoma. Int J Cancer.
102:338–342. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Shi GM, Ke AW, Zhou J, Wang XY, Xu Y, Ding
ZB, Devbhandari RP, Huang XY, Qiu SJ, Shi YH, et al: CD151
modulates expression of matrix metalloproteinase 9 and promotes
neoangiogenesis and progression of hepatocellular carcinoma.
Hepatology. 52:183–196. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kwon M, Lee SJ, Wang Y, Rybak Y, Luna A,
Reddy S, Adem A, Beaty BT, Condeelis JS and Libutti SK: Filamin A
interacting protein 1-like inhibits WNT signaling and MMP
expression to suppress cancer cell invasion and metastasis. Int J
Cancer. 135:48–60. 2014. View Article : Google Scholar :
|
|
38
|
Liu T, Zhou W, Zhang F, Shi G, Teng H,
Xiao J and Wang Y: Knockdown of IRX2 inhibits osteosarcoma cell
proliferation and invasion by the AKT/MMP9 signaling pathway. Mol
Med Rep. 10:169–174. 2014.PubMed/NCBI
|