Introduction
Cancer cachexia is a severe, debilitating and
life-threatening syndrome, characterized by a marked loss of
skeletal muscle and adipose tissue and appetite, a reduction in
physical functions, and profound involuntary weight loss despite
adequate nutritional intake (1–3).
Weight loss is more frequently experienced by 80–90% of pancreatic
and gastrointestinal cancer patients than by sarcoma and breast
cancer patients, and severe cachexia with >10% weight loss is
closely correlated with high mortality, impaired quality of life
and poor response to chemotherapy or radiotherapy (4). In addition, cachectic patients are
more prone to chemotherapy-related toxic side effects. Therefore,
it is important to control the cachectic state in cancer patients,
since cachexia is the main cause of cancer-related death in
approximately 25–30% of patients.
Although the mechanisms of cancer cachexia are not
completely known, recent studies have indicated that persistent
production of proinflammatory cytokines and catabolic factors
secreted from both tumor cells and host cells, such as macrophages,
plays an essential role in the induction and progression of cancer
cachexia (5,6). Among procachectic cytokines,
interleukin-6 (IL-6) is considered a key mediator in the
pathogenesis of cancer cachexia. In previous studies, it has been
reported that IL-6-secreting cells induce wasting of both muscle
and fat, and IL-6 is responsible for muscle wasting in cachectic
mice with CT-26 or Yomoto uterine cancer (7). Treatment with monoclonal antibodies to
IL-6, or to the IL-6 receptor, markedly suppresses the development
of cachexia in tumor-bearing mice and in patients with
IL-6-overexpressing lung cancer, suggesting that blocking IL-6
function might be an effective intervention for the management of
cachectic patients (8,9).
Several clinical trials to treat cancer cachexia
have been performed, involving drugs that stimulate appetite,
reduce proinflammatory cytokine production and inhibit
tumor-induced protein degradation. However, current medical
treatments are limited due to low efficacy and high toxicity. Among
them, medroxyprogesterone acetate, approved in Europe for treatment
of cancer and acquired immune deficiency syndrome (AIDS)-related
cachexia, is widely used in the treatment of hormone-related cancer
as a supportive therapy (10,11).
However, this treatment also has unwanted side effects, including
diabetes, osteoporosis, mood swings and thromboembolism (12). Therefore, it is essential to find
novel agents capable of preventing cancer-induced cachexia with
minimal adverse effects.
Sosiho-tang (SO, known as Xiaochaihu-tang in
Chinese, and as Sho-saiko-to in Japanese) is an Oriental
traditional herbal formula comprised of seven medicinal herbs,
including Bupleurum root, Glycyrrhizae radix et rhizoma, Ginseng,
radix, Pinellia tuber, Scutellaria root, Zingiberis rhizoma crudus
and Zizyphi fructus. SO has long been used to treat chronic liver
diseases such as hepatitis and liver cirrhosis in Korea, China and
Japan (13). In addition, SO
prevents inflammation and cancer progression (14–17),
suggesting that it might also prevent cancer-induced cachexia
through suppression of inflammatory responses caused by cancer.
In the present study, we examined the effects of
oral administration of SO on the severity of key parameters of
cachexia in CT-26-bearing mice. In addition, we also investigated
the efficacy of SO on the production of proinflammatory cytokines
and muscle wasting, and further elucidated the in vitro
mechanism of its anti-cachectic activity using murine cell lines,
including the CT-26 colon carcinoma, the J774A.1 macrophage, and
the C2C12 myoblast cell lines.
Materials and methods
Cells
Murine colon carcinoma CT-26 cells and murine
myoblast C2C12 cells were purchased from the American Type Culture
Collection (Manassas, VA, USA). Murine macrophage-like J774A.1
cells [Korean Cell Line Bank (KCLB): no. 40067] were obtained from
the KCLB (Seoul, Korea). The cells were maintained in Dulbecco's
modified Eagle's medium (DMEM) or Roswell Park Memorial Institute
(RPMI)-1640 medium (Lonza, Walkersville, MD, USA) supplemented with
10% (v/v) heat-inactivated fetal bovine serum (FBS; Cellgro,
Manassas, VA, USA) and penicillin (100 U/ml)/streptomycin (100
µg/ml) (Cellgro) in a humidified 5% CO2 incubator
at 37°C. To induce myogenic differentiation, the C2C12 cells at a
density of 70–80% were cultured in DMEM containing 5% horse serum
(HS; Gibco-BRL, Grand Island, NY, USA) for 5–7 days.
Animals
Six-week-old male BALB/c mice were purchased from
Taconic Farms (Samtako Bio Korea, Osan, Korea) and housed under
specific pathogen-free conditions under a 12-h light-dark cycle at
22±1°C and 55±5% humidity. All animal experiments were approved by
the Animal Care and Use Committee of the Korea Institute of
Oriental Medicine (KIOM, Daejeon, Korea) with reference numbers
#13–100, #14–074 and #15–011. Experiments were performed according
to the guidelines of the Animal Care and Use Committee at KIOM.
Antibodies and reagents
Antibodies against p21, cyclin-dependent kinase
(CDK)2, cyclin D, p38, p-p38 (Thr180/Tyr182), p-IκBα (Ser32),
p-IKKα/β (Ser176/180), signal transducer and activator of
transcription (STAT)3, p-STAT3 (Tyr705), p65, p-p65 (Ser536),
inducible nitric oxide synthase (iNOS), and TBP were purchased from
Cell Signaling Technology (Danvers, MA, USA). Anti-α-tubulin
antibody was obtained from Santa Cruz Biotechnology (Santa Cruz,
CA, USA). Antibody against myosin heavy chain was purchased from
R&D Systems (Minneapolis, MN, USA). Horseradish peroxidase
(hRP)-conjugated anti-mouse and anti-rabbit antibodies were
purchased from Cell Signaling Technology. Recombinant murine tumor
necrosis factor-α (rMu TNF-α) was purchased from Promokine
(heidelberg, Germany).
Preparation of SO
The composition of herbal extract SO is listed in
Table I. All herbs were obtained
from Yeongcheon herbal Market (Yeongcheon, Korea), validated by
Professor Ki hwan Bae (Chungnam National University, Daejeon,
Korea), and stored in the herbal bank at KIOM prior to use. A total
of 1,674.5 g of chopped SO was heat-extracted in 16.745 liters of
distilled water for 3 h at 115°C using a Cosmos-600 Extractor
(Gyungseo, Incheon, Korea). The decoction was filtered through
standard testing sieves (150 µm; Retsch, haan, Germany),
lyophilized, and stored in desiccators at 4°C. The final amount of
lyophilized SO powder was 416.783 g, and the yield was 24.89%. For
in vitro experiments, the SO powder was dissolved in 10%
dimethyl sulfoxide to 50 mg/ml, filtered through a 0.22-µm
disk filter, and then stored at -20°C prior to use.
 | Table IHerbal composition of Sosiho-tang. |
Table I
Herbal composition of Sosiho-tang.
| Name of herb | Amount
(g) | Location of
origin |
|---|
| Bupleurum root | 12.00 | Yeongcheon,
Korea |
| Glycyrrhizae radix et
rhizoma | 2.00 | China |
| Ginseng radix | 4.00 | Geumsan, Korea |
| Pinellia tuber | 4.00 | Uiseong, Korea |
| Scutellaria
root | 8.00 | Suncheon,
Korea |
| Zingiberis rhizoma
crudus | 1.49 | Yeongcheon,
Korea |
| Zizyphi
fructus | 2.00 | Yeongcheon,
Korea |
| Total amount | 33.49 | |
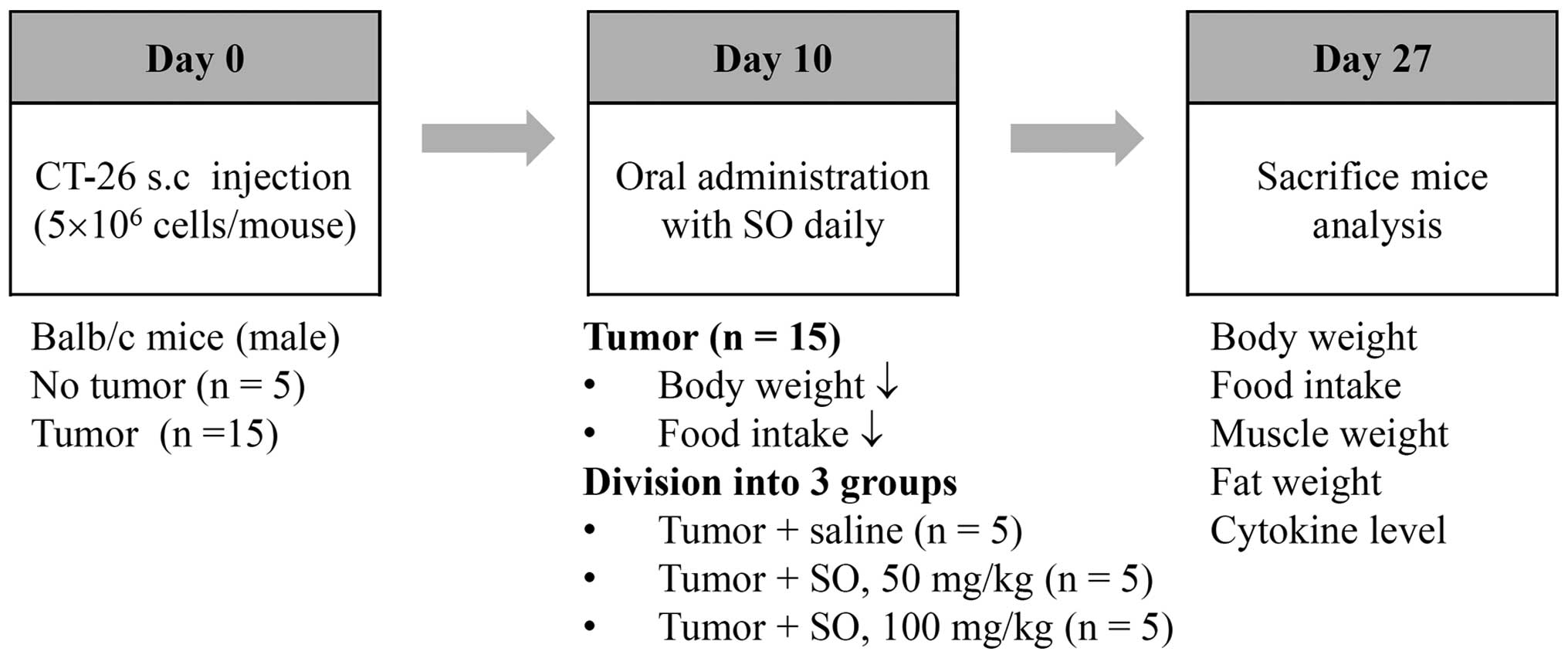
Experimental design of cancer
cachexia
The CT-26 cells were subcutaneously inoculated into
the abdominal region of 7-week-old male BALB/c mice
(5×106 cells/mouse). To confirm the induction of
cancer-mediated cachexia, the body weights and tumor volumes of the
mice were measured once every 2 days throughout the experiment.
Significant weight losses in the tumor-bearing mice were observed
between 8–10 days after the tumor inoculations, and the mice were
fed with saline or SO at doses of 50 and 100 mg/kg from day 10 to
day 27 after tumor inoculation. The administered dose was
calculated based on the amount used in human adults (33.49 g/60 kg
of body weight/day) and the yield of powdered extract (24.89%). The
age-matched healthy control mice having no tumors were treated with
saline. During the experiments, food intake was calculated as the
mean value of five mice per cage. At the time of sacrifice, mice
were euthanized by intraperitoneal injection with a 2:1 mixture of
zoletil (Virbac, Magny-en-Vexin, France) and rompun (Bayer, Seoul,
Korea), and then the tumor, epididymal fat, gastrocnemius muscle,
and heart were dissected and weighed. In addition, serum samples
were obtained for measuring levels of IL-6, tumor necrosis factor
(TNF)-α, and IL-1β. After exsanguination, the remaining viscera
were removed and the carcass weight was measured.
Preparation of CT-26 conditioned medium
(CM)
The CT-26 cells were plated into 100-mm culture
dishes at a density of 5×104 cells/cm2 and
treated with or without SO for 24 h under complete medium
conditions. After washing three times with phosphate-buffered
saline (PBS), the cells were additionally washed twice with
serum-free medium, and then incubated for another 24 h in
serum-free DMEM. The resulting CM was centrifuged to remove debris,
filtered using a 0.22-µm disk filter, and then stored in a
freezer. The CM was diluted at a 1:3 or 1:5 ratio with either DMEM
containing 10% FBS and antibiotics [growth medium (GM)] for
myoblast treatment or DMEM containing 5% hS and antibiotics
[differentiation medium (DM)] for the myotube treatment. Prior to
dilution, an appropriate quantity of FBS, hS, or antibiotics for
compensation were added to the CM.
Myoblast proliferation assay
To examine the C2C12 myoblast proliferation, cells
were plated into a 96-well culture plate at a density of
1×103 cells/well, and then treated with SO-treated or
-untreated CM at 37°C for 48 h. At the indicated time points, cell
proliferation was determined using the Cell Counting Kit-8 (Dojindo
Laboratories, Kumamoto, Japan) according to the manufacturer's
protocol.
Determination of NO production
The cells were pretreated with the indicated
concentrations of SO for 1 h and then stimulated with LPS for 24 h.
The collected culture supernatant was mixed with an equal volume of
Griess reagent (1% sulfanil-amide, 0.1% naphthylethylenediamine
dihydrochloride and 2.5% phosphoric acid), and incubated at room
temperature for 5 min, and then the absorbance was measured at 570
nm.
Reverse transcription-polymerase chain
reaction (RT-PCR)
The total RNA was isolated using an RNA extraction
solution (BioAssay Co., Daejeon, Korea) according to the
manufacturer's instructions, and the RNA concentrations were
quantitated using a NanoDrop ND-1000 spectrophotometer (NanoDrop
Technologies, Wilmington, DE, USA). The RNA (3 µg) was
reverse transcribed using a 1st Strand cDNA Synthesis
kit (BioAssay Co.) and then the cDNA samples were analyzed by
semiquantitative PCR using specific primers (Table II). PCR products were visualized by
electrophoresis using agarose gels and staining with GreenLight™
(BioAssay Co.), and band intensities were analyzed using ImageJ
software (National Institutes of health, Bethesda, MD, USA).
 | Table IIPrimers used for PCR. |
Table II
Primers used for PCR.
| Target gene | Sequences |
|---|
| iNOS | F:
5′-CCTCCTCCACCCTACCAAGT-3′
R: 5′-CACCCAAAGTGCTTCAGTCA-3′ |
| IL-6 | F:
5′-CATGTTCTCTGGGAAATCGTGG-3′
R: 5′-AACGCACTAGGTTTGCCGAGTA-3′ |
| TNF-α | F:
5′-CATGTTCTCTGGGAAATCGTGG-3′
R: 5′-AACGCACTAGGTTTGCCGAGTA-3′ |
| IL-1α | F:
5′-GGTTAAATGACCTGCAACAGGA-3′
R: 5′-TCTTTGGTGGCAATAAACAGC-3′ |
| GAPDH | F:
5′-TCATGACCACAGTCCATGCC-3′
R: 5′-TCCACCACCCTGTTGCTGTA-3′ |
Western blot analysis
For whole cell lysates, cells were harvested, washed
and lysed in M-PER Mammalian Protein Extraction Reagent (Thermo
Scientific, Rockford, IL, USA). The cytosolic and nuclear fractions
were obtained using NE-PER Nuclear and Cytoplasmic Extraction
Reagent (Thermo Scientific). The lysates were separated using
sodium dodecyl sulfate polyacrylamide gel electrophoresis
(SDS-PAGE), followed by transfer onto a polyvinylidene fluoride
(PVDF) membrane (Bio-Rad, hercules, CA, USA), then blocked with 3%
bovine serum albumin (BSA) in Tris-buffered saline containing 0.05%
Tween-20 (TBST) and immunoblotted using specific antibodies at 4°C
overnight. After washing with TBST, the membranes were reacted with
secondary antibodies conjugated with HRP for 1 h at room
temperature, and the target proteins were visualized using the
SuperSignal WestFemto Maximum Sensitivity Substrate (Pierce,
Rockford, IL, USA) and the ImageQuant LAS 4000 Mini (GE healthcare,
Piscataway, NJ, USA). The relative band intensities were measured
using ImageJ software.
Enzyme-linked immunosorbent assay
(ELISA)
The levels of murine IL-6, TNF-α and IL-1β in the
culture supernatants and sera were determined using an ELISA
antibody kit (eBioscience, San Jose, CA, USA) according to the
manufacturer's instructions.
Statistical analysis
Data are presented as means ± standard deviation
(SD). Differences between groups were analyzed by the Student's
t-test using the SigmaPlot software (ver 8.0; SPSS Inc., Chicago,
IL, USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
Oral administration of SO alleviates
cachexia symptoms in CT-26 tumor-bearing mice and suppresses tumor
growth
To examine whether SO is an effective treatment for
cancer-induced cachexia, mice were administered SO from 10 days
after CT-26 cell inoculation, when mice showed reductions in body
weight and food intake by ~10–12% compared to the normal mice
(Fig. 1). Normal mice exhibited
gradually increased body weight during the experimental period by
21.6%, while control mice had increased body weight by 4.3%
(Fig. 2A). SO administration at
doses of 50 and 100 mg/kg significantly increased body weights, by
13.1 and 13.7%, respectively, showing recovery of body weights to
~93.5 and 93.9%, respectively, of the normal mice. In previous
studies, it has been reported that SO inhibits cell proliferation
via cell cycle arrest at G0/G1, and induces
apoptosis of various types of cancer cells, including
hepatocellular carcinoma, Lewis lung carcinoma, cholangiocarcinoma,
ovarian cell carcinoma and renal cell carcinoma (15,16).
In the present study, SO administration at 50 and 100 mg/kg
significantly retarded CT-26 tumor growth, by 34.29 and 38.81%,
respectively, compared with the control mice on day 27. Control
mice had a mean tumor weight of 2.88±0.89 g, while mice treated
with 50 and 100 mg/kg of SO had mean tumor weights of 2.52±0.61 and
2.26±0.19 g, reflecting 12.56 and 21.37% reductions, respectively
(Fig. 2B). The mean values of food
intake/mouse/day during the experiment for normal, control, and 50-
and 100-mg/kg SO-treated mice were 3.56±0.11, 3.13±0.24, 3.32±0.16
and 3.37±0.18 g, respectively, indicating that SO aids in improving
appetite (data not shown). In addition, administration of SO
significantly prevented the loss of final body weight, carcass
weight, heart weight and wasting of epididymal adipose tissue and
gastrocnemius muscle in the CT-26 tumor-bearing mice. Furthermore,
serum IL-6 levels were markedly elevated in the tumor-bearing
control mice compared to the non-tumor-bearing normal mice, and
these values were significantly reduced by SO administration
(Fig. 2C). The serum levels of
TNF-α and IL-1β were below the detection limit in all groups (data
not shown). These results indicate that SO reduces tumor burden and
delays the process of CT-26 tumor-induced cachexia.
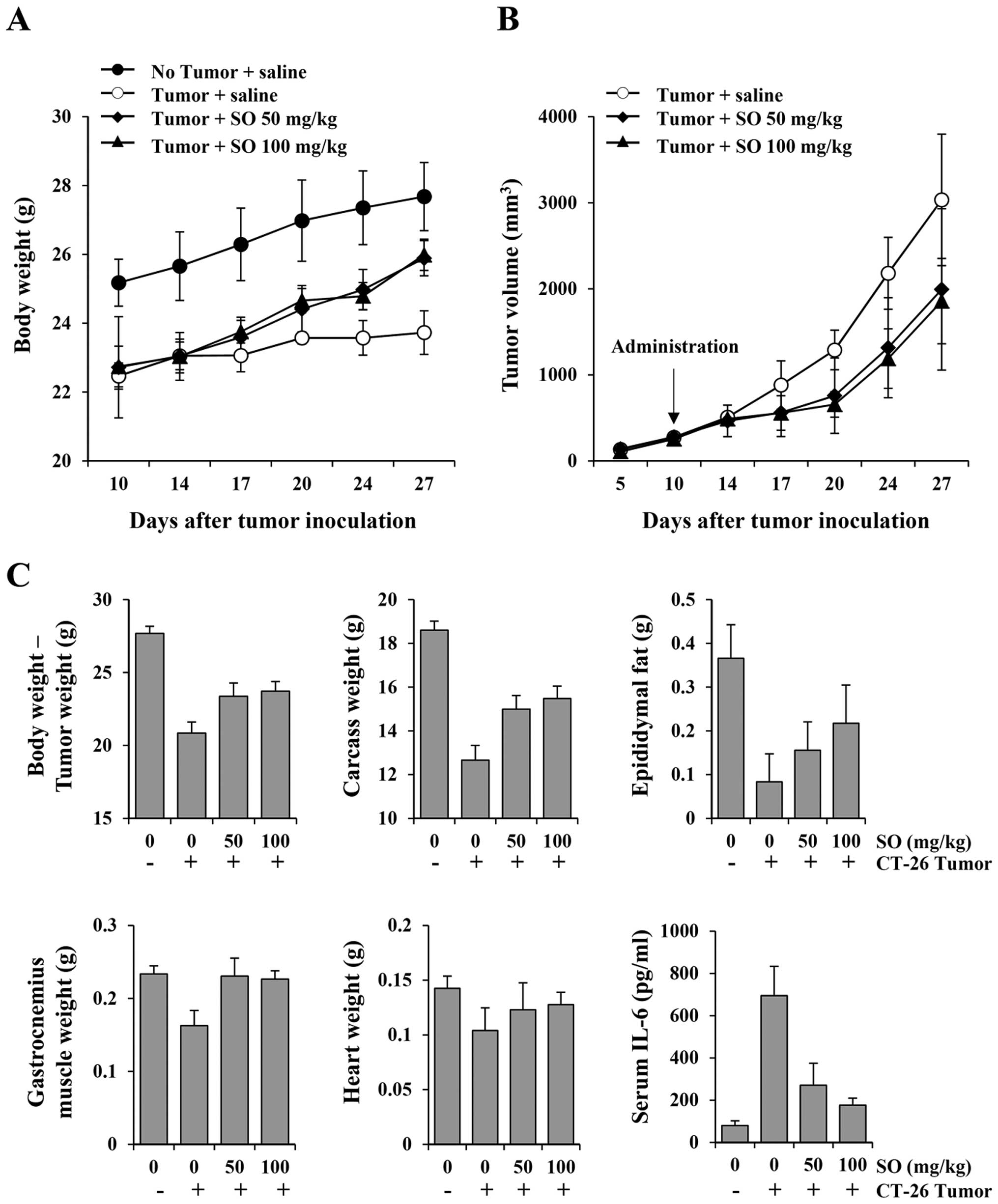 | Figure 2Effects of SO administration on body
weight, tumor growth, nutritional parameters and serum interleukin
(IL)-6 levels in CT-26 tumor-bearing mice. On day 10 after tumor
inoculation, the mice were administered daily with SO at doses of
50 and 100 mg/kg, or saline for 17 consecutive days. The healthy
control mice with no tumors were also treated daily with saline.
The body weight, tumor size, and food intake were measured on days
14, 17, 20, 24 and 27 (A and B). After mice were sacrificed, the
carcass, epididymal fat, gastrocnemius muscle, and heart were
weighed, and serum IL-6 levels were measured using an enzyme-linked
immunosorbent assay (ELISA) (C). Animal experiments were performed
three times. Representative results are shown. |
SO downregulates LPS-induced NO
production, iNOS expression and inflammatory cytokine production in
murine macrophage J774A.1 cells
Fig. 2C shows that
the serum level of IL-6 was markedly decreased by SO administration
in the CT-26-bearing mice compared to the control mice. In cancer
patients, the tumor induces a chronic host inflammatory response,
characterized by the production of cytokines such as IL-6, IL-1β,
IFN-γ and TNF-α. These cytokines are known to be involved in the
induction of cancer-related muscle wasting through activation of
iNOS expression and NO production (18–20).
Therefore, we examined whether SO could suppress iNOS expression,
NO generation and inflammatory cytokine production in the
LPS-stimulated J774A.1 cells. SO almost completely blocked the
LPS-induced increase in iNOS expression at both the mRNA and
protein levels (Fig. 3A).
Furthermore, SO at 10, 50 and 100 µg/ml inhibited the NO
production by 59.62, 76.43 and 90.42%, respectively, in a
dose-dependent manner, compared with the untreated controls
(Fig. 3B). The mRNA levels of IL-6,
IL-1α and TNF-α, as well as the protein levels of IL-6, IL-1β and
TNF-α, were strongly increased by LPS stimulation, and were
efficiently suppressed by the SO treatment (Fig. 3C and D). Similar to observations in
J774A.1 cells, SO suppressed the LPS-induced NO production, iNOS
expression, and cytokine production in peritoneal macrophages (data
not shown). SO at the concentrations used in the experiments did
not affect the viability of J774A.1 cells or primary peritoneal
macrophages, excluding the possibility of cytotoxic effects.
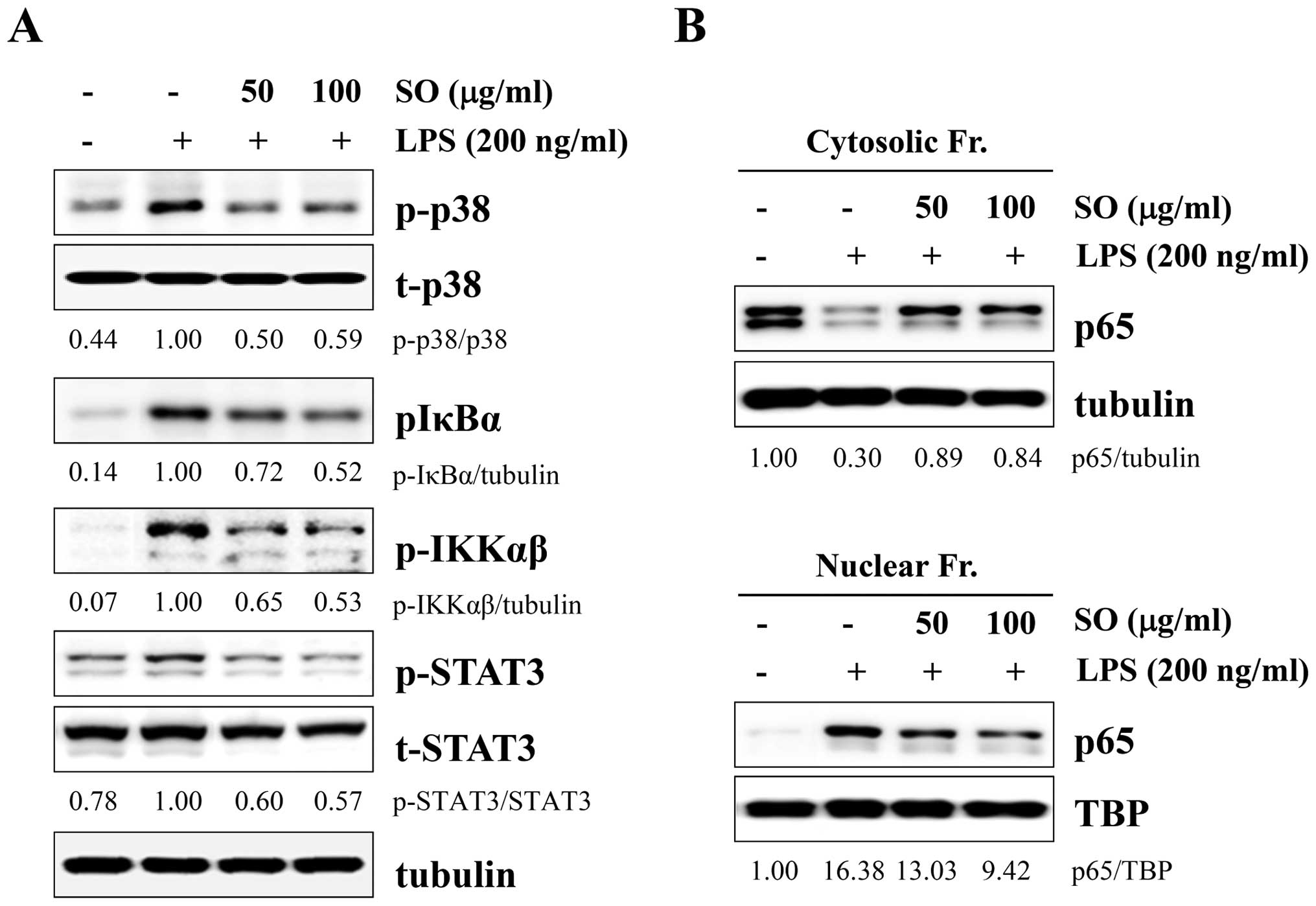
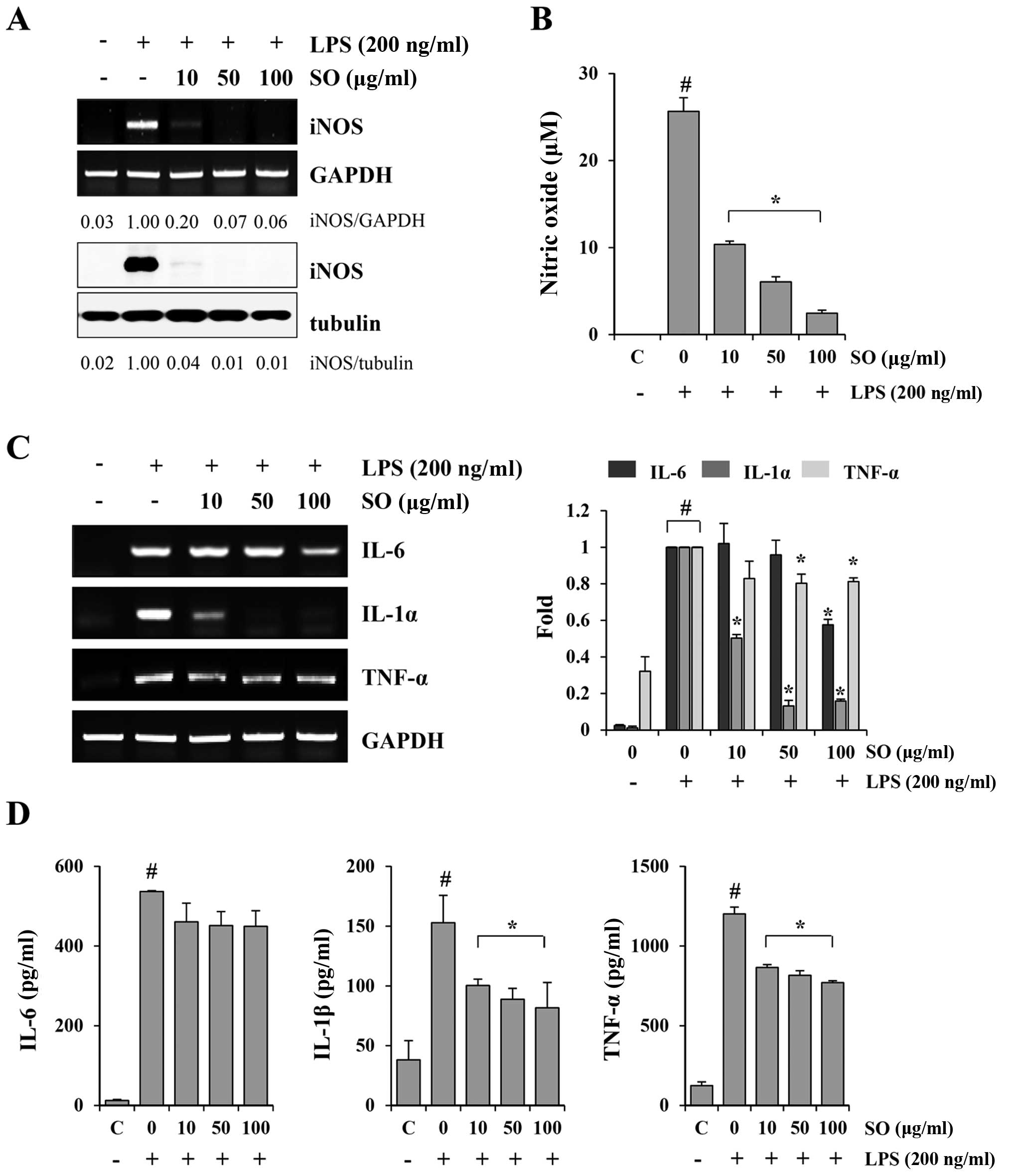 | Figure 3The inhibitory effects of SO on
lipopolysaccharide (LPS)-induced nitric oxide (NO) production and
inflammatory cytokine production in murine macrophage J774A.1
cells. (A) The J774A.1 cells were pretreated with indicated
concentrations of SO for 1 h, and then stimulated with 200 ng/ml
LPS for 24 h. The inducible nitric oxide synthase (iNOS) mRNA and
protein levels were examined by RT-PCR and western blotting,
respectively. The band intensities relative to LPS-stimulated cells
were calculated using the ImageJ software. The levels of
glyceraldehyde 3-phosphate dehydrogenase (GAPDH) and tubulin were
measured for normalization. Data are representative of three
independent experiments. (B) The culture supernatants were
collected and analyzed for NO production. The control cells were
treated with vehicle alone. The data are representative of three
independent experiments performed in triplicate and are expressed
as means ± SD. #P<0.05 vs. untreated control,
*P<0.05 vs. SO-untreated control cells. (C) The
J774A.1 cells pretreated with SO for 1 h were stimulated with 200
ng/ml LPS for 6 h. The mRNA levels for interleukin (IL)-6, IL-1α,
and tumor necrosis factor (TNF)-α were analyzed by RT-PCR, and
relative fold increases were calculated after normalization with
GAPDH using ImageJ software. The data show means ± SD of two
independent experiments. #P<0.05 vs. untreated
control, *P<0.05 vs. SO-untreated control cells. (D)
After stimulation of SO-pretreated cells with LPS for 24 h, the
levels of IL-6, IL-1β, and TNF-α in the culture supernatants were
quantitated by ELISA. Data are representative of three independent
experiments performed in triplicate and are expressed as means ±
SD. #P<0.05 vs. untreated control,
*P<0.05 vs. SO-untreated control cells. |
SO strongly blocks LPS-induced p38,
NF-κB, and STAT3 activation in J774A.1 cells
Because mitogen-activated protein kinase (MAPK),
NF-κB, and STAT3 activations are closely related with
proinflammatory cytokines, we examined whether these pathways were
affected by SO treatment. After LPS stimulation, the levels of
phosphorylated p38, IκBα, IKKαβ, and STAT3 were significantly
decreased by SO treatment, in a dose-dependent manner (Fig. 4A). However, the levels of
phosphorylated ERK and JNK were not affected (data not shown).
Because NF-κB activation requires nuclear translocation of p65, we
measured p65 levels in the cytosolic and nuclear fractions. In
control cells, the p65 subunit translocated from the cytosol to the
nucleus by LPS stimulation, whereas SO treatment effectively
prevented p65 nuclear translocation in a dose-dependent manner
(Fig. 4B).
SO attenuates CT-26-mediated skeletal
muscle atrophy in murine C2C12 myotubes
In cancer cachexia, proinflammatory cytokines
including IL-6, IL-1β, IFN-γ and TNF-α have been implicated in the
progression of skeletal muscle wasting (21). In addition to these humoral factors,
tumor-derived factors such as myostatin and proteolysis-inducing
factor collectively promote skeletal muscle wasting (22–24).
In previous studies, it has been reported that CT-26 CM inhibited
C2C12 myoblast proliferation and differentiation and stimulated
C2C12 myotube wasting, while myostatin secreted from tumors acted
as a key contributor (22). To
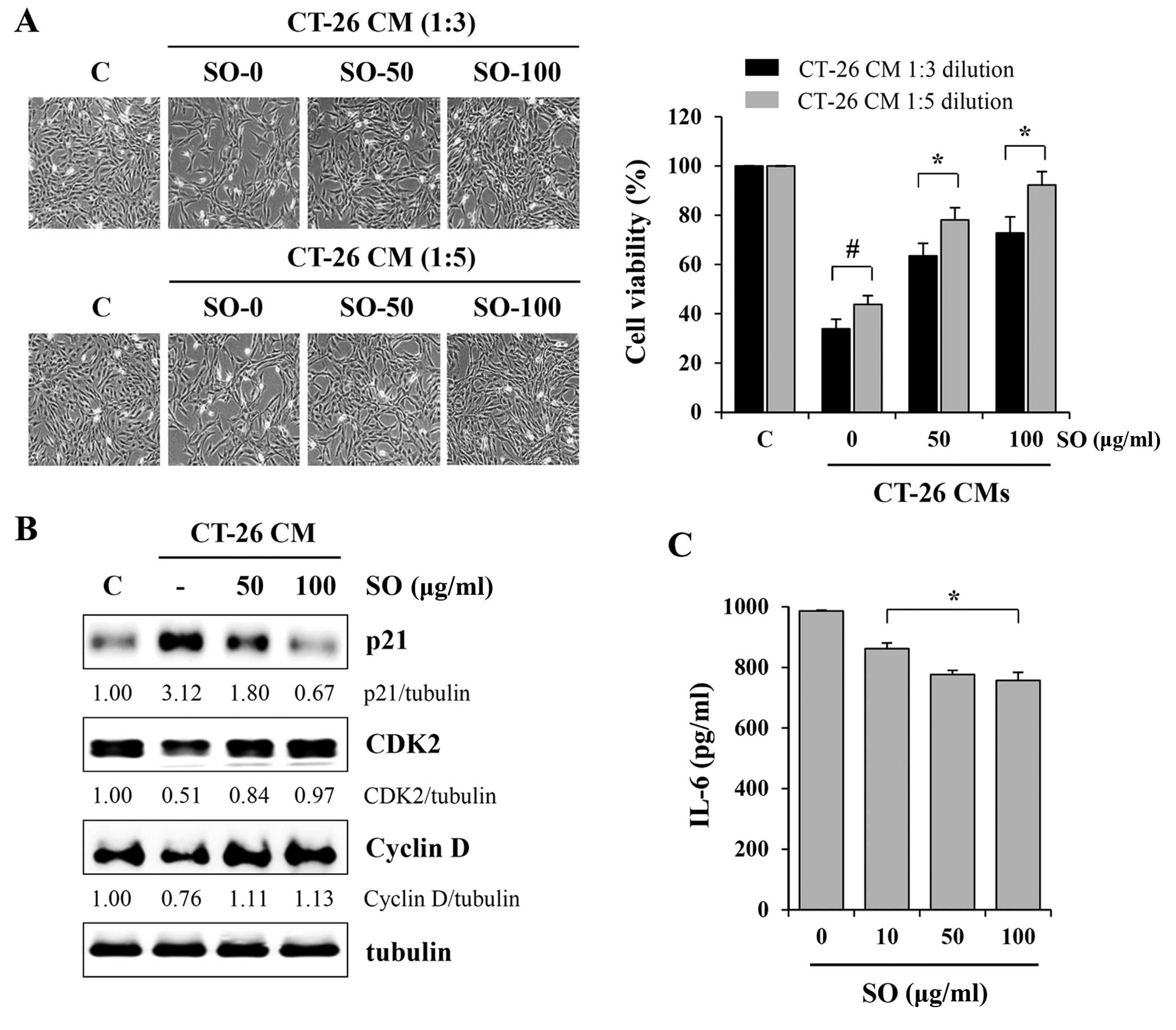
examine the effects of SO on tumor-induced muscle wasting, we
evaluated C2C12 myoblast proliferation after exposure to SO-treated
or -untreated CT-26 CM at 1:3 or 1:5 dilutions with GM. Control CM
significantly retarded C2C12 myoblast growth by ~65 and 55% at 1:3
and 1:5 dilutions, respectively, compared to GM (Fig. 5A). However, compared with the
control CM, SO-treated CM at 100 µg/ml did not cause a
significant inhibition of C2C12 myoblast proliferation, resulting
in 28.2 and 7.7% inhibition at 1:3 and 1:5 dilutions, respectively,
compared to GM. Since CT-26 CM inhibits myoblast proliferation by
cell cycle arrest, we next examined the effect of SO-treated or
-untreated CM on the expression of cell cycle-related proteins in
C2C12 myoblasts. Consistent with previous studies, the level of p21
was dramatically upregulated after exposure to control CM, while
the levels of CDK2 and cyclin D were decreased. However, changes in
these proteins in C2C12 myoblasts exposed to SO-treated CM were
insignificant, consistent with their effects on cell proliferation
(Fig. 5B). Notably, the levels of
IL-6 in CT-26 CM were significantly decreased by SO treatment, in a
dose-dependent manner (Fig. 5C). We
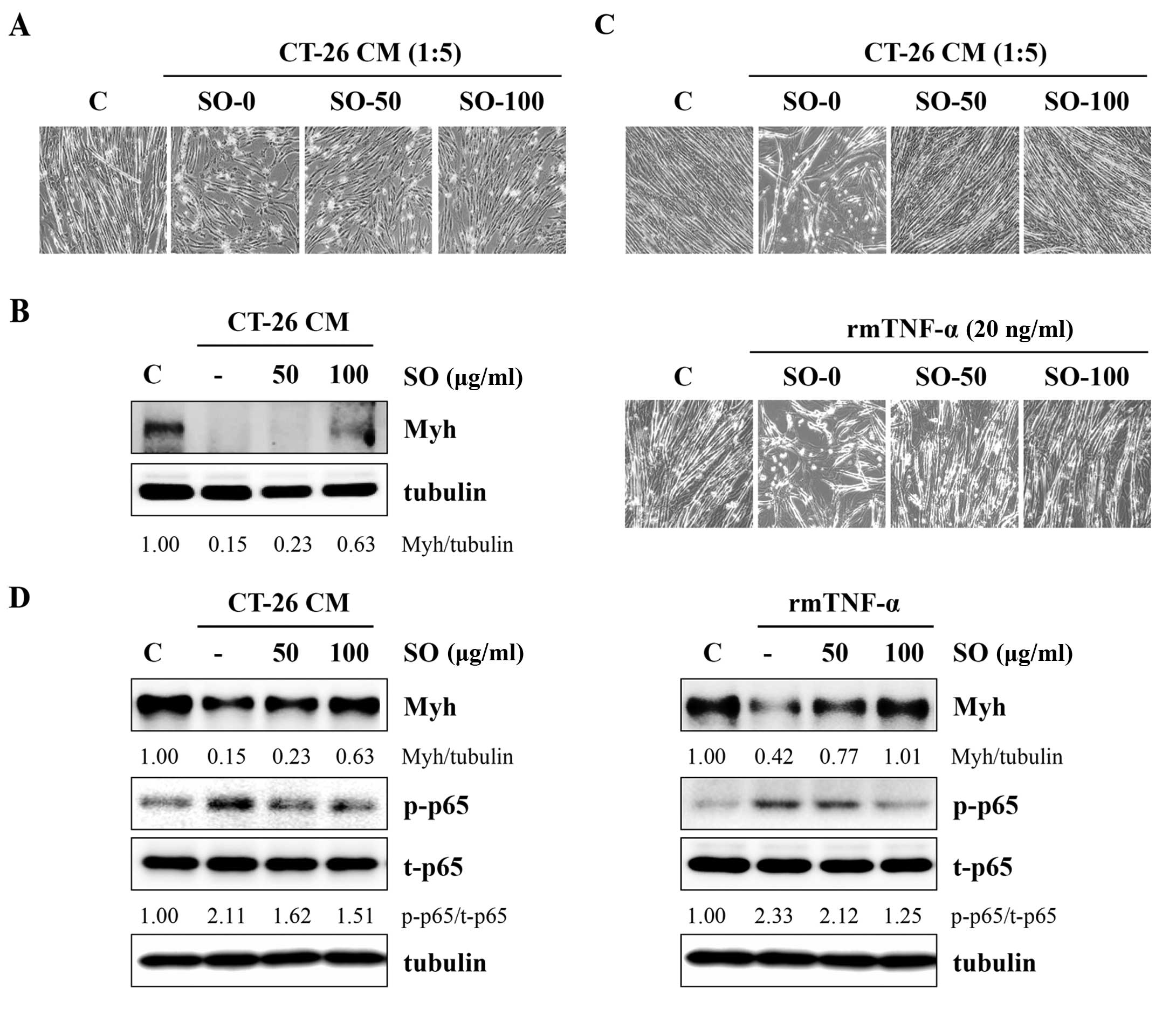
next examined whether SO attenuated the CT-26-mediated inhibition
of C2C12 myoblast differentiation. Fig.
6A shows that C2C12 myoblasts differentiating in control CM
(diluted to a ratio of 1:5 with DM) exhibited reduced myotube
numbers as compared with the untreated control, whereas SO-treated
CM slightly attenuated the impairment of C2C12 myoblast
differentiation. The immunoblot analysis revealed that Myh
expression in myoblasts differentiating in control CM was markedly
decreased compared with DM, while SO significantly prevented a
CT-26-mediated reduction in Myh expression (Fig. 6B). To examine the effect of SO on
C2C12 myotube wasting, differentiated C2C12 myotubes were incubated
in SO-treated or -untreated CT-26 CM at 1:5 dilutions with DM for
48 h. The myotubes incubated with control CM exhibited a
considerable muscle wasting appearance, whereas myotubes incubated
with SO-treated CM did not exhibit muscle wasting, maintaining
almost intact myotubes in a similar manner to the DM-treated
controls (Fig. 6C). The Myh
expression in myotubes was also reduced by control CM, while it was
significantly conserved in myotubes incubated with SO-treated CM
compared with DM-treated controls. In addition, control CM
increased p65 phosphorylation in myotubes, while SO-treated CM did
not (Fig. 6D). Previous studies
reported that skeletal muscle atrophy was induced by TNF-α, as
shown by a significant decrease in cell surface myotubes derived
from C2C12 cells and Myh expression (25). We confirmed that TNF-α induced
myotube wasting and SO significantly attenuated TNF-α-mediated
skeletal muscle atrophy in C2C12 cells (Fig. 6C and D).
Discussion
Cachexia is a complex metabolic syndrome
characterized by anorexia, loss of skeletal muscle mass and adipose
tissue, significant reduction in body weight and asthenia. It
occurs in many chronic diseases, including cancer, AIDS, renal
failure, diabetes and chronic obstructive pulmonary disease. Half
of all cancer patients, and 80–90% of patients with tumors of
pancreatic and gastric origin, exhibit a cachexia syndrome, which
has a profound impact on quality of life, and causes more severe
chemotherapy-related adverse events and a decreased lifespan. Most
importantly, the majority of terminal cancer patients suffer from
cachexia, and 22% of them die as a result of this disorder
(1–3,26).
Numerous cytokines secreted from the tumor or the
host inflammatory cells, including TNF-α, IL-1, IL-6 and IFN-γ,
have been postulated to act as procachectic factors that mimic
leptin signaling and suppress ghrelin signaling, leading to
sustained anorexia. Furthermore, these cytokines are also involved
in the induction of cancer-mediated skeletal muscle wasting by
inhibition of protein synthesis and/or acceleration of protein
degradation. Skeletal muscle depletion is closely associated with
worse outcomes in patients undergoing surgery, chemotherapy, and
radiotherapy; therefore, reversal of skeletal muscle mass and body
weight loss by inhibiting procachectic cytokines may be useful for
the management of cancer patients and for an overall increase of
well-being (3,6,18–20).
The current management strategies for cancer
cachexia using appetite stimulants, progestational agents and
orexigenic agents are limited due to their poor in vivo
efficacy and high toxicity. Instead, herbal medicines have received
increasing attention for use as adjuvants to enhance the efficacy
and diminish complications during chemotherapy or radiation
therapy. Recent studies have shown that administration of
Rikkunshito, a Japanese Kampo medicine composed of eight medicinal
plants, attenuated anorexia-cachexia, prolonged survival and
promoted anticancer efficacy by potentiating ghrelin signaling in
tumor-bearing mice (27,28). Hochuekkito, a Kampo formula
comprised of 10 medicinal plants, significantly attenuated marked
reductions in carcass weight, food and water intake, and weight of
gastrocnemius muscle and epididymal fat tissue caused by the CT-26
adenocarcinoma, by suppressing IL-6 production in macrophages
(29). In addition,
Sipjeondaebo-Tang (Shi-Quan-Da-Bu-Tang in Chinese and
Juzen-Taiho-To in Japanese), composed of 10 species of herbs,
showed therapeutic effects on cancer anorexia and cachexia by
modulating body and muscle weight, food intake and cytokine and
hormone production (30). Some
natural herbs and their components, including Rhizoma coptidis,
berberine and quercetin showed anti-cachectic effects by inhibiting
tumor growth and suppressing inflammation (31,32).
SO is a traditional Oriental herbal prescription
that has long been used to cure alternating chills and fever from
half-exterior, half-interior lesser yang disease, and has been used
to treat patients with liver diseases, including hepatic fibrosis,
chronic hepatitis and hepatocellular carcinoma (14–16).
SO is currently prescribed for the management of fever and sore
throat. Previous studies demonstrated that SO prevents
depressive-like behavior in rodents by elevating serotonin and
5-hydroxyindoleacetic acid levels in the prefrontal cortex and
hippocampus, inhibits thrombus formation by anti-platelet activity,
and suppresses anaphylactic reaction in mast cells (33–35).
Recently, we demonstrated that SO has anti-inflammatory activity in
LPS-stimulated RAW 264.7 cells, by suppression of NF-κB activation
and MAPK phosphorylation (17).
The present study examined whether SO administration
exerted inhibitory effects on the induction of cachexia in CT-26
adenocarcinoma-bearing mice, and then determined the mechanism zof
action for this anti-cachectic activity. Our data showed that SO
oral administration significantly inhibited tumor growth and
recovered body weight compared with saline-treated control mice. SO
prevented loss of skeletal muscle/fat tissue and increase in serum
IL-6 levels caused by the CT-26 tumor. We also observed that SO
suppressed the production of procachectic inflammatory cytokines,
including IL-6, IL-1 and TNF-α, in macrophages through inhibition
of NO generation and suppression of p38, NF-κB and STAT3
activation. In addition, SO prevented CT-26-mediated muscle atrophy
involving proliferation, differentiation and wasting in murine
C2C12 myoblasts and myotubes.
In summary, the present results demonstrate that SO
reduces tumor burden and systemic inflammatory responses, followed
by prevention of muscle and fat degradation, indicating that SO is
a safe and effective herbal medicine for treating cancer patients
with cachexia by protecting against loss of skeletal muscle mass
during catabolic conditions.
Acknowledgments
The present study was supported by Grant K15280
awarded to the Korea Institute of Oriental Medicine (KIOM) from the
Ministry of Science, ICT and Future Planning (MSIP), Republic of
Korea
References
|
1
|
Fearon KC, Voss AC and Hustead DS; Cancer
Cachexia Study Group: Definition of cancer cachexia: Effect of
weight loss, reduced food intake, and systemic inflammation on
functional status and prognosis. Am J Clin Nutr. 83:1345–1350.
2006.PubMed/NCBI
|
|
2
|
Tazi E and Errihani H: Treatment of
cachexia in oncology. Indian J Palliat Care. 16:129–137. 2010.
|
|
3
|
Aoyagi T, Terracina KP, Raza A, Matsubara
H and Takabe K: Cancer cachexia, mechanism and treatment. World J
Gastrointest Oncol. 7:17–29. 2015.PubMed/NCBI
|
|
4
|
Ozola Zalite I, Zykus R, Francisco
Gonzalez M, Saygili F, Pukitis A, Gaujoux S, Charnley RM and Lyadov
V: Influence of cachexia and sarcopenia on survival in pancreatic
ductal adenocarcinoma: A systematic review. Pancreatology.
15:19–24. 2015. View Article : Google Scholar
|
|
5
|
Grabiec K, Burchert M, Milewska M,
Błaszczyk M and Grzelkowska-Kowalczyk K: Systemic and local
mechanisms leading to cachexia in cancer. Postepy hig Med Dosw
(Online). 67:1397–1409. 2013.In Polish. View Article : Google Scholar
|
|
6
|
Onesti JK and Guttridge DC: Inflammation
based regulation of cancer cachexia. BioMed Res Int.
2014:1684072014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tamura S, Ouchi KF, Mori K, Endo M,
Matsumoto T, Eda H, Tanaka Y, Ishitsuka H, Tokita H and Yamaguchi
K: Involvement of human interleukin 6 in experimental cachexia
induced by a human uterine cervical carcinoma xenograft. Clin
Cancer Res. 1:1353–1358. 1995.PubMed/NCBI
|
|
8
|
Enomoto A, Rho MC, Fukami A, hiraku O,
Komiyama K and hayashi M: Suppression of cancer cachexia by
20S,21-epoxy-resi bufogenin-3-acetate-a novel nonpeptide IL-6
receptor antagonist. Biochem Biophys Res Commun. 323:1096–1102.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ando K, Takahashi F, Kato M, Kaneko N, Doi
T, Ohe Y, Koizumi F, Nishio K and Takahashi K: Tocilizumab, a
proposed therapy for the cachexia of Interleukin6-expressing lung
cancer. PLoS One. 9:e1024362014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Madeddu C, Macciò A, Panzone F, Tanca FM
and Mantovani G: Medroxyprogesterone acetate in the management of
cancer cachexia. Expert Opin Pharmacother. 10:1359–1366. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mantovani G, Macciò A, Lai P, Massa E,
Ghiani M and Santona MC: Cytokine involvement in cancer
anorexia/cachexia: Role of megestrol acetate and
medroxyprogesterone acetate on cytokine downregulation and
improvement of clinical symptoms. Crit Rev Oncog. 9:99–106. 1998.
View Article : Google Scholar
|
|
12
|
Inui A: Cancer anorexia-cachexia syndrome:
Current issues in research and management. CA Cancer J Clin.
52:72–91. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lee JK, Kim JH and Shin HK: Therapeutic
effects of the oriental herbal medicine Sho-saiko-to on liver
cirrhosis and carcinoma. Hepatol Res. 41:825–837. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shimizu I: Sho-saiko-to: Japanese herbal
medicine for protection against hepatic fibrosis and carcinoma. J
Gastroenterol Hepatol. 15:D84–D90. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mizushima Y, Kashii T, Tokimitsu Y and
Kobayashi M: Cytotoxic effect of herbal medicine Sho-saiko-to on
human lung-cancer cell-lines in vitro. Oncol Rep. 2:91–94.
1995.PubMed/NCBI
|
|
16
|
Yano H, Mizoguchi A, Fukuda K, Haramaki M,
Ogasawara S, Momosaki S and Kojiro M: The herbal medicine
Sho-saiko-to inhibits proliferation of cancer cell lines by
inducing apoptosis and arrest at the G0/G1 phase. Cancer Res.
54:448–454. 1994.PubMed/NCBI
|
|
17
|
Oh YC, Cho WK, Jeong YH, Im GY, Lee KJ,
Yang HJ and Ma JY: Anti-inflammatory effect of Sosihotang via
inhibition of nuclear factor-κB and mitogen-activated protein
kinases signaling pathways in lipopolysaccharide-stimulated RAW
264.7 macrophage cells. Food Chem Toxicol. 53:343–351. 2013.
View Article : Google Scholar
|
|
18
|
Barton BE: IL-6-like cytokines and cancer
cachexia: Consequences of chronic inflammation. Immunol Res.
23:41–58. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
MacDonald N: Cancer cachexia and targeting
chronic inflammation: A unified approach to cancer treatment and
palliative/supportive care. J Support Oncol. 5:157–162.
2007.PubMed/NCBI
|
|
20
|
Deans C and Wigmore SJ: Systemic
inflammation, cachexia and prognosis in patients with cancer. Curr
Opin Clin Nutr Metab Care. 8:265–269. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bonetto A, Aydogdu T, Kunzevitzky N,
Guttridge DC, Khuri S, Koniaris LG and Zimmers TA: STAT3 activation
in skeletal muscle links muscle wasting and the acute phase
response in cancer cachexia. PLoS One. 6:e225382011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lokireddy S, Wijesoma IW, Bonala S, Wei M,
Sze SK, McFarlane C, Kambadur R and Sharma M: Myostatin is a novel
tumoral factor that induces cancer cachexia. Biochem J. 446:23–36.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Costelli P, Muscaritoli M, Bonetto A,
Penna F, Reffo P, Bossola M, Bonelli G, Doglietto GB, Baccino FM
and Rossi Fanelli F: Muscle myostatin signalling is enhanced in
experimental cancer cachexia. Eur J Clin Invest. 38:531–538. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jespersen J, Kjaer M and Schjerling P: The
possible role of myostatin in skeletal muscle atrophy and cachexia.
Scand J Med Sci Sports. 16:74–82. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Langen RC, Schols AM, Kelders MC, Wouters
EF and Janssen-Heininger YM: Inflammatory cytokines inhibit
myogenic differentiation through activation of nuclear
factor-kappaB. FASEB J. 15:1169–1180. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Donohoe CL, Ryan AM and Reynolds JV:
Cancer cachexia: Mechanisms and clinical implications.
Gastroenterol Res Pract. 2011:6014342011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fujitsuka N, Asakawa A, Amitani H, Hattori
T and Inui A: Efficacy of ghrelin in cancer cachexia: Clinical
trials and a novel treatment by Rikkunshito. Crit Rev Oncog.
17:277–284. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Fujitsuka N and Uezono Y: Rikkunshito, a
ghrelin potentiator, ameliorates anorexia-cachexia syndrome. Front
Pharmacol. 5:2712014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yae S, Takahashi F, Yae T, Yamaguchi T,
Tsukada R, Koike K, Minakata K, Murakami A, Nurwidya F, Kato M, et
al: Hochuekkito (TJ-41), a kampo formula, ameliorates cachexia
induced by colon 26 adenocarcinoma in mice. Evid Based Complement
Alternat Med. 2012:9769262012. View Article : Google Scholar
|
|
30
|
Choi YK, Jung KY, Woo SM, Yun YJ, Jun CY,
Park JH, Shin YC, Cho SG and Ko SG: Effect of Sipjeondaebo-tang on
cancer-induced anorexia and cachexia in CT-26 tumor-bearing mice.
Mediators Inflamm. 2014:7365632014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Iizuka N, Miyamoto K, hazama S, Yoshino S,
Yoshimura K, Okita K, Fukumoto T, Yamamoto S, Tangoku A and Oka M:
Anticachectic effects of Coptidis rhizoma, an anti-inflammatory
herb, on esophageal cancer cells that produce interleukin 6. Cancer
Lett. 158:35–41. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Camargo CA, da Silva ME, da Silva RA,
Justo GZ, Gomes-Marcondes MC and Aoyama H: Inhibition of tumor
growth by quercetin with increase of survival and prevention of
cachexia in Walker 256 tumor-bearing rats. Biochem Biophys Res
Commun. 406:638–642. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kim HM, Kim YY, Moon HS, Lee EH, Moon SJ
and An NH: Inhibitory effect of anaphylactic reaction of
Sosiho-Tang. Immunopharmacol Immunotoxicol. 20:567–578. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lee JJ, Kim T, Cho WK and Ma JY:
Antithrombotic and anti-platelet activities of Soshiho-tang
extract. BMC Complement Altern Med. 13:1372013. View Article : Google Scholar
|
|
35
|
Su GY, Yang JY, Wang F, Xiong ZL, Hou Y,
Zhang K, Song C, Ma J, Song SJ, Teng HF, et al: Xiaochaihutang
prevents depressive-like behaviour in rodents by enhancing the
serotonergic system. J Pharm Pharmacol. 66:823–834. 2014.
|