Introduction
Breast cancer, a pathologically and clinically
heterogeneous disease, is the most frequent malignancy diagnosed in
women (1). Invasion and metastasis
of breast cancer are the primary causes of treatment failure and
mortality in women (2). Moreover,
invasion and metastasis of cancer cells are complex multistep
processes that involve cell adhesion, proteolytic enzyme
degradation of the extracellular matrix (ECM) and production of
growth factors that influence cell migration (3). Degradation of the basement membrane
mainly by matrix metalloproteinases (MMPs) is considered to be a
factor of crucial importance for breast cancer cell invasion and
metastasis (4–6).
MMPs comprise a large family of zinc-dependent
endopeptidases that have the capacity to cleave ECM. Owing to their
matrix-degrading abilities and high expression in advanced tumors,
the activity of MMPs has been shown to be required for breast
cancer cell invasion and angiogenesis (4,7,8). Among
the MMPs, MMP-1 has been reported to be upregulated in breast
cancer cell lines with an enhanced ability of tumor growth,
invasion and distant metastasis (9,10).
Induction of MMP-1 by transcription factors, such as δ-crystallin
enhancer factor 1 (δEF1) has been reported to contribute to
enhanced cell migration and invasion (10). Therefore, factors with the ability
to block MMP-1 activation may have therapeutic potential for the
treatment human cancer.
Bone morphogenetic protein-6 (BMP-6) belongs to the
TGF-β superfamily. The traditional BMP-6 signaling pathway is
Smad-dependent (11). In addition
to the Smad pathway, BMP-6 is also known to activate and crosstalk
with other pathways, such as the MAPK pathway (12). In addition to its effect on inducing
endochondral bone formation, BMP-6 has been shown to be involved in
numerous biological processes. In our previous studies, we found
that BMP-6 induced E-cadherin and microRNA-21 expression in
MDA-MB-231 breast cancer cells, which are critical genes involved
in breast cancer invasion and metastasis (13,14).
Moreover, we confirmed that BMP-6 inhibits MMP-9 expression by
regulating heme oxygenase-1 in MCF-7 breast cancer cells (15). MMP-9 is particularly known to play a
critical role in breast cancer invasion and distant metastasis
(16). Taken together, these
results suggest that BMP-6 may play an important role in breast
cancer invasion and metastasis.
In the present study, we demonstrated that the
expression of BMP-6 was absent in breast cancer tissues. Moreover,
BMP-6 inhibited MMP-1 expression in MDA-MB-231 cells at the
transcriptional level, an effect that was mediated via reduction of
the recruitment of the AP-1 components, c-Jun/c-Fos to the
endogenous MMP-1 promoter. BMP-6 also blocked MDA-MB-231 cell
invasion by inhibiting MMP-1 expression.
Materials and methods
Immunohistochemical analysis
Formalin-embedded breast cancer samples were
obtained from the Department of Surgical Pathology of Tangshan
People's Hospital. Immunohistochemical analysis was performed on
paraffin-embedded sections using the EnVision kit (Dako, Glostrup,
Denmark) following the manufacturer's protocols. Sections were
boiled in retrieval solutions to expose the antigens. Polyclonal
anti-BMP-6 (ab134723) and control primary antibodies were applied
to the sections at a dilution of 1:50. The section-affixed slides
were counterstained with hematoxylin, dehydrated and mounted.
Cell culture
Human breast cancer cell line MDA-MB-231 was
maintained in Dulbecco's modified Eagle's medium (DMEM)-high
glucose medium (Gibco) supplemented with 10% fetal bovine serum
(FBS) (HyClone), penicillin and streptomycin. MDA-MB-231 cells were
plated at a density of 2×104 cells/well into 24-well
plates for use in the luciferase assays, and at a density of
8×104 into 6-well plates for western blotting and
quantitative CHIP assays.
Plasmid construction and
transfection
The constitutive vectors, pcDNA6B-BMP-6 (17), hMMP-1-WT and hMMP-1-mAP1 (10) were previously described. The
full-length MMP-1 CDS was PCR amplified using the following
primers: forward, 5′-AGCAAGCTTAGATGCACAGCTTTCCTCCAC-3′ and reverse,
5′-AGTCTCGAGTCAATTTTTCCTGCAGTTGAA-3′. The
HindIII/XhoI site-tagged potential target sequence
was then cloned into the HindIII/XhoI site of the
pcDNA-6B vector.
The MDA-MB-231 cells were plated into 6-well plates
and transfected with pcDNA6B-BMP-6 or pcDNA6B using Lipofectamine
2000 (Invitrogen). Transfected cells were resuspended in 10 ml of
DMEM containing 10% FBS and seeded in a 10-cm-dish.
Blaticidin-resistant clones were isolated over a period of 3–4
weeks. Overexpression of BMP-6 was confirmed by quantitative RT-PCR
and western blotting.
Preparation of small interfering
RNAs
The siRNA target sequences of human BMP-6 and MMP-1
were, 5′-GCGACACCACAAAGAGTTCTT-3′ and 5′-GAGTACAACTTACATCG TG-3′ as
previously reported (9,18). Sense and antisense oligonucleotides
with the internal loop were synthesized (Takara). These were
annealed and ligated into the BamHI and HindIII sites
of pSilencer 4.1-CMV neo (Ambion) to construct the BMP-6- and
MMP-1-specific siRNA expression plasmids, according to the
manufacturer's instructions. pSilencer 4.1-CMV neo expressing
scrambled siRNAs (Ambion) were used as controls.
Scratch-wound assay
231-control and 231-BMP-6 cells were seeded into a
6-well plate. For the scratch-wound healing assay, linear scratches
were made in cell sheets with a pipette tip. After 24 and 48 h of
growth, images were captured of the cells using a microscope.
Migration and invasion assays
Transwell 24-well chambers with 8-mm pore size
(Costar) were used as recommended by the manufacturer. The
membranes were precoated with collagen matrix (Sigma), which was
reconstituted by adding 0.5 ml serum-free medium to the well for 2
h. To assess the invasive ability of the cells, 2.5×104
cells in 0.5 ml medium containing 1% FBS were placed into the upper
compartment of the wells and 0.75 ml of medium containing 10% FBS
was placed in the lower compartment. The Transwell chambers were
incubated for 16 h at 37°C in a 5% CO2 incubator. Cell
penetration through the membrane was detected by staining the cells
on the porous membrane with 0.25% crystal violet. To quantify the
data, we washed the chamber twice with phosphate-buffered saline
(PBS), and then used 33% acetic acid to wash off the excess crystal
violet. Crystal violet remaining on the membranes was measured on a
spectrophotometer at A570.
RNA extraction and quantitative
RT-PCR
Total RNA was extracted from MDA-MB-231 cells that
were treated with BMP-6 protein using the TRIzol reagent
(Invitrogen). Total RNA (0.5 µg) from each sample was used
for first-strand cDNA synthesis (M-MLV reverse transcriptase;
Promega, Madison, WI, USA). Specific products of human MMP-1
(10), MMP-2 (10), MMP-9 (10), MMP-14 (19), TIMP-1 (20) and TIMP-2 (20) were amplified by quantitative PCR
using the primers as reported. GAPDH was used as an internal
control. Verification of the expression levels of genes was
performed by quantitative RT-PCR using EvaGreen (Botium).
ELISA
MMP-1 protein expression levels were measured with
an MMP-1 ELISA kit (cat. #QIA55; Calbiochem) according to the
manufacturer's instructions. To prepare samples for ELISA, cells
were grown to 80% confluency and transfected with MMP-1-pcDNA6B,
si-MMP-1 or treated with 2 µM MMP-1 inhibitor (cat. #
444250; EMD Chemicals, Gibbstown, NJ, USA) for 24 h; supernatants
from the 48-h incubation were collected and concentrated 10-fold,
using Amicon Ultra-4 centrifugal filters (Merck Millipore,
Billerica, MA, USA). MMP-1 amounts were calculated as ng/ml
protein.
Western blotting and antibodies
Preparation of total cell extracts and western
blotting with the appropriate antibodies was performed as
previously described (13). The
following antibodies were used: rabbit polyclonal Ab against MMP-1
(sc-30069; Santa Cruz), mouse monoclonal Ab against actin (A-4700;
Sigma), rabbit polyclonal Ab against c-Fos (sc-52), and rabbit
polyclonal Ab against c-Jun (sc-1694) (both from Santa Cruz).
Luciferase assay
MDA-MB-231 cells were co-transfected with wild-type
or mutant human MMP-1 promoter constructs into 24-well plates using
Lipofectamine 2000. Cells were treated with TPA (100 ng/ml),
curcumin (20 µM) or BMP-6 (100 ng/µl) for 6 h after
transfection. Lysates were prepared and the luciferase activity was
then measured using the Dual-Luciferase Reporter Assay system
(Promega) according to the manufacturer's instructions. Luciferase
activity was normalized using the Renilla luciferase
activity.
Quantitative chromatin
immunoprecipitation (q-CHIP)
ChIP assays were performed using reagents
commercially obtained from Upstate Biotechnology (Lake Placid, NY,
USA), essentially according to the manufacturer's instructions. The
antibodies and primers used in these experiments were previously
reported (10).
Results
BMP-6 expression is absent in a certain
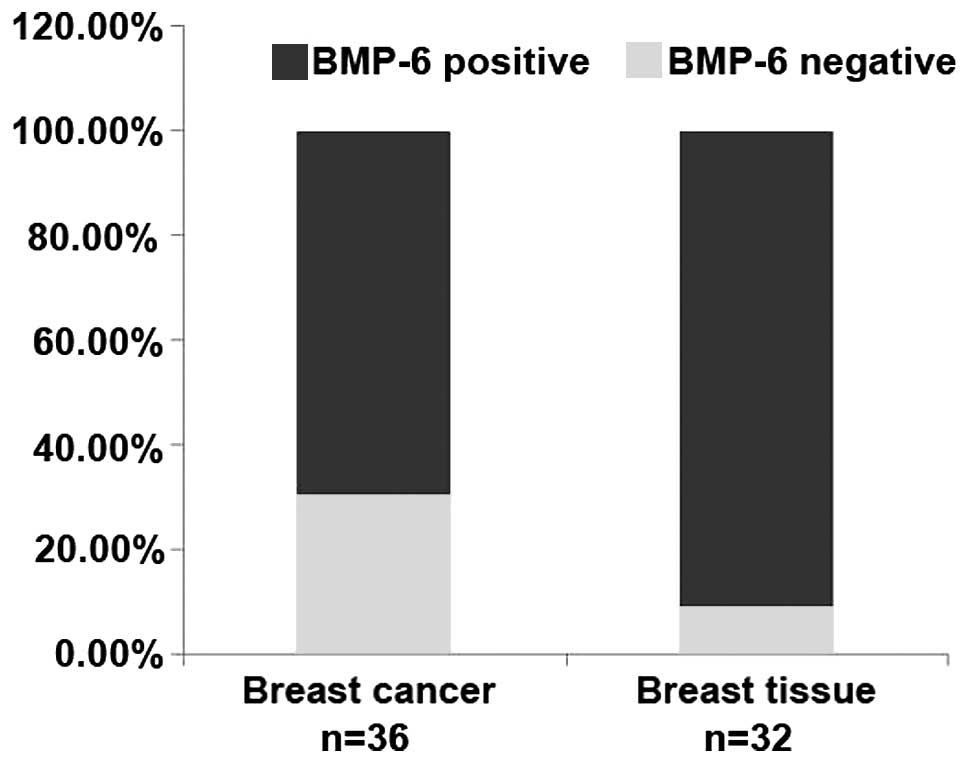
percentage of breast cancer tissues
To better understand the expression of BMP-6 in
breast cancer, we collected 36 cases of advanced breast tumor
specimens with lymph node metastasis, and 32 cases of paired or
unpaired normal tissues. We detected the BMP-6 expression level in
breast cancer and normal breast tissues by immunohistochemical
staining. The expression of BMP-6 was represented by the numbers or
percentages of negative cases. The results showed that the rate of
BMP-6-negative expression was 30.56% in breast cancer, but was
9.58% in the normal tissues (Table
I and Fig. 1). These results
from clinical samples suggest that the absence of BMP-6 expression
in a certain percentage of the breast cancer tissues may be closely
related with the metastasis of certain breast cancer cases.
 | Table IExpression of BMP-6 is different in
advanced breast tumor specimens and normal tissues. |
Table I
Expression of BMP-6 is different in
advanced breast tumor specimens and normal tissues.
| BMP-6 staining
intensity scores | No. of breast
cancer specimens (%) | No. of normal
tissue specimens (%) |
|---|
| − | 11 (30.56) | 3 (9.58) |
| + | 21 (58.33) | 22 (67.75) |
| ++ | 4 (11.11) | 7 (21.88) |
BMP-6 inhibits the migration and invasion
of MDA-MB-231 cells
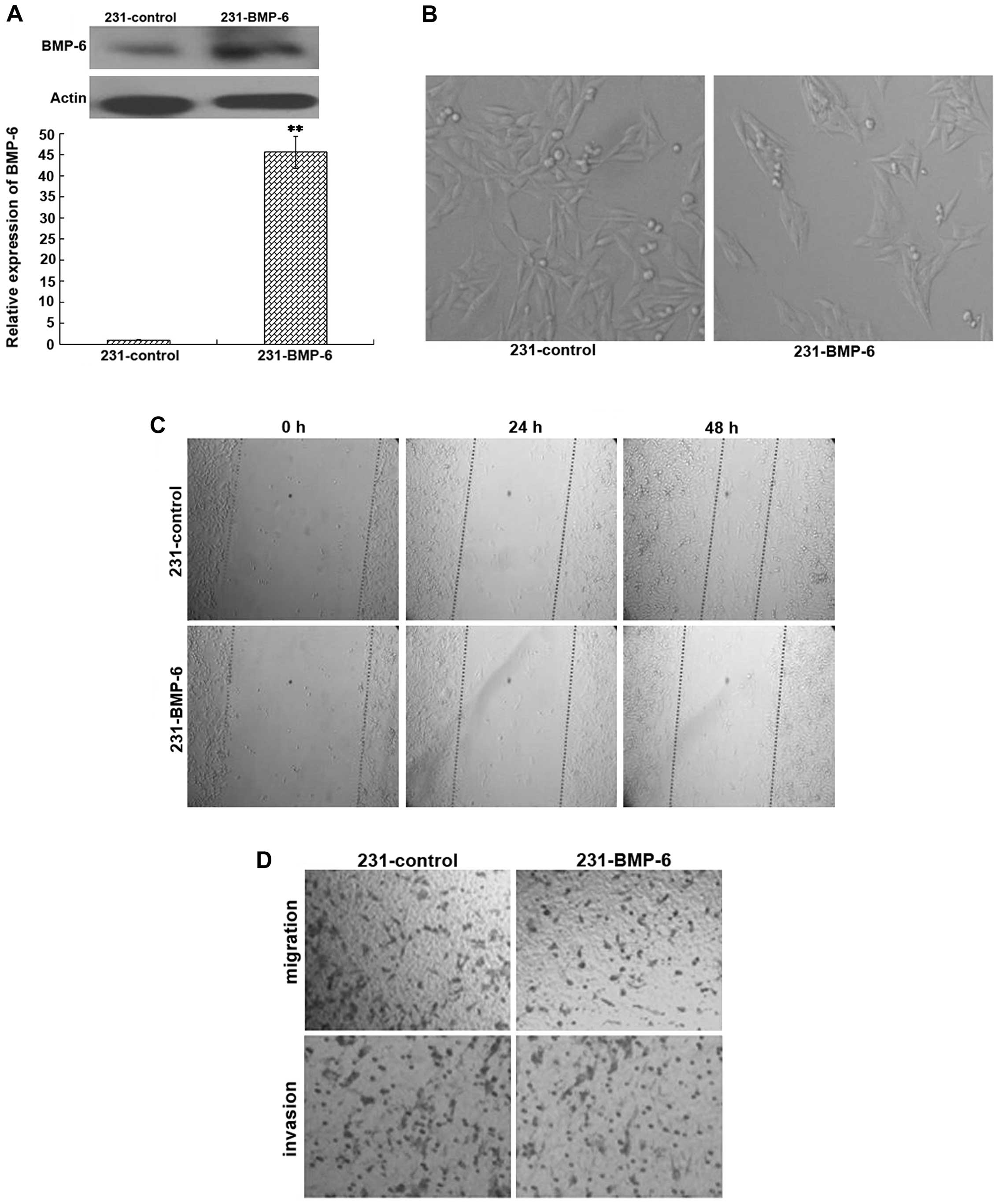
To confirm the potential role of BMP-6 in cell
migration and invasion in vivo, MDA-MB-231 cells were stably
trans-fected with the BMP-6 expression plasmid (231-BMP-6) or the
empty vector (231-control). The overexpression of BMP-6 was
confirmed by quantitative RT-PCR and western blotting (Fig. 2A). The morphology of 231-control and
231-BMP-6 breast cancer cells was detected under a light
microscope. As shown in Fig. 2B,
the 231-BMP-6 cells were arranged more closely than the 231-control
cells. The results of the wound healing test showed that the motile
ability of the 231-BMP cells was weaker than that of the
231-control cells (Fig. 2C).
Furthermore, we examined the effect of BMP-6 on the ability of
breast cancer cells to migrate through an artificial basal membrane
using the Boyden chamber assay. The results of cell migration and
invasion assays indicated that MDA-MB-231 cells overexpressing
BMP-6 presented a distinct decrease in the number of migrating and
invading cells in comparison to the empty-vector control (Fig. 2D).
BMP-6 inhibits MMP-1 expression at the
transcriptional level
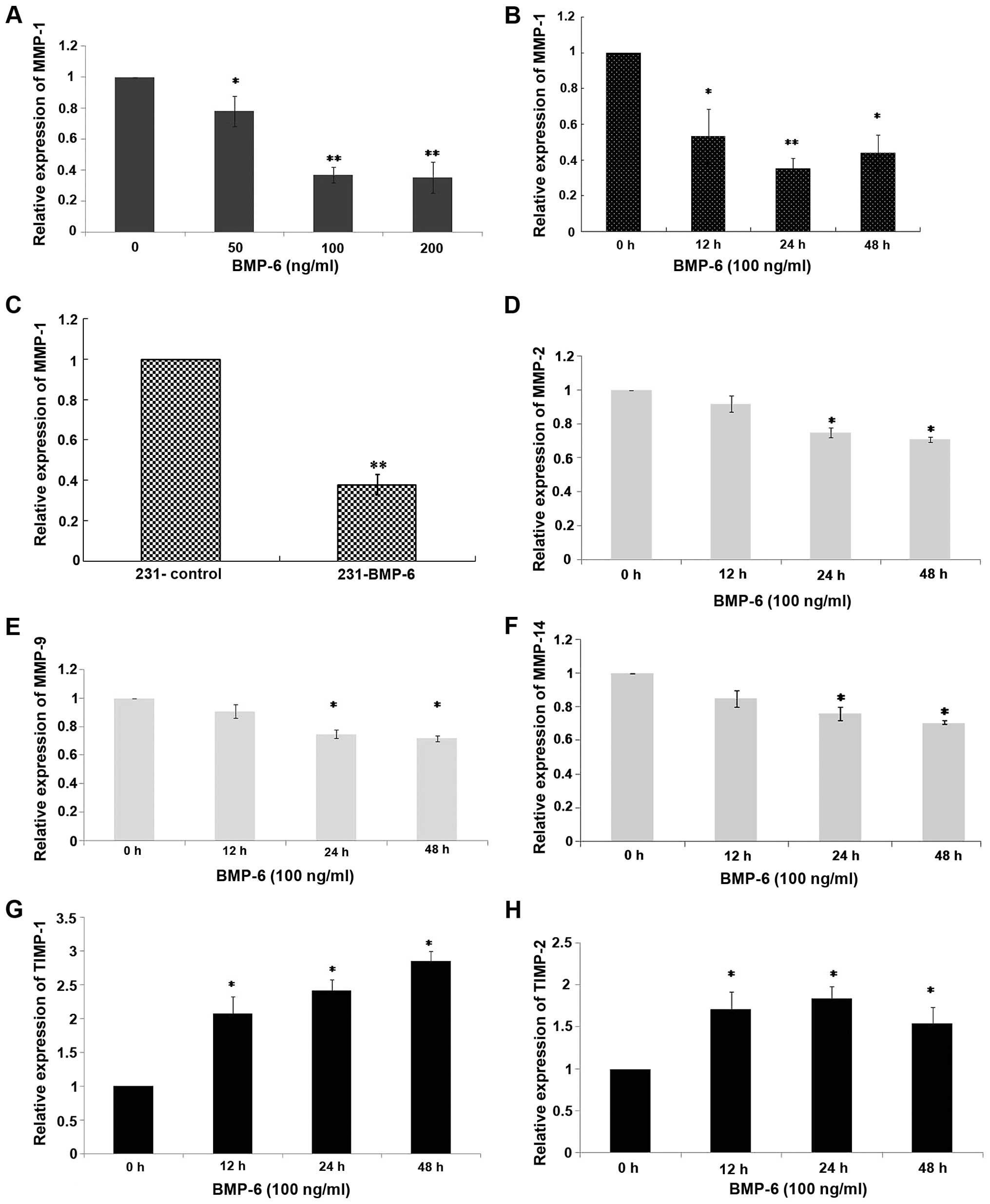
To understand the molecular changes, we performed
quantitative RT-PCR to examine the effect of BMP-6 on MMPs and
TIMPs in the MDA-MB-231 cells. MMP-1 has been described as a
mediator that controls the ability of breast cancer growth,
invasion and distant metastasis (9,10). In
the present study, MDA-MB-231 cells were treated with different
doses (50, 100 and 200 ng/ml) of BMP-6 protein. Total RNAs were
collected at 24 h. As shown in Fig.
3A, BMP-6 down-regulated MMP-1 expression in a dose-dependent
manner. Then, MDA-MB-231 cells were treated with BMP-6 (100 ng/ml).
Total RNAs were collected at 0, 12, 24 and 48 h. In the MDA-MB-231
cells treated with BMP-6 (100 ng/ml) for 12 h, an up to 40%
decrease in the expression of MMP-1 mRNA was noted when compared to
the basal level (Fig. 3B). The
inhibition was further decreased to ~60% between 24 and 48 h
(Fig. 3B). The expression of MMP-1
was significantly lower in the 231-BMP-6 cells compared with the
level in the control group at both the mRNA and protein levels
(Figs. 3C, 5A and B). Additionally, we examined
BMP-6-regulated expression levels of another three MMP members,
MMP-2, MMP-9 and MMP-14 in the MDA-MB-231 cells. However, as shown
in Fig. 3D–F, BMP-6 slightly
downregulated MMP-2, MMP-9 and MMP-14 expression at the mRNA level.
The MMP system is counterbalanced by a group of four tissue
inhibitors of metalloproteinases (TIMPs) that have varying
specificities for individual MMPs (21). Thus, we further assessed the
potential effect of BMP-6 on TIMP-1, TIMP-2, TIMP-3 and TIMP-4. The
results of quantitative RT-PCR showed that BMP-6 only slightly
upregulated the mRNA levels of TIMP-1 and TIMP-2 (Fig. 3G and H). BMP-6 resulted in an up to
3-fold increase in the expression of TIMP-1 mRNA at 48 h, compared
to the basal level (Fig. 3G).
TIMP-3 and TIMP-4 mRNA levels were not significantly affected (data
not shown). The above observations suggest there may be a dual
effect of BMP-6 in promoting MDA-MB-231 cell migration, through
concurrently downregulating MMPs and upregulating TIMP-1 and
TIMP-2. However, inhibition of MMP-1, which was specifically and
significantly mediated by BMP-6, contributed to induce the
metastasis of breast cancer.
BMP-6 inhibits MMP-1 activity through
AP-1
Having found that BMP-6 inhibits MMP-1 expression in
MDA-MB-231 cells, we next assessed whether BMP-6 is a true
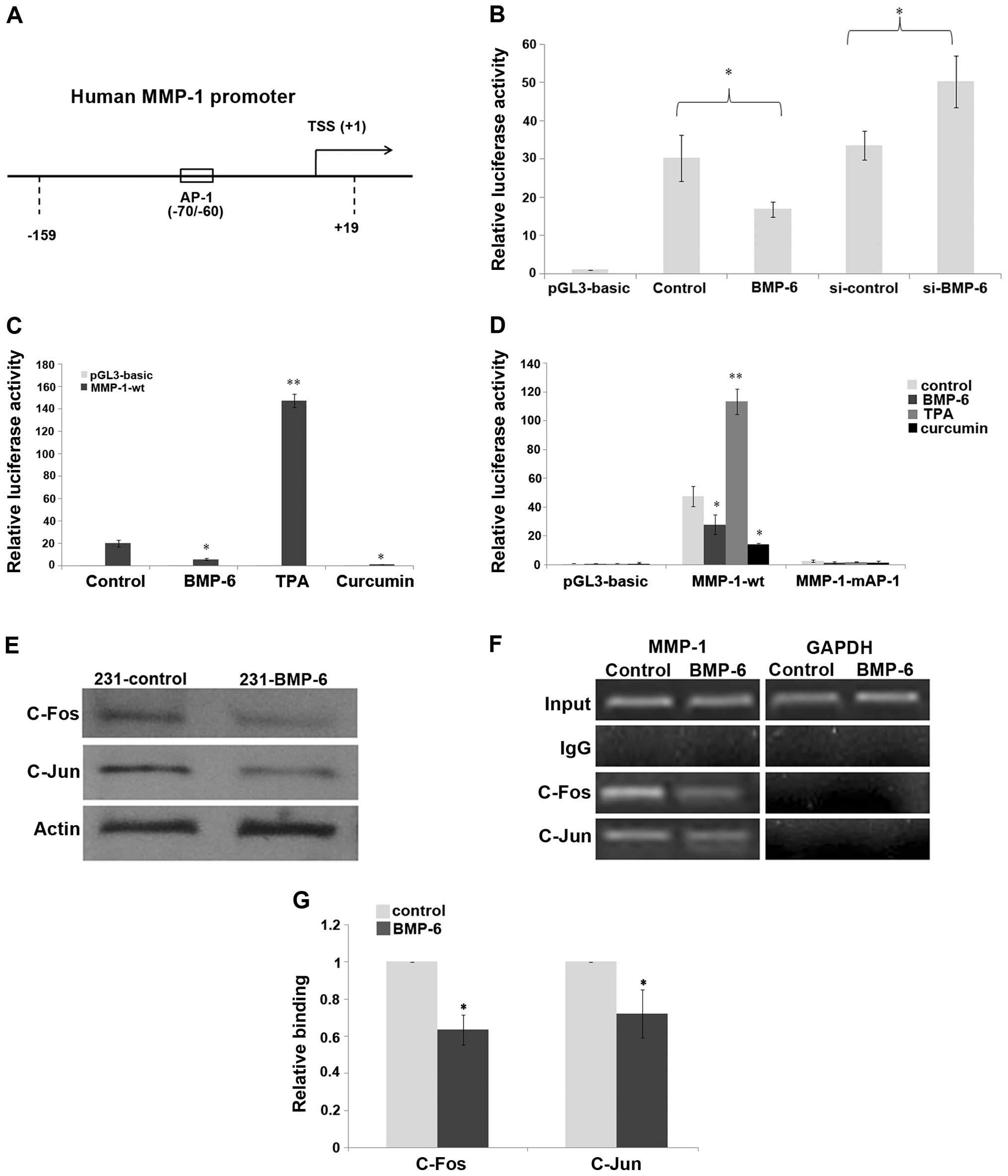
suppressor of MMP-1 transcription using reporter gene assays. As
shown in Fig. 4B, a reporter gene
assay showed that BMP-6 significantly inhibited human MMP-1
promoter activity of the wild-type −159/+19 reporter gene. The
inhibition was >50% following BMP-6 treatment, compared to that
of the control. However, knockdown of BMP-6 using RNA interference
resulted in increased luciferase activity of the human MMP-1
promoter, compared to that of the si-control (Fig. 4B). In order to clarify the
regulatory mechanism by which BMP-6 affects MMP-1 expression, we
analyzed the MMP-1 promoter using the online tools TRANSFAC
(http://www.generegulation.com) and
thTESS (http://www.cbil.upenn.edu/cgi-bin/tess/tess). Based on
our online analysis, we found that there was an AP-1 binding site
(CATGAGTCAG) at the position −70/−60 of the MMP-1 promoter
(Fig. 4A). Our previous results
indicated that BMP-6 inhibited the expression of microRNA-21 via
reduction in the expression of δEF1 and c-Fos/c-Jun (22). In the present study, luciferase
assay results indicated that TPA (activator of the AP-1 signaling)
upregulated the promoter activity of MMP-1, whereas curcumin
(inhibitor of AP-1 signaling) repressed its activity in the
MDA-MB-231 cells (Fig. 4C),
suggesting a potential effect of the AP-1 pathway to mediate MMP-1
transcription. Thus, the AP-1 element on the human MMP-1 promoter
was mutated to generate the MMP-1-mAP-1 construct (10). The results of the luciferase assay
demonstrated that the mutation of the AP-1 element on human MMP-1
promoter totally depleted its response to TPA, curcumin and BMP-6
(Fig. 4D). We investigated whether
BMP-6 affects c-Fos or c-Jun expression at the protein level.
Western blotting experiments demonstrated that both c-Fos and c-Jun
expression was obviously inhibited in the 231-BMP-6 cells (Fig. 4E). Importantly, the ChIP assays
indicated that c-Fos/c-Jun was able to bind to the MMP-1 promoter
during basal conditions in an AP-1 site-dependent manner (Fig. 4F). The result of the q-CHIP assay
showed that the binding of c-Fos/c-Jun to the MMP-1 promoter was
decreased by BMP-6 induction (Fig.
4G).
BMP-6 inhibits breast cancer cell
invasion through MMP-1 in vitro
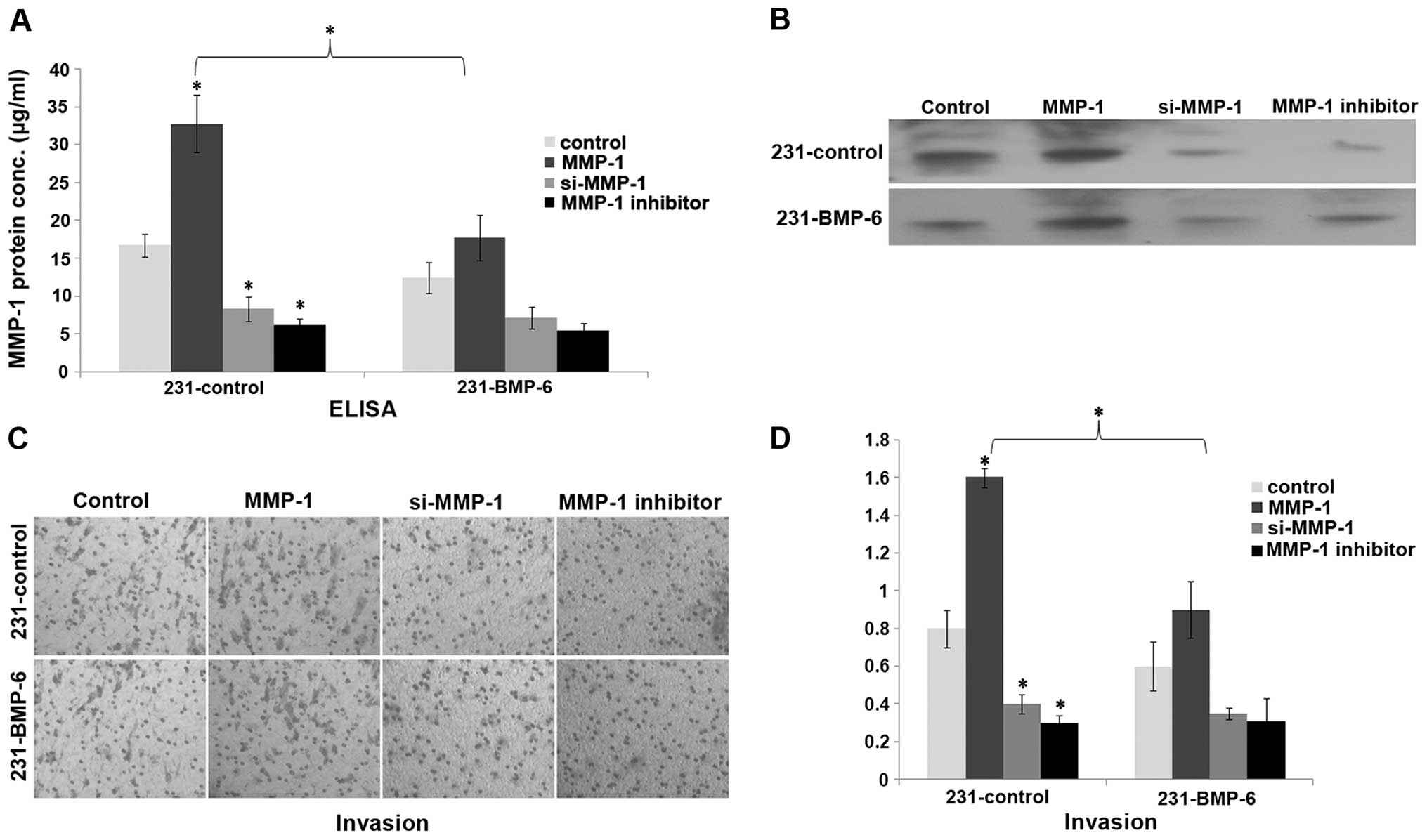
Having clarified how BMP-6 inhibits MMP-1
expression, we investigated whether MMP-1 influences the biological
effect of BMP-6 in breast cancer cells. MMP-1 has been reported to
induce invasion in MDA-MB-231 cells (9). We investigated whether MMP-1 could
rescue BMP-6-inhibited breast invasion in vitro. 231-control
and 231-BMP-6 cells were transfected with the MMP-1 expression
plasmid or an siRNA expression plasmid targeting MMP-1 or treated
with an MMP-1 inhibitor. Expression of MMP-1 was confirmed by ELISA
and western blotting (Fig. 5A and
B). Boyden chamber assay showed that MMP-1 induced MDA-MB-231
cell invasion in vitro. In contrast, MMP-1 knockdown by RNA
interference or the MMP-1 inhibitor exhibited an opposite effect
(Fig. 5C and D). Moreover, MMP-1
decreased BMP-6-inhibited invasion of MDA-MB-231 cells (Fig. 5C and D).
Discussion
Breast cancer is a leading cause of cancer-related
mortality among women worldwide, and is the second most common
metastatic cancer, frequently metastasizing to the bone, lung,
liver and brain (23). Moreover,
distant metastases account for most incidences of breast cancer
recurrence and are often the cause of death in breast cancer
patients (24,25). Due to clinical importance, the
detection and treatment of breast cancer metastases have been
urgently researched. We previously uncovered that BMP-6 exhibits a
potential inhibitory effect on breast cancer invasion and
metastasis (13,26). However, little is known concerning
the mechanisms of BMP-6 in breast cancer metastasis. In the present
study, we provided novel evidence that BMP-6 suppressed breast
cancer metastasis by downregulating MMP-1 expression in MDA-MB-231
cells. We further demonstrated that this effect was mediated
through decreasing the binding of c-Fos/c-Jun to the MMP-1
promoter. Since MMP-1 has been confirmed as a factor that
facilitates breast cancer metastasis, our research provides a novel
function of BMP-6, namely, acting as an MMP-1 inhibitor in breast
cancer progression. This regulatory mechanism by BMP-6 has clinical
significance in breast cancer progression and metastasis
research.
It has been known that MMPs are expressed in nearly
all tumors, where they facilitate tumor growth, invasion and
metastasis (7,8). In breast cancer, short hairpin
RNA-mediated stable knockdown of MMP-1 or induction of MMP-1 by
over-expression of δ-crystallin enhancer factor 1 (δEF1) was found
to significantly regulate the invasive ability of MDA-MB-231 cells
(9,10). These findings are consistent with
our present study that inhibition of MMP-1 by BMP-6 exhibited an
anti-tumorigenic effect to inhibit MDA-MB-231 cell invasion in the
Boyden chamber assay. Collectively, these observations suggest that
MMP-1 inhibitors, such as BMP-6, may play roles as agents that
inhibit tumor invasion and metastasis, with the potential for
further therapeutic development.
Recently, a number of studies have shown that δEF1
is closely associated with the malignant progression of breast
cancer (10,27,28).
We previously reported that δEF1 functions primarily as an inducer
of EMT, and δEF1 promotes osteolytic metastasis of MDA-MB-231
breast cancer cells by regulating MMP-1 expression (10,13,28).
Moreover, BMP-6 has been implicated as an antimetastatic factor,
involving the transcriptional repression of δEF1 in MDA-MB-231
cells (22). In the present study,
we demonstrated that BMP-6 inhibited the metastasis of MDA-MB-231
cells by downregulating MMP-1 expression. Our results collectively
indicate a mechanism of the BMP-6/δEF1/MMP-1 cascade involved in
the regulation of breast cancer metastasis. Factors that regulate
δEF1 or BMP-6 levels would subsequently change the expression of
MMP-1, thus altering the metastatic ability of breast cancer
cells.
In conclusion, we provide novel findings of a
potential mechanism for BMP-6-regulated metastasis of breast
cancer, which in effect is mediated through the reduction in MMP-1
expression in a paracrine manner. Thus, BMP-6 activation may
decrease the metastatic ability of breast cancer cells. However,
the interactions among cellular factors occurring in the metastasis
of breast cancer are far more complex in vivo. Further
investigation in vivo is required to elucidate the exact
role of BMP-6 in breast cancer metastasis.
Acknowledgments
The present study was funded by grants from the
National Natural Science Foundation of China (no. 81302323), the
Natural Science Foundation of Hebei Province (C2014209140,
C2013209024 and H2013209040), the Science and Technology Research
Project of the higher Education Institutions in Hebei Province,
China (QN20131059), the General Higher Education Young Talents
Program of Hebei Province (BJ2014027), the PhD Research Startup
Foundation of North China University of Science and Technology, and
the Foundation Project of Tangshan Normal University.
References
|
1
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Global cancer statistics, 2002. CA Cancer J Clin. 55:74–108. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Liotta LA, Steeg PS and Stetler-Stevenson
WG: Cancer metastasis and angiogenesis: An imbalance of positive
and negative regulation. Cell. 64:327–336. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hanahan D and Folkman J: Patterns and
emerging mechanisms of the angiogenic switch during tumorigenesis.
Cell. 86:353–364. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nabeshima K, Inoue T, Shimao Y and
Sameshima T: Matrix metalloproteinases in tumor invasion: Role for
cell migration. Pathol Int. 52:255–264. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Duffy MJ: The role of proteolytic enzymes
in cancer invasion and metastasis. Clin Exp Metastasis. 10:145–155.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sreenath T, Matrisian LM,
Stetler-Stevenson W, Gattoni-Celli S and Pozzatti RO: Expression of
matrix metalloproteinase genes in transformed rat cell lines of
high and low metastatic potential. Cancer Res. 52:4942–4947.
1992.PubMed/NCBI
|
|
7
|
Chabottaux V and Noel A: Breast cancer
progression: Insights into multifaceted matrix metalloproteinases.
Clin Exp Metastasis. 24:647–656. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Egeblad M and Werb Z: New functions for
the matrix metallopro-teinases in cancer progression. Nat Rev
Cancer. 2:161–174. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu H, Kato Y, Erzinger SA, Kiriakova GM,
Qian Y, Palmieri D, Steeg PS and Price JE: The role of MMP-1 in
breast cancer growth and metastasis to the brain in a xenograft
model. BMC Cancer. 12:5832012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hu F, Wang C, Guo S, Sun W, Mi D, Gao Y,
Zhang J, Zhu T and Yang S: δEF1 promotes osteolytic metastasis of
MDA-MB-231 breast cancer cells by regulating MMP-1 expression.
Biochim Biophys Acta. 1809:200–210. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tang SJ, Hoodless PA, Lu Z, Breitman ML,
McInnes RR, Wrana JL and Buchwald M: The Tlx-2 homeobox gene is a
downstream target of BMP signalling and is required for mouse
mesoderm development. Development. 125:1877–1887. 1998.PubMed/NCBI
|
|
12
|
Derynck R and Zhang YE: Smad-dependent and
Smad-independent pathways in TGF-beta family signalling. Nature.
425:577–584. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yang S, Du J, Wang Z, Yuan W, Qiao Y,
Zhang M, Zhang J, Gao S, Yin J, Sun B, et al: BMP-6 promotes
E-cadherin expression through repressing deltaEF1 in breast cancer
cells. BMC Cancer. 7:2112007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Meng F, Henson R, Wehbe-Janek H, Ghoshal
K, Jacob ST and Patel T: MicroRNA-21 regulates expression of the
PTEN tumor suppressor gene in human hepatocellular cancer.
Gastroenterology. 133:647–658. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang C, Hu F, Guo S, Mi D, Shen W, Zhang
J, Qiao Y, Zhu T and Yang S: BMP-6 inhibits MMP-9 expression by
regulating heme oxygenase-1 in MCF-7 breast cancer cells. J Cancer
Res Clin Oncol. 137:985–995. 2011. View Article : Google Scholar
|
|
16
|
Bendrik C, Robertson J, Gauldie J and
Dabrosin C: Gene transfer of matrix metalloproteinase-9 induces
tumor regression of breast cancer in vivo. Cancer Res.
68:3405–3412. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yan JD, Yang S, Lü SJ, Lei RY and Zhu TH:
Expression of recombinant human BMP6 in CHO cells by fused to the
signal peptide and propeptide of another homologue protein. Sheng
Wu Gong Cheng Xue Bao. 23:413–417. 2007.In Chinese. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xia Y, Yu PB, Sidis Y, Beppu H, Bloch KD,
Schneyer AL and Lin HY: Repulsive guidance molecule RGMa alters
utilization of bone morphogenetic protein (BMP) type II receptors
by BMP2 and BMP4. J Biol Chem. 282:18129–18140. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Peng Z, Fernandez P, Wilder T, Yee H,
Chiriboga L, Chan ES and Cronstein BN: Ecto-5′-nucleotidase
(CD73)-mediated extracellular adenosine production plays a critical
role in hepatic fibrosis. Nucleosides Nucleotides Nucleic Acids.
27:821–824. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fukui T, Suga T, Iida RH, Morito M, Luan
X, Diekwisch TG, Nakamura Y and Yamane A: BMP-2 regulates the
formation of oral sulcus in mouse tongue by altering the balance
between TIMP-1 and MMP-13. Anat Rec. 293:1408–1415. 2010.
View Article : Google Scholar
|
|
21
|
Nagase H, Visse R and Murphy G: Structure
and function of matrix metalloproteinases and TIMPs. Cardiovasc
Res. 69:562–573. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Du J, Yang S, An D, Hu F, Yuan W, Zhai C
and Zhu T: BMP-6 inhibits microRNA-21 expression in breast cancer
through repressing deltaEF1 and AP-1. Cell Res. 19:487–496. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Oxmann D, Held-Feindt J, Stark AM,
Hattermann K, Yoneda T and Mentlein R: Endoglin expression in
metastatic breast cancer cells enhances their invasive phenotype.
Oncogene. 27:3567–3575. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nagaiah G and Abraham J: Circulating tumor
cells in the management of breast cancer. Clin Breast Cancer.
10:209–216. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jemal A, Siegel R, Ward E, Hao Y, Xu J and
Thun MJ: Cancer statistics, 2009. CA Cancer J Clin. 59:225–249.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yang S, Du J, Wang Z, Yan J, Yuan W, Zhang
J and Zhu T: Dual mechanism of deltaEF1 expression regulated by
bone morphogenetic protein-6 in breast cancer. Int J Biochem Cell
Biol. 41:853–861. 2009. View Article : Google Scholar
|
|
27
|
Funahashi J, Kamachi Y, Goto K and Kondoh
H: Identification of nuclear factor delta EF1 and its binding site
essential for lens-specific activity of the delta 1-crystallin
enhancer. Nucleic Acids Res. 19:3543–3547. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Eger A, Aigner K, Sonderegger S, Dampier
B, Oehler S, Schreiber M, Berx G, Cano A, Beug H and Foisner R:
DeltaEF1 is a transcriptional repressor of E-cadherin and regulates
epithelial plasticity in breast cancer cells. Oncogene.
24:2375–2385. 2005. View Article : Google Scholar : PubMed/NCBI
|