Introduction
For the treatment of lung cancer, surgical
resection, consisting of a lobectomy and complete lymph node
dissection, has long been considered the standard treatment for
resectable tumors (1). However, it
is estimated that only one-third of patients with non-small cell
lung cancers (NSCLCs) are suitable candidates for curative
resection (2). However, for small
cell lung cancers (SCLCs), a histological subtype of lung cancers
with unique biological and oncological features that differ
significantly from NSCLCs, standard treatment usually consists of
systemic chemotherapy and radiotherapy. Surgery in association with
chemotherapy and radiotherapy is only indicated for a small subset
of SCLCs presenting with limited disease progression (3). Although SCLCs are sensitive to
radiotherapy and chemotherapy, patients remain in remission for a
relatively short duration and the emergence of rapidly growing
drug-resistant metastases are frequent in such patients. Therefore,
treatments for SCLCs are more likely to incorporate
multidisciplinary synthetic therapies (4).
Previous studies have indicated that human NCI-H446
SCLC cells are more sensitive to thermal treatment compared to
NSCLC cells, and thermal treatment can augment the cytotoxicity of
chemotherapeutic drugs (5).
Radiofrequency ablation (RFA) is an example of a thermotherapy
technique that is advanced, minimally invasive, and is used to
treat several types of neoplasms. Its application to the treatment
of lung tumors has received great interest (6). Lung tumors may be ideal targets for
RFA, since the surrounding air in the adjacent normal lung
parenchyma can provide an insulating effect, subsequently
concentrating the RF energy within the tumor tissue (7). However, according to a previous study,
RFA is associated with increased rates of local recurrence for lung
tumors with large volumes, since it is difficult to reach
sufficiently high temperatures in larger tumors and achieve
complete tumor destruction, as the heat source is farther removed
from the deeper sections of the tumor (8). Moreover, a rich tumor blood supply is
another factor that influences local recurrence, as an abundant
blood supply may help to dissipate the thermal energy and weaken
the effects of ablation (8).
However, as ablative devices are improved, satisfactory ablative
margins will likely be achieved. Various scholars suggest that even
when the tumor is completely destroyed/removed by RFA, residual
viable tumor cells within the periphery of the ablated area may
repopulate the tumor during a recurrence (9). Therefore, a new view was proposed that
local recurrence of the tumor is a product of the biological
behaviors of residual tumor cells, since proliferation and
angiogenesis potential change with RFA. Overproliferation is mainly
observed around areas of tissue necrosis and is associated with a
hypoxic tissue microenvironment (10). Therefore, altering the biological
characteristics of the hypoxic tissue microenvironment that
develops following RFA may influence the local recurrence rate.
Therefore, our goal was to observe the tissue microenvironment
following RFA and its relationship to recurrence.
The most common patterns of recurrence were
classified as local, intrapulmonary or distant. Local recurrence
was defined as a recurrence within or at the margin of the ablation
site. An intrapulmonary recurrence was defined as the presence of a
new tumor within the same lobe as the initially ablated tumor that
is separated from the original ablation zone. Distant recurrence
was defined as newly identified lung cancer metastases within the
other unablated lobe of the ipsilateral lung (11). According to these three patterns, we
divided the right lobe that underwent RFA treatment into three
distinct regions. We examined the transition zone (TZ) located at
the margin of the ablation zone; tumors appearing here were
considered a local recurrence. We also examined the reference zone
(RZ) of the ablated lobe, which consisted of the unablated part in
the lobe that underwent RFA treatment; tumors appearing here were
considered an intrapulmonary recurrence. Finally, we examined the
RZ on the ipsilateral side of the unablated lobe; tumors appearing
here were considered a distant recurrence. We established a nude
mouse model with a metastatic human SCLC to study the impact of RFA
on the overproliferation of SCLC cell clusters within these three
regions.
Hypoxia inducible factor (HIF)-1α is a transcription
factor whose expression can be induced by hypoxia and which
regulates multiple biological processes including angiogenesis,
cell proliferation and migration (12). Our pervious results indicated that
HIF-1α can regulate the expression of multiple cytokines and
promote the proliferation (13) and
angiogenesis potential (14) of
SCLCs. Therefore, we aimed to ascertain whether intervention
strategies aimed at inhibiting HIF-1α expression could limit or
prevent the overproliferation of residual SCLC cells or reduce the
angiogenesis potential of the tissues in the TZ to ultimately
decrease the tumor recurrence rate following RFA.
Materials and methods
Animals and surgical procedures
Male congenital athymic BALB/c nude mice were
obtained from the Experimental Animal Center of the Shanghai Jiao
Tong University School of Medicine. Mice were maintained under
pathogen-free conditions in accordance with established
institutional guidance and approved protocols. All experiments were
carried out using 6- to 8-week-old mice weighing 16–22 g. Animals
were housed under standard laboratory conditions. All surgical
procedures were performed under isoflurane inhalation anesthesia.
Buprenorfine was injected intramuscularly prior to surgery for
perioperative analgesia. All animals received humane care during
the experiment under a protocol that was in accordance with
institutional guidelines for animal research and was approved by
the Ethics and Research Committee of Anhui Medical University.
Cell culture and induction of a nude
mouse model with metastatic human SCLCs
The NCI-H446 cell line expressing firefly luciferase
(Luc) was obtained from the Shanghai Institutes for Biological
Sciences Cell Bank and cultured in RPMI-1640 medium (Sigma-Aldrich
Co., St. Louis, MO, USA) supplemented with 10% fetal bovine serum
(FBS; HyClone, Thermo Fisher Scientific, Grand Island, NY, USA) and
100 g/ml kanamycin at 37°C in humidified atmosphere containing 5%
CO2 and 20% O2.
All animals were acclimated for at least 7 days
prior to the intravenous (i.v.) injection of NCI-H446-Luc. On day
1, NCI-H446-Luc cells were collected and resuspended in
phosphate-buffered saline (PBS; Thermo Fisher Scientific) at a
concentration of 5×107 cells/ml. Tail vein injections of
100 µl of cell solution were administered to each mouse.
Tumor metastasis was monitored by in vivo imaging at 1 or 3
weeks following the injection. Chloral hydrate (100 µl) was
administered by intraperitoneal (i.p.) injection, and the procedure
commenced after 5 min. After anesthesia, mice were imaged one at a
time for 3 min each using an IVIS Lumina II in vivo imaging
system (Caliper Life Sciences, a PerkinElmer Co., Hopkinton, MA,
USA). Images were collected for further analysis.
RFA treatment and drug intervention
A multipole RF ablation instrument and bipolar
ablation needle were purchased from Beijing Blade Opto-Electronic
Technology Development Co., Ltd. In nude mice, a bipolar electrode
(outer diameter 1.0 mm, active length 10 mm) was used for RFA at 2
W for 45 sec with complete puncture of the right lung upper lobe,
which corresponded to a total energy output of 90 J. RF was applied
for 5 min with the generator output titrated to maintain a
designated tip temperature (70°C±2, 90 mA±20).
YC-1,
3-(5′-hydroxy-methyl-2′-furyl)-1-benzylindazole (Sigma-Aldrich
Co.), possesses antiplatelet activity and decreases hypoxia-induced
HIF-1α accumulation and stability (15). Therefore, YC-1 was dissolved in
saline and administered via i.p. injections at a preoperative dose
of 100 mg/kg body weight, followed by postoperative doses of 30
mg/kg body weight.
PTK787/ZK-222584 (PTK/ZK; MedChem Express, Monmouth
Junction, NJ, USA) is a non-selective vascular endothelial growth
factor receptor tyrosine kinase inhibitor (16). PTK/ZK was dissolved in
polyethyleneglycol 400 and administered twice daily by i.p.
injections at a dose of 50 mg/kg from 1 day prior to RFA.
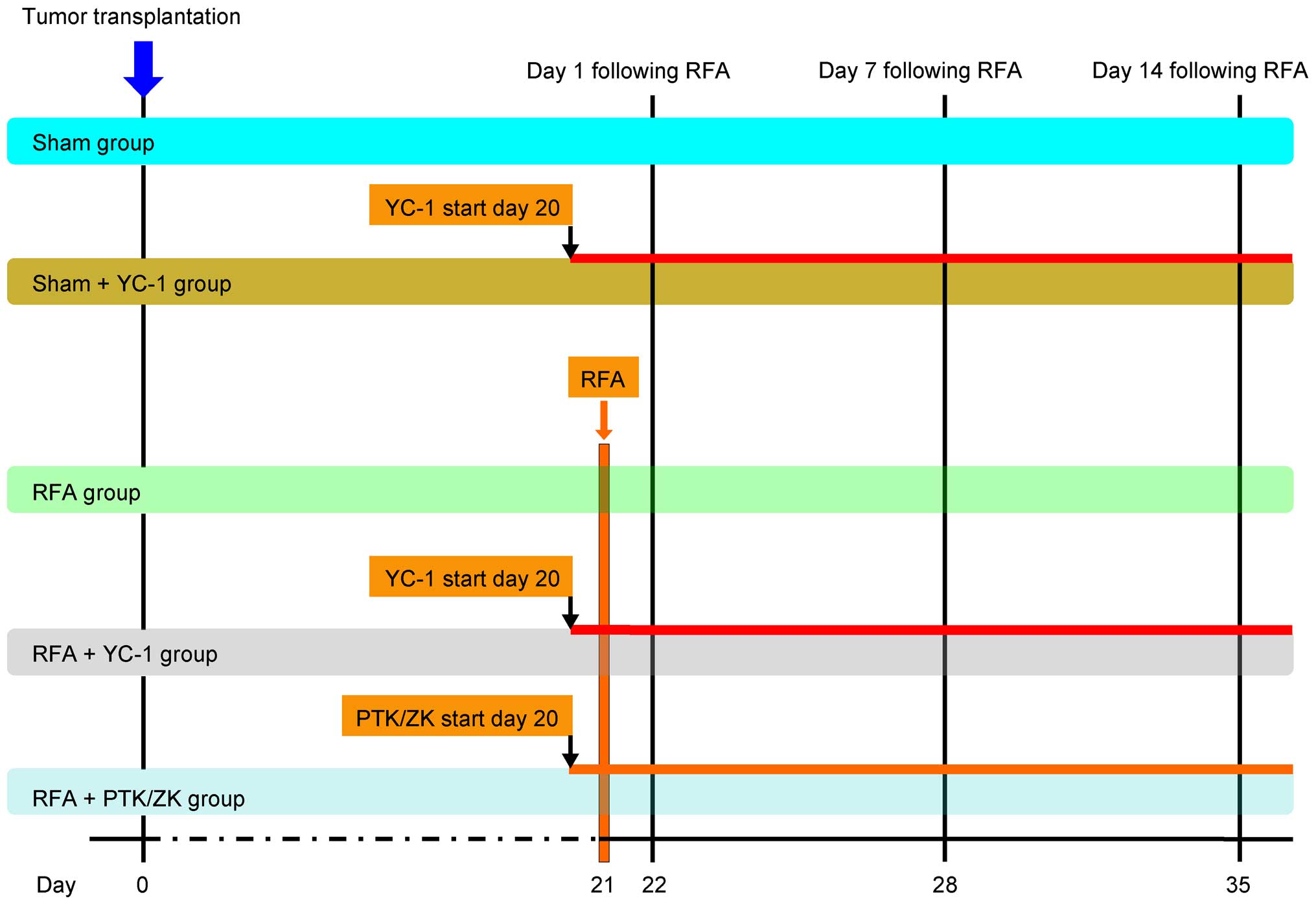
Experimental design and tumor assay
The effect of RFA on the perinecrotic
overproliferation of SCLC micrometastases in nude mouse lungs was
assessed. This nude mouse model of human SCLC micrometastases was
previously established (17).
Altogether 150 mice (evenly divided into female and male genders)
with established SCLC micrometastases were involved in the
experiment and the experimental analysis, which was carried out in
three stages at day 1, 7 and 14 following the RFA treatment. In
each stage, 50 mice were randomized into five groups: the sham
group; sham + YC-1 group; RFA group; RFA + YC-1 group; RFA + PTK/ZK
group, and each group had 10 mice. The treatment plan is shown in
Fig. 1. Mice were individually
sacrificed at day 1, 7 and 10 following RFA treatment
(n=10/group/time point). Then, the lungs were harvested and fixed
in 4% buffered formaldehyde and embedded in paraffin for
morphological assessment of tumor proliferation.
For tumor assays, we applied the methods of Nijkamp
et al (16). Tumor load in
the lung was scored as the pneumonic replacement area (PRA), which
is the percentage of lung tissue that had been replaced by tumor
tissue. The PRA in the TZ and RZ was measured. We defined the TZ
based on preliminary histologic data, showing histologic
characteristics in the TZ that were most abundant in the first 2 mm
stretching from the edge of the central necrosis zone (CZ). RZ was
defined as the remaining part of the ipsilateral lung tissue and
included the RZ of the right lung upper lobe (RUL) and the RZ of
the right lung lower lobe (RLL). All analyses were performed by at
least two independent observers that were blinded to the treatment,
and used an automated microscope with an interactive video overlay
system (Leica Q-Prodit; Leica Microsystems, Rijswijk, The
Netherlands). The scar tissue induced by RFA was excluded from
analysis. For analysis of treatment with YC-1 and PTK/ZK, we
compared the PRA ratios, the ratio between the PRA in the TZ and
the PRA in the RZ. PRA ratios were used to reflect the level of
tumor proliferation.
Immunohistochemistry detection for
identification of human SCLC metastasis and HIF-1α, and CD34
expression in tumor tissues
All tumor tissues were sectioned into 4-µm
slices, deparaffinized, and endogenous peroxidases were inhibited
with 0.3% hydrogen peroxide in methanol for 30 min. Antigen
retrieval was achieved by treating sections with 0.05% protease XIV
for 5 min at 37°C. Sections were then incubated with a mouse
anti-human NSE, HIF-1α and CD34 primary antibody (NSE, 1:50;
HIF-1α, 1:1,000; CD34, 1:200 dilution; Wuhan Boster Biological
Engineering Technology Co. Ltd.) overnight at 4°C. The slides were
then incubated with a biotin-conjugated rabbit anti-mouse secondary
antibody (1:1,000; Wuhan Boster Biological Engineering Technology
Co. Ltd.) at room temperature for 45 min. The sections were
subsequently incubated with a streptavidin-biotin-peroxidase
complex (Vectastain ABC kit; Vector Laboratories, Burlingame, CA,
USA) at room temperature for 45 min. The reaction was visualized by
applying chromogen diaminobenzi-dine (DAB) for 10 sec. Finally, the
slides were counterstained with hematoxylin and mounted. The slides
were examined with a Nikon Eclipse Ti microscope under a ×40
objective (Nikon Instruments, Melville, NY, USA). Images of all
fields directly surrounding the lesion in one representative
slide/tumor (6–13 fields/slide) were selected. The percentage of
positive areas in each field was calculated with ImageJ software
(National Institutes of Health, Bethesda, MD, USA).
Statistical analysis
Statistical differences between groups were analyzed
by a Student's t-test and ANOVA for parametric data. Data are
expressed as mean ± SEM. p<0.05 or p<0.01 was set as the
level of statistical significance.
Results
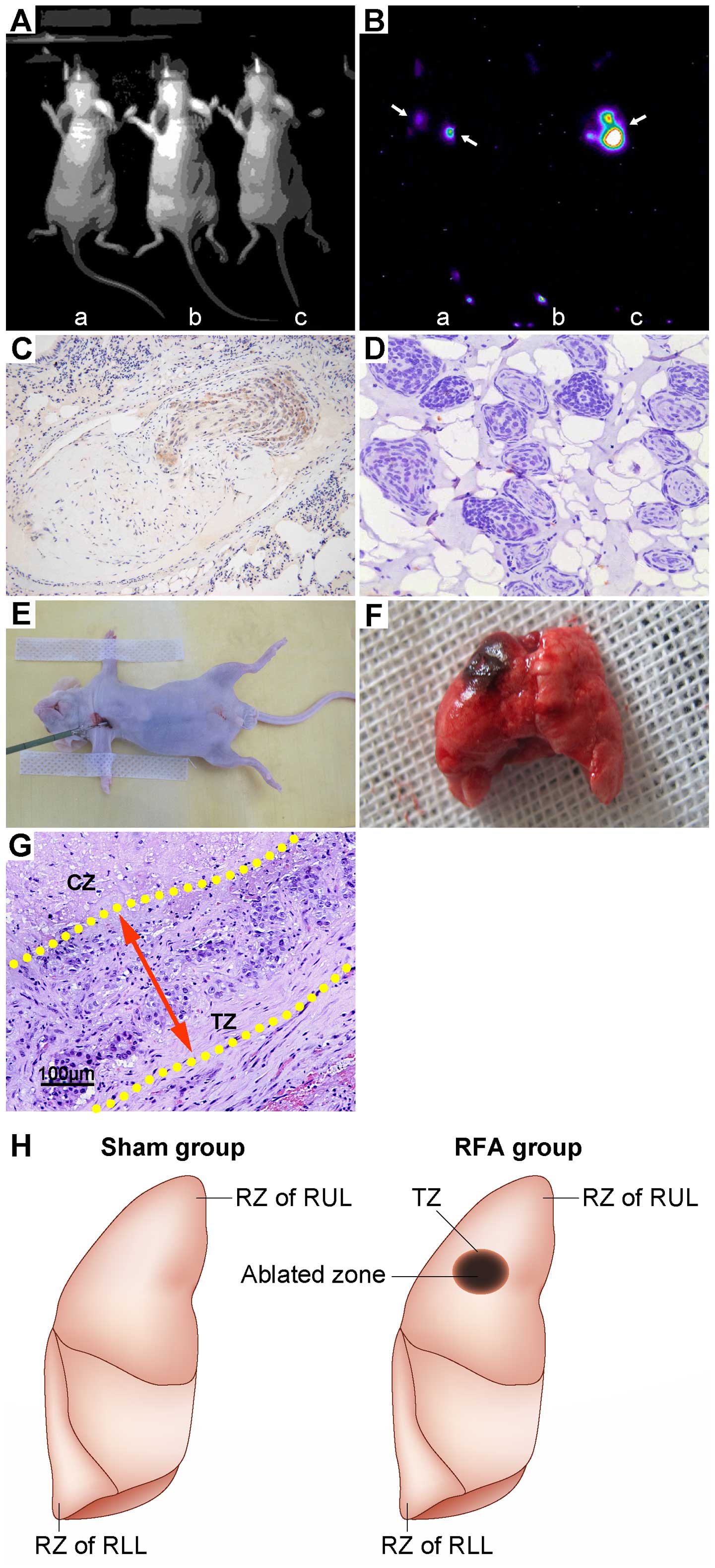
RFA treatment effects and the TZ in lung
tissue are morphologically distinct
We implanted a luciferase-labeled subline of the
human variant SCLC cell line NCI-H446 orthotopically into the right
lung of nude mice to establish an SCLC metastasis model. Three
weeks following tail vein injection of SCLC cells, signs of
systemic failure became increasingly more apparent, which included
emaciation, lethargy and hunching of the back. Imaging was
performed to assess fluorescence in the lung to locate and quantify
the luciferase-labeled cancer cells, which could be used as an
overall metric to quantify metastatic behavior (Fig. 2A and B). Histological observations
revealed that the SCLC tumor tissue tended to cluster close to the
pulmonary bronchium and vessel. In addition, human NSE expression
was evaluated as the positive area of DAB staining observed within
the tumor tissue. Since NSE is a specific marker of human SCLC
cells, we used NSE expression to verify that the transplantation
tumor was indeed derived from human SCLC cells (Fig. 2C). By 3 weeks following injection,
human SCLC metastasis became prominent and pervasive throughout the
lung tissue (Fig. 2D). All the mice
were then anesthetized and subjected to RFA treatment in the RUL.
The depth of needle percutaneous puncture was 0.5 cm and this
formed the ablation area (Fig. 2E and
F). Histological sections showed that the TZ stretched 2 mm
outside the necrotic central area and any local recurrences often
occurred in this area (Fig. 2G).
With thermal ablation, the TZ and RZ in the RUL exhibited a
different microenvironment as compared to RZ in the RLL,
representing a different region of recurrence (Fig. 2H).
RFA accelerates the outgrowth of human
SCLC micrometastases in the TZ and RZ of the lobe that underwent
RFA
The first parameter analyzed was PRA, or the
percentage of lung tissue that had been replaced by tumor tissue.
Measuring the tumor size reflects proliferation. The PRA value of
the RZ in the RLL showed that at day 1, 7 and 14 following RFA
treatment, no significant changes could be observed when comparing
the RFA group with the sham group (day 1 RFA, 14.8±2.98% vs. sham,
14.5±2.62%, p=0.96; day 7 RFA, 19.9±3.98% vs. sham, 19.2±3.52%,
p=0.87; day 14 RFA, 27.9±4.39% vs. sham, 28.5±4.47%, p=0.89). These
data indicated that RFA did not affect the outgrowth of metastases
in the lobe not undergoing RFA treatment (Fig. 3A–C; Table IA–C). However, the RZ in the RUL
showed a significantly higher PRA value in the RFA group when
compared with the sham group (day 1 RFA, 19.6±3.46% vs. sham,
15.9±3.43%, p=0.036; day 7 RFA, 25.6±4.42% vs. sham, 18.9±3.99%
p=0.021; day 14 RFA, 40.3±5.69% vs. sham, 29.8±4.53%, p=0.019)
(Fig. 3A–C; Table IA–C).
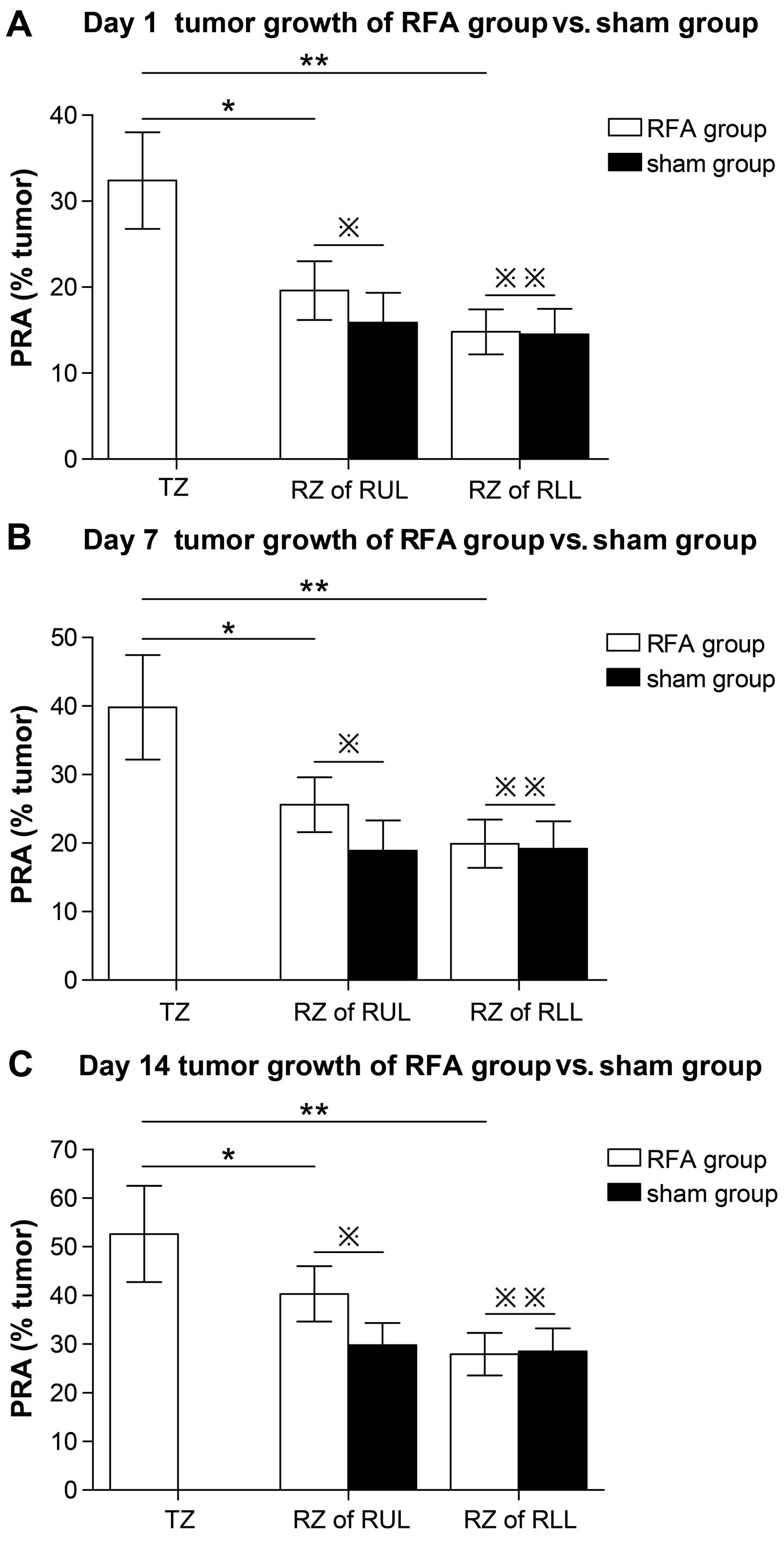 | Figure 3Accelerated perilesional outgrowth of
established micrometastases following RFA in the TZ and RZ. (A–C)
Tumor growth at day 1, 7 and 14 following RFA treatment, expressed
as the PRA in the TZ, defined as the area extending 2 mm outside
the necrotic lesion in the RUL and RZ defined as the remaining part
of the RUL and free area of the RLL. (A) Day 1:
※※p=0.96, RZ of RFA group vs. RZ of sham group in RLL;
※p=0.036, RZ of RFA group vs. RZ of sham group in RUL;
**p=0.000, TZ vs. RZ in RLL; and *p=0.008, TZ
vs. RZ in RUL. (B) Day 7: ※※p=0.87, RZ of RFA group vs.
RZ of sham group in RLL; ※p=0.021, RZ of RFA group vs.
RZ of sham group in RUL; **p=0.001, TZ vs. RZ in RLL and
*p=0.011, TZ vs. RZ in RUL. (C) Day 14:
※※p=0.89, RZ of RFA group vs. RZ of sham group in RLL
and ※p=0.019, RZ of RFA group vs. RZ of sham group in
RUL; **p=0.006, TZ vs. RZ in RLL and
*p=0.026, TZ vs. RZ in RUL. |
 | Table IHPR value (%) in TZ and RZ at days 1,
7 and 14 following RFA. |
Table I
HPR value (%) in TZ and RZ at days 1,
7 and 14 following RFA.
| A, HPR value (%) in
TZ and RZ at day 1 following RFA |
|---|
|
|---|
| Group | TZ | RZ in RUL | RZ in RLL | P-value |
|---|
| RFA | 32.4±5.62 | 19.6±3.46 | 14.8±2.98 | p=0.000 TZ vs. RZ
in RLL of RFA group |
| | | | p=0.008 TZ vs. RZ
in RUL of RFA group |
| Sham | | 15.9±3.43 | 14.5±2.62 | p=0.036 HPR of RZ
in RUL RFA group vs. sham group |
| | | | p=0.96 HPR of RZ in
RLL RFA group vs. sham group |
RFA induced a significant increase in the amount of
tumor tissue in the TZ surrounding the ablated region as compared
with the RZ in the same lobe (day 1 TZ, 32.4±5.62% vs. RZ,
19.6±3.46%, p=0.008; day 7 TZ, 39.8±7.62% vs. RZ, 25.6±4.42%, p=
0.011; day 14 TZ, 52.6±9.89% vs. RZ, 40.3±5.69%, p=0.026). There
was an ~2-fold increase in the TZ compared with the RZ in the RLL
(day 1 TZ, 32.4±5.62% vs. RZ, 14.8±2.98%, p=0.000; day 7 TZ,
39.8±7.62% vs. RZ, 19.9±3.98%, p=0.001; day 14 TZ, 52.6±9.89% vs.
RZ, 27.9±4.39%, p=0.006) (Fig.
3A–C; Table IA–C).
These results indicated that RFA treatment induced
the proliferation of tumors in the ablated lobe, but did not affect
the tumor on the ipsilateral side of the other lobe. In the ablated
lobe, the tumor in the TZ surrounding the ablated region had a
higher proliferative capacity than the tumor in the distant areas.
The tumor microenvironment may have been altered by RFA treatment,
which could help to account for these differences. Therefore, we
explored the expression of tumor-relevant regulatory factors within
the tumor microenvironment.
Accelerated tumor proliferation in the TZ
and RZ is associated with HIF-1α expression
Based on the immunohistochemical results (Fig. 4A–C) and semi-quantitative analysis,
the highest HIF-1α expression level occurred in the TZ at day 1
following RFA treatment (TZ, 31.4±4.43% vs. RZ in RUL, 24.5±5.79%,
p= 0.028; TZ, 31.4±4.43% vs. RZ in RLL, 10.9±3.21%, p=0.001)
(Fig. 4D; Table IIA). At day 1, 7 and 14 following
RFA treatment, HIF-1α expression in the RZ of the ablated lobe was
significantly higher in the RFA than the sham group (RFA group vs.
sham group; day 1, 24.5±5.97% vs. 11.7±3.03%, p=0.019; day 7,
20.3±4.35% vs. 10.3±3.32%, p=0.011; day 14, 12.5±3.96% vs.
6.7±2.13%, p=0.017), although the expression level in the RZ of RLL
showed no significant difference (day 1, p=0.89; day 7, p=0.92; day
14, p=0.96 (Fig. 4D; Table IIA–C). Meanwhile, in the RFA group,
thermal stimulus more significantly increased HIF-1α expression in
the RZ of the ablated lobe in RUL than RZ of the non-ablated lobe
in RLL at day 1, 7 and 14 (RZ of RUL vs. RZ of RLL: day 1,
24.5±5.79% vs. 10.9±3.21%, p=0.005; day 7, 20.3±4.35% vs.
9.1±2.98%, p=0.011; day 14, 12.5±3.96% vs. 6.4±2.78%, p=0.021)
(Fig. 4D; Table IIA–C). Following treatment with
YC-1, an inhibitor of HIF-1α, the PRA value in the TZ significantly
decreased at days 1, 7 and 14 following RFA (RFA group vs. RFA +
YC-1 group: day 1, 32.4±5.62% vs. 10.8±2.34%, p=0.000; day 7,
39.8±7.62% vs. 16.6±3.98%, p=0.000; day 14, 52.6±9.89% vs.
23.5±4.74%, p=0.001) (Fig. 4E;
Table IIIA). In addition to the
TZ, tumor proliferation capacity was also inhibited by YC-1 in the
RZ of the RUL and RLL, regardless of RFA treatment (Fig. 4F and G; Table IIIA and B; data not shown). These
data suggest that tumor proliferation was universally induced by
HIF-1α and can be suppressed by inhibiting HIF-1α. Thermal stimulus
had the greatest impact on HIF-1α expression; HIF-1α reached its
highest expression levels in the TZ surrounding the central
necrotic zone. In addition, RFA treatment induced HIF-1α expression
in the distant region (RZ) of the ablation zone.
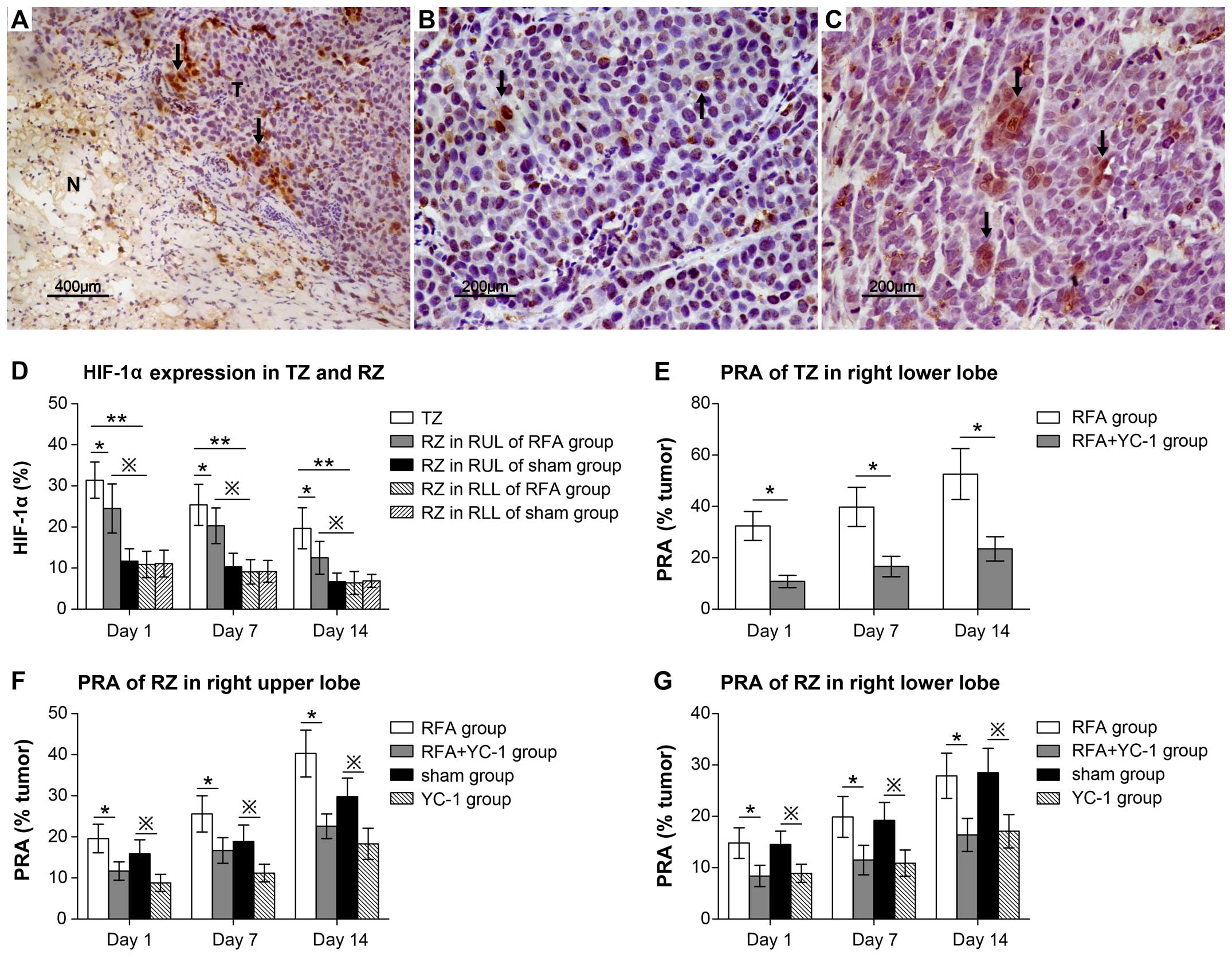 | Figure 4Regardless of the TZ or RZ, tumor
growth was induced by HIF-1α at day 1, 7 and 14 following RFA
treatment. (A-C) Immunostaining showed the HIF-1α expression as
brown tumor cells (arrows) in tumor tissue (T) of TZ (A) adjacent
to the necrosis ablated zone (N), RZ in RUL (B) and RZ in RLL (C).
(D) Results of the semi-quantitative analysis and comparison of the
HIF-1α expression level in the TZ and RZ of the RUL and RLL at day
1, 7 and 14: day 1, *p=0.028 TZ vs. RZ in RUL and
**p=0.001 TZ vs. RZ in RLL; day 7, *p=0.037
TZ vs. RZ in RUL and **p=0.007 TZ vs. RZ in RLL; day 14,
*p=0.014 TZ vs. RZ in RUL and **p=0.000 TZ
vs. RZ in RLL. In addition these, the HIF-1α expression level was
higher in the RZ of the RUL than the RZ of the RLL. Day 1,
※p=0.005 RZ in RUL vs. RZ in RLL; day 7,
※p=0.011 RZ in RUL vs. RZ in RLL; day 14,
※p=0.021 RZ in RUL vs. RZ in RLL. (E) Treatment with
YC-1 decreased the PRA value significantly: *p≤0.001,
RFA group vs. RFA + YC-1 group at day 1, 7 and 14. (F and G) PRA
value of RZ in RUL and RLL decreased in the RFA + YC-1 group at
days 1, 7 and 14: *p<0.01, RFA group vs. RFA + YC-1
group. Meanwhile in the sham group, the PRA value also decreased
after treatment with YC-1: ※p<0.05 sham vs. YC-1
group. |
 | Table IIHIF-1α expression (%) in TZ and RZ at
days 1, 7 and 14 following RFA. |
Table II
HIF-1α expression (%) in TZ and RZ at
days 1, 7 and 14 following RFA.
| A, HIF-1α
expression (%) in TZ and RZ at at day 1 following RFA |
|---|
| Group | TZ | RZ in RUL | RZ in RLL | P-value |
|---|
| RFA | 31.4±4.43 | 24.5±5.97 | 10.9±3.21 | p=0.001 RFA group:
TZ vs. RZ in RLL |
| | | | p=0.028 RFA group:
TZ vs. RZ in RUL |
| | | | p=0.019 RZ in RUL:
RFA group vs. sham group |
| Sham | | 11.7±3.03 | 11.1±3.24 | p=0.89 RZ in RLL:
RFA group vs. sham group |
| | | | p=0.005 RFA group:
RZ in RUL vs. RZ in RLL |
 | Table IIIPRA value (%) in TZ, RZ of RUL and RZ
of RLL at days 1, 7 and 14. |
Table III
PRA value (%) in TZ, RZ of RUL and RZ
of RLL at days 1, 7 and 14.
| A, PRA value (%) in
TZ |
|---|
|
|---|
| Group | Day 1 | Day 7 | Day 14 | P-value |
|---|
| RFA | 32.4±5.62 | 39.8±7.62 | 52.6±9.89 | Day 1, p=0.000 RFA
group vs. RFA + YC-1 group |
| | | | Day 7, p=0.000 RFA
group vs. RFA + YC-1 group |
| RFA + YC-1 | 10.8±2.34 | 16.6±3.98 | 23.5±4.74 | Day 14, p=0.001 RFA
group vs. RFA + YC-1 group |
Tissue angiogenesis potential is also
regulated by HIF-1α, but has no effect on tumor proliferation in
the TZ
As demonstrated, accelerated tumor growth in the TZ
or RZ of the ablated lobe following RFA was found to be associated
with activation of HIF-1α. Our pervious study indicated that HIF-1α
can induce the expression of multiple angiogenic cytokines, while
upregulating the angiogenesis potential of SCLC cells. Therefore,
we set out to determine whether tissue angiogenesis acts as the
driving force in RFA-stimulated micrometastasis growth. To assess
the effects on the growth of micrometastases by angiogenesis, we
analyzed the microvessel density (MVD) as CD34-positive stained
areas to compare the angiogenesis potential of the TZ and RZ in the
RUL and RLL (Fig. 5A–C). At days 1,
7 and 14 following RFA treatment, the TZ exhibited a higher
angiogenesis potential in the CD34-positive area compared with the
RZ in the RUL (such as: day 1 TZ, 156.7±30.99% vs. RZ,
127.8±24.34%, p=0.011) and RZ in RLL (such as: day 1, TZ,
156.7±30.99% vs. RZ, 119.8±19.90%, p=0.002) (Fig. 5D, Table
IVA–C). Treatment with YC-1 reduced CD34 expression in all
zones following RFA (day 1 TZ, 156.7±30.99% vs. 82.2±20.67% p=0.00;
RZ in RUL: 127.8±24.34% vs. 43.1±8.65% p=0.00; RZ in RLL:
119.8±19.90% vs. 37.8±7.12% p=0.000) (Fig. 5D; Table
IVA–C). However, treatment with the vascular specific inhibitor
PTK/ZK did not alter tumor growth in the TZ (day 1 PRA of TZ:
32.4±5.62 vs. 31.6±5.37, p=0.87), which was different from the
results of the RZ in the RUL and RLL (Fig. 5E; Table
VA–C; data not shown). Our data indicated that HIF-1α regulates
tumor growth by inducing tissue angiogenesis in the RZ of the RUL
and the RLL. However, in the TZ in RFA-treated mice, tumor growth
was not associated with tissue angiogenesis potential, although
regulated by HIF-1α.
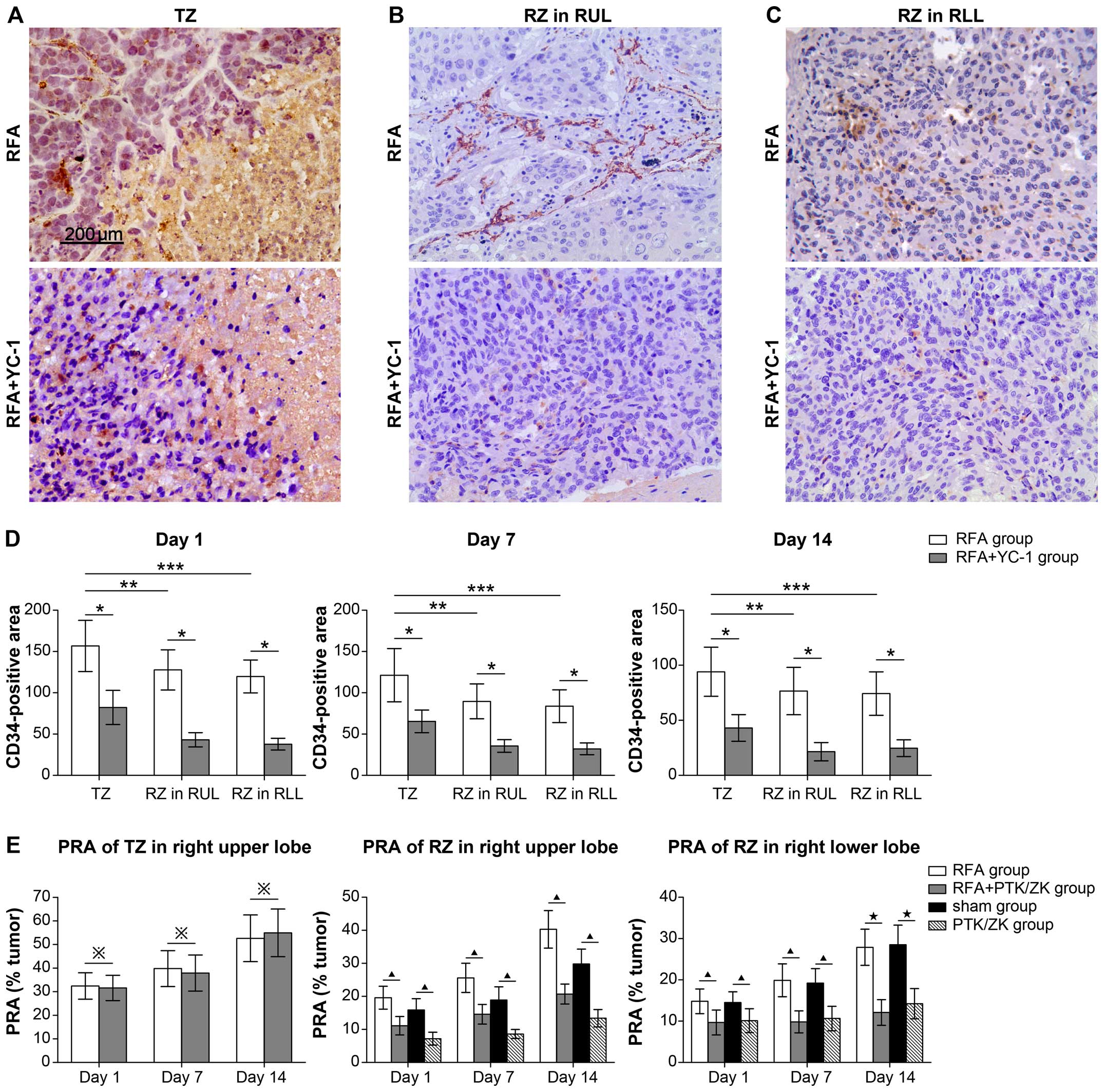 | Figure 5Angiogenesis potential is higher in
the TZ than that in the RZ of the RUL and RLL, but has no effect on
tumor proliferation. (A-C) Immunostaining showed CD34-positive
staining in the TZ (A), RZ in RUL (B) and RZ in RLL (C) of the RFA
group and RFA + YC-1 group. (D) Based on the semi-quantitative
analysis of the CD34-positive stained area, it was noted that the
MVD value of the TZ was higher than that of the RZ in the RUL and
RLL at day 1, 7 and 14 following RFA treatment, but when treated
with YC-1, MVD values of the TZ, RZ in RUL and RLL were decreased.
*P<0.01, RFA group vs. RFA + YC-1 group in TZ, RZ in
RUL and RZ in RLL; **P<0.05, RFA group: TZ vs. RZ in
RUL; ***P<0.05, RFA group: TZ vs. RZ in RLL. (E) The
effect of PTK/ZK on perilesional outgrowth of established
micrometastases evaluated 1, 7 and 14 days. After RFA treatment,
tumor growth was expressed as the PRA in the TZ and RZ. In the RFA
and sham groups, treatment with PTK/ZK reduced tumor growth in the
RZ of the RUL and RLL. ※p>0.05, RFA group vs. RFA +
PTK/ZK group; ▲p<0.05, RFA group vs. RFA + PTK/ZK
group or sham group vs. PTK/ZK group; ★P<0.01, RFA
group vs. RFA + PTK/ZK group or sham group vs. PTK/ZK group. |
 | Table IVMVD (mm2) in TZ and RZ at
days 1, 7 and 14 following RFA. |
Table IV
MVD (mm2) in TZ and RZ at
days 1, 7 and 14 following RFA.
| A, MVD
(mm2) in TZ and RZ at day 1 following RFA |
|---|
|
|---|
| Group | TZ | RZ in RUL | RZ in RLL | P-value |
|---|
| RFA | 156.7±30.99 | 127.8±24.34 | 119.8±19.9 | TZ: p=0.000 RFA
group vs. RFA + YC-1 group |
| | | | RZ in RUL: p=0.004
RFA group vs. RFA + YC-1 group |
| | | | RZ in RLL: p=0.000
RFA group vs. RFA + YC-1 group |
| RFA + YC-1 | 82.2±20.67 | 43.1±8.65 | 37.8±7.12 | p=0.047 RFA group:
TZ vs. RZ in RLL |
| | | | p=0.025 RFA group:
TZ vs. RZ in RUL |
 | Table VPRA value (%) in TZ, in RZ of RUL and
in RZ of RLL at days 1, 7 and 14. |
Table V
PRA value (%) in TZ, in RZ of RUL and
in RZ of RLL at days 1, 7 and 14.
| A, PRA value (%) in
TZ |
|---|
|
|---|
| Group | Day 1 | Day 7 | Day 14 | P-value |
|---|
| RFA | 32.4±5.62 | 39.8±7.62 | 52.6±9.89 | Day 1: p=0.079 RFA
group vs. RFA + YC-1 group |
| | | | Day 7: p=0.088 RFA
group vs. RFA + YC-1 group |
| RFA + PTK/ZK | 31.6±5.37 | 37.9±7.64 | 54.5±10.1 | Day 14: p=0.091 RFA
group vs. RFA + YC-1 group |
In conclusion, we found the following. i) Following
RFA treatment, the TZ formed between the central necrotic zone and
the unaffected RZ along with the micrometastasis in this zone
exhibited stronger proliferative activity and higher HIF-1α
expression levels than the other zones. The growth of
micrometastases in the TZ was induced by HIF-1α, but was not
associated with the tissue angiogenesis potential. ii) In the RZ of
the ablated or unablated lobe, HIF-1α induced the growth of
micrometastases by regulating tissue angiogenesis potential.
Discussion
Radiofrequency ablation (RFA) is a thermal ablative
technique and a relatively new modality of treatment, which may be
applicable in high-risk patients with lung cancer (18). Compared with radiotherapy and
chemotherapy, RFA is focused exclusively on the tumor area and does
not damage the normal surrounding tissue. Compared with surgical
intervention, RFA is minimally invasive. For advanced lung cancers,
including small cell lung cancer (SCLC), RFA also can enhance
palliative treatment (19). In
general, RFA appears to be a safe procedure with limited/minimal
morbidity and mortality (20). The
most common operation complication appears to be the occasional
development of a pneumothorax or hemothorax. However, with the
progression in hemostasis techniques and surgical repair materials,
the incidence of these complications will be significantly reduced
(21). Although RFA has been
increasingly more widely used for the treatment of lung cancer,
postoperative recurrence is still a major shortcoming of this
therapy (8). Various scholars argue
that recurrences may be managed or mitigated based on a careful
distinction of the recurrence site. Based on this concept, when
regional hilar mediastinal lymphatic metastasis or distant relapse
occurs, patients should be considered as affected by systemic tumor
factors; initial ablation was probably complete, but the stage was
underestimated and the patients should be managed by systemic
therapy. If a tumor recurrence occurs in the local area surrounding
the ablation zone, patients should be considered as being affected
by the local tumor microenvironmental changes, and ablation was
probably incomplete (22).
For the recurrence of malignant tumors following
RFA, previous research indicates that increasing tumor size is
associated with an increased rate of local recurrence, which
emphasizes the importance of obtaining satisfactory ablative
margins (23). However, even when
complete tumor destruction is achieved and satisfactory ablation
margins have been created, residual tumor cells could survive in
the transition zone (TZ) between the necrosis induced by RFA and
the normal tissue (24). Therefore,
a new opinion was put forward that the biological characteristics
of the microenvironment in the TZ are critically important in the
timing and prognosis of tumor local recurrences (25). Stoeckelhuber et al proposed
that a clear demarcation between the ablation zones and TZs should
be the criterion used to judge the success of RFA treatment.
Applicable parameters of RFA, such as ablation temperature, time
and the depth of needle-electrode puncture were selected as
sentinels according to the results of immunohistochemisty and
hematoxylin and eosin (H&E) staining. The parameters of RFA
that result in the maximum diameter of the ablation zone and the
maximum width of the TZ were selected for treatment (26). Thermal destruction therapies will
unequivocally produce a TZ, such that the growth of residual tumor
cells that are located in the TZ may be accelerated and cause local
recurrences (27).
In general, hyperthermia can directly inhibit the
proliferation of tumor cells (28,29),
while the tumor cells surviving in the TZ following RFA may
overproliferate. There are two factors that may account for this
phenomenon. First, hyperthermia treatment creates a local hypoxic
microenvironment, which is often observed in the TZ surrounding the
necrosis zone. Nikfarjam et al (33) found that following RFA treatment of
liver metastases, a highly localized hypoxia-driven acceleration of
tumor growth occurs in the TZ. However, angiogenesis is not the
driving force behind RFA-stimulated tumor growth, indicating that
other hypoxia-activated pathways are likely important (16). HIF-1α is a master regulator of the
essential adaptive responses to hypoxia, which is highly expressed
under hypoxic conditions and is maintained at a constant low
concentration under normoxic condition (30).
In the present study, HIF-1α immunostaining was
mainly localized immediately adjacent to the necrotic area, and
strong HIF-1α staining was observed in tumor cells or the
extracellular matrix of the TZ following RFA. After treatment with
a specific inhibitor of HIF-1α (YC-1), HIF-1α expression in the TZ
decreased, while the angiogenesis potential was also significantly
reduced. Angiogenesis potential is critical to tumor proliferation
(31), as indicated by the results
of our previous studies in SCLC cells (14). HIF-1α promotes angiogenesis
potential by upregulating VEGF-A expression. VEGF-A is one of the
best-studied downstream effectors of HIF-1α, and it plays a pivotal
role in the stimulation of hypoxia-driven angiogenesis (32). Several angiogenic factors, including
VEGF-A, are upregulated primarily adjacent to the ablation zone
(33). PTK787/ZK-222584, a specific
inhibitor that reduces VEGF-A expression, has been widely used in
the clinical treatment of SCLCs (34). However, in the present study, while
PTK/ZK largely inhibited the angiogenic response, it had no
significant effect on tumor proliferation in the TZ, in sharp
contrast to YC-1, which reduced proliferation. For this reason, RFA
is an important method for tumor hyperthermia and can destroy tumor
cells by generating high temperatures. Heat shock proteins (HSPs),
synthesized under heat stress, can facilitate recovery of tumor
cells from heat damage (35). For
example, HSP90 expression is upregulated in response to stresses
such as hypoxia and hyperthermia, followed by modulation of
numerous targets aimed at promoting cell survival, including HIF-1α
(36). HSP70 plays an important
role in protecting cell death from a variety of stresses and the
upregulation of inducible HSP70 expression can stabilize HIF-1α
expression through p85α mediation (37). Thus, it is speculated that
upregulation of expression of HIF-1α in TZ and RZ of RUL may be
induced by HSPs (HSP90 or HSP70) following RFA treatment. VEGF is
one of the best studied downstream effectors of HIF and plays a
pivotal role in the stimulation of hypoxia-driven angiogenesis
(14), Meanwhile, HSP70-HSP90 heat
shock protein can also stimulate degradation of endothelial VEGFR
and inhibit the angiogenesis of tumors (38). Therefore, we believe that the
angiogenesis in TZ is regulated to a great extent by HSP and cannot
independently affect the growth of tumors in the TZ. Thus,
inhibition of angiogenesis in the TZ following RFA treatment had no
significant effect on the overproliferation of residual SCLC cells.
Hence, HSPs/HIF-1α may play a vital role in regulating the
proliferation rate of residual SCLC cells in TZ and the underlying
specific molecular mechanism involved in the regulation of HIF-1α
expression by HSPs will be investigated in our future study.
RFA has been proposed as an acceptable alternative
for the treatment of SCLCs. Although this technique and the
conditions of ablation have been improved by applying multipolar
ablation or widening the ablation margin, local recurrence is still
common. Multidisciplinary synthetic therapy, which is RFA combined
with another treatment has subsequently gained appeal. Recently,
adjuvant treatments used in combination with thermal destruction
therapies such as RFA have mainly involved the addition of
cytotoxic agents (39). In
preclinical tests, the combination of RFA with doxorubicin-based
chemotherapy created an increase in coagulation size and reduced
the risk of recurrence (40). As
accelerated overproliferation (elicited by RFA) was associated with
hypoxia in our study, hypoxia-activated prodrugs may be efficacious
in the adjuvant treatment of SCLC following RFA. Molecular-targeted
therapy against HIF-1α may also prove to be a suitable adjuvant
treatment for RFA. In addition, hyperthermia may elicit an
antitumor T cell response by presenting tumor antigens to the
immune system, ultimately resulting in the inhibition of tumor
proliferation (41). Our future
study will focus on developing methods to induce an antitumor
immune response. Our study provides a theoretical basis for the
development of new multidisciplinary synthetic therapies for SCLCs
typically treated with RFA alone.
Abbreviations:
|
RFA
|
radiofrequency ablation
|
|
SCLC
|
small cell lung cancer
|
|
TZ
|
transition zone
|
|
RZs
|
reference zones
|
|
RUL
|
right upper lobe
|
|
HIF
|
hypoxia inducible factor
|
|
RLL
|
right lower lobe
|
|
NSCLCs
|
non-small cell lung cancers
|
|
SCLCs
|
small cell lung cancers
|
|
CZ
|
central necrosis zone
|
|
PRA
|
pneumonic replacement area
|
|
DAB
|
diaminobenzidine
|
Acknowledgments
The present study was funded by the National Nature
Science Foundation of China (no. 81302028). The authors would like
to thank the Duoease Scientific Service Center for excellent
language editing service and suggestions for Figure revision.
References
|
1
|
Varela G and Thomas PA: Surgical
management of advanced non-small cell lung cancer. J Thorac Dis.
6(Suppl 2): S217–S223. 2014.PubMed/NCBI
|
|
2
|
Schreiner W, Semrau S, Fietkau R and Sirbu
H: Oligometastatic non-small cell lung cancer - surgical options
and therapy strategies. Zentralbl Chir. 139:335–341. 2014.In
German. PubMed/NCBI
|
|
3
|
Bolca C, Dănăilă O, Paleru C and Cordoş I:
Role of surgery in small cell lung cancer. Pneumologia. 62:236–238.
2013.In Romanian.
|
|
4
|
Hosono MN, Hosono M, Endo K, Ueda R and
Onoyama Y: Effect of hyperthermia on tumor uptake of radiolabeled
anti-neural cell adhesion molecule antibody in small-cell lung
cancer xenografts. J Nucl Med. 35:504–509. 1994.PubMed/NCBI
|
|
5
|
Kawahara T, Ito H, Terao H, Kato Y, Uemura
H, Kubota Y and Matsuzaki J: Effectiveness of ureteroscopy-assisted
retrograde nephrostomy (UARN) for percutaneous nephrolithotomy
(PCNL). PLoS One. 7:e521492012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Alexander ES and Dupuy DE: Lung cancer
ablation: Technologies and techniques. Semin Intervent Radiol.
30:141–150. 2013. View Article : Google Scholar :
|
|
7
|
Roberton BJ, Liu D, Power M, Wan JM,
Stuart S, Klass D and Yee J: Pulmonary ablation: A primer. Can
Assoc Radiol J. 65:177–185. 2014. View Article : Google Scholar
|
|
8
|
Lanuti M, Sharma A, Willers H, Digumarthy
SR, Mathisen DJ and Shepard JA: Radiofrequency ablation for stage I
non-small cell lung cancer: Management of locoregional recurrence.
Ann Thorac Surg. 93:921–927; discussion 927–988. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Goldberg SN, Gazelle GS, Compton CC,
Mueller PR and Tanabe KK: Treatment of intrahepatic malignancy with
radiofrequency ablation: Radiologic-pathologic correlation. Cancer.
88:2452–2463. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yamada S, Utsunomiya T, Morine Y, Imura S,
Ikemoto T, Arakawa Y, Kanamoto M, Iwahashi S, Saito Y, Takasu C, et
al: Expressions of hypoxia-inducible factor-1 and epithelial cell
adhesion molecule are linked with aggressive local recurrence of
hepatocellular carcinoma after radiofrequency ablation therapy. Ann
Surg Oncol. 21(Suppl 3): S436–S442. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Beland MD, Wasser EJ, Mayo-Smith WW and
Dupuy DE: Primary non-small cell lung cancer: Review of frequency,
location, and time of recurrence after radiofrequency ablation.
Radiology. 254:301–307. 2010.
|
|
12
|
Fraga A, Ribeiro R and Medeiros R: Tumor
hypoxia: The role of HIF. Actas Urol Esp. 33:941–951. 2009.In
Spanish. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wan J, Ma J, Mei J and Shan G: The effects
of HIF-1alpha on gene expression profiles of NCI-H446 human small
cell lung cancer cells. J Exp Clin Cancer Res. 28:1502009.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wan J, Chai H, Yu Z, Ge W, Kang N, Xia W
and Che Y: HIF-1α effects on angiogenic potential in human small
cell lung carcinoma. J Exp Clin Cancer Res 30. 77:2011. View Article : Google Scholar
|
|
15
|
Tsui L, Fong TH and Wang IJ: YC-1
targeting of hypoxia-inducible factor-1α reduces RGC-5 cell
viability and inhibits cell proliferation. Mol Vis. 18:1594–1603.
2012.
|
|
16
|
Nijkamp MW, van der Bilt JD, de Bruijn MT,
Molenaar IQ, Voest EE, van Diest PJ, Kranenburg O and Borel Rinkes
IH: Accelerated perinecrotic outgrowth of colorectal liver
metastases following radiofrequency ablation is a hypoxia-driven
phenomenon. Ann Surg. 249:814–823. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Holleran JL, Miller CJ, Edgehouse NL,
Pretlow TP and Culp LA: Differential experimental micrometastasis
to lung, liver, and bone with lacZ-tagged CWR22R prostate carcinoma
cells. Clin Exp Metastasis. 19:17–24. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bott MJ and Crabtree T: Treatment of stage
I lung cancer in high-risk and inoperable patients: SBRT vs. RFA
vs. sublobar resection. Ann Cardiothorac Surg. 3:167–169.
2014.PubMed/NCBI
|
|
19
|
Baisi A, Raveglia F, De Simone M and
Cioffi U: Palliative role of percutaneous radiofrequency ablation
for severe hemoptysis in an elderly patient with inoperable lung
cancer. J Thorac Cardiovasc Surg. 140:1196–1197. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kelekis AD, Thanos L, Mylona S, Ptohis N,
Malagari K, Nikita A, Christodoulidou J and Kelekis N: Percutaneous
radiofrequency ablation of lung tumors with expandable needle
electrodes: Current status. Eur Radiol. 16:2471–2482. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kashima M, Yamakado K, Takaki H, Kodama H,
Yamada T, Uraki J and Nakatsuka A: Complications after 1000 lung
radiofrequency ablation sessions in 420 patients: A single center's
experiences. AJR Am J Roentgenol. 197:W576–W580. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Baisi A, Raveglia F, De Simone M and
Cioffi U: Recurrence after radiofrequency ablation for stage I
non-small cell lung cancer. Ann Thorac Surg. 94:1788–1789. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mulier S, Ni Y, Jamart J, Ruers T, Marchal
G and Michel L: Local recurrence after hepatic radiofrequency
coagulation: Multivariate meta-analysis and review of contributing
factors. Ann Surg. 242:158–171. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nijkamp MW, Borren A, Govaert KM,
Hoogwater FJ, Molenaar IQ, van Diest PJ, Kranenburg O and Borel
Rinkes IH: Radiofrequency ablation of colorectal liver metastases
induces an inflammatory response in distant hepatic metastases but
not in local accelerated outgrowth. J Surg Oncol. 101:551–556.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Okhunov Z, Roy O, Duty B, Waingankar N,
Herati A, Morgenstern N, Sheikh-Fayyaz S and Kavoussi LR: Clinical
evaluation of a novel bipolar radiofrequency ablation system for
renal masses. BJU Int. 110:688–691. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Stoeckelhuber BM, Noack F, Kapsimalakou S,
Rudolf I, Bergmann-Koester CU, Helmberger T and Stoeckelhuber M:
Radiofrequency ablation in breast tissue: Experimental study for
evaluation of radiofrequency effects in the bovine udder and review
of the literature. J Vasc Interv Radiol. 20:1477–1482. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Frich L, Bjørnland K, Pettersen S, Clausen
OP and Gladhaug IP: Increased activity of matrix metalloproteinase
2 and 9 after hepatic radiofrequency ablation. J Surg Res.
135:297–304. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Takagi H, Azuma K, Tsuka T, Imagawa T,
Osaki T and Okamoto Y: Antitumor effects of high-temperature
hyperthermia on a glioma rat model. Oncol Lett. 7:1007–1010.
2014.PubMed/NCBI
|
|
29
|
Chen EY, Samkoe KS, Hodge S, Tai K, Hou H,
Petryk AA, Strawbridge R, Hoopes PJ and Khan N: Modulation of
hypoxia by magnetic nanoparticle hyperthermia to augment
therapeutic index. Adv Exp Med Biol. 812:87–95. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Brocato J, Chervona Y and Costa M:
Molecular responses to hypoxia-inducible factor 1α and beyond. Mol
Pharmacol. 85:651–657. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Mittal K, Ebos J and Rini B: Angiogenesis
and the tumor micro-environment: Vascular endothelial growth factor
and beyond. Semin Oncol. 41:235–251. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Shahneh FZ, Baradaran B, Zamani F and
Aghebati-Maleki L: Tumor angiogenesis and anti-angiogenic
therapies. Hum Antibodies. 22:15–19. 2013.PubMed/NCBI
|
|
33
|
Nikfarjam M, Muralidharan V and Christophi
C: Altered growth patterns of colorectal liver metastases after
thermal ablation. Surgery. 139:73–81. 2006. View Article : Google Scholar
|
|
34
|
Pati S, Orsi SA, Moore AN and Dash PK:
Intra-hippocampal administration of the VEGF receptor blocker
PTK787/ZK222584 impairs long-term memory. Brain Res. 1256:85–91.
2009. View Article : Google Scholar :
|
|
35
|
Selvarajah GT, Bonestroo FA, Kirpensteijn
J, Kik MJ, van der Zee R, van Eden W, Timmermans-Sprang EP, Slob A
and Mol JA: Heat shock protein expression analysis in canine
osteosarcoma reveals HSP60 as a potentially relevant therapeutic
target. Cell Stress Chaperones. 18:607–622. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Katschinski DM, Le L, Heinrich D, Wagner
KF, Hofer T, Schindler SG and Wenger RH: Heat induction of the
unphosphorylated form of hypoxia-inducible factor-1alpha is
dependent on heat shock protein-90 activity. J Biol Chem.
277:9262–9267. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Guo W, Yang Z, Xia Q, Liu J, Yu Y, Li J,
Zuo Z, Zhang D, Li X, Shi X, et al: Arsenite stabilizes HIF-1α
protein through p85α-mediated up-regulation of inducible Hsp70
protein expression. Cell Mol Life Sci. 68:475–488. 2011. View Article : Google Scholar
|
|
38
|
Bruns AF, Yuldasheva N, Latham AM, Bao L,
Pellet-Many C, Frankel P, Stephen SL, Howell GJ, Wheatcroft SB,
Kearney MT, et al: A heat-shock protein axis regulates VEGFR2
proteolysis, blood vessel development and repair. PLoS One.
7:e485392012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ahmed M, Liu Z, Lukyanov AN, Signoretti S,
Horkan C, Monsky WL, Torchilin VP and Goldberg SN: Combination
radiofrequency ablation with intratumoral liposomal doxorubicin:
Effect on drug accumulation and coagulation in multiple tissues and
tumor types in animals. Radiology. 235:469–477. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Veenendaal LM, van Hillegersberg R,
Smakman N, van der Bilt JD, van Diest PJ, Kranenburg O and Borel
Rinkes IH: Synergistic effect of interstitial laser coagulation and
doxorubicin in a murine tumor recurrence model of solitary
colorectal liver metastasis. Ann Surg Oncol. 13:168–175. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Isbert C, Ritz JP, Roggan A, Schuppan D,
Rühl M, Buhr HJ and Germer CT: Enhancement of the immune response
to residual intrahepatic tumor tissue by laser-induced
thermotherapy (LITT) compared to hepatic resection. Lasers Surg
Med. 35:284–292. 2004. View Article : Google Scholar : PubMed/NCBI
|