Introduction
Cytotoxic T lymphocyte antigen-4 (CTLA-4) is an
inhibitory molecule found on T cells. Immunotherapy by blocking
CTLA-4 can enhance T-cell activation and proliferation and improve
antitumor immune responses (1).
However, anti-CTLA-4 antibody therapy is not sufficient for the
treatment of metastatic malignant tumors (2), likely owing to the effects of the
tumor microenvironment (TME) (3).
The TME has a more stable genetic background and has been shown to
participate in regulating tumor immune escape and mediating the
sensitivity of tumor cells to anticancer drugs (4–7).
Matrix metalloproteinases (MMPs) are the primary factor regulating
the TME through degradation of the extracellular matrix and
promotion of tumor angiogenesis (8). Moreover, MMPs play a key role in
promoting the occurrence and development of cancer (9). However, the specific effects of
combined inhibition of CTLA-4 and MMPs in breast cancer are
unknown.
Therefore, in this study, we constructed a breast
cancer model in mice using a highly metastatic breast cancer cell
line. We used this model to evaluate the therapeutic effects of
anti-CTLA-4 antibody therapy alone or combination with an MMP
inhibitor (MMPI) and to explore the role of the TME in tumor
treatment.
Materials and methods
Murine mammary carcinoma cells and the
animal model
The 4T1 murine mammary carcinoma cell line harboring
the luciferase construct was provided by the Pathology Department
of the College of Basic Medical Sciences, Jilin University, China.
4T1 cells were cultured in RPMI-1640 medium (Gibco, USA)
supplemented with 10% fetal bovine serum (FBS; Hyclone, USA) and
incubated at 37°C in an atmosphere containing 5% CO2. In
the logarithmic growth phase, 4T1 cells were collected and diluted
to a concentration of 5×106 cells/ml with
phosphate-buffered saline (PBS). Next, 0.2 ml of the cell
suspension was injected subcutaneously into the right flanks of
6–8-week-old female BALB/c mice, provided by the Laboratory Animal
Center of the College of Basic Medical Sciences, Jilin University.
All procedures followed the animal care regulations of the
University Health Network and were approved by the Research Ethic
Board of Jilin University.
Treatment of tumor-bearing mice
Tumor nodules reached 50 mm3 on day 3.
Mice were randomly divided into four groups as follows: vehicle,
anti-CTLA-4 antibody alone (a-CTLA-4); MMPI alone, and combination
therapy (a-CTLA-4 plus MMPI). Mice were treated with 100 µg
anti-CTLA-4 antibody (clone, 9H10; BioXCell, West Lebanon, NH, USA)
by intraperitoneal (i.p.) injection once every two day and/or with
0.1 MMPI (1 mg/kg) by subcutaneous injection once every two days.
The MMPI potassium ferricyanide {K3[Fe(CN)6
(10,11), was kindly provided by Professor
Xuexun Fang (Key Laboratory for Molecular Enzymology and
Engineering of Ministry of Education, Jilin University, China).
Mice in the vehicle group were treated with an equal volume of
normal saline and PBS. The dosages used in the combined treatment
group were equal to those used in the monotherapy groups. The
longest (a) and shortest (b) diameters of the tumor were measured
with calipers every 3 days, and tumor volume was estimated by the
formula V = ab2/2. On days 7, 21 and 35, tumors were
measured using an Ultrasound Biomicroscopy InviVue instrument
(PanoView β1500; Taiwan).
Biophotonic imaging of animals
Mice were anesthetized by i.p. injection of 4%
chloral hydrate and then subjected to i.p. injection with 200
µl D-luciferin (15 mg/ml; GoldBio, St. Louis, MO, USA).
After 10 min, the mice were imaged using a small animal in
vivo optical imaging system (IVIS Spectrum; Caliper Life
Sciences, USA).
Hematoxylin and eosin (H&E)
staining
Mice were sacrificed. The lungs and livers were
immediately placed in Bouin's fixation and 10% formaldehyde. After
48 h, metastatic lesions on the surface of the lungs (faint yellow
in color) were counted without the use of a microscope. The lungs
and livers of each mouse were then embedded in paraffin, sectioned,
stained with H&E staining, and examined histologically for
evidence of metastatic lesions within the lung and liver tissue.
For each lung and liver, three consecutive sections, separated by
200 µm, were collected. The sections was randomized and
coded, and the total number of metastatic foci was counted.
Functional tests for the liver and
kidneys
Peripheral blood was obtained from each mouse and
centrifuged at 500 × g for 20 min after standing at room
temperature for 20 min. The supernatant was collected as serum.
Total protein, serum albumin, serum globulin, albumin/globulin,
alanine aminotransferase (ALT), aspartate aminotransferase (AST),
ALT/AST, alkaline phosphatase, urea, creatinine, and uric acid in
serum were measured using an automatic biochemical analyzer
(Beckman Coulter, USA).
Flow cytometry analysis
The spleens of mice were placed in cold RPMI-1640
medium immediately and then cut into small pieces with eye
scissors. Single-cell suspensions of spleen tissues were acquired
after filtration with 300- and 100-mesh filters. Single-cell
suspensions of bone marrow tissues were obtained from the thigh
bones of mice after being washed with cold PBS. The red blood cells
were then disrupted within the cell suspension. For treatment,
2×106 cells were stimulated with phorbol 12-myristate
13-acetate (PMA; Sigma, St. Louis, MO, USA; 50 ng/ml) plus 2
µg/ml ionomycin (Sigma) and monensin (Sigma; 5 µg/ml)
for 5 h. The cells were then collected for Th17 cytokine staining.
Subsequently, they were fixed and/or permeabilized using
fixation/permeabilization kits (BD, USA) according to the
manufacturer's instructions, and incubated with anti-interleukin
(IL)-17A-Alexa Fluor (BD), anti-CD4-PE (BD), anti-CD4-APC (BD),
anti-CD25-PE-CyTM7 (BD), anti-FoxP3-PE (eBioscience, San Diego, CA,
USA), anti-CD3e-FITC (BD), anti-CD4-PE-CyTM7 (BD),
anti-CD8-PE (BD), anti-CD11b-APC (BD), and anti-Gr-1-PE (BD)
antibodies for 40 min at 4°C. The cells were incubated with rat
anti-mouse CD16/CD32 (BD) for 30 min before CD11b and Gr-1 staining
at 4°C. Flow cytometry (BD) was performed, and data were analyzed
using FlowJo software.
Immunohistochemistry analysis
For immunohistochemical analysis of microvessel
density (MVD), 2-µm-thick sections of formalin-fixed,
paraffin-embedded mammary tumor tissue sections were deparaffinized
in xylene and rehydrated in graded alcohols. For antigen retrieval,
slides were immersed and boiled for 5 min in citrate buffer (pH
6.0). Slides were incubated with a peroxidase inhibitor and horse
serum blocking solution for 30 min and then subjected to overnight
staining with rat anti-mouse CD34 primary monoclonal antibodies
(1:100 dilution; ab81289; Abcam, USA) at 4°C. Slides were then
washed three times with PBS-T, incubated with horse anti-rat
secondary antibodies for 30 min at room temperature, washed again
with PBS-T three times, and incubated in DAB peroxide substrate
solution. Counterstaining was performed with Harris hematoxylin
counterstain. MVD was determined as the mean number of microvessels
under five microscopic fields (200×).
Immunofluorescence analysis
Immunofluorescence staining of slides was performed
as described for immunohistochemistry until antigen retrieval.
Then, slides were treated with or without 1% Triton X-100 for 20
min, followed by blocking with 5% bovine serum albumin (BSA) for 1
h at room temperature. Subsequently, slides were incubated with
anti-CD3e-FITC (BD), anti-CD8a-PE (BD), anti-CD4-PE (BD),
anti-CD11b-FITC (BD), anti-Gr-1-PE (BD), anti-Foxp3-PE (BD), and
anti-IL-17A-Alexa Fluor 647 (BD) for 30 min at room temperature.
Slides were then washed three times with PBS-T, incubated with
Hoechst-33342 for 5 min at room temperature, washed again with
PBS-T three times, and sealed with glycerin.
Statistical analysis
All values were expressed as means ± standard
deviations (SDs). All statistical analyses were performed using
Student's t-tests, non-parametric tests, or Spearman correlation
analyses. Differences or correlations with P-values of ≤0.05 were
considered significant.
Results
Addition of the MMPI enhances the
inhibitory effects of the anti-CTLA-4 antibody on breast cancer
growth
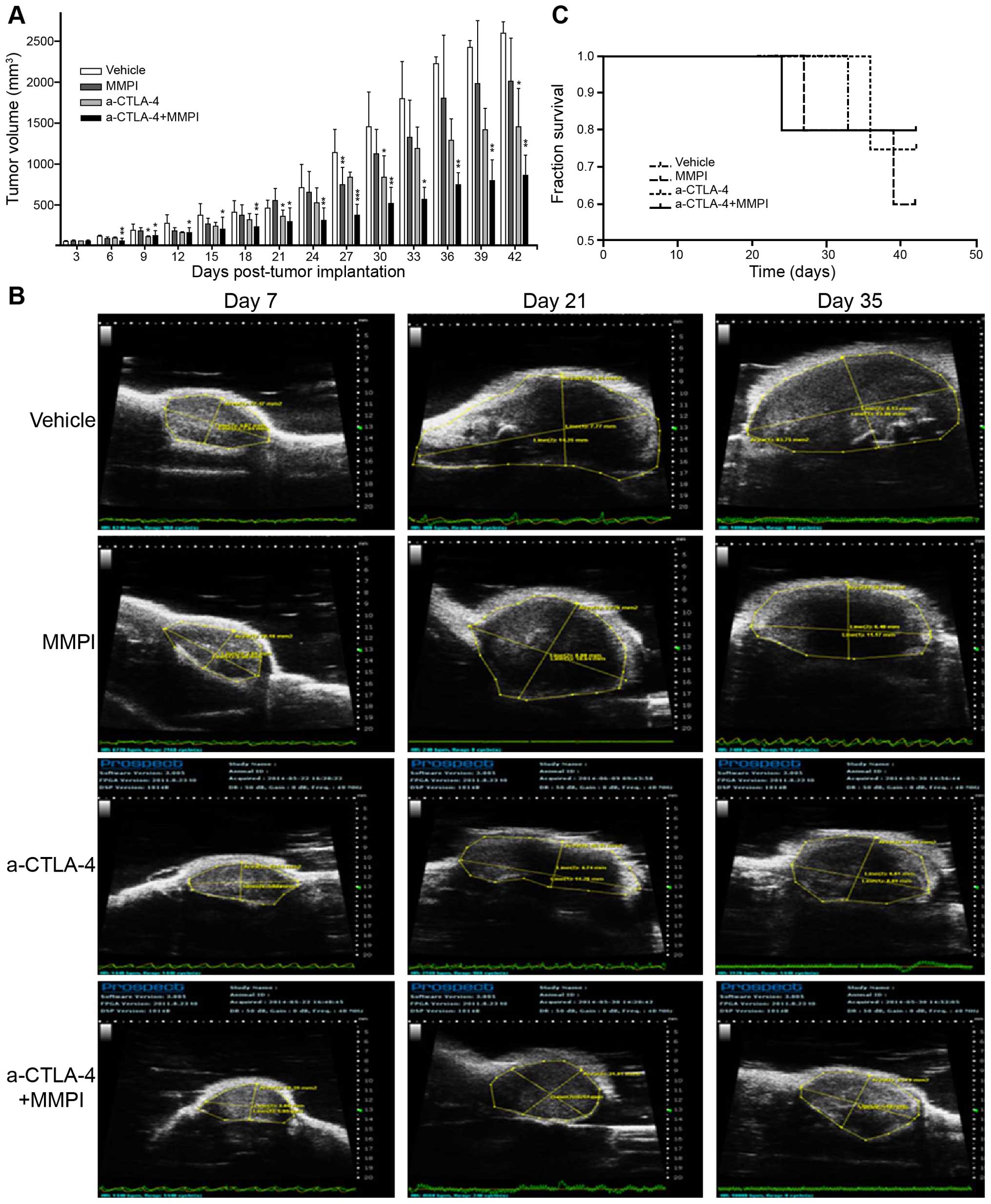
First, we established a breast cancer model by
subcutaneous inoculation of 4T1 cells in order to easily monitor
tumor growth using ultrasound and calipers. By day 3, tumor nodules
reached ~50 mm3; therefore, treatments, including
anti-CTLA-4 antibody and MMPI, were applied beginning on day 3. Our
results demonstrated that treatment with the MMPI or MMPI plus
anti-CTLA-4 antibody inhibited the growth of transplanted tumors
compared with vehicle or anti-CTLA-4 antibody alone to varying
degrees. Indeed, according to analysis of the total days, combined
treatment caused a significant reduction in the growth of breast
tumors compared with that in vehicle-treated mice (P<0.05;
Fig. 1A). Additionally,
ultrasonography on days 7, 21 and 35 revealed that tumors in mice
treated with anti-CTLA-4 antibody and MMPI were significantly
smaller than those in mice treated with vehicle, MMPI alone, or
anti-CTLA-4 antibody alone (Fig.
1B). However, the combination treatment had no effect on the
survival of tumor-bearing mice (P>0.05; Fig. 1C).
Treatment with the MMPI enhances the
effects of the anti-CTLA-4 antibody on breast cancer
metastasis
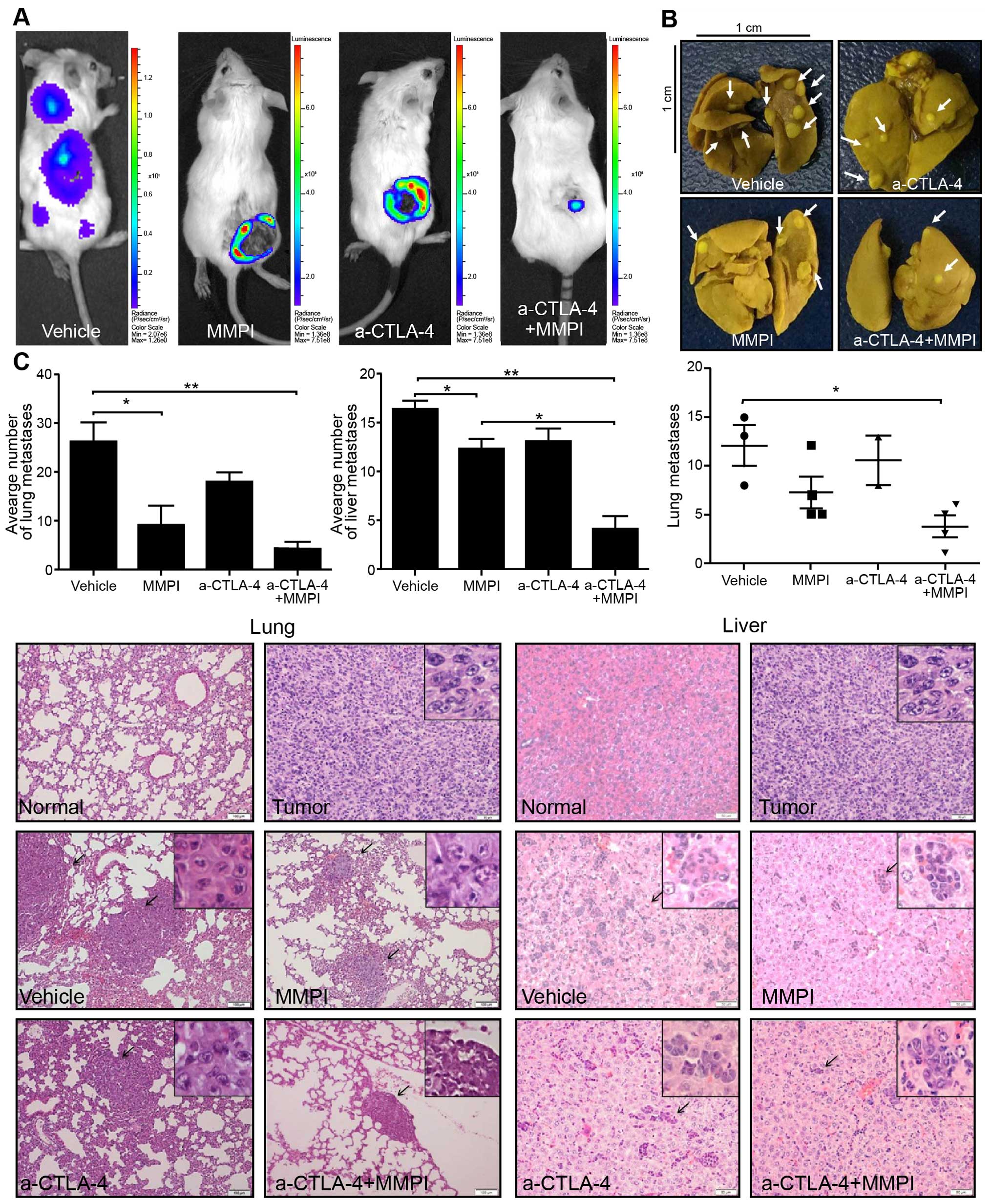
In order to visualize tumor metastasis in live mice,
we constructed a mouse model of breast cancer using 4T1 cells
stably expressing luciferase. D-luciferin was applied on day 42 for
detection of tumor metastasis using a small animal in vivo
optical imaging system. The results showed that the mice in the
vehicle group had metastatic lesions, whereas the mice in the other
groups had no metastatic lesions (Fig.
2A).
Observation of fixed lungs showed that the number of
tumor metastases on the lung surface was significantly reduced
after combined treatment (P<0.05; Fig. 2B). However, there was no significant
reduction after treatment with anti-CTLA-4 antibody alone or MMPI
alone (P>0.05; Fig. 2B).
Additionally, analysis of paraffin-embedded lung and liver sections
stained with H&E revealed that both tissues had reduced numbers
of metastases after the combined treatment (P<0.001; Fig. 2C). However, there was no significant
difference in the number of metastases between the anti-CTLA-4
antibody group and vehicle group (Fig.
2C).
Effects of the MMPI and anti-CTLA-4
antibody on the function of the liver and kidney
MMPIs have been reported to have toxic effects.
Therefore, in this study, we examined changes in liver and kidney
function in mice after treatment with the MMPI. The results showed
that the liver and kidney functions of tumor-bearing mice treated
with the MMPI did not differ from those of control mice (P>0.05;
Fig. 3), indicating that the MMPI
used in this study did not damage the liver or kidneys of mice.
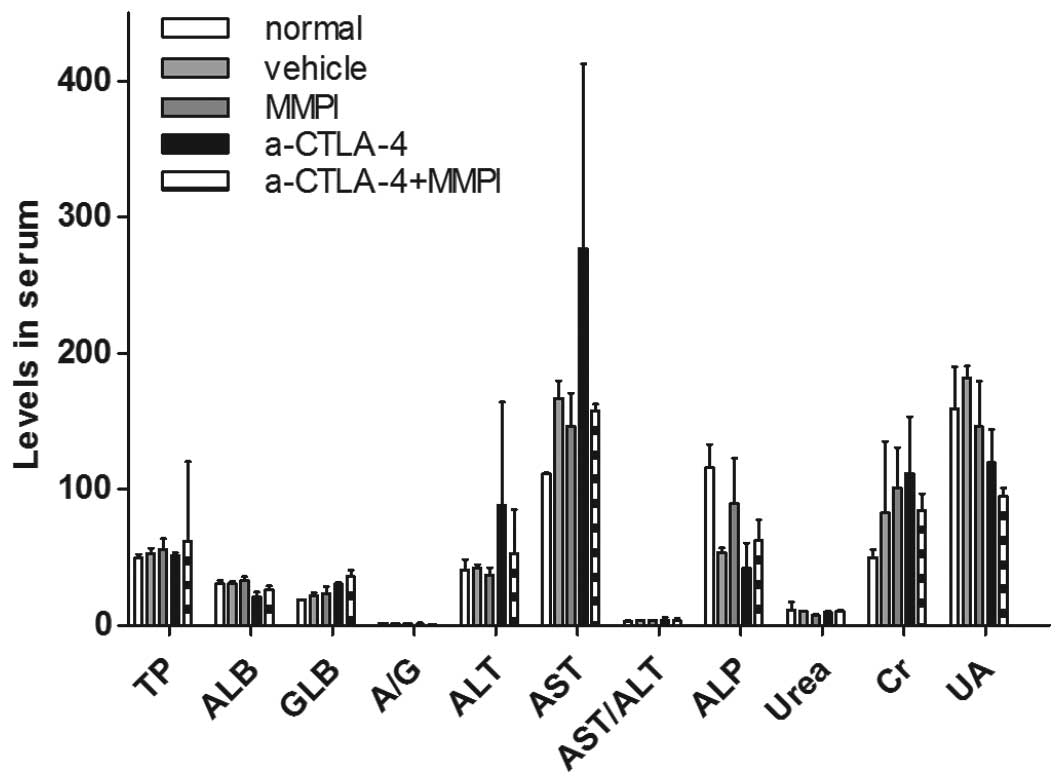 | Figure 3Functional analysis of the liver and
kidneys after treatment with the MMP inhibitor and anti-CTLA-4
antibody. Blood serum was obtained from tumor-bearing mice after
treatment and normal 6–8-week-old female BALB/c mice (n=3). Total
protein (TP, g/l), serum albumin (ALB, g/l), serum globulin (GLB,
g/l), A/G, alanine aminotransferase (ALT, U/l), aspartate
aminotransferase (AST, U/l), ALT/AST and alkaline phosphatase (ALP,
U/l) were measured using an automatic biochemical analyzer to
investigate the function of the livers. Urea (mM/l), creatinine
(Cr, µM/l), and uric acid (UA, µM/l) were measured to
determine the function of the kidneys. The average of each index
was determined, and the value for each treatment group was compared
with that of the normal group (Student's t-test, nonparametric
test). |
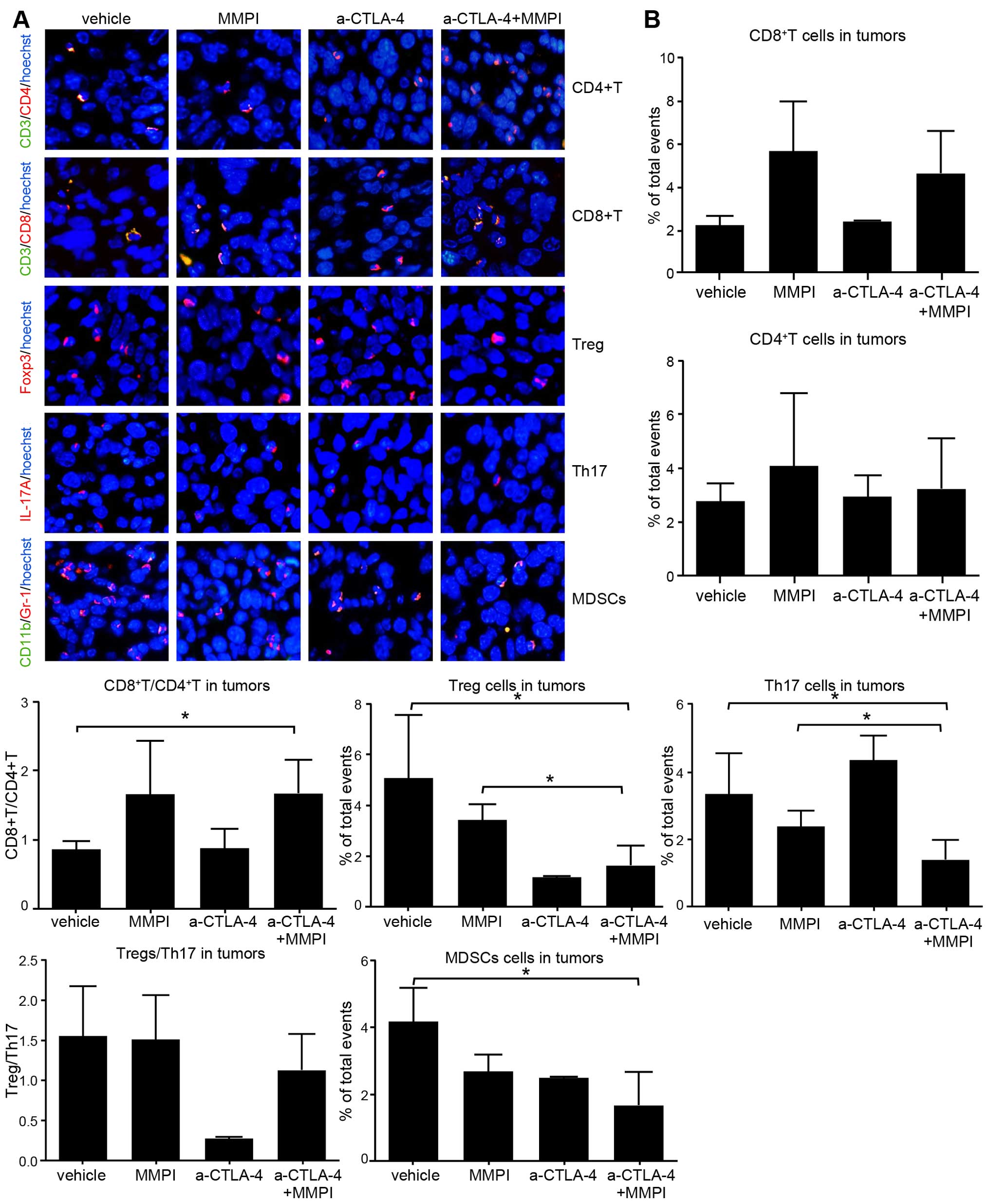
Treatment with the MMPI and anti-CTLA-4
antibody improves the immune microenvironment in mice
Our data showed that MMPI could enhance the
therapeutic effects of anti-CTLA 4 antibodies in a model of breast
cancer in mice. The mechanism through which the anti-CTLA-4
antibody improves the antitumor immune response involves prompt
T-cell activation and proliferation. Therefore, in order to
determine whether the MMPI affected the immune microenvironment of
tumor-bearing mice to enhance the therapeutic effects of the
anti-CTLA-4 antibody, we used flow cytometry to measure changes in
the percentages of CD4+ T cells, CD8+ T
cells, Tregs, Th17 cells, and MDSCs in the spleens of mice and to
evaluate changes of MDSCs in the bone marrow. The results showed
that there were no significant differences in the number of
CD4+ and CD8+ T cells between the groups
(Fig. 4). However, the ratio of
CD8+ T cells to CD4+ T cells was
significantly increased after MMPI treatment (P<0.05; Fig. 4). Moreover, combined treatment
caused significant decreases in Tregs and the Treg/Th17 ratio in
mouse spleens (P<0.01; Fig. 4),
and the percentage of MDSCs in spleens and the bone marrow was
significantly reduced after combination treatment (P<0.01;
Fig. 4).
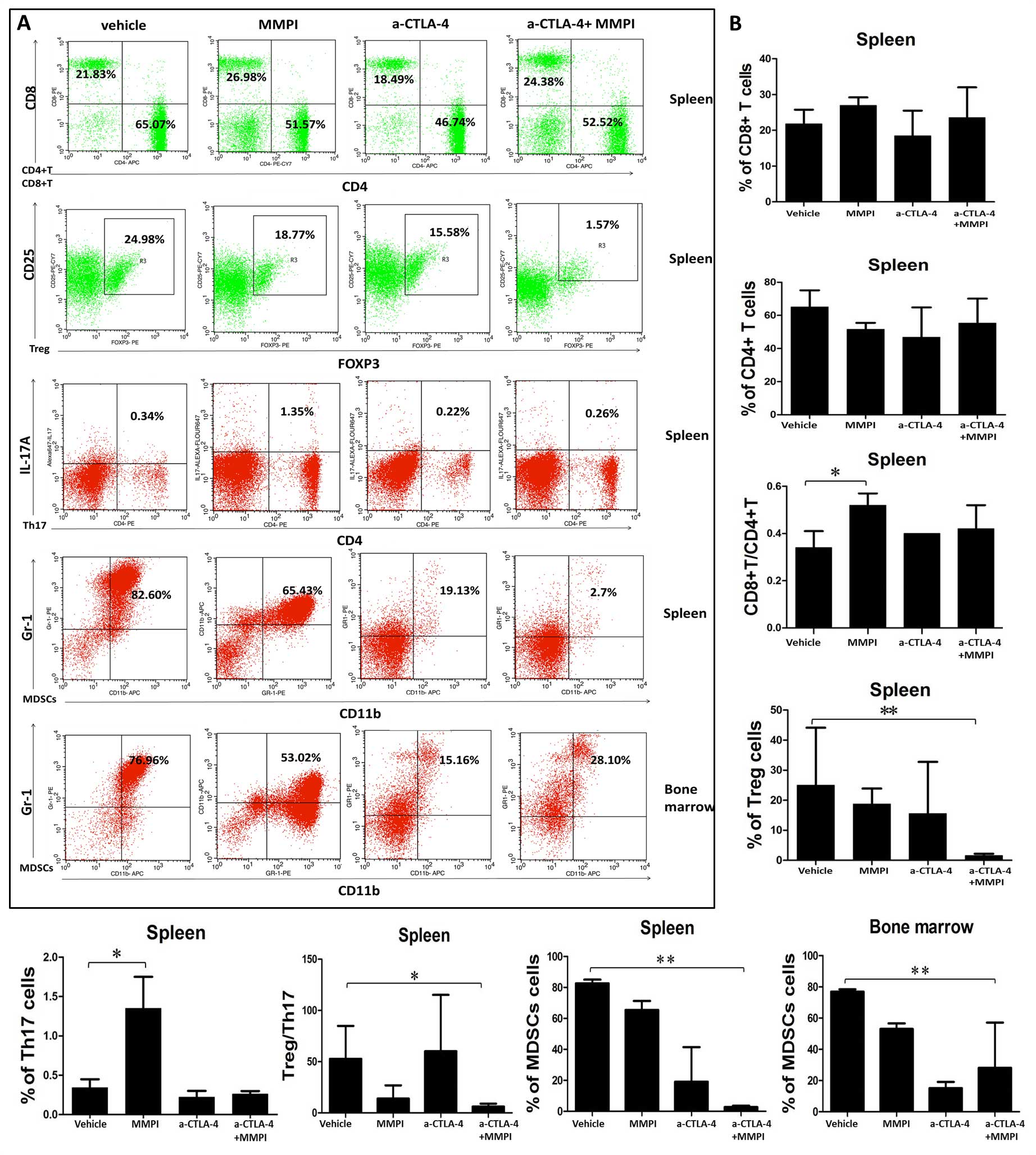 | Figure 4Analysis of immune cells in the
spleen and bone marrow after treatment with the MMP inhibitor and
anti-CTLA-4 antibody. (A) Immune cells obtained from spleen and
bone marrow were, respectively, analyzed using flow cytometry. CD4,
CD8, Treg, Th17, and MDSCs were stained with CD3/CD4, CD3/CD8,
CD4/CD25/FOXP3, CD4/IL-17A, and CD11b/Gr-1. MDSCs were blocked with
CD16/CD32 before staining. Statistical significance was determined
using two-sample Student's t-tests. *P<0.05;
**P<0.01; ***P<0.001. |
Thus, taken together, these data suggested that the
anticancer mechanism of the MMPI may be related to the increased
proportion of CD8+ T cells, relieving immune suppression
and enhancing the antitumor function of the immune system.
Treatment with the MMPI and anti-CTLA-4
antibody improves the TME
The primary target of MMPIs is the TME, which is
generally enriched in immunosuppressive cells. Thus, we examined
the infiltration of immune cells into tumor tissues using
immunofluorescence. The results showed that while there were no
obvious increases in the percentage of CD4+ and
CD8+ T cells in the TME, the ratio of CD8+ T
cells to CD4+ T cells was increased after MMPI treatment
and after combined treatment as compared with that in
vehicle-treated mice (P<0.05; Fig.
5). Moreover, the percentages of Tregs, Th17 cells, and MDSCs
in the tumors were significantly decreased after combined treatment
(P<0.05 for Tregs and Th17 and P<0.01 for MDSCs; Fig. 5). However, the Treg/Th17 ratio did
not differ significantly compared with that in vehicle-treated
mice. Therefore, these data showed that immunosuppression of the
TME was improved to a certain extent after combination treatment
with the MMPI and anti-CTLA-4 antibody.
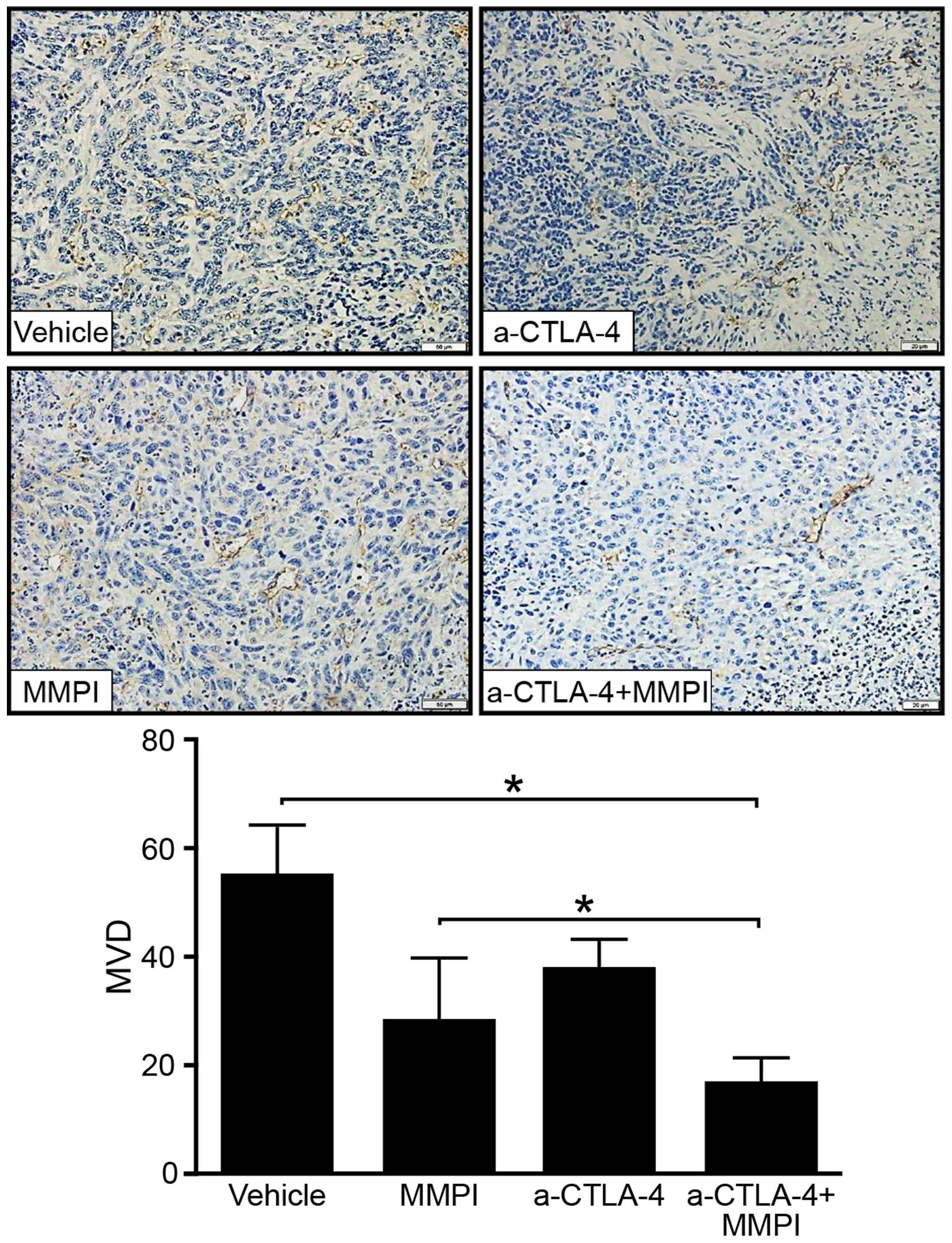
Combined treatment with the MMPI and
anti-CTLA-4 antibody reduced the MVD within the TME
Tumor vessels are important elements of the TME, and
MVD within the tumor is often associated with tumor progression. We
selected paraffin-embedded sections of tumors, stained them with
anti-CD34 antibodies to mark tumor stromal vascular endothelial
cells, and determined the MVD. Immunohistochemical staining showed
that the MVD was higher in tumors from vehicle-treated mice than in
tumors from mice treated with the MMPI and anti-CTLA-4 antibody
(P<0.01; Fig. 6). Thus, the MMPI
may reduce immunosuppression and inhibit neovascularization in the
TME.
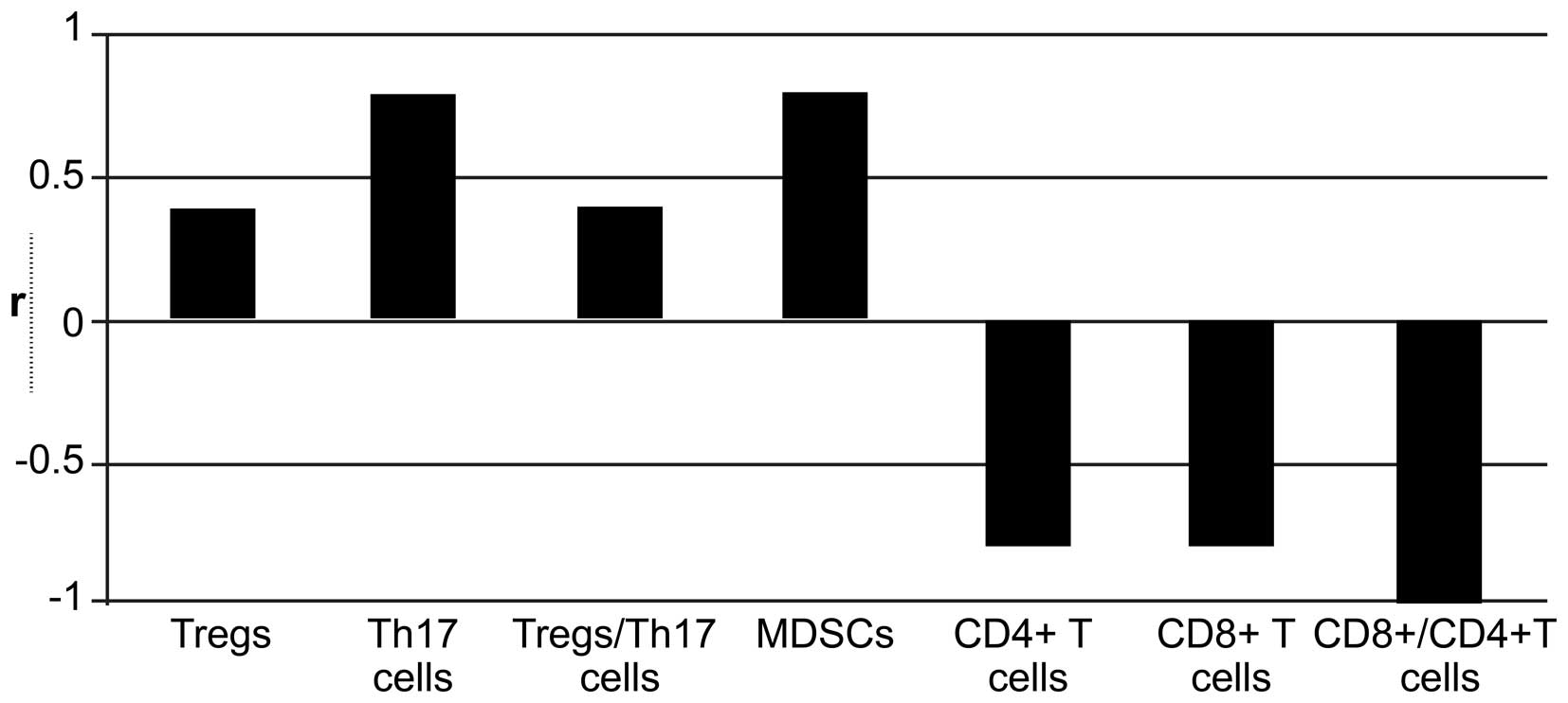
Correlation between immune cell and MVD
within the TME
Immune cells are associated with angiogenesis in the
tumor stroma (12). Therefore, we
next analyzed the relationship between infiltration of immune cells
and MVD within the tumor tissue. Spearman correlation analyses
showed that CD8+ T cells, CD4+ T cells, and
the CD8+/CD4+ T cell ratio were negatively
correlated with MVD in tumor tissues (correlation coefficients:
−0.800, −0.800, and −0.100, respectively; P>0.05), whereas
Tregs, the Treg/Th17 ratio, Th17 cells, and MDSCs were positively
correlated with MVD (correlation coefficients: 0.400, 0.400, 0.800
and 0.800); the correlation between MDSCs and MVD was statistically
significant (P<0.05; Fig.
7).
Discussion
In this study, we examined the therapeutic effects
of combined treatment with an MMPI and anti-CTLA-4 antibody in a
breast cancer model in mice. Our data demonstrated that addition of
MMPI enhanced the effects of anti-CTLA-4 antibody treatment in a
mouse model of breast cancer by delaying tumor growth and reducing
metastases. These results may have important implications in the
development of novel therapeutic strategies for the treatment of
breast cancer.
Murine breast cancer 4T1 cell can spontaneously
metastasize to other organs after subcutaneous injection into
female BALB/c mice. This process is similar to the occurrence and
development of human breast cancer; therefore, transplantation of
4T1 cells into mice is considered an ideal model for studying human
breast cancer (13). The
application of anti-CTLA-4 antibodies alone is ineffective for the
treatment of breast cancer in mice (14). Therefore, successful immunotherapy
using anti-CTLA-4 antibodies would require identification of the
appropriate combination therapy. Recent studies have shown that the
TME mediates immune escape and regulates the sensitivity of tumors
to anticancer drugs; therefore, targeting of the TME may represent
a novel method for the treatment of malignant tumors.
MMPs play an important role in tumor progression by
regulating the TME. MMPs promote angiogenesis, tumor growth, and
tumor spread through the degradation of extracellular matrix,
thereby changing the adhesion between cells, promoting cell
movement, and regulating the immune response of the tumor (15). Based on the overexpression of MMPs
in tumor tissues and the overactivation of MMPs observed in tumors
(16), application of an MMPI could
block the activity of MMPs, improve the TME, and inhibit tumor
growth (17). Indeed, many animal
experiments and clinical trials have shown that MMPIs have
different degrees of inhibitory effects on tumor growth but are
often accompanied by obvious side effects (18,19).
Therefore, identification of an MMPI with low toxicity and high
efficacy is necessary. In the present study, we used the MMPI
potassium ferricyanide, a member of a class of inorganic compounds
identified through enzyme kinetics experiments in a previous study.
This compound exhibits low toxicity, has weak stimulation in the
skin and eyes, and produces toxic gases only when exposed the
strong acids. Potassium ferricyanide specifically inhibits MMP-14,
MMP-2, and MMP-13 through non-competitive inhibition. Our results
of combination therapy with the MMPI (potassium ferricyanide) and
anti-CTLA-4 antibody showed that anti-CTLA-4 antibody alone
exhibited poor efficacy, consistent with a study by Demaria et
al (14), combined treatment
with the MMPI and anti-CTLA-4 antibody significantly inhibited the
growth and metastasis of breast cancer. Therefore, inhibition of
MMPs may improve the therapeutic effects of the anti-CTLA-4
antibody.
Tumor cells are often able to evade immune
surveillance and induction of immune tolerance and are thought to
be associated with various types of immune cells, including MDSCs,
Tregs, and Th17 cells (20–22). The antitumor immune response is
markedly suppressed by immunosuppressive cells within the body;
these cells tend to be found in tumors after treatment with an
immunological stimulus, facilitating the development of a
microenvironment promoting tumor immunosuppression (23). Tregs play a role in
immunosuppression mainly through CTLA-4 on the cell surface
(24). MDSCs can produce arginine
to inhibit T-cell function and can secrete transforming growth
factor (TGF)-β and IL-10 to inhibit T-cell activation (25). Our data suggested that the
percentage of Tregs and MDSCs was reduced in spleens and tumors
after the combination treatment. Tregs are primarily developed from
naïve CD4+ T cells, and TGF-β is the key factor involved
in Treg development; indeed, TGF-β can induce the expression of
Foxp3, a transcription factor that facilitates the transformation
of naïve CD4+ T cells into Tregs (26). MMP-14 induces TGF-β1 protein
expression (27); therefore, MMPI
may regulate the expression of TGF-β, thus reducing the number of
Tregs (28). Additionally, the
growth of MDSCs, a large group of naïve cells derived from the bone
marrow, can be inhibited by MMP-2 through upregulation of a number
of immune suppressor genes, including IL-10, IL-14, IL-11, and
chemokine ligand CCL-5 (29).
Because the MMPI used in our study could inhibit the activity of
MMP-2, we expect that the MMPI may have inhibited the growth of
MDSCs, thereby reducing MDSC numbers. Our data also suggested that
MDSCs in bone marrow was declined after combined treatment. It was
reported that TGF-β signaling pathway is an important factor in
regulation of bone marrow-derived MDSCs (28,30).
Thus, the inhibition of MMPs may have influence on mature MDSCs.
Exosomes may be another factor by which tumor exosomes can switch
the differentiation pathway of myeloid cells to the MDSC pathway
and of which tumor exosomes will reduce after treatment (31).
Blood vessels in tumors are an important part of the
TME and can support the growth of tumors, promote the spread of the
tumor cells, and regulate the TME. The tumor vasculature is
abnormal, exhibiting circuity and expansion in structure,
morphological and structural abnormalities in perithelial cells
that are loosely connection or even absent, an incomplete basement
membrane, and increased MVD (33).
The abnormal blood vessel formation can create an abnormal TME,
further affecting the proliferation, invasion, survival, and
function of immune cells (33,34).
Therefore, the TME is generally characterized by a lack of
antitumor immune cells and the accumulation of immunosuppressive
cells. Additionally, sustained low concentrations of
anti-angiogenic agents can reduce the tumor vascular density,
reshape the abnormal blood vessels in the TME, balance vascular
perfusion, increase the effects of T-cell infiltration, and improve
the immunosuppressive effects of the TME (35,36).
Our data showed that the anti-CTLA-4 antibody alone
could not effectively reduce the MVD in tumors; however, combined
treatment with the MMPI and anti-CTLA-4 antibody significantly
reduced the MVD in tumor tissues. MMPs can promote angiogenesis by
degrading the vascular basement membrane, thereby regulating
angiogenic factors. MMP-14 plays an important role within the TME
and affects angiogenesis by regulating the expression of vascular
endothelial growth factor (VEGF) and the biological activities of
TGF-β (37). Therefore, the MMPI
used in this study may inhibit the angiogenesis-promoting effects
of MMPs, thereby reducing the MVD in the TME. In addition, immune
cells, particularly immunosuppressive cells, regulate angiogenesis
in the TME. Our data suggested that Tregs, Th17 cells, and MDSCs
were positively correlated with MVD in the TME; these cells may
promote tumor angiogenesis (12).
Moreover, hypoxia can promote the infiltration of Tregs into the
TME by mediating VEGF-A expression, subsequently promoting
endothelial cell recruitment and amplification (38). In ovarian cancer, Tregs are an
important source of VEGF in the TME, and removal of Tregs can
effectively reduce the production of VEGF. Additionally, Tregs are
required for angiogenesis in lung tissue (39). In an alternatively pathway, MDSCs
stimulate the activation of signal transducer and activator of
transcription 3 (STAT3) in tumor cells via secretion of IL-28 and
induction of tumor-associated factors. The tumor cells then promote
the formation of tubular structures by endothelial cells (40). Importantly, MDSCs are factors
involved in mediating the effects of anti-angiogenic therapy in
cancer (41). Angiogenesis is also
affected by the expression of IL-17A, which is secreted by Th17
cells and is positively associated with the MVD in gastric cancer,
liver cancer, and breast cancer (42). IL-17A indirectly participates in
angiogenesis by promoting the secretion of VEGF, prostaglandin E2
(PGE2), and chemotactic factors from tumor cells. IL-17A can also
directly affect endothelial cells and endothelial progenitor cells
to promote angiogenesis (43).
Therefore, the observed reduction in Tregs, Th17 cells, and MDSCs
in response to the MMPI in our study may explain the reduction in
the MVD. Reducing the MVD through application of the MMPI could
also further inhibit immune cell infiltration.
In a recent study in mice, inhibition of MMP-14
improved tumor blood perfusion and oxygen supply by increasing the
expression of inducible nitric oxide synthase (iNOS) and heat-shock
protein 90 (HSP90) and also resulted in bending of the blood
vessels within the tumor (44).
Vascular remodeling can improve the abnormal state of tumor blood
vessels, enhance vascular perfusion, and increase drug delivery. In
addition to MMP-14, MMP-2 plays an important role in promoting
tumor vascular remodeling (45).
Moreover, MMPI has been shown to enhance the therapeutic effects of
chemotherapy drugs on tumors by promoting remodeling of the tumor
vasculature and increasing drug delivery (46). Thus, the MMPI we used may promote
tumor vascular remodeling to a certain extent.
CD4+ and CD8+ T cells are the
main effector cells of antitumor immune responses and can combine
with tumor associated antigens, MHC-I and MHC-II molecules to
induce tumor cells death through secretion of cytokines and other
cytotoxic effects. Thus, the number of CD4+ and
CD8+ T cells can reflect the antitumor immunity of
tumor-bearing mice. Unexpectedly, we did not see an increase in the
number of CD4+ and CD8+ T cells after
treatment. However, the ratio of CD8+ T cells to
CD4+ T cells (CD8+/CD4+ T cells)
was increased in tumor after combined treatment and in spleen after
MMPI treatment. The different cell sources may be the cause for the
different results. The characteristics of 4T1 cells themselves and
the reduction in Tregs, a type of CD4+ T cell, may
explain this unexpected result.
In conclusion, our data showed that the MMPI used in
this study inhibited angiogenesis in the TME and suppressed the
infiltration of immunosuppressive cells. Therefore, the MMPI may
regulate blood vessels and immunosuppressive cells, together or
separately, within the TME, thereby making the TME less favorable
for tumor growth and enhancing the anticancer effects of
anti-CTLA-4 antibody. Notably, tumor neovascularization and tumor
infiltrating immune cells are two main components in TME. The main
target of CTLA-4 antibody and MMPI is the immune cells and blood
vessels, respectively. Thus, we only examined the two main factors
most relevant to tumor progression in the TME. There may be other
factors related to these processes that have not yet been
identified. Further studies are needed to determine the functions
of immunosuppressive cells regulating blood vessels in the TME.
Acknowledgments
We thank Professor Xuexun Fang (Key Laboratory for
Molecular Enzymology and Engineering of Ministry of Education,
Jilin University, China) for providing the MMPI potassium
ferricyanide {K3[Fe(CN)6. This study was
supported by the Jilin Provincial Science and Technology Projects
(grant no. 20130102084JC) and the Frontier Interdisciplinary
Program of Norman Bethune Health Science Center of Jilin University
(grant no. 2013101005).
Abbreviations:
|
MMP
|
matrix metalloproteinase
|
|
MMPI
|
matrix metalloproteinase inhibitor
|
|
CTLA-4
|
cytotoxic T lymphocyte antigen-4
|
|
TME
|
tumor microenvironment
|
|
Tregs
|
regulatory T cells
|
|
MDSCs
|
myeloid-derived suppressor cells
|
|
MVD
|
microvessel density
|
|
ALT
|
alanine aminotransferase
|
|
AST
|
aspartate aminotransferase
|
|
IL
|
interleukin
|
|
BSA
|
bovine serum albumin
|
|
VEGF
|
vascular endothelial growth factor
|
|
TGF
|
transforming growth factor
|
|
STAT3
|
signal transducer and activator of
transcription
|
|
PGE2
|
prostaglandin E2
|
References
|
1
|
Lotem M, Merims S, Frank S, Ospovat I and
Peretz T: Ctla-4 blockade: A new hope for the immunotherapy of
malignant melanoma. Harefuah. 151:585–588. 6042012.In Hebrew.
|
|
2
|
Prieto PA, Yang JC, Sherry RM, Hughes MS,
Kammula US, White DE, Levy CL, Rosenberg SA and Phan GQ: CTLA-4
blockade with ipilimumab: Long-term follow-up of 177 patients with
metastatic melanoma. Clin Cancer Res. 18:2039–2047. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
McAllister SS and Weinberg RA: The
tumour-induced systemic environment as a critical regulator of
cancer progression and metastasis. Nat Cell Biol. 16:717–727. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Baginska J, Viry E, Paggetti J, Medves S,
Berchem G, Moussay E and Janji B: The critical role of the tumor
microenvironment in shaping natural killer cell-mediated anti-tumor
immunity. Front Immunol. 4:4902013. View Article : Google Scholar
|
|
5
|
Whiteside TL: Induced regulatory T cells
in inhibitory microenvironments created by cancer. Expert Opin Biol
Ther. 14:1411–1425. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Borriello L and DeClerck YA: Tumor
microenvironment and therapeutic resistance process. Med Sci
(Paris). 30:445–451. 2014.In French. View Article : Google Scholar
|
|
7
|
Hida K, Akiyama K, Ohga N, Maishi N and
Hida Y: Tumour endothelial cells acquire drug resistance in a
tumour microenvironment. J Biochem. 153:243–249. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kessenbrock K, Plaks V and Werb Z: Matrix
metalloproteinases: Regulators of the tumor microenvironment. Cell.
141:52–67. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shuman Moss LA, Jensen-Taubman S and
Stetler-Stevenson WG: Matrix metalloproteinases: Changing roles in
tumor progression and metastasis. Am J Pathol. 181:1895–1899. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Thomas D, Ritz MF, Malviya AN and Gaillard
S: Intracellular acidification mediates the proliferative response
of PC12 cells induced by potassium ferricyanide and involves MAP
kinase activation. Int J Cancer. 68:547–552. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Martinus RD, Linnane AW and Nagley P:
Growth of rho 0 human Namalwa cells lacking oxidative
phosphorylation can be sustained by redox compounds potassium
ferricyanide or coenzyme Q10 putatively acting through the plasma
membrane oxidase. Biochem Mol Biol Int. 31:997–1005.
1993.PubMed/NCBI
|
|
12
|
Stockmann C, Schadendorf D, Klose R and
Helfrich I: The impact of the immune system on tumor: Angiogenesis
and vascular remodeling. Front Oncol. 4:692014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tao K, Fang M, Alroy J and Sahagian GG:
Imagable 4T1 model for the study of late stage breast cancer. BMC
Cancer. 8:2282008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Demaria S, Kawashima N, Yang AM, Devitt
ML, Babb JS, Allison JP and Formenti SC: Immune-mediated inhibition
of metastases after treatment with local radiation and CTLA-4
blockade in a mouse model of breast cancer. Clin Cancer Res.
11:728–734. 2005.PubMed/NCBI
|
|
15
|
Fink K and Boratyński J: The role of
metalloproteinases in modification of extracellular matrix in
invasive tumor growth, metastasis and angiogenesis. Postepy Hig Med
Dosw Online. 66:609–628. 2012.In Polish. View Article : Google Scholar
|
|
16
|
Benson CS, Babu SD, Radhakrishna S,
Selvamurugan N and Ravi Sankar B: Expression of matrix
metalloproteinases in human breast cancer tissues. Dis Markers.
34:395–405. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Overall CM and Kleifeld O: Tumour
microenvironment - opinion: Validating matrix metalloproteinases as
drug targets and anti-targets for cancer therapy. Nat Rev Cancer.
6:227–239. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li NG, Tang YP, Duan JA and Shi ZH: Matrix
metalloproteinase inhibitors: A patent review (2011–2013). Expert
Opin Ther Pat. 24:1039–1052. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li W, Saji S, Sato F, Noda M and Toi M:
Potential clinical applications of matrix metalloproteinase
inhibitors and their future prospects. Int J Biol Markers.
28:117–130. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Srivastava MK, Zhu L, Harris-White M,
Huang M, St John M, Lee JM, Salgia R, Cameron RB, Strieter R,
Dubinett S, et al: Targeting myeloid-derived suppressor cells
augments antitumor activity against lung cancer. Immunotargets
Ther. 2012:7–12. 2012.PubMed/NCBI
|
|
21
|
Radosavljević GD, Jovanović IP, Kanjevac
TV and Arsenijević NN: The role of regulatory T cells in the
modulation of anti-tumor immune response. Srp Arh Celok Lek.
141:262–267. 2013.In Serbian. View Article : Google Scholar
|
|
22
|
Prabhala RH, Pelluru D, Fulciniti M,
Prabhala HK, Nanjappa P, Song W, Pai C, Amin S, Tai YT, Richardson
PG, et al: Elevated IL-17 produced by TH17 cells promotes myeloma
cell growth and inhibits immune function in multiple myeloma.
Blood. 115:5385–5392. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Becker JC, Andersen MH, Schrama D and Thor
Straten P: Immune-suppressive properties of the tumor
microenvironment. Cancer Immunol Immunother. 62:1137–1148. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Walker LS: Treg and CTLA-4: Two
intertwining pathways to immune tolerance. J Autoimmun. 45:49–57.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nagaraj S, Youn JI and Gabrilovich DI:
Reciprocal relationship between myeloid-derived suppressor cells
and T cells. J Immunol. 191:17–23. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li Z, Li D, Tsun A and Li B:
FOXP3+ regulatory T cells and their functional
regulation. Cell Mol Immunol. 12:558–565. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kumar S, Pan CC, Bloodworth JC, Nixon AB,
Theuer C, Hoyt DG and Lee NY: Antibody-directed coupling of
endoglin and MMP-14 is a key mechanism for endoglin shedding and
deregulation of TGF-β signaling. Oncogene. 33:3970–3979. 2014.
View Article : Google Scholar :
|
|
28
|
Krstic J and Santibanez JF: Transforming
growth factor-beta and matrix metalloproteinases: Functional
interactions in tumor stroma-infiltrating myeloid cells. Sci World
J. 2014:5217542014. View Article : Google Scholar
|
|
29
|
Guedez L, Jensen-Taubman S, Bourboulia D,
Kwityn CJ, Wei B, Caterina J and Stetler-Stevenson WG: TIMP-2
targets tumor-associated myeloid suppressor cells with effects in
cancer immune dysfunction and angiogenesis. J Immunother.
35:502–512. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Fan Q, Gu D, Liu H, Yang L, Zhang X, Yoder
MC, Kaplan MH and Xie J: Defective TGF-β signaling in bone
marrow-derived cells prevents hedgehog-induced skin tumors. Cancer
Res. 74:471–483. 2014. View Article : Google Scholar :
|
|
31
|
Xiang X, Poliakov A, Liu C, Liu Y, Deng
ZB, Wang J, Cheng Z, Shah SV, Wang GJ, Zhang L, et al: Induction of
myeloid-derived suppressor cells by tumor exosomes. Int J Cancer.
124:2621–2633. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hida K, Kawamoto T, Ohga N, Akiyama K,
Hida Y and Shindoh M: Altered angiogenesis in the tumor
microenvironment. Pathol Int. 61:630–637. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Goubran HA, Kotb RR, Stakiw J, Emara ME
and Burnouf T: Regulation of tumor growth and metastasis: The role
of tumor microenvironment. Cancer growth Metastasis. 7:9–18. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Mittal K, Ebos J and Rini B: Angiogenesis
and the tumor microenvironment: Vascular endothelial growth factor
and beyond. Semin Oncol. 41:235–251. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chauhan VP, Stylianopoulos T, Martin JD,
Popović Z, Chen O, Kamoun WS, Bawendi MG, Fukumura D and Jain RK:
Normalization of tumour blood vessels improves the delivery of
nanomedicines in a size-dependent manner. Nat Nanotechnol.
7:383–388. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Trédan O, Lacroix-Triki M, Guiu S,
Mouret-Reynier MA, Barrière J, Bidard FC, Braccini AL, Mir O,
Villanueva C and Barthélémy P: Angiogenesis and tumor
microenvironment: Bevacizumab in the breast cancer model. Target
Oncol. 10:189–198. 2015. View Article : Google Scholar
|
|
37
|
Sounni NE, Paye A, Host L and Noël A:
MT-MMPS as regulators of vessel stability associated with
angiogenesis. Front Pharmacol. 2:1112011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Pucino V, De Rosa V, Procaccini C and
Matarese G: Regulatory T cells, leptin and angiogenesis. Chem
Immunol Allergy. 99:155–169. 2014. View Article : Google Scholar
|
|
39
|
D'Alessio FR, Zhong Q, Jenkins J,
Moldobaeva A and Wagner EM: Lung angiogenesis requires CD4 (+)
forkhead homeobox protein-3 (+) regulatory T cells. Am J Respir
Cell Mol Biol. 52:603–610. 2015. View Article : Google Scholar
|
|
40
|
Mucha J, Majchrzak K, Taciak B, Hellmén E
and Król M: MDSCs mediate angiogenesis and predispose canine
mammary tumor cells for metastasis via IL-28/IL-28RA (IFN-λ)
signaling. PLoS One. 9:e1032492014. View Article : Google Scholar
|
|
41
|
Finke J, Ko J, Rini B, Rayman P, Ireland J
and Cohen P: MDSC as a mechanism of tumor escape from sunitinib
mediated anti-angiogenic therapy. Int Immunopharmacol. 11:856–861.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Yang B, Kang H, Fung A, Zhao H, Wang T and
Ma D: The role of interleukin 17 in tumour proliferation,
angiogenesis, and metastasis. Mediators Inflamm. 2014:6237592014.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Numasaki M, Fukushi J, Ono M, Narula SK,
Zavodny PJ, Kudo T, Robbins PD, Tahara H and Lotze MT:
Interleukin-17 promotes angiogenesis and tumor growth. Blood.
101:2620–2627. 2003. View Article : Google Scholar
|
|
44
|
Ager EI, Kozin SV, Kirkpatrick ND, Seano
G, Kodack DP, Askoxylakis V, Huang Y, Goel S, Snuderl M, Muzikansky
A, et al: Blockade of MMP14 activity in murine breast carcinomas:
Implications for macrophages, vessels, and radiotherapy. J Natl
Cancer Inst. 107:1072015. View Article : Google Scholar
|
|
45
|
Romanchikova N, Trapencieris P, Zemītis J
and Turks M: A novel matrix metalloproteinase-2 inhibitor
triazolylmethyl aziridine reduces melanoma cell invasion,
angiogenesis and targets ERK1/2 phosphorylation. J Enzyme Inhib Med
Chem. 29:765–772. 2014. View Article : Google Scholar
|
|
46
|
Weisshardt P, Trarbach T, Dürig J, Paul A,
Reis H, Tilki D, Miroschnik I, Ergün S and Klein D: Tumor vessel
stabilization and remodeling by anti-angiogenic therapy with
bevacizumab. Histochem Cell Biol. 137:391–401. 2012. View Article : Google Scholar
|