Introduction
Lung cancer is one of the leading causes of
cancer-related mobility worldwide affecting millions of people
every year (1,2). Lung cancer consists of two major
types: small cell lung cancer (SCLC) and non-small cell lung cancer
(NSCLC), of which NSCLC account for 80% of them (3). Though various therapeutic agents for
the treatment of NSCLC have been developed (4), poor prognosis was always discovered in
patients on account of the frequent metastasizing characteristic of
this malignancy (5).
The Notch signaling pathway has been identified as
critical in governing cell fate determination by directly
regulating transcriptional programs, including differentiation,
proliferation, self-renewal, and apoptosis (6–8).
Notch-1 signaling is activated when the complex of ligand and
receptor binding is formed between directly contacting cells
(9,10). Then Notch1 is cleaved by r-secretase
and translocates the Notch-1 intracellular domain (NICD) from the
plasma membrane into the nucleus to regulate related target genes
including hairy enhance of split (Hes) family and hairy enhancer of
split related with YRPW motif (Hey) family (11,12).
Previous studies have reported that aberrant activation of Notch
could cause a variety of solid malignancies including ovarian,
breast, lung, and renal cancer, suggesting Notch may be an
attractive target for therapeutic intervention in cancer (13,14).
Notch activation complex kinase (NACK) is the first
identified as both a co-activator and a target gene of the Notch
pathway. NACK regulates Notch transcriptional activity by
interaction with the Notch transcriptional activation complex on
DNA, which is required for Notch-mediated tumorigenesis (15). Recent studies have showed that
knockdown of NACK resulted in inhibiting tumor growth in human
esophageal carcinoma cells (15).
However, the role and underlying mechanism of NACK in NSCLC still
remains unknown.
In the present study, we analyzed the expression of
NACK among NSCLC patients, and further investigated the role of
NACK on tumor growth of NSCLC by small RNA interference method both
in vitro and in vivo. We found that NACK expression
was significantly increased in NSCLC tumors and cell lines. High
NACK expression was associated with different clinicopathological
parameters and poor prognosis. Knocking down NACK could directly
inhibit cell proliferation and increase cell apoptosis.
Downregulation of Hes1, HeyL and Notch1 was discovered in the NACK
knockdown in NSCLC cells. Our study may provide a better
understanding of the underlying molecular mechanism of NACK in the
regulation of NSCLC.
Materials and methods
Tissue sample collection
In this study, NSCLC tumor tissues and paired
adjacent normal tissues were obtained from 35 patients who
underwent percutaneous lung puncture or biopsy of lung cancer
tissue at The Second Affiliated Hospital of Xi'an Jiaotong
University (Xi'an, China) between October 2013 and May 2014. All
NSCLC cases were clinically and pathologically confirmed by two
independent experts. All tissues were frozen at −80°C for further
analysis. The follow-up data for 60 months were recorded by
communicating with the patients or their relatives, the median
duration of follow-up was 59 months (range, 1–60 months). Informed
consent was obtained from each patient, and this study was approved
by the Human Ethics Committee of Second Affiliated Hospital of
Xi'an Jiaotong University.
Cell lines and cell culture
The human lung adenocarcinoma cell lines A549 and
H1299 and normal human bronchial epithelial cell line (NHBE) were
obtained from the Shanghai GeneChem Co., Bank (Shanghai, China).
All cell culture reagents were purchased from Invitrogen and
supplied with 1% penicillin/streptomycin (Sigma-Aldrich, ON,
Canada). Cells were grown in Dulbecco's modified Eagle's medium
(DMEM) supplemented with 10% FBS (Hyclone, Logan, UT, USA), and
maintained in monolayer culture at 37°C in an incubator of
humidified air with 5% CO2.
Plasmid construction and transient
transfection of NSCLC cell lines
For NACK, the sequences for siRNA were design as
follows: sense strand, 5′-UCG CAU UGA CCA UUC AAA CUG GUG G-3′ and
antisense, 5′-CCA CCA GUU UGA AUG GUC AAU GCG A-3′. The scramble
siRNA was random sequenced by Blast website. The cells were
harvested at 48 h post-transfection. A549 and H1299 cells were used
in this experiment. Twenty-four hours before transfection,
1×104 cells/well, in 5 ml medium, were plated in 60 mm
dish (Nunc; Thermo Fisher Scientific, Waltham, MA, USA), and then
transfected with plasmids of NACK-siRNA or scramble siRNA as
negative control using Lipofectamine 2000 as described in the
manufacturer's instruction.
Luciferase reporter assay
For reporter assay, A549 and H1299 cells were
cultured in 24-well plates. Hes1-luciferase reporter plasmids were
constructed by subcloning the 5-upsteam elements of human Hes1 into
the pGL3-enhancer vector (Promega, Madison, WI, USA). Hes1
promoter-specific primers (−747−+66): Forward, 5′-CGA GCT CAG CGG
CAA CTT TAG ATG TG-3′; and reverse, 5′-CCC AAG CTT GTT GAC ACT GGC
TGG GGT A-3′. After 24 h, the cells were co-transfected with the
siRNA expression plasmid targeting NACK (NACK siRNA). Fourty-eight
hours later, luciferase activities were measured using
Dual-Luciferase Reporter Assay system (Promega) according to
manufacturer's instructions. Firefly luciferase activity was
normalized to Renilla luciferase activity.
RNA extraction and quantitative RT-PCR
analysis
Total RNA was isolated from cells or tissues by
TRIzol reagent (15596-026; Life Technologies) following the
manufacturer's instructions. Quantitative real-time reverse
transcriptase-PCR assay (qRT-PCR) was performed using a SYBR-Green
method with a Master Mix buffer system. Gene expression in human
was normalized to GAPDH. The primers sequences were as follows:
GAPDH forward, 5′-CCG ATT TCT CCT CCG GGT G-3′ and reverse, 5′-TGG
TCA TGA GTC CTT CCA CG-3′; NACK forward, 5′-TCT CTT GTG AAG GAA CCG
GC-3′ and reverse, 5′-CCG GCT TGT AAG TCC TGG TT-3′; Notch1
forward, 5′-GGG CCT CAA GTG AGC GGA C-3′ and reverse, 5′-GGT GAG
GGG TCG AGA AGT GA-3′; Hes1 forward, 5′-TTT CTT CCA GAC TTC CGC
CC-3′ and reverse, 5′-GGA CAA TGC CTC CCA ATC CA-3′; and HeyL
forward, 5′-AGA CCG CAT CAA CAG TAG CC-3′ and reverse, 5′-TCA GGC
AGC TGC TAC CAA TC-3′. The PCR conditions were as follows: 95°C for
2 min, 95°C for 15 sec, 60°C for 30 sec for 40 cycles. The relative
expression levels were calculated by the value of
2−ΔΔCt. All experiments were repeated at least three
times.
Western blotting
The tissues and cells were washed twice with
ice-cold PBS and lysed in RIPA lysis buffer (Solarbio, Beijing,
China). The proteins were separated on SDS-polyacrylamide gels
(SDS-PAGE) and electrophoretically transferred to PVDF membranes
(Roche Diagnostics). Then the membranes were blocked in 5% milk,
and incubated with the appropriate primary antibody overnight. The
primary antibodies NACK (1:1,000; AbMax Company, China), Notch1
(1:1,000), Hes1 (1:1,000), and HeyL (1:1,000) (all from Abcam, UK),
were incubated with HRP-conjugated secondary antibodies (Abcam) at
1:1,000 for 1 h, then the protein bands were detected using the
enhanced chemiluminescence detection system (Pierce; Thermo
Scientific, Rockford, IL, USA).
Cell proliferation assay
The proliferation of NSCLC cells was evaluated by
Cell Counting Kit-8 (CCK-8) assay (Beyotime, Shanghai, China)
according to manufacturer's instruction. Briefly, cells transfected
with negative-siRNA or NACK-siRNA were seeded into a 96-well plate
(100 μl/well) and incubated at 37°C, 5% CO2 for 5
days. CCK-8 (10%) was added to the well at 24 h intervals and
maintained for 2 h according to the manufacturer's protocol, and
then the absorbance was measured at 450 nm by a microplate reader
(BioTek ELx800; BioTek, USA).
Colony formation assay
Non-small cell lung cancer cells (A549 and H1299)
were transfected with negative-siRNA or NACK-siRNA for 24 h, then
the transfected cells were resuspended, counted and seeded at a
density of 600 cells/well in a 6-well plate. The cells were
continually cultured for 10 days with a change of media every other
day. On day 10, cells were fixed with 3.7% methanol, stained with
0.1% crystal violet the number of colonies were counted under a
microscope (Olympus, Beijing, China).
Are of Migration and invasion assays
Cell motility was assessed by migration and invasion
assays. A 24-well Transwell insert containing a pore size of 8
μm polycarbonate membrane (Corning, Inc., Corning, NY, USA)
was used to determine the effect of NACK on A549 and H1299
migration and invasion in vitro. Briefly, the transfected
cells were cultured in serum-free medium for 12 h, then cells were
resuspended in serum-free medium and placed in the upper chambers
at the density of 2×104. The lower chamber was filled
with medium containing 10% fetal bovine serum as the
chemoattractant and incubated 24 h for migration assay and 48 h for
the invasion assay, respectively. For migration assay, the number
of cells on the lower surface of the membrane was counted in 5
fields under a microscope. For invasion assay, the upper chambers
of the inserts were covered with Matrigel. After 48 h incubation,
cells on the lower surface of the member was stained with 0.1%
crystal violet. The number of cells on the lower surface of the
membrane was counted in 5 fields under a microscope.
Apoptosis
NSCLC cells transfected as negative-siRNA or
NACK-siRNA were collected and washed with PBS twice. After
centrifuge, the cells were resuspended with 1X staining buffer at
the dose of 1×106 cell/ml, then cells were dyed with 5
μl Annexin V-APC in the dark, at room temperature for 15
min. Flow cytometry was applied with FACSCalibur flow cytometer and
analysis was performed with FlowJo software (Tree Star).
Xenografts
Four weeks old female nude mice were purchased from
the Animal Center of The Fourth Military Medical University (Xi'an,
China) and randomly divided into three groups with 10 per group,
receiving subcutaneous injection of NACK-siRNA, negative-siRNA, or
control cells, respectively. of Tumor volume was measured using
calipers every 4 days for 28 days and calculated as follows: Tumor
size [volume (mm3) = width2 (mm)/2 × length
(mm)]. Following 28-day post-inoculation, the animals were
euthanized and the tumors were collected for further analyzing. The
animal experiment was reviewed and approved by the Animal Care and
Use Committee of the Second Affiliated Xi'an Jiaotong University
(Xi'an, China).
Statistical analysis
All assays were repeated three times to insure
reproducibility. The significance of results obtained from the
control and treated groups were analyzed using the Student's
t-test. The results are given as mean ± SD. p<0.05 was
considered statistically significant.
Results
Overexpression of NACK in NSCLC tumor
tissues and cell lines
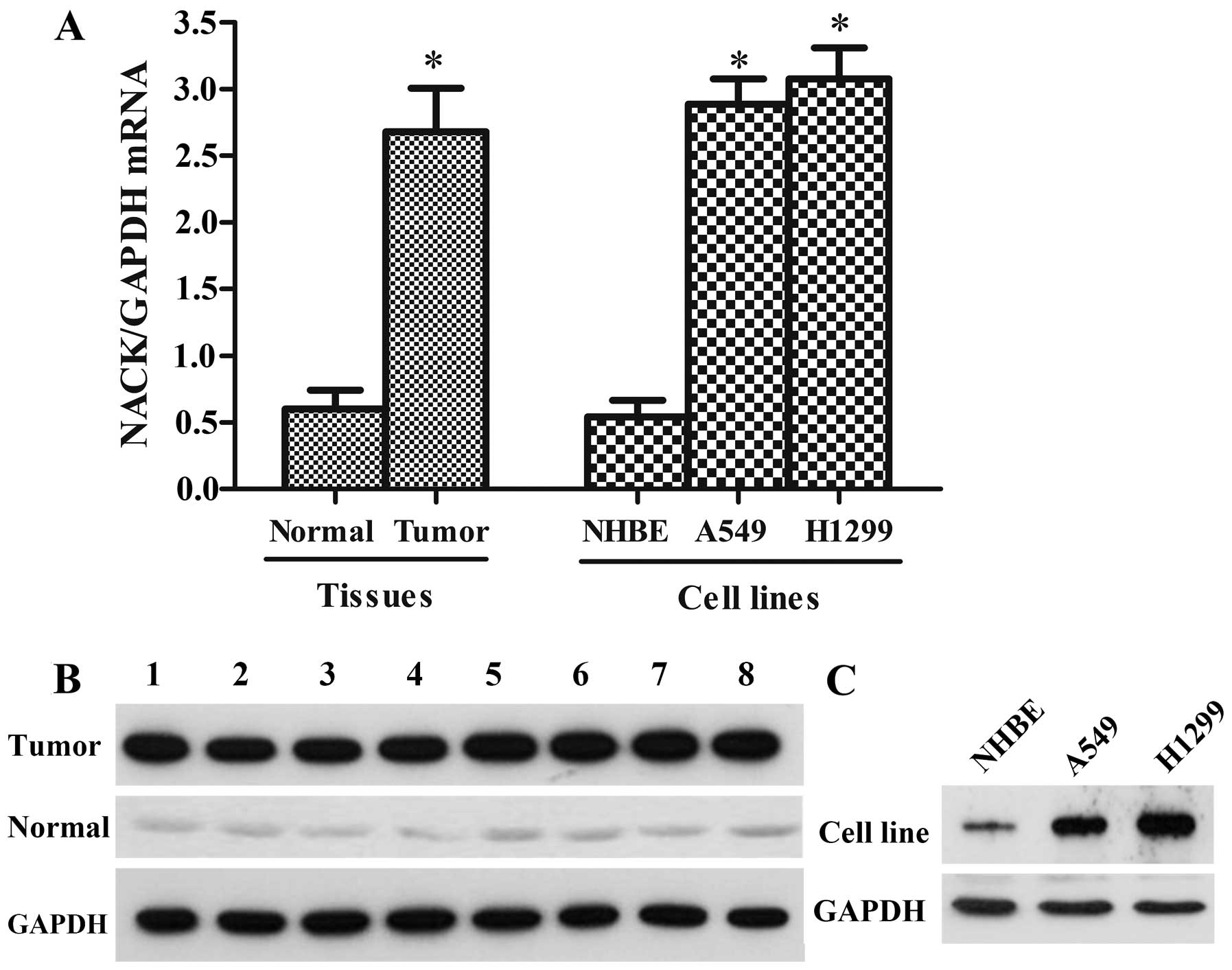
The expression level of NACK in 35 NSCLC tissue
samples and corresponding adjacent non-cancerous tissues were
detected by the quantitative RT-PCR (qRT-PCR) and western blot
assay. As shown in Fig. 1A and B,
NACK was discovered highly expressed both at mRNA and protein
levels in the NSCLC tissue samples compared with the paired
adjacent normal tissues. Furthermore, the expression level of NACK
was assessed in NSCLC cell lines (A549 and H1299) and NHBE, the
result showed that NACK was greatly higher in A549 and H1299 cell
lines than the normal cell line NHBE (Fig. 1A and C), indicating that NACK may
play a positive role in NSCLC progression.
High NACK expression is associated with
different clinicopathological parameters
To determine the potential role of NACK in the
progress of NSCLC, the relationship between NACK expression and
clinicopathological parameters in NSCLC were analyzed by Pearson's
Chi-square. NSCLC cases were divided into two groups according to
the mRNA level of NACK (low < median; high > median). The
high expression of NACK was observed in 26/35 (74.29%) of NSCLC
samples, and the expression of NACK was significantly correlated
with the degree of differentiation (p=0.034), lymphatic metastasis
(N stage) (p=0.007) and clinical stage (p=0.014) (Table I). However, the high level of NACK
expression was not associated with gender, age, smoking, tumor size
or histological type (p>0.05). Hence, NACK may be used as a
potential unfavorable prognostic biomarker in NSCLC.
 | Table IAssociation between NACK expression
and clinicopathological factors in patient with NSCLC. |
Table I
Association between NACK expression
and clinicopathological factors in patient with NSCLC.
| Variables | No. of cases | NACK expression
| P-value |
|---|
| Low (%) | High (%) |
|---|
| Gender | | | | |
| Male | 19 | 5 (26.32) | 14 (73.68) | 0.471 |
| Female | 16 | 4 (25.00) | 12 (75.00) | |
| Age (years) | | | | |
| >60 | 24 | 5 (20.83) | 19 (79.17) | 0.583 |
| ≤60 | 11 | 4 (36.36) | 7 (63.64) | |
| Smoking | | | | |
| Yes | 22 | 4 (18.18) | 18 (81.82) | 0.192 |
| No | 13 | 5 (38.46) | 8 (61.54) | |
| Tumor size
(cm) | | | | |
| >3 | 21 | 6 (28.57) | 15 (71.43) | 0.538 |
| ≤3 | 14 | 3 (21.43) | 11 (78.57) | |
| Histological
type | | | | |
| AC | 26 | 6 (23.08) | 20 (76.92) | 0.139 |
| SCC | 7 | 2 (28.57) | 5 (71.43) | |
| Other | 2 | 1 (50.00) | 1 (50.00) | |
|
Differentiation | | | | |
| Well | 11 | 4 (36.36) | 7 (63.64) | 0.034a |
| Moderate | 15 | 4 (26.67) | 11 (73.33) | |
| Poor | 9 | 1 (11.11) | 8 (88.89) | |
| T stage | | | | |
| T1–2 | 23 | 6 (26.09) | 17 (73.91) | 0.316 |
| T3–4 | 12 | 3 (25.00) | 9 (75.00) | |
| N stage | | | | |
| N0 | 17 | 5 (29.41) | 12 (70.59) | 0.007b |
| N1, 2, 3 | 18 | 4 (22.22) | 14 (77.78) | |
| Clinical stage | | | | |
| I–II | 21 | 5 (23.81) | 16 (76.19) | 0.014a |
| III–IV | 14 | 4 (28.57) | 10 (71.43) | |
NACK as a prognostic marker in NSCLC
patients
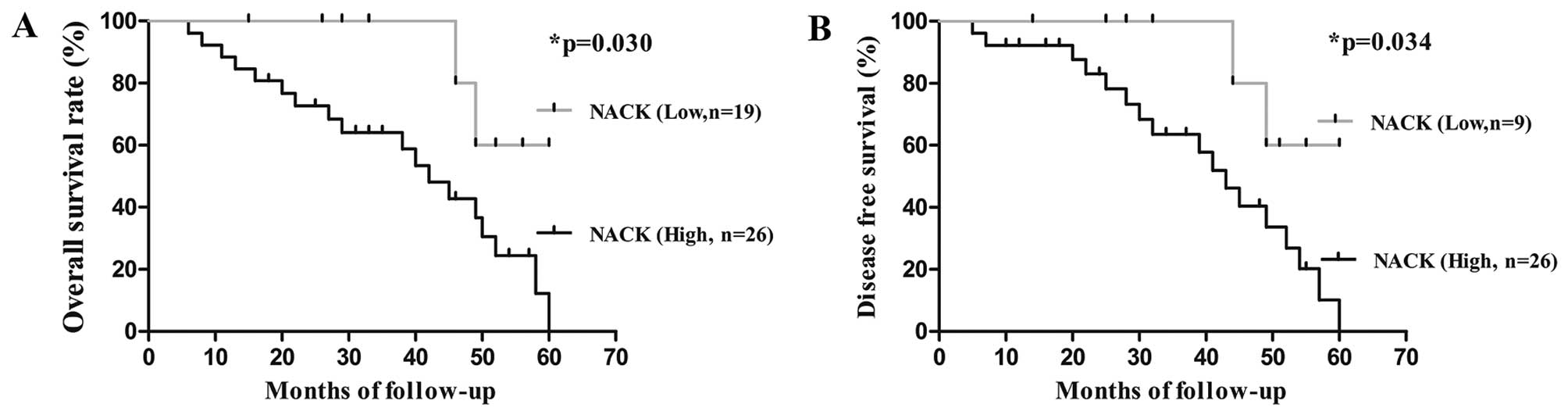
To investigate the correlation between NACK
expression and the survival rates of NSCLC patients, Kaplan-Meier
survival analysis was performed until the patients died or end of
the research. Patients were divided into two groups depending on
NACK expression level (low and high). We observed that the
cumulative 4-year overall survival rate (OS) and decreased survival
rate (DFS) of patients with high level of NACK detection were 30.7
and 34.6%, respectively, when compared to low NACK expression
patients (77.8 and 77.8%, respectively) (Fig. 2). Hence, the level of NACK
expression was an unfavorable predictive factor for prognosis of
NSCLC patients.
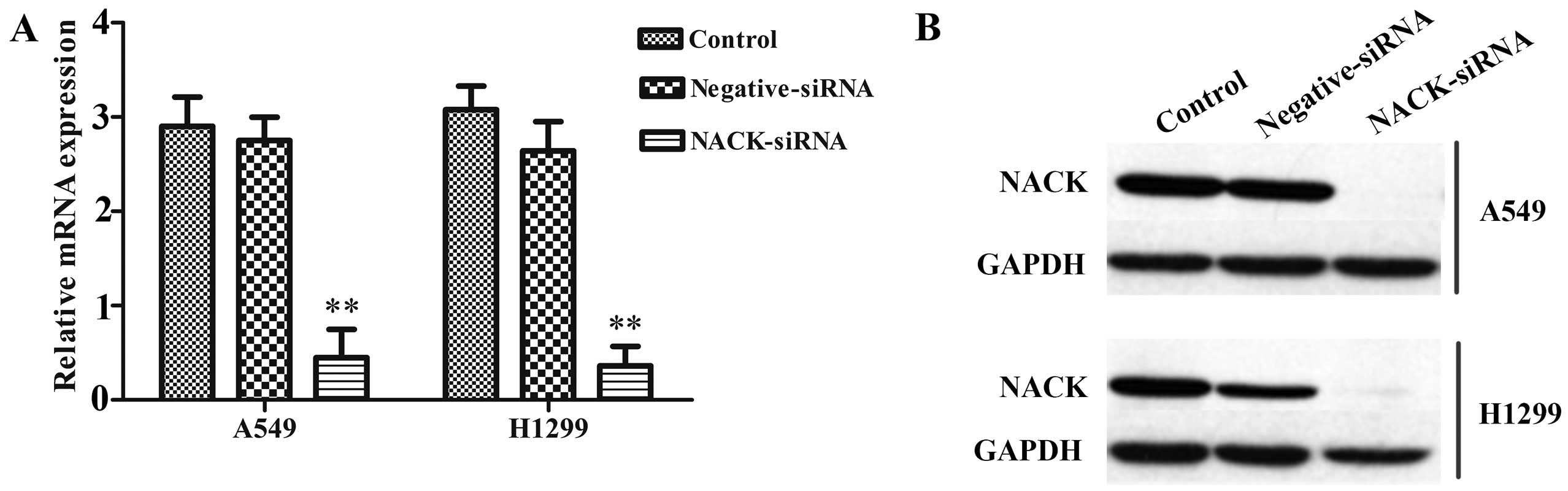
Knockdown of NACK inhibits the expression
of NACK in NSCLC cells
The efficient inhibition of NACK expression was
examined by qRT-PCR and western blotting, the result showed that
after transfected with NACK-siRNA, the expression of NACK was
significantly decreased at mRNA level (Fig. 3A) and hardly observed at protein
level (Fig. 3B) either in A549 or
H1299 cells. No significant difference was observed between
negative-siRNA and control groups.
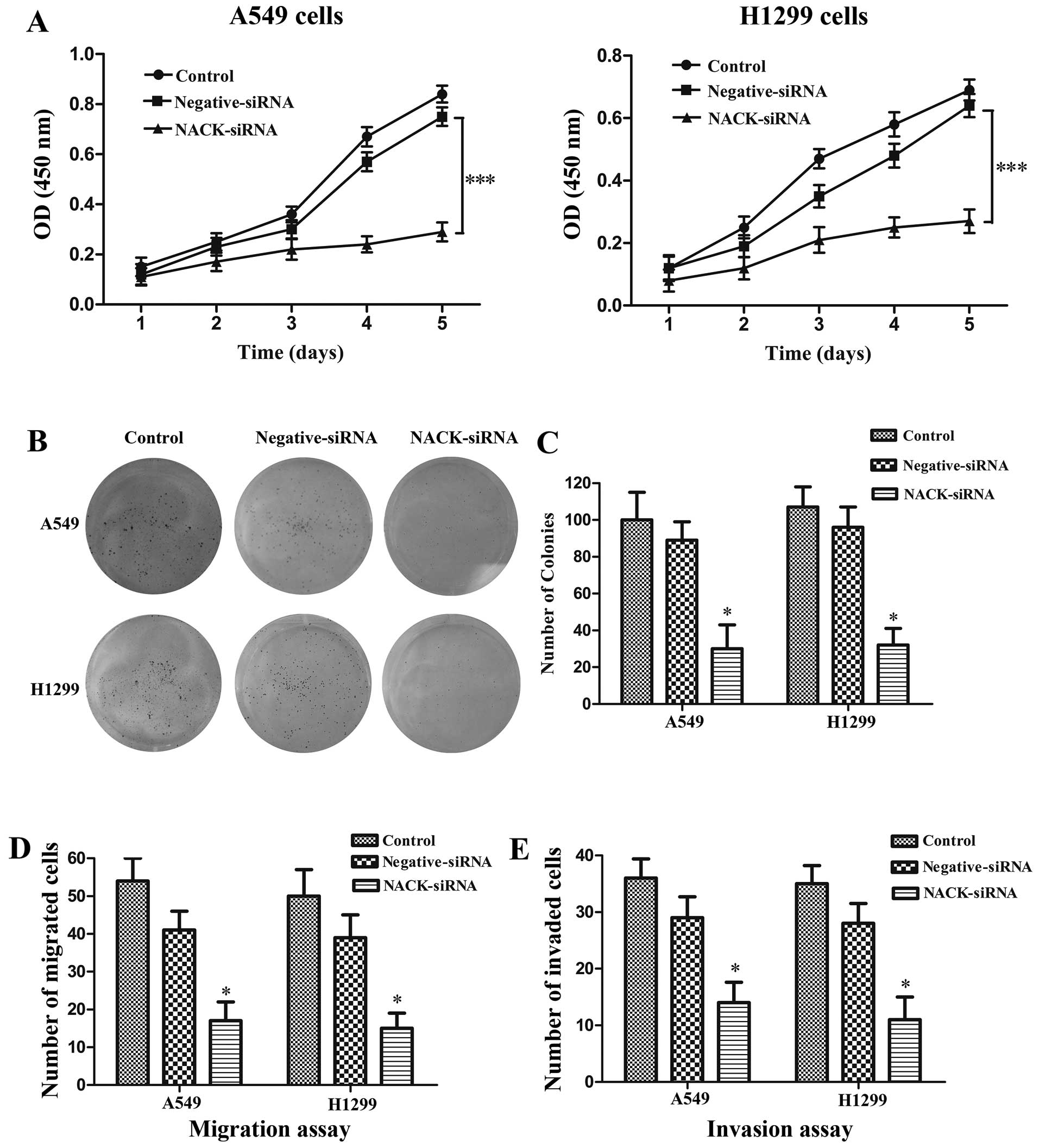
NACK is required for cell proliferation
in NSCLC
Cell proliferation assay and cell invasion assay
were performed in NSCLC cell lines A549 and H1299 with NACK
knockdown by siRNA. CCK-8 assay verified that the cells transfected
with NACK-siRNA showed a significant decrease in cell proliferation
when compared with control group both in A549 (p<0.001) and
H1299 (p<0.001) cells at the 5th day (Fig. 4A). The colony formation assay was
used to evaluate the tumor growth of a transfected cells. Our data
indicated that when A549 and H1299 cells were treated with
NACK-siRNA, the ability of colony formation was remarkably
decreased in A549 (p<0.05) and H1299 (p<0.05) cells (Fig. 4B and C).
NACK knockdown inhibits the migration and
invasion of NSCLC cells
To investigate whether NACK regulates the migration
and invasion of NSCLC cell, NSCLC cells were transfected with
either NACK-siRNA or negative-siRNA. Cell migration and invasion
were evaluated by using a Transwell insert. The results
demonstrated that knockdown of NACK significantly reduced the
number of migration at 24 h (p<0.01) and invasion at 48 h
(p<0.01) (Fig. 4D and E),
suggesting that NACK is necessary for NSCLC cell metastasis during
the tumor progress.
Induction of apoptosis by interference of
NACK
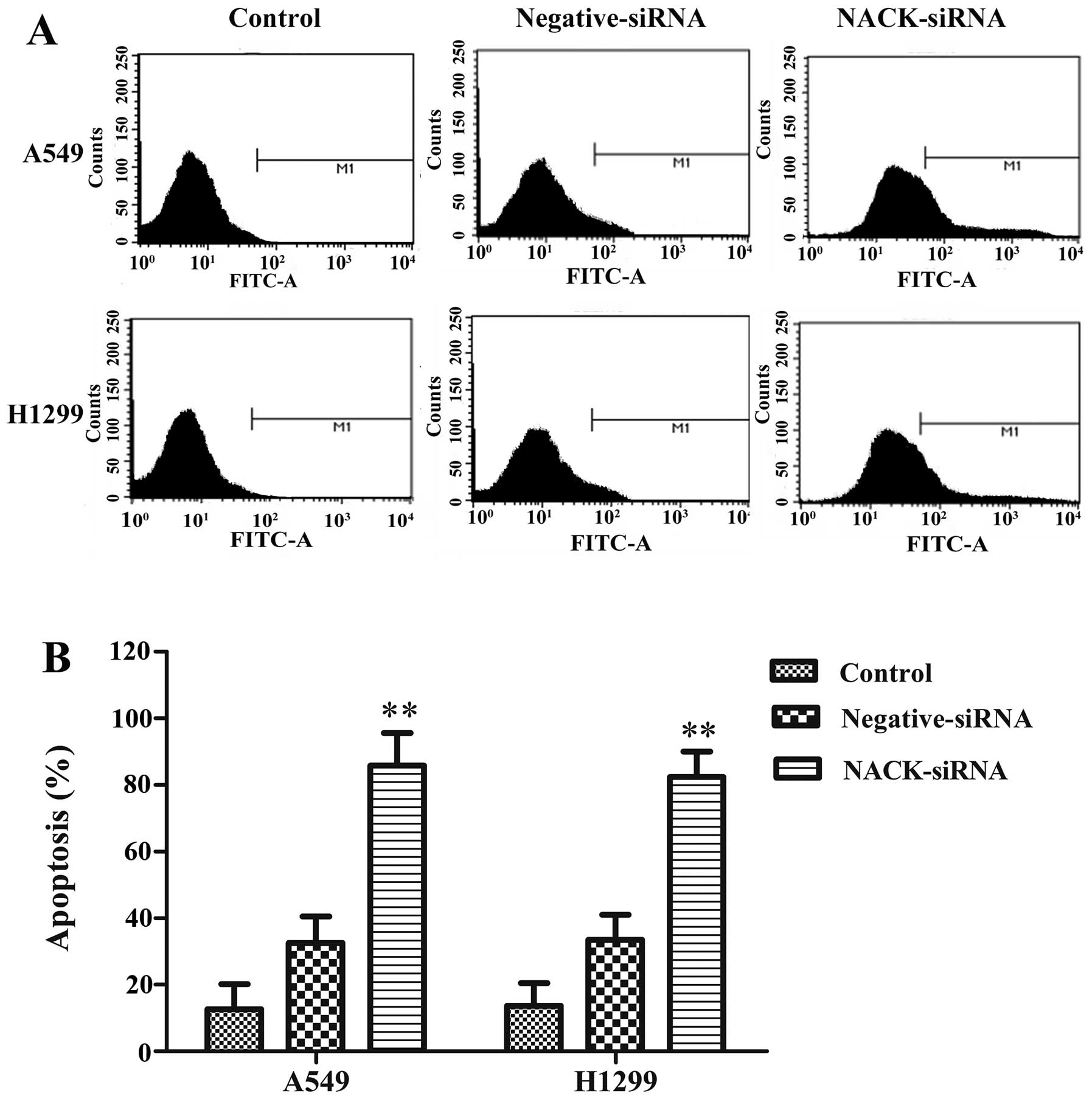
We further investigated whether interference NACK
could induce apoptosis in A549 and H1299 cells. FITC assay results
indicated that the apoptosis rates were significantly increased
both in A549 and H1299 cells after knocking down NACK (Fig. 5). These results suggest the
interference of NACK inhibits NSCLC progression through apoptosis
induction in vitro.
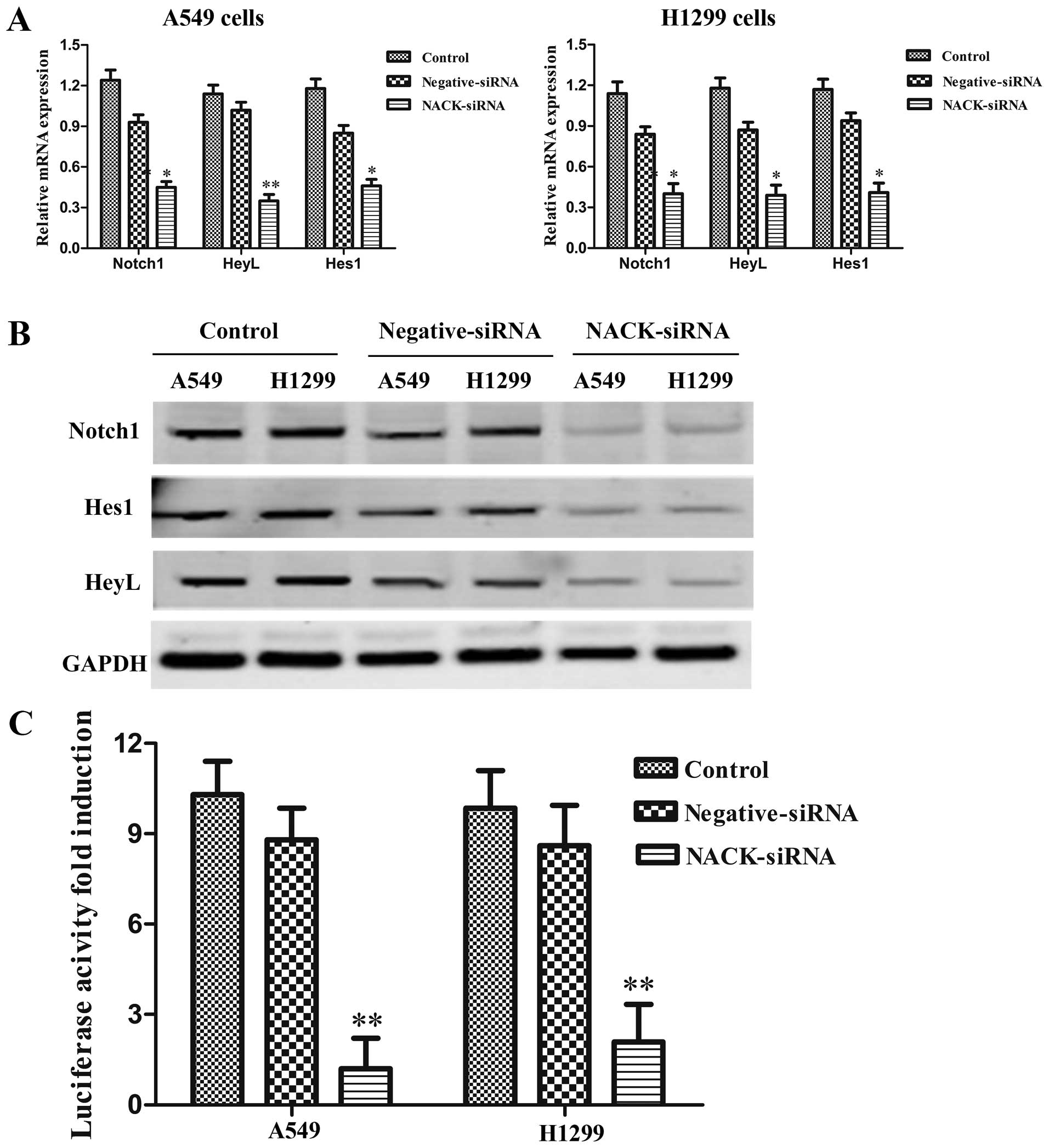
Knocking down NACK inhibits the
transcriptional activity of Notch signaling pathway in NSCLC
NACK was identified as a direct target gene of Notch
pathway in human esophageal adenocarcinoma cells (15). Considering the specific action of
the Notch pathway in different types of tumors, we investigated the
role of NACK on the regulation of Notch pathway in NSCLC by
transfecting NACK-siRNA into A549 and H1299 cells to knock down
NACK expression. The results showed that the expression of the
Notch target genes Hes1 and HeyL were reduced significantly at mRNA
level (Fig. 6A). Western blot
results also showed that transfection with NACK-siRNA reduced
expression of Notch1, Hes1 and HeyL protein both in A549 and H1299
cells (Fig. 6B). Furthermore, as
luciferase reporter assay result showed, co-transfection of
NACK-siRNA with Hes1 reporter construct strongly reduced luciferase
activity in both A549 and H1299 cells (Fig. 6C) when compared to the cells
co-transfected with negative-siRNA. The data demonstrated that the
transcriptional activity of Notch1 signaling pathway could be
inhibited by knocking down the expression of NACK in NSCLC.
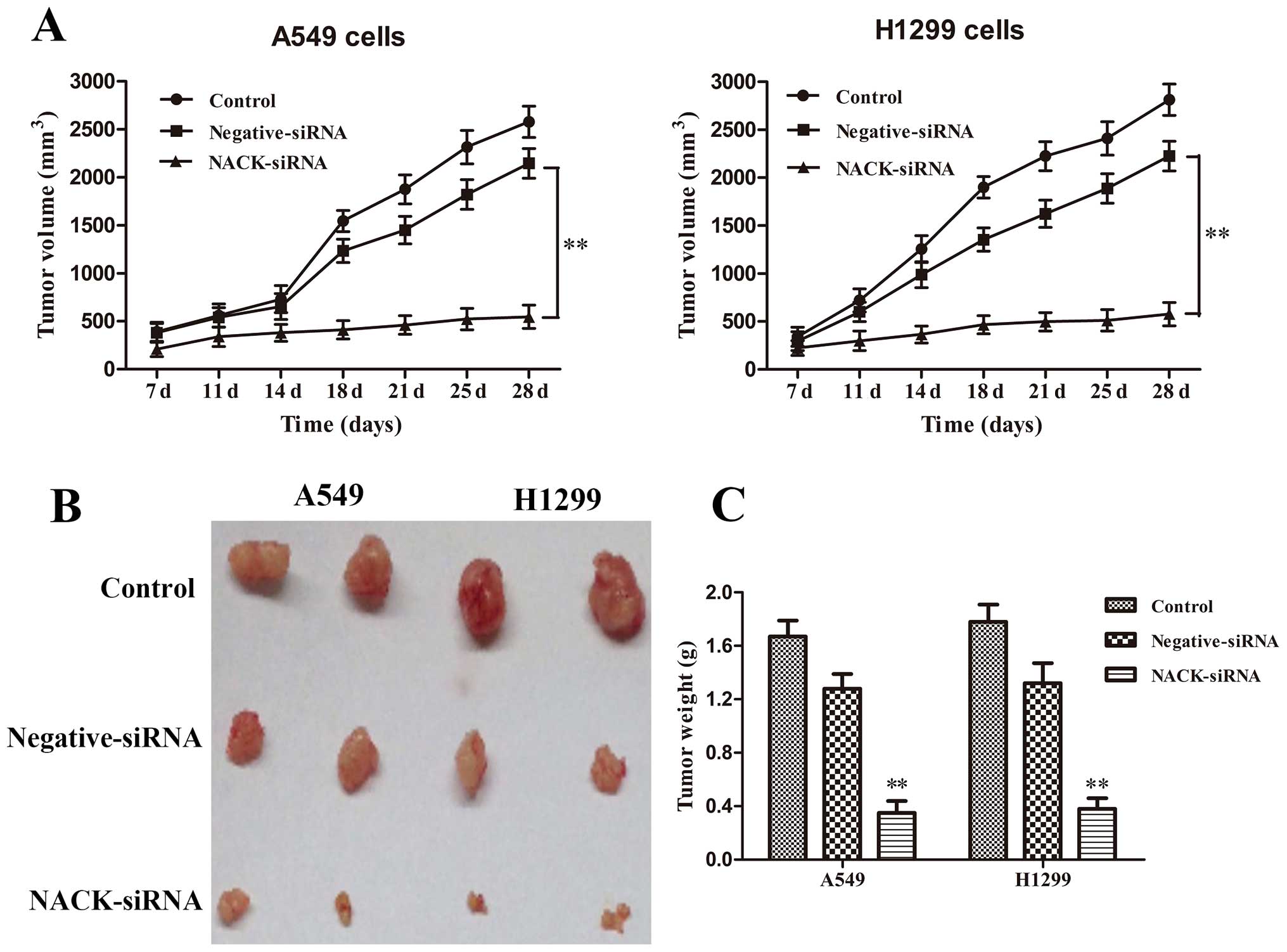
Interference of NACK inhibits NSCLC
progression in vivo
To address whether changes of NACK expression could
influence the growth of tumor in vivo, A549 and H1299 cells
infected with negative-siRNA or NACK-siRNA were injected
subcutaneously into the nude mice, and tumor size was measured
every 4 days for 28 days. The results revealed that the knockdown
of NACK inhibited tumorigenesis in vivo. The average tumor
volume (Fig. 7A) and weight of mice
(Fig. 7B and C) in NCAK-siRNA group
at day 28 was significantly decreased (p<0.01) compared to that
of mice in control group. These results demonstrated that the
interference of NACK inhibits NSCLC progression in vivo.
Discussion
Notch signaling is an evolutionarily conserved
intercellular communication mechanism critical for cell survival,
proliferation, differentiation, as well as maintaining stem cell
quiescence and identity (16–18).
Moreover, it has great relevance to multiple aspects of cancer
biology, from cancer stem cells, angiogenesis to tumor immunity
(19–23). Jiang et al, observed strong
Notch-1 immunoreactivity in NSCLC, which correlated with Jagged-1
and VEGF expression (24). Under
hypoxia, Notch-1 provides important survival signals to NSCLC cells
(25). Baumgart et al,
reported that Notch-1 also acts as a regulator of EGFR expression
through incorporating with ADAM17 in NSCLC (26). Ji et al, discovered that
Notch-1 downregulation inhibited cell growth and induced apoptosis
in NSCLC by δ-tocotrienol (27).
Furthermore, Notch-1 promoted NSCLC tumor progression through
direct up-regulation of insulin-like growth factor 1 receptor
(IGF1-R) (28) facilitating
expression of the survivin (29).
NACK, a kind of atypical kinase (30), was first named Pragmin due to its
ability to stimulate the activity of RhoA (31). Recently, Weaver et al,
reported the crucial role of NACK as a novel regulator of Notch
transcription and as the Notch-mediated tumor proliferation of
mammary epithelial cells and esophageal adenocarcinoma cells
(15). Therefore, we hypothesized
that interference of NACK may inhibit the progression of NSCLC.
Thus, the relationship between NACK expression and tumorigenesis of
NSCLC is first demonstrated in this study.
Through detecting the expression of NACK in 35 tumor
samples from NSCLC patients and analyzing their clinicopathological
parameters, we found that NACK was remarkably over-expressed in
NSCLC tumor tissues both at transcriptional and translational
levels. Furthermore, high NACK expression was associated with tumor
differentiation, lymphatic metastasis, clinical stage and poor
survival prognosis in NSCLC patients, which indicated that NACK may
be an independent prognostic factor for NSCLC.
RNA interference method as a powerful technology
(32) was used in this study to
knock down NACK expression. Cell indefinite proliferation, invasion
and metastasis are the major causes of NSCLC occurrence (33). From in vivo and in
vitro studies, we found that the interference of NACK markedly
inhibited the proliferation, invasion and metastasis of NSCLC
cells, indicating that NACK is necessary for the proliferation of
tumor cells, which was consistent with a previous study (15). Apoptosis plays a vital role in
cancer development by the dysregulation of cell death and cell
growth (34). Based on the flow
cytometry assay, the interference of NACK significantly induces
apoptosis rate in NSCLC cells, demonstrating that NACK may be a
potential agent for the treatment of NSCLC (35,36).
Hes1 and HeyL, members of Hes and Hey families,
respectively, have been reported as the direct downstream targets
of the Notch1 signaling (37–39).
In the present study, we found that their expression was markedly
reduced when NACK was knocked down. Moreover, luciferase reporter
assay also showed down-regulation of Notch1 signaling with NACK
silencing in NSCLS cells. The Notch1 target genes Hes1 and HeyL are
involved in regulating cell proliferation, apoptosis,
differentiation and metabolism (40,41).
Taken together, we hypothesized that the interference of NACK by
siRNA inhibits tumorigenesis of NSCLC directly via targeting the
Notch1 signaling pathway. However, the mechanisms of interference
of NACK inhibiting tumorigenesis is not clear, and further research
is necessary.
In conclusion, we provide the first evidence to
demonstrate that NACK was robustly expressed in a subset of NSCLC
samples and interference of NACK inhibits NSCLC progression through
failing to activate Notch1 signaling complexes. Thus, NACK may be
an attractive target to help develop novel therapeutic methods
against NSCLC. Insightful studies are still needed to disclose
other signaling pathways involved in regulating NSCLC.
Acknowledgments
We would like to thank Dr Xijing He and Dr Bin Zhou
for their insightful comments and suggestions.
References
|
1
|
Siegel R, Ma J, Zou Z and Jemal A: Cancer
statistics, 2014. CA Cancer J Clin. 64:9–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nguyen KS, Neal JW and Wakelee H: Review
of the current targeted therapies for non-small-cell lung cancer.
World J Clin Oncol. 5:576–587. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Boolell V, Alamgeer M, Watkins DN and
Ganju V: The evolution of therapies in non-small cell lung cancer.
Cancers (Basel). 7:1815–1846. 2015. View Article : Google Scholar
|
|
4
|
Rovigatti U: Cancer modelling in the NGS
era - Part I: Emerging technology and initial modelling. Crit Rev
Oncol Hematol. 96:274–307. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gottfried M, Keizman D, Mishaeli M and
Rabinovich NM: The current approach to advanced lung cancer.
Harefuah. 154:521–540. 2015.In Hebrew.
|
|
6
|
Wang Z, Li Y, Banerjee S and Sarkar FH:
Emerging role of Notch in stem cells and cancer. Cancer Lett.
279:8–12. 2009. View Article : Google Scholar :
|
|
7
|
Kristoffersen K, Villingshøj M, Poulsen HS
and Stockhausen MT: Level of Notch activation determines the effect
on growth and stem cell-like features in glioblastoma multiforme
neurosphere cultures. Cancer Biol Ther. 14:625–637. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jang MS, Miao H, Carlesso N, Shelly L,
Zlobin A, Darack N, Qin JZ, Nickoloff BJ and Miele L: Notch-1
regulates cell death independently of differentiation in murine
erythroleukemia cells through multiple apoptosis and cell cycle
pathways. J Cell Physiol. 199:418–433. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li L, Zhao F, Lu J, Li T, Yang H, Wu C and
Liu Y: Notch-1 signaling promotes the malignant features of human
breast cancer through NF-κB activation. PLoS One. 9:e959122014.
View Article : Google Scholar
|
|
10
|
Leong KG and Karsan A: Recent insights
into the role of Notch signaling in tumorigenesis. Blood.
107:2223–2233. 2006. View Article : Google Scholar
|
|
11
|
Leong KG and Gao WQ: The Notch pathway in
prostate development and cancer. Differentiation. 76:699–716. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Miele L and Osborne B: Arbiter of
differentiation and death: Notch signaling meets apoptosis. J Cell
Physiol. 181:393–409. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang Z, Li Y, Banerjee S and Sarkar FH:
Exploitation of the Notch signaling pathway as a novel target for
cancer therapy. Anticancer Res. 28:3621–3630. 2008.
|
|
14
|
Yin L, Velazquez OC and Liu ZJ: Notch
signaling: Emerging molecular targets for cancer therapy. Biochem
Pharmacol. 80:690–701. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Weaver KL, Alves-Guerra MC, Jin K, Wang Z,
Han X, Ranganathan P, Zhu X, DaSilva T, Liu W, Ratti F, et al: NACK
is an integral component of the Notch transcriptional activation
complex and is critical for development and tumorigenesis. Cancer
Res. 74:4741–4751. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Androutsellis-Theotokis A, Leker RR,
Soldner F, Hoeppner DJ, Ravin R, Poser SW, Rueger MA, Bae SK,
Kittappa R and McKay RD: Notch signalling regulates stem cell
numbers in vitro and in vivo. Nature. 442:823–826. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bi P and Kuang S: Notch signaling as a
novel regulator of metabolism. Trends Endocrinol Metab. 26:248–255.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Guruharsha KG, Kankel MW and
Artavanis-Tsakonas S: The Notch signalling system: Recent insights
into the complexity of a conserved pathway. Nat Rev Genet.
13:654–666. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Galluzzo P and Bocchetta M: Notch
signaling in lung cancer. Expert Rev Anticancer Ther. 11:533–540.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nickoloff BJ, Osborne BA and Miele L:
Notch signaling as a therapeutic target in cancer: A new approach
to the development of cell fate modifying agents. Oncogene.
22:6598–6608. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ranganathan P, Weaver KL and Capobianco
AJ: Notch signalling in solid tumours: A little bit of everything
but not all the time. Nat Rev Cancer. 11:338–351. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Takebe N, Harris PJ, Warren RQ and Ivy SP:
Targeting cancer stem cells by inhibiting Wnt, Notch, and Hedgehog
pathways. Nat Rev Clin Oncol. 8:97–106. 2011. View Article : Google Scholar
|
|
23
|
Wael H, Yoshida R, Kudoh S, Hasegawa K,
Niimori-Kita K and Ito T: Notch1 signaling controls cell
proliferation, apoptosis and differentiation in lung carcinoma.
Lung Cancer. 85:131–140. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jiang X, Zhou JH, Deng ZH, Qu XH, Jiang HY
and Liu Y: Expression and significance of Notch1, Jagged1 and VEGF
in human non-small cell lung cancer. Zhong Nan Da Xue Xue Bao Yi
Xue Ban. 32:1031–1036. 2007.In Chinese.
|
|
25
|
Chen Y, De Marco MA, Graziani I, Gazdar
AF, Strack PR, Miele L and Bocchetta M: Oxygen concentration
determines the biological effects of NOTCH-1 signaling in
adenocarcinoma of the lung. Cancer Res. 67:7954–7959. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Baumgart A, Seidl S, Vlachou P, Michel L,
Mitova N, Schatz N, Specht K, Koch I, Schuster T, Grundler R, et
al: ADAM17 regulates epidermal growth factor receptor expression
through the activation of Notch1 in non-small cell lung cancer.
Cancer Res. 70:5368–5378. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ji X, Wang Z, Geamanu A, Sarkar FH and
Gupta SV: Inhibition of cell growth and induction of apoptosis in
non-small cell lung cancer cells by delta-tocotrienol is associated
with notch-1 down-regulation. J Cell Biochem. 112:2773–2783. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Eliasz S, Liang S, Chen Y, De Marco MA,
Machek O, Skucha S, Miele L and Bocchetta M: Notch-1 stimulates
survival of lung adenocarcinoma cells during hypoxia by activating
the IGF-1R pathway. Oncogene. 29:2488–2498. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen Y, Li D, Liu H, Xu H, Zheng H, Qian
F, Li W, Zhao C, Wang Z and Wang X: Notch-1 signaling facilitates
survivin expression in human non-small cell lung cancer cells.
Cancer Biol Ther. 11:14–21. 2011. View Article : Google Scholar
|
|
30
|
Manning G, Whyte DB, Martinez R, Hunter T
and Sudarsanam S: The protein kinase complement of the human
genome. Science. 298:1912–1934. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tanaka H, Katoh H and Negishi M: Pragmin,
a novel effector of Rnd2 GTPase, stimulates RhoA activity. J Biol
Chem. 281:10355–10364. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Nguyen TA and Fruehauf JH: Transkingdom
RNA interference (tkRNAi): A novel method to induce therapeutic
gene silencing. Methods Mol Biol. 514:27–34. 2009. View Article : Google Scholar
|
|
33
|
Geng J, Li X, Zhou Z, Wu CL, Dai M and Bai
X: EZH2 promotes tumor progression via regulating VEGF-A/AKT
signaling in non-small cell lung cancer. Cancer Lett. 359:275–287.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Koff JL, Ramachandiran S and
Bernal-Mizrachi L: A time to kill: Targeting apoptosis in cancer.
Int J Mol Sci. 16:2942–2955. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Drosopoulos K and Pintzas A: Multifaceted
targeting in cancer: The recent cell death players meet the usual
oncogene suspects. Expert Opin Ther Targets. 11:641–659. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Taylor K, Micha D, Ranson M and Dive C:
Recent advances in targeting regulators of apoptosis in cancer
cells for therapeutic gain. Expert Opin Investig Drugs. 15:669–690.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Fischer A and Gessler M: Delta-Notch - and
then? Protein interactions and proposed modes of repression by Hes
and Hey bHLH factors. Nucleic Acids Res. 35:4583–4596. 2007.
View Article : Google Scholar :
|
|
38
|
Nakagawa O, McFadden DG, Nakagawa M,
Yanagisawa H, Hu T, Srivastava D and Olson EN: Members of the HRT
family of basic helix-loop-helix proteins act as transcriptional
repressors downstream of Notch signaling. Proc Natl Acad Sci USA.
97:13655–13660. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Iso T, Kedes L and Hamamori Y: HES and
HERP families: Multiple effectors of the Notch signaling pathway. J
Cell Physiol. 194:237–255. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Al-Hussaini H, Subramanyam D, Reedijk M
and Sridhar SS: Notch signaling pathway as a therapeutic target in
breast cancer. Mol Cancer Ther. 10:9–15. 2011. View Article : Google Scholar
|
|
41
|
Candy PA, Phillips MR, Redfern AD, Colley
SM, Davidson JA, Stuart LM, Wood BA, Zeps N and Leedman PJ:
Notch-induced transcription factors are predictive of survival and
5-fluorouracil response in colorectal cancer patients. Br J Cancer.
109:1023–1030. 2013. View Article : Google Scholar : PubMed/NCBI
|