Introduction
Esophageal squamous cell carcinoma (ESCC) is a
malignancy associated with high mortality in China.
Multidisciplinary treatment (surgery, radiotherapy and
chemotherapy) is generally used to treat locally advanced and
metastatic ESCC. Unfortunately ESCC is frequently resistant to
radiotherapy and chemotherapy. The 5-year survival of ESCC patients
is only ~20.9% (1). Better
understanding of prognostic indicators in ESCC is needed.
Heme oxygenase-1 (HO-1) is a stress-induced gene
with anti-inflammatory, anti-apoptosis, antioxidation and drug
resistance inducing properties. The expression of HO-1 in normal
human tissues is extremely low. Increased expression of HO-1 has
been seen after exposure to alcohol and spicy foods. Increased HO-1
expression has been reported in a variety of tumors and has been
associated with ESCC tumor invasion, metastases,
chemotherapy-induced apoptosis and worse patient prognosis
(2,3). HO-1 expression is thought to be
regulated by upstream expression of nuclear-related factor 2
(Nrf-2). Cell growth is inhibited with inhibition of HO-1 and
increased Nrf-2 expression (4).
Increased HO-1 expression is also associated with increased removal
of reactive oxygen species (ROS) and maintenance of the internal
cellular environment (5).
We previously reported that overexpression of HO-1
can significantly impede the apoptosis of ESCC cells (6). It is not known whether decreasing HO-1
expression induces apoptosis or impact ROS removal. In order to
better understand HO-1 control, we evaluated the expression of
HO-1, HIF-1α and EGFR protein in human ESCC tissue using
immunochemistry. We also evaluated intracellular ROS levels and
apoptosis-related HO-1 protein levels in ESCC cell lines.
Materials and methods
Patients and tissue specimens
Medical records at the Union Hospital Affiliated to
Huazhong University of Science and Technology were reviewed for
patients with ESCC. All patients had histologic confirmation of
their diagnosis. Clinical features were collected including patient
age, clinical stage, tumor grade, presence of mediastinal lymph
node metastases. Tumor blocks were obtained for immunohistochemical
evaluation of HO-1, HIF-1α and EGFR expression in the tumor.
Clinical staging was performed using WHO 2003 AJCC Sixth Edition
PTNM staging criteria. No patient received chemotherapy or
radiotherapy before biopsy or surgical resection. The use of ESCC
specimens was approved by the local Ethics Committee. All patients
have given their informed consent for the present study.
Immunohistochemical staining
Tumor blocks were obtained from the department of
Pathology and immunohistochemical staining was performed as
previously described (7). The
protein expression of HO-1, HIF-1α and EGFR protein were scored
according to the number of cells exhibiting cytoplasmic and nuclear
staining using the following classification system: I, no staining;
II, nuclear staining in 10% of cells and/or weak cytoplasmic
staining; III, nuclear staining in 10–50% of cells and/or distinct
cytoplasmic staining; IV, nuclear staining in 50% of cells and/or
strong cytoplasmic staining. Tumors with I or II amounts of
staining were considered negative for expression and tumors with
III or IV were considered positive.
Cell culture and transfection with HO-1
small interfering RNA (siRNA)
The human ESCC cell lines TE-13 and Eca109 were a
gift from the He Bei, Medical University Affiliated Cancer
Hospital. HO-1-siRNA and the empty vector containing a nonsense RNA
sequence were purchased from Guangzhou RiboBio Co., Ltd.,
Guangzhou, China. The RNA transfection kit was from Guangzhou
RiboBio Co, Ltd. TE-13 and Eca109 were cultured in Dulbecco's
modified Eagle's medium (DMEM) median containing 10% fetal bovine
serum. The two cell lines were maintained in a humidified incubator
at 37°C in a 5% CO2 atmosphere. Four experimental groups
were examined including negative untreated cell line controls, cell
line controls transfected with a nonsense RNA sequence, cell lines
transfected with si-HO-1 and cell lines transfected with
si-HO-1-NAC (a powerful antioxidant, NAC namely N-acetyl cysteine).
Transfection was performed using 100 nM siRNA. Logarithmic growth
phase cells were harvested and plated at a density of
2×105 cells/well in 6-well plates. Cells were grown and
the expression of HO-1 in transfected cells quantified using
real-time PCR.
RNA isolation and reverse-transcription
PCR
Cell lines were harvested at different time points
after transfection. Total RNA was extracted from cell lines using
TRIzol reagent (Invitrogen). The purity and concentration of RNA
was determined using a NanoDrop Spectrophotometer (ND-2000;
NanoDrop Technology, Wilmington, DE, USA). cDNA was produced using
a reverse-transcription kit (Takara Bio, Inc.). Real-time
quantitative PCR was performed using the SYBR-Green Prime Script
RT-PCR kit (Takara Bio, Inc.) and the Real-time PCR detection
system (Applied Biosystems). GAPDH was used as an internal control.
The primers for HO-1 were: forward, GTCAGGCAGAGGGTGATAGAAG and
reverse, GTGTAAGGACCCATCGGAGAAG. The primers for GAPDH were:
forward, TCCCATCACCATCTTCCAG and reverse, GAGCCCCAGCCTTCTCCAT. The
results of three independent experiments were analyzed using the
2−∆∆Ct method.
MTT assay
TE-13 and Eca109 cells were seeded into 96-well
plates at a density of 8×103 cells/well, in a volume of
180 µl culture medium. The four previously described
treatment groups were evaluated. Cells in each group were cultured
to 70% confluency before transfection. MTT (20 µl) was then
added to each well after 24 h, 48 h and 72 h of incubation and
incubated for 4 h. The media were then removed and 150 µl
dimethylsulfoxide (DMSO) added to each well. The incubation plates
were gently mixed for 10 min before viability analysis. Cell
viability was determined as absorbance at 490 nm at 24 h, 48 h and
72 h after MTT treatment. Four wells of each cell line were
evaluated for each experiment. The results of three independent
experiments are reported.
Flow cytometric analysis
TE-13 and Eca109 cells were inoculated into 6-well
plates at a density of 2×105 cells/well. Procedures of
interfering HO-1 in TE-13 and Eca109 cells were executed according
to manual of infection reagent kit. Flow cytometric analysis was
performed as previously described (6). The results of three independent
experiments were reported.
Western blot analysis
HO-1 interference in two cell lines was treated with
transfection reagent kit represented above. Protein lysates were
obtained 48 h after transfection. Protein (100 µg) was
loaded into each gel lane prior to electrophoresis on 12% SDS
polyacrylamide gels. Electrophoresed protein was transferred to
polyvinylidene defluoride (PVDF) membranes. The membranes were
blocked using 5% skimmed milk at room temperature for 1.5 h, and
then incubated at 4°C overnight with primary antibodies directed
against HO-1, Bax, Bcl-2, A-caspase-3/-9 or β-actin (1:500
dilution; Abnova Corporation, Taipei City, Taiwan).
Peroxidase-conjugated secondary antibodies were used to visualize
the primary antibodies with an enhanced chemiluminescence reagent
(Beyotime). Protein expression was quantitated using densitometry
analysis and normalized against β-actin.
Statistical analysis
SPSS 17.0 software (SPSS, Inc., Chicago, IL, USA)
was used to evaluate data. Data are reported as the mean ± standard
deviation (SD). Statistical significance was determined using a
two-sided, unpaired t-test. P-value <0.05 was considered to
indicate a statistically significant result.
Results
In total, 143 male patients with ESCC were
identified at the Union Hospital Affiliated to Huazhong University
of Science and Technology between April 2006 and October 2007. The
mean patient age was 59.5±1.4 years old (range, 40–83 years
old).
Protein expression of HO-1, HIF-1α and
EGFR in human ESCC tumors
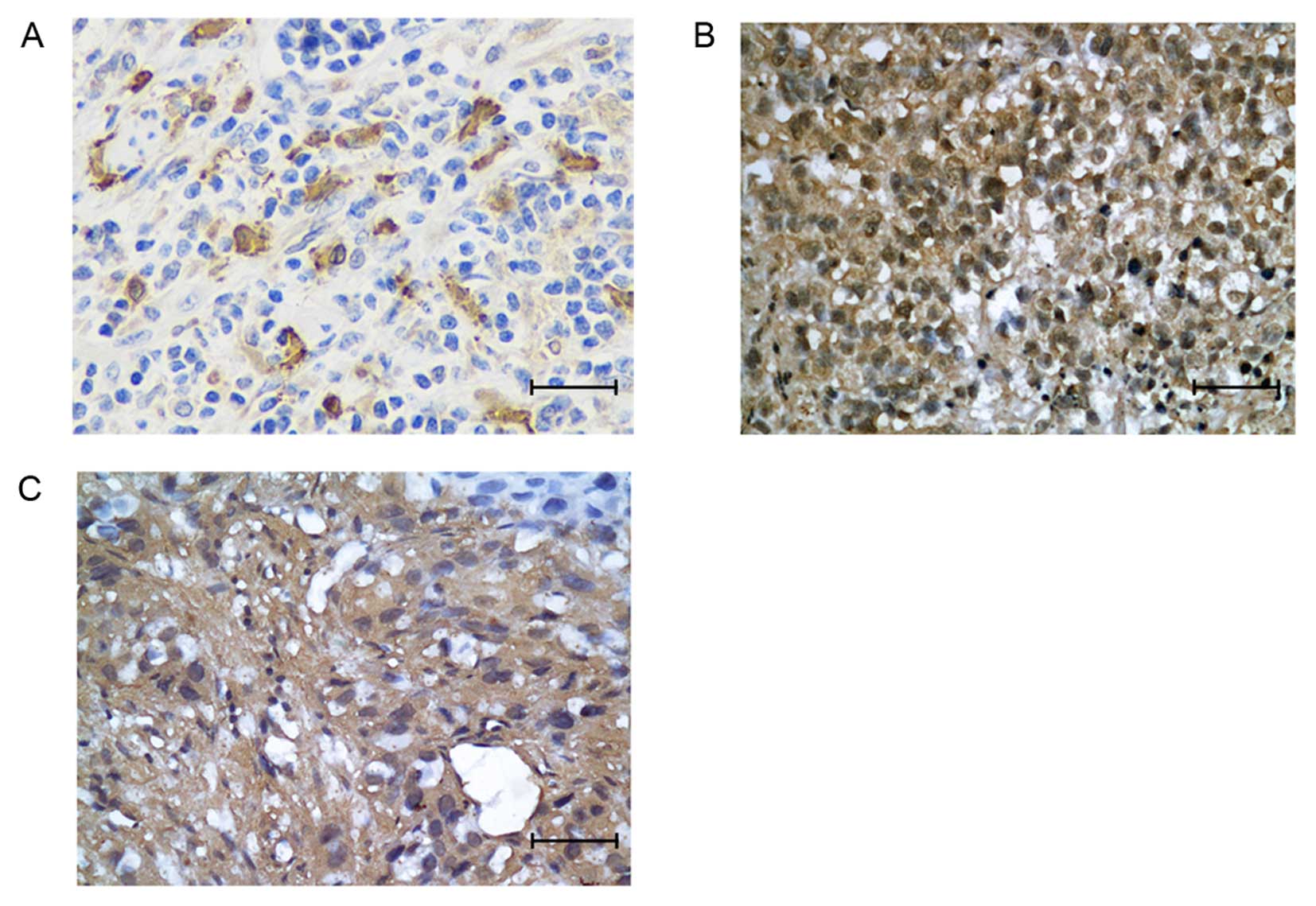
Immunohistochemical probing of fixed resected ESCC
tissues was performed. HO-1 was expressed in 58 of 143 (40.6%)
patient tumors. The HO-1 protein was mainly localized to the
cytoplasm of tumor cells (Fig. 1A).
HIF-1α expression was observed in 43.4% (62/143) of patient tumors,
EGFR in 58% (83/143). HIF-1α was mainly located in the nucleus and
EGFR was mainly localized in cytoplasm (Fig. 1B and C).
Correlation between HO-1 expression and
HIF-1α, EGFR expression in ESCC tumors
HO-1 expression was positively correlated with
HIF-1α and EGFR expression in the 143 ESCC tumors (P<0.001 for
both, Table I).
 | Table IRelationship between HO-1 expression
and HIF-1α, EGFR expression in ESCC tumors (N=143). |
Table I
Relationship between HO-1 expression
and HIF-1α, EGFR expression in ESCC tumors (N=143).
| Related gene | HO-1 expression
| χ2 | P-value |
|---|
| Negative | Positive |
|---|
| HIF-1α | | | | |
| Negative | 66 | 15 | 37.646 | <0.001 |
| Positive | 19 | 43 | | |
| EGFR | | | | |
| Negative | 44 | 16 | 10.914 | <0.001 |
| Positive | 41 | 42 | | |
Correlation between HO-1 expression in
ESCC tumors and clinicopathological characteristics
There was no association between HO-1 expression and
patient clinical stage (P=0.641) or age (P=0.409) (Table II). Increasing tumor histologic
grade was associated with increasing expression of HO-1 (P=0.001).
There was no correlation between HO-1 expression and mediastinal
lymph node metastases (P=0.415).
 | Table IICorrelation between HO-1 and
clinicopathological characteristics. |
Table II
Correlation between HO-1 and
clinicopathological characteristics.
| Clinicopathological
characteristics | HO-1
| χ2 | P-value |
|---|
| N | Positive rate
(%) |
|---|
| Age (years) | | | | |
| <60 | 65 | 25 (38.5) | 0.218 | 0.641 |
| ≥60 | 78 | 33 (42.3) | | |
| Clinical stage | | | | |
| II | 39 | 18 (46.2) | | |
| III | 53 | 23 (43.5) | 1.788 | 0.409 |
| IV | 51 | 17 (33.3) | | |
| Grade | | | | |
| G1 | 32 | 9 (28.1) | | |
| G2 | 50 | 13 (26.0) | 15.068 | 0.001 |
| G3 | 61 | 36 (59.0) | | |
| Mediastinal lymph
node metastasis | | | | |
| Yes | 73 | 32 (43.8) | 0.664 | 0.415 |
| No | 70 | 26 (37.1) | | |
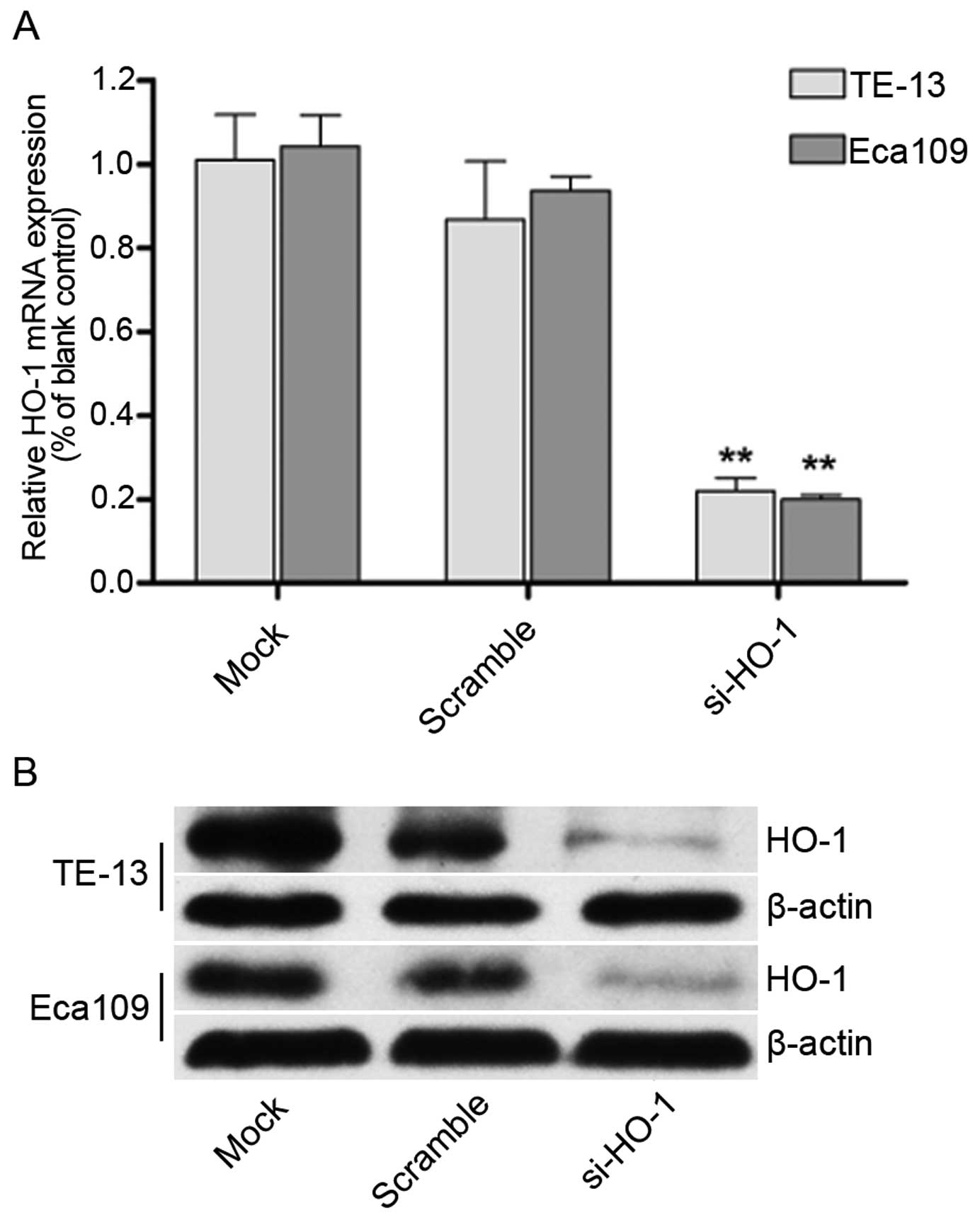
Effect of transfection with HO-1 siRNA on
HO-1 mRNA and protein expression in TE-13 and Eca109 cell
lines
Real-time quantitative PCR (Fig. 2A) and western blotting (Fig. 2B) demonstrated decreased expression
of HO-1 in the TE-13 and Eca109 cell lines. There was no change in
expression of the untransfected control cell lines or the cell
lines transfected with nonsense mRNA. Significantly less HO-1
expression was seen in the HO-1 siRNA transfected cell lines
(P<0.01 for both cell lines).
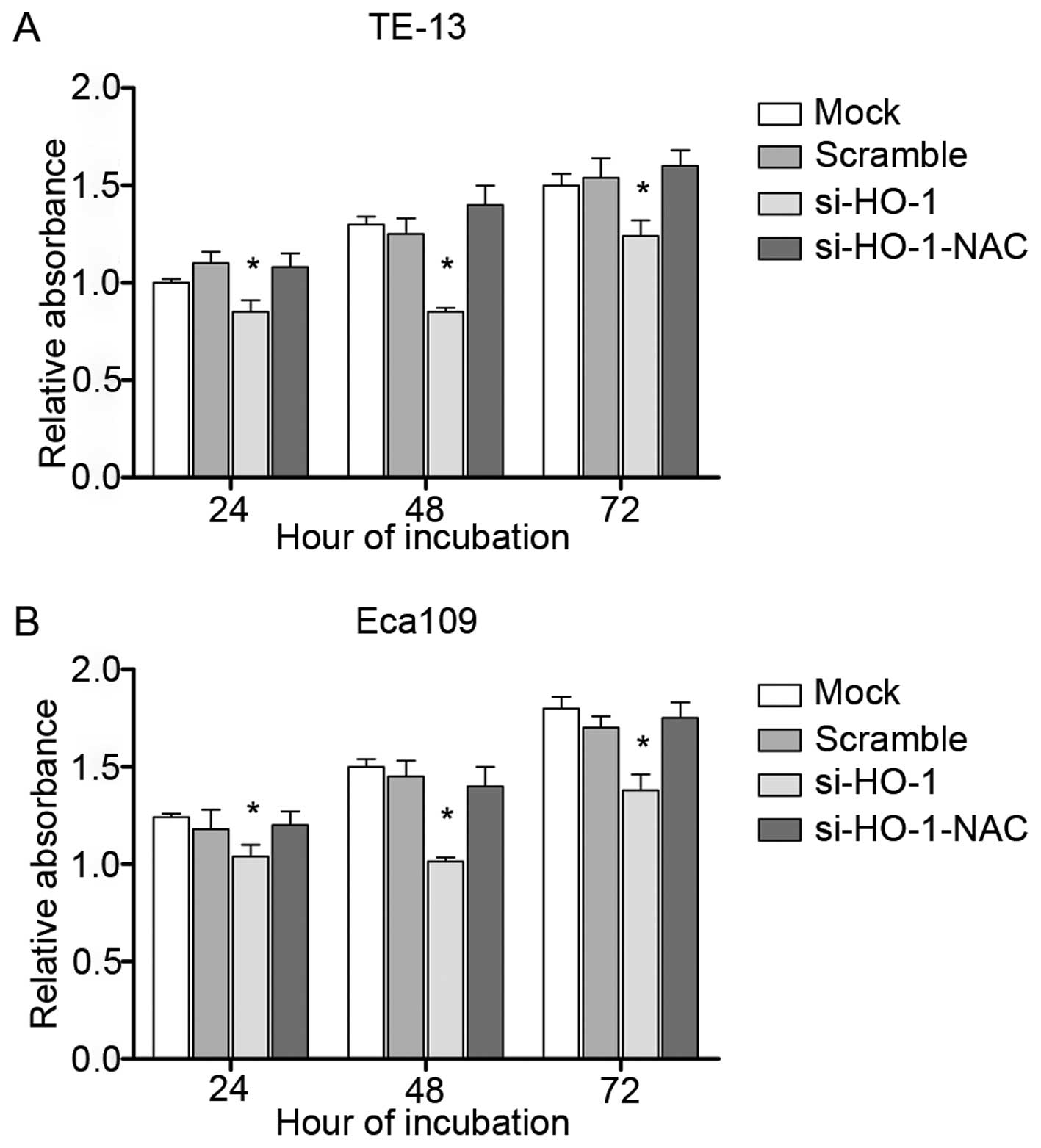
Effect of blocking HO-1 expression on
cell line viability
MTT assays were performed to assess cell viability.
All four treatment groups had decreased cell viability at 24 h, 48
h and 72 h in TE-13 (Fig. 3A) and
Eca109 cell line (Fig. 3B) after
treatment, compared to their respective controls. The lowest cell
viability was observed at 48 h. si-HO-1-treated cells had less cell
viability at each time point, compared to untransfected cells and
cells transfected with nonsense RNA (P<0.05). The cell viability
of si-HO-1-NAC-treated cells was similar to that of the two control
groups (P>0.05).
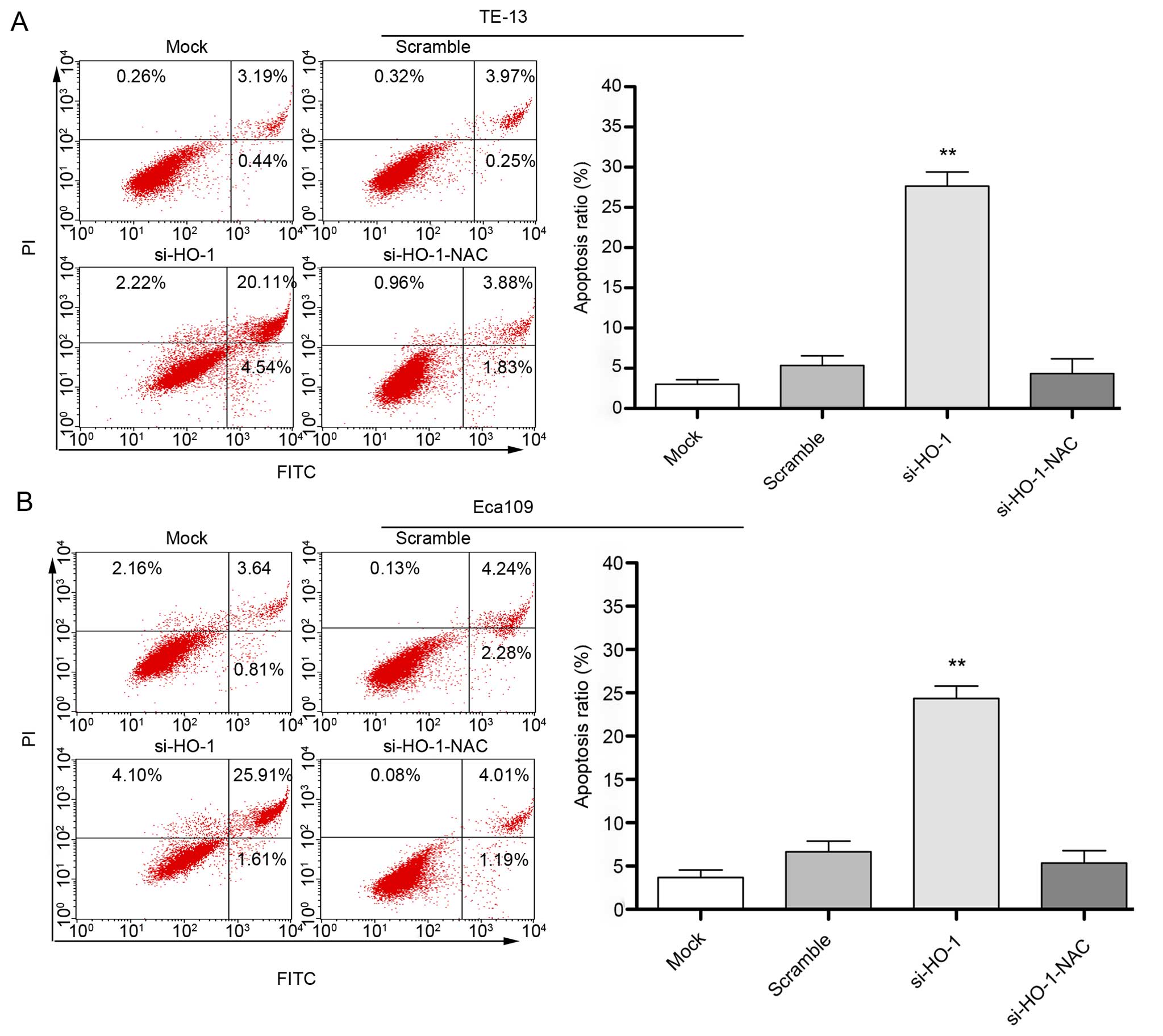
Effect of blocking HO-1 expression on
cell line apoptosis
Flow cytometry after 48 h treatment showed the
apoptosis rate of untransfected cells transfected with nonsense
RNA, cells transfected with si-HO-1 and cells transfected with
si-HO-1-NAC was (3.8±1.2)%, (6.8±1.9)%, (27.4±1.6)% and (4.1±1.5)%,
respectively (Fig. 4A) in the TE-13
cell line. The apoptosis rate in the same treatment groups was
(2.6±1.0)%, (5.5±1.6)%, (24.2±2.1)% and (3.9±1.7)%, respectively
(Fig. 4B) in the Eca109 cell line.
The rate of apoptosis was greatest in the si-HO-1-treated cell
lines (P<0.001) compared to the two control treatment groups in
both cell lines. NAC had a protective effect in cells transfected
with si-HO-1 (P<0.001).
Effect of blocking HO-1 expression on
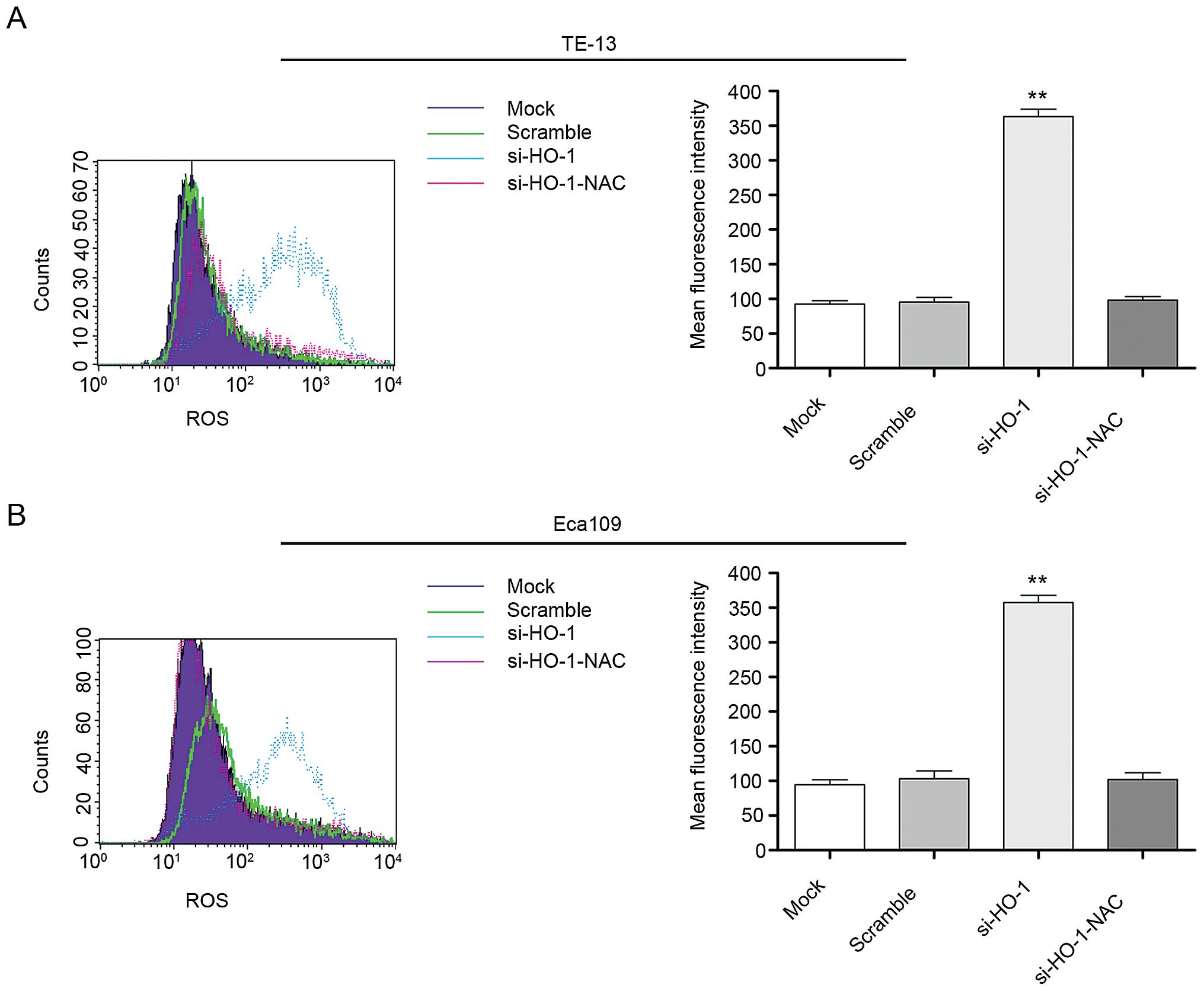
cell line intracellular ROS levels
Mean fluorescent intensity (MFI) of intracellular
ROS in all four cell line treatment groups was evaluated at 48 h
using flow cytometry. The MFI of the untreated cell line controls,
cell line controls transfected with nonsense RNA, cell lines
transfected with si-HO-1 and cell lines transfected with
si-HO-1-NAC was 98.1±4.7, 99.4±5.2, 360.5±8.8 and 105.1±4.0,
respectively, in TE-13 cells (Fig.
5A) and 95.3±3.9, 103.7±6.3, 350.1±7.2 and 101.4±5.7,
respectively, in Eca109 cells (Fig.
5B). The MFI of the si-HO-1 transfected cell lines were
significantly greater than that of the two control groups
(P<0.001 for both cell lines). The MFI of the si-HO-1-NAC
transfected group was significantly less than that of the si-HO-1
transfected cell lines (P<0.001 for both cell lines).
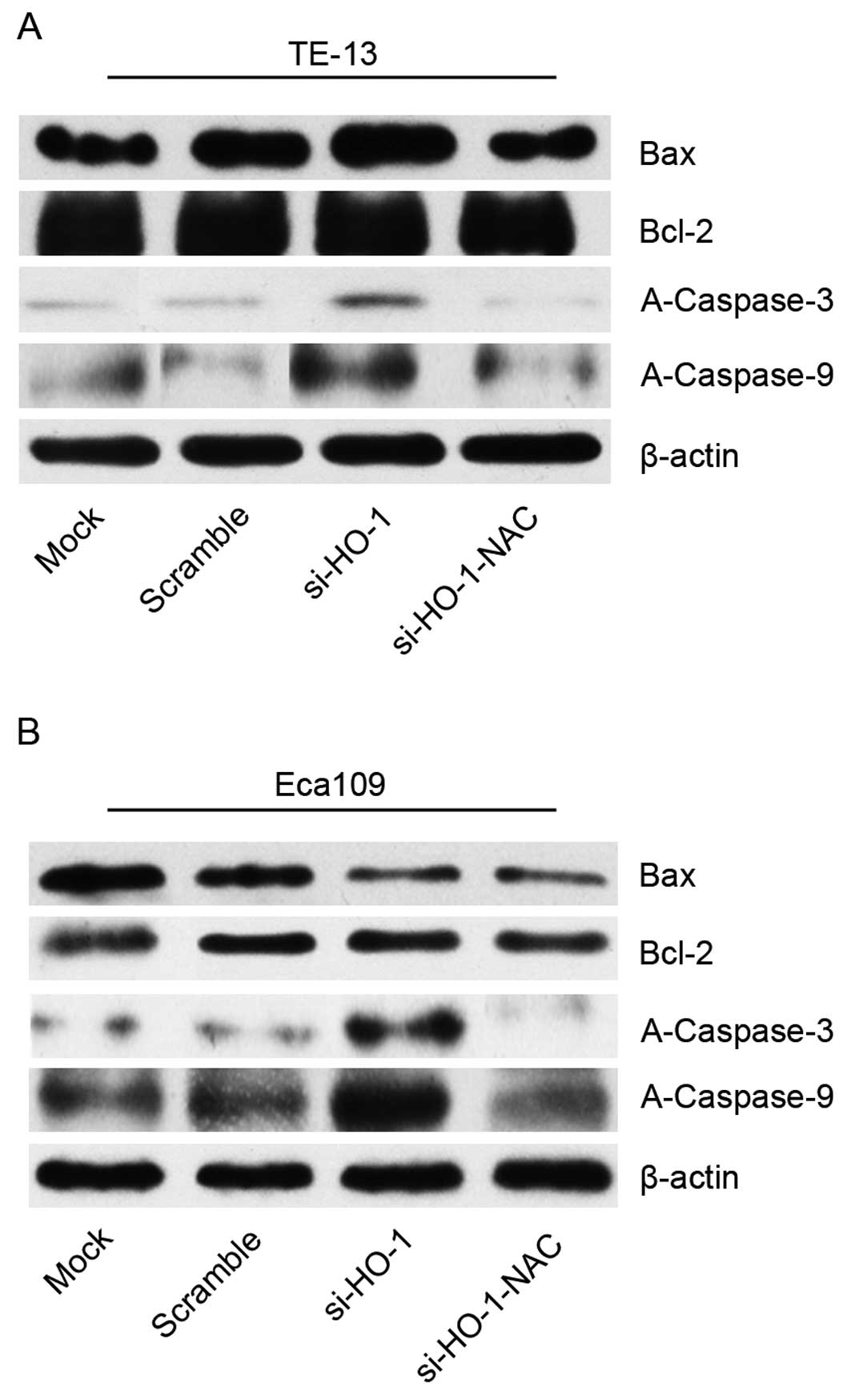
Effect of blocking HO-1 expression on
cell line protein expression of Bax, Bcl-2, A-caspase-3 and -9
Protein expression was evaluated using western blot
analyses of cell line protein isolated 48 h after treatment. There
was no statistical difference in Bax or Bcl-2 expression of cell
lines transfected with si-HO-1 or si-HO-1-NAC, compared to control
(scramble). There was no statistical difference in the Bax/Bcl-2
expression ratio of cell lines transfected with si-HO-1 or
si-HO-1-NAC.
Expression of A-caspase-3 and -9 was increased in
the si-HO-1 group, compared to the two control groups (P<0.05
for both groups). The si-HO-1-NAC transfected cell lines had
significantly less expression of A-caspase-3 and -9 than the
si-HO-1 transfected cell lines (P<0.05) (Fig. 6).
Discussion
HO-1 is an enzyme with strong antioxidant activity.
The products of HO-1, biliverdin, carbon monoxide and ferrous iron,
also have various antioxidant properties. HO-1 thus plays a vital
role in the antioxidant system (8).
Increased HO-1 expression has been observed in breast, gastric and
lung cancer. Moreover, it has been reported that overexpression of
HO-1 could inhibit tumor cellular apoptosis and promote tumor cell
growth (9–11).
We found that HO-1, HIF-1α and EGFR were expressed
in 40.6%, 43.4% and 58%, respectively, of 143 ESCC tumors. We also
found that HO-1 expression was related to tumor grade, but not
related to clinical stage or the presence of mediastinal lymph node
metastases. These findings suggest HO-1 may have a role in ESCC
progression.
As in general, hypoxia exists in cancer
microenvironment also in ESCC. Under hypoxia condition, HIF-1α
acting as a key transcription factor is highly expressed and
oxygen-free radicals accumulate. Evidence has shown that the
expression of HO-1 was increased by HIF-1α (12,13).
Moreover, oxidation stress exists in tumors (14). Additionally, we found that HO-1 is
positively correlated with HIF-1α. As a result, we inferred that
HIF-1α prompted the expression of HO-1, and that the oxidation
stress resulted from hypoxia also contributed to the increased
expression of HO-1. High expression of EGFR can suppress tumor cell
apoptosis and promote angiogenesis, proliferation and metastasis of
tumor cells. EGFR could induce expression of HO-1 in colon cancer;
EGFR-induced colon cancer cell proliferation was inhibited by the
decreased expression of HO-1 (15).
Thus, we deduced that EFGR could stimulate the expression of HO-1,
as well. Taken together, we concluded that the expression of
HIF-1α, EGFR and HO-1 were rather high and the expression of HO-1
was induced by HIF-1α and EGFR in ESCC tumor progression.
We previously reported that ethanol increased HO-1
expression and decreased apoptosis in ESCC cells (6). In the present study, we demonstrated
that decreasing HO-1 expression in ESCC cell lines decreased
cellular proliferation and increased apoptosis. We then
investigated whether this finding was associated with intracellular
ROS levels or activation of apoptosis signaling pathways.
Decreasing cell line HO-1 expression was found to be associated
with increased intracellular ROS levels. Lin et al have
reported that decreased HO-1 expression in renal carcinoma cells
was associated with increased intracellular ROS production and that
this was associated with damage to cellular DNA (16). ROS is a major product of
oxidation-reduction reactions in human cells. Various ROS are
always found in normally functioning cells and are thought to be a
normal part of cell growth, proliferation and differentiation. ROS
are usually found in very high levels in tumor cells (14). These high intracellular levels could
affect normal cellular function and may contribute to tumor
progression (17,18)
We hypothesized that the decrease in HO-1 expression
and ROS levels may affect the expression of apoptosis-related
proteins. Bcl-2 and Bax are two important components of the
mitochondrial apoptotic pathway. Bcl-2 inhibits the release of
cytochrome c from mitochondria to cytosol, which plays a
vital role in inhibiting apoptosis. Bax has an opposing effect on
the action of Bcl-2. ROS activates the mitochondrial apoptotic
pathway by increasing Bax expression and decreasing Bcl-2
expression. Hambright et al have reported that NAC, a
specific ROS scavenger, could inhibit the upregulation of Bax and
downregulation of Bcl-2 by altering ROS levels in melanoma cells
(19). However, we found no
significant changes in Bax or Bcl-2 expression, or in the Bax/Bcl-2
expression ratio in ESCC cell lines we examined.
The powerful antioxidant NAC was used to scavenge
ROS in our experiment. Treatment with NAC after transfection with
si-HO-1 was associated with a significant decrease in MFI,
increased cellular proliferation, and decreased apoptosis. These
findings support a relationship between HO-1 expression and these
events.
ROS are largely generated in mitochondria. The
mitochondrial apoptosis pathway is a major apoptotic pathway that
acts through the production of ROS. Caspases are responsible for
the deliberate disassembly of cells into apoptotic bodies during
apoptosis. Caspases-3, -8 and -9 appear to be regulators of this
process. Caspase-9 activates disassembly in response to events that
trigger the release of cytochrome c from mitochondria.
Caspase-3 activity appears to regulate the speed of this response.
The study of caspase-3−/− and caspase-9−/−
mice suggested the caspase pathway used for disassembly is cell
type specific (20–22). Our findings suggest that control of
HO-1 expression contributes to this process.
si-HO-1 transfected cell lines examined by us had
significantly greater expression of A-caspase-3 and -9 than
controls. Treatment of these cell lines with NAC, significantly
decreased the expression of A-caspase-3 and -9. These findings
suggest the mitochondrial apoptosis pathway may not be the only
mechanism of controlling ROS-mediated apoptosis and supports the
role of HO-1 as a mediator of this alternate pathway. Choi et
al reported that ROS can impact the expression of Fas, Fas-L
and caspases-3, -8 and -9 in gastric cancer cells (23). This finding further supports the
presence of a ROS-mediated non-mitochondrial pathway for cellular
apoptosis.
In summary, we found increased tissue expression of
HO-1 in ESCC. This expression was correlated with tumor grade and
expression of EGFR and HIF-1α. Blocking HO-1 expression in ESCC
cell lines resulted in decreased cellular proliferation, increased
ROS levels, and increased cellular apoptosis. HO-1 appears to have
a role in tumor progression via a mitochondrion-independent
pathway.
Acknowledgments
The present study was supported by a grant from the
Hubei Province Natural Science Foundation (grant no. 2014CFB390)
and Wuhan Municipal Science and Technology Bureau (grant no.
2013060602010238).
References
|
1
|
Zeng H, Zheng R, Guo Y, Zhang S, Zou X,
Wang N, Zhang L, Tang J, Chen J, Wei K, et al: Cancer survival in
China, 2003–2005: A population-based study. Int J Cancer.
136:1921–1930. 2015. View Article : Google Scholar
|
|
2
|
Heasman SA, Zaitseva L, Bowles KM,
Rushworth SA and Macewan DJ: Protection of acute myeloid leukaemia
cells from apoptosis induced by front-line chemotherapeutics is
mediated by haem oxygenase-1. Oncotarget. 2:658–668. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tibullo D, Barbagallo I, Giallongo C, La
Cava P, Parrinello N, Vanella L, Stagno F, Palumbo GA, Li Volti G
and Di Raimondo F: Nuclear translocation of heme oxygenase-1
confers resistance to imatinib in chronic myeloid leukemia cells.
Curr Pharm Des. 19:2765–2770. 2013. View Article : Google Scholar
|
|
4
|
Na HK and Surh YJ: Oncogenic potential of
Nrf2 and its principal target protein heme oxygenase-1. Free Radic
Biol Med. 67:353–365. 2014. View Article : Google Scholar
|
|
5
|
Wegiel B, Nemeth Z, Correa-Costa M, Bulmer
AC and Otterbein LE: Heme oxygenase-1: A metabolic nike. Antioxid
Redox Signal. 20:1709–1722. 2014. View Article : Google Scholar :
|
|
6
|
Hu JL, Xiao L, Li ZY, Wang Q, Chang Y and
Jin Y: Upregulation of HO-1 is accompanied by activation of p38MAPK
and mTOR in human oesophageal squamous carcinoma cells. Cell Biol
Int. 37:584–592. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yang SL, Liu LP, Jiang JX, Xiong ZF, He QJ
and Wu C: The correlation of expression levels of HIF-1α and HIF-2α
in hepatocellular carcinoma with capsular invasion, portal vein
tumor thrombi and patients' clinical outcome. Jpn J Clin Oncol.
44:159–167. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang S, Hannafon BN, Wolf RF, Zhou J,
Avery JE, Wu J, Lind SE and Ding WQ: Characterization of
docosahexaenoic acid (DHA)-induced heme oxygenase-1 (HO-1)
expression in human cancer cells: The importance of enhanced BTB
and CNC homology 1 (Bach1) degradation. J Nutr Biochem. 25:515–525.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Noh SJ, Bae JS, Jamiyandorj U, Park HS,
Kwon KS, Jung SH, Youn HJ, Lee H, Park BH, Chung MJ, et al:
Expression of nerve growth factor and heme oxygenase-1 predict poor
survival of breast carcinoma patients. BMC Cancer. 13:5162013.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yin Y, Liu Q, Wang B, Chen G, Xu L and
Zhou H: Expression and function of heme oxygenase-1 in human
gastric cancer. Exp Biol Med. 237:362–371. 2012. View Article : Google Scholar
|
|
11
|
Degese MS, Mendizabal JE, Gandini NA,
Gutkind JS, Molinolo A, Hewitt SM, Curino AC, Coso OA and
Facchinetti MM: Expression of heme oxygenase-1 in non-small cell
lung cancer (NSCLC) and its correlation with clinical data. Lung
Cancer. 77:168–175. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Miyake M, Fujimoto K, Anai S, Ohnishi S,
Kuwada M, Nakai Y, Inoue T, Matsumura Y, Tomioka A, Ikeda T, et al:
Heme oxygenase-1 promotes angiogenesis in urothelial carcinoma of
the urinary bladder. Oncol Rep. 25:653–660. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ben Mosbah I, Mouchel Y, Pajaud J, Ribault
C, Lucas C, Laurent A, Boudjema K, Morel F, Corlu A and Compagnon
P: Pretreatment with mangafodipir improves liver graft tolerance to
ischemia/reperfusion injury in rat. PloS One. 7:e502352012.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen W, Balakrishnan K, Kuang Y, Han Y, Fu
M, Gandhi V and Peng X: Reactive oxygen species (ROS) inducible DNA
cross-linking agents and their effect on cancer cells and normal
lymphocytes. J Med Chem. 57:4498–4510. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lien GS, Wu MS, Bien MY, Chen CH, Lin CH
and Chen BC: Epidermal growth factor stimulates nuclear factor-κB
activation and heme oxygenase-1 expression via c-Src, NADPH
oxidase, PI3K, and Akt in human colon cancer cells. PloS One.
9:e1048912014. View Article : Google Scholar
|
|
16
|
Lin PH, Lan WM and Chau LY: TRC8
suppresses tumorigenesis through targeting heme oxygenase-1 for
ubiquitination and degradation. Oncogene. 32:2325–2334. 2013.
View Article : Google Scholar
|
|
17
|
Trachootham D, Alexandre J and Huang P:
Targeting cancer cells by ROS-mediated mechanisms: A radical
therapeutic approach? Nat Rev Drug Discov. 8:579–591. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sanchez-Alvarez R, Martinez-Outschoorn UE,
Lin Z, Lamb R, Hulit J, Howell A, Sotgia F, Rubin E and Lisanti MP:
Ethanol exposure induces the cancer-associated fibroblast phenotype
and lethal tumor metabolism: Implications for breast cancer
prevention. Cell Cycle. 12:289–301. 2013. View Article : Google Scholar :
|
|
19
|
Hambright HG, Meng P, Kumar AP and Ghosh
R: Inhibition of PI3K/AKT/mTOR axis disrupts oxidative
stress-mediated survival of melanoma cells. Oncotarget.
6:7195–7208. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Josefsson EC, Burnett DL, Lebois M,
Debrincat MA, White MJ, Henley KJ, Lane RM, Moujalled D, Preston
SP, O'Reilly LA, et al: Platelet production proceeds independently
of the intrinsic and extrinsic apoptosis pathways. Nat Commun.
5:34552014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Oh BS, Shin EA, Jung JH, Jung DB, Kim B,
Shim BS, Yazdi MC, Iranshahi M and Kim SH: Apoptotic effect of
galbanic acid via activation of caspases and inhibition of Mcl-1 in
H460 non-small lung carcinoma cells. Phytother Res. 29:844–849.
2015. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jiang L, Li L, He X, Yi Q, He B, Cao J,
Pan W and Gu Z: Overcoming drug-resistant lung cancer by paclitaxel
loaded dual-functional liposomes with mitochondria targeting and
pH-response. Biomaterials. 52:126–139. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Choi YH, Kang YJ, Kim SH, Sung B, Kim DH,
Hwang SY, Kim M, Lim HS, Yoon JH, Moon HR, et al: MHY-449 induces
apoptotic cell death through ROS- and caspase-dependent pathways in
AGS human gastric cancer cells. Cancer Res. 75(Suppl 15):
S17732015. View Article : Google Scholar
|