Introduction
Breast cancer is the most frequently diagnosed
cancer and the leading cause of cancer death in female worldwide,
accounting for 23% (1.38 million) of the total new cancer cases and
14% (458,400) of the total cancer deaths in 2008 (1). At present, chemotherapy is one of the
main methods to treat cancer, and drug-resistance is the main
obstacle to ensure optimal outcomes. So drug-resistance is one of
the key blocks on the way to cure cancer.
Vinorelbine (Navelbine®) is a
semisynthetic vinca alkaloid that interferes with microtubule
assembly and induces a cell cycle arrest at mitosis due to its
microtubule targeting activity. Vinorelbine has a broad spectrum of
antitumor activity (2), it has been
shown to be active as a single agent in metastatic breast cancer,
with response rates of 40–60% in chemonaive disease and with good
tolerability (3). Vinorelbine tends
to be reserved for anthracycline-resistant disease in the second-
or third-line therapy. Although drug resistance is common in the
second- or third-line therapy, there are few studies of
vinorelbine-resistant breast cancer.
To illustrate the mechanism of drug resistance, many
research teams have successfully established a variety of
drug-resistant tumor cell lines (4–7).
Currently, the most typical method to establish a drug-resistant
tumor cell line in vitro is stimulating tumor cells with a
certain concentration of chemotherapy drug continuously while
increasing the concentration gradually (8–10), but
some studies state that this method has limitations (11,12).
As the purpose to establish a drug-resistant tumor cell line is to
provide a proper research tool for overcoming drug-resistance in
clinic, the method should imitate the clinical chemotherapy
setting, which commonly contains several cycles of 21 to 28 days
discontinuous administration (13,14).
In this study, two vinorelbine-resistant breast
cancer cell lines, BC-DS (BCap37-dose stimulated) and BC-TS
(BCap37-time stimulated), were developed from the chemo-sensitive
human breast cancer cell line BCap37 by different screening
strategies. By investigating their biological characterization and
drug-resistant traits, we found differences between them in
different levels. This indicates that different drug-resistant cell
lines can be developed with a certain drug even from the same cell
line.
Materials and methods
Cell lines and mice
Human breast cancer cell lines BCap37, BC-DS and
BC-TS, were cultured in RPMI-1640 medium supplemented with 10%
fetal bovine serum (FBS), and frozen in liquid nitrogen. After
thawing, experiments were done within two weeks. Female, aged 5–6
weeks, athymic nude (nu/nu) mice were purchased from Shanghai SLAC
Animal Facility. All animal care and experiments were conducted
according to Zhejiang University Animal Care Committee
guidelines.
Observation of morphology under
microscope
BCap37, BC-DS and BC-TS cells were sub-cultured into
6-cm dishes for 48 h to reach logarithmic growth phase. Cells were
first observed and photographed under an inverted microscope. Then
Giemsa staining (Jiangcheng Biotech, Nanjing, China) was carried
out.
Cell growth rate in vitro
Cell lines was plated into ten 6-cm dishes with a
density of 4×104 cells/dish. Three cell counts for each
cell line were made every 24 h for 10 days. Cell growth curves were
made with cell number for ordinate and time for abscissa. Doubling
time (Td) was calculated based on the formula:
Td = T × lg2/lg (N1/N0).
N1 (N0) stands for the cell number at
T1 (T0) time during logarithmic growth phase.
T = T1 − T0.
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay
Cells were seeded at the amount of
5×103/well at 96-well tissue culture plates. After 12 h
of incubation, a series of drug concentration gradients were added
to the wells, 6 repeats for one concentration. Sixty-nine hours
later, MTT solution was added. Another 3 h later, the medium
containing MTT was replaced with 150 µl of DMSO in each well
to dissolve the formazan crystals. The absorbance was detected at
560 nM using a microplate reader (Bio-Rad, Sunnyvale, CA, USA).
Cell growth rate and drug resistance in
vivo
To establish human breast xenografts, BCap37, BC-DS
and BC-TS (0.2 ml PBS containing 1×106 cells) were
injected into the right armpits of the homozygous nude athymic mice
(female, 5–6-weeks old). Each cell line had two groups. The control
groups were treated with PBS, while the treatment groups were
treated with vinorelbine (5 mg/kg, intraperitoneal injection). The
injections were repeated every 6 days for 6 injection cycles. Width
(a) and length (b) of the tumors were measured every 3 days. Tumor
volume was calculated with the formula: V = (π/6) × ab2.
When the mice were terminated, the tumor tissues were removed and
weighted. Data are representative of two separate experiments.
Western blotting
Cellular proteins were prepared with a protein lysis
buffer (Beyotime, Haimen, China) and its concentration was measured
by BCA protein assay kit (KeyGen Biotech, Nanjing, China). Equal
samples (15 µl containing 45 µg) of protein were
separated on 6–10% SDS-PAGE gels and then transferred to
polyvinylidene difluoride membranes. Then these membranes were
blocked for 1 h at room temperature with 5% non-fat dry milk in
Tris-buffered saline (150 mM NaCl, 20 mM Tris-HCl, pH 7.5).
Membranes were washed in PBST and respectively incubated with
anti-MDR1, anti-tubulin primary antibodies (Santa Cruz
Biotechnology, Santa Cruz, CA, USA) at 4°C. After overnight
incubation, the membranes were washed with PBST, and incubated with
horseradish peroxidase-conjugated goat anti-mouse IgG followed by
enhanced chemiluminescent staining using the ECL system. Tubulin
was used for normalization of protein loading.
Cell cycle arrest assay
Three cell lines were treated with 20 nM vinorelbine
for 48 h and then harvested by trypsinization. After
centrifugation, cells were fixed in 70% ethanol at 4°C overnight
and then resuspended in propidium iodide staining solution
containing 20 mg/ml propidium iodide and 0.5 mg/ml RNase in PBS at
room temperature for 30 min before analysis by flow cytometry. Flow
cytometric analysis was performed with a Beckman Coulter flow
cytometer (Beckman Coulter, Miami, FL, USA) with an excitation at
488 nm and an emission at 630 nm (15).
Annexin V/PI assay
Three cell lines were treated with 20 nM vinorelbine
for 48 h and then harvested by trypsinization. After
centrifugation, cells were washed with PBS and incubated in the
dark for 10 min at room temperature in 100 µl binding buffer
(10 mM HEPES, pH 7.4; 140 mM NaCl; 2.5 mM CaCl2)
(Beyotime) containing Annexin V-FITC (40 µl/ml) and PI (1
µg/ml). After incubation, 400 µl binding buffer was
added to each sample and cells were kept on ice (16). Flow cytometric analysis was
performed with a Beckman Coulter flow cytometer (Beckman Coulter).
The 488-nm laser was used for excitation and FITC was detected in
FL-1 by a 525/30-bp filter while PI was detected in FL-2 by a
575/30-bp filter (16).
In vitro migration assay
Migration assays were performed in a 24-well
Transwell chamber (Corning, Cambridge, MA, USA). Three cell lines
were harvested at logarithmic growth phase and plated into upper
chambers (0.2 ml serum-free medium containing 1×105
cells). The lower parts of the chambers were filled with 0.5 ml of
RPMI-1640 medium containing 10% FBS. After 24 h of incubation, the
migration cells were stained and enumerated.
Rhodamine 123 efflux assay
Each cell line had four groups. The control groups
were incubated with Rhodamine 123 (10 µg/ml) for 30 min. In
treatment 1, 3 h of incubation was added with 5 µM verapamil
before Rhodamine 123. Treatment 2 was followed by 2 h of incubation
with RPMI-1640 medium based on treatment 1. Treatment 3 was
followed by 2 h of incubation with 5 µM verapamil based on
treatment 1. After washing with 4°C PBS, and harvesting by
trypsinization, intracellular Rhodamine 123 fluorescence intensity
was determined with Coulter Epics V instrument (Beckman Coulter,
Fullerton, CA, USA).
Statistical analysis
Data are presented as mean ± standard error of three
independent experiments. Two-sided Student's t-test was used to
determine the statistical difference between various experimental
and control groups. Differences were considered statistically
significant at p<0.05 (17).
Results
Establishment and morphological
characterization of BC-DS and BC-TS
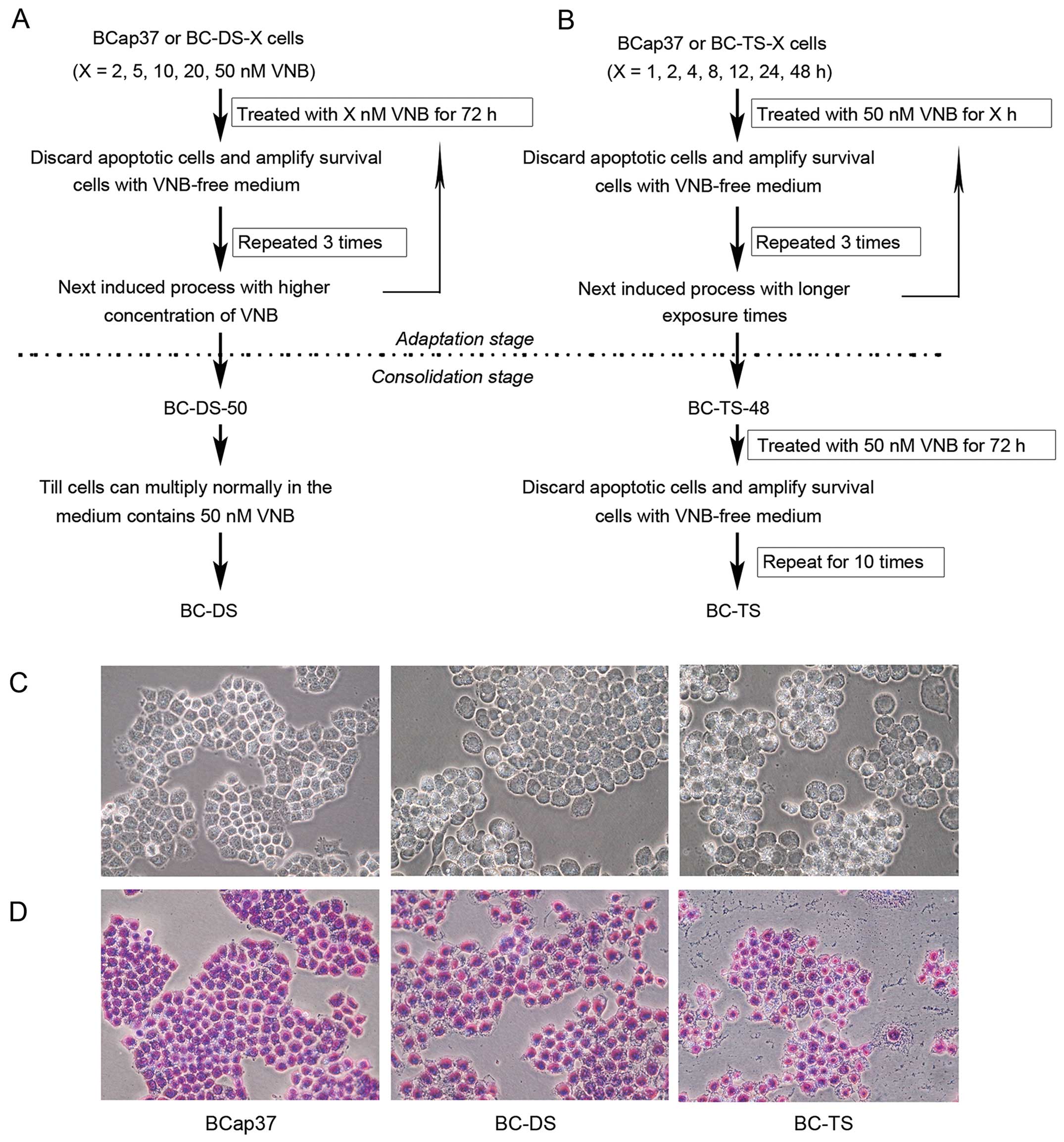
Two vinorelbine-resistant sublines BC-DS and BC-TS,
were successfully established from the human breast cancer cell
line BCap37, with different 'two-stage screening methods'. BC-DS
cells were selected based on continuous exposure to vinorelbine
using a dose-stepwise incremental strategy (Fig. 1A). In the adaptation stage, BCap37
cells were exposed to vinorelbine from 2 to 50 nM (2, 5, 10, 20, 50
nM) for 72 h step by step. In the consolidation stage, previously
selected cells were continuously cultured in medium containing 50
nM vinorelbine. When they multiplied normally, we had established
the BC-DS cell line.
BC-TS were selected based on a strategy of pulsed
exposure to vinorelbine with time-stepwise increments (Fig. 1B). In the adaptation stage, BCap37
cells were exposed to 50 nM vinorelbine for 1 to 48 h (1, 2, 4, 12,
24, 48 h) step by step. In the consolidation stage, previously
selected cells were exposured ten times to 50 nM vinorelbine for 72
h. The resulting cell line was named as BC-TS.
Morphological characterizations of BC-DS and BC-TS
are different from the parental BCap37 cells (Fig. 1C and D). Bcap37 cells grew closely
and had clearly demarcated colony edges while BC-DS and BC-TS had
larger cell size and the cells grew loosely with various
shapes.
The biological characterizations of BC-DS
and BC-TS differed at different levels compared to the parental
Bcap37
Biological characterization of breast cancer cells
may change during the establishment of drug-resistant sublines.
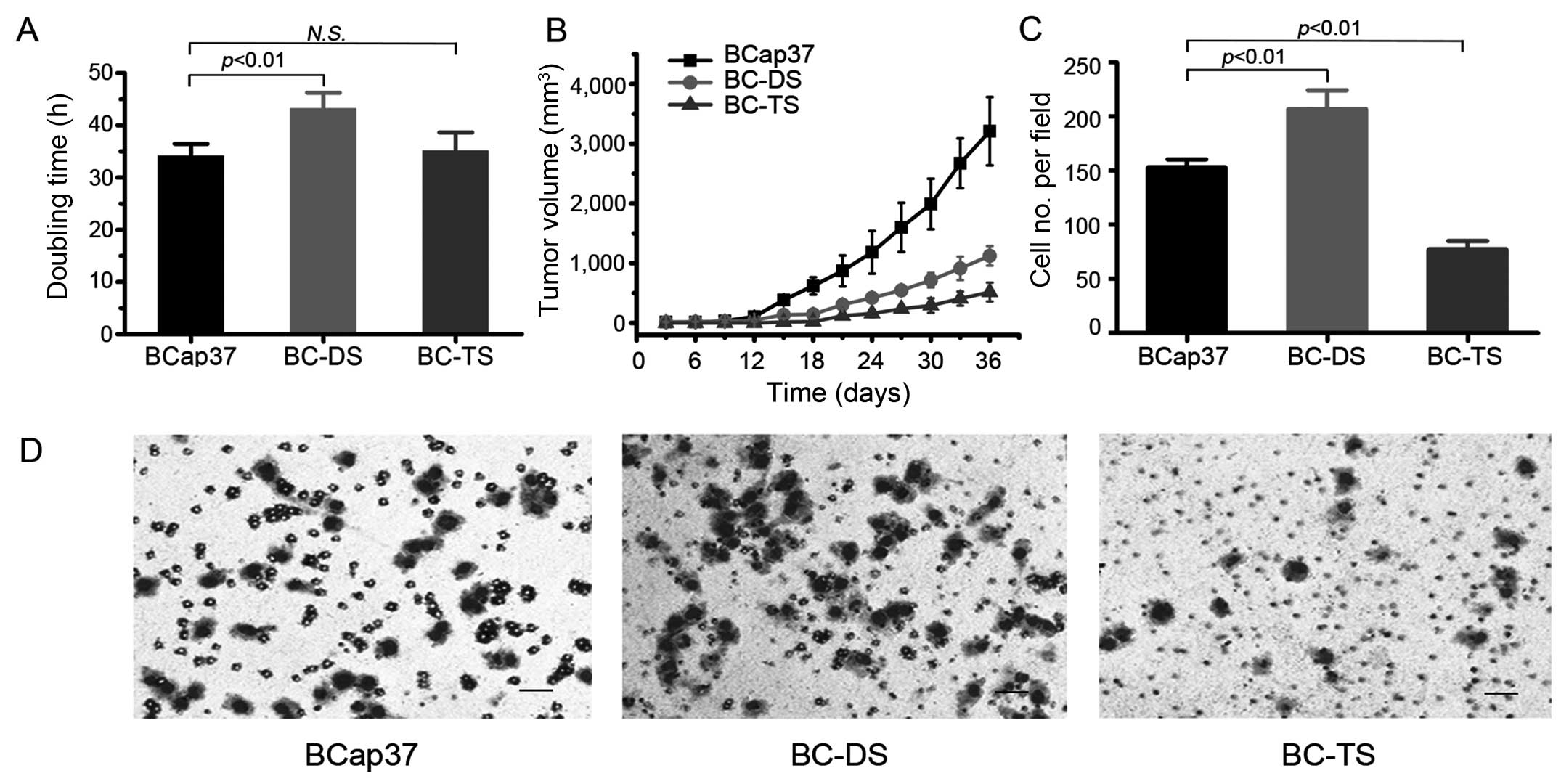
Thus, we examined the growth rate of BCap37, BC-DS and BC-TS both
in vitro and in vivo (Fig. 2A and B). Based on the data from
in vitro growth assays, the doubling time of BCap37, BC-DS
and BC-TS were 34.1±2.3, 43.8±3.3 and 35.0±3.9 h, respectively.
Thus, BC-DS had slower proliferation rate compared to BCap37. While
BC-TS had exactly the same proliferation rate as BCap37, however,
its adaptability became worse, as it needed more time to reach
logarithmic growth phase.
To study the proliferation ability of BCap37, BC-DS
and BC-TS cells in vivo, we further established tumor
xenograft models with homozygous nude athymic mice. In vivo,
BCap37 still has the most aggressive proliferation based on the
in vitro data, and BC-TS needed the longest time to adapt
(Fig. 2B).
To analyze the migratory activity of BCap37, BC-DS
and BC-TS cells, Transwell assay was conducted (Fig. 2C and D). We counted the migrated
cells of these three cell lines. Compared with parental BCap37
cells, there were 35.3% more cells migrating successfully in BC-DS,
but 48.4% less in BC-TS. The results indicate that the migration
ability of BC-DS was enhanced, but that of BC-TS was
attenuated.
BC-DS and BC-TS resist vinorelbine in
vitro, but the resistant characterizations are both unstable
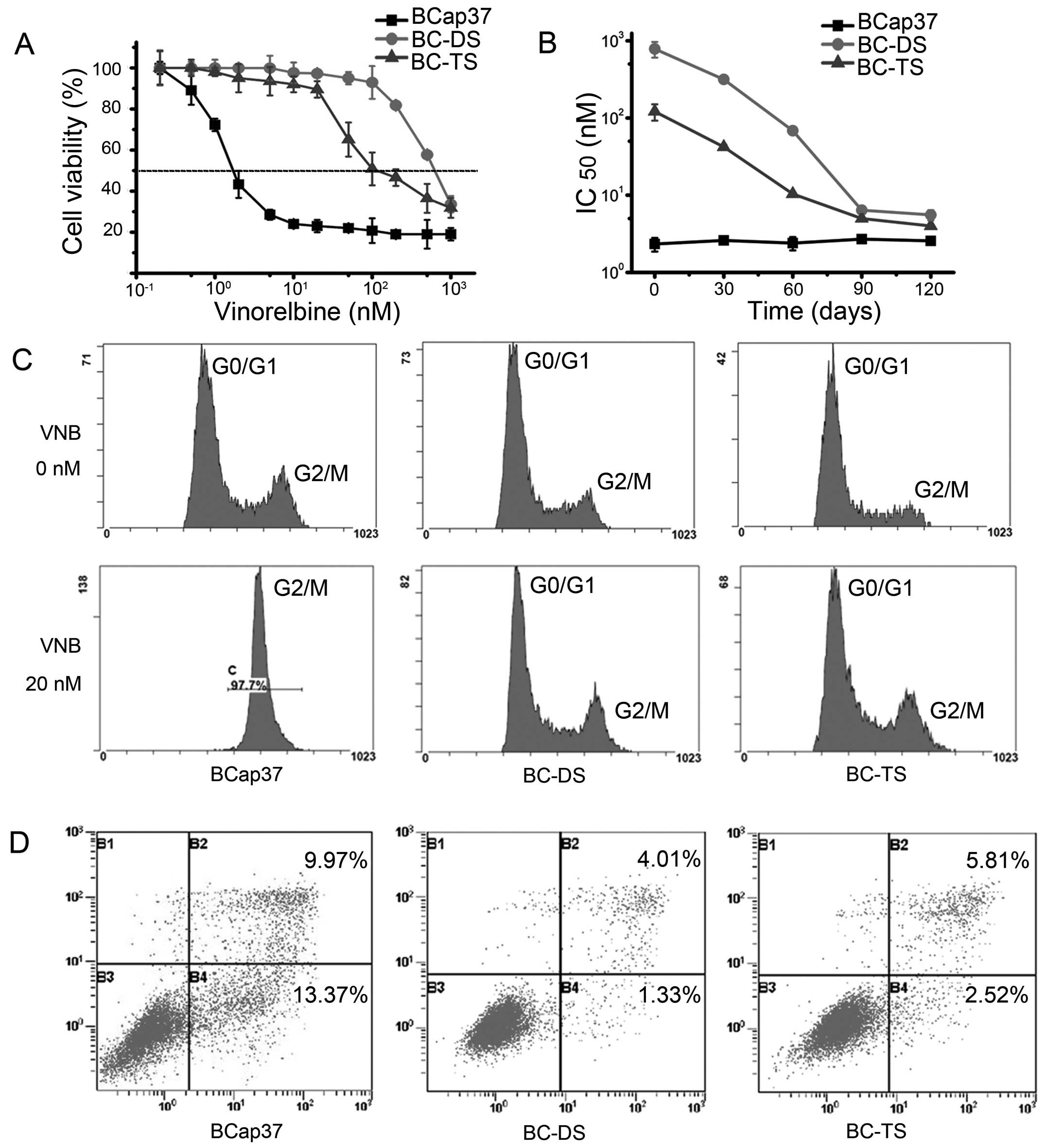
BC-DS and BC-TS cell lines were selected as
vinorelbine-resistant. We used MTT assay to examine their
sensitivity to vinorelbine in vitro. The IC50
value of 72 h vinorelbine exposure for BCap37, BC-DS and BC-TS was
2.3±0.4, 729±100 and 120±21 nM, respectively (Fig. 3A and Table I). Thus, BC-DS and BC-TS were about
317-fold and 52-fold more resistant to vinorelbine than the
parental BCap37.
 | Table IDrug sensitivity of BCap37, BC-DS and
BC-TS. |
Table I
Drug sensitivity of BCap37, BC-DS and
BC-TS.
| Drug | BCap37
| BC-DS
| BC-TS
|
|---|
| IC50
(nM)a | IC50
(nM) | RIb | IC50
(nM) | RI |
|---|
| Vinorelbine | 2.3±0.4 | 729±100d | 316.96 | 120±21d | 52.17 |
| Paclitaxel | 4.1±0.2 | 701±73d | 170.98 | 85±7d | 20.73 |
| Doxorubicin | 231.6±19.7 | 1,354±76d | 5.85 | 412±18.2c | 1.78 |
| Methotrexate | 18.2±0.8 | 4.3±0.9d | 0.24 | 17.1±0.2 | 0.94 |
| 5-Fluorouracil | 9,144±945 | 16,850±2,616 | 1.84 | 11,380±593 | 1.24 |
| Cisplatin | 1,097±77 | 1,755±148c | 1.60 | 1,678±83c | 1.53 |
To observe if BC-DS and BC-TS had stable
vinorelbine-resistant characterizations, we cultured the three cell
types in vinorelbine-free medium and detected the IC50
values every 30 days. As presented in Fig. 3B, the IC50 values at 72 h
vinorelbine exposure decreased markedly in BC-DS and BC-TS with
time. It took about 90 days for them to lose the
vinorelbine-resistance.
We also used flow cytometric analyses, which
indicated that both BC-DS and BC-TS were much more resistant to
vinorelbine-induced cell cycle arrest and apoptosis. The cell cycle
of BCap37 was obviously arrested in 20 nM vinorelbine for 48 h,
while BC-DS and BC-TS were slightly affected in the same conditions
(Fig. 3C). Annexin V/PI assay
showed the percentage of apoptotic cells in BCap37 was 23.34, while
it was only 5.34 and 8.33 for BC-DS and BC-TS, respectively
(Fig. 3D).
BC-DS and BC-TS resist vinorelbine in
vivo
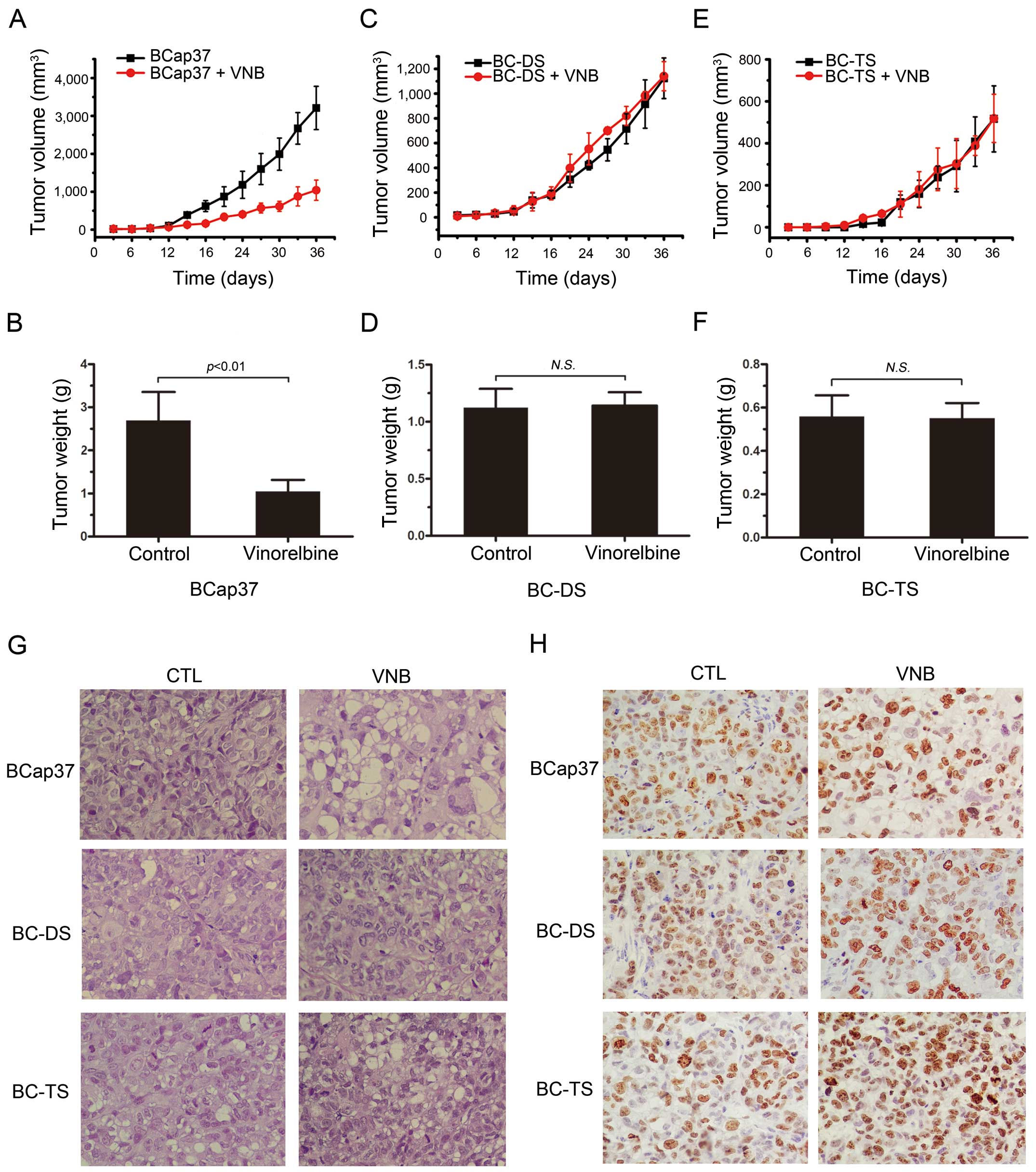
Sensitivity of BC-DS and BC-TS to vinorelbine was
also observed in vivo. Vinorelbine had dramatic inhibiting
effect of tumor growth on BCap37 (Fig.
4A and B), but little on BC-DS (Fig. 4C and D) and BC-TS (Fig. 4E and F). Corresponding tissue
sections were stained with H&E or for the proliferation marker
Ki-67. Compared to BCap37 cells, fewer BC-DS and BC-TS cells
exhibited vacuolization and apoptotic features (Fig. 4G), but more BC-DS and BC-TS cells
were Ki-67 positive (Fig. 4H),
which proved their resistance to vinorelbine in vivo.
BC-DS and BC-TS exhibit different
phenotypes of multidrug resistance
Acquired multidrug resistance (MDR) is the main
mechanism of chemotherapeutic drug resistance. We next examined
their sensitivity to other chemotherapeutic agents including
paclitaxel, doxorubicin, methotrexate, 5-fluorouracil and
cisplatin. The MTT assay showed BC-DS and BC-TS exhibited
significantly higher resistance than BCap37 to vinorelbine,
paclitaxel, doxorubicin and cisplatin (Table I). As to 5-fluorouracil, there was a
slight increase, but not significant in drug resistance on both
BC-DS and BC-TS, whereas to methotrexate, BC-DS became more
sensitive while BC-TS stayed the same. These findings suggested
that BC-DS and BC-TS may represent two distinct MDR phenotypes.
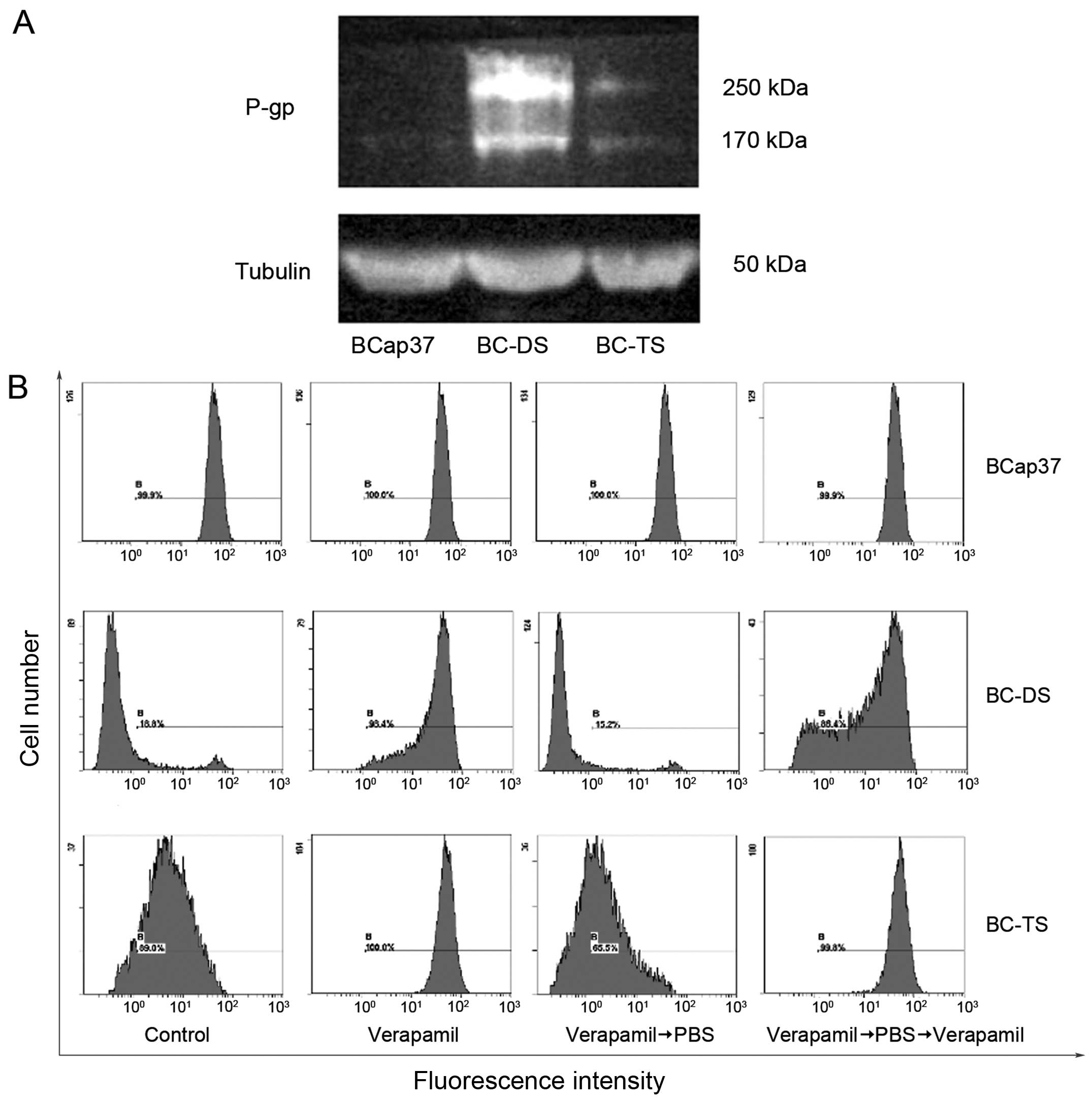
BC-DS and BC-TS express P-glycoprotein
(P-gp) at different level
Multidrug transporter P-gp could induce multidrug
resistance after exposure to any drug tested (18). To determine whether P-gp was one of
the main reasons responding for multidrug resistance of BC-DS and
BC-TS in our study, we detected its expression in three cell lines
through western blotting. Compared with BCap37, BC-DS had a remark
able increase in P-gp expression while it was slight for BC-TS
(Fig. 5A). This finding may explain
the ability of BC-DS cells to tolerate a much higher concentration
of vinorelbine than BC-TS.
Furthermore, to investigate whether intracellular
drug accumulation was significantly decreased in BC-DS and BC-TS,
Rhodamine 123 was used as a molecular probe in drug efflux assay.
Verapamil is a calcium channel blocker and also a P-gp inhibitor
that can reverse MDR (19).
According to the assay (Fig. 5B),
no significant change in Rhodamine 123 retention was observed in
BCap37 cells with or without verapamil co-treatment. On the
contrary, verapamil significantly inhibited Rhodamine 123 efflux in
both BC-DS and BC-TS cell lines. Furthermore, quantity of Rhodamine
123 changed more in BC-DS than BC-TS before and after verapamil
co-treatment, which indirectly indicated greater expression of P-gp
in the BC-DS cell line.
Interestingly, there were also unknown bands
observed around 250 kDa, the concentration of which was similar to
P-gp, the expression was the most in BC-DS and the least in BCap37
(Fig. 5A), temporarily, it was
named M250. The tight connection between M250 and P-gp strongly
indicated M250 to be a potential tumor resistance-associated
protein similar to P-gp.
Discussion
In this study, we have successfully established two
vinorelbine-resistant sublines, BC-DS and BC-TS, from the human
breast cancer cell line BCap37, with different 'two-stage screening
methods'.
Compared to the parental BCap37 cells, both BC-DS
and BC-TS were less active, which was consistent with other
literature (20,21,12).
While BC-DS and BC-TS could resist vinorelbine in vitro and
in vivo, they also gained multidrug resistance to
paclitaxel, doxorubicin and cisplatin. Other researchers also
discovered multidrug-resistant phenomena while investigating
chemotherapy-resistant cancer cell lines they established (22–24).
Interestingly, our study also showed that BC-DS became more
sensitive to methotrexate (MTX). As MTX is one of the first-line
antineoplastic drugs for breast cancer with relatively low price
for patient, combination of MTX and vinorelbine could be a new
treatment strategy. However, only few investigations were
previously reported demonstrating the strategy to be a
well-tolerated and effective regimen for patients with advanced
breast cancer (25–27). Therefore, further research is needed
to prove the safety and efficacy of this strategy.
MDR by increased efflux transporters, including
ATP-binding cassette transporters is associated with upregulated
ABCB1 expression and the main cause of treatment failure (18,20,28),
can be observed in the majority of cancers (29). The expression of P-gp was found to
be upregulated strongly in BC-DS and slightly in BC-TS, which may
result in the difference of their maximum tolerated concentration
to chemotherapeutic agents. Felipe et al (10) reported that P-gp was overexpressed
in the epirubicin-resistant gastric cancer line they established
using dose-stepwise incremental strategy. Monoclonal anti-body,
antagonist or depleting agent against P-gp is promising to optimize
the therapeutic effect of vinorelbine.
The results showed that vinorelbine-resistant
characterization of both BC-DS and BC-TS were unstable. After being
cultured in drug-free medium for two to three months, they became
sensitive to vinorelbine again. Previous studies found cell lines
established by dose-stepwise incremental strategy may be
genetically unstable (11).
Twentyman et al (20)
adopted a pulsatile approach and found that the cell line was
unstable during the first 3 weeks of drug-free growth, but with no
loss of resistance if maintained in drug-containing media. However
Jiang et al (17) reported
that a cell line displayed stable resistant property using the
pulsatile approach.
Moreover, different from BC-TS, BC-DS exhibited
significantly enhanced migratory properties. It was also reported
that drug-resistant cell line developed by time-stepwise increments
administration exhibited enhanced migration (17). These contradictions may be due to
the different drugs and parental cells used in the establishment,
which suggested that different administration strategies with a
single drug could induce distinct phenotypes of drug-resistant cell
lines. It was the drugs and parental cells, not strategies that
decided the final characterization of the produced variants.
BC-DS and BC-TS were distinct from each other and
represented two different MDR phenotypes. Exposure may over time
induce genetic events, which confer a drug-resistant phenotype on
cells that were not intrinsically resistant at the start.
Alternatively, resistant cells can be selected from a culture on
the strength of an intrinsic mutation conferring resistance in that
cell or group of cells, thus establishing them as the dominant
clone in the culture (11). We
speculate that BC-DS acquired drug-resistance, while BC-TS was
intrinsically drug-resistant.
In clinical treatment, patients received vinorelbine
intravenous 25–30 or 60–80 mg/m2 orally in days 1 and 8
of a 21-day cycle (30–32), which was similar to the way we
developed BC-TS cells. As discussed above, BC-TS shows lower
migratory behavior and resistant ability compared to the
conventional continuous exposure strategy developed in breast
cancer BC-DS cells. In this aspect, our research showed that pulsed
exposure is a better clinical medication strategy. Furthermore, the
different phenotypes of multidrug resistance showed by BC-DS and
BC-TS, are meaningful guidance for clinical drug combination.
BC-DS and BC-TS also provide opportunity for
undertaking large-scale expression profile screening to identify
novel biomarkers of chemotherapy resistance in breast cancer. The
molecular weight of P-gp is 170 kDa, which is the most important
MDR-associated protein (33). When
we detected P-gp expression using western blotting, the 170-kDa
bands appeared as we expected. However, there was also an unknown
protein (M250), the concentration of which was similar to P-gp
(Fig. 4A). This indicated M250 to
be a potential tumor resistance-associated protein similar to P-gp.
Part of our further research will focus on the mechanism and signal
pathways of M250.
In summary, by using different screening strategies,
we established two novel MDR cell lines, BC-DS and BC-TS, from
chemo-sensitive human breast cancer cell line BCap37. Although
BC-DS and BC-TS shared the same origin, they differed in many
aspects. BC-TS cells show lower migratory behavior and resistant
ability compared to the conventional continuous exposure strategy
developed in breast cancer BC-DS cells, which verifies that pulsed
exposure is a better clinical medication strategy. The unknown
protein M250, we found in drug-resistant cancer cells, may be a
potential tumor resistance-associated protein, which deserves
further research.
Acknowledgments
This research was supported by grants NSFC-81372462,
NSFC-81302288 and National Key Project on New Drug Developmental
Program-2014ZX09507009026 (W. Fan).
Abbreviations:
|
VNB
|
vinorelbine
|
|
BC-DS
|
BCap37 dose stimulated
|
|
BC-TS
|
BCap37 time stimulated
|
|
MDR
|
multiple drug resistance
|
|
P-gp
|
P-glycoprotein
|
|
MTX
|
methotrexate
|
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Xu YC, Wang HX, Tang L, Ma Y and Zhang FC:
A systematic review of vinorelbine for the treatment of breast
cancer. Breast J. 19:180–188. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gregory RK and Smith IE: Vinorelbine - a
clinical review. Br J Cancer. 82:1907–1913. 2000.PubMed/NCBI
|
|
4
|
Calcagno AM, Fostel JM, To KK, Salcido CD,
Martin SE, Chewning KJ, Wu CP, Varticovski L, Bates SE, Caplen NJ,
et al: Single-step doxorubicin-selected cancer cells overexpress
the ABCG2 drug transporter through epigenetic changes. Br J Cancer.
98:1515–1524. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yang JX, Luo Y, Qiu HM and Tang WX:
Characterization and resistance mechanisms of cisplatin-resistant
human hepatocellular carcinoma cell line. Saudi Med J. 30:35–40.
2009.PubMed/NCBI
|
|
6
|
Zhou Y, Ling XL, Li SW, Li XQ and Yan B:
Establishment of a human hepatoma multidrug resistant cell line in
vitro. World J Gastroenterol. 16:2291–2297. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li L, Luan Y, Wang G, Tang B, Li D, Zhang
W, Li X, Zhao J, Ding H, Reed E, et al: Development and
characterization of five cell models for chemoresistance studies of
human ovarian carcinoma. Int J Mol Med. 14:257–264. 2004.PubMed/NCBI
|
|
8
|
Shin KH, Ku JL, Kim WH, Lee SE, Lee C, Kim
SW and Park JG: Establishment and characterization of seven human
renal cell carcinoma cell lines. BJU Int. 85:130–138. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Han T, Zhu X, Wang J, Zhao H, Ma Q, Zhao
J, Qiu X and Fan Q: Establishment and characterization of a
cisplatin-resistant human osteosarcoma cell line. Oncol Rep.
32:1133–1139. 2014.PubMed/NCBI
|
|
10
|
Felipe AV, Moraes AA, de Oliveira J, da
Silva TD and Forones NM: Establishment and partial characterization
of an epirubicin-resistant gastric cancer cell line with
upregulated ABCB1. Asian Pac J Cancer Prev. 15:6849–6853. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Watson MB, Lind MJ and Cawkwell L:
Establishment of in-vitro models of chemotherapy resistance.
Anticancer Drugs. 18:749–754. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yang LY and Trujillo JM: Biological
characterization of multi-drug-resistant human colon carcinoma
sublines induced/selected by two methods. Cancer Res. 50:3218–3225.
1990.PubMed/NCBI
|
|
13
|
Ellis GK, Barlow WE, Gralow JR, Hortobagyi
GN, Russell CA, Royce ME, Perez EA, Lew D and Livingston RB: Phase
III comparison of standard doxorubicin and cyclophosphamide versus
weekly doxorubicin and daily oral cyclophosphamide plus granulocyte
colony-stimulating factor as neoadjuvant therapy for inflammatory
and locally advanced breast cancer: SWOG 0012. J Clin Oncol.
29:1014–1021. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Pagliaro LC, Williams DL, Daliani D,
Williams MB, Osai W, Kincaid M, Wen S, Thall PF and Pettaway CA:
Neoadjuvant paclitaxel, ifosfamide, and cisplatin chemotherapy for
metastatic penile cancer: A phase II study. J Clin Oncol.
28:3851–3857. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang W, Heideman L, Chung CS, Pelling JC,
Koehler KJ and Birt DF: Cell-cycle arrest at G2/M and growth
inhibition by apigenin in human colon carcinoma cell lines. Mol
Carcinog. 28:102–110. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Foldbjerg R, Olesen P, Hougaard M, Dang
DA, Hoffmann HJ and Autrup H: PVP-coated silver nanoparticles and
silver ions induce reactive oxygen species, apoptosis and necrosis
in THP-1 monocytes. Toxicol Lett. 190:156–162. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jiang D, Sui M, Zhong W, Huang Y and Fan
W: Different admin-istration strategies with paclitaxel induce
distinct phenotypes of multidrug resistance in breast cancer cells.
Cancer Lett. 335:404–411. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gottesman MM, Fojo T and Bates SE:
Multidrug resistance in cancer: Role of ATP-dependent transporters.
Nat Rev Cancer. 2:48–58. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Krishna R and Mayer LD: Multidrug
resistance (MDR) in cancer. Mechanisms, reversal using modulators
of MDR and the role of MDR modulators in influencing the
pharmacokinetics of anticancer drugs. Eur J Pharm Sci. 11:265–283.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Twentyman PR, Fox NE, Wright KA and
Bleehen NM: Derivation and preliminary characterisation of
adriamycin resistant lines of human lung cancer cells. Br J Cancer.
53:529–537. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yu DS, Ma CP and Chang SY: Establishment
and characterization of renal cell carcinoma cell lines with
multidrug resistance. Urol Res. 28:86–92. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hour TC, Chen J, Huang CY, Guan JY, Lu SH,
Hsieh CY and Pu YS: Characterization of chemoresistance mechanisms
in a series of cisplatin-resistant transitional carcinoma cell
lines. Anticancer Res. 20:3221–3225. 2000.PubMed/NCBI
|
|
23
|
Uchiyama-Kokubu N and Watanabe T:
Establishment and characterization of adriamycin-resistant human
colorectal adenocarcinoma HCT-15 cell lines with multidrug
resistance. Anticancer Drugs. 12:769–779. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chung YM, Park S, Park JK, Kim Y, Kang Y
and Yoo YD: Establishment and characterization of
5-fluorouracil-resistant gastric cancer cells. Cancer Lett.
159:95–101. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Abrahamova J, Wagnerova M, Kubala E, Malec
V, Simova E, Sirakova I, Pavlikova E, Machova D, Kocak I, Pavlikova
I, et al: Vinorelbine, epirubicin, and methotrexate (VEM) as
primary treatment in locally advanced breast cancer. Oncologist.
6:347–352. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Elomaa I, Joensuu H and Blomqvist C:
Vinorelbine, methotrexate and fluorouracil (VMF) as first-line
therapy in metastatic breast cancer: A randomized phase II trial.
Ann Oncol. 14:699–703. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Subramanyan S, Abeloff MD, Bond SE,
Davidson NE, Fetting JH, Gordon GB and Kennedy MJ: A phase I/II
study of vinorelbine, doxorubicin, and methotrexate with leucovorin
rescue as first-line treatment for metastatic breast cancer. Cancer
Chemother Pharmacol. 43:497–502. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Richter M, Molnár J and Hilgeroth A:
Biological evaluation of bishydroxymethyl-substituted cage dimeric
1,4-dihydropyridines as a novel class of P-glycoprotein modulating
agents in cancer cells. J Med Chem. 49:2838–2840. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Cordon-Cardo C, O'Brien JP, Boccia J,
Casals D, Bertino JR and Melamed MR: Expression of the multidrug
resistance gene product (P-glycoprotein) in human normal and tumor
tissues. J Histochem Cytochem. 38:1277–1287. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ardavanis A, Tryfonopoulos D, Orphanos G,
Ioannidis G, Karamouzis M and Rigatos G: First-line chemotherapy
with fluorouracil-epirubicin-navelbine (FEN) combination in
advanced breast cancer. Anticancer Res. 25:4493–4498.
2005.PubMed/NCBI
|
|
31
|
Wang J, Xu B, Yuan P, Ma F and Li Q, Zhang
P, Cai R, Fan Y, Luo Y and Li Q: Capecitabine combined with
docetaxel versus vinorelbine followed by capecitabine maintenance
medication for first-line treatment of patients with advanced
breast cancer: Phase 3 randomized trial. Cancer. 121:3412–3421.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Stravodimou A, Zaman K and Voutsadakis IA:
Vinorelbine with or without trastuzumab in metastatic breast
cancer: A retrospective single institution series. ISRN Oncol.
2014:2898362014.PubMed/NCBI
|
|
33
|
Breier A, Barancík M, Sulová Z and Uhrík
B: P-glycoprotein - implications of metabolism of neoplastic cells
and cancer therapy. Curr Cancer Drug Targets. 5:457–468. 2005.
View Article : Google Scholar : PubMed/NCBI
|