Introduction
Gastric cancer is the fourth most diagnosed cancer
and the second leading cause of cancer-related death worldwide
(1). A pivotal risk factor for
gastric carcinogenesis is Helicobacter pylori (H.
pylori) infection (2). H.
pylori infection is among the most common infectious diseases.
It is considered to be carcinogenic and accounts for approximately
80% of gastric carcinomas (3,4). H.
pylori infection also influences the clinical treatment outcome
of gastric cancer (5). However, the
association between gastric cancer and H. pylori infection
and its functional mechanism in gastric cancer remain largely
unknown. A better understanding of this molecular mechanism may
improve therapeutic prevention and treatment for gastric
cancer.
H. pylori is a Gram-negative bacteria that
contributes to gastric tumorigenesis mainly through its virulence
factor cytotoxin-associated gene A (CagA) (6). The CagA protein has been suggested as
an oncogenic protein for gastric cancer (7,8). It
has been reported that H. pylori infection helps activate
multiple oncogenic pathways, such as PI3K/Akt (9–11),
WNT/β-catenin (12), and Janus
kinase signal transducers, as well as activators of the
transcription 3 signaling pathway (13). H. pylori infection also
deactivates tumor suppressors, such as p53 (14,15).
Therefore, focusing on oncogenic signaling pathways in H.
pylori infection-induced gastric carcinogenesis could support
novel and promising therapeutic strategies for gastric cancer.
Frizzled7 (FZD7) is a critical receptor for the
WNT/β-catenin signaling pathway (16,17).
Various studies have reported that FZD7 plays an important role in
many types of human cancers, including renal cell carcinoma
(18), cervical cancer (19), ovarian cancer (17), breast cancer (20), colon cancer (21), and hepatocellular carcinoma
(22). It participates in
carcinogenesis mainly through promoting the activation of the
WNT/β-catenin signaling pathway. Aberrant activation of the
WNT/β-catenin signaling pathway occurs frequently in gastric cancer
(23). H. pylori infection
also has been implicated in this WNT/β-catenin signaling pathway
(12). These findings suggest that
FZD7 may be involved in the WNT/β-catenin signaling pathway that is
induced by H. pylori infection. However, little is known
regarding the role of FZD7 in gastric carcinogenesis induced by
H. pylori infection.
MicroRNAs (miRNAs) are a class of small, non-coding
RNAs that negatively regulate gene expression through binding the
3′-untranslated region (UTR) of target gene (24,25).
Therefore, miRNAs mediate cancer cell proliferation,
differentiation, and metastasis by targeting various genes and
regulating numerous signaling pathways (26). Several studies have suggested miRNAs
as novel candidates for cancer therapy (27). Although the role of miRNAs in H.
pylori-induced gastric carcinogenesis has been widely
investigated in recent years, their precise role and target genes
remain unclear. In this study, we investigated the role of FZD7 in
H. pylori-induced gastric carcinogenesis and demonstrated a
targeting relationship between FZD7 and miR-27b, a well
characterized tumor-suppressive miRNA (28,29).
We found that FZD7 was highly upregulated by H. pylori
infection and was associated with H. pylori
infection-induced cell proliferation. miR-27b suppressed H.
pylori infection-induced cell proliferation and the WNT
signaling pathway by directly targeting and negatively regulating
FZD7 expression. Our study demonstrated that miR-27b/FZD7 is
implicated in H. pylori-induced gastric carcinogenesis by
affecting the WNT signaling pathway.
Materials and methods
Cell lines
The gastric epithelial-derived cancer cell lines AGS
and BGC-823 and human embryonic kidney cell line HEK-293T were
obtained from the American Type Culture Collection (ATCC; Manassas,
VA, USA). AGS and BGC-823 cells were grown in RPMI-1640 medium
(Gibco, Rockville, MD, USA) and HEK-293T cells were cultured in
Dulbecco's modified Eagle's medium (DMEM; Gibco). All cultured
media were supplemented with 10% fetal bovine serum (Gibco) and 1%
penicillin-streptomycin mix (Sigma, St. Louis, MO, USA) in a
humidified atmosphere containing 5% CO2 at 37°C.
H. pylori culture
Standard strain H. pylori 43504, was
purchased from the ATCC and cultured on rain-heart infusion plates
containing 5% goat blood and incubated for 3–4 days in a humidified
CO2 incubator containing 10% CO2, 5%
O2, and 85% N2 at 37°C. Thereafter, H.
pylori was collected and re-suspended in RPMI-1640 for a
concentration of 3×108 colony forming units/ml. Gastric
cancer cells were then infected with H. pylori at a
multiplicity of infection (MOI) of 1:25, 1:50, and 1:100 and
incubated for 6 and 12 h.
RNA extraction and real-time quantitative
PCR analysis
Total RNA from cells was harvested with a miRNeasy
kit (Qiagen, Dusseldorf, Germany). For analysis of FZD7 mRNA
expression, RNA was reverse transcribed into cDNA using M-MLV
Reverse Transcriptase (BioTeke, Beijing, China) and RT-PCR was
performed using SYBR Green PCR Master Mix (Applied Biosystems,
Carlsbad, CA, USA). For analysis of miR-27b expression, RNA was
converted into cDNA using miScript reverse transcription kit
(Qiagen), and real-time quantitative PCR (RT-qPCR) was conducted
using TaqMan miRNA Reverse Transcription kit (Applied Biosystems).
GAPDH or U6 small nuclear RNA was used as an internal reference for
relative gene expression quantitation. The RT-qPCR reactions were
performed in triplicate, and relative gene expression level was
determined by using the 2−ΔΔCt method.
Western blot analysis
An equal amount of protein from different samples
was isolated by sodium dodecyl sulfate-polyacrylamide gel
electrophoresis and then transferred onto a PVDF membrane (Bio-Rad,
Hercules, CA, USA). The transferred protein on the membrane was
confirmed by Ponceau staining solution. Then, the membrane was
blocked in 3% nonfat milk for 1 h at 37°C. Primary antibodies,
including anti-FZD7 and anti-GAPDH (Santa Cruz Biotechnology, Santa
Cruz, CA, USA), were added and incubated at 4°C overnight. After
three washes with Tris-buffered saline with Tween-20, horseradish
peroxidase-conjugated secondary antibodies (1:5,000; Bioss Inc.,
Beijing, China) were added and incubated for 1 h. The membrane was
then developed by use of enhanced chemiluminescence (Pierce,
Rockford, IL, USA). Relative protein expression was quantitated by
using Image-Pro Plus 6.0 software.
Cell transfection
FZD7 was knocked down by transfection of FZD7 siRNA
(Santa Cruz Biotechnology), according to the manufacturer's
instruction. Briefly, FZD7 siRNA and transfection reagent were
mixed in transfection medium for 45 min. Cells were washed with
transfection medium and then incubated with the FZD7 siRNA mixture
for 6 h. Normal growth media were added and incubated for 24 h. The
old media were then replaced with fresh normal-growth media and
further incubated for 24–72 h. Negative control (NC) siRNA was used
as a control. For miRNA transfection, miR-27b mimics and NC miRNA
(GenePharma, Shanghai, China) were transfected into cells using
Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) at a
concentration of 20 nM and incubated for 48 h. For FZD7
overexpression, pcDNA3.1/FZD7 vectors were transiently transfected
into cells using Lipofectamine 2000 (Invitrogen) for 48 h. The
transfection efficiency was finally detected by RT-qPCR or western
blot analysis.
Cell proliferation assay
For the MTT assay, cells were seeded into 96-well
plates and transfected with FZD7 siRNA, miR-27b mimics, or
pcDNA3.1/FZD7 vectors for 48 h, followed by H. pylori
infection for 12 h. MTT solution (5 mg/ml) was added at 20
µl/well. Dimethyl sulfoxide was added at 200 µl/well
to dissolve the formazan products. The optical density of the
solution was detected using a microplate reader (Bio-Tek
Instruments, Winooski, VT, USA) at a wavelength of 490 nm. For the
colony formation assay, cells transfected with FZD7 siRNA, miR-27b
mimics, or pcDNA3.1/FZD7 vectors followed by H. pylori
infection were seeded into a 6-well plate in a growth medium
containing 0.3% noble agar (200 cells/well) to form natural
colonies for 14 days. The cells were washed with phosphate buffer
saline and fixed with 4% paraformaldehyde. The plates were stained
with crystal violet (Sigma), and the number of colonies was counted
and averaged.
Luciferase reporter assay
The targeted relationship between miR-27b and FZD7
3′-UTR was detected using a dual-luciferase reporter assay.
Briefly, the cDNA fragments of FZD7 3′-UTR containing the miR-27b
targeted site were inserted into pmirGLO vectors (Promega, Madison,
WI, USA). HEK-293T cells were transfected with pmirGLO-FZD7 3′-UTR
and miR-27b mimics or NC miRNA and incubated for 48 h. Cells were
harvested and lysed. The activity of firefly and Renilla
luciferase was tested with a Dual-Luciferase reporter assay kit
(Promega). The WNT signaling activity was examined using Tcf
luciferase reporter assays. Briefly, gastric cells were
co-transfected with a TOPFlash firefly luciferase reporter vector
(Addgene, Cambridge, MA, USA) and phRL-TK Renilla luciferase
vectors (Promega) together with FZD7 siRNA, miR-27b mimics, or
pcDNA3.1/FZD7 vectors, followed by H. pylori infection.
After 48 h of transfection, cells were lysed and the activity of
firefly and Renilla luciferase was detected,
respectively.
Statistical analysis
Quantitative data are shown as mean ± standard
deviation. Statistical analyses were processed with SPSS version
11.5 software (SPSS Inc., Chicago, IL, USA) with one-way analysis
of variance. A p-value of <0.05 was regarded as statistically
significant.
Results
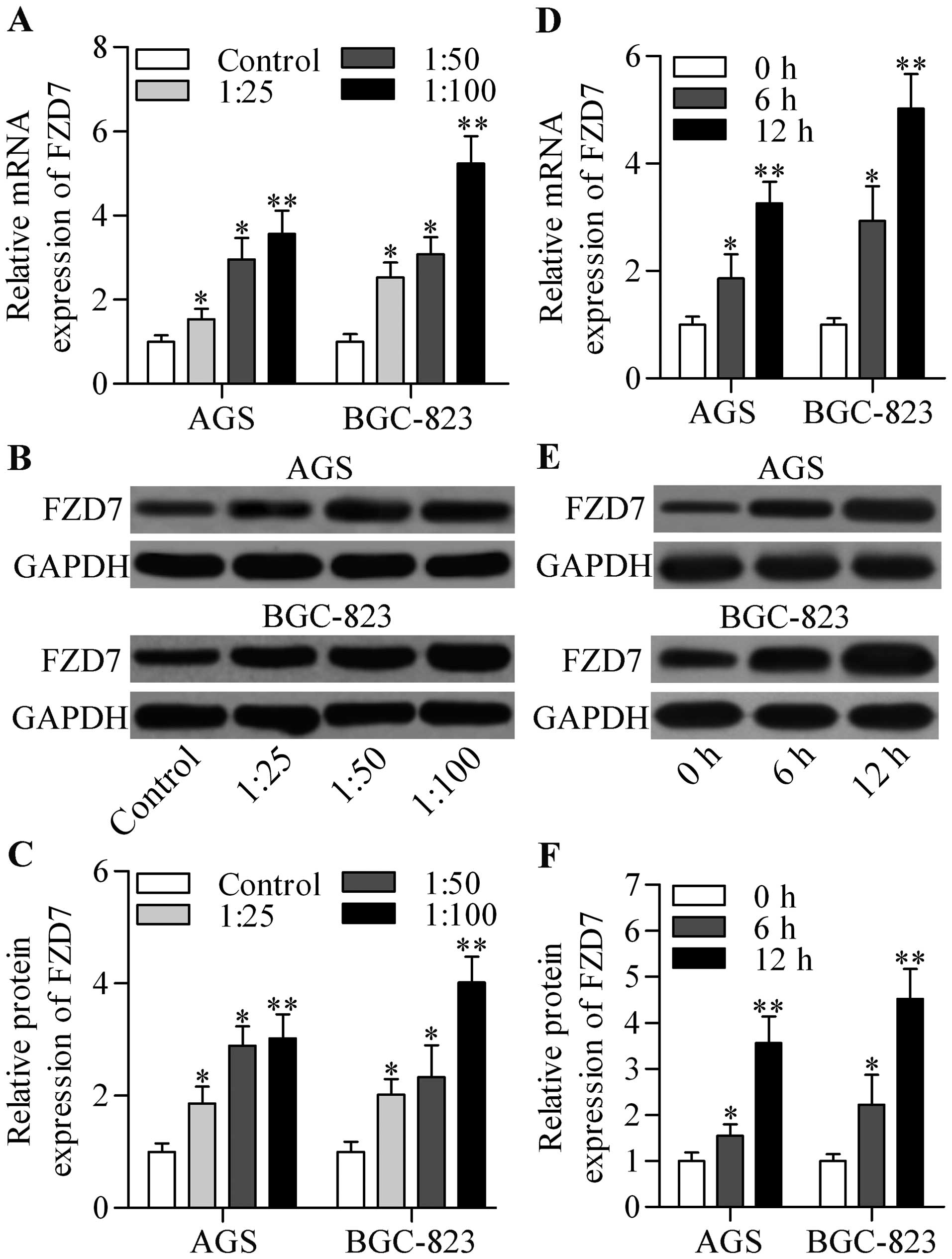
H. pylori infection promotes FZD7
expression in gastric cancer cells
To explore whether FZD7 is involved in
carcinogenesis related to H. pylori infection, we examined
its expression level in two gastric cancer cell lines, AGS and
BGC-823, infected with H. pylori. The results showed that
both the mRNA (Fig. 1A) and protein
(Fig. 1B and C) expression level of
FZD7 were highly upregulated in AGS and BGC-823 cells by H.
pylori infection in a dose-dependent manner. Further data
showed that the mRNA (Fig. 1D) and
protein (Fig. 1E and F) expression
levels of FZD7 increased in a time-dependent manner. These results
indicate that FZD7 was induced by H. pylori in gastric
cancer cells.
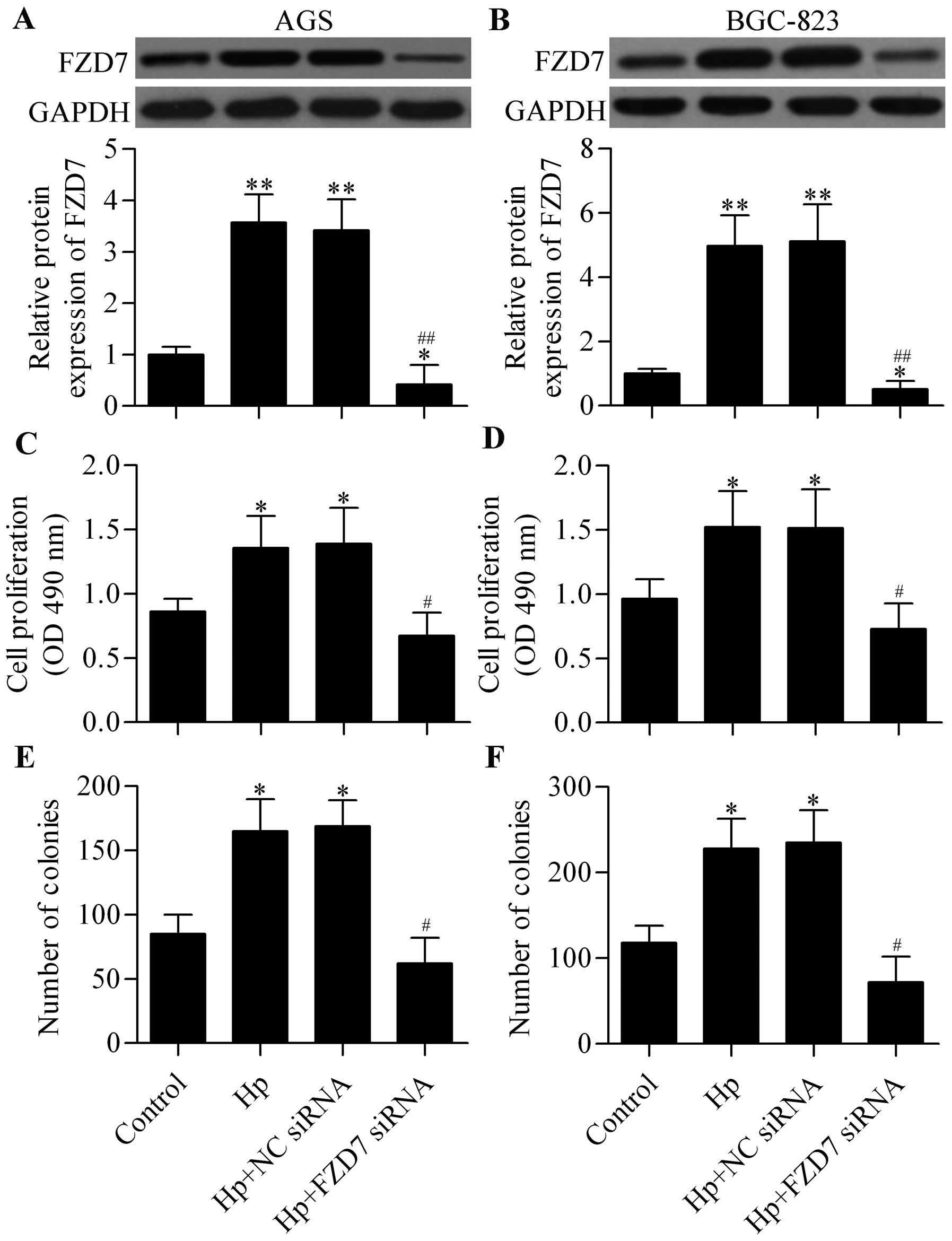
Knockdown of FZD7 inhibits H. pylori
infection-induced cell proliferation of gastric cancer cells
To investigate the potential biological role of FZD7
in H. pylori-induced carcinogenesis, we silenced the
expression of FZD7 by using FZD7 siRNA (Fig. 2A and B) and detected its effect on
gastric cancer cell proliferation with H. pylori infection.
MTT results showed that H. pylori infection significantly
promoted gastric cancer cell proliferation and that FZD7 knockdown
could reverse this promotion (Fig. 2C
and D). Similarly, H. pylori infection markedly
upregulated the colony formation of gastric cancer cells, and FZD7
knockdown reversed this upregulation (Fig. 2E and F). These results suggest that
the increased expression of FZD7 induced by H. pylori
infection might contribute to H. pylori-related gastric
carcinogenesis.
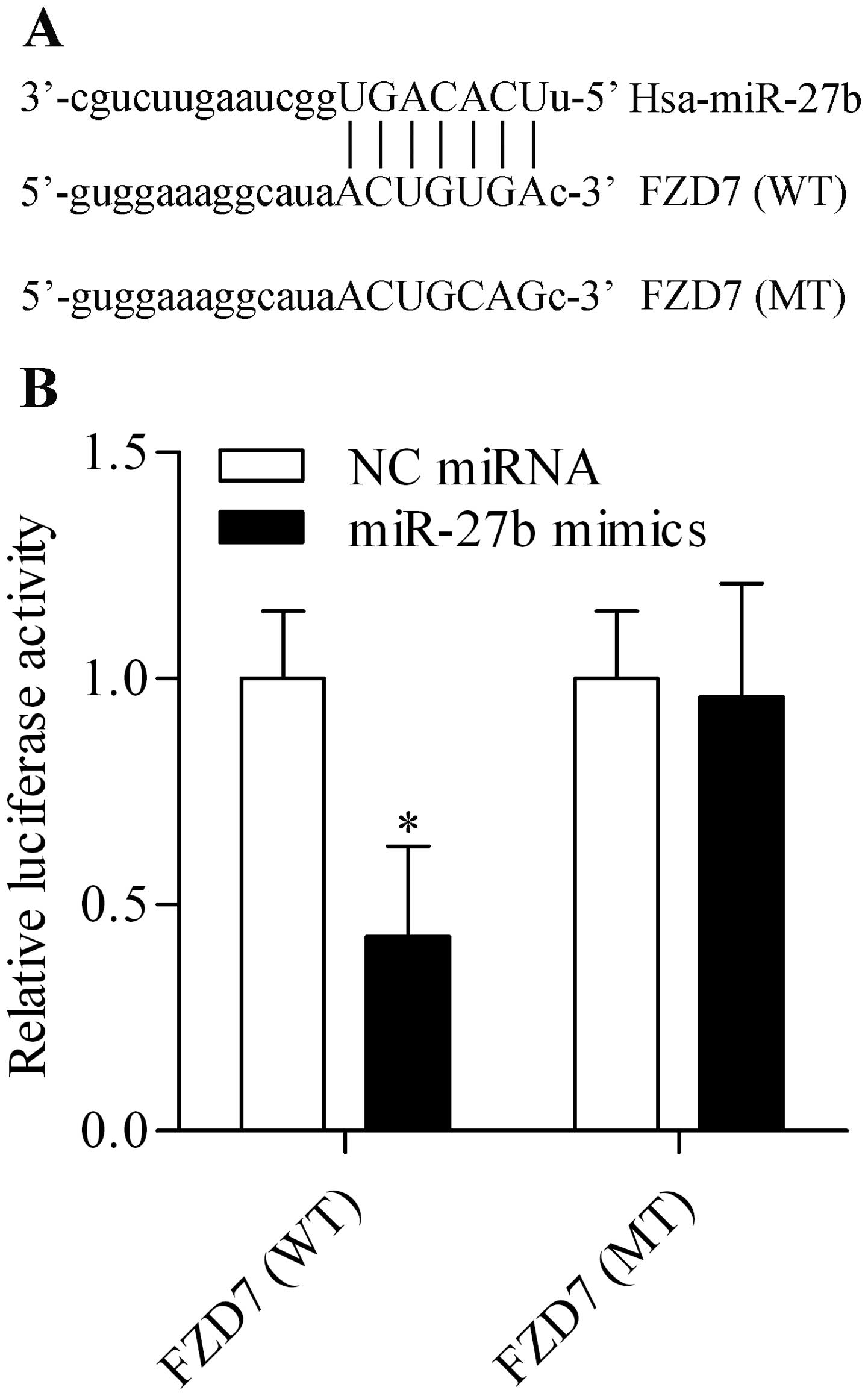
miR-27b directly targets the 3′-UTR of
FZD7 and negatively regulates FZD7 expression
miRNAs have emerged as novel tools for cancer
treatment because of their regulatory function on gene expression
(30). We sought to identify and
characterize novel miRNA that could target and regulate FZD7
expression and were thus involved in gastric carcinogenesis related
to H. pylori. Through bioinformatic analysis, we found that
miR-27b harbored a putative binding site for FZD7 3′-UTR (Fig. 3A). This miRNA attracted our
interests because of its critical role in tumorigenesis of various
cancer types (29). To validate
whether FZD7 is a bona fide target of miR-27b, we performed a
dual-luciferase reporter assay using two luciferase reporter
vectors containing the putative miR-27b binding sites in the
wild-type FZD7 3′-UTR (WT) and mutant 3′-UTR (MT). The results
showed that co-transfection of HEK-293T cells with miR-27b mimics
and luciferase reporter vectors containing the WT FZD7 3′-UTR led
to a significant decrease in luciferase activity, compared to NC
miRNA transfection (Fig. 3B). In
contrast, the luciferase activity of luciferase reporter vectors
containing the MT FZD7 3′-UTR was not affected by miR-27b mimics
transfection (Fig. 3B). The data
imply that miR-27b could target the 3′-UTR of FZD7 directly. Next,
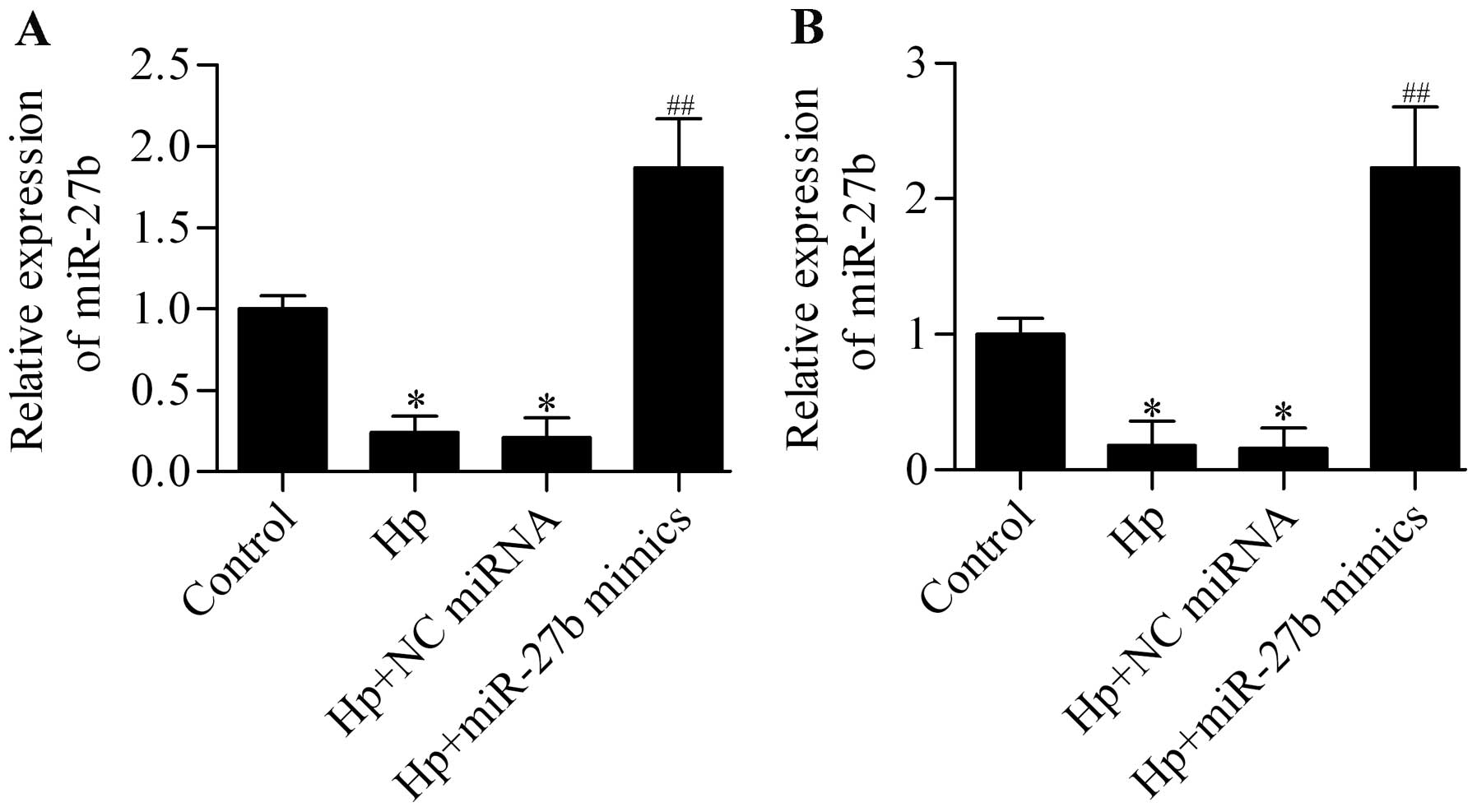
we detected the expression of miR-27b in H. pylori-infected
cells and found that miR-27b expression was significantly decreased
by H. pylori infection in AGS and BGC-823 cells, which could
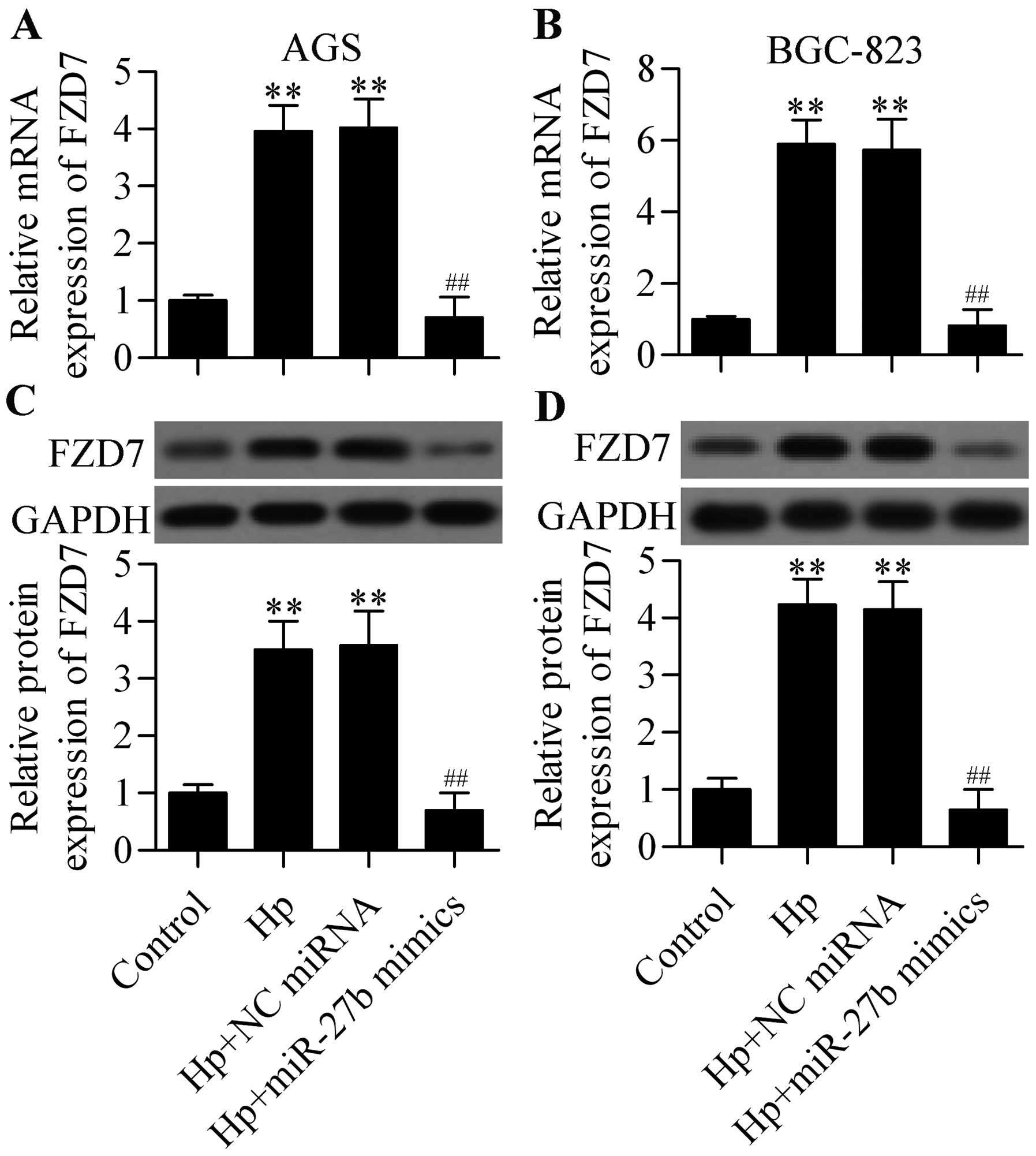
be upregulated by transfection of miR-27b mimics (Fig. 4). To investigate whether miR-27b
regulated FZD7 expression, we overexpressed miR-27b by transfection
of miR-27b mimics in AGS and BGC-823 cells. The results showed that
both the mRNA (Fig. 5A and B) and
protein (Fig. 5C and D) expression
of FZD7 that was upregulated by H. pylori infection was
significantly decreased by miR-27b overexpression in AGS and
BGC-823 cells. Taken together, these results demonstrated that
miR-27b regulated FZD7 expression.
The regulation of FZD7 by miR-27b is involved in
H. pylori-induced cell proliferation. As previously described,
H. pylori infection significantly promoted the expression of
FZD7, and FZD7 was verified as a direct target gene of miR-27b. We
thus speculated that miR-27b may play an important role in H.
pylori-induced cell proliferation by regulating FZD7
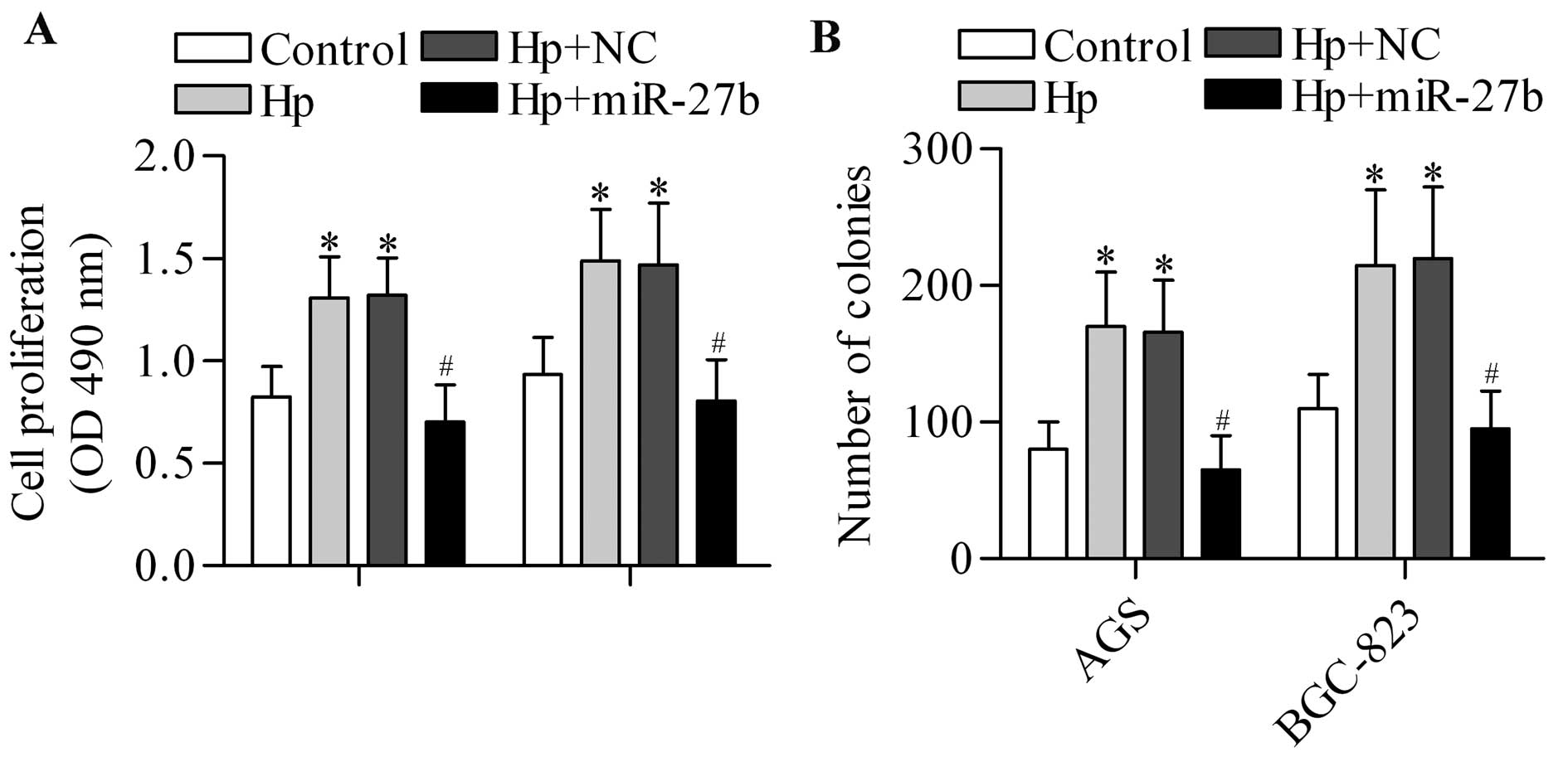
expression. To test the hypothesis, we overexpressed miR-27b in
H. pylori-infected cells and detected its effect on cell
proliferation. As expected, the results showed that the cell
proliferation (Fig. 6A) and colony
formation (Fig. 6B) of gastric
cancer cells promoted by H. pylori infection could be
significantly reversed by miR-27b overexpression. However,
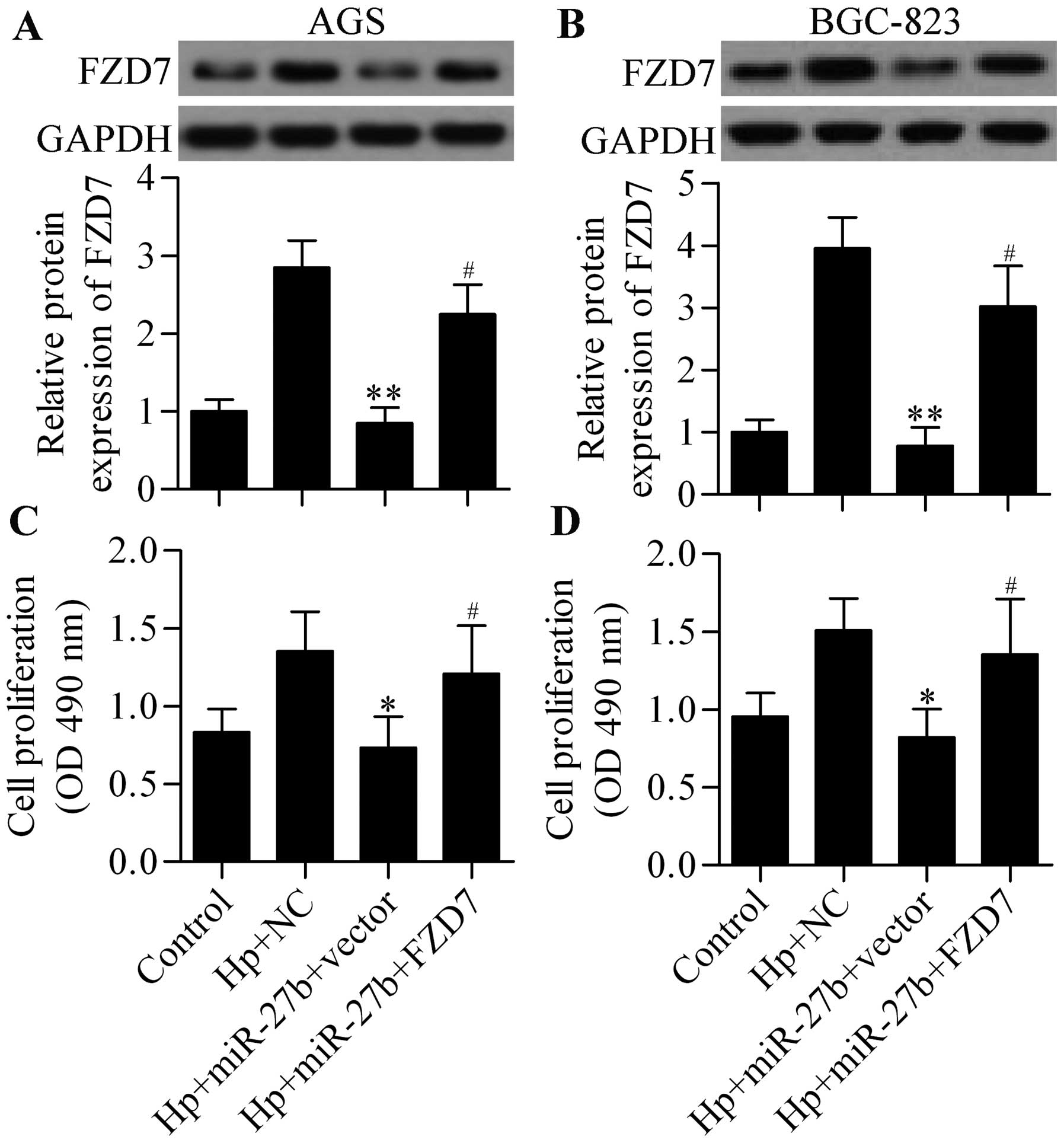
restoring expression of FZD7 (Fig. 7A
and B) in H. pylori-infected cells significantly
reversed the inhibitory effect of miR-27b overexpression on H.
pylori-induced cell proliferation (Fig. 7C and D). In summary, these results
indicate that miR-27b inhibited H. pylori-induced cell
proliferation directly through targeting miR-27b.
Suppression of FZD7 by FZD7 siRNA or
miR-27b overexpression inhibits the activation of WNT signaling
pathway
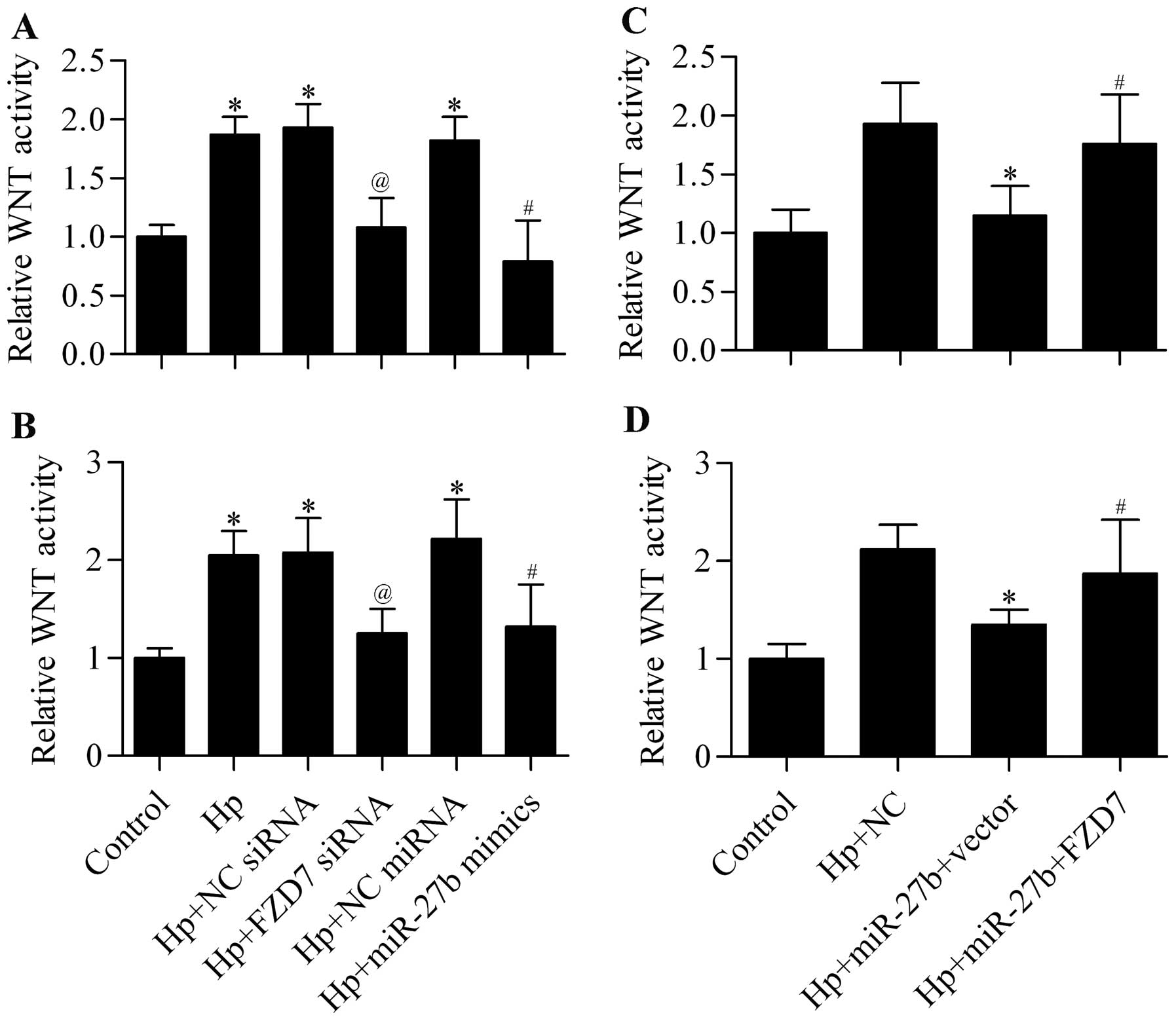
A previous study has reported that H. pylori
infection is associated with activation of the WNT signaling
pathway that contributes to carcinogenesis (12). We speculated that the increased
expression of FZD7 induced by H. pylori infection might
contribute to the activated WNT signaling pathway. To test this
hypothesis, we suppressed the expression of FZD7 by FZD7 siRNA or
miR-27b overexpression and detected their effect on the WNT
signaling pathway. The results showed that H. pylori
infection significantly increased the activity of the WNT signaling
pathway, but this promotion was significantly decreased by FZD7
knockdown or miR-27b overexpression in AGS (Fig. 8A) and BGC-823 (Fig. 8B) cells. Further data showed that
the inhibitory effect of miR-27b on WNT activity was significantly
reversed by restoration of FZD7 expression (Fig. 8C and D). Taken together, the
promoted FZD7 expression induced by H. pylori infection
contributed to the activated WNT signaling pathway, which could be
directly inhibited by miR-27b overexpression.
Discussion
In this study, we demonstrated that H. pylori
infection significantly upregulated the expression of FZD7 in
gastric cancer cells and that this promotion could be markedly
reversed by miR-27b overexpression. FZD7 is the critical
co-receptor for the WNT signaling pathway. Our result revealed that
suppression of FZD7 could block the WNT activation induced by H.
pylori infection. Therefore, H. pylori infection may
induce the repression of miR-27b, resulting in overexpression of
FZD7 and activation of the WNT signaling pathway, which play
important roles in H. pylori-induced gastric
tumorigenesis.
H. pylori promotes gastric tumorigenesis by
facilitating proliferation, angiogenesis, and invasion of cancer
cells (31). Various genes and
signaling pathways are involved in this process (6). However, the precise molecular
mechanism underlying this process remains poorly understood. In
this study, we found that FZD7, the critical co-receptor for the
WNT signaling pathway, was highly induced by H. pylori in
AGS and BGC-823 gastric cancer cells. These results indicate that
FZD7 may play an important role in H. pylori-induced gastric
tumorigenesis. Moreover, we found that knockdown of FZD7
significantly decreased the gastric cancer cell proliferation
induced by H. pylori infection. The results confirmed the
pivotal role of FZD7 in H. pylori-induced gastric
tumorigenesis. As an important regulator for the WNT signaling
pathway, FZD7 has drawn particular interest in tumorigenesis.
Studies have revealed that FZD7 is highly expressed in
hepatocellular carcinoma and that it contributes to aberrant
activation of the WNT signaling pathway (22,32,33).
Furthermore, a pharmacological inhibitor of FZD7 showed antitumor
effects linked to the inactivated WNT signaling pathway (34). FZD7 is involved in regulating the
cell proliferation, invasion, and metastasis of colon cancer cells
through the WNT signaling pathway (21,35).
Similarly, FZD7 plays critical roles in breast cancer (36) and cervical cancer (19). In gastric cancers, it was found that
FZD7 was overexpressed and was associated with activation of the
WNT signaling pathway (37).
Importantly, FZD7-positive gastric cancers are associated with
lower survival rates (38). All
these findings suggest that FZD7 is dysregulated in cancer
development and is positively associated with tumorigenesis.
Indeed, FZD7 is considered an emerging and promising molecular
target for cancer therapy (39).
Increasing evidence has suggested that miRNAs are
emerging tools for cancer therapy because of their negative
regulatory effect on target genes (30). Targeting FZD7 by specific miRNA may
be a promising and effective therapeutic strategy for cancer.
Several studies have tested the hypothesis. For example, miR-23b
was found to inhibit the tumorigenic potential of colon cancer
cells by regulating pro-metastatic targets, including FZD7
(40). miR-27a suppressed the
multiple drug resistance of hepatocellular carcinoma cells by
targeting FZD7 and inhibiting the FZD7-mediated WNT signaling
pathway (41). Other miRNAs,
including miR-199a (42),
miR-142-3p (43), and miR-126
(44), were found to be capable of
directly targeting and regulating FZD7 expression in cancer cells,
implying that these miRNAs are novel and promising approaches for
cancer therapy. In this study, we identified miR-27b as a novel
miRNA that targets and regulates FZD7 expression. Our results
showed that overexpression of miR-27b could inhibit cell
proliferation induced by H. pylori by suppressing FZD7
expression and by mimicking the effect of FZD7 knockdown via FZD7
siRNA. Additionally, restoring the expression of FZD7 could block
the effect of miR-27b overexpression, further confirming that FZD7
was a functional downstream target gene of miR-27b. Recent studies
have demonstrated that miR-27b functioned as a tumor suppressor
through suppressing the expression of various oncogenic proteins.
miR-27b was found to inhibit colorectal cancer progression and
angiogenesis by targeting vascular endothelial growth factor C
(45). miR-27b suppressed non-small
cell lung cancer cell proliferation and invasion by targeting Sp1
transcription factor (29) or LIM
kinase 1 (46). Cyclin A2 (47) and ectonucleotide
pyrophosphatase/phosphodiesterase family member 1 (48) are also direct target genes of
miR-27b in regulating tumorigenesis. Other evidence suggests that
miR-27b functions as a tumor suppressor (28,49–51).
Most recently, a study demonstrated that miR-27b could target and
inhibit cAMP responsive element binding protein 1 which is
associated with metastasis and poor outcome in gastric cancer
(52). However, this study did not
further investigate the function role of miR-27b on gastric cancer.
By investigating the expression and functional role of miR-27b in
gastric cancer, our study demonstrated that miR-27b might be a
tumor suppressor for H. pylori-induced gastric tumorigenesis
by modulating FZD7 expression. We found that miR-27b could inhibit
gastric cancer cell proliferation by targeting FZD7 and inhibiting
the FZD7-mediated WNT signaling pathway involved in H.
pylori-induced gastric tumorigenesis.
In this study, we also observed that H.
pylori infection activated the WNT signaling pathway. Other
studies have confirm the activation of WNT/β-catenin by H.
pylori (12). However, the
underlying mechanism remains obscure. In normal conditions,
β-catenin mainly interacts with E-cadherin at the cell membrane,
whereas CagA of H. pylori disrupts the complex, resulting in
cytoplasmic and nuclear accumulation of β-catenin for WNT signaling
activation (9). Interestingly, in
AGS cells lacking the expression of E-cadherin, H. pylori
still upregulates the cytoplasmic and nuclear accumulation of
β-catenin, implying that other mechanism are involved in H.
pylori-induced WNT signaling activation (9,12).
Sokolova et al found that H. pylori inhibited the
activity of GSK-3β, leading to increased accumulation of β-catenin
(53). It has also been reported
that H. pylori infection induced activation of low-density
lipoprotein receptor-related protein 6, another co-receptor of the
WNT signaling pathway (54). In
this study, we found that H. pylori infection induced the
expression of FZD7, the co-receptor for WNT signaling pathway.
Thus, we speculate that the increased expression of FZD7 may
contribute to the activated WNT signaling pathway induced by H.
pylori infection. Subsequently, we demonstrated that
suppression of FZD7 by siRNA or miR-27b significantly blocked the
activation of WNT induced by H. pylori infection. Our study
thus provides novel insight into the aberrant WNT signaling pathway
induced by H. pylori infection that involves FZD7.
Our study indicates that H. pylori infection
triggers the high expression of FZD7 in gastric cancer cells and
may contribute to the cell proliferation and WNT activation
processes induced by H. pylori. Furthermore, we demonstrated
that the expression of FZD7 and WNT activation could be inhibited
by miR-27b overexpression. We conclude that the miR-27b-FZD7-WNT
signaling pathway may be a promising molecular target for the
treatment of gastric cancer associated with H. pylori
infection.
Acknowledgments
This study was supported by the National Natural
Science Foundation of China (no. 81200583 and 81070328).
Abbreviations:
|
miRNAs
|
microRNAs
|
|
H. pylori,
|
Helicobacter pylori
|
|
3′-UTR
|
3′-untranslated region
|
|
FZD7
|
Frizzled7
|
|
CagA
|
cytotoxin-associated gene A
|
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Uemura N, Okamoto S, Yamamoto S, Matsumura
N, Yamaguchi S, Yamakido M, Taniyama K, Sasaki N and Schlemper RJ:
Helicobacter pylori infection and the development of gastric
cancer. N Engl J Med. 345:784–789. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wu WK, Cho CH, Lee CW, Fan D, Wu K, Yu J
and Sung JJ: Dysregulation of cellular signaling in gastric cancer.
Cancer Lett. 295:144–153. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shen J, Xiao Z, Wu WK, Wang MH, To KF,
Chen Y, Yang W, Li MS, Shin VY, Tong JH, et al: Epigenetic
silencing of miR-490-3p reactivates the chromatin remodeler SMARCD1
to promote Helicobacter pylori-induced gastric carcinogenesis.
Cancer Res. 75:754–765. 2015. View Article : Google Scholar
|
|
5
|
Fukase K, Kato M, Kikuchi S, Inoue K,
Uemura N, Okamoto S, Terao S, Amagai K, Hayashi S and Asaka M;
Japan Gast Study Group: Effect of eradication of Helicobacter
pylori on incidence of metachronous gastric carcinoma after
endoscopic resection of early gastric cancer: An open-label,
randomised controlled trial. Lancet. 372:392–397. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yong X, Tang B, Li BS, Xie R, Hu CJ, Luo
G, Qin Y, Dong H and Yang SM: Helicobacter pylori virulence factor
CagA promotes tumorigenesis of gastric cancer via multiple
signaling pathways. Cell Commun Signal. 13:302015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hayashi T, Senda M, Morohashi H, Higashi
H, Horio M, Kashiba Y, Nagase L, Sasaya D, Shimizu T, Venugopalan
N, et al: Tertiary structure-function analysis reveals the
pathogenic signaling potentiation mechanism of Helicobacter pylori
oncogenic effector CagA. Cell Host Microbe. 12:20–33. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ohnishi N, Yuasa H, Tanaka S, Sawa H,
Miura M, Matsui A, Higashi H, Musashi M, Iwabuchi K, Suzuki M, et
al: Transgenic expression of Helicobacter pylori CagA induces
gastrointestinal and hematopoietic neoplasms in mouse. Proc Natl
Acad Sci USA. 105:1003–1008. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Suzuki M, Mimuro H, Kiga K, Fukumatsu M,
Ishijima N, Morikawa H, Nagai S, Koyasu S, Gilman RH, Kersulyte D,
et al: Helicobacter pylori CagA phosphorylation-independent
function in epithelial proliferation and inflammation. Cell Host
Microbe. 5:23–34. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tabassam FH, Graham DY and Yamaoka Y:
Helicobacter pylori activate epidermal growth factor receptor- and
phos-phatidylinositol 3-OH kinase-dependent Akt and glycogen
synthase kinase 3beta phosphorylation. Cell Microbiol. 11:70–82.
2009. View Article : Google Scholar
|
|
11
|
Sokolova O, Vieth M, Gnad T, Bozko PM and
Naumann M: Helicobacter pylori promotes eukaryotic protein
translation by activating phosphatidylinositol 3 kinase/mTOR. Int J
Biochem Cell Biol. 55:157–163. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Franco AT, Israel DA, Washington MK,
Krishna U, Fox JG, Rogers AB, Neish AS, Collier-Hyams L,
Perez-Perez GI, Hatakeyama M, et al: Activation of beta-catenin by
carcinogenic Helicobacter pylori. Proc Natl Acad Sci USA.
102:10646–10651. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bronte-Tinkew DM, Terebiznik M, Franco A,
Ang M, Ahn D, Mimuro H, Sasakawa C, Ropeleski MJ, Peek RM Jr and
Jones NL: Helicobacter pylori cytotoxin-associated gene A activates
the signal transducer and activator of transcription 3 pathway in
vitro and in vivo. Cancer Res. 69:632–639. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wei J, Noto J, Zaika E, Romero-Gallo J,
Correa P, El-Rifai W, Peek RM and Zaika A: Pathogenic bacterium
Helicobacter pylori alters the expression profile of p53 protein
isoforms and p53 response to cellular stresses. Proc Natl Acad Sci
USA. 109:E2543–E2550. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wei J, Noto JM, Zaika E, Romero-Gallo J,
Piazuelo MB, Schneider B, El-Rifai W, Correa P, Peek RM and Zaika
AI: Bacterial CagA protein induces degradation of p53 protein in a
p14ARF-dependent manner. Gut. 64:1040–1048. 2015. View Article : Google Scholar :
|
|
16
|
Abu-Elmagd M, Garcia-Morales C and Wheeler
GN: Frizzled7 mediates canonical Wnt signaling in neural crest
induction. Dev Biol. 298:285–298. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Asad M, Wong MK, Tan TZ, Choolani M, Low
J, Mori S, Virshup D, Thiery JP and Huang RY: FZD7 drives in vitro
aggressiveness in Stem-A subtype of ovarian cancer via regulation
of non-canonical Wnt/PCP pathway. Cell Death Dis. 5:e13462014.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xu R, Zeng S, Xie W, Sun C, Chen YL, Chen
MJ and Zhang L: The expression and function of Frizzled-7 in human
renal cell carcinoma. Clin Transl Oncol. Aug 5–2015.(Epub ahead of
print). http://dx.doi.org/10.1007/s12094-015-1362-3.
|
|
19
|
Deng B, Zhang S, Miao Y, Zhang Y, Wen F
and Guo K: Down-regulation of Frizzled-7 expression inhibits
migration, invasion, and epithelial-mesenchymal transition of
cervical cancer cell lines. Med Oncol. 32:1022015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Simmons GE Jr, Pandey S,
Nedeljkovic-Kurepa A, Saxena M, Wang A and Pruitt K: Frizzled 7
expression is positively regulated by SIRT1 and β-catenin in breast
cancer cells. PLoS One. 9:e988612014. View Article : Google Scholar
|
|
21
|
Ueno K, Hazama S, Mitomori S, Nishioka M,
Suehiro Y, Hirata H, Oka M, Imai K, Dahiya R and Hinoda Y:
Down-regulation of frizzled-7 expression decreases survival,
invasion and metastatic capabilities of colon cancer cells. Br J
Cancer. 101:1374–1381. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Merle P, Kim M, Herrmann M, Gupte A,
Lefrançois L, Califano S, Trépo C, Tanaka S, Vitvitski L, de la
Monte S, et al: Oncogenic role of the frizzled-7/beta-catenin
pathway in hepato-cellular carcinoma. J Hepatol. 43:854–862. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
White BD, Chien AJ and Dawson DW:
Dysregulation of Wnt/β-catenin signaling in gastrointestinal
cancers. Gastroenterology. 142:219–232. 2012. View Article : Google Scholar :
|
|
24
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Winter J, Jung S, Keller S, Gregory RI and
Diederichs S: Many roads to maturity: microRNA biogenesis pathways
and their regulation. Nat Cell Biol. 11:228–234. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Dalmay T and Edwards DR: MicroRNAs and the
hallmarks of cancer. Oncogene. 25:6170–6175. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Esquela-Kerscher A and Slack FJ: Oncomirs
- microRNAs with a role in cancer. Nat Rev Cancer. 6:259–269. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chiyomaru T, Seki N, Inoguchi S, Ishihara
T, Mataki H, Matsushita R, Goto Y, Nishikawa R, Tatarano S, Itesako
T, et al: Dual regulation of receptor tyrosine kinase genes EGFR
and c-Met by the tumor-suppressive microRNA-23b/27b cluster in
bladder cancer. Int J Oncol. 46:487–496. 2015.
|
|
29
|
Jiang J, Lv X, Fan L, Huang G, Zhan Y,
Wang M and Lu H: MicroRNA-27b suppresses growth and invasion of
NSCLC cells by targeting Sp1. Tumour Biol. 35:10019–10023. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jiang C, Chen X, Alattar M, Wei J and Liu
H: MicroRNAs in tumorigenesis, metastasis, diagnosis and prognosis
of gastric cancer. Cancer Gene Ther. 22:291–301. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hatakeyama M: Oncogenic mechanisms of the
Helicobacter pylori CagA protein. Nat Rev Cancer. 4:688–694. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Merle P, de la Monte S, Kim M, Herrmann M,
Tanaka S, Von Dem Bussche A, Kew MC, Trepo C and Wands JR:
Functional consequences of frizzled-7 receptor overexpression in
human hepatocellular carcinoma. Gastroenterology. 127:1110–1122.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kim M, Lee HC, Tsedensodnom O, Hartley R,
Lim YS, Yu E, Merle P and Wands JR: Functional interaction between
Wnt3 and Frizzled-7 leads to activation of the Wnt/beta-catenin
signaling pathway in hepatocellular carcinoma cells. J Hepatol.
48:780–791. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Nambotin SB, Lefrancois L, Sainsily X,
Berthillon P, Kim M, Wands JR, Chevallier M, Jalinot P, Scoazec JY,
Trepo C, et al: Pharmacological inhibition of Frizzled-7 displays
anti-tumor properties in hepatocellular carcinoma. J Hepatol.
54:288–299. 2011. View Article : Google Scholar
|
|
35
|
Ueno K, Hiura M, Suehiro Y, Hazama S,
Hirata H, Oka M, Imai K, Dahiya R and Hinoda Y: Frizzled-7 as a
potential therapeutic target in colorectal cancer. Neoplasia.
10:697–705. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yang L, Wu X, Wang Y, Zhang K, Wu J, Yuan
YC, Deng X, Chen L, Kim CC, Lau S, et al: FZD7 has a critical role
in cell proliferation in triple negative breast cancer. Oncogene.
30:4437–4446. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kirikoshi H, Sekihara H and Katoh M:
Up-regulation of Frizzled-7 (FZD7) in human gastric cancer. Int J
Oncol. 19:111–115. 2001.PubMed/NCBI
|
|
38
|
Schmuck R, Warneke V, Behrens HM, Simon E,
Weichert W and Röcken C: Genotypic and phenotypic characterization
of side population of gastric cancer cell lines. Am J Pathol.
178:1792–1804. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
King TD, Zhang W, Suto MJ and Li Y:
Frizzled7 as an emerging target for cancer therapy. Cell Signal.
24:846–851. 2012. View Article : Google Scholar :
|
|
40
|
Zhang H, Hao Y, Yang J, Zhou Y, Li J, Yin
S, Sun C, Ma M, Huang Y and Xi JJ: Genome-wide functional screening
of miR-23b as a pleiotropic modulator suppressing cancer
metastasis. Nat Commun. 2:5542011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Chen Z, Ma T, Huang C, Zhang L, Lv X, Xu
T, Hu T and Li J: miR-27a modulates the MDR1/P-glycoprotein
expression by inhibiting FZD7/β-catenin pathway in hepatocellular
carcinoma cells. Cell Signal. 25:2693–2701. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Song J, Gao L, Yang G, Tang S, Xie H, Wang
Y, Wang J, Zhang Y, Jin J, Gou Y, et al: miR-199a regulates cell
proliferation and survival by targeting FZD7. PLoS One.
9:e1100742014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Deng B, Zhang Y, Zhang S, Wen F, Miao Y
and Guo K: Micro-RNA-142-3p inhibits cell proliferation and
invasion of cervical cancer cells by targeting FZD7. Tumour Biol.
36:8065–8073. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Li Z, Chen P, Su R, Li Y, Hu C, Wang Y,
Arnovitz S, He M, Gurbuxani S, Zuo Z, et al: Overexpression and
knockout of miR-126 both promote leukemogenesis. Blood.
126:2005–2015. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Ye J, Wu X, Wu D, Wu P, Ni C, Zhang Z,
Chen Z, Qiu F, Xu J and Huang J: miRNA-27b targets vascular
endothelial growth factor C to inhibit tumor progression and
angiogenesis in colorectal cancer. PLoS One. 8:e606872013.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Wan L, Zhang L, Fan K and Wang J: miR-27b
targets LIMK1 to inhibit growth and invasion of NSCLC cells. Mol
Cell Biochem. 390:85–91. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Wang B, Li D, Kovalchuk A, Litvinov D and
Kovalchuk O: Ionizing radiation-inducible miR-27b suppresses
leukemia proliferation via targeting cyclin A2. Int J Radiat Oncol
Biol Phys. 90:53–62. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Takahashi RU, Miyazaki H, Takeshita F,
Yamamoto Y, Minoura K, Ono M, Kodaira M, Tamura K, Mori M and
Ochiya T: Loss of microRNA-27b contributes to breast cancer stem
cell generation by activating ENPP1. Nat Commun. 6:73182015.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Goto Y, Kojima S, Nishikawa R, Enokida H,
Chiyomaru T, Kinoshita T, Nakagawa M, Naya Y, Ichikawa T and Seki
N: The microRNA-23b/27b/24-1 cluster is a disease progression
marker and tumor suppressor in prostate cancer. Oncotarget.
5:7748–7759. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Mu W, Hu C, Zhang H, Qu Z, Cen J, Qiu Z,
Li C, Ren H, Li Y, He X, et al: miR-27b synergizes with anticancer
drugs via p53 activation and CYP1B1 suppression. Cell Res.
25:477–495. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Veliceasa D, Biyashev D, Qin G, Misener S,
Mackie AR, Kishore R and Volpert OV: Therapeutic manipulation of
angiogenesis with miR-27b. Vasc Cell. 7:62015. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Wang YW, Chen X, Gao JW, Zhang H, Ma RR,
Gao ZH and Gao P: High expression of cAMP-responsive
element-binding protein 1 (CREB1) is associated with metastasis,
tumor stage and poor outcome in gastric cancer. Oncotarget.
6:10646–10657. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Sokolova O, Bozko PM and Naumann M:
Helicobacter pylori suppresses glycogen synthase kinase 3beta to
promote beta-catenin activity. J Biol Chem. 283:29367–29374. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Gnad T, Feoktistova M, Leverkus M,
Lendeckel U and Naumann M: Helicobacter pylori-induced activation
of beta-catenin involves low density lipoprotein receptor-related
protein 6 and Dishevelled. Mol Cancer. 9:312010. View Article : Google Scholar : PubMed/NCBI
|