Introduction
Thyroid cancers are the most common malignant tumors
of the endocrine system. Metastasis of tumor cells frequently
contributes to the failure of treatments in patients diagnosed with
thyroid cancer. Substantial research has been devoted to
elaborating the relationship between glycan alterations and
invasive properties of malignant cells. The changes of glycans on
cell glycoproteins play a physiological role in regulating
metastatic efficiency of tumor cells (1). It is known that alteration in cell
surface sialylated antigens affects many cellular properties
(2–5). Sialyltransferases, a subset of
glycosyltransferases, have been recognized to be involved in
various diseases by catalyzing the biosynthesis of different
glycoconjugates and saccharide structures (6). They use CMP-Neu5Ac as an activated
sugar donor to catalyze the transfer of sialic acid residues to
terminal positions of glycoprotein and glycolipid carbohydrate
groups (7).
Sialyltransferases (ST) are a group of enzymes
responsible for the transfer of sialic acid from cytidine 5-prime
monophospho-N-acetylneuraminic acid (CMP-NeuAc) to terminal
positions of glycoprotein and glycolipid carbohydrate groups. ST
consisting of 20 members that are subjected into three subfamilies:
α-2, 3-sialyltransferases, α-2, 6-sialyltrans-ferases, α-2,
8-sialyltransferases (8). α-2,
6-sialyltransferases mediate the transfer of sialic acid with an
α-2, 6-linkage to it with terminal Gal (ST6Gal 1–2) (9) or GalNAc residues (ST6GalNAc 1–6).
Changes in specific ST6GalNAc family expression have been reported
to be altered in several tumors. High level of ST6GalNAcI
expression was associated with the tumorigenicity of MDA-MB-231
breast cancer cells (10).
Suppression of ST6GalNAcII mRNA is not only associated with the
pathological phenotype of IgA nephropathy but also with the poor
prognosis in IgA nephropathy patients (11,12).
ST6GalNAcIV promotes lung cancer metastasis through adhesion to
galectins (13). ST6GalNAc V plays
a positive role in mediating brain metastasis of breast cancer
cells (14).
The phosphatidylinositol-3-kinase (PI3K)/Akt pathway
is one of the core intracellular signaling pathways, it plays a
crucial role in many cellular processes including proliferation,
differentiation, apoptosis, cell cycle progression, cell motility
and tumorigenesis, tumor growth, angiogenesis (15,16).
Furthermore, aberrant activation of PI3K/Akt pathway has been
reported to be a significant indicator of proliferation, invasion,
metastasis in thyroid cancer. Activation of PI3K/AKT/mTOR pathway
sustains malignant features of MTC cell models (17). The proliferation and invasion of
thyroid cancer cells are inhibited by curcumin via downregulation
of PI3K/Akt signaling pathway (18). However, it remained largely unknown
whether there is a certain correlation between ST6GalNAcII and
PI3K/Akt pathway in the progression of invasion, metastasis in
thyroid cancer.
Therefore, we undertook to characterize the
expression of ST6GalNAcII in FTC-238 and FTC-133 cell lines and
thyroid cancer tissue samples. Besides, we investigated the
correlation between ST6GalNAcII and PI3K/Akt pathway and their role
in the thyroid cancer metastasis.
Materials and methods
Cell culture and tissues
Human follicular thyroid carcinoma cell lines
FTC-133 and FTC-238 were purchased from Guangzhou Jennio Biotech
Co. (China). The cell lines were grown in Dulbecco's modified
Eagle's medium (DMEM)/Ham's F12 medium containing 2 mM glutamine,
10% fetal bovine serum (FBS), 100 U/ml penicillin, and 100 Ag/ml
streptomycin in a humidified CO2 incubator at 37°C.
All specimens were obtained from the General Surgery
Department of The Second Affiliated Hospital of Dalian Medical
University (Liaoning, China) and included 101 samples of follicular
thyroid cancer and the corresponding peritumoral tissues (3 cm from
the tumor edge). For the use of these clinical materials for
research purposes, prior consents from the patients and approval
from the Ethics Committees of The Second Affiliated Hospital of
Dalian Medical University were obtained, and all the procedures
have been performed in compliance with the Helsinki Declaration.
All specimens had confirmed pathological diagnosis and were staged
according to the 2013 thyroid carcinomas staging system of the
International Union against Cancer (UICC). These tissues were
snap-frozen in liquid nitrogen and stored at −80°C until used.
RNA isolation and real-time PCR
analysis
Real-time PCR was used to analyze gene expression.
Total RNA was isolated using the RNeasy Mini kit (Qiagen, Valencia,
CA, USA), and cDNA was synthesized using the QuantiTect Reverse
Transcription kit (Qiagen) according to the manufacturer's
protocol. Real-time PCR was carried out on an ABI Prism 7500 Fast
Real-Time PCR system (Applied Biosystems, Foster City, CA, USA)
using QuantiTect SYBR Green PCR kit (Qiagen). The primer sequences
used for amplification were as follows: forward,
5′-CTTTGCCCTGTACTTCTCG-3′ and reverse, 5′-CAGCACTGGAATGGAGAGA-3′
for ST6GalNAcII; and forward, 5′-CTCCTCCACCTTTGACGCTG-3′ and
reverse, 5′-TCCTCTTGTGCTCTTGCTGG-3′ for GAPDH. The relative
expression level of target gene was normalized to that of the
respective GAPDH.
Western blot analysis
Extracted proteins were electrophoresed under
reducing conditions in 10% sodium dodecylsulfate-polyacrylamide
gels, and then blotted onto a polyvinylidene difluoride membrane.
After blocking with 5% skimmed milk in PBS containing 0.1% Tween-20
(PBST), the membrane was incubated with antibody (1/1,000 diluted;
Abcam) and then with peroxidase-conjugated anti-rabbit IgG
(1/10,000 diluted; GE Healthcare UK Ltd., Little Chalfont, UK). A
GAPDH antibody (1/200 diluted; Santa Cruz Biotechnology) was used
as a control. All bands were detected using ECL Western Blot kit
(Amersham Biosciences, UK), and the bands were analyzed with
LabWorks™ (ver 4.6, UVP; Bio-Imaging Systems).
Deregulation of ST6GalNAcII by RNAi
FTC-238 cells were incubated in appropriate
antibiotic-free medium with 10% fetal bovine serum (Gibco),
transferred to a 6-well tissue culture, and incubated at 37°C in a
CO2 incubator to obtain 60–80% confluence. The cell
cultures were stably co-transfected with a plasmid vector
containing the puromycin-resistance marker and the specific short
hairpin RNA (shRNA) (ST6GalNAcII), respectively, which was prepared
according to the protocol. Scrambled shRNA was used as the negative
control. The transfection efficiency calculated by the percentage
of fluorescent cells was about 82%, and cell viability was 89% by
trypan blue dye exclusion assay. Four weeks later, we used
puromycin to screen the cells stably expressing shRNA. Several
colonies were picked and expanded for further study. The knockdown
had no effects on the cell morphology.
Overexpression of ST6GalNAcII
The human ST6GalNAcII coding sequences were obtained
from Takara Company (Dalian, China) and were inserted into the
pEGFP-N2 vector (Invitrogen, Carlsbad, CA, USA) at the sites of
EcoRI, XhoI. Cells were transfected with 5 µg
of target gene expression vector or empty vector (EV) in 100-mm
dishes using PolyFect transfection reagent (Qiagen) according to
the manufacturer's instruction. After 4 weeks of screening, the
cell lines stably expressing ST6GalNAcII (FTC-133/ST6GalNAcII) and
empty vector (FTC-133/mock) were established. Then cells were
collected for gene expression assay and for further explorations.
The cell transfection efficiency was 79% and the survival rate was
87%.
In vitro extracellular matrix invasion
assays
Invasiveness of tumor cells was examined using
24-well Transwell units (Corning, Corning, NY, USA) with 8-mm pore
size poly-carbonate filter coated with Matrigel (BD Biosciences) to
form a continuous thin layer. Cells (3×105) were
harvested in serum-free medium containing 0.1% BSA and added to the
upper chamber. The lower chamber contained 500 ml 90% RPMI-1640 and
10% FBS. At the end of incubation, the cells on the upper surface
of the filter were completely removed by wiping with a cotton swab.
The filters were fixed in methanol and stained with Wright-Giemsa.
Cells invading the Matrigel that reached the lower surface of the
filter were counted with light microscope at a magnification of
×400. In migration assay, the upper chamber was not coated with
Matrigel. Samples were acquired in triplicate and data expressed as
the average cell number in five fields.
In vivo tumorigenicity assay
The tumorigenicity of ST6GalNAcII in vivo was
investigated using a xenograft tumor model in the nude mice.
Forty-eight 5-week-old male athymic nude mice were provided with
sterilized food and water and equally divided into three groups.
Approximately, 1×107 cells (with or without ST6GalNAcII
shRNA interference and control shRNA) were subcutaneously
inoculated into the right flank of each nude mouse. Once bearing
palpable tumors (about 4 weeks after tumor cell inoculation), mice
were sacrificed and their tumors were isolated, weighed, and
photographed. Experiments were repeated three times.
Inhibition of the PI3K/Akt signaling
LY294002 (Sigma) was used to suppress the activity
of the PI3K/Akt signaling in FTC-238 cells. Briefly, the tumor
cells (1×104 cells/well) were incubated with dimethyl
sulfoxide (DMSO) or the PI3K inhibitor LY294002 (20 mmol/l)
dissolved in DMSO, and collected after 24 h. Tumorigenicity was
analyzed when PI3K/Akt signaling was blocked in xenograft tumor
model. Sixty female athymic nude mice (5-week-old) were divided
into 4 groups and 1×107 FTC-238 cells (with DMSO,
LY294002, control shRNA, Akt shRNA, respectively) were injected
subcutaneously into the axillary regions of each nude mouse,
respectively. Once bearing palpable tumors (about 4 weeks after
tumor cell inoculation), mice were sacrificed and their tumors were
isolated and weighed. Changes in protein expression were measured
by western blot analysis.
Immunohistochemical (IHC) staining
analysis
Tumors were removed from the mice and
immunohistochemical (IHC) staining was conducted using
formalin-fixed paraffin-embedded sections of tissues by the
avidin-biotin-peroxidase complex (ABC) method. Four-micron sections
of formalin-fixed paraffin-embedded tissues were cut with a
microtome and dried overnight at 37°C on a silicanized slide (Dako,
Carpinteria, CA, USA). Samples were deparaffinized in xylene at
room temperature for 80 min and washed with a graded ethanol/water
mixture and then with distilled water. The samples were soaked in a
citrate buffer and then microwaved at 100°C for 10 min. The
following steps were used. Before addition of the primary
antibodies, endogenous peroxidase activity was blocked by
incubation in methanol containing 1% H2O2 for
20 min, followed by 60 min incubation with normal donkey serum to
reduce background staining. The primary antibodies, goat
anti-human, ST6GalNAcII antibodies (Abcam, Cambridge, UK), were
incubated at 4°C for 8 h, followed by incubation with the
biotinylated secondary antibodies (donkey anti-goat IgG; Santa Cruz
Biotechnology) for 30 min and ABC complex for 30 min. The primary
and secondary antibodies were used at 1:80 and 1:100 dilutions,
respectively. The peroxidase binding sites were demonstrated by the
diaminobenzidine method. A phosphate-buffered solution instead of
the primary antibody was used in the protocols for negative
controls. The level of expression level is measured by Image-Pro
Plus software 6.0.
Statistical analysis
Each experiment was performed at least in
triplicate, and the measurements were performed in three
independent experiments. The data were expressed as mean ± standard
deviation (SD) from the triple tests of each group. SPSS 17.0
software was used for statistical analysis and Student's t-test was
selected to determine the significance of differences among the
examined groups. P<0.05 was considered to be statistically
significant.
Results
Differential expression of ST6GalNAcII in
follicular thyroid carcinoma cell lines
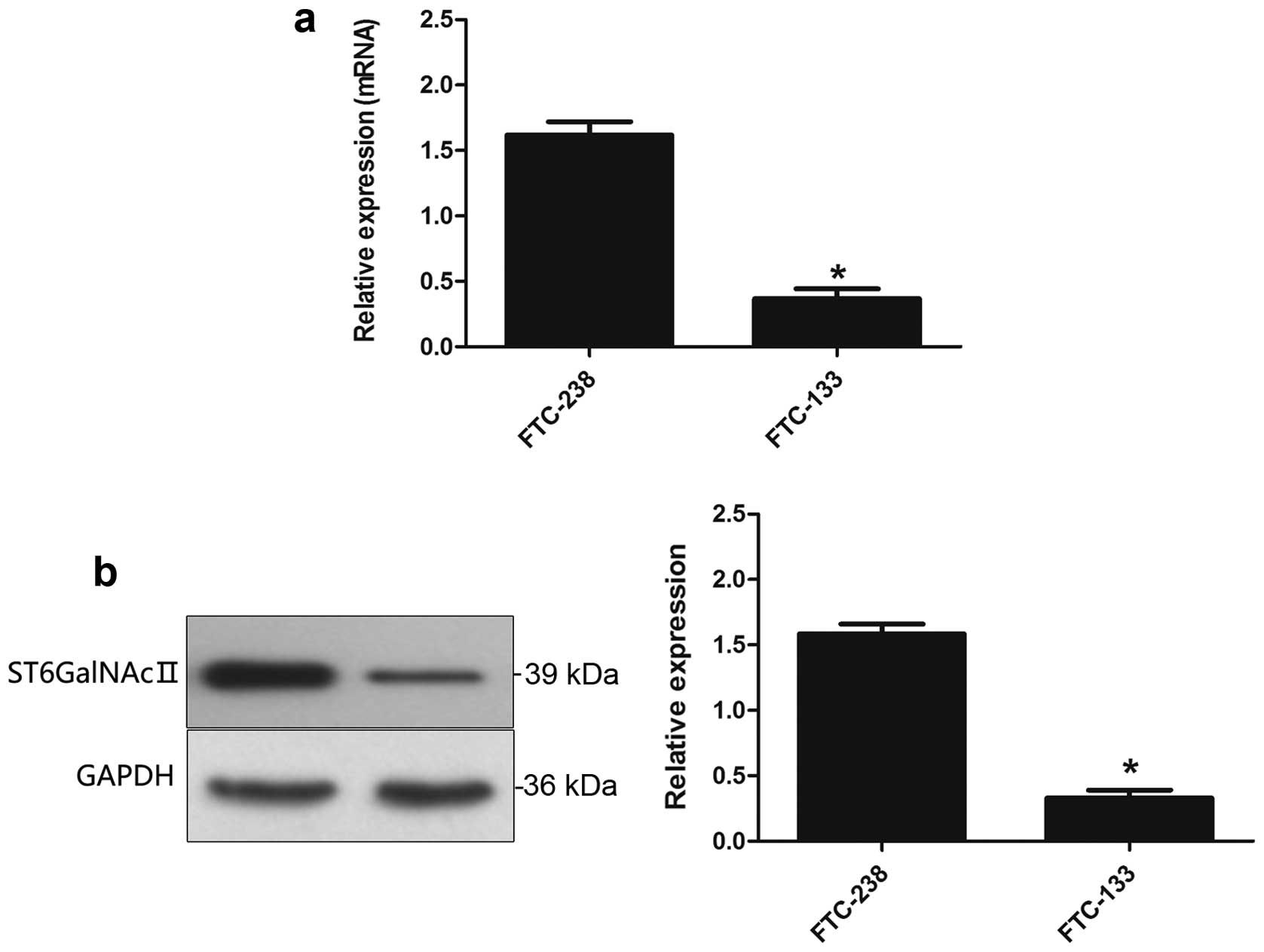
Real-time PCR and western blot analysis was used to
evaluate the expression level of ST6GalNAcII in mRNA (Fig. 1a) and protein expression (Fig. 1b). These data indicated that
ST6GalNAcII may be associated with metastasis of human follicular
thyroid carcinoma cells (FTC-238).
Silence of ST6GalNAcII inhibits the
invasive ability of follicular thyroid cancer cells in vitro and in
vivo
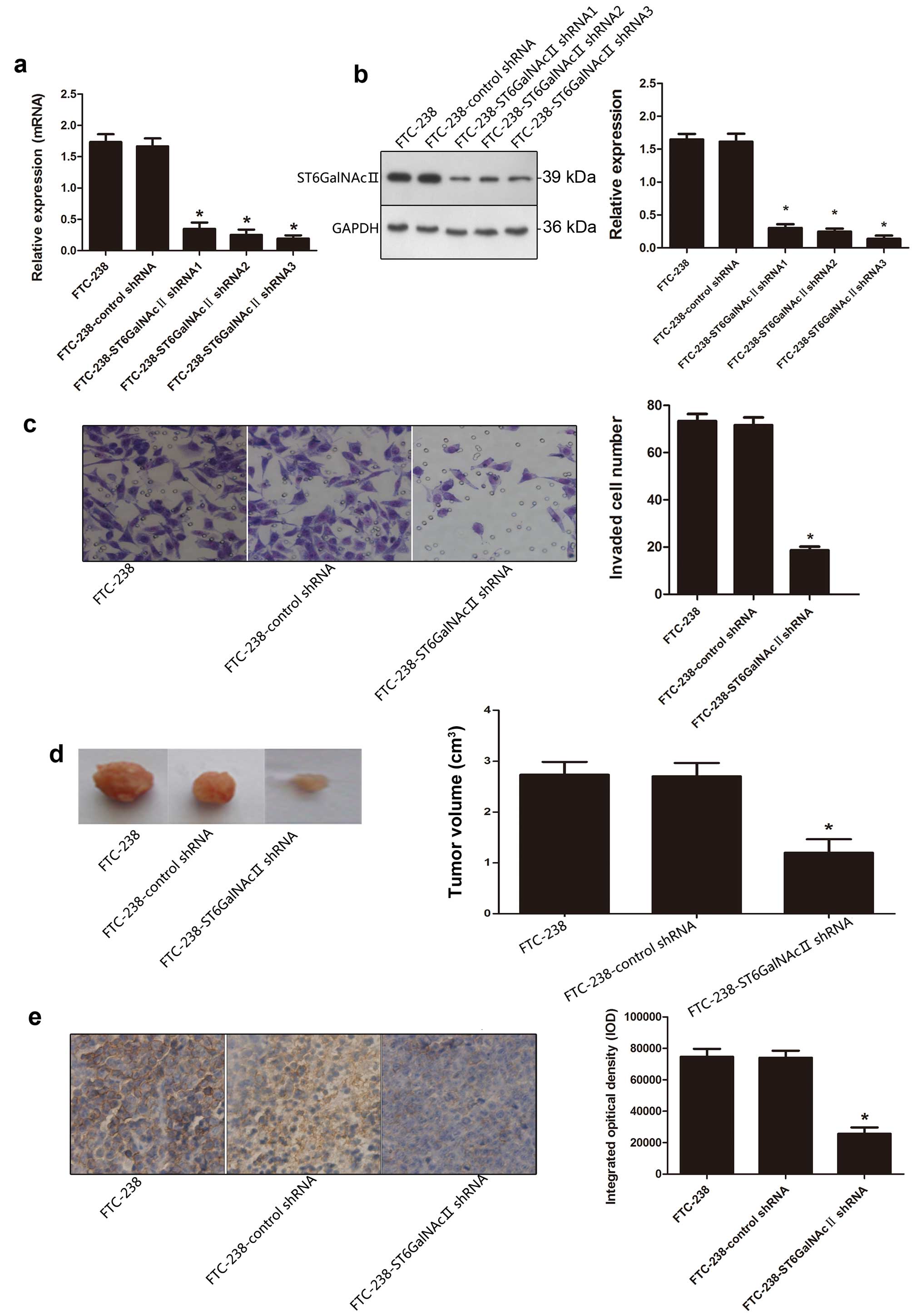
Owing to the high expression of ST6GalNAcII in
FTC-238 cells, we silenced ST6GalNAcII with shRNA, in order to
elucidate the effect of ST6GalNAcII on the invasion and metastasis
of thyroid cancer cells. As shown in Fig. 2a and b, the expression level of
ST6GalNAcII was significantly reduced in FTC-238 transfectants
compared to the control transfectants (P<0.05).
After ST6GalNAcII shRNA transfection, Transwell
invasion assay was performed to further evaluate the invasion
capability of cells with ST6GalNAcII knockdown on tumor cells in
vitro. The invasion capability of FTC-238 cells transfected
with ST6GalNAcII shRNA showed a significant reduction, as compared
with control shRNA group, suggesting that cell invasion capability
was inhibited by treatment with ST6GalNAcII shRNA (Fig. 2c).
Nude mice bearing FTC-238, FTC-238-control shRNA and
FTC-238-ST6GalNAcII shRNA1 xenografts were used to analyze the
differences by measuring tumor volumes. Fig. 2d showed a significant reduction of
mean tumor volume in nude mice bearing FTC-238-ST6GalNAcII shRNA,
as compared with control shRNA group.
IHC staining analysis of the tumor section revealed
that ST6GalNAcII was reduced in tumors derived from
FTC-238-ST6GalNAcII shRNA cells compared to control group (Fig. 2e).
The above results indicated that knockdown of
ST6GalNAcII could inhibit the invasion ability of follicular
thyroid carcinoma cells.
Overexpression of ST6GalNAcII enhances
the invasive ability of follicular thyroid cancer cells in vitro
and in vivo
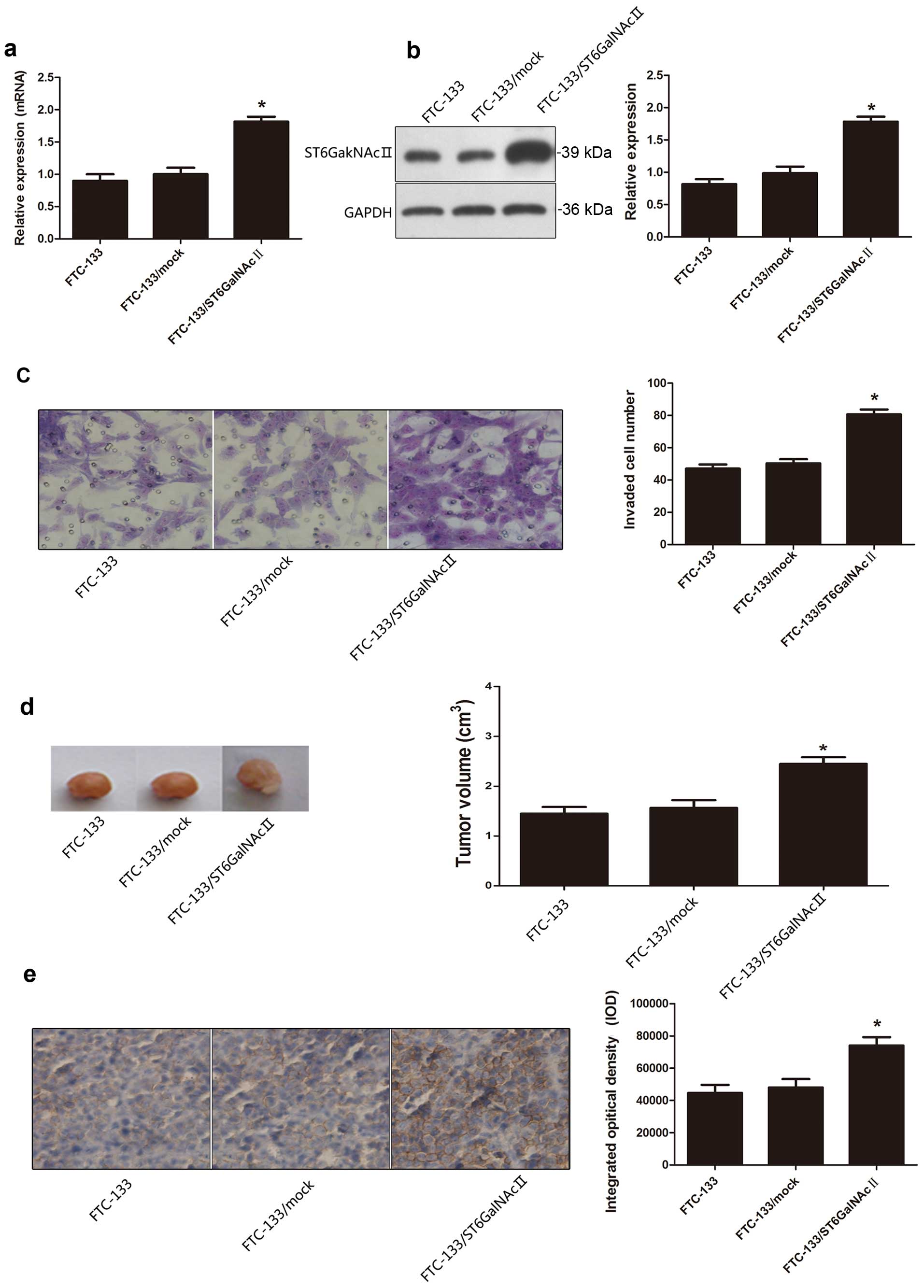
After elucidating whether the effect of ST6GalNAcII
suppresses the invasion ability of FTC-238, we transfected FTC-133
cells with ST6GalNAcII expression vector to determine the effect of
overexpression of ST6GalNAcII on tumor cell invasion ability of
FTC-133. As shown in Fig. 3a and b,
increased levels of mRNA and protein of were detected in FTC-133
transfectants.
Transwell invasion assay revealed that the invasion
capability of FTC-133 cells transfected with ST6GalNAcII expression
vector was obviously increased compared with the FTC-133/mock cells
(Fig. 3c).
Nude mice were inoculated with tumor cells FTC-133,
FTC-133/mock, and FTC-133/ST6GalNAcII. Tumor volumes were increased
obviously in nude mice bearing FTC-133/ST6GalNAcII, as compared to
the FTC-133/mock group (Fig.
3d).
High expression levels of ST6GalNAcII in tumor cells
of FTC-133/ST6GalNAcII, was also detected using IHC staining, as
shown in Fig. 3e.
Therefore, the upregulation of the ST6GalNAcII gene
was able to increase the invasion ability of follicular thyroid
carcinoma cells.
ST6GalNAcII regulates the activity of
PI3K/Akt signaling pathway in thyroid cells
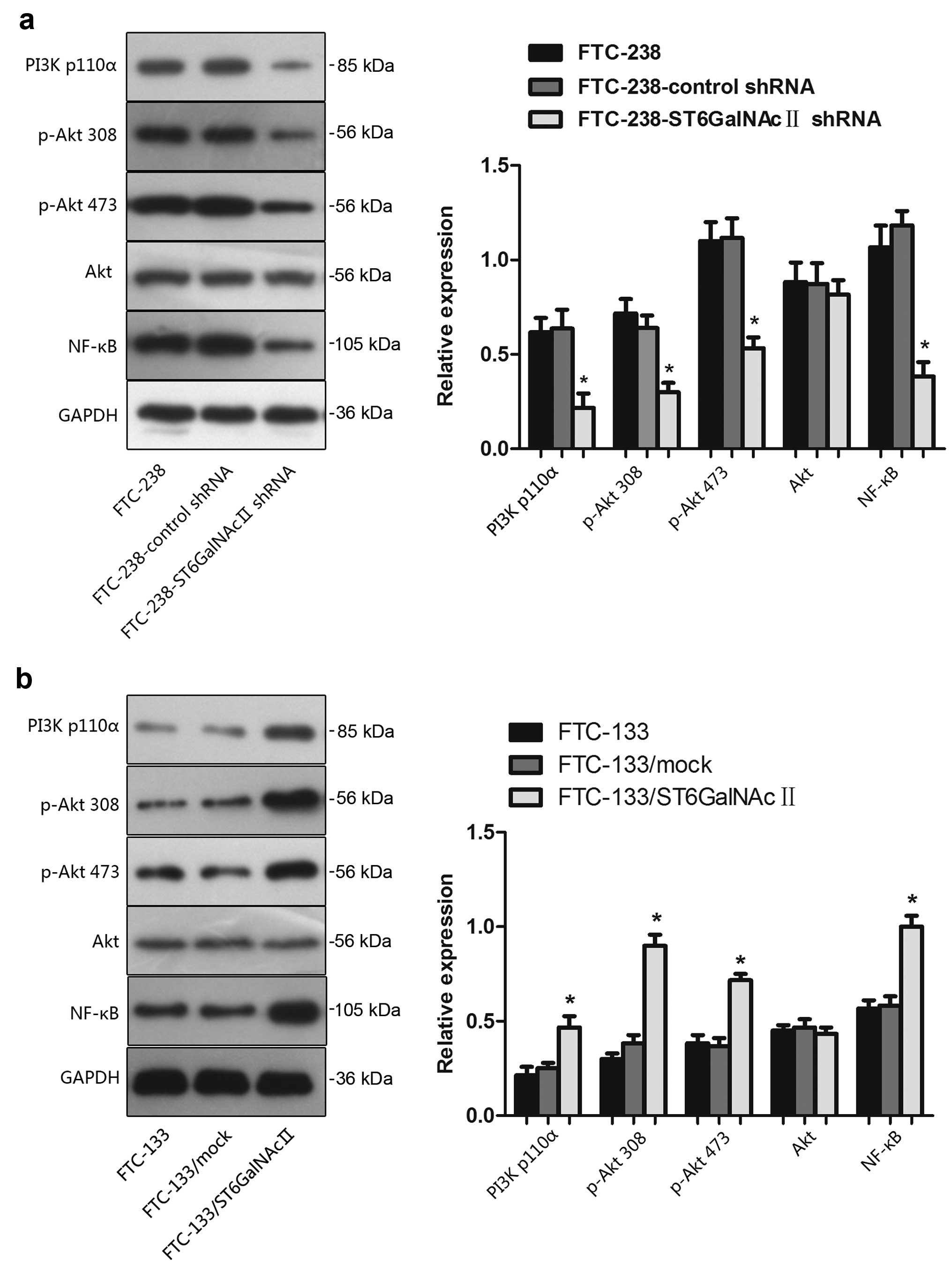
Having established the pivotal role of PI3K/Akt
pathway in tumor cells, we investigated whether ST6GalNAcII
activated the PI3K/Akt pathway and whether this pathway played a
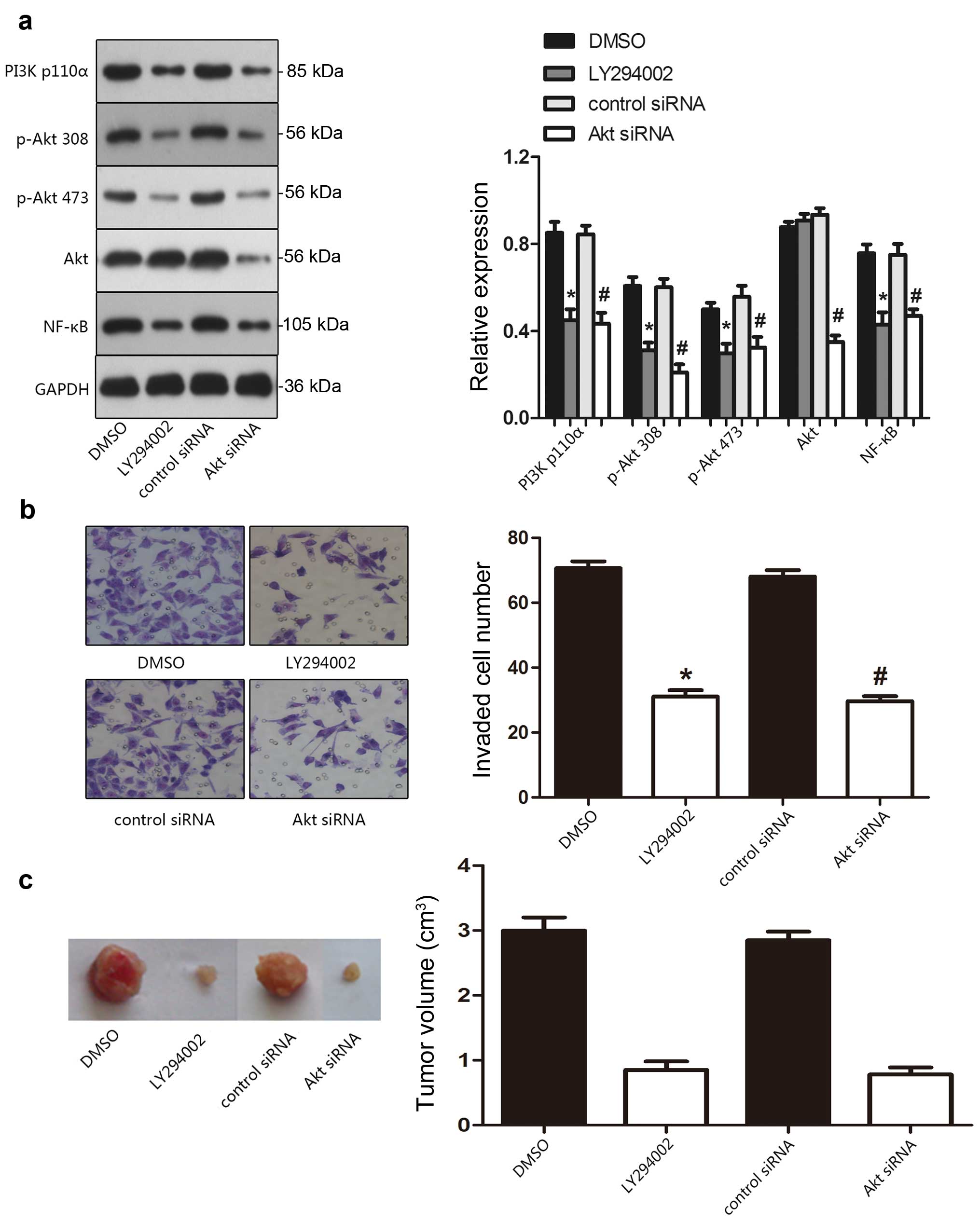
central role in ST6GalNAcII-mediated cell invasion. Fig. 4a shows that following the decreased
expression level of ST6GalNAcII, the expression and activity of the
PI3K/Akt pathway was inhibited. PI3K expression decreased the
protein and phosphorylation levels of Akt. The degree of
phosphorylation of Akt at Ser473 and Thr308 and its downstream
effector NF-κB was also downregulated after ST6GalNAcII silencing.
No variation could be detected in the total amount of Akt protein,
indicating a true decrease in the phosphorylation status. In
addition, as illustrated in Fig.
4b, overexpression of ST6GalNAcII in FTC-133 cells showed the
reverse tendency. The above suggested that the variation of
ST6GalNAcII expression levels alters the PI3K/Akt signaling
pathway.
PI3K/Akt inhibition mediates the invasion
ability of FTC-238 cells both in vitro and in vivo
After inhibition of PI3K and Akt by LY294002 and Akt
siRNA, the invasion ability was significantly inhibited in FTC-238
cells. By western blotting, FTC-238 cells treated with LY294002,
and Akt siRNA treatment exhibited apparently decreased expression
levels of the main signal molecules of PI3K/Akt pathway (Fig. 5a). As shown in Fig. 5b, the inhibition of PI3K/Akt pathway
made the FTC-238 cells less invasive. In Transwell invasion assay,
the invasive capability of FTC-238 cells transfected with Akt siRNA
obviously decreased in the Transwell migration and invasion assay.
Similar results were also observed in vivo analysis where
reduced tumor weight was measured in the mouse group bearing
FTC-238 tumors with impaired PI3K/Akt signaling (Fig. 5b). Altered expression levels of the
main signal molecules of the PI3K/Akt pathway in the mouse group
bearing FTC-238 tumors with LY294002 or Akt shRNA treatment were
also validated using IHC staining. These data implicated a role of
PI3K/Akt signaling in regulating the invasive properties of FTC-238
cells.
Clinical implications of ST6GalNAcII
expression in thyroid carcinoma
The ST6GalNAcII expression status was detected in
thyroid cancer with the corresponding pericarcinomatous tissue
samples by immunohistochemistry staining (Table I). The data as shown in Table I are number of cases, and the
expression of ST6GalNAcII was classified as high if >30% of
tumor cells are stained and as low if <30% of cancer cells were
stained. It is shown that follicular thyroid cancer tissues had a
higher expression level of ST6GalNAcII compared with transitional
tissues (P=0.004). There was no significant association between
ST6GalNAcII expression and age, or distant metastasis in follicular
thyroid carcinoma patients (P>0.05). Interestingly, the
expression of ST6GalNAcII was also closely correlated with
histological grade, lymph node metastasis, and clinical stage
(P=0.004, 0.002, 0.006, respectively).
 | Table ICorrelation between the
clinicopathological characteristics and expression of ST6GalNAcII
protein in thyroid carcinoma. |
Table I
Correlation between the
clinicopathological characteristics and expression of ST6GalNAcII
protein in thyroid carcinoma.
| Characteristics | n | ST6GalNAcII
| P-value |
|---|
| ST6GalNAcII(high)
(%) | ST6GalNAcII(low)
(%) |
|---|
| Group |
| Cancer tissue | 101 | 73 (72.3) | 28 (27.3) | 0.004 |
| Pericarcinomatous
tissues | 101 | 53 (52.5) | 48 (47.5) | |
| Age (years) |
| ≥50 | 35 | 20 (57.1) | 15 (42.9) | 0.736 |
| <50 | 66 | 40 (60.6) | 26 (39.4) | |
| Histological
grade |
| G1 | 75 | 33 (44.0) | 42 (56.0) | 0.004 |
| G2, G3 | 26 | 20 (76.9) | 6 (23.1) | |
| Lymph node
metastasis |
| Absent | 49 | 23 (46.9) | 26 (53.1) | 0.002 |
| Present | 52 | 40 (76.9) | 12 (23.1) | |
| Distant
metastasis |
| Yes | 19 | 14 (73.7) | 5 (26.3) | 0.112 |
| No | 82 | 44 (53.7) | 38 (46.3) | |
| Clinical stage |
| I–II | 45 | 20 (44.4) | 25 (55.6) | 0.006 |
| III–IV | 56 | 40 (71.4) | 16 (28.6) | |
Discussion
Metastasis remains the major cause of mortality and
relapse for most solid malignancies. Some research is currently
underway to identify the signaling pathways and molecular
mechanisms of metastasis in thyroid cancer (19–22).
In this study, we investigated the expression of ST6GalNAcII, via
PI3K/Akt signaling pathway, to assess whether it effectively
regulated the invasiveness of follicular thyroid carcinoma cell
lines FTC-238 (lung metastasis) and FTC236 (lymph node metastasis)
and FTC-133 (primary tumor). We further analyzed the differential
expression of ST6GalNAcII, which was reported to be related with
clinicopathological characteristics of human follicular thyroid
cancer.
Alteration in the expression pattern of glycogens is
correlated with the invasive potential of various types of cancer
(23,24). The biosynthetic pathway of
sialylated glycans highlights the importance of sialyltransferases.
Using real-time PCR analysis, we revealed that the expression
profile of ST6GalNAcII gene was remodeled between FTC-238 and
FTC-133 and FTC-236. As depicted in Fig. 1a, FTC-238 cells showed a higher
expression of ST6GalNAcII mRNA compared to FTC-133 and FTC-236
cells. This result suggested that ST6GalNAcII gene was active in
the cells with high metastatic potential. Our previous report also
indicated the crucial role of ST6GalNAcII gene in promoting high
metastatic potential cell invasion in breast carcinoma (25). The altering expression of
ST6GalNAcII gene in the two follicular thyroid cell lines may be
more important as indicators and functional regulators of tumor
metastasis.
Recent findings show that the overactivation of the
PI3K/Akt signaling pathway plays a crucial role in regulating tumor
invasive ability (26,27). We investigated the molecular
mechanism by which ST6GalNAcII-mediated PI3K/Akt signaling pathway
regulates follicular thyroid cancer cells invasiveness. Our study
explored a novel mechanism that the invasion and chemosensitivity
of human hepatocarcinoma cells can be regulated by the activation
of ST6GAL1 or ST8SIA2-mediated PI3K/Akt (28). In this study, we indicated that
ST6GalNAcII exerts the role of tumor metastasis of human follicular
thyroid carcinoma through activation of the PI3K/Akt/NF-κB signal
pathway. We demonstrated that FTC-238 cells exhibited higher
PI3K/Akt activity than FTC-133. In addition, the invasive
properties of FTC-238 cells were reversed by the inhibition of the
PI3K/Akt pathway (Fig. 2e). These
results indicated that ST6GalNAcII-modulated follicular thyroid
carcinoma cell invasion was, at least in part,
PI3K/Akt-dependent.
With altered mRNA expression in carcinoma tissues,
sialyltransferases are regarded as prognostic factors and potential
targets for therapeutic approaches (29,30).
In this study, we utilized immunohistochemistry to evaluate protein
expression of ST6GalNAcII in follicular thyroid cancer specimens of
101 cases. The result illustrated that follicular thyroid cancer
tissues had a higher expression level of ST6GalNAcII than in the
normal thyroid tissues (Table I).
In addition, we found the expression of ST6GalNAcII was associated
with histological grade, lymph node metastasis and clinical stage
(Table I). The results from the
clinical samples indicated that the altered level of ST6GalNAcII
may play an important role in promoting invasion and metastasis of
follicular thyroid cancer. Furthermore, it may be possible to
utilize ST6GalNAcII as a useful biomarker for clinical diagnosis of
thyroid cancer metastasis.
In conclusion, our work reveals differential
expression of ST6GalNAcII gene in two human follicular thyroid
carcinoma cell lines and follicular thyroid cancer specimens.
ST6GalNAcII elucidated the unusual properties of invasion in
thyroid cancer cells via modulating the PI3K/AKt signaling pathway.
Besides, the elevated expression of ST6GalNAcII was associated with
histological grade, lymph node metastasis and clinical stage of
follicular thyroid cancer. Seeking for agents that simultaneously
inhibit ST6GalNAcII gene may be a promising strategy for blocking
follicular thyroid carcinoma metastasis in patients.
References
|
1
|
Dall'Olio F and Chiricolo M:
Sialyltransferases in cancer. Glycoconj J. 18:841–850. 2001.
View Article : Google Scholar
|
|
2
|
Harvey BE, Toth CA, Wagner HE, Steele GD
Jr and Thomas P: Sialyltransferase activity and hepatic tumor
growth in a nude mouse model of colorectal cancer metastases.
Cancer Res. 52:1775–1779. 1992.PubMed/NCBI
|
|
3
|
Majuri ML, Niemelä R, Tiisala S, Renkonen
O and Renkonen R: Expression and function of alpha 2,3-sialyl- and
alpha 1,3/1,4-fucosyltransferases in colon adenocarcinoma cell
lines: Role in synthesis of E-selectin counter-receptors. Int J
Cancer. 63:551–559. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yogeeswaran G and Salk PL: Metastatic
potential is positively correlated with cell surface sialylation of
cultured murine tumor cell lines. Science. 212:1514–1516. 1981.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Dennis J, Waller C, Timpl R and
Schirrmacher V: Surface sialic acid reduces attachment of
metastatic tumour cells to collagen type IV and fibronectin.
Nature. 300:274–276. 1982. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ohtsubo K and Marth JD: Glycosylation in
cellular mechanisms of health and disease. Cell. 126:855–867. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kim YJ, Kim KS, Kim SH, Kim CH, Ko JH,
Choe IS, Tsuji S and Lee YC: Molecular cloning and expression of
human Gal beta 1,3GalNAc alpha 2,3-sialytransferase (hST3Gal II).
Biochem Biophys Res Commun. 228:324–327. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Harduin-Lepers A, Mollicone R, Delannoy P
and Oriol R: The animal sialyltransferases and
sialyltransferase-related genes: A phylogenetic approach.
Glycobiology. 15:805–817. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Takashima S, Tsuji S and Tsujimoto M:
Characterization of the second type of human beta-galactoside alpha
2,6-sialyl-transferase (ST6Gal II), which sialylates Galbeta
1,4GlcNAc structures on oligosaccharides preferentially. Genomic
analysis of human sialyltransferase genes. J Biol Chem.
277:45719–45728. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Julien S, Adriaenssens E, Ottenberg K,
Furlan A, Courtand G, Vercoutter-Edouart AS, Hanisch FG, Delannoy P
and Le Bourhis X: ST6GalNAc I expression in MDA-MB-231 breast
cancer cells greatly modifies their O-glycosylation pattern and
enhances their tumourigenicity. Glycobiology. 16:54–64. 2006.
View Article : Google Scholar
|
|
11
|
Ding JX, Xu LX, Zhu L, Lv JC, Zhao MH,
Zhang H and Wang HY: Activity of α2,6-sialyltransferase and its
gene expression in peripheral B lymphocytes in patients with IgA
nephropathy. Scand J Immunol. 69:174–180. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xu LX and Zhao MH: Aberrantly glycosylated
serum IgA1 are closely associated with pathologic phenotypes of IgA
nephropathy. Kidney Int. 68:167–172. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Reticker-Flynn NE and Bhatia SN: Aberrant
glycosylation promotes lung cancer metastasis through adhesion to
galectins in the metastatic niche. Cancer Discov. 5:168–181. 2015.
View Article : Google Scholar :
|
|
14
|
Bos PD, Zhang XH, Nadal C, Shu W, Gomis
RR, Nguyen DX, Minn AJ, van de Vijver MJ, Gerald WL, Foekens JA, et
al: Genes that mediate breast cancer metastasis to the brain.
Nature. 459:1005–1009. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Engelman JA, Luo J and Cantley LC: The
evolution of phosphatidylinositol 3-kinases as regulators of growth
and metabolism. Nat Rev Genet. 7:606–619. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bellacosa A, Kumar CC, Di Cristofano A and
Testa JR: Activation of AKT kinases in cancer: Implications for
therapeutic targeting. Adv Cancer Res. 94:29–86. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tamburrino A, Molinolo AA, Salerno P,
Chernock RD, Raffeld M, Xi L, Gutkind JS, Moley JF, Wells SA Jr and
Santoro M: Activation of the mTOR pathway in primary medullary
thyroid carcinoma and lymph node metastases. Clin Cancer Res.
18:3532–3540. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xu X, Qin J and Liu W: Curcumin inhibits
the invasion of thyroid cancer cells via down-regulation of
PI3K/Akt signaling pathway. Gene. 546:226–232. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Vu-Phan D, Grachtchouk V, Yu J, Colby LA,
Wicha MS and Koenig RJ: The thyroid cancer PAX8-PPARG fusion
protein activates Wnt/TCF-responsive cells that have a transformed
phenotype. Endocr Relat Cancer. 20:725–739. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Milosevic Z, Pesic M, Stankovic T, Dinic
J, Milovanovic Z, Stojsic J, Dzodic R, Tanic N and Bankovic J:
Targeting RAS-MAPK-ERK and PI3K-AKT-mTOR signal transduction
pathways to chemosensitize anaplastic thyroid carcinoma. Transl
Res. 164:411–423. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Geraldo MV, Yamashita AS and Kimura ET:
MicroRNA miR-146b-5p regulates signal transduction of TGF-β by
repressing SMAD4 in thyroid cancer. Oncogene. 31:1910–1922. 2012.
View Article : Google Scholar
|
|
22
|
Soula-Rothhut M, Coissard C, Sartelet H,
Boudot C, Bellon G, Martiny L and Rothhut B: The tumor suppressor
PTEN inhibits EGF-induced TSP-1 and TIMP-1 expression in FTC-133
thyroid carcinoma cells. Exp Cell Res. 304:187–201. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sawada M, Moriya S, Saito S, Shineha R,
Satomi S, Yamori T, Tsuruo T, Kannagi R and Miyagi T: Reduced
sialidase expression in highly metastatic variants of mouse colon
adenocarcinoma 26 and retardation of their metastatic ability by
sialidase overexpression. Int J Cancer. 97:180–185. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lin S, Kemmner W, Grigull S and Schlag PM:
Cell surface alpha 2,6 sialylation affects adhesion of breast
carcinoma cells. Exp Cell Res. 276:101–110. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ren D, Jia L, Li Y, Gong Y, Liu C, Zhang
X, Wang N and Zhao Y: ST6GalNAcII mediates the invasive properties
of breast carcinoma through PI3K/Akt/NF-κB signaling pathway. IUBMB
Life. 66:300–308. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhou F, Cui C, Ge Y, Chen H, Li Q, Yang Z,
Wu G, Sun S, Chen K, Gu J, et al: Alpha2,3-Sialylation regulates
the stability of stem cell marker CD133. J Biochem. 148:273–280.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li Z: CD133: A stem cell biomarker and
beyond. Exp Hematol Oncol. 2:172013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhao Y, Li Y, Ma H, Dong W, Zhou H, Song
X, Zhang J and Jia L: Modification of sialylation mediates the
invasive properties and chemosensitivity of human hepatocellular
carcinoma. Mol Cell Proteomics. 13:520–536. 2014. View Article : Google Scholar :
|
|
29
|
López-Morales D, Velázquez-Márquez N,
Valenzuela O, Santos-López G, Reyes-Leyva J and Vallejo-Ruiz V:
Enhanced sialyltransferases transcription in cervical
intraepithelial neoplasia. Invest Clin. 50:45–53. 2009.PubMed/NCBI
|
|
30
|
Wang PH, Lee WL, Juang CM, Yang YH, Lo WH,
Lai CR, Hsieh SL and Yuan CC: Altered mRNA expressions of
sialyl-transferases in ovarian cancers. Gynecol Oncol. 99:631–639.
2005. View Article : Google Scholar : PubMed/NCBI
|