Introduction
Cervical cancer is the second leading cause of
cancer-related deaths in women, and the leading cause in some
developing countries (1). The
occurrence and development of cervical cancer are closely
associated with the human immune system. The activation,
inactivation and dysfunction of the immune system have drawn
increasingly more research interest. Suppressed immune function may
result in accelerated disease progression, and immune escape exerts
an important effect on tumor progression. Recent research on T cell
negative regulatory pathways have confirmed the important role of
negative co-stimulatory molecules in the tumor immune response.
This negative signal is mainly provided by the B7 family, which has
become an intense focus of research in cancer immunotherapy. B7-H3
and B7-H4 are newly identified members of the family that are
abnormally expressed in tumors. Both molecules have similar
patterns of expression. Specifically, the mRNAs are widely
expressed in multiple non-lymphoid tissues, including the
intestines, stomach, lungs and kidneys, whereas the proteins are
only highly expressed in a variety of malignant tumors, such as
prostate cancer (2), pancreatic
cancer (3,4), lung cancer (5,6), renal
cell carcinoma (7,8), and ovarian cancer (9,10), but
not in any of the normal peripheral tissues. In a previous study
involving 102 patients with cervical cancer, the rate of expression
of B7-H4 was 69.6% (71/102), although the level of expression was
not correlated with the clinicopathologic parameters of the tumor
(11). A subsequent long-term
follow-up study revealed poor prognosis in patients with B7-H4
expression in tumor tissues (11).
Regulatory T (Treg) cells have significant effects
on the blood, tissues and organs, and play an important role in
maintaining the stability of the immune system, and tumor immune
tolerance and escape due to an immunosuppressive ability (12). Foxp3 is a specific transcription
factor expressed in Treg cells. It has been recently reported that
the number of Treg cells is significantly increased in some tumors
(13,14) that penetrate into the tumor
microenvironment, and are involved in the process of tumor immune
escape by preventing natural killer (NK) cells and cytotoxic
lymphocytes (CTLs) from attacking cancer cells (15). Foxp3 is also expressed in tumor
cells, such as pancreatic (16),
prostate (17) and gastric cancers
(18); however, it has been shown
that the expression of Foxp3 varies in different tumor cells, and
is even different in the same types of tumors. We speculate that
tumor cells expressing Foxp3 may participate in tumor immune escape
by stimulating Treg cells. IL-2 is a key antitumor cytokine
released during an immune response, and is regulated by lymphocytes
and other cells upon stimulation by antigen or mitogen. IL-2
directly affects the function of immune cells and local immune
status. IL-2 is an important component of cellular immunity that
activates CTLs and promotes the differentiation of activated T
cells. IL-2 stimulates the proliferation of T cells and enhances
their killing effect, and thereby plays an important anti-viral and
antitumor role. It has also been shown that the antitumor effect of
T cells is reduced with a decreased level of IL-2. Expression of
B7-H3 and B7-H4 may decrease the efficacy of immunotherapy by
suppressing the antitumor effect of T cells and by promoting the
function of Treg cells.
In the present study, we sought to identify whether
B7-H3, B7-H4, Foxp3 and IL-2 were overexpressed in cervical cancer
cells, and to investigate any correlation between the expression of
these proteins. What is more, their correlation with
clinicopathologic features was analyzed to assess the impact on the
progression of the tumor. Furthermore, the prognostic significance
of these proteins in cervical cancer was evaluated to provide
additional information for the treatment of cervical cancer.
Materials and methods
Patients and follow-up
Before this analysis, patient information was
anonymized and de-identified. All participants provided written
informed consent. The entire analysis of tissues was approved by
the Institutional Review Board of Shengjing Hospital of China
Medical University (ethics approval code, 2013PS47K).
Paraffin cervical tumor tissues from 108 patients
treated between 2007 and 2012 in the Department of Gynecology and
Obstetrics of Shengjing Hospital affiliated with the China Medical
University were obtained. All tissue sections were independently
examined by two pathologists to make a final histopathologic
diagnosis using the World Health Organization criteria. The
classification of cancer clinical stage was in accordance with the
2009 International Federation of Gynecology and Obstetrics (FIGO)
criteria.
All of the patients were diagnosed with cancer for
the first time. No chemotherapeutic treatments were administered
before surgery. The follow-up was carried out according to National
Comprehensive Cancer Network (NCCN) guidelines: every month in the
first year; every third month in the second year; twice in the
third, fourth and fifth years; and annually thereafter. After
treatment, all patients were followed up until May 2015.
Immunohistochemistry
Immunohistochemistry was used to determine levels of
expression. Four antibodies, including murine monoclonal B7-H3
(ab105922, 1:700 dilution), rabbit monoclonal B7-H4 (ab108336,
1:500 dilution), murine monoclonal Foxp3 (ab22510, 1:50 dilution),
and rabbit monoclonal IL-2 antibodies (ab92381, 1:300 dilution)
were purchased from Abcam Co. (Cambridge, UK). The staining
procedure was performed as described in the manual of an
ultrasensitive streptavidin-peroxidase kit (kit 9701; Maixin Bio,
Fuzhou, China). Colorectal carcinoma tissue samples known to be
positive tissue slices were used as positive controls, while
tissues treated with phosphate-buffered saline (PBS) substituted
for the primary antibody were used as a negative control.
Evaluation of immunostaining
Immunostaining was scored by two pathologists.
Scores representing the percentage of positive cells were as
follows: 0, no positive cells; 1, 1–25% positive cells; 2, 26–50%
positive cells; 3, 51–75% positive cells; and 4, 76–100% positive
cells. Staining intensity was scored as follows: 0, no staining; 1,
weak staining; 2, moderate staining; and 3, strong staining. These
two scores were then multiplied to yield the final score, resulting
in the following scores: 0–2, (−); 3–4, (+); 5–8, (++); and 9–12,
(+++). Two independent observers, who were blinded to the patient
materials, scored each section to control for observer error.
Statistical analysis
The correlations between the levels of expression of
B7-H3, B7-H4, Foxp3 and IL-2 in different pathologic lesions and
the clinicopathologic characteristics were analyzed using a
Chi-squared (χ2) test. Correlation analysis of B7-H3,
B7-H4, Foxp3 and IL-2 protein expression was performed using the
Spearman's rank order correlation coefficient. Cancer-related death
was used as an endpoint, and Kaplan-Meier survival curves were
plotted and compared using the log-rank test for univariate overall
survival analysis. The Cox regression model was used for
multivariate overall survival analysis. All statistical analyses
were performed using SPSS 17.0 software (SPSS, Inc., Chicago, IL,
USA). Analyses were performed with two-sided tests; a p-value
<0.05 was considered statistically significant.
Results
Patient characteristics
The age range of the 108 patients was 22–67 years
(mean, 43.75 years; median, 44.00 years). There were two histologic
types of cancer tissue: squamous cell carcinoma, n=98; and
adenocarcinoma, n=10. Tumor differentiation was as follows: well
differentiated, n=17; moderately differentiated, n=77; and poorly
differentiated, n=14. According to FIGO stage, there were 55
patients in stage I, 51 patients in stage II, and 2 patients in
stage III. All of the clinical features, including lymph node
metastasis and tumor size, are shown in Table I.
 | Table IClinical and pathological
characteristics. |
Table I
Clinical and pathological
characteristics.
| Characteristics | n (%) |
|---|
| Differentiation | |
| Well differentiated
(WICC) | 17 (15.74) |
| Moderately
differentiated (MICC) | 77 (71.30) |
| Poorly
differentiated (PICC) | 14 (12.96) |
| Histology | |
| Squamous cell
carcinoma (SCC) | 98 (90.74) |
| Adenosquamous cell
carcinoma (ACC) | 10 (9.26) |
| Lymph node (LN)
metastasis | |
| Absent (−) | 44 (40.74) |
| Present (+) | 64 (59.26) |
| Tumor size
(cm) | |
| ≤4 | 80 (74.07) |
| >4 | 28 (25.93) |
| FIGO stage | |
| Ia | 2 (1.85) |
| Ib | 53 (49.08) |
| IIa | 44 (40.74) |
| IIb | 7 (6.48) |
| III | 2 (1.85) |
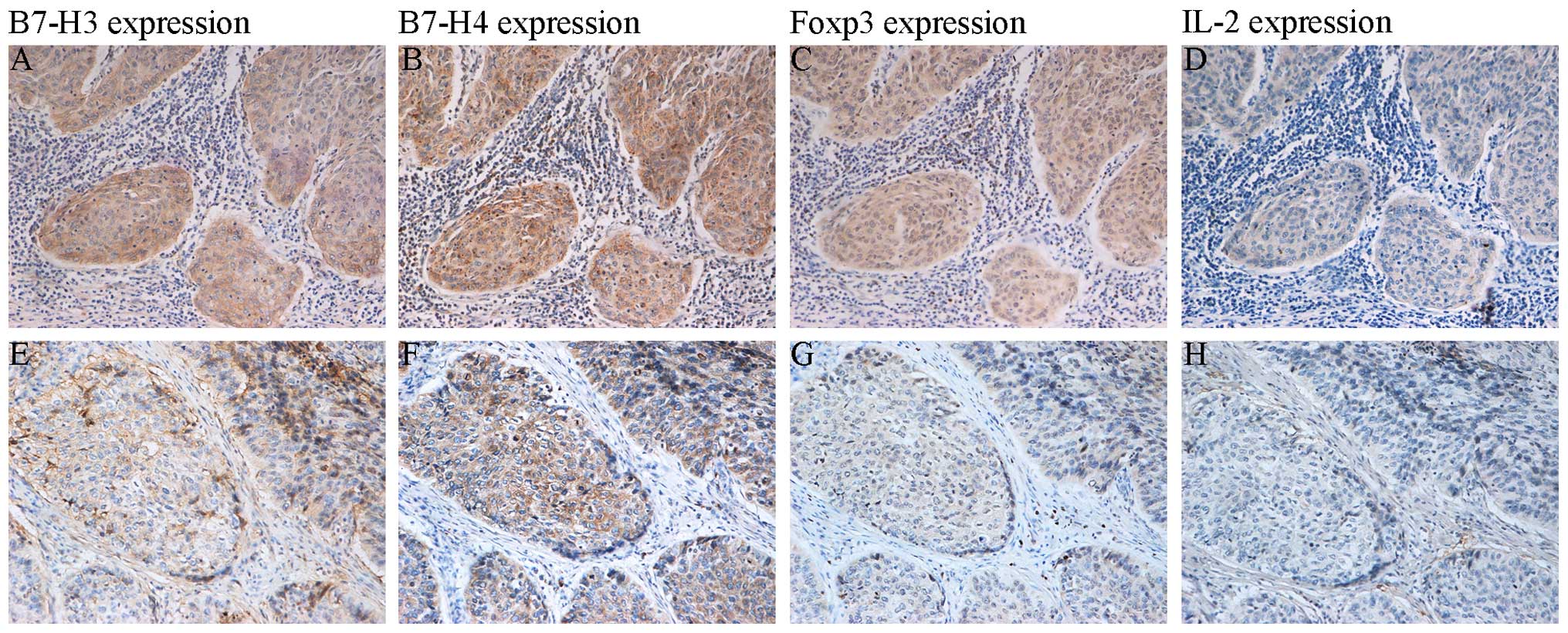
Expression of B7-H3, B7-H4, Foxp3 and
IL-2 in cervical cancer (Fig.
1)
In the cervical cancer tissues, B7-H3 and B7-H4 were
mainly expressed in the cytoplasm of the cancer cells, with minimal
expression in the nucleus; there was also a small amount of
interstitial expression. Foxp3 expression was located in the T cell
nucleus, and the cytoplasm and nucleus of malignant cervical cancer
cells. IL-2 staining was detected in the cytoplasm. Among the 108
specimens, the positive expression rates were as follows: B7-H3,
72.22% (78/108); B7-H4, 80.56% (87/108); Foxp3, 91.67% (99/108);
and IL-2, 50.00% (54/108).
Relationship between B7-H3, B7-H4, Foxp3
and IL-2 levels and the clinicopathologic features of the cervical
cancer tissues (Table II)
Te expression profiles of B7-H3, B7-H4 and Foxp3
were similar. B7-H3 levels were significantly associated with tumor
size (P=0.013). B7-H4, Foxp3 and IL-2 levels were significantly
associated with FIGO stage (P=0.023, 0.014 and 0.036, respectively)
and tumor size (P=0.045, 0.010 and 0.021, respectively). The levels
of expression of B7-H3, B7-H4 and Foxp3 were not correlated with
age, histologic type, differentiation and lymph node
metastasis.
Association between B7-H3, B7-H4, Foxp3
and IL-2 expression
There was a positive correlation between the
expression of B7-H3 and B7-H4 with Foxp3, and between B7-H3 and
B7-H4 expression in cervical cancer; however, Spearman's rank
correlation analysis showed that B7-H3 or B7-H4 protein expression
was negatively correlated with IL-2 expression in the cervical
cancer (the Spearman's correlation coefficient, r, is shown in
Table III). The expression of
B7-H3, B7-H4, Foxp3 and IL-2 in the same tissue is shown in
Fig. 2.
 | Table IIICorrelation between B7-H3, B7-H4,
Foxp3 and IL-2 expression in cervical cancer tissues. |
Table III
Correlation between B7-H3, B7-H4,
Foxp3 and IL-2 expression in cervical cancer tissues.
| Spearman
correlation analysis (n=108) | B7-H3 | B7-H4 | Foxp3 | IL-2 |
|---|
| B7-H3 | | | | |
| r | 1.000 | 0.489 | 0.366 | −0.296 |
| P-value | – | <0.001a | <0.001a | 0.002a |
| B7-H4 | | | | |
| r | 0.489 | 1.000 | 0.483 | −0.327 |
| P-value | <0.001a | – | <0.001a | 0.001a |
| Foxp3 | | | | |
| r | 0.366 | 0.483 | 1.000 | −0.105 |
| P-value | <0.001a | <0.001a | – | 0.280 |
| IL-2 | | | | |
| r | −0.296 | −0.327 | −0.105 | 1.000 |
| P-value | 0.002a | 0.001a | 0.280 | - |
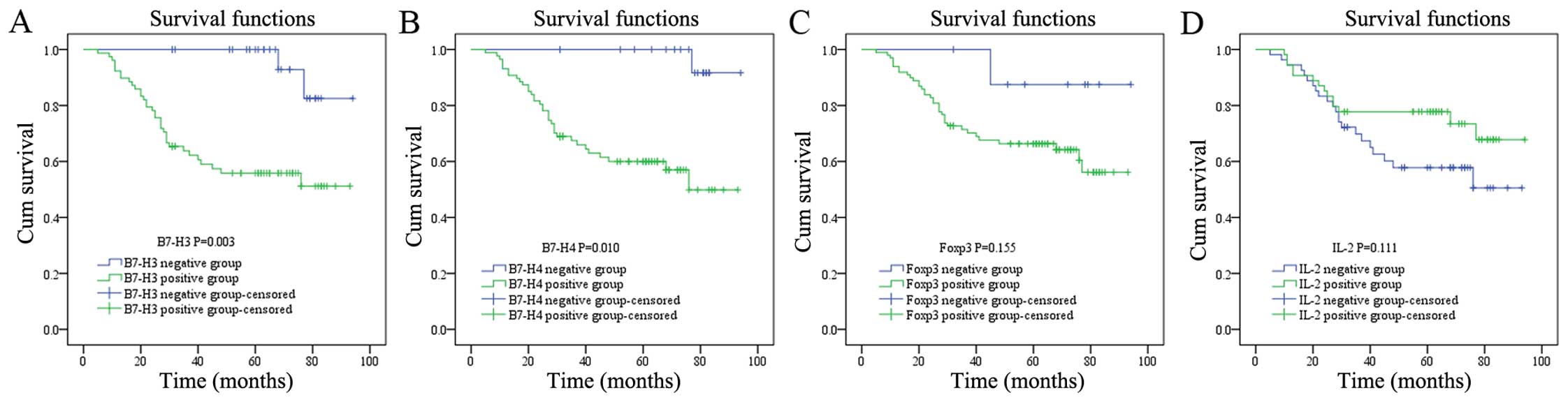
The prognostic significance of B7-H3,
B7-H4, Foxp3 and IL-2 in cervical cancer
Between January 2007 and May 2015, 36 (33.33%) of
the 108 patients died and 72 (66.67%) were alive. The overall
survival for patients with B7-H3/B7-H4/Foxp3-positive expression
was significantly lower (Fig. 3).
Univariate and multivariate analyses showed that there was a
significant correlation between the expression of B7-H3 and B7-H4
and prognosis, but no correlation was noted between Foxp3 and IL-2
expression and prognosis in the cervical cancer patients (Table IV).
 | Table IVAnalysis of B7-H3, B7-H4, Foxp3 or
IL-2 expression in relation to prognosis of patients with cervical
cancer. |
Table IV
Analysis of B7-H3, B7-H4, Foxp3 or
IL-2 expression in relation to prognosis of patients with cervical
cancer.
| β-value | SE | P-value | OR value | 95% CI |
|---|
| Univariate
analysis |
| B7-H3 | 2.174 | 0.729 | 0.003a | 8.793 | 2.108–36.674 |
| B7-H4 | 2.643 | 1.024 | 0.010a | 14.049 | 1.890–104.443 |
| Foxp3 | 1.445 | 1.016 | 0.155 | 4.241 | 0.579–31.038 |
| IL-2 | −0.548 | 0.343 | 0.111 | 0.578 | 0.295–1.133 |
| Multivariate
analysis |
| B7-H3 | 1.882 | 0.749 | 0.012a | 6.567 | 1.512–28.528 |
| B7-H4 | 2.101 | 1.053 | 0.046a | 8.172 | 1.038–64.340 |
Age, FIGO stage, histologic type, histologic
findings, lymph node metastasis, and tumor size were included in
the multivariate overall survival analysis using Cox proportional
hazards analysis; however, the variables were not significantly
associated.
Discussion
Cervical cancer is the second most common cancer in
women. In recent years, there has been an increase in the number of
studies involving the negative co-stimulatory factors, B7-H3 and
B7-H4. The negative co-stimulatory effect of B7-H3 and B7-H4 on T
cells has been confirmed (19–21).
We speculated that both factors share one or several receptors due
to their high similarity; however, the putative receptor has not
been identified. Although the abnormal expression of both factors
in a variety of tumor tissues has been previously reported, the
expression in cervical cancer has been seldom studied. Wang et
al (22) demonstrated that
B7-H4 is not expressed in normal cervical epithelium, weakly
expressed in CINII-III, but highly expressed in cervical cancer
cells (P<0.05). Additionally, B7-H4 was found to be moderately
expressed in mesenchymal cells in cervical cancer. In other
studies, B7-H4 expression has been detected in the tumor
microenvironment, such as tumor-associated macrophages (2,23–25).
In a study involving 102 cases of cervical cancer conducted by Liu
et al (11), the rate of
expression of B7-H4 in cervical cancer cells was reported to be
high (69.6%, 71/102), and B7-H4 was positively expressed in the
cytoplasm and on the membrane of cervical cancer cells.
Nevertheless, no correlation between B7-H4 expression and
clinicopathologic factors was identified, including age, histologic
type, and lymph node metastasis. In a study involving a large
number of patients with prostate cancer, Zang et al
(2) revealed the abnormally high
expression of B7-H3 and B7-H4 in tumor tissues with positive
expression rates of 93 and 99%, respectively. The expression of
B7-H3 and B7-H4 was also detected in the tumor microenvironment,
such as macrophages and perivascular areas (small vessel
endothelial cells). Consistent with these previous findings, our
study demonstrated rates of expression of 72.22% (78/108) and
80.56% (87/108) for B7-H3 and B7-H4, respectively. Both proteins
were also highly expressed in the tumor stroma. Moreover, it was
shown that B7-H3 expression in cervical cancer was only correlated
with tumor size among all of the clinicopathologic features
evaluated and B7-H4 expression was correlated with tumor size and
FIGO stage. Patients with a high level of B7-H3 and B7-H4
expression tended to have a worse prognosis; moreover, the
difference in the prognosis between these patients and other
patients was statistically significant. Furthermore, B7-H3 and
B7-H4 expression were identified as independent risk factors for
the clinical prognosis of cervical cancer.
Currently, the findings on the prognostic effect of
B7-H3 are controversial. Most reports suggest that a high level of
B7-H3 expression in tumor cells is associated with the progression
and prognosis of the disease (2,8,10,26–29),
which was consistent with our results. Other studies demonstrated
no correlation (30,31). Low expression of B7-H3 in tumor
stroma may facilitate escape of the antitumor immune response,
whereas high B7-H3 expression prevents tumor progression. B7-H3 was
also shown to suppress the antitumor immune response, which is in
contrast to the previous finding. Another hypothesis suggests that
interstitial B7-H3 inhibits the progression of tumors through an
unknown non-immune mechanism. Overall, the effect of B7-H3 as a
prognostic marker in cancer is uncertain. The inconsistent findings
in the literature may be associated with the different methodology
used in these studies or different functions of B7-H3 in different
forms of cancer.
Regulatory T cells (Treg cells) are a class of
immunosuppressive cells involved in tumor immune escape. Foxp3
(transcription factor forkhead box P3) is the specific surface
marker of Treg cells (32). The
increased number of Foxp3+ Treg cells in tumor tissues
is correlated with poor prognosis (13,14).
Geng et al (33) reported
that the expression of B7-H1 and B7-H4 in gastric cancer is
positively correlated with interstitial Foxp3 expression. Wang
et al (22) demonstrated
that the proportion of CD25+ Foxp3+ T cells
in CD4+ T cells is significantly increased after a 48-h
co-culture with B7-H4, suggesting that B7-H4 can promote the
proliferation of Treg cells. Luo et al (34) found in a further study that Foxp3
had a high expression in cervical cancer cells and tumor
interstitium, moreover was able to facilitate the proliferation and
invasiveness of SiHa cells, alter the cell cycle and inhibit their
apoptosis. In our study, it was shown that the expression of B7-H3
and B7-H4 in cervical cancer cells was positively correlated with
Foxp3 expression. On the one hand, B7-H3 and B7-H4 may mediate
tumor immune escape by increasing the number of Treg cells, thereby
promoting the progression of tumors. On the other hand, high
expression levels of B7-H3 and B7-H4 upregulate Foxp3 expression in
cervical cancer cells, which regulates the occurrence and
development of tumors by affecting the proliferation and apoptosis
of cancer cells. From the perspective of immunotherapy, inhibition
of B7-H3 and B7-H4 expression may reduce or block the proliferation
of Treg cells, leading to antitumor effects. Nevertheless, the
mechanism underlying the stimulatory effect of B7-H3 and B7-H4 on
Foxp3 expression has yet to be elucidated. Our results suggest that
high expression of Foxp3 is associated with prognosis in cervical
cancer patients. Rather, as discussed above, not only an increase
in Treg cell numbers but also a high level of expression in tumor
cells may be clearly correlated with prognosis. IL-2 is a key
cytokine involved in the immune response. IL-2 regulation promotes
the differentiation of T cells and enhances the killing effect.
Decreased IL-2 expression has a lower regulatory effect on the
proliferation and differentiation of T lymphocytes, leading to a
reduced antitumor effect of T cells and dysfunction of cellular
immunity. Our study showed that the expression of B7-H3, B7-H4 and
Foxp3 is negatively correlated with IL-2 expression, suggesting
that the immune inhibition of B7-H3, B7-H4 and Foxp3 may be
achieved by promoting the proliferation of Treg cells, upregulating
Foxp3 expression in cervical cancer cells, or directly suppressing
the secretion of IL-2. It was also shown that the immune response
in cervical cancer patients with B7-H3, B7-H4 and Foxp3 expression
was less than patients with negative expression of B7-H3, B7-H4 and
Foxp3.
In conclusion, several studies, including our study,
have reported abnormally high expression of B7-H3 and B7-H4 in
various human malignant cells, which may be a promising target for
cancer immunotherapy (7,29,35–40).
An association of B7-H3 and B7-H4 expression with reduced survival
in cervical cancer patients was found. Although immunotherapy based
on inhibition of T cell signaling by blocking CTLA-4 has been
applied in the treatment of hormone-refractory prostate cancer
(41), multiple molecular
therapeutic targets are involved for effective immunomodulation.
Based on our results, we suggest that blocking B7-H4 may be more
efficient compared with blocking B7-H3 in the treatment of cervical
cancer.
Acknowledgments
This study was supported by the National Nature
Science Foundation of China (grant no. 81372776), the Science and
Technology Program of Liaoning Province (grant no. 2011225009), the
Higher Specialized Research Fund for the Doctoral Program (grant
no. 20122104110014), and the Free Researcher Project of Shengjing
Hospital (grant no. 201302).
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zang X, Thompson RH, Al-Ahmadie HA, Serio
AM, Reuter VE, Eastham JA, Scardino PT, Sharma P and Allison JP:
B7-H3 and B7x are highly expressed in human prostate cancer and
associated with disease spread and poor outcome. Proc Natl Acad Sci
USA. 104:19458–19463. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tsiaousidou A, Lambropoulou M,
Chatzitheoklitos E, Tripsianis G, Tsompanidou C, Simopoulos C and
Tsaroucha AK: B7H4, HSP27 and DJ-1 molecular markers as prognostic
factors in pancreatic cancer. Pancreatology. 13:564–569. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhao X, Li DC, Zhu XG, Gan WJ, Li Z, Xiong
F, Zhang ZX, Zhang GB, Zhang XG and Zhao H: B7-H3 overexpression in
pancreatic cancer promotes tumor progression. Int J Mol Med.
31:283–291. 2013.
|
|
5
|
Sun Y, Wang Y, Zhao J, Gu M, Giscombe R,
Lefvert AK and Wang X: B7-H3 and B7-H4 expression in non-small-cell
lung cancer. Lung Cancer. 53:143–151. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Schneider T, Hoffmann H, Dienemann H,
Schnabel PA, Enk AH, Ring S and Mahnke K: Non-small cell lung
cancer induces an immunosuppressive phenotype of dendritic cells in
tumor microenvironment by upregulating B7-H3. J Thorac Oncol.
6:1162–1168. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Krambeck AE, Thompson RH, Dong H, Lohse
CM, Park ES, Kuntz SM, Leibovich BC, Blute ML, Cheville JC and Kwon
ED: B7-H4 expression in renal cell carcinoma and tumor vasculature:
Associations with cancer progression and survival. Proc Natl Acad
Sci USA. 103:10391–10396. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Crispen PL, Sheinin Y, Roth TJ, Lohse CM,
Kuntz SM, Frigola X, Thompson RH, Boorjian SA, Dong H, Leibovich
BC, et al: Tumor cell and tumor vasculature expression of B7-H3
predict survival in clear cell renal cell carcinoma. Clin Cancer
Res. 14:5150–5157. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Simon I, Zhuo S, Corral L, Diamandis EP,
Sarno MJ, Wolfert RL and Kim NW: B7-h4 is a novel membrane-bound
protein and a candidate serum and tissue biomarker for ovarian
cancer. Cancer Res. 66:1570–1575. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zang X, Sullivan PS, Soslow RA, Waitz R,
Reuter VE, Wilton A, Thaler HT, Arul M, Slovin SF, Wei J, et al:
Tumor associated endothelial expression of B7-H3 predicts survival
in ovarian carcinomas. Mod Pathol. 23:1104–1112. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu W, Shibata K, Koya Y, Kajiyama H,
Senga T, Yamashita M and Kikkawa F: B7-H4 overexpression correlates
with a poor prognosis for cervical cancer patients. Mol Clin Oncol.
2:219–225. 2014.PubMed/NCBI
|
|
12
|
Mailloux AW and Young MR: Regulatory
T-cell trafficking: From thymic development to tumor-induced immune
suppression. Crit Rev Immunol. 30:435–447. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lu X, Liu J, Li H, Li W, Wang X, Ma J,
Tong Q, Wu K and Wang G: Conversion of intratumoral regulatory T
cells by human gastric cancer cells is dependent on transforming
growth factor-β1. J Surg Oncol. 104:571–577. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Woo EY, Chu CS, Goletz TJ, Schlienger K,
Yeh H, Coukos G, Rubin SC, Kaiser LR and June CH: Regulatory
CD4(+)CD25(+) T cells in tumors from patients with early-stage
non-small cell lung cancer and late-stage ovarian cancer. Cancer
Res. 61:4766–4772. 2001.PubMed/NCBI
|
|
15
|
Wang HY and Wang RF: Regulatory T cells
and cancer. Curr Opin Immunol. 19:217–223. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hinz S, Pagerols-Raluy L, Oberg HH,
Ammerpohl O, Grüssel S, Sipos B, Grützmann R, Pilarsky C,
Ungefroren H, Saeger HD, et al: Foxp3 expression in pancreatic
carcinoma cells as a novel mechanism of immune evasion in cancer.
Cancer Res. 67:8344–8350. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang L, Liu R, Li W, Chen C, Katoh H, Chen
GY, McNally B, Lin L, Zhou P, Zuo T, et al: Somatic single hits
inactivate the X-linked tumor suppressor FOXP3 in the prostate.
Cancer Cell. 16:336–346. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang LH, Su L and Wang JT: Correlation
between elevated FOXP3 expression and increased lymph node
metastasis of gastric cancer. Chin Med J (Engl). 123:3545–3549.
2010.
|
|
19
|
Zang X, Loke P, Kim J, Murphy K, Waitz R
and Allison JP: B7x: A widely expressed B7 family member that
inhibits T cell activation. Proc Natl Acad Sci USA.
100:10388–10392. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sica GL, Choi IH, Zhu G, Tamada K, Wang
SD, Tamura H, Chapoval AI, Flies DB, Bajorath J and Chen L: B7-H4,
a molecule of the B7 family, negatively regulates T cell immunity.
Immunity. 18:849–861. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Prasad DV, Richards S, Mai XM and Dong C:
B7S1, a novel B7 family member that negatively regulates T cell
activation. Immunity. 18:863–873. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang X, Wang T, Xu M, Xiao L, Luo Y, Huang
W, Zhang Y and Geng W: B7-H4 overexpression impairs the immune
response of T cells in human cervical carcinomas. Hum Immunol.
75:1203–1209. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kryczek I, Wei S, Zhu G, Myers L, Mottram
P, Cheng P, Chen L, Coukos G and Zou W: Relationship between B7-H4,
regulatory T cells, and patient outcome in human ovarian carcinoma.
Cancer Res. 67:8900–8905. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kryczek I, Zou L, Rodriguez P, Zhu G, Wei
S, Mottram P, Brumlik M, Cheng P, Curiel T, Myers L, et al: B7-H4
expression identifies a novel suppressive macrophage population in
human ovarian carcinoma. J Exp Med. 203:871–881. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Galazka K, Opławski M, Windorbska W,
Skret-Magierlo J, Koper K, Basta P, Mach P, Dutch-Wicherek M, Mazur
A and Wicherek L: The immunohistochemical analysis of antigens such
as RCAS1 and B7H4 in the cervical cancer nest and within the
fibroblasts and macrophages infiltrating the cancer
microenvironment. Am J Reprod Immunol. 68:85–93. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yamato I, Sho M, Nomi T, Akahori T,
Shimada K, Hotta K, Kanehiro H, Konishi N, Yagita H and Nakajima Y:
Clinical importance of B7-H3 expression in human pancreatic cancer.
Br J Cancer. 101:1709–1716. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Arigami T, Narita N, Mizuno R, Nguyen L,
Ye X, Chung A, Giuliano AE and Hoon DS: B7-h3 ligand expression by
primary breast cancer and associated with regional nodal
metastasis. Ann Surg. 252:1044–1051. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sun TW, Gao Q, Qiu SJ, Zhou J, Wang XY, Yi
Y, Shi JY, Xu YF, Shi YH, Song K, et al: B7-H3 is expressed in
human hepatocellular carcinoma and is associated with tumor
aggressiveness and postoperative recurrence. Cancer Immunol
Immunother. 61:2171–2182. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Roth TJ, Sheinin Y, Lohse CM, Kuntz SM,
Frigola X, Inman BA, Krambeck AE, McKenney ME, Karnes RJ, Blute ML,
et al: B7-H3 ligand expression by prostate cancer: A novel marker
of prognosis and potential target for therapy. Cancer Res.
67:7893–7900. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Quandt D, Fiedler E, Boettcher D, Marsch
WC and Seliger B: B7-h4 expression in human melanoma: Its
association with patients' survival and antitumor immune response.
Clin Cancer Res. 17:3100–3111. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Boland JM, Kwon ED, Harrington SM,
Wampfler JA, Tang H, Yang P and Aubry MC: Tumor B7-H1 and B7-H3
expression in squamous cell carcinoma of the lung. Clin Lung
Cancer. 14:157–163. 2013. View Article : Google Scholar
|
|
32
|
Strauss L, Bergmann C, Szczepanski M,
Gooding W, Johnson JT and Whiteside TL: A unique subset of
CD4+CD25highFoxp3+ T cells
secreting interleukin-10 and transforming growth factor-beta1
mediates suppression in the tumor microenvironment. Clin Cancer
Res. 13:4345–4354. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Geng Y, Wang H, Lu C, Li Q, Xu B, Jiang J
and Wu C: Expression of costimulatory molecules B7-H1, B7-H4 and
Foxp3+ Tregs in gastric cancer and its clinical
significance. Int J Clin Oncol. 20:273–281. 2015. View Article : Google Scholar
|
|
34
|
Luo Q, Zhang S, Wei H, Pang X and Zhang H:
Roles of Foxp3 in the occurrence and development of cervical
cancer. Int J Clin Exp Pathol. 8:8717–8730. 2015.PubMed/NCBI
|
|
35
|
Chen L: Co-inhibitory molecules of the
B7-CD28 family in the control of T-cell immunity. Nat Rev Immunol.
4:336–347. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zang X and Allison JP: The B7 family and
cancer therapy: Costimulation and coinhibition. Clin Cancer Res.
13:5271–5279. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Choi IH, Zhu G, Sica GL, Strome SE,
Cheville JC, Lau JS, Zhu Y, Flies DB, Tamada K and Chen L: Genomic
organization and expression analysis of B7-H4, an immune inhibitory
molecule of the B7 family. J Immunol. 171:4650–4654. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Tringler B, Zhuo S, Pilkington G, Torkko
KC, Singh M, Lucia MS, Heinz DE, Papkoff J and Shroyer KR: B7-h4 is
highly expressed in ductal and lobular breast cancer. Clin Cancer
Res. 11:1842–1848. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Tringler B, Liu W, Corral L, Torkko KC,
Enomoto T, Davidson S, Lucia MS, Heinz DE, Papkoff J and Shroyer
KR: B7-H4 overexpression in ovarian tumors. Gynecol Oncol.
100:44–52. 2006. View Article : Google Scholar
|
|
40
|
Miyatake T, Tringler B, Liu W, Liu SH,
Papkoff J, Enomoto T, Torkko KC, Dehn DL, Swisher A and Shroyer KR:
B7-H4 (DD-O110) is overexpressed in high risk uterine endometrioid
adenocarcinomas and inversely correlated with tumor T-cell
infiltration. Gynecol Oncol. 106:119–127. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Small EJ, Tchekmedyian NS, Rini BI, Fong
L, Lowy I and Allison JP: A pilot trial of CTLA-4 blockade with
human anti-CTLA-4 in patients with hormone-refractory prostate
cancer. Clin Cancer Res. 13:1810–1815. 2007. View Article : Google Scholar : PubMed/NCBI
|