Introduction
Laryngeal squamous cell carcinoma (LSCC) is the most
common type of laryngeal carcinoma (LC) and accounts for more than
90% of LC cases. In 2015, the estimated number of new cases of LC
was 13,560 in the United States, and about 3,640 Americans were
likely to die from LC (1). During
the past 2 decades, clinicians have seen a worrying decline in the
survival rate of LC patients, partly due to lymph node metastases
(LNM). Recent studies showed that the 5-year overall survival was
39–44% in patients with advanced LC, and the 2-year local relapse
rate was 27.5% in stage III LC (2,3).
Although a variety molecules associated with the oncogenesis and
the treatment of LSCC have been identified, the potential
therapeutic targets and the precise molecular mechanisms of LSCC
proliferation and metastasis remain to be elucidated.
In the past few years, miRNAs have been identified
in most carcinomas and have become potential biomarkers for cancer
diagnosis and treatment (4–6). miRNAs are small, single-stranded
non-coding RNA molecules that regulate gene expression by binding
to miRNA at the 3′-untranslated region (7). As of May 2014, 2,578 miRNAs have been
identified in the human genome (4).
Deregulated miRNAs have been shown to play pivotal roles in the
multistep process of carcinogenesis via regulated oncogenes or
tumor suppressor genes. For example, miR-10b and miR-320b enhance
cell proliferation and invasion in pancreatic cancer and colorectal
cancer, respectively (8,9), and miR-218 and miR-195 inhibit cell
proliferation, migration, and invasion in gliomas and non-small
cell lung cancer (10,11).
miRNAs also play important roles in LSCC (12,13).
miR-27a was significantly upregulated in LSCC, promoted cell
viability and colony formation, and repressed apoptosis by
targeting PLK2 in Hep-2 cells (13). Our previous study (14) reported the identification via miRNA
microarray and verification by real-time PCR (RT-PCR) of 10 miRNAs
that were expressed differentially in LSCC patients with vs. those
without LNM, including 9 upregulated miRNAs (miR-365a-3p,
miR-143-5p, miR-634, miR-223-3p, miR-409-5p, miR-1224-3p,
miR-192-5p, miR-30d-5p, and miR-1249-3p) and 1 downregulated miRNA
(miR-494-3p).
Based in part on findings from our previous study,
we analyzed the effects of miR-365a-3p, miR-143-5p, and miR-494-3p
in LSCC in vitro. However, only miR-365a-3p can affect cell
apoptosis, mitosis, migration, and invasion. We also found that
miR-365a-3p promoted LSCC xenograft tumor growth and metastases in
liver and hepatic lymph nodes in mouse models. Moreover,
miR-365a-3p was demonstrated to mediate the PI3K/AKT signaling
pathway via p-AKT (Ser473) in LSCC.
Materials and methods
Cell culture and transient infection
Human Hep-2 cell lines (American Type Culture
Collection, ATCC; Manassas, VA, USA) were cultured in RPMI-1640
medium (Gibco-Life Technologies, Carlsbad, CA, USA) with 10% fetal
bovine serum at 37°C and 5% CO2 in a
humidified-atmosphere incubator. For overexpression and inhibition
of miRNAs (all from GE Healthcare Dharmacon, Lafayette, CO, USA),
miR-365a-3p inhibitor (IH-300666-05-0002), miR-143-5p inhibitor
(IH-301057-02-0002), miR-494-3p mimic (C-300761-05-0002), normal
control (NC)-inhibitor (IN-001005-01-05), and NC-mimic
(CN-001000-01-05) were transiently transfected in Hep-2 cells by
Lipofectamine 2000 (Invitrogen-Life Technologies, Carlsbad, CA,
USA) following the manufacturer's protocol.
RNA extraction and real-time PCR
analysis
Total RNAs of the transfected Hep-2 cells were
extracted with TRIzol (Invitrogen-Life Technologies) according to
the manufacturer's protocol. Single-stranded cDNA of the miRNAs was
synthesized by reverse transcription using the miScript Reverse
Transcription kit (Qiagen, Valencia, CA, USA). The expression level
of transfected miRNAs was assessed with RT-PCR (TaqMan MicroRNA
Assay; Life Technologies). The PCR primers for miR-365a-3p (sense,
GCCCCTAAAAATCCTT and antisense, GTGCAGGGTCCGAGGT); miR-143-5p
(sense, AAAAGAAAGAAAACACCC and antisense, GTGC AGGGTCCGAGGT);
miR-494-3p (sense, GAAACATACAC GGGAAACC and antisense,
GTGCAGGGTCCGAGGT); and U6 (sense, TGCGGGTGCTCGCTTCGCAGC and
anti-sense, CCAGTGCAGGGTCCGAGGT) were obtained from Life
Technologies. U6 served as an internal reference. The relative
expression levels of miR-365a-3p, miR-143-5p, and miR-494-3p in
Hep-2 cells were calculated using the 2−ΔΔCt method.
Cell apoptosis assay
Hep-2 cells were seeded in 6-well plates, grown to
about 80% confluence, and transiently transfected with the prepared
miRNA inhibitors and mimics. NC-mimic and NC-inhibitor were used as
controls. The transfected cells were trypsinized, washed twice in
prechilled phosphate-buffered saline (PBS), and collected after
transfection at 48 and 72 h. Subsequently, the cells were
resuspended with 300 µl of 1X binding buffer. Then, 5
µl of Annexin V-FITC conjugate and 5 µl of propidium
iodide (PI) solution (both from BD Biosciences, San Diego, CA, USA)
were added, and cells were incubated at room temperature for 15 min
in the dark. Flow cytometric analysis for cell apoptosis was
performed with a FACSCalibur flow cytometer (BD Biosciences)
according to the manufacturer's instructions.
Cell cycle assay
The Hep-2 cells were treated according to the
procedures for cell apoptosis assay. NC-mimic and NC-inhibitor were
used as controls. Subsequently, the transfected cells were
resuspended with 400 µl PBS and fixed with prechilled 80%
ethanol at 4°C for 24 to 48 h for cell cycle analysis. Then, the
fixed cells were washed with PBS and resuspended in staining
solution (Boehringer Mannheim, Mannheim, Germany) containing 50
µg/ml of RNase A and 65 µg/ml of PI solution and
incubated at 37°C for 30 min. Cell cycle flow cytometric analysis
was performed with a FACSCalibur flow cytometer.
Transwell migration and invasion
assay
For the migration assay, the transiently transfected
Hep-2 cells with the miRNA inhibitors and mimics described above
were seeded in the upper Transwell chamber (Sigma-Aldrich, St.
Louis, MO, USA) coated with 8-µm pore Transwells (Millipore,
Billerica, MA, USA). After incubation for 24 h, the non-migrated
cells in the upper chamber were removed. The invaded cells were
fixed with methanal, stained with Giemsa staining solution, and
then counted by an inverted light microscope. The Hep-2 cells
invasion assays were performed using the Matrigel invasion chamber
(Sigma-Aldrich) according to the manufacturer's protocol. The
seeding, staining, and counting of Hep-2 cells were performed as
the migration assay. In the above two assays, the medium of upper
Transwell chamber contains serum-free culture, and of the lower
chamber contains 10% fetal bovine serum served as the
chemoattractant.
Lentivirus-mediated inhibition of
miR-365a-3p
To establish the stable miR-365a-3p and miR-365a-3p
inhibitor cell lines, Hep-2 cells were transfected with
NC-pLenti6/TR inhibitor (Lv-NC-inhibitor) and pLenti6/TR
miR-365a-3p inhibitor (Lv-miR-365a-3p inhibitor), respectively,
followed by selection for 42 days in fresh complete medium
supplemented with 3 µg/ml puromycin (Invitrogen-Life
Technologies). Single colonies were selected and amplified, and the
expression level of miR-365a-3p was detected by RT-PCR.
Analysis of tumorigenesis and metastatic
potential of miR-365a-3p in vivo
For the in vivo tumorigenesis assay,
2×106 stable transfected Hep-2 cells with
Lv-NC-inhibitor were injected into the enterocoelia of 10 nude mice
(Laboratory Animal Center of the Capital Medical University,
Beijing, China), aged 6 weeks, so were the 2×106 stable
transfected Hep-2 cells with Lv-miR-365a-3p inhibitor. The mice
were sacrificed on the day 42 after injection, and the final tumors
were isolated. Tumor size was measured with a digital caliper, and
tumor volume was calculated with the formula: tumor volume =
(length × width2) × 0.5. For the tumor-metastasis assay
in vivo, all the organs of the sacrificed mice were examined
at necropsy. Lungs, livers, and the corresponding lymph nodes were
stained with hematoxylin and eosin and were examined by two
pathologists.
All mice used in this experiment were bred and
maintained in sterile cages and were handled according to the
National Institutes of Health Animal Care and Use Committee
Regulations. All experimental procedures were approved by the
Animal Care and Use Committee of Capital Medical University
(Beijing, China).
Protein extraction and western blot
analysis
Cells were lysed in RIPA buffer with a protease
inhibitor cocktail (both from Millipore). Protein was extracted and
analyzed by standard western blot analyses. The primary antibodies,
including total ERK, p-ERK1/2, total AKT, p-Akt (Ser473), and p-Akt
(Thr308) were obtained from Life Technologies. GAPDH was used as an
endogenous normalizer. The membrane was incubated with alkaline
phosphatase secondary antibodies (Thermo Fisher Scientific,
Waltham, MA, USA). Then the intensities of enhanced bands of
immunoreactive protein was quantified (Image-Pro Plus 6.0; Media
Cybernetics, Rockville, MD, USA).
Statistical analyses
All of the experiments were repeated in triplicate,
and one representative experiment was selected for data analysis.
The statistical analyses were performed with SPSS 17.0 (IBM,
Chicago, IL, USA). The statistical significance was tested using
two-tailed Student's t-test. A p-value of <0.05 was considered
statistically significant.
Results
miR-365a-3p inhibitor facilitates
apoptosis and suppresses mitosis in Hep-2 cells
Our previous study determined that miR-365a-3p and
miR-143-5p were upregulated and miR-494-3p was downregulated in
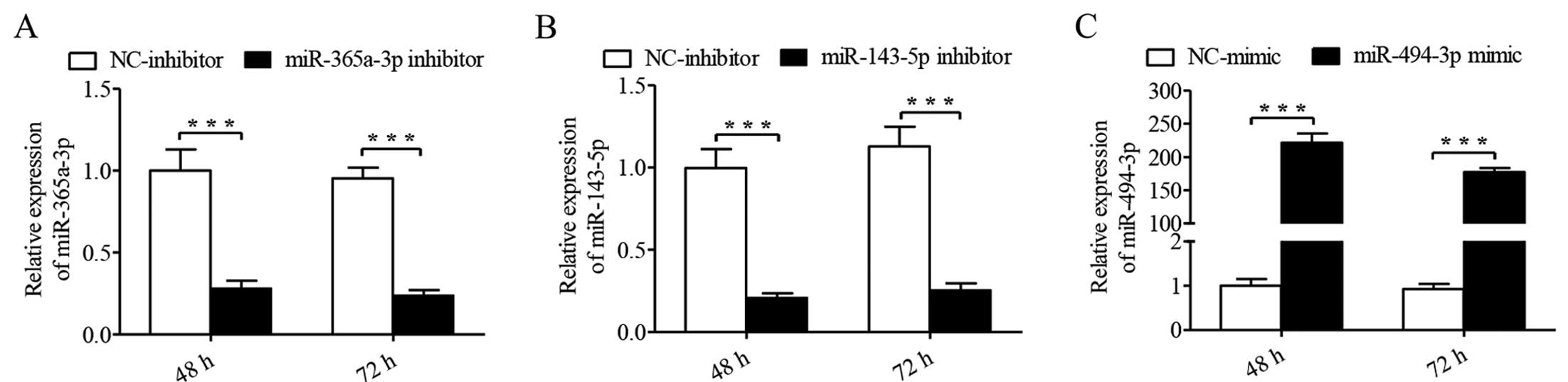
LSCC patients with LNM. In the present study, we successfully
transfected Hep-2 cells with miR-365a-3p inhibitor (P<0.001,
Fig. 1A), miR-143-5p inhibitor
(P<0.001, Fig. 1B), and
miR-494-3p mimics (P<0.001, Fig.
1C) for 48 and 72 h.
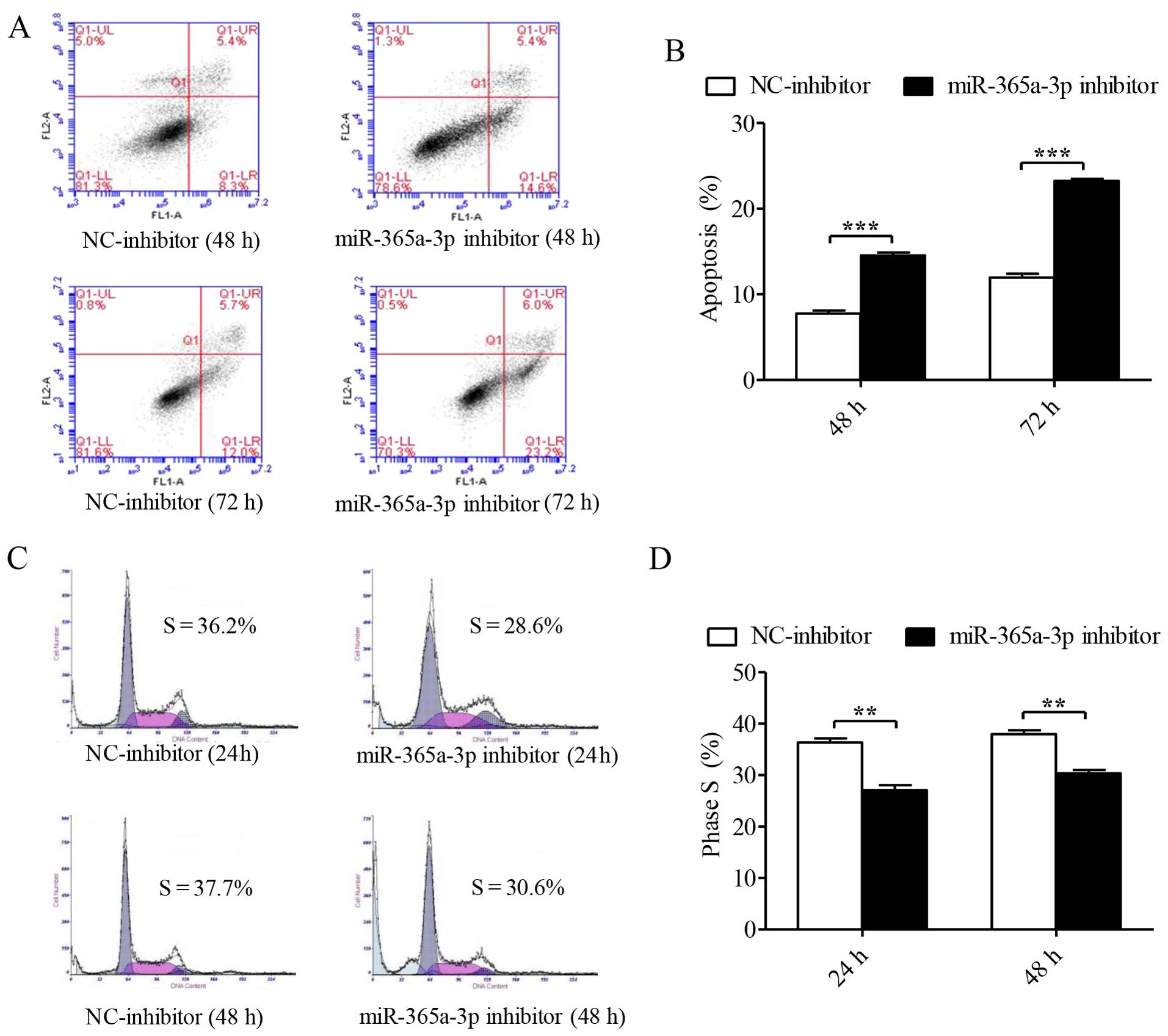
We first assessed the roles of these miRNAs in
apoptosis in Hep-2 cells by flow cytometric analysis. At 48 and 72
h after transient transfection, analyses based on Annexin V-FITC/PI
apoptosis revealed that with the decreased expression of
miR-365a-3p, the number of Hep-2 cells that expressed apoptotic
protein Annexin V was elevated (representative data are shown in
Fig. 2A). The result indicates that
miR-365a-3p inhibitor significantly facilitated the apoptosis of
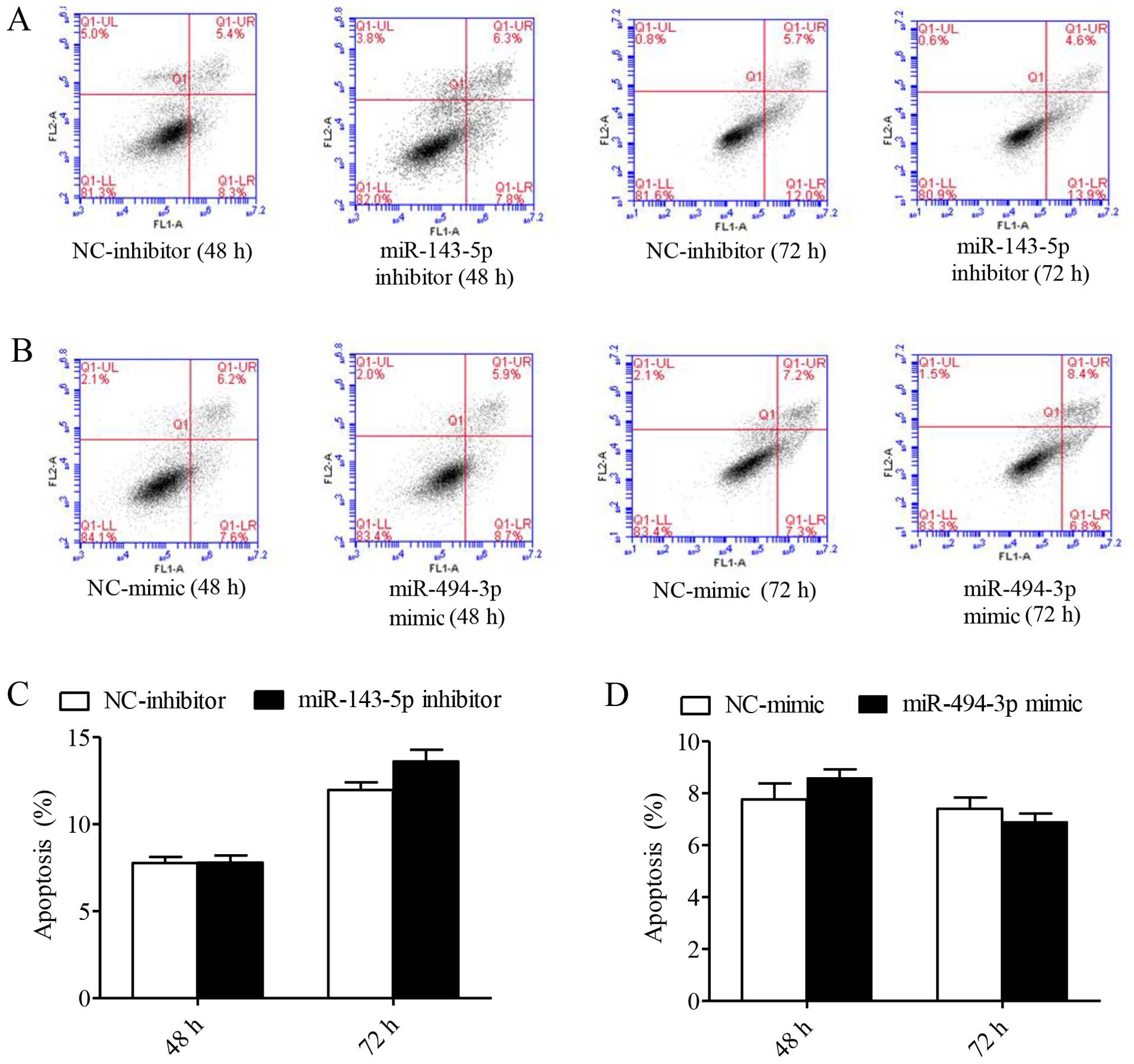
Hep-2 cells at 48 and 72 h (P<0.001, Fig. 2B). In contrast, miR-143-5p inhibitor
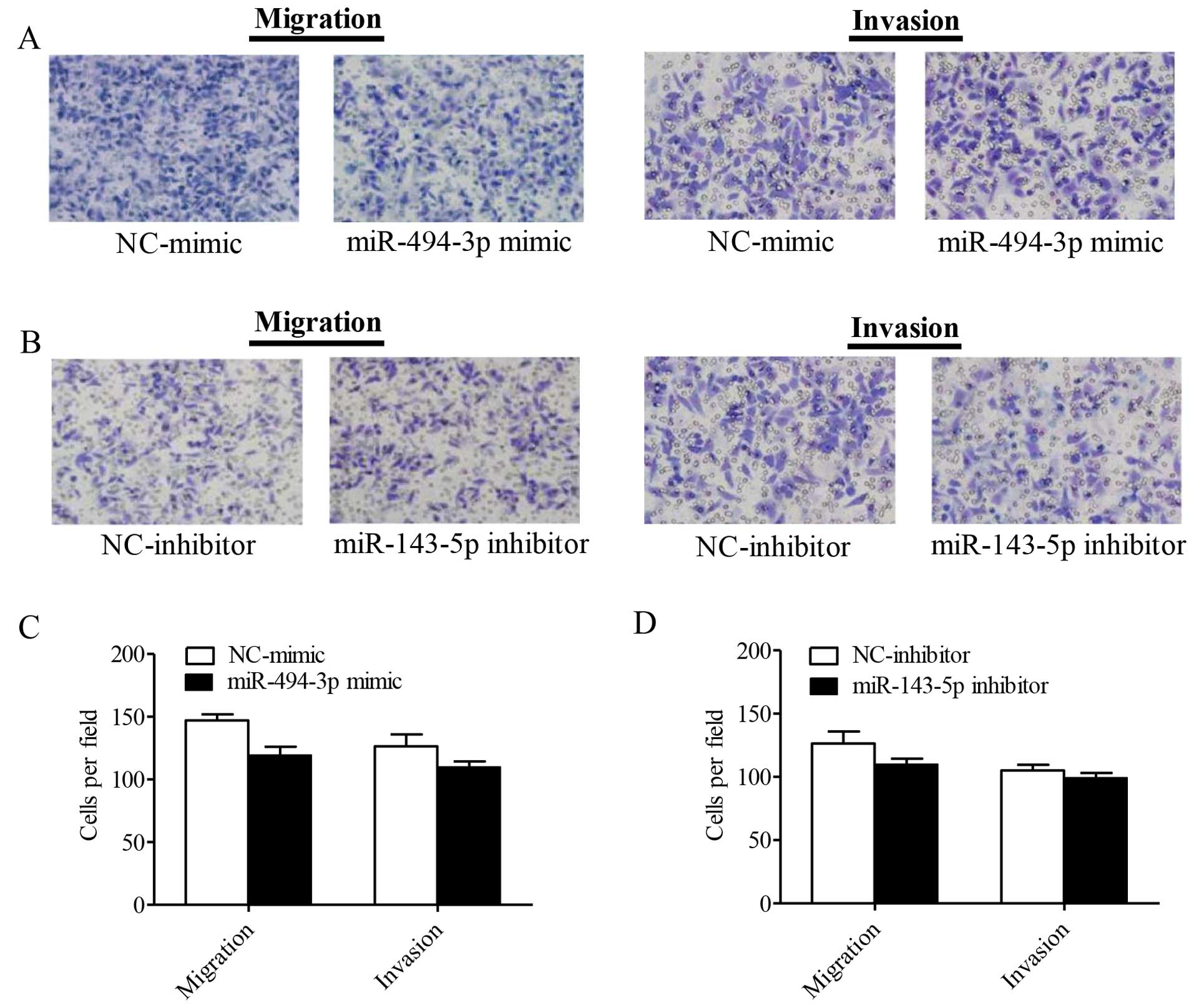
and miR-494-3p mimics had no effect on Hep-2 cells (Fig. 3).
To characterize the influences of these miRNAs on
the cell cycle, we used flow cytometry with PI staining to measure
the DNA content in order to analyze the cell cycle distribution in
Hep-2 cells. The percentages of cells in the S1 phase infected with
the NC-inhibitor at 24 and 48 h after transfection were 36.2 and
37.7% and with the miR-365a-3p inhibitor were 28.6 and 30.6%,
respectively (representative data are shown in Fig. 2C). These findings suggest that the
miR-365a-3p inhibitor significantly suppressed cell cycle
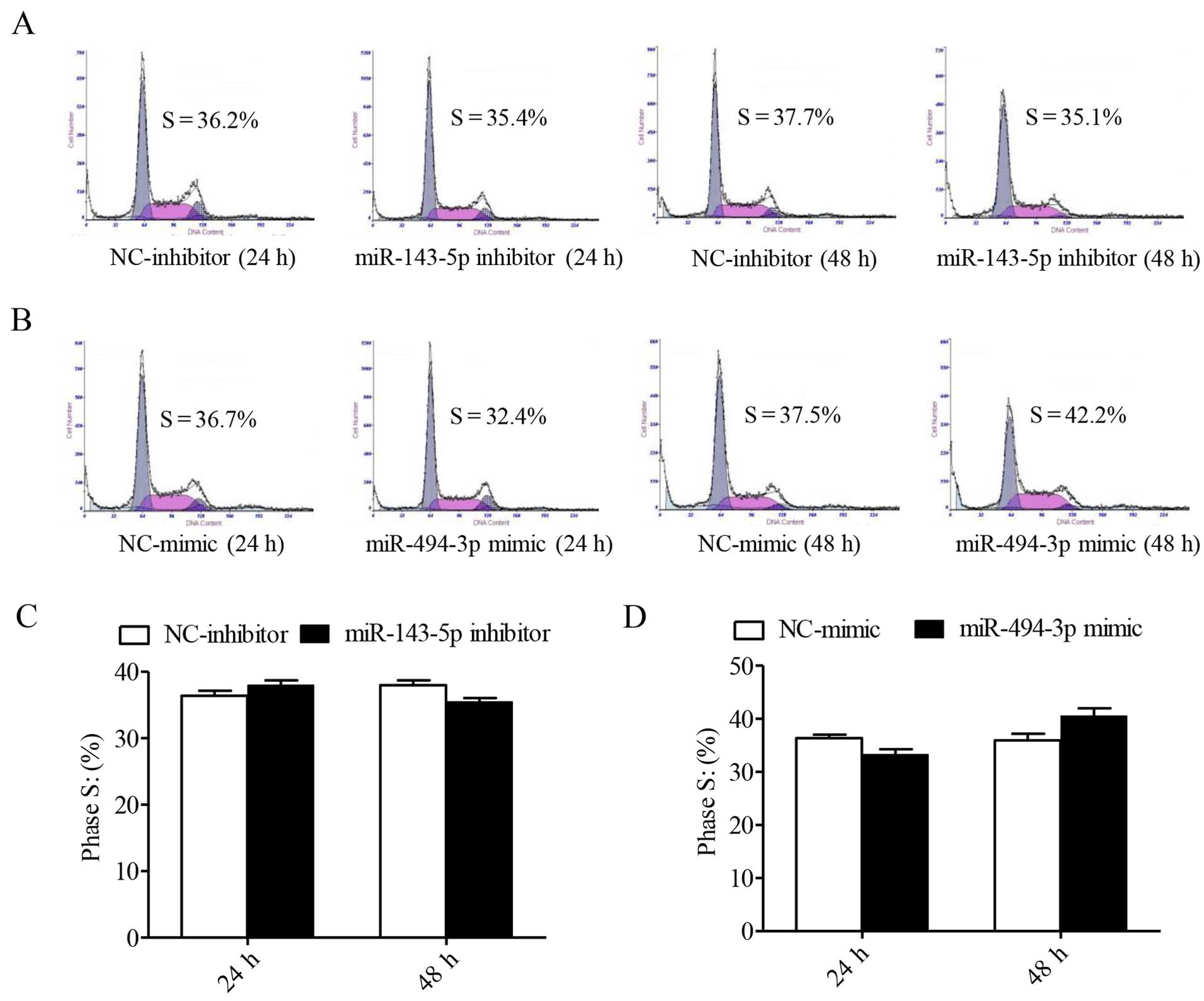
progression in Hep-2 cells, as shown by the histogram in Fig. 2D(P=0.002). However, Hep-2 cells
infected with the miR-143-5p inhibitor and the miR-494-3p mimics
showed no significant differences compared with the homologous
controls (Fig. 4).
miRNA-365 promotes migration and invasion
of Hep-2 cells
To determine the biological functions of these miRNA
mimics and inhibitors in LSCC metastasis, we performed Transwell
migration and invasion assays on Hep-2 cells transfected with
miR-365a-3p inhibitor, miR-143-5p inhibitor, and miR-494-3p mimics
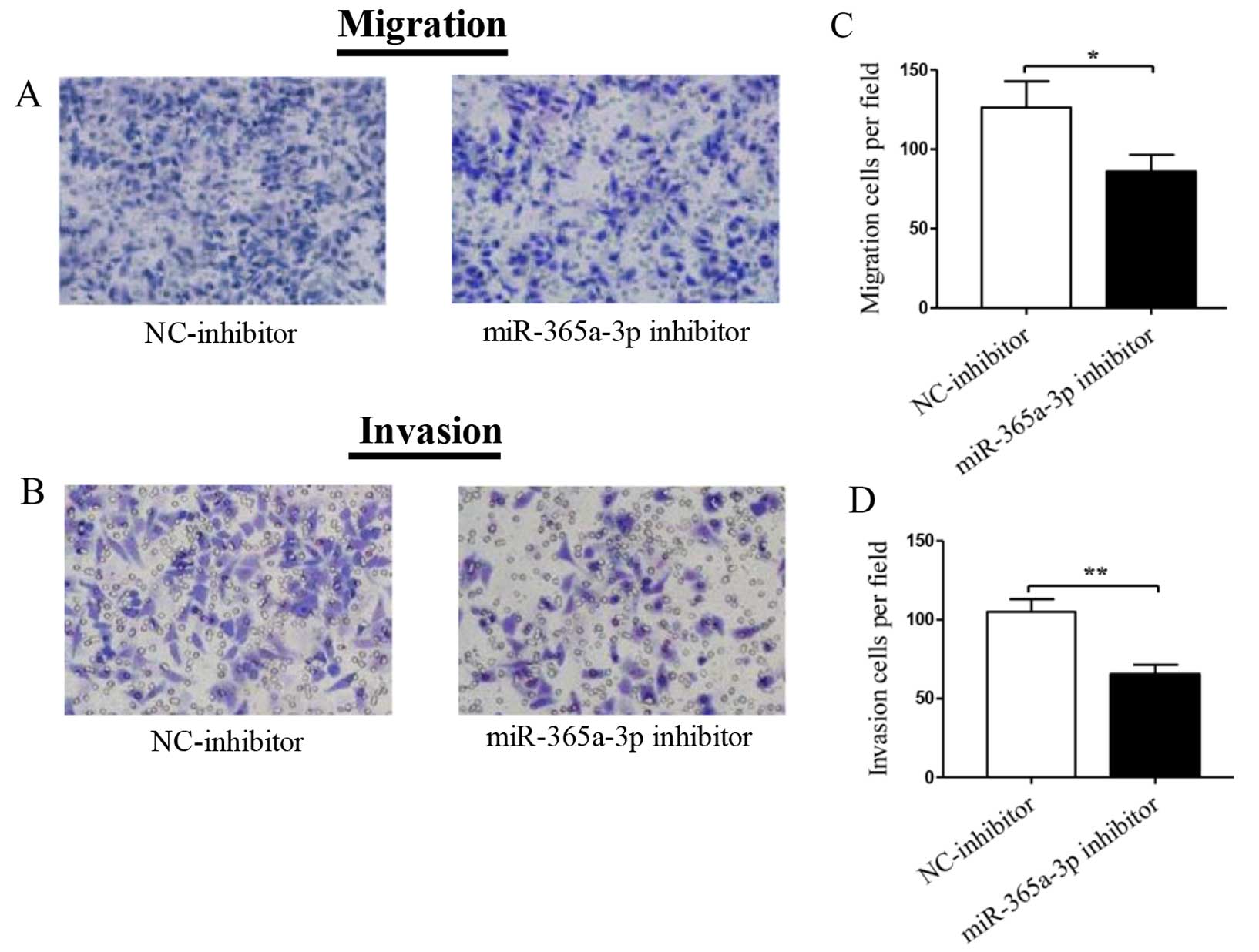
at 24 h after transfection. As shown in Fig. 5A, transfection of the miR-365a-3p
inhibitor led to significantly decreased cell migration of Hep-2
cells, as shown in the histogram in Fig. 5C (P=0.023). Similarly, Transwell
invasion assays using Matrigel further demonstrated that only Hep-2
cells transfected with the miR-365a-3p inhibitor were significantly
reduced compared to the parental control cells (P=0.0024) (Fig. 5B and D). As shown in Fig. 6, the miR-143-5p inhibitor and the
miR-494-3p mimics had no significant influence on the cell
migration and invasion of Hep-2 cells.
miR-365a-3p promotes LSCC tumor growth
and metastasis in vivo
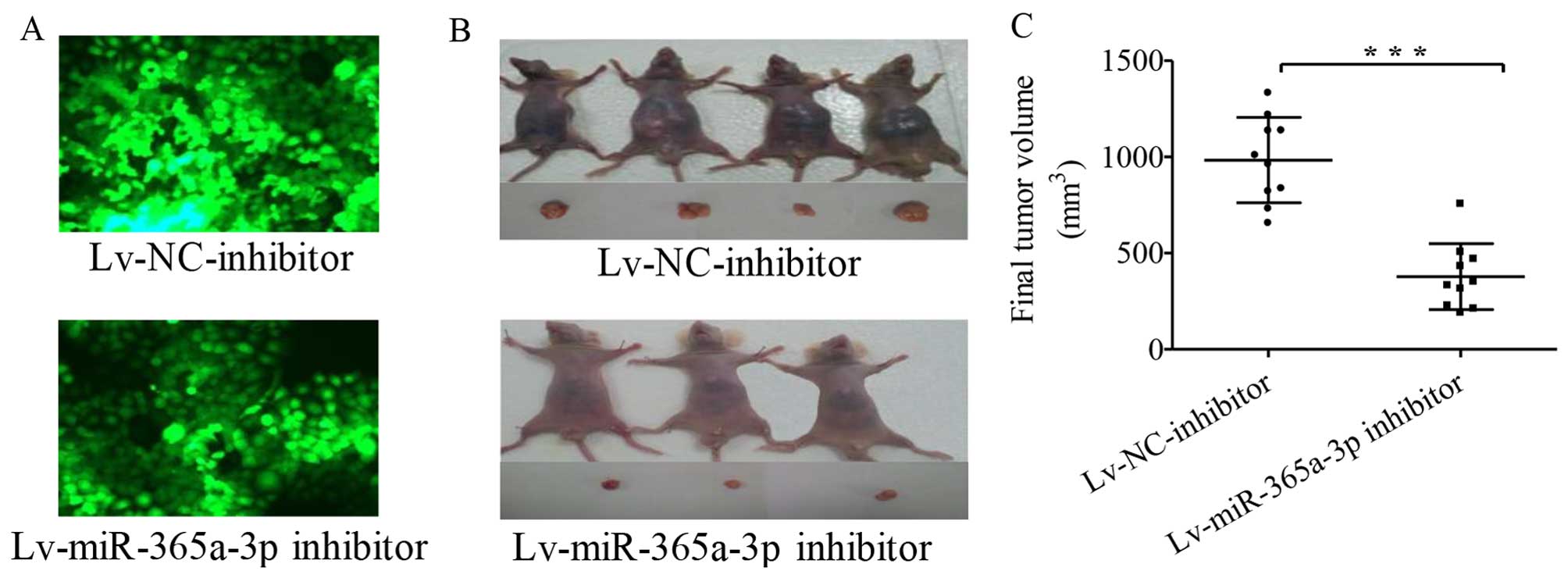
To further view the potential effects of
miR-365a-3p, we established stably transfected Hep-2 cells
containing an Lv-NC-inhibitor and an Lv-miR-365a-3p inhibitor
(Fig. 7A) and injected the
inhibitors into the enterocoelia of nude mice. The mice were
euthanized, and the tumors were harvested on day 42 after
injection. Tumors formed from Hep-2 cells stably transfected with
the Lv-NC-inhibitor grew much faster than tumors from the cells
that stably expressed the Lv-miR-365a-3p inhibitor (Fig. 7B). Accordingly, the final tumor
volumes in the two groups were calculated and compared, as shown in
Fig. 7C (P<0.0001 for the
difference).
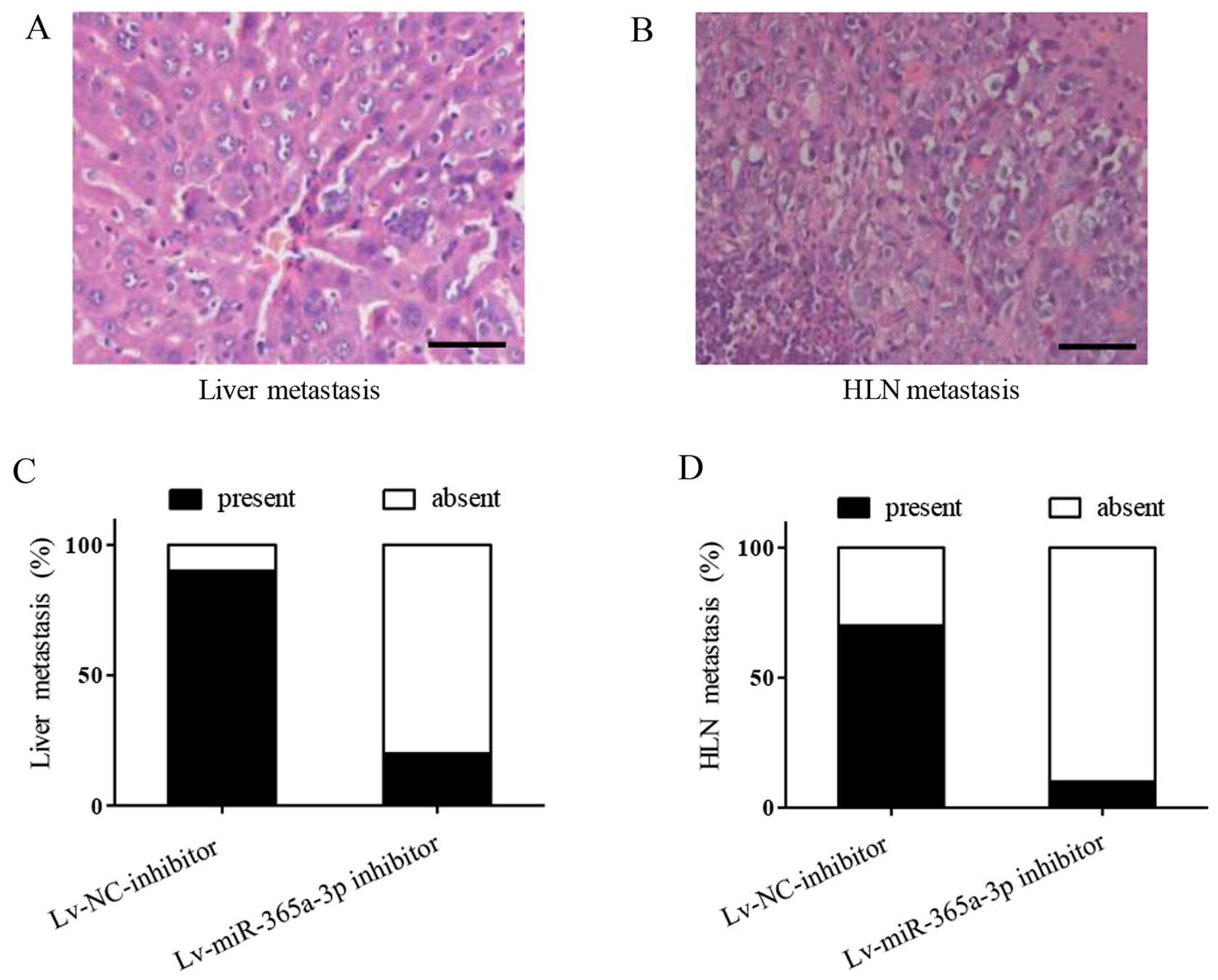
We further explored the metastasis in vivo
using a body vision microscope. We found that tumors were
metastasized in the livers (Fig.
8A) and hepatic lymph nodes (Fig.
8B). In particular, metastases to the livers in the
Lv-NC-inhibitor group occurred at a significantly higher frequency
than those in the Lv-miR-365a-3p inhibitor group. As shown in the
stacked bars of Fig. 8C, 9 of 10 mice in the Lv-NC-inhibitor group
showed liver metastases, but in the Lv-miR-365a-3p inhibitor group
only 2 of 10 mice showed liver metastases. As expected, there was a
similar trend of metastases in the hepatic lymph nodes in the
Lv-NC-inhibitor and the Lv-miR-365a-3p inhibitor groups: 7 in 10
mice and only 1 in 10 mice showed hepatic lymph node metastases,
respectively (Fig. 8D). These
findings indicate that miR-365a-3p promotes LSCC tumor growth and
metastases in vivo.
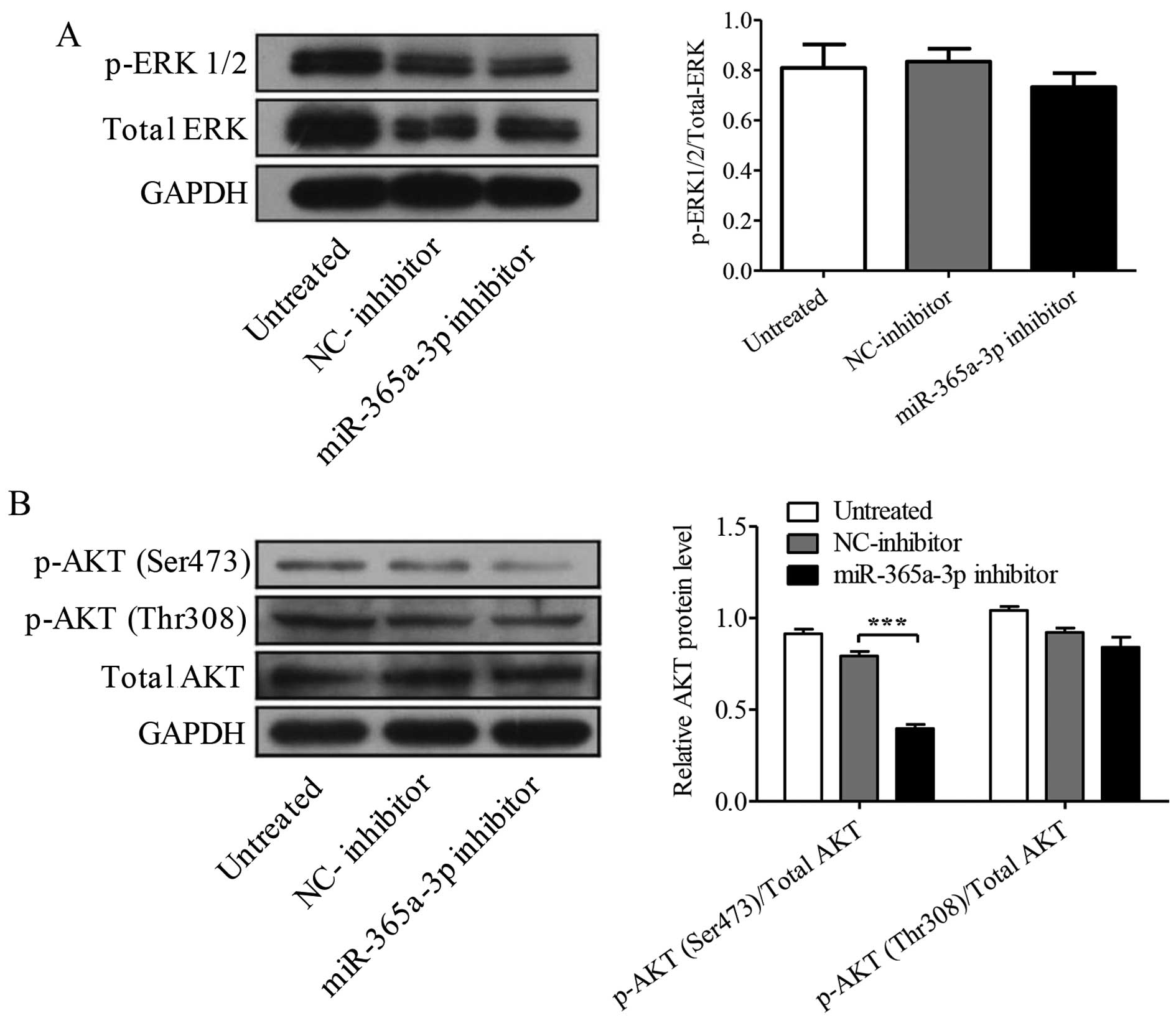
miR-365a-3p inhibitor downregulates p-AKT
(Ser473)
ERK and AKT play key roles in proliferation,
migration, and invasion in malignant tumor cells. Accordingly, we
performed western blotting to examine the protein levels of
p-ERK1/2, p-AKT (Thr308), and p-AKT (Ser473). The results showed
that the levels of p-ERK1/2 have no significant difference after
miR-365a-3p inhibitor transfection compared with the levels of
NC-inhibitor (P=0.082, Fig. 9A).
Interestingly, miR-365a-3p inhibitor significantly decreased the
expression of p-AKT (Ser473) but not p-AKT (Thr308) compared to the
expression in the Hep-2 cells transfected with matched NC-inhibitor
miRNA (Ser473, P<0.001; Thr308, P<0.245, Fig. 9B).
Discussion
Aberrant expression of miRNAs has been demonstrated
to contribute to LSCC tumorigenesis and progression (13,15).
For the first time, this study analyzed the roles of miR-365a-3p,
miR-143-5p, and miR-494-3p in Hep-2 cells. Because of the
overexpression of miR-365a-3p and miR-143-5p and the lower
expression of miR-494-3p in LSCC patients with LNM (14), we transfected Hep-2 cells with
miR-365a-3p inhibitor, miR-143-5p inhibitor, and miR-494-3p mimics.
Interestingly, the loss-of-function results demonstrated that only
the miR-365a-3p inhibitor significantly facilitated apoptosis and
suppressed mitosis, migration, and invasion of Hep-2 cells, and we
found that miR-365a-3p promotes LSCC xenograft tumor growth and
metastasis in vivo. These data suggest that miR-365a-3p may
be an oncomiR in LCSS, and miR-365a-3p inhibition may have
potential in the treatment of laryngeal carcinoma.
The mature miR-365 is derived from two separate RNA
precursors, hsa-miR-365a (previously named hsa-miR-365-1,
MI0000767) and hsa-miR-365b (previously named hsa-miR-365-2,
MI0000769), and mature hsa-miR-365a-3p (MIMAT0000710) is sheared
from hsa-miR-365a (www.mirbase.org). miR-365 has been confirmed to act as
an oncogene or tumor suppressor in different carcinomas (16,17),
although this finding has not previously been reported in LSCC.
Gastaldi et al (18) found
that the expression of miR-365a was decreased in chemically-induced
mouse skin carcinomas, which suggests a tumor suppressor role of
miR-365a although miR-365a-3p has been found to be an oncogene
(19). A recent study revealed that
miR-365b was upregulated in cutaneous squamous cell carcinoma and
induced subcutaneous tumors in vivo (16). The study also found that
anti-miR-365b oligonucleotide inhibited cutaneous tumor formation
in nude mice, along with apoptosis and G1 phase arrest in cancer
cells. Hamada et al also found that miR-365 was highly
expressed in ductal adenocarcinoma of the pancreas and contributed
to the epithelial-mesenchymal transition. miR-365 can target
apoptosis-promoting protein BAX and adaptor protein Src homology 2
domain containing 1, and then influences the survival of pancreatic
cancer cells (20). Obviously, the
expression levels of miR-365 are diverse in different malignant
tumors. For example, in human gastric cancer the low expression of
miR-365 correlated with poorly differentiated histology, advanced
stage, and deep invasion, as well as the deregulation of
phosphorylated Akt, p53, and cyclin D1. In mouse gastric cancers,
the activation of Akt led to downregulated transcription of miR-365
and promoted gastric cancer cell proliferation (17). These data demonstrate that miR-365
was downregulated in gastric cancer. In addition, miR-365 was also
found to be downregulated and accompanied the overexpression of
NKX2-1 protein in lung cancer tissues. Furthermore, the miR-365
mimic significantly reduced the proliferation of lung cancer cells
(21). The discrepancy of these
findings may be due to the dynamic tumor microenvironment, stages
of progression, and different types of cancers studied.
PI3K/AKT and MAPK signaling pathways are often
hyperactivated in many malignant tumors, including LSCC, and the
dependence of carcinoma cells on the two activated pathways has
been used successfully in the clinic (22–25).
AKT and ERK play key roles in the PI3K/AKT pathway and MAPK
pathway, respectively (26,27). Recent studies have also found that
AKT and ERK are highly correlated with miRNAs in various human
malignancies (28–30). Therefore, to explore the underlying
signaling pathways of miR-365a-3p-induced LCSS cell proliferation,
migration, and invasion, we examined the expression of p-AKT
(Thr308), p-AKT (Ser473), and p-ERK1/2. The data showed that only
the expression of p-AKT (Ser473) was significantly influenced by
the miR-365a-3p inhibitor. The serine/threonine kinase Akt encoded
by the protein kinase B gene is a downstream effector of PI3K.
Studies have shown that activation of Akt signaling is responsible
for cancer cell proliferation, invasiveness, and metastasis
(17,31). AKT activation is initiated by
docking of the PH domain of AKT to PIP3 on the cellular membrane,
exposing two critical amino acid residues for phosphorylation
(32). Both phosphorylation events,
of Ser473 by the protein kinase PDK1 and of Thr308 by the mTORC2
complex, are required for full activation of AKT (33). However, the levels of AKT
phosphorylation on either Ser473 or Thr308 correlate differently
with tumor cell growth and proliferation in distinct carcinomas
(34–37). In our study, p-AKT (Ser473) was
significantly decreased by the miR-365a-3p inhibitor, but p-AKT
(Ser308) had no such influence. The data suggested that miR-365a-3p
can mediate the PI3K/AKT signaling pathway by upregulating p-AKT
(Ser473) to promote growth and metastasis in LSCC. This result is
consistent with a recent study that showed that patients with head
and neck squamous cell carcinomas with higher levels of p-AKT
(Ser473) had worse survival, but the survival of patients with
higher levels of p-AKT (Thr308) was not affected (25).
Dysregulated miR-143 and miR-494 have important
roles in many carcinomas (38,39),
although this association has not been reported in LSCC patients. A
recent study of epithelial cancers, including esophagus and lung
cancer, indicated that downregulation of miR-143 contributes to
epithelial cancer development, and its re-expression suppresses
cellular proliferation and triggers apoptosis of epithelial cancer
cells (38). miR-494 was reported
to be significantly upregulated and to negatively modulate the
expression of its target gene PTEN, in human cervical cancer
cell lines and tumor samples (39).
These findings identified the essential roles that miR-143 and
miR-494 play in many carcinomas. However, our experiment shows that
the two miRNAs did not have significant influence on Hep-2 cell
apoptosis, mitosis, migration, and invasion in vitro. This
is partly explained by the observation that miRNAs may have
different roles cancer type-dependently.
In view of the low 5-year survival rate and the
limited improvements in the treatment of LSCC during the past 20
years (1,2), finding novel molecular therapeutic
targets for treating LSCC is essential. Because miRNAs play pivotal
roles in the development of carcinoma, it is conceivable that miRNA
mimics or inhibitors may become a new class of molecular
target-based therapies for various cancers (40–42).
Their effects and regulatory mechanisms in LSCC remain uncertain,
but the present study revealed that miR-365a-3p, a novel oncomiR,
activates the PI3K/AKT signaling pathway via p-AKT (Ser473) and can
promote LSCC growth and metastasis. Therefore, miR-365a-3p may
become a potential therapeutic target for the treatment of
LSCC.
Acknowledgments
This study was supported by the National Natural
Science Foundation of China (#81071785), the Clinical Research
Special Foundation by the Wu Jieping Medical Foundation
(320.6750.12398), the Beijing Municipal Science and Technology
Project (D131100005313014), the Scientific Research Project of
Beijing Children's Hospital, Capital Medical University (2012ZD01),
and the Science and Technology Planning Project of Beijing
(Z151100003715006).
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Megwalu UC and Sikora AG: Survival
outcomes in advanced laryngeal cancer. JAMA Otolaryngol Head Neck
Surg. 140:855–860. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Connor KL, Pattle S, Kerr GR and Junor E:
Treatment, comorbidity and survival in stage III laryngeal cancer.
Head Neck. 37:698–706. 2015. View Article : Google Scholar
|
|
4
|
Berindan-Neagoe I, Monroig PC, Pasculli B
and Calin GA: MicroRNAome genome: A treasure for cancer diagnosis
and therapy. CA Cancer J Clin. 64:311–336. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Valeri N, Braconi C, Gasparini P, Murgia
C, Lampis A, Paulus-Hock V, Hart JR, Ueno L, Grivennikov SI, Lovat
F, et al: MicroRNA-135b promotes cancer progression by acting as a
downstream effector of oncogenic pathways in colon cancer. Cancer
Cell. 25:469–483. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Drayton RM, Dudziec E, Peter S, Bertz S,
Hartmann A, Bryant HE and Catto JW: Reduced expression of miRNA-27a
modulates cisplatin resistance in bladder cancer by targeting the
cystine/glutamate exchanger SLC7A11. Clin Cancer Res. 20:1990–2000.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kong YW, Ferland-McCollough D, Jackson TJ
and Bushell M: microRNAs in cancer management. Lancet Oncol.
13:e249–e258. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ouyang H, Gore J, Deitz S and Korc M:
microRNA-10b enhances pancreatic cancer cell invasion by
suppressing TIP30 expression and promoting EGF and TGF-β actions.
Oncogene. 33:4664–4674. 2014. View Article : Google Scholar :
|
|
9
|
Zhou J, Zhang M, Huang Y, Feng L, Chen H,
Hu Y, Chen H, Zhang K, Zheng L and Zheng S: MicroRNA-320b promotes
colorectal cancer proliferation and invasion by competing with its
homologous microRNA-320a. Cancer Lett. 356:669–675. 2015.
View Article : Google Scholar :
|
|
10
|
Tu Y, Gao X, Li G, Fu H, Cui D, Liu H, Jin
W and Zhang Y: MicroRNA-218 inhibits glioma invasion, migration,
proliferation, and cancer stem-like cell self-renewal by targeting
the polycomb group gene Bmi1. Cancer Res. 73:6046–6055. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yongchun Z, Linwei T, Xicai W, Lianhua Y,
Guangqiang Z, Ming Y, Guanjian L, Yujie L and Yunchao H:
MicroRNA-195 inhibits non-small cell lung cancer cell
proliferation, migration and invasion by targeting MYB. Cancer
Lett. 347:65–74. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sun X, Liu B, Zhao XD, Wang LY and Ji WY:
MicroRNA-221 accelerates the proliferation of laryngeal cancer cell
line Hep-2 by suppressing Apaf-1. Oncol Rep. 33:1221–1226.
2015.PubMed/NCBI
|
|
13
|
Tian Y, Fu S, Qiu GB, Xu ZM, Liu N, Zhang
XW, Chen S, Wang Y, Sun KL and Fu WN: MicroRNA-27a promotes
proliferation and suppresses apoptosis by targeting PLK2 in
laryngeal carcinoma. BMC Cancer. 14:678–689. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tai J, Xiao X, Huang ZG, Yu ZK, Chen XH,
Zhou WG, Chen XJ, Rao YS, Fang JG and Ni X: MicroRNAs regulate
epithelial-mesenchymal transition of supraglottic laryngeal cancer.
Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 48:499–503. 2013.In
Chinese. PubMed/NCBI
|
|
15
|
Xu Y, Wang K, Gao W, Zhang C, Huang F, Wen
S and Wang B: MicroRNA-106b regulates the tumor suppressor RUNX3 in
laryngeal carcinoma cells. FEBS Lett. 587:3166–3174. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhou M, Liu W, Ma S, Cao H, Peng X, Guo L,
Zhou X, Zheng L, Guo L, Wan M, et al: A novel onco-miR-365 induces
cutaneous squamous cell carcinoma. Carcinogenesis. 34:1653–1659.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Guo SL, Ye H, Teng Y, Wang YL, Yang G, Li
XB, Zhang C and Yang X, Yang ZZ and Yang X: Akt-p53-miR-365-cyclin
D1/cdc25A axis contributes to gastric tumorigenesis induced by PTEN
deficiency. Nat Commun. 4:2544–2554. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gastaldi C, Bertero T, Xu N,
Bourget-Ponzio I, Lebrigand K, Fourre S, Popa A, Cardot-Leccia N,
Meneguzzi G, Sonkoly E, et al: miR-193b/365a cluster controls
progression of epidermal squamous cell carcinoma. Carcinogenesis.
35:1110–1120. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tarasov VA, Matishov DG, Shin EF, Boĭko
NV, Timoshkina NN, Makhotkin MA, Lomonosov AM and Kirpiĭ AA:
Inheritable changes in miRNAs expression in HeLa cells after X-ray
and mitomycin C treatment. Genetika. 50:909–917. 2014.In
Russian.
|
|
20
|
Hamada S, Masamune A, Miura S, Satoh K and
Shimosegawa T: miR-365 induces gemcitabine resistance in pancreatic
cancer cells by targeting the adaptor protein SHC1 and
pro-apoptotic regulator BAX. Cell Signal. 26:179–185. 2014.
View Article : Google Scholar
|
|
21
|
Kang SM, Lee HJ and Cho JY: MicroRNA-365
regulates NKX2-1, a key mediator of lung cancer. Cancer Lett.
335:487–494. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Smith MP, Sanchez-Laorden B, O'Brien K,
Brunton H, Ferguson J, Young H, Dhomen N, Flaherty KT, Frederick
DT, Cooper ZA, et al: The immune microenvironment confers
resistance to MAPK pathway inhibitors through macrophage-derived
TNFα. Cancer Discov. 4:1214–1229. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mittal S, Sharma A, Balaji SA, Gowda MC,
Dighe RR, Kumar RV and Rangarajan A: Coordinate hyperactivation of
Notch1 and Ras/MAPK pathways correlates with poor patient survival:
Novel therapeutic strategy for aggressive breast cancers. Mol
Cancer Ther. 13:3198–3209. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bjerke GA, Yang CS, Frierson HF, Paschal
BM and Wotton D: Activation of Akt signaling in prostate induces a
TGFβ-mediated restraint on cancer progression and metastasis.
Oncogene. 33:3660–3667. 2014. View Article : Google Scholar :
|
|
25
|
Freudlsperger C, Horn D, Weißfuß S,
Weichert W, Weber KJ, Saure D, Sharma S, Dyckhoff G, Grabe N,
Plinkert P, et al: Phosphorylation of AKT(Ser473) serves as an
independent prognostic marker for radiosensitivity in advanced head
and neck squamous cell carcinoma. Int J Cancer. 136:2775–2785.
2015. View Article : Google Scholar
|
|
26
|
Fresno Vara JA, Casado E, de Castro J,
Cejas P, Belda-Iniesta C and González-Barón M: PI3K/Akt signalling
pathway and cancer. Cancer Treat Rev. 30:193–204. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
McKay MM and Morrison DK: Integrating
signals from RTKs to ERK/MAPK. Oncogene. 26:3113–3121. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Du J, Liu S, He J, Liu X, Qu Y, Yan W, Fan
J, Li R, Xi H, Fu W, et al: MicroRNA-451 regulates stemness of side
population cells via PI3K/Akt/mTOR signaling pathway in multiple
myeloma. Oncotarget. 6:14993–15007. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ihle MA, Trautmann M, Kuenstlinger H, Huss
S, Heydt C, Fassunke J, Wardelmann E, Bauer S, Schildhaus HU,
Buettner R, et al: miRNA-221 and miRNA-222 induce apoptosis via the
KIT/AKT signalling pathway in gastrointestinal stromal tumours. Mol
Oncol. 9:1421–1433. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang W, Ren F, Wu Q, Jiang D, Li H and Shi
H: MicroRNA-497 suppresses angiogenesis by targeting vascular
endothelial growth factor A through the PI3K/AKT and MAPK/ERK
pathways in ovarian cancer. Oncol Rep. 32:2127–2133.
2014.PubMed/NCBI
|
|
31
|
Xue G, Restuccia DF, Lan Q, Hynx D,
Dirnhofer S, Hess D, Rüegg C and Hemmings BA: Akt/PKB-mediated
phosphorylation of Twist1 promotes tumor metastasis via mediating
cross-talk between PI3K/Akt and TGF-β signaling axes. Cancer
Discov. 2:248–259. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Stephens L, Anderson K, Stokoe D,
Erdjument-Bromage H, Painter GF, Holmes AB, Gaffney PR, Reese CB,
McCormick F, Tempst P, et al: Protein kinase B kinases that mediate
phosphatidylinositol 3,4,5-trisphosphate-dependent activation of
protein kinase B. Science. 279:710–714. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Freudlsperger C, Burnett JR, Friedman JA,
Kannabiran VR, Chen Z and Van Waes C: EGFR-PI3K-AKT-mTOR signaling
in head and neck squamous cell carcinomas: Attractive targets for
molecular-oriented therapy. Expert Opin Ther Targets. 15:63–74.
2011. View Article : Google Scholar
|
|
34
|
Tsurutani J, Fukuoka J, Tsurutani H, Shih
JH, Hewitt SM, Travis WD, Jen J and Dennis PA: Evaluation of two
phosphorylation sites improves the prognostic significance of Akt
activation in non-small-cell lung cancer tumors. J Clin Oncol.
24:306–314. 2006. View Article : Google Scholar
|
|
35
|
Lin A, Piao HL, Zhuang L, Sarbassov D, Ma
L and Gan B: FoxO transcription factors promote AKT Ser473
phosphorylation and renal tumor growth in response to pharmacologic
inhibition of the PI3K-AKT pathway. Cancer Res. 74:1682–1693. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Beck JT, Ismail A and Tolomeo C: Targeting
the phosphatidylinositol 3-kinase (PI3K)/AKT/mammalian target of
rapamycin (mTOR) pathway: An emerging treatment strategy for
squamous cell lung carcinoma. Cancer Treat Rev. 40:980–989. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Gallay N, Dos Santos C, Cuzin L, Bousquet
M, Simmonet Gouy V, Chaussade C, Attal M, Payrastre B, Demur C and
Récher C: The level of AKT phosphorylation on threonine 308 but not
on serine 473 is associated with high-risk cytogenetics and
predicts poor overall survival in acute myeloid leukaemia.
Leukemia. 23:1029–1038. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhang J, Sun Q, Zhang Z, Ge S, Han ZG and
Chen WT: Loss of microRNA-143/145 disturbs cellular growth and
apoptosis of human epithelial cancers by impairing the MDM2-p53
feedback loop. Oncogene. 32:61–69. 2013. View Article : Google Scholar
|
|
39
|
Yang YK, Xi WY, Xi RX, Li JY, Li Q and Gao
YE: Micro-RNA-494 promotes cervical cancer proliferation through
the regulation of PTEN. Oncol Rep. 33:2393–2401. 2015.PubMed/NCBI
|
|
40
|
Zhao L, Liu W, Xiao J and Cao B: The role
of exosomes and 'exosomal shuttle microRNA' in tumorigenesis and
drug resistance. Cancer Lett. 356:339–346. 2015. View Article : Google Scholar
|
|
41
|
Won YS, Jeong JS, Kim SJ, Ju MH and Lee
SW: Targeted anti-cancer effect through microRNA-181a regulated
tumor-specific hTERT replacement. Cancer Lett. 356:918–928. 2015.
View Article : Google Scholar
|
|
42
|
Serpico D, Molino L and Di Cosimo S:
microRNAs in breast cancer development and treatment. Cancer Treat
Rev. 40:595–604. 2014. View Article : Google Scholar
|