Introduction
Liver cancer is the most prevalent cancer in the
clinic in China with a very high mortality rate of 26.04/100,000
cases. Treatment with conventional chemotherapeutics frequently
produces unsatisfactory results mainly due to the acquisition of
multidrug resistance (MDR) by the tumor cells. It has been
suggested that MDR is caused by the upregulation of permeability
P-glycoprotein (P-gp), which leads to enhanced capability of cancer
cells to pump chemotherapeutics out, thus preventing the
intracellular accumulation of drugs. In addition, other factors,
such as upregulation of anti-apoptotic proteins and abnormal
apoptotic pathway are also involved in MDR (1). However, in recent years, a large
number of epidemiological studies have revealed a close correlation
between insulin resistance (IR) and liver cancer IR (2–4), a
pathological condition in which insulin function is impaired in
peripheral target tissues. This has been associated with various
diseases including NIDDM, obesity, hypertension, dyslipidemia and
atherosclerotic cardiovascular disease (5). Recent epidemiological and clinical
evidence also links IR with cancer, including liver cancer
(6,7). As the target organ of insulin, a
variety of pathological processes, such as tumorigenesis and
inflammation, could lead to blockade in the insulin signaling
pathway in liver cells and cause IR (8), which has been shown to be an
independent risk factor affecting the survival and post-surgical
relapse of cancer patients (9).
However, the exact mechanisms of IR-induced tumor cell resistance
to chemotherapeutics are still poorly understood.
Liver cells contain a large number of endoplasmic
reticula (ER) for protein synthesis. It has been suggested that IR
could trigger various cellular responses, such as enhanced
oxidative stress and unfolded protein responses, and upregulation
of glucose regulated protein 78 (GRP78), to control damage and
restore normal ER functions and protein folding (10).
Our previous study demonstrated that IR may lead to
chemotherapeutic drug tolerance in liver cancer cells (11,12),
which could be caused by ER stress response (13). To further elucidate the mechanisms
of IR-induced resistance to chemotherapeutics in liver tumor cells,
the present study established an IR HepG2 cell line and
investigated the possible involvement of the PERK signaling
pathway. Our results demonstrated that IR can lead to liver cancer
cell resistance to various chemotherapeutic drugs, including
cisplatin. IR in HepG2 cells promoted the unfolded protein
response; increased the expression of ER chaperone GRP78;
phosphorylated PERK kinase to activate the PERK ER stress pathway;
significantly upregulated the expression of B-cell lymphoma 2
(Bcl-2) and P-gp, all of which ultimately inhibited the
caspase-3-dependent apoptosis pathway and promoted the survival of
liver cancer cells. Notably, this entire process failed to
significantly enhance the expression of CCAAT-enhancer-binding
protein homologous protein (CHOP), indicating that IR only
activated the unfolded protein response which is an early ER
protective stress reaction.
Therefore, we hypothesized that IR promoted liver
tumor cell tolerance to various chemotherapeutics through
activation of the ER stress response PERK pathway and the
upregulation of Bcl-2 and P-gp.
Materials and methods
Chemicals and antibodies
Insulin was purchased from Sigma-Aldrich (St. Louis,
MO, USA) and diluted to the appropriate concentrations using 2%
glacial acetic acid. The primers used to measure InsR, Glut-2 and
β-actin were synthesized by Takara Corporation (Japan). SYBR Premix
Ex Taq and Prime Script RT reagents were also purchased from
Takara. Cisplatin (DDP), 5-fluorouracil (5-FU), mitomycin C (MMC)
and vincristine sulfate (VCR) were obtained from Sigma-Aldrich. The
glucose content in the culture supernatant was measured with the
GOD-POD method by Randox Laboratories (Crumlin, UK) using a Hitachi
7600 automatic biochemical analyzer (Hitachi, Tokyo, Japan).
Antibodies for insulin receptor, glucose
transporter-2 and P-gp were purchased from Abcam (Cambridge, UK).
Antibodies for caspase-3, Bcl-2, PERK and β-actin were purchased
from Cell Signaling Technology (Boston, MA, USA). Antibodies for
phosphorylated PERK and CHOP were obtained from Santa Cruz
Biotechnology (Santa Cruz, CA, USA).
Cell culture and induction for IR
Human hepatocarcinoma HepG2 cells from the American
Type Culture Collection (ATCC; Manassas, VA, USA) were maintained
in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10%
fetal bovine serum (FBS) from HyClone (Logan, UT, USA) at 37°C with
5% CO2. IR was induced in HepG2 cells according to a
previously described method (12).
Briefly, the cells were incubated in serum-free DMEM for 6 h, and
then treated with 0.5 µmol/l insulin for 72 h. The resultant
cells were named as HepG2/IR cells. HepG2/IR cells were then
treated with PH (10 mmol/l) for 24 h to reverse IR, and the
resultant cells were called HepG2/IR/PH cells.
Glucose oxidase-peroxide enzyme (GOD-POD)
assay
HepG2 cells were stimulated with insulin as
described above and cultured in DMEM without phenol red for 24 h.
Culture supernatants were then collected for the GOD-POD assay to
measure glucose levels and calculate glucose consumption (12).
Periodic acid-Schiff (PAS) reaction
assay
The PAS assay was used to assess glycogen synthesis
(14). HepG2 and HepG2/IR cells
were cultured on cover slides and fixed in 10% formaldehyde. Cells
were then incubated with 1% periodate solution, washed, and then
incubated in the dark with Schiff reagent for 20 min at 60°C. The
reactions were terminated when the color of the dye had changed
from pink to purple-red. Optical density (OD) (as a measure of
glycogen synthesis) was analyzed using an AX80 optical microscope
(Olympus, Japan).
Ultrastructure
The morphological features were assessed using
electron microscopy (15). Briefly,
HepG2 and HepG2/IR cells were immobilized in 3% glutaraldehyde,
embedded and sectioned. The ultrastructure was observed using a JEM
1230 transmission electron microscope (Jeol, Japan).
MTT assays
HepG2, HepG2/IR and HepG2/IR/PH cells were harvested
and plated into 96-well plates at a density of 1×105
cells/ml, and then treated with 1.00–128.00 mg/l DDP, 12.50–1,600.0
mg/l 5-FU, 1.00–128.00 mg/l VCR and 0.08–10.00 mg/l mitomycin C
(MMC) (all from Sigma-Aldrich), respectively, for 44 and 68 h. Drug
cytotoxicity was then assessed using the MTT assay. OD was recorded
at 490 nm using a PowerWave X plate reader (Bio-Tek, USA). Cell
proliferation inhibition rates were calculated using the following
formula: Cell proliferation inhibition rate = [(OD control − OD
experiment)/OD experiment] × 100%. The half-maximal inhibitory
concentration (IC50) was also calculated as previously
reported (12). MTT was also used
in glucose consumption assays to calculate cell number thus that
glucose consumption could be normalized to cell number.
Flow cytometric analysis (FCM)
An Annexin V/propidium iodide (PI) double staining
assay from Invitrogen (Carlsbad, CA, USA) was used to determine
cell apoptosis. HepG2 and HepG2/IR cells were treated with DDP,
5-FU, VCR and MMC for 48 h, then collected and suspended in binding
buffer (400 µl) and incubated with Annexin V-FITC and PI for
0.5 h, and then suspended in binding buffer. The samples were
analyzed by a Coulter Epics XL flow cytometer (Beckman Coulter,
USA). The early apoptotic index was calculated as the percentage of
Annexin V-positive and PI-negative cells. The later period index
was assessed using the percentage of Annexin V-positive and
PI-positive cells.
For cell cycle analysis, 1×106 cells were
collected and fixed overnight in 70% ethanol at 4°C. Cells were
then washed with phosphate-buffered saline (PBS) and stained with
PI for 30 min at room temperature in the dark. Cell cycle analysis
was performed with a Coulter Epics XL flow cytometer (15).
Real-time quantitative RT-PCR
Total RNA was extracted from different cells using
TRIzol reagent from Invitrogen. All primers used for the PCR
amplification of β-actin, InsR and glut-2 genes were designed and
synthesized by Takara as shown in Table
I. For qRT-PCR, 400 ng of RNA was used for cDNA synthesis with
the PrimeScript™ RT Master Mix according to the manufacturer's
protocol [Perfect Real-Time and qPCR was performed with the SYBR
Premix Ex Taq (Tli RNase H Plus)] (both from Takara) using a
Rotor-Gene 3000 quantitative PCR amplifier (Corbett, Australia).
The PCR cycling conditions consisted of an initial denaturing phase
at 95°C for 30 sec followed by 35 cycles of 95°C for 5 sec and 60°C
for 30 sec. Data were analyzed with Rotor-Gene 6.0 software and
relative gene expression was expressed as 2−ΔΔCt = (Ct
target gene − Ct housekeeping gene)experimental group − (Ct target
gene − Ct housekeeping gene)control group. β-actin was used as the
housekeeping gene for all the calculations.
 | Table IPrimer sequences for qRT-PCR. |
Table I
Primer sequences for qRT-PCR.
| Gene | | Primer
sequence |
|---|
| InsR | 5′ primer |
5′-TACCCTTCAAGAGATGATT-3′ |
| 3′primer |
5′-CAGAAGAAGTGGTGAAGAC-3′ |
| Glut-2 | 5′primer |
5-TGGGCTGAGGAAGAGACTGT-3′ |
| 3′primer |
5′-AGAGACTGAAGGATGGCTCG-3′ |
| β-actin | 5′ primer |
5′-TGCTCCTCCTGAGCGCAAGTA-3′ |
| 3′ primer |
5′-CCACATCTGCTGGAAGGTGGA-3′ |
Western blotting
Briefly, HepG2 cells under different treatments were
lysed, and the protein concentrations were assessed using the
Bradford assay from Roche (Basel, Switzerland). Proteins were
separated using SDS-PAGE electrophoresis and transferred to
polyvinylidene difluoride (PVDF) membranes. The membranes were
blocked with non-fat milk and probed with primary antibodies
(anti-insulin receptor, anti-glut-2, anti-caspase3, anti-bcl-2,
anti-GRP78, anti-PERK, anti-p-PERK, anti-P-gp or anti-β-actin)
followed by IRDye 800CW or IRDye 700DX-conjugated secondary
antibodies from LI-COR Biosciences (Lincoln, NE, USA). Protein
bands were visualized using an Odyssey double-color infrared laser
imaging system (LI-COR). Relative expression was then calculated to
reflect true protein expression (average infrared fluorescence
intensity of the target protein/β-actin).
Statistical analysis
Data are expressed as means ± SD. Statistical
analysis was performed using the Student's t-test with SPSS 15.0
(SPSS, Inc., Chicago, IL, USA). P-value <0.05 was considered to
indicate a statistically significant result.
Results
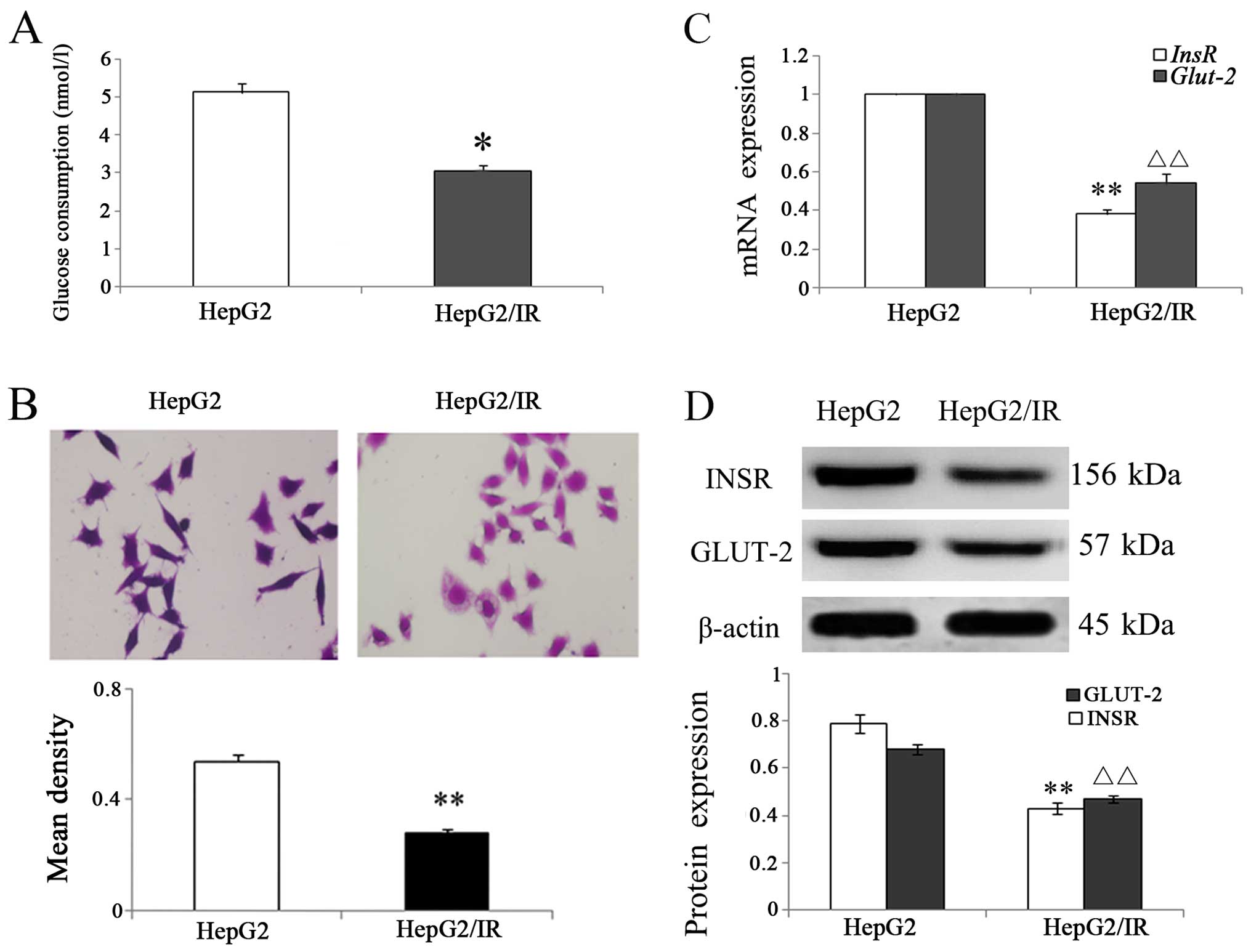
IR in HepG2/IR cells
IR was induced in HepG2 cells by incubation with 0.5
µmol/l insulin for 72 h to establish the HepG2/IR cell
model. GOD-POD assay and PAS staining were used to measure the
glucose consumption and glycogen synthesis. The relative glucose
consumption in the HepG2/IR cells was significantly decreased when
compared with the parental HepG2 cells (P<0.01; Fig. 1A), and PAS staining revealed that
the OD of intracellular glycogen in the HepG2/IR cells was
decreased 45.10% compared with the HepG2 cells (Fig. 1B). These results indicated that IR
in HepG2/IR cells is associated with decreased glucose consumption
and glycogen synthesis.
Results from the qRT-PCR analysis showed that the
InsR mRNA level in HepG2/IR cells decreased to 62.00% and
Glut-2 mRNA decreased to 58.23% of the levels in the
parental HepG2 cells, respectively (Fig. 1C). Western blot analysis further
confirmed the data from qRT-PCR with both INSR and GLUT-2 protein
levels decreased (Fig. 1D).
MDR in HepG2/IR cells
HepG2/IR, HepG2/IR/PH and HepG2 cells were treated
with DDP (1–64 mg/l), 5-FU (12.5–1,600 mg/l), VCR (1–128 mg/l) and
MMC (0.08–10 mg/l) for 48 and 72 h. MTT assay showed that cell
proliferation was inhibited in all three groups, but HepG2/IR cells
exhibited the least sensitivity towards the chemotherapeutics
followed by HepG2/IR/PH and HepG2 cells. The IC50 values
of HepG2/IR cells for DDP at 48 and 72 h were 1.66 and 1.59 times
that of the parental HepG2 cells. However, after IR reversal with
10 mmol/l PH, the IC50 values of HepG2/IR/PH cells were
0.69 and 0.74 times that of the HepG2 cells, respectively. Similar
trends, as shown in Fig. 2A, were
also observed among the three different cell lines towards 5-FU,
VCR and MMC. When cells were treated with different
chemotherapeutics at concentrations of IC50 values for
48 h, cell apoptosis was analyzed with flow cytometry using Annexin
V/PI double staining. The results were consistent with data from
the MTT assay (Fig. 2B). When HepG2
and HepG2/IR cells were treated with DDP at 16 mg/l for 48 h, the
hypodiploid peak was significantly reduced from 8.3% in HepG2 to
0.4% in the HepG2/IR cells (Fig.
2C). At the same time, western blot analysis showed that the
expression of apoptotic protein caspase-3 decreased to 33.16% in
the HepG2/IR cells compared to that parental levels in the HepG2
cells (Fig. 2D). Further analysis
with TEM revealed cytoplasmic vacuolization and other morphological
changes in the HepG2 cells associated with apoptosis. In the
HepG2/IR cells, only a small number of vacuoles were observed
(Fig. 2E).
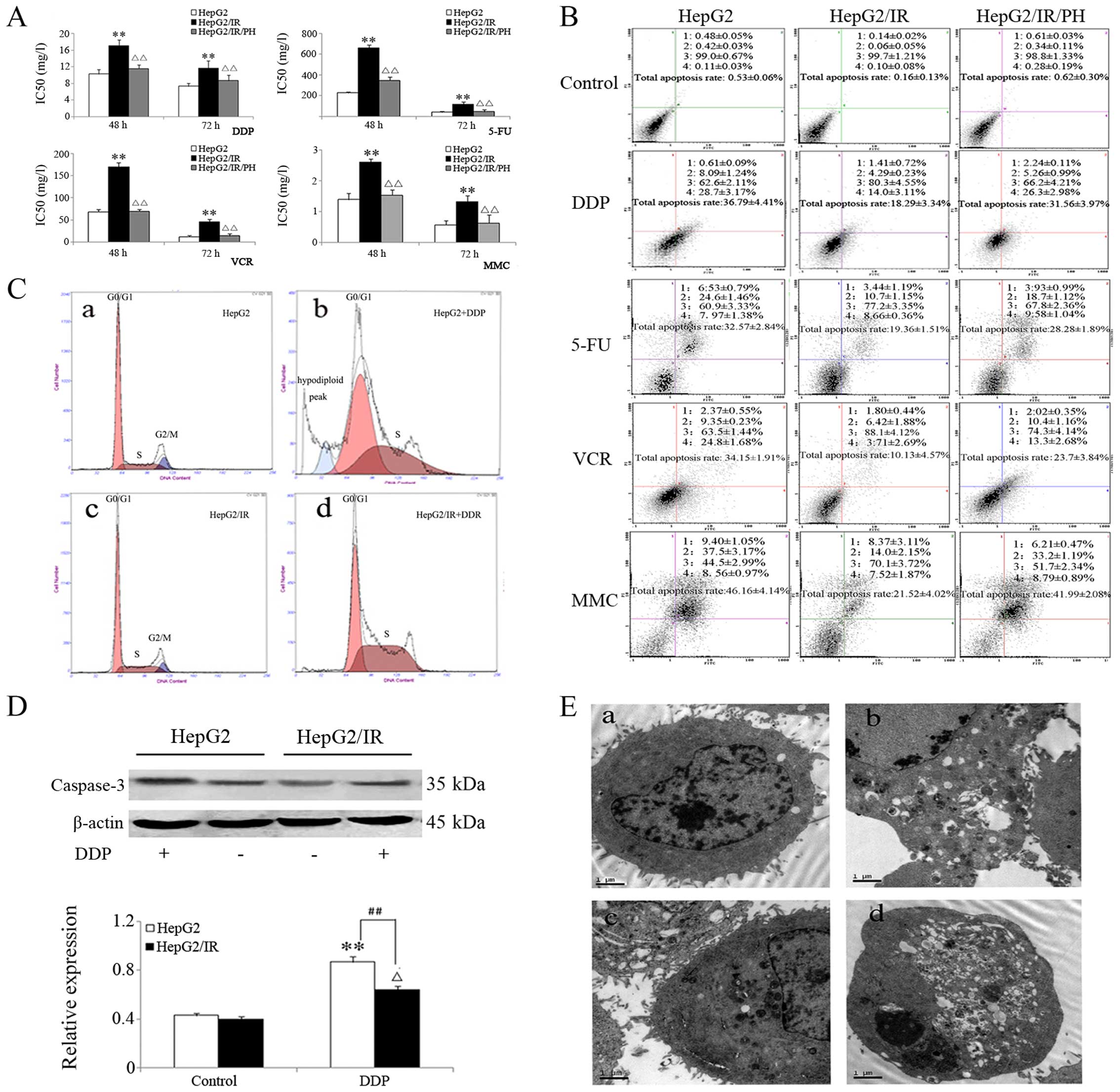 | Figure 2Analysis of multidrug resistance in
the HepG2/IR cells. (A) Inhibition of proliferation in HepG2/IR,
HepG2/IR/PH and HepG2 cells by various chemotherapeutic drugs at 48
and 72 h using the MTT assay. (B) Cell apoptosis at 48 h following
treatment with different chemotherapeutic drugs using Annexin/PI
double staining. (C) Cell cycle analysis of HepG2/IR and HepG2
cells at 48 h following DDP treatment. a, HepG2 cells; b, HepG2
cells treated with DDP for 48 h; c, HepG2/IR cells; and d, HepG2/IR
cells treated with DDP for 48 h. As indicated in the image, HepG2
cells treated with DDP showed a clear hypodiploid peak, which was
absent from the HepG2/IR cells. (D) Western blot analysis of
caspase-3 levels in the HepG2/IR and HepG2 cells before and after
DDP treatment (upper panel). Lower panel shows the densitometric
data from the western blotting. (E) Morphological changes
associated with apoptosis in the HepG2/IR and the HepG2 cells after
DDP treatment under electron microscopy (original magnification,
×10,000): a, HepG2 cells; b, HepG2 cells treated with DDP; c,
HepG2/IR cells; and d, HepG2/IR cells treated with DDP. All
experiments were repeated three times, and data are presented as
mean ± SD of triplicate experiments. *, ∆P<0.05;
**, ∆∆, ##P<0.01. |
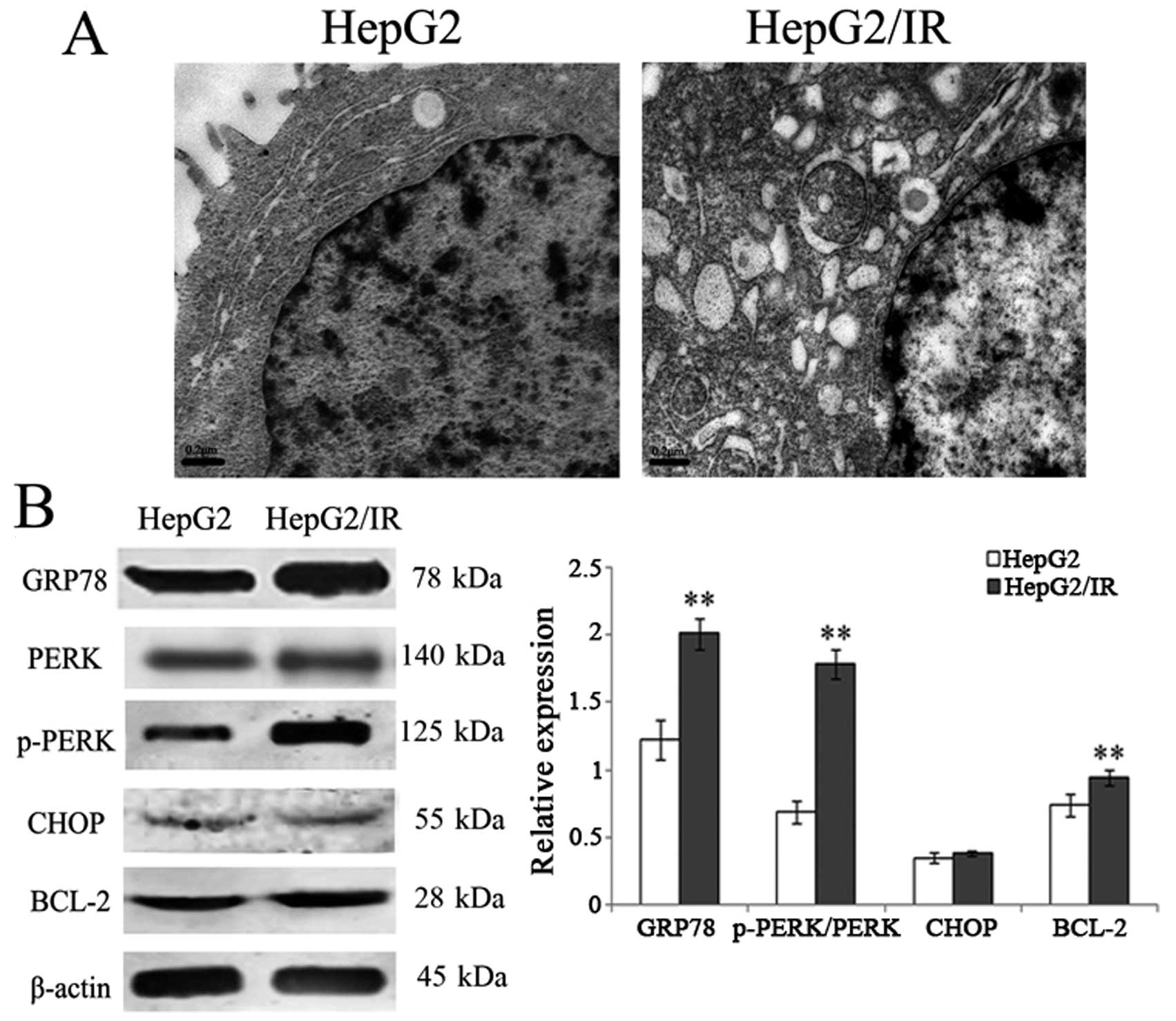
Activation of the PERK signaling pathway
in the HepG2/IR cells
To investigate ultrastructural changes in HepG2
cells after DDP treatment, cells were observed under an electron
microscope. The results showed that the ultrastructure
significantly changed in the HepG2/IR cells, such as ER expansion,
ER degranulation, mitochondrial swelling, increased cell surface
protrusions, and enlarged cell surface protrusions (Fig. 3A), indicating ER stress responses in
the HepG2/IR cells.
To further elucidate the relationship between ER
stress responses and apoptosis resistance in HepG2/IR, we further
examined the proteins involved in the ER unfolded protein response,
including chaperone protein GRP78/Bip, PERK and phosphorylated-PERK
which are important in the ER stress-related PERK pathway and CHOP
which is important in the ER stress-related apoptosis pathway. In
addition, we also observed a change in the expression of apoptosis
resistance of the Bcl-2 protein. The results showed that the
expression level of GRP78 increased 64.75% and the p-PERK/PERK
ratio increased by 1.59-fold in the HepG2/IR cells compared to the
parental HepG2 cells. Notably, no significant change in CHOP
expression was observed in the HepG2/IR cells. Furthermore, the
expression of Bcl-2 also increased 27.03% (Fig. 3B).
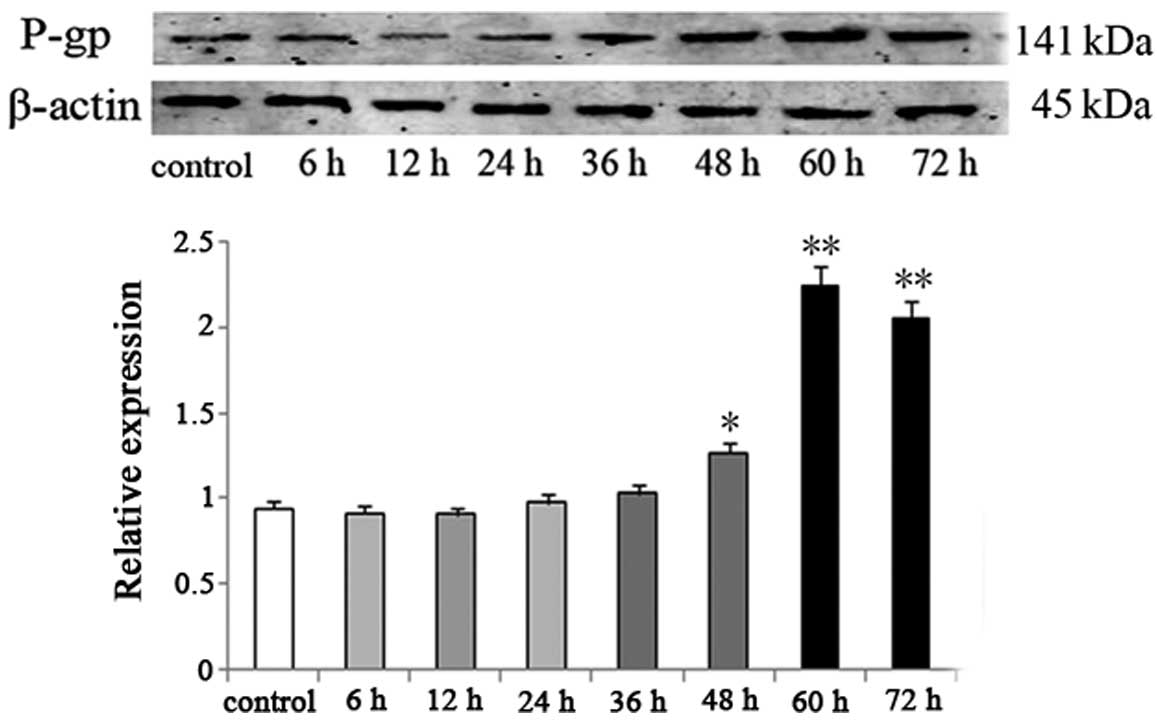
Upregulation of P-gp in the HepG2 cells
during the induction of IR
P-gp has a drug reverse transport function, which
can lead to high expression of the MDR gene in tumor cells. It is
known that IR-induced hyperinsulinemia promotes the expression of
P-gp, resulting in anticancer drug resistance in tumor cells
(16). In addition, P-gp also plays
protective roles as a chaperone for the transport of unfolded
proteins during the ER stress reaction (17,18).
During the process of IR induction in the HepG2 cells, the
expression of MDR protein P-gp was significantly upregulated. In
comparison with the HepG2 cells, P-gp expression in the HepG2/IR
cells increased 33.7% at 48 h (P<0.05) and continued to increase
until reaching a peak at >100% at ~60 h (P<0.01) (Fig. 4).
Discussion
Hepatocellular carcinoma (HCC) is the fifth most
common malignancy and the third-leading cause of cancer-related
death worldwide. Since HCC is often diagnosed at an advanced stage
when it is no longer amenable to curative surgery, chemotherapy is
the main treatment option in the clinic (19–21).
However, the efficacy of first-line chemotherapeutics commonly used
in the clinic for HCC treatment, such as doxorubicin and cisplatin,
is not ideal (22). Thus, it has
become imperative to elucidate the mechanisms underlying the
resistance of HCC towards chemotherapeutics in order to find a more
effective treatment strategy.
Liver is an insulin sensitive organ and plays a
critical role in the regulation of glucose metabolism and whole
body energy homeostasis. The sensitivity of liver cells towards
insulin can be reduced to create an IR condition when the insulin
signaling pathway is blocked under pathological conditions
(8). Mounting evidence suggests
that IR is closely correlated with the initiation, progression and
prognosis of various malignancies (2). It has been suggested that IR is a high
risk factor for liver tumorigenesis and post-surgery relapse
(23,24). However, the role of IR in
hepatocarcinoma has not been fully understood.
Recent studies suggest that the activation of the
PI3K/Akt signaling pathway, the main pathway involved with IR,
could cause tumor tolerance to various chemotherapeutic drugs
(25). Alleviation of IR has been
shown to increase patient sensitivity to chemotherapeutic drugs,
reducing the progression of tumors and improving patient prognosis
(26–28), indicating a possible correlation
between IR and tumor tolerance to chemotherapeutics. In the present
study, a comparison between high-dose insulin-induced IR HepG2/IR
cells and parental HepG2 cells indicated that IR conferred tumor
resistance to various chemotherapeutic drugs, such as cisplatin,
5-fluorouracil, vincristine and mitomycin. In addition, this
resistance to chemotherapeutics was reversed by PH, an insulin
sensitizer. Our results suggested that IR decreased the sensitivity
of HepG2 tumor cells towards chemotherapeutic drugs. Our results
also showed that the hypodiploid peak and the expression of
apoptotic protein caspase-3 in the HepG2/IR cells were
significantly reduced after treatment with DDP, indicating that
IR-induced inhibition of caspase-3-mediated cell apoptosis could be
involved in the occurrence of resistance to chemotherapeutics.
Previous studies suggest that IR triggers endoplasmic reticulum
(ER) stress response (29–31). Imbalance in ER homeostasis could
promote the accumulation of unfolded proteins to induce ER stress
response, which is critical for cell survival. In contrast, chronic
and severe ER stress response could also activate the apoptosis
signal transduction pathway. Our ultrastructural data also showed
signs of ER stress response in HepG2 cells after treatment with
chemotherapeutics.
Emerging evidence suggests that ER stress is
important in the drug resistance of tumor cells due to its
involvement in cell survival. GRP78/Bip, an ER stress chaperone,
was shown to help protein folding and transport during ER
stress-induced unfolded protein response. External stress could
induce a high expression of GRP78/Bip to maintain ER homeostasis
and cell survival (32,33). In the present study, the levels of
GRP78/Bip, phosphorylated PERK and Bcl-2 in the HepG2/IR cells were
significantly increased, which was consistent with previous
observations. PERK, a member of the ER I transmembrane protein
family, is a major player in the ER stress pathway. Under normal
circumstances, PERK is in an inactive state by binding to GRP78.
Accumulation of unfolded proteins can facilitate the dissociation
of PERK from GRP78 which in turn causes the auto-phosphorylation of
PERK. Phosphorylated PERK helps to block protein synthesis and to
upregulate the expression of Bcl-2, leading to the survival of
cells (34–36). In contrast, CHOP/GADD153 could be
upregulated to start the ER apoptosis pathway when the accumulated
misfolded protein from long-term ER stress exceeds the capacity of
the unfolded protein response (37). However, our data showed that no
significant change in CHOP expression was observed between HepG2/IR
and the parental HepG2 cells, suggesting that the development of IR
only promoted the protective ER stress response and the activation
of the PERK pathway was one of the mechanisms leading to the
tolerance to chemotherapeutic drugs of the IR liver tumor
cells.
P-gp, a drug transporter via ATP hydrolysis, has
been linked with multidrug resistance (38). P-gp protein also functions as an ER
chaperone protein to participate in the ER stress response by
directly or indirectly transporting unfolded or misfolded protein
and it could also provide protection for cells through inhibition
of caspase-dependent cell apoptosis (17,18).
In the present study, the expression levels of the P-gp protein
were studied in the HepG2 cells at 6, 12, 24, 48 and 72 h post
high-dose insulin exposure. Our results showed that the expression
level of P-gp was gradually increased during IR induction and
peaked at ~72 h, indicating that P-gp could be involved in the
acquisition of drug tolerance in HepG2/IR cells.
In summary, our data demonstrated that multiple
mechanisms are involved with the development of chemotherapeutic
drug tolerance in HepG2/IR liver cancer cells, such as ER unfolded
protein stress response, activation of the PERK signaling pathway,
upregulation of P-gp and anti-apoptotic protein Bcl-2, leading to
the inhibition of the chemotherapeutic-induced caspase-3 pathway.
However, further studies are needed to elucidate the full spectrum
of the regulatory pathways of IR-mediated multidrug tolerance in
cancer cells.
Acknowledgments
The present study was funded by The Project of the
Youth Science and Technology Fund of Gansu Province of China (grant
no. 1308RJYA055), the Scientific Research Initiation Funds for
Doctor of the Second Hospital Affiliated to Lanzhou University, and
the Program for Changjiang Scholars and Innovative Research Team in
University (PCSIRT: IRT1137).
References
|
1
|
Huesker M, Folmer Y, Schneider M, Fulda C,
Blum HE and Hafkemeyer P: Reversal of drug resistance of
hepatocellular carcinoma cells by adenoviral delivery of anti-MDR1
ribozymes. Hepatology. 36:874–884. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nicolucci A: Epidemiological aspects of
neoplasms in diabetes. Acta Diabetol. 47:87–95. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Donadon V, Balbi M, Perciaccante A,
Casarin P and Zanette G: Insulin resistance and hyperinsulinemia in
patients with chronic liver disease and hepatocellular carcinoma.
Clin Med Insights Endocrinol Diabetes. 2:25–33. 2009.
|
|
4
|
Wang YG, Wang P, Wang B, Fu ZJ, Zhao WJ
and Yan SL: Diabetes mellitus and poorer prognosis in
hepatocellular carcinoma: A systematic review and meta-analysis.
PLoS One. 9:e954852014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
DeFronzo RA and Ferrannini E: Insulin
resistance. A multifaceted syndrome responsible for NIDDM, obesity,
hypertension, dyslipidemia, and atherosclerotic cardiovascular
disease. Diabetes Care. 14:173–194. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tsugane S and Inoue M: Insulin resistance
and cancer: Epidemiological evidence. Cancer Sci. 101:1073–1079.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Farrell G: Insulin resistance, obesity,
and liver cancer. Clin Gastroenterol Hepatol. 12:117–119. 2014.
View Article : Google Scholar
|
|
8
|
Leclercq IA, Da Silva Morais A, Schroyen
B, Van Hul N and Geerts A: Insulin resistance in hepatocytes and
sinusoidal liver cells: Mechanisms and consequences. J Hepatol.
47:142–156. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Feng YH, Lin CY, Huang WT, Wu CL, Fang JL
and Tsao CJ: Diabetes mellitus impairs the response to
intra-arterial chemotherapy in hepatocellular carcinoma. Med Oncol.
28:1080–1088. 2011. View Article : Google Scholar
|
|
10
|
Quan X, Wang J, Liang C, Zheng H and Zhang
L: Melatonin inhibits tunicamycin-induced endoplasmic reticulum
stress and insulin resistance in skeletal muscle cells. Biochem
Biophys Res Commun. 463:1102–1107. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jong CJ, Ito T, Azuma J and Schaffer S:
Taurine depletion decreases GRP78 expression and downregulates
Perk-dependent activation of the unfolded protein response. Adv Exp
Med Biol. 803:571–579. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li LJ, Li GD, Wei HL, Chen J, Liu YM, Li
F, Xie B, Wang B and Li CL: Insulin resistance reduces sensitivity
to Cis-platinum and promotes adhesion, migration and invasion in
HepG2 cells. Asian Pac J Cancer Prev. 15:3123–3128. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li L, Li G, Wei H, Sun J, Chen J, Xie B,
Wang B, Gu J, Li C, Tian B, et al: The endoplasmic reticulum stress
response is associated with insulin resistance-mediated drug
resistance in HepG2 cells. Neoplasma. 62:180–190. 2015. View Article : Google Scholar
|
|
14
|
Kurakula K, Hamers AA, van Loenen P and de
Vries CJ: 6-Mercaptopurine reduces cytokine and Muc5ac expression
involving inhibition of NFκB activation in airway epithelial cells.
Respir Res. 16:732015. View Article : Google Scholar
|
|
15
|
Li CL, Wei HL, Chen J, Wang B, Xie B, Fan
LL and Li LJ: Arsenic trioxide induces autophagy and antitumor
effects in Burkitt's lymphoma Raji cells. Oncol Rep. 32:1557–1563.
2014.PubMed/NCBI
|
|
16
|
Zhou G and Kuo MT: NF-kappaB-mediated
induction of mdr1b expression by insulin in rat hepatoma cells. J
Biol Chem. 272:15174–15183. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ledoux S, Yang R, Friedlander G and
Laouari D: Glucose depletion enhances P-glycoprotein expression in
hepatoma cells: Role of endoplasmic reticulum stress response.
Cancer Res. 63:7284–7290. 2003.PubMed/NCBI
|
|
18
|
Morand JP, Macri J and Adeli K: Proteomic
profiling of hepatic endoplasmic reticulum-associated proteins in
an animal model of insulin resistance and metabolic dyslipidemia. J
Biol Chem. 280:17626–17633. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Marrero JA, Kudo M and Bronowicki JP: The
challenge of prognosis and staging for hepatocellular carcinoma.
Oncologist. 15(Suppl 4): S23–S33. 2010. View Article : Google Scholar
|
|
20
|
Johnson PJ: Systemic chemotherapy of liver
tumors. Semin Surg Oncol. 19:116–124. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Roberts LR: Sorafenib in liver cancer -
just the beginning. N Engl J Med. 359:420–422. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Thorgeirsson SS and Grisham JW: Molecular
pathogenesis of human hepatocellular carcinoma. Nat Genet.
31:339–346. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Nagaoki Y, Hyogo H, Aikata H, Tanaka M,
Naeshiro N, Nakahara T, Honda Y, Miyaki D, Kawaoka T, Takaki S, et
al: Recent trend of clinical features in patients with
hepatocellular carcinoma. Hepatol Res. 42:368–375. 2012. View Article : Google Scholar
|
|
24
|
Huo TI, Wu JC, Lui WY, Lee PC, Huang YH,
Chau GY, Tsay SH, Chang FY and Lee SD: Diabetes mellitus is a
recurrence-independent risk factor in patients with hepatitis B
virus-related hepatocellular carcinoma undergoing resection. Eur J
Gastroenterol Hepatol. 15:1203–1208. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Oki E, Baba H, Tokunaga E, Nakamura T,
Ueda N, Futatsugi M, Mashino K, Yamamoto M, Ikebe M, Kakeji Y, et
al: Akt phosphorylation associates with LOH of PTEN and leads to
chemoresistance for gastric cancer. Int J Cancer. 117:376–380.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kourelis TV and Siegel RD: Metformin and
cancer: New applications for an old drug. Med Oncol. 29:1314–1327.
2012. View Article : Google Scholar
|
|
27
|
Oliveras-Ferraros C, Cufí S,
Vazquez-Martin A, Menendez OJ, Bosch-Barrera J, Martin-Castillo B,
Joven J and Menendez JA: Metformin rescues cell surface major
histocompatibility complex class I (MHC-I) deficiency caused by
oncogenic transformation. Cell Cycle. 11:865–870. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Shen Z, Ye Y, Bin L, Yin M, Yang X, Jiang
K and Wang S: Metabolic syndrome is an important factor for the
evolution of prognosis of colorectal cancer: Survival, recurrence,
and liver metastasis. Am J Surg. 200:59–63. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yoshiuchi K, Kaneto H, Matsuoka TA, Kohno
K, Iwawaki T, Nakatani Y, Yamasaki Y, Hori M and Matsuhisa M:
Direct monitoring of in vivo ER stress during the development of
insulin resistance with ER stress-activated indicator transgenic
mice. Biochem Biophys Res Commun. 366:545–550. 2008. View Article : Google Scholar
|
|
30
|
Misra UK and Pizzo SV: Up-regulation of
GRP78 and antiapoptotic signaling in murine peritoneal macrophages
exposed to insulin. J Leukoc Biol. 78:187–194. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kaneto H, Nakatani Y, Kawamori D,
Miyatsuka T, Matsuoka TA, Matsuhisa M and Yamasaki Y: Role of
oxidative stress, endoplasmic reticulum stress, and c-Jun
N-terminal kinase in pancreatic beta-cell dysfunction and insulin
resistance. Int J Biochem Cell Biol. 38:782–793. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Al-Rawashdeh FY, Scriven P, Cameron IC,
Vergani PV and Wyld L: Unfolded protein response activation
contributes to chemoresistance in hepatocellular carcinoma. Eur J
Gastroenterol Hepatol. 22:1099–1105. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lin JA, Fang SU, Su CL, Hsiao CJ, Chang
CC, Lin YF and Cheng CW: Silencing glucose-regulated protein 78
induced renal cell carcinoma cell line G1 cell-cycle arrest and
resistance to conventional chemotherapy. Urol Oncol.
32:29.e1–29.e11. 2014. View Article : Google Scholar
|
|
34
|
Nagelkerke A, Bussink J, van der Kogel AJ,
Sweep FC and Span PN: The PERK/ATF4/LAMP3-arm of the unfolded
protein response affects radioresistance by interfering with the
DNA damage response. Radiother Oncol. 108:415–421. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Atkins C, Liu Q, Minthorn E, Zhang SY,
Figueroa DJ, Moss K, Stanley TB, Sanders B, Goetz A, Gaul N, et al:
Characterization of a novel PERK kinase inhibitor with antitumor
and antiangiogenic activity. Cancer Res. 73:1993–2002. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Jiang HY and Wek RC: Phosphorylation of
the α-subunit of the eukaryotic initiation factor-2 (eIF2α) reduces
protein synthesis and enhances apoptosis in response to proteasome
inhibition. J Biol Chem. 280:14189–14202. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Feng J, Chen X and Sun X, Wang F and Sun
X: Expression of endoplasmic reticulum stress markers GRP78 and
CHOP induced by oxidative stress in blue light-mediated damage of
A2E-containing retinal pigment epithelium cells. Ophthalmic Res.
52:224–233. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Gagliano T, Gentilin E, Benfini K, Di
Pasquale C, Tassinari M, Falletta S, Feo C, Tagliati F, Uberti ED
and Zatelli MC: Mitotane enhances doxorubicin cytotoxic activity by
inhibiting P-gp in human adrenocortical carcinoma cells. Endocrine.
47:943–951. 2014. View Article : Google Scholar : PubMed/NCBI
|