Introduction
Esophageal carcinoma is the eighth most common
cancer worldwide, with 482,000 new cases in 2008, and is the sixth
most common cause of cancer-related deaths with 406,000 deaths
(1). China has one of the highest
incidences of esophageal carcinoma in the world. The predominant
type of esophageal carcinoma is esophageal squamous cell carcinoma
(ESCC), and the crude mortality rate in 2004–2005 was 15.2/100,000
(2). Despite advances in surgery
and chemotherapy, the majority of patients have a poor prognosis
since they are initially diagnosed in an advanced stage of disease,
and frequently present with recurrence and are not responsive to
further chemotherapy (3). The ESCC
mortality and incidence rates are similar, and the 5-year overall
survival rate is less than 15% (4).
These facts emphasize the need to develop new effective therapies
for ESCC.
One of the most encouraging candidates is melanoma
differentiation-associated gene-7 (mda-7), also known as
interleukin-24 (IL-24), which was identified by subtraction
hybridization of cDNA libraries prepared from terminally
differentiated human melanoma cells treated with human fibroblast
interferon and mezerein (5). IL-24
is considered to be a tumor suppressor due to its ability to
selectively kill and induce apoptosis in a wide range of cancer
cells, both in vitro and in vivo, with minimal
toxicity to normal cells (6). Of
importance, IL-24 also exerts immunomodulatory effects (7), displays anti-angiogenic properties,
and enhances tumor cell sensitivity to chemotherapy agents and
radiotherapy (6). Dr P.D. Fisher,
the discoverer of IL-24, suggested that it may be the long
sought-after and proverbial ʻmagical bulletʼ for a diverse set of
cancers (8). Although the
biological activity of IL-24 was identified by adenovirus
expressing IL-24 (Ad-IL-24), overexpression of recombinant human
IL-24 (rhIL-24) protein in tumor cells is the most critical factor,
and the recombinant protein has advantages over Ad with regard to
tumor treatment, such as lower cytotoxicity, higher activity and
specificity, and easier usage. The rhIL-24 protein is usually
expressed in different expression systems, such as bacteria
(9) and mammalian cells (7), but seldom in yeast (10) or insects (11). However, secreted recombinant human
IL-24 (srhIL-24) purified from mammalian cell supernatant has the
highest antitumor activity at the lowest concentration in diverse
cancer cells, such as melanoma (12–14),
pancreatic (15), lung (14,16),
prostate (14), cervical (14) and breast cancer (17). srhIL-24 has many functions apart
from its inhibitory activity in tumor cells, similar to Ad-IL-24.
Caudell et al (7)
demonstrated in human peripheral blood mononuclear cells that
srhIL-24 plays a role as a pro-Th1 cytokine and induces secretion
of IL-6, IFN-γ, TNF-α, IL-1β, IL-12 and GM-CSF, which may evoke an
antitumor immune response. srhIL-24 has potent anti-angiogenic
activity in vitro and in vivo (16). In addition, srhIL-24 protein
modulates the sensitivity of melanoma cells to the EGFR inhibitor
erlotinib (18) and temozolomide
(13) similar to Ad-IL-24,
suggesting that a combination treatment of rhIL-24-mediated
molecular therapy may be a novel treatment strategy for human
melanoma. Secreted rhIL-24 by normal cells inhibits invasion and
sensitizes specific cancer cells containing functional IL-20R to
radiation (19).
With regard to ESCC, Ma et al (20) reported that Ad-IL-24 enhanced
agent-mediated cytotoxicity in certain cell lines (TE-1, TE-2,
TE-10, TE-11, YES-2, YES-4, YES-5, YES-6 and T.Tn cells), and the
combination with 5-fluorouracil, cisplatin, mitomycin and etoposide
produced greater anti-tumor effects than monotherapy. Our previous
study showed that the human IL-24 peptide created by
computer-guided design contributed to suppression of proliferation
in ESCC Eca-109 cells (21). It is
not clear whether rhIL-24 protein could inhibit ESCC cells and how
it functions. In the present study, we prepared a stable
site-specific integrated cell line, Flp-In™CHO/IL-24 (FCHO/IL-24),
with high expression levels of secreted rhIL-24 and tested the
antitumor activity of srhIL-24. We identified that srhIL-24 had
high potent antitumor activity in ESCC Eca-109 cells in
vitro and in vivo and activated STAT3, which may be
mediated by the receptor pathway. The data strongly suggest that
srhIL-24 may represent a potential treatment for ESCC.
Materials and methods
Reagents
Anti-IL-24 (AF1965 and MAB1965) and IL-20R
antibodies (MAB11762, AF1788 and MAB2770) were purchased from
R&D Systems, China (Shanghai, China). The pSTAT3 (Tyr705)
rabbit antibody (D3A7) and anti-STAT3α rabbit antibody (D1A5) were
purchased from Cell Signaling Technology (Beverly, MA, USA). The
anti-PCNA (ZM-0213), Ki-67 (ZA-0502) and secondary antibodies were
purchased from Zhongshan Golden Bridge Biotechnology (Beijing,
China). The anti-CD34 antibody (BA0532) was purchased from Boster
Biological Technology Ltd. (Wuhan, China). The anti-His6
antibody was a product of Beijing B&M Biotechnology (Beijing,
China). The IL-24 ELISA kit (F01531) was purchased from Xitang
Biotechnology (Shanghai, China). The Flp-In system plasmids
(pCDNA5/FRT and pOG44) and pCEP4-IL-24 containing IL-24 full cDNA
were kindly gifted by Dr Zhiwei Sun of the Beijing Institute of
Biotechnology and Dr Jean-Christophe Renauld from Luding Institute
for Cancer Research in Belgium, respectively.
Cell culture
Flp-In™CHO (FCHO) cells obtained from Invitrogen
(Carlsbad, CA, USA) were maintained in F12 nutrient mixture medium
supplemented with 10% fetal bovine serum (FBS), 100 µg/ml
penicillin/streptomycin and 100 µg/ml Zeocin. Human melanoma
A375 cells and lung cancer A549 cells were purchased from the
Institute of Basic Medical Sciences, Chinese Academy of Medical
Sciences. ESCC Eca-109 cells, the human embryo lung fibroblast cell
line HEL and human embryonic kidney 293 cells (HEK293) were kindly
provided by Dr XiaoFei Zheng of the Beijing Institute of Radiation
Medicine. A375, A549, Eca-109, HEL and HEK293 cells were checked by
short tandem repeat (STR) analysis at the Beijing Microread Gene
Technology (Beijing, China) and maintained in Dulbecco's modified
Eagle's medium (DMEM) supplemented with 10% FBS, 100 µg/ml
penicillin/streptomycin, 2 mmol/l L-glutamine and HEPES buffer.
Production of secreted rhIL-24
protein
The full-length cDNA of IL-24 with an additional
His6-tag at the COOH-terminus was cloned into the
pcDNA™5/FRT. The recombinant plasmid pcDNA5/FRT-IL-24 was
co-transfected with pOG44 into FCHO cells using the Lipofectamine
2000 method. The stable cells, FCHO/IL-24, were selected with 500
µg/ml hygromycin and identified by western blotting. Three
other random-integrated cell lines (HEK293/pSecTag2A-IL-24,
HEK293/pcDNA3.1myc/His-IL-24 and HEK293/pCEP4-IL-24) were created
by transfecting the plasmids containing the full-length cDNA of
IL-24 (pSec-Tag2A-IL-24, pcDNA3.1myc/His-IL-24 and pCEP4-IL-24)
into HEK293 cells, respectively. The stable cell lines were
isolated by hygromycin (400 µg/ml) or G418 (600
µg/ml). To identify and compare the expression level of
srhIL-24, four types of engineered cells were seeded in 100-mm
plates (1.5×106) in 10 ml DMEM, respectively. The
supernatant was collected at 1–4 days and assayed via ELISA. To
purify the srhIL-24 protein on a large scale, FCHO/IL-24 cells were
grown to 90% confluency in 150-mm tissue culture plates for 72 h.
The supernatant was collected, centrifuged to remove cell debris,
supplemented with protease inhibitors [1 µg/ml leupeptin, 1
µg/ml pepstatin and 0.5 mmol/l phenylmethylsulphonyl
fluoride (PMSF)], mixed with Ni-NTA slurry and incubated at 4°C
overnight. The Ni-NTA slurry-containing supernatant was allowed to
pass through a column to collect the beads. The beads were washed
with binding buffer (20 mmol/l sodium phosphate, 0.5 mol/l NaCl, 20
mmol/l imidazole, pH 7.4), and rhIL-24 was eluted by elution buffer
(250 mmol/l imidazole in binding buffer). The eluted fractions
identified as containing secreted rhIL-24 by western blotting were
pooled, desalted and concentrated. The final protein concentration
was determined by ELISA. The size of rhIL-24 was determined by
western blotting.
Western blotting
Cells lysates were treated with RIPA lysis buffer
and the protein concentrations were determined with the Pierce BCA
protein assay kit (Thermo Scientific, Waltham, MA, USA). Total
protein (20–50 µg) or 50 µl supernatant was separated
by 15% SDS-PAGE and transferred to a nitrocellulose membrane. After
incubation in 5% non-fat milk solution, the membranes were
incubated in the anti-IL-24 antibody (1:400; AF1965) or
anti-His6-tag antibody (1:1,000) at 4°C overnight. The
activation of STAT3 in tumor cells was detected using the
anti-pSTAT3 antibody (1:1,000) and anti-STAT3α (1:1,000) antibody.
The proteins were visualized on Kodak X-ray film (Rochester, NY,
USA) by application of the enhanced chemiluminescence western
blotting detection system (Thermo Scientific).
ELISA
The ELISA reaction to detect rhIL-24 was conducted
in 96-well plates according to the manufacturer's instructions.
Briefly, samples (100 µl) were diluted, added to 96-well
plates and incubated at 37°C for 2 h. The plate was reacted with a
biotinylated antibody against IL-24 for 1 h at 37°C and then
HRP-streptavidin for 30 min at room temperature. The reaction was
developed with the addition of TMB peroxidase substrate and stopped
with 1 N H2SO4. The optical density (OD)
values were read on a Bio-Rad microplate reader Model 550
(Hercules, CA, USA) at 450 nm. The concentration of IL-24 was
determined via comparison with a standard curve.
Cell viability assay
Cell viability was assessed by MTT assay. Cells were
seeded in 96-well plates (2,500 cells/well) for 24 h at 37°C and
treated with purified rhIL-24 at different final concentrations (0,
1, 5, 10, 20 and 50 ng/ml). On day 4 after treatment, the medium
was removed and MTT was added to each well. The cells were
maintained at 37°C for 4 h; then 150 µl of dimethyl
sulfoxide (DMSO) was added to each well and mixed thoroughly. The
absorbance was read on a Bio-Rad microplate reader Model 550 at 490
nm. MTT absorbance of the untreated control cells was set to 1 to
calculate the relative number of viable cells. The experiments were
repeated at least three times to ensure reproducibility and
statistical significance.
Clone formation assay
Cells (Eca-109, A375, A549 and HEL) were seeded at
400 cells/10-cm dish in triplicate and treated with purified
rhIL-24 at final different concentrations (0, 1, 5, 10, 20 and 50
ng/ml) 24 h later. After 2 weeks of incubation, the clones were
fixed with 4% formaldehyde, stained with Giemsa and colonies of
>50 cells were enumerated.
Apoptosis assay
Cell apoptosis was detected via the Annexin V
binding assay and FACS analysis. Cells (Eca-109, A375, A549 and
HEL) were seeded at 4.5×104 cells/well in 6-well plates
and treated with purified rhIL-24 at different final concentrations
(0, 1, 10 and 40 ng/ml) on the following day. On day 4 after
treatment, the cells were stained with FITC-labeled Annexin V and
PI according to the manufacturer's instructions (KGA105-50; Annexin
V-FITC apoptosis detection kit; Nanjing KeyGen Biotech, Nanjing,
China) and FACS assay was performed immediately after staining. For
the xenograft tumor tissue, the apoptosis of a section was analyzed
by TUNEL staining using the DeadEnd™ Colorimetric TUNEL system
(G7130; Promega Corporation, Beijing, China) following the
manufacturer's protocol. Hematoxylin was applied as a counter
stain. In each sample, the number of apoptotic tumor cells from 50
different fields was evaluated at high magnification (×400).
Evaluation of tumor growth in vivo
The effect of rhIL-24 in Eca-109 cells in
vivo was investigated by xenograft tumors in nude mice as
previously described (16). All
applicable international, national and/or institutional guidelines
for the care and use of animals were followed. All procedures
performed in studies involving animals were in accordance with
ethical standards of the Center of Biomedical Analysis of Tsinghua
University where the studies were conducted. First, the cell number
of Eca-109-induced tumors in athymic BALB/c female nude mice was
identified by injecting s.c. 1×106, 2×106 or
5×106 cells into the lower right flank of mice,
respectively. Each group consisted of three animals, and tumor
growth was monitored for 30 days. Tumor length and width were
measured using a Vernier caliper every other day without knowledge
of the treatment groups, and the tumor volume was calculated by
ʻlength × width2/2ʼ. At the same time, FCHO and
FCHO/IL-24 cells were tested for their ability to induce tumors.
Aliquots of cells (1×106 and 2×106) were
injected s.c., as described above. Then, Eca-109
(5×107/ml), FCHO (2×107/ml) and FCHO/IL-24
cells (2×107/ml) were resuspended in sterile
phosphate-buffered saline (PBS) at different cell concentrations,
respectively. There were 8 animals in the experimental group and
each animal received 7×106 cells (200 µl) into
the lower right flank, which consisted of 5×106 Eca-109
cells and 2×106 FCHO/IL-24 cells. Each animal in the
control group received a mixture of Eca-109 and FCHO cells, as
described above. After 30 days, the animals were euthanized, and
the tumors were analyzed by immunohistochemical staining with
H&E, PCNA or Ki-67, CD34, IL-24 and TUNEL staining. Finally,
Eca-109 cells were injected s.c. into the lower right flanks of
nude mice. When the size of the tumors reached 50–100
mm3 (nearly 7 days), the animals were divided randomly
into two groups. The animals in the experimental group were
injected s.c. into the upper right flank with 200 µl
Matrigel containing FCHO/IL-24 cells (2×106/animal), and
the mice in the control group were injected with Matrigel
containing FCHO cells. The tumor volume was monitored and the tumor
sections were analyzed as described above.
Immunohistochemical analysis
Immunohistochemical labeling was performed on 10%
formalin-fixed, paraffin-embedded tumor tissues (cut into
4-µm secions). After being deparaffinized in xylene and
rehydrated in graded concentrations of ethanol, the sections were
recovered in citrate buffer and blocked with 3%
H2O2 and 10% serum. The primary antibodies
were added and incubated overnight at 4°C at various dilutions as
follows: IL-24 (1:20; AF1965), PCNA (1:500; ZM-0213), Ki-67 (1:100;
ZA-0502) and CD34 (1:100; BA-0532). Then, the slides were incubated
with a secondary antibody and the horseradish
peroxidase-streptavidin complex reagent for 30 min, respectively
(Beijing Golden Bridge Biotechnology, Beijing, China). The
immunolabeling was developed with the chromogen
3,3′-diaminobenzidine tetra-hydrochloride (DAB). Hematoxylin was
applied as a counter stain. Tissue sections stained without the
primary antibody served as the negative controls. The staining of
tissue sections was analyzed and quantified, and the results were
interpreted in a blinded manner.
Statistical analysis
All experiments were performed at least three times.
Statistical comparisons between groups were performed using an
unpaired Student's t-test with the Statistical Package for the
Social Sciences (SPSS) software version 15 (SPSS, Inc., Chicago,
IL, USA). p<0.05 or p<0.01 was considered to indicate a
statistically significant result.
Results
Purification of rhIL-24 from the
site-specific-integrated cell line FCHO/IL-24 with a high
expression level of rhIL-24
Although the protein expressed in mammalian cells
has a high biological function at a ng/ml concentration, the
expression level is lower than that in E. coli (22). To identify whether IL-24 cDNA
site-specifically integrated at a specific genomic location is an
advantage over random integration, we established a stable
site-specific cell line, FCHO/IL-24, that secretes rhIL-24 using
the Flp-In system (for generating stable mammalian expression cell
lines by Flp recombinase-mediated integration; Invitrogen,
Carlsbad, CA, USA). After culturing for 72 h, the supernatant and
lysates of FCHO/IL-24, FCHO/mock and FCHO cells were collected and
examined by western blotting using anti-human IL-24 (AF1965) and
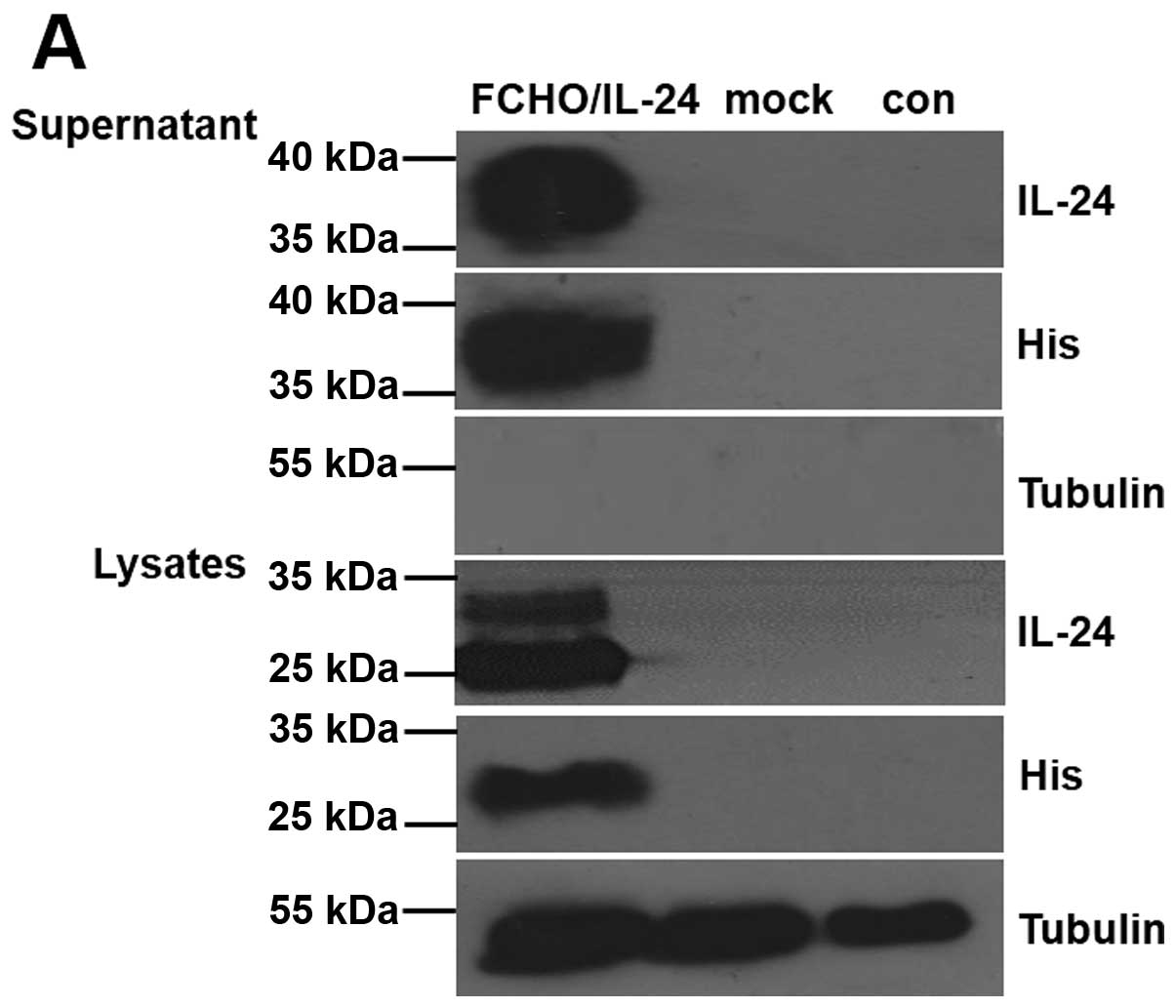
anti-His6 antibodies. As shown in Fig. 1A, both the anti-IL-24 and the
anti-His6 antibodies recognized the recombinant
glycosylated protein in the supernatant (MW, 40 kDa) and the
doublet intracellular protein in the lysates (MW, 23 and 30 kDa) of
FCHO/IL-24 cells, as in previous studies (23,24).
The samples of FCHO/mock and FCHO did not display any detectable
rhIL-24 expression. Thus, we successfully established the
FCHO/IL-24 cell line to constitutively express secreted rhIL-24
protein.
At the same time, we established 3 random-integrated
cell lines, HEK293/pSecTag2A-IL-24, HEK293/pcDNA3.1myc/His-IL-24
and HEK293/pCEP4-IL-24, which could stably secrete rhIL-24 into the
supernatant, as determined by western blotting assays (data not
shown). To compare the expression level of srhIL-24 via ELISA, we
collected the supernatant from the four cell types at different
times (1–4 days). As shown in Fig.
1B, compared with the three random-integrated cell lines,
FCHO/IL-24 secreted srhIL-24 protein at the highest level with a
marked difference (p<0.05 or p<0.01). The expression level of
FCHO/IL-24 was 1.7, 5.7, 7.4 and 8.0 ng/ml at the respective times,
which was more than that of HEK293/pCEP4-IL-24 at 1.4, 4.0, 6.1 and
7.1 ng/ml, respectively. The difference between the two cell lines
was particularly notable (p<0.05) on day 2. This experiment was
repeated at least three times. Thus, the expression level of the
site-specific stable cell line FCHO/IL-24 was higher than the
levels in the three random-integrated cell lines. The rhIL-24
protein in the supernatant of FCHO/IL-24 cells was eluted by 250
mmol/l imidazole using Ni2+ affinity chromatography and
recognized by the anti-IL-24 antibody (data not shown). The final
concentration of purified srhIL-24 was 500 ng/ml after treatment of
ultrafiltration, as determined by ELISA.
Secreted rhIL-24 inhibits ESCC Eca-109
cell growth and induces apoptosis in a dose-dependent manner
We showed that Eca-109, A375 and HEL cells were
IL-20R-positive by immunofluorescence, while the A549 cell line
showed positive staining for IL-20R2, and the two other receptor
subunits were not expressed (21).
Therefore, A375 cells were used as a positive control for the
expression of IL-20 receptor complexes, which are the target
receptors of IL-24; A549 cells were used as a negative control for
receptor expression. The control for primary normal cells was HEL,
which showed positive expression of IL-20R. The effects of rhIL-24
on cell growth were analyzed on day 4 using the MTT assay. Purified
rhIL-24 was added at different concentrations (0, 1, 5, 10, 20 and
50 ng/ml) to the cell culture media, and PBS was used as the
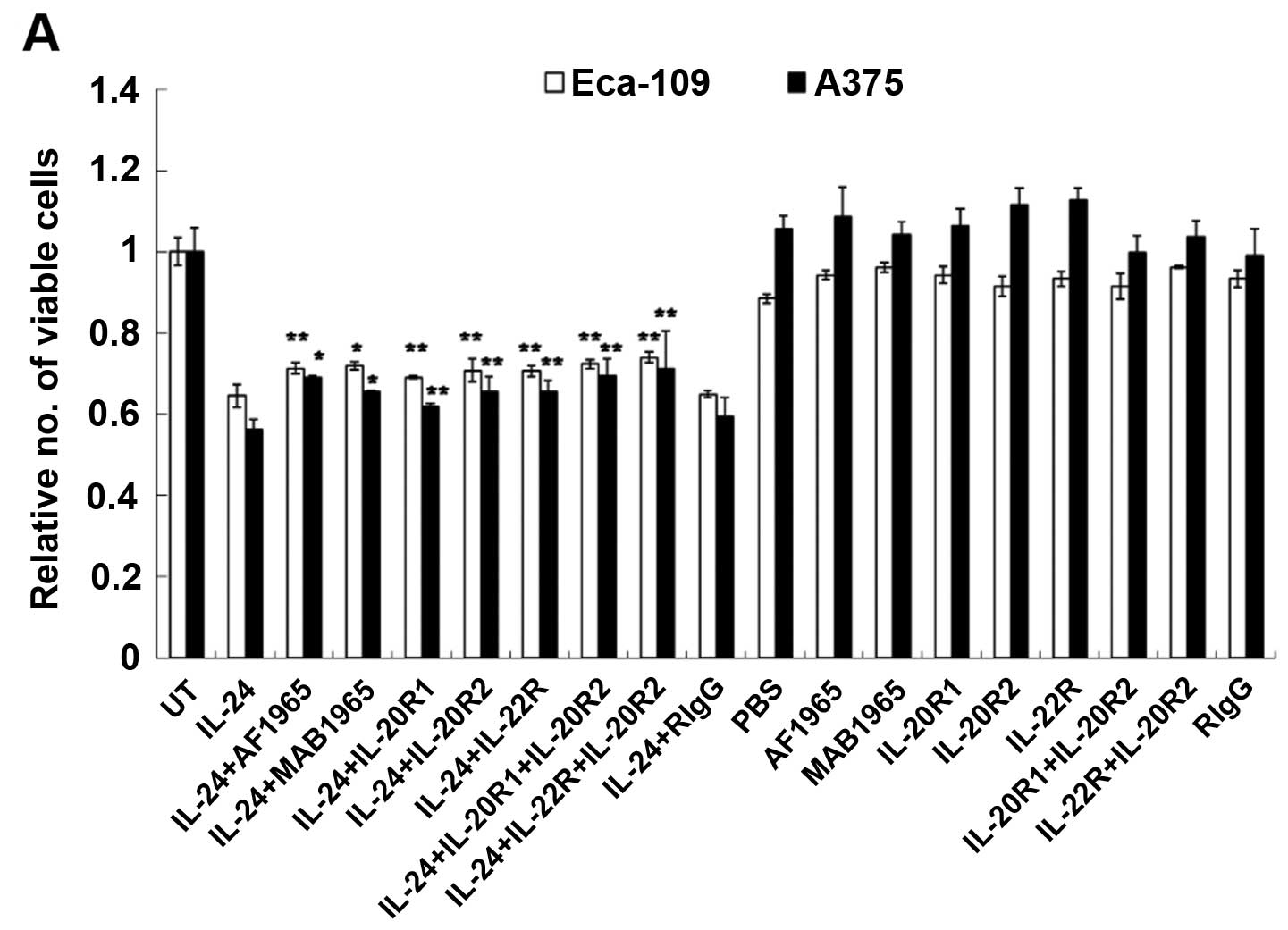
untreated control. As shown in Fig.
2A, compared with the PBS-treated cells, rhIL-24 induced a
significant decrease in the cell viability of the Eca-109 and A375
cells. In these two cell lines, a decrease in cell viability was
evident even after treatment with 1 ng/ml (p<0.05), and
increasing concentrations of rhIL-24 produced a more significantly
pronounced effect (p<0.01). The highest percentage of inhibition
of rhIL-24 in the Eca-109 and A375 cells was 34.69 and 36.21% at 50
ng/ml, respectively. However, treatment with rhIL-24 at the same
concentrations (1–50 ng/ml) did not decrease the viability of the
other two cell lines (A549 and HEL). These results showed that the
observed inhibitory activity in the MTT assays was attributable to
rhIL-24. This effect was corroborated by a clone formation assay
(Fig. 2B). These data demonstrated
that rhIL-24 inhibited the growth and clone formation of the
Eca-109 and A375 cells in a dose-dependent manner and had no effect
on A549 and HEL cells.
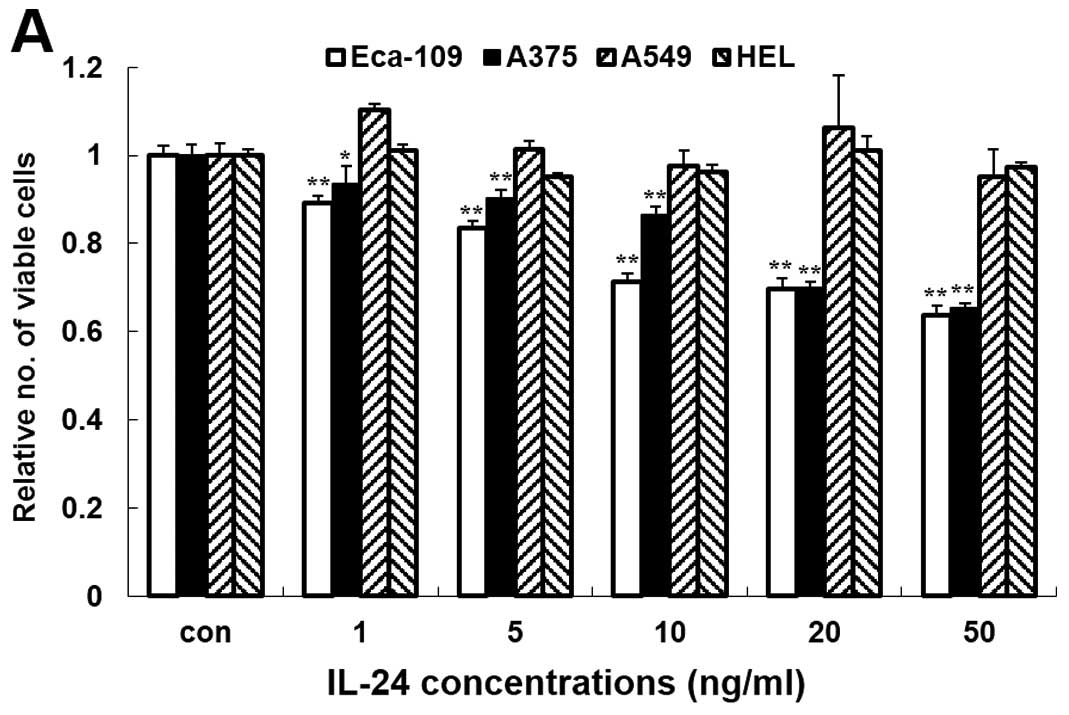 | Figure 2Secreted rhIL-24 inhibits ESCC
Eca-109 cell growth and induces apoptosis in a dose-dependent
manner. (A) Cells (Eca-109, A375, A549 or HEL) were treated with
purified rhIL-24 (1, 5, 10, 20 and 50 ng/ml) for 4 days, and cell
viability was evaluated by MTT assay. The numbers represent the
ratio of the specific treatments indicated vs. control cells;
columns, average of three independent experiments (n=3);
**p<0.01 or *p<0.05 represents a
significant difference compared with the control group. (B) The
effect of srhIL-24 protein on colony formation in cancer and normal
cells. A375, Eca-109, A549 or HEL cells were treated with srhIL-24
protein (0, 1, 5, 10, 20 and 50 ng/ml) for 2 weeks, and the colony
formation rate was evaluated as the ratio of clone number to cell
number. Columns, the mean of three independent experiments
(n>3), and **p<0.01 represents a significant
reduction compared with the untreated group. (C) srhIL-24 protein
induces apoptosis in the A375 and Eca-109 cells. The indicated
cells were seeded in 6-well plates at a density of
4.5×105/well and the next day they were treated with
different concentrations of rhIL-24 (0, 1, 10 and 40 ng/ml). Four
days after treatment, the cells were harvested for Annexin V
FITC/PI staining and FASC analysis. The data are shown as one of
three independent experiments. |
Previous studies have extensively demonstrated that
secreted rhIL-24 can trigger apoptosis in a broad spectrum of
tumors (13,15,16).
Annexin V binding and FACS assays were performed to determine
whether secreted rhIL-24 induced apoptosis in the Eca-109 and A375
cells. As shown in Fig. 2C, both
cancer cell lines underwent significant apoptosis after secreted
rhIL-24 treatment in a dose-dependent manner. The apoptosis rates
of Eca-109 and A375 cells were 34.5 and 64.5% when rhIL-24 was
added at a final concentration of 40 ng/ml, respectively. The
results indicated that the secreted rhIL-24 protein robustly
induced apoptosis in the ESCC Eca-109 and melanoma A375 cells in a
dose-dependent manner compared with PBS treatment.
rhIL-24 protein-mediated killing of
Eca-109 cells and activation of STAT3 may be related to IL-20
receptors
To confirm that the inhibitory effect was due to
treatment with rhIL-24, cell viability was assessed using MTT assay
4 days after treatment with rhIL-24 (20 ng/ml) plus various
neutralizing antibodies against IL-24 (AF1965 or MAB1965) or IL-20R
(IL-20R1, IL-20R2 or IL-22R1) at a concentration of 200 ng/ml. As
shown in Fig. 3A, the anti-IL-24
antibody (AF1965 or MAB1965) significantly inhibited (p<0.05 or
p<0.01) IL-24-mediated cell killing in the Eca-109 cells, as did
the anti-IL-20R antibodies (IL-20R1, IL-20R2 or IL-22R1)
(p<0.01). The anti-IL-24 or anti-IL-20R antibodies also
significantly reduced the killing of A375 cells (p<0.05 or
p<0.01) (Fig. 3A). Treatment
with immunoglobulin (Ig), a nonspecific control, had no effect on
the cell killing by rhIL-24. This result suggested that
rhIL-24-mediated cell death was caused by extracellular mechanisms.
At the same time, an anti-IL-24 or an anti-IL-20R antibody was not
able to fully abrogate cell killing. These results suggest the
possibility that other unappreciated functional receptor molecules
and signaling pathways may allow them to antagonize the growth
suppression of secreted rhIL-24 protein on cancer cells.
Recent studies have demonstrated the activation of
STAT3 in tumor cells upon receptor engagement by srhIL-24 (12,23).
As shown in Fig. 3B, western
blotting analysis showed that the addition of srhIL-24 (final
concentration of 20 ng/ml) in the culture media of Eca-109 cells
increased the expression levels of the pSTAT-3 protein at 4 h, but
not at 1 h, as in A375 cells. On the basis of these studies, we
identified that activity of srhIL-24 on the proliferation of
Eca-109 and A375 cells and activation of STAT3, may be mediated by
the receptor pathway.
rhIL-24 inhibits the tumorigenicity of
Eca-109 cells in nude mice
We firstly identified the cell number of Eca-109
cells to induce tumors in nude mice. After 5–7 days following s.c.
injection of 5×106 Eca-109 cells into nude mice, the
tumors reached 50-100 mm3, which was more rapid than the
animals that received 1×106 or 2×106 cells
(data not shown). At the same time, we verified whether parental
FCHO cells and gene engineered FCHO/IL-24 cells could induce tumors
in nude mice. Injection of 1×106 or 2×106 of
FCHO or FCHO/IL-24 cells alone for 30 days did not induce tumors in
the nude mice (data not shown). Then, the animals in the
experimental group were injected s.c. with a total of
7×106 cells containing a mixture of Eca-109 and
FCHO/IL-24 cells (2.5:1 ratio). For animals in the control group,
Eca-109 and FCHO cells were mixed and injected s.c. as described
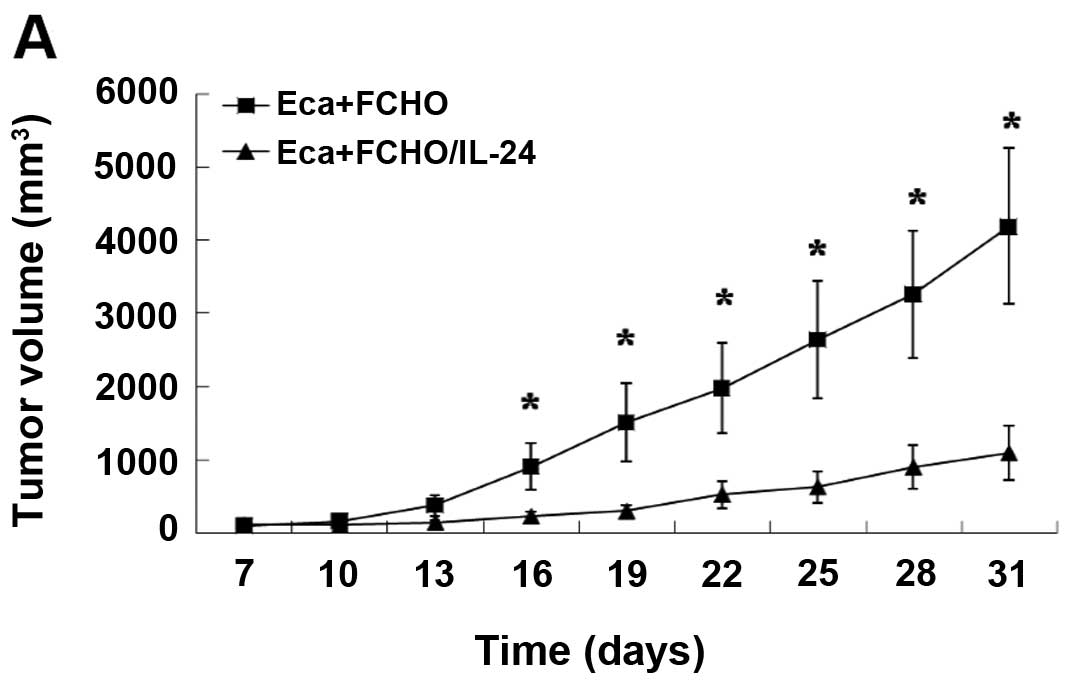
above. After 30 days, the growth of tumors was significantly
suppressed by 67% (mean value) in the animals of the experimental
group compared with the control group (Fig. 4A; p<0.05). There was no
significant differences in the tumor cell mitotic index or tissue
infiltration and tumor cell proliferative index between the two
group as determined by H&E and PCNA staining, respectively
(Fig. 4B and C). However, less
vascularization as determined by CD34 staining was detected in
tumor tissues of the experimental group than that noted in the
control group (Fig. 4D and E;
p<0.01). The reduction in CD34 staining indicated that secreted
rhIL-24 inhibited angiogenesis in the tumor tissues of nude mice.
Compared with tumor tissues of the control group, a higher number
of TUNEL-positive stained cells were observed in tumor cells of the
experimental group (Fig. 4F and G;
p<0.01). Immunohistochemical analysis with an anti-IL-24
antibody in the tumor sections of the experimental group
demonstrated IL-24 protein expression (Fig. 4H). In contrast, IL-24 was not
detected in the tumor sections of the control group. Finally, we
ascertained whether the growth of formed tumor xenografts could be
inhibited by rhIL-24 protein secreted by FCHO/IL-24 cells. When the
tumors reached 50-100 mm3 following s.c. injection of
Eca-109 cells (5×106) in the lower right flank of nude
mice, 2×106 FCHO/IL-24 cells resuspended in Matrigel
were injected s.c. in the upper right flank of the experimental
group animals. The animals of the control group received the same
number of FCHO cells resuspended in Matrigel at the same site. The
day of implantation of FCHO or FCHO/IL-24 cells was recorded as the
first day and tumor volume was measured as described above.
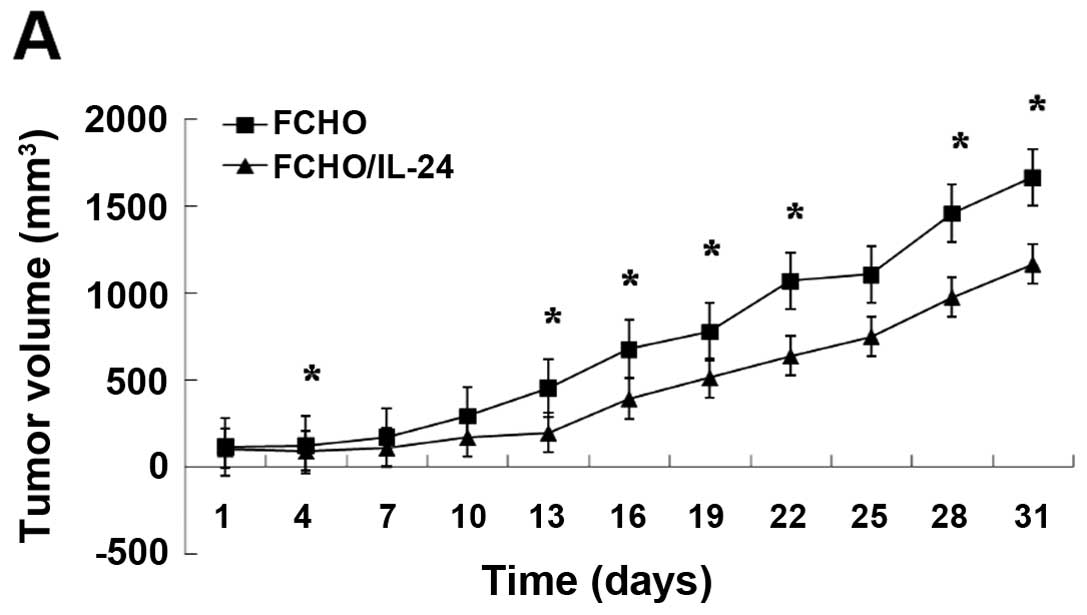
Compared with the tumors of the control group, the growth of
Eca-109 tumor xenografts in the experimental group was
significantly inhibited by 29% (mean value) (Fig. 5A; p<0.05) after 30 days. Similar
to the findings described above, there was no significant
difference in staining by the H&E or anti-Ki-67 antibody in the
tumor tissues between the two groups (Fig. 5B and C). In addition, the tumor
tissues of the experimental group showed significantly less
CD34-positive staining (Fig. 5D and
E; p<0.01) and more TUNEL-positive staining (Fig. 5F and G; p<0.01) than that of the
control group. The IL-24 protein was expressed in the tumor
sections of the experimental group, but not in the tumor sections
of the control group (Fig. 5H).
These results demonstrated that srhIL-24 inhibited tumor growth by
reducing angiogenesis and inducing apoptosis in vivo.
Discussion
Since 1995, numerous publications have shown that
IL-24 selectively kills a large variety of cancer cells in
vivo and in vitro and leaves healthy cells unharmed. Due
to the lack of post-translational modification, particularly
glycosylation, rhIL-24 protein expressed from E. coli plays
a role at very high concentrations (µg/ml) (25), or has no activity (26) on tumor cells. In contrast, rhIL-24
from mammalian cells plays an antitumor role at a substantially
lower concentration (ng/ml) (17,23),
but the expression level is lower. At present, there are several
reported methods to prepare rhIL-24 from mammalian cells, such as
overexpression of plasmid pCEP4-IL-24 in HEK293 cells (7,16,27)
and infection of immortal normal human (14,19) or
tumor cells (13) with Ad-IL-24. In
the present study, we aimed to improve the expression level of
srhIL-24 protein using a site-specific integrated cell line.
Compared with three random-integrated cell lines,
HEK293/pSecTag2A-IL-24, HEK293/pcDNA3.1myc/His-IL-24 and
HEK293/pCEP4-IL-24, the expression level of srhIL-24 from the
site-specific FCHO/IL-24 cells was the highest, with markedly
differences and reached 7.4 and 8.0 ng/ml on day 3 and 4,
respectively. This may be the first study to compare the expression
level of srhIL-24 from different gene engineered cell lines.
Caudell et al transfected plasmid pCEP4-IL-24 into HEK293
cells and the concentration of rhIL-24 in the crude supernatant of
an aliquot of 106 cells in 24 h was 30–50 ng/ml
(7). Since there was no detailed
information concerning the cell culture, such as the positive ratio
of cells overexpressing rhIL-24, the culture medium volume, cell
density or plate size, we were unable to compare our results with
theirs. They obtained purified srhIL-24 protein with a
concentration of 300 ng/ml as determined by ELISA (7) and ours was 500 ng/ml. Then, we
analyzed the biological function of srhIL-24 in vitro on
ESCC Eca-109 cells. srhIL-24 inhibited the growth and colony
formation (1–50 ng/ml) and induced apoptosis (1–40 ng/ml) in a
dose-dependent manner. The concentration of srhIL-24 was similar to
other studies (13,14,23,27).
In contrast to Ad-IL-24, rhIL-24 protein did not have any
biological effect in vitro on tumor cells lacking expression
of the IL-20R complexes, such as human lung cancer A549 cells
(14,27), and we obtained similar results. At
present, the mechanism involved in srhIL-24 protein from mammalian
cells in regards to the preferential killing activity against
cancer cells is in a receptor-dependent fashion (15,23).
srhIL-24 binds to the IL-20R complexes consisted of two sets of
heterodimeric chains, IL-20R1/IL-20R2 or IL-22R1/IL-20R2 (28). Our previous results (21) showed that Eca-109, A375 and HEL
cells expressed three types of subunits and A549 cells just
positively stained for IL-20R2. In the present study, the results
using anti-IL-24 or anti-IL-20R neutralizing antibodies (200 ng/ml)
indicated that srhIL-24 killed Eca-109 and A375 cells in a
receptor-mediated manner, and two types of IL-20R complex were
involved. Upon ligand binding, both receptors induced the
phosphorylation of STAT3 and its trans-location to the
nucleus, demonstrating that the JAK-STAT signaling pathway has
indeed been activated (28). The
concentration of rhIL-24 was usually 10–50 ng/ml, and the target
cells were treated from 30 min to 4 h (12,23).
From our results, the STAT3 in Eca-109 cells or A375 cells was
apparently phosphorylated after cells were treated with srhIL-24
(20 ng/ml) for 4 h. Apart from the phosphorylation of STAT3,
srhIL-24 also functions through different pathways, such as
increasing IL-24 mRNA stability and endogenous IL-24 protein
expression in normal and cancer cells (14), inducing a bystander antitumor effect
through an ER stress mechanism and increasing reactive oxygen
species (ROS) production similar to Ad-IL-24 infection (14), upregulating the tumor-suppressor
proteins p53 (13,17), p27Kip1 (17) and cell cycle regulator p21 (13,23).
Numerous studies have identified the antitumor
activity of IL-24 in vivo by direct injection of Ad-IL-24
(29) into tumor-bearing nude mice,
and several using adeno-associated virus IL-24 (30) or plasmid (31). With regard to rhIL-24 protein, there
were no studies that directly injected the recombinant protein, no
matter whether the rhIL-24 was expressed in E. coli or
mammalian cells. The possible reasons are lower activity and lower
expression level, respectively. In this experiment, the ultimate
collection of rhIL-24 protein was not sufficient for in vivo
investigations; therefore, we injected s.c. FCHO/IL-24 cells to
identify the antitumor function of srhIL-24 in vivo
according to Ramesh et al (16). They mixed 5×105 tumor
cells (A549) with an equal number (5×105) of
HEK-293/IL-24 cells, and every animal received 106 cells
(ratio, 1:1). In the present study, the ratio of tumor (Eca-109)
and FCHO/IL-24 or FCHO cells was 2.5:1, and every animal received
7×106 cells. Following injection of a mixture of
FCHO/IL-24 and Eca-109 cells, and also injection of FCHO/IL-24
cells resuspended in Matrigel into tumor-bearing nude mice, tumor
growth was significantly inhibited in the experimental animals that
received FCHO/IL-24 cells compared with the control animals that
received FCHO cells after 30 days. However, when FCHO/IL-24 and
Eca-109 cells were mixed and injected at the same time, the effect
of srhIL-24 was more evident. The reason may be that FCHO-IL-24
cells induced the apoptosis of Eca-109 cells as soon as the two
types of cells were mixed. The mechanism by which sIL-24 inhibited
tumor growth in vivo possibly involves at least three
functions: tumoricidal effects, anti-angiogenic activity and
modulation of immune responses.
In summary, we demonstrated that rhIL-24 was
secreted at a higher level from the site-specific integrated
FCHO/IL-24 cells than three random-integrated cell lines. The
purified srhIL-24 inhibited proliferation and induced apoptosis of
ESCC Eca-109 cells in vitro and in vivo and activated
STAT3, which may be mediated by the receptor pathway. Further
preliminary research should be performed. Studies should be carried
out to treat tumors using rhIL-24 and should aim to improve the
expression level, the suspension culture of engineeried cells in
medium-free fetal bovine serum, to improve the half-life of
purified rhIL-24 protein by polyethyleneglycol modification, and to
identify more effective delivery systems. These insights should
pave the way for enhanced translational applications of rhIL-24 for
the therapy of human cancers.
Acknowledgments
The present study was supported by the Beijing
Natural Science Foundation (grant no. 7142117) to Q.F. Ma and the
National High Technology Research and Development Program of China
(863 Program, grant no. 2014AA021605) to Z.L. Wang.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wang JB, Fan JH, Liang H, Li J, Xiao HJ,
Wei WQ, Dawsey SM, Qiao YL and Boffetta P: Attributable causes of
esophageal cancer incidence and mortality in China. PLoS One.
7:e422812012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Burrows WM: Gastrointestinal function and
related problems following esophagectomy. Semin Thorac Cardiovasc
Surg. 16:142–151. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Xu YW, Peng YH, Chen B, Wu ZY, Wu JY, Shen
JH, Zheng CP, Wang SH, Guo HP, Li EM, et al: Autoantibodies as
potential biomarkers for the early detection of esophageal squamous
cell carcinoma. Am J Gastroenterol. 109:36–45. 2014. View Article : Google Scholar :
|
|
5
|
Jiang H, Lin JJ, Su ZZ, Goldstein NI and
Fisher PB: Subtraction hybridization identifies a novel melanoma
differentiation associated gene, mda-7, modulated during human
melanoma differentiation, growth and progression. Oncogene.
11:2477–2486. 1995.PubMed/NCBI
|
|
6
|
Gupta P, Su ZZ, Lebedeva IV, Sarkar D,
Sauane M, Emdad L, Bachelor MA, Grant S, Curiel DT, Dent P, et al:
mda-7/IL-24: Multifunctional cancer-specific apoptosis-inducing
cytokine. Pharmacol Ther. 111:596–628. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Caudell EG, Mumm JB, Poindexter N,
Ekmekcioglu S, Mhashilkar AM, Yang XH, Retter MW, Hill P, Chada S
and Grimm EA: The protein product of the tumor suppressor gene,
melanoma differentiation-associated gene 7, exhibits
immunostimulatory activity and is designated IL-24. J Immunol.
168:6041–6046. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fisher PB: Is mda-7/IL-24 a ʻmagic bulletʼ
for cancer? Cancer Res. 65:10128–10138. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Su ZZ, Lebedeva IV, Sarkar D,
Gopalkrishnan RV, Sauane M, Sigmon C, Yacoub A, Valerie K, Dent P
and Fisher PB: Melanoma differentiation associated gene-7,
mda-7/IL-24, selectively induces growth suppression, apoptosis and
radiosensitization in malignant gliomas in a p53-independent
manner. Oncogene. 22:1164–1180. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang J, Fan X, Bao L and Du L:
Construction of eukaryotic expression vector of EGFRi-IL-24
recombinant gene. Sheng Wu Yi Xue Gong Cheng Xue Za Zhi.
27:395–399. 2010.In Chinese. PubMed/NCBI
|
|
11
|
Sieger KA, Mhashilkar AM, Stewart A,
Sutton RB, Strube RW, Chen SY, Pataer A, Swisher SG, Grimm EA,
Ramesh R, et al: The tumor suppressor activity of MDA-7/IL-24 is
mediated by intracellular protein expression in NSCLC cells. Mol
Ther. 9:355–367. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ekmekcioglu S, Ellerhorst JA, Mumm JB,
Zheng M, Broemeling L, Prieto VG, Stewart AL, Mhashilkar AM, Chada
S and Grimm EA: Negative association of melanoma
differentiation-associated gene (mda-7) and inducible nitric oxide
synthase (iNOS) in human melanoma: MDA-7 regulates iNOS expression
in melanoma cells. Mol Cancer Ther. 2:9–17. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zheng M, Bocangel D, Ramesh R, Ekmekcioglu
S, Poindexter N, Grimm EA and Chada S: Interleukin-24 overcomes
temozolo-mide resistance and enhances cell death by down-regulation
of O6-methylguanine-DNA methyltransferase in human
melanoma cells. Mol Cancer Ther. 7:3842–3851. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sauane M, Su ZZ, Gupta P, Lebedeva IV,
Dent P, Sarkar D and Fisher PB: Autocrine regulation of mda-7/IL-24
mediates cancer-specific apoptosis. Proc Natl Acad Sci USA.
105:9763–9768. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chada S, Bocangel D, Ramesh R, Grimm EA,
Mumm JB, Mhashilkar AM and Zheng M: mda-7/IL24 kills pancreatic
cancer cells by inhibition of the Wnt/PI3K signaling pathways:
Identification of IL-20 receptor-mediated bystander activity
against pancreatic cancer. Mol Ther. 11:724–733. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ramesh R, Mhashilkar AM, Tanaka F, Saito
Y, Branch CD, Sieger K, Mumm JB, Stewart AL, Boquoi A, Dumoutier L,
et al: Melanoma differentiation-associated gene 7/interleukin
(IL)-24 is a novel ligand that regulates angiogenesis via the IL-22
receptor. Cancer Res. 63:5105–5113. 2003.PubMed/NCBI
|
|
17
|
Zheng M, Bocangel D, Doneske B, Mhashilkar
A, Ramesh R, Hunt KK, Ekmekcioglu S, Sutton RB, Poindexter N, Grimm
EA, et al: Human interleukin 24 (MDA-7/IL-24) protein kills breast
cancer cells via the IL-20 receptor and is antagonized by IL-10.
Cancer Immunol Immunother. 56:205–215. 2007. View Article : Google Scholar
|
|
18
|
Lebedeva IV, Su ZZ, Sarkar D and Fisher
PB: Restoring apoptosis as a strategy for cancer gene therapy:
Focus on p53 and mda-7. Semin Cancer Biol. 13:169–178. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Su Z, Emdad L, Sauane M, Lebedeva IV,
Sarkar D, Gupta P, James CD, Randolph A, Valerie K, Walter MR, et
al: Unique aspects of mda-7/IL-24 antitumor bystander activity:
Establishing a role for secretion of MDA-7/IL-24 protein by normal
cells. Oncogene. 24:7552–7566. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ma G, Kawamura K, Shan Y, Okamoto S, Li Q,
Namba M, Shingyoji M, Tada Y, Tatsumi K, Hiroshima K, et al:
Combination of adenoviruses expressing melanoma
differentiation-associated gene-7 and chemotherapeutic agents
produces enhanced cyto-toxicity on esophageal carcinoma. Cancer
Gene Ther. 21:31–37. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ma Q, Deng X, Jin B, Zhang Y, Luo D, Song
H, Wang P, Zhang C, Li X, Shi Y, et al: A novel human
interleukin-24 peptide created by computer-guided design
contributes to suppression of proliferation in esophageal squamous
cell carcinoma Eca-109 cells. Oncol Rep. 33:193–200. 2015.
|
|
22
|
Yang J, Zhang W, Liu K, Jing S, Guo G, Luo
P and Zou Q: Expression, purification, and characterization of
recombinant human interleukin 24 in Escherichia coli. Protein Expr
Purif. 53:339–345. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chada S, Mhashilkar AM, Ramesh R, Mumm JB,
Sutton RB, Bocangel D, Zheng M, Grimm EA and Ekmekcioglu S:
Bystander activity of Ad-mda7: Human MDA-7 protein kills melanoma
cells via an IL-20 receptor-dependent but STAT3-independent
mechanism. Mol Ther. 10:1085–1095. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Aggarwal S, Takada Y, Mhashilkar AM,
Sieger K, Chada S and Aggarwal BB: Melanoma
differentiation-associated gene-7/IL-24 gene enhances NF-kappa B
activation and suppresses apoptosis induced by TNF. J Immunol.
173:4368–4376. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yacoub A, Mitchell C, Hong Y,
Gopalkrishnan RV, Su ZZ, Gupta P, Sauane M, Lebedeva IV, Curiel DT,
Mahasreshti PJ, et al: MDA-7 regulates cell growth and
radiosensitivity in vitro of primary (non-established) human glioma
cells. Cancer Biol Ther. 3:739–751. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kreis S, Philippidou D, Margue C,
Rolvering C, Haan C, Dumoutier L, Renauld JC and Behrmann I:
Recombinant interleukin-24 lacks apoptosis-inducing properties in
melanoma cells. PLoS One. 2:e13002007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mumm JB, Ekmekcioglu S, Poindexter NJ,
Chada S and Grimm EA: Soluble human MDA-7/IL-24: Characterization
of the molecular form(s) inhibiting tumor growth and stimulating
monocytes. J Interferon Cytokine Res. 26:877–886. 2006. View Article : Google Scholar
|
|
28
|
Dash R, Bhutia SK, Azab B, Su ZZ, Quinn
BA, Kegelmen TP, Das SK, Kim K, Lee SG, Park MA, et al:
mda-7/IL-24: A unique member of the IL-10 gene family promoting
cancer-targeted toxicity. Cytokine Growth Factor Rev. 21:381–391.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Saeki T, Mhashilkar A, Swanson X, Zou-Yang
XH, Sieger K, Kawabe S, Branch CD, Zumstein L, Meyn RE, Roth JA, et
al: Inhibition of human lung cancer growth following
adenovirus-mediated mda-7 gene expression in vivo. Oncogene.
21:4558–4566. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tamai H, Miyake K, Yamaguchi H, Takatori
M, Dan K, Inokuchi K and Shimada T: AAV8 vector expressing IL24
efficiently suppresses tumor growth mediated by specific mechanisms
in MLL/AF4-positive ALL model mice. Blood. 119:64–71. 2012.
View Article : Google Scholar
|
|
31
|
Gu J, Chen X, Xin H, Fang X and Sha X:
Serum-resistant complex nanoparticles functionalized with
imidazole-rich polypeptide for gene delivery to pulmonary
metastatic melanoma. Int J Pharm. 461:559–569. 2014. View Article : Google Scholar
|