Introduction
Melanoma, a malignancy that arises from melanocytes,
accounts for ~10% of all skin tumors (1). It is the most aggressive skin cancer
and is characterized by abnormal proliferation of melanocytes
(2). Due to its high metastatic
potential and strong resistance to radiation, immunotherapy and
chemotherapy, the search for novel anti-melanoma therapies is
urgent (2). Currently, it is widely
accepted that metabolic changes are one of the hallmarks of cancer
(3). Accordingly in recent years,
cancer therapeutics are focused on two metabolic fields: glycolytic
metabolism and bioactive sphingolipid synthesis (4).
Increased glycolysis in tumor cells compared to
normal tissues is observed in most types of cancers and supports
the increased energy and biosynthetic demands of tumor cells
(5). This is in accordance with the
Warburg hypothesis which posits that aerobic glycolysis is a major
source of energy in malignant cells (6). Therefore, the glycolytic metabolism is
an important target for regulating tumor progression.
Ceramide, a backbone of the sphingolipid family, is
not only a component of the membrane structure, but also is an
essential mediator of cellular functions, such as growth,
differentiation and apoptosis (7–10).
Disturbances in ceramide synthesis and signaling have been
implicated in many types of cancers (8,11,12).
In human melanoma cell lines, resistance to stress-induced
apoptosis has been associated with low ceramide levels (13). Additionally, an increased
intracellular level of ceramide was found to inhibit the cell
proliferation and promote the apoptosis of tumor cells (9). Thus, strategies targeting ceramide
synthesis in cancer cells have been developed as novel approaches
for anticancer chemotherapy (14,15).
Ceramide can be produced via two distinct pathways,
one of which is de novo by a family of genes known as
ceramide synthases (CerSs), which consists of six members, CerS1 to
CerS6 (16,17). In addition to regulating
sphingolipid synthesis, CerS activity has also been shown to
regulate numerous functions of cell biology, including cell growth,
apoptosis, autophagy and particularly cancer development (18–21).
As an important member of the CerS family, various studies have
suggested that CerS6 is involved in cancer etiology. For example,
knockdown of CerS6 resulted in a specific decrease in intracellular
C16-ceramide, protected colon adenocarcinoma cells against
TRAIL-mediated apoptosis and interfered with translocation of
active caspase-3 into the nucleus. In contrast, increased CerS6
expression sensitized the cells to TRAIL (22). Senkal et al demonstrated the
anti-apoptotic role of CerS6 in head and neck squamous cell
carcinoma (HNSCC) (23).
Downregulation of CerS6 in HNSCC resulted in the induction of ER
stress, while overexpression of CerS6 increased HNSCC tumor
development and growth (24).
However, little is known concerning the exact effect of CerS6 on
the malignant behavior of melanoma.
In the present study, we found that the expression
of CerS6 in three melanoma cell lines, including WM35, WM451 and
SK-MEL-28 (SK-28) was low. We further overexpressed and knocked
down CerS6 in the three melanoma cell lines. The effect of CerS6 on
the invasion, proliferation, and glycolysis in melanoma cells was
evaluated, respectively. Furthermore, we detected the genes that
had altered expression after CerS6 silencing by human gene chip,
and found that the expression levels of GLUT1 and WNT5A in the
CerS6-silenced cells were greatly altered, which was confirmed by
qPCR. Moreover, the role of human glucose transporter GLUT1 in
regulating the WNT5A expression and the invasion and proliferation
of the melanoma cells was then analyzed. The present study may
provide a novel therapeutic target for clinical melanoma
treatment.
Materials and methods
Cell culture
Human melanoma cell lines, including WM35 (25), WM451 (25) and SK28 (26), HaCaT (27) and Hm (25) cells were purchased from the Chinese
Academy of Sciences (Shanghai, China). Cells were cultured in
Dulbecco's modified Eagle's medium (DMEM; HyClone, UK) with 10%
fetal bovine serum (FBS; Gibco, USA) in an 37°C atmosphere of 5%
CO2.
Real-time RT-PCR assay
Total RNA of the cells was extracted using TriPure
reagent (Roche, Shanghai, China). A reverse transcription kit
(Fermentas, USA) was used to convert RNA into cDNA, according to
the manufacturer's instructions. For mRNA detection, real-time PCR
was conducted using a qPCR detection kit on ABI 7500 thermocycler
(both from Life Technologies). β-actin was used as an internal
reference. The PCR reaction conditions were 95°C for 5 min,
followed by 40 cycles of 95°C for 15 sec and 60°C for 30 sec. The
primer sequences were as follows: human MCT1 (F, CCA ACC CTA AGA
TTA CTT CAC A and R, TCT GCC ATG ATA GCA ACA A); human CerS6 (F,
TGG TGC GGC TCA TCT TC and R, CAT CCC AGT CCA GTT GCT T); human
WNT5A (F, ACC GCT TTG CCA AGG AGT TCG and R, GCC TCG TTG TTG TGC
AGG TTC AT); human GLUT1 (F, GCA TCG TCG TCG GCA TCC T and R, GGT
TCT CCT CGT TGC GGT TG).
ELISA
The activity of glycolysis-related enzymes was
detected using an ELISA kit (Huamei, Wuhan, China) in accordance
with the manufacturer's instructions.
Transfection
Melanoma cells were cultured as described above
before transfection. To knock down the endogenous expression of
CerS6, a CerS6 inhibitor (the recombinant CerS6-shRNA) was used,
while to upregulate the expression of CerS6, a recombinant plasmid
with overexpression of CerS6 (both from GeneChem Co., Ltd.,
Shanghai, China) was used.
GLUT1-siRNA was used to regulate the
expression of GLUT1
Transfection was performed using Lipofectamine™ 2000
(Invitrogen, USA) according to the manufacturer's protocol.
Briefly, the diluted Lipofectamine 2000 was added into the diluted
plasmid. After incubation at room temperature for 20 min, the above
mixture was added into the cell suspension, which was then
incubated at 37°C, in 5% CO2 for 6 h. After that, the
transfection mixture was replaced with DMEM with 10% FBS. Cells
were then cultured for 48 h before the following assays.
Western blotting
Cells were lysed in cold RIPA buffer (Life
Technologies). The protein concentration was determined using the
BCA protein assay kit (Pierce Chemical, Rockford, IL, USA). Protein
was separated with 10% SDS-PAGE, transferred to a polyvinylidene
difluoride (PVDF) membrane (Life Technologies), and then blocked in
5% non-fat dried milk (Yili, Beijing, China) in Dulbecco's
phosphate-buffered saline (DPBS) (Life Technologies) for 4 h. The
PVDF membrane was then incubated with a monoclonal antibody (1:100)
or human anti-β-actin monoclonal antibody (1:100) (both from Abcam,
Cambridge, MA, USA) as an internal reference for 3 h at room
temperature, and then washed with DPBS for 10 min. After that, the
PVDF membrane was incubated with a secondary antibody (1:20,000;
Abcam) for 1 h at room temperature. After being washed with DPBS
for 15 min, the immune complexes on the PVDF membrane were detected
using the ECL western blotting kit (Pierce Chemical). Image-Pro
Plus software 6.0 (Media Cybernetics, Inc., Rockville, MD, USA) was
used to analyze the relative protein expression, represented as the
density ratio vs. β-actin.
GeneChip experiments
Total RNA was isolated from the human melanoma cell
lines with TRIzol reagent (Invitrogen). A reverse transcription kit
(Fermentas) was used to convert RNA into cDNA according to the
manufacturer's instructions. The gene expression detection was
performed by PrimeView™ Human Gene Expression Array (Affymetrix,
Inc., Santa Clara, CA, USA).
Detection of cell proliferation
Cell proliferation was detected by MTT assay. Cells
(10,000/group) were plated into 96-well plates and cultured at 37°C
in 5% CO2 for 24, 48 and 72 h. MTT (20 µl) (5
mg/ml; Life Technologies) was added into the plates and incubated
at 37°C for 4 h, followed by addition of 150 µl of dimethyl
sulfoxide (DMSO). After incubation at room temperature for 10 min,
the optical density (OD) at 570 nm was detected to evaluate the
formazan production using a Multiskan FC enzyme immunoassay
analyzer (Thermo Fisher Scientific).
Colony formation assay
Cells (200/well) were cultured in a 6-well plate.
Plates were incubated at 37°C for 2 weeks. The cells were then
gently washed, followed by staining with crystal violet. Viable
colonies containing at least 50 cells were counted. Colony
formation rate was determined as: the number of colonies/the seeded
cell numbers.
Cell migration assay
Transwell chambers (BD, USA) were used for migration
analysis. A total of 5×105 cells/ml suspended cells was
prepared in serum-free media, and 300 µl of the cell
suspension was added into the upper chamber. Meanwhile, 500
µl DMEM with 10% FBS was added into the lower chamber. After
that, the cells were incubated at 37°C for 48 h. A cotton-tipped
swab was used to carefully wipe off the cells that did not migrate
through the pores. The filters were then fixed in 90% alcohol and
stained by 0.1% crystal violet. The filters were then observed
under an inverted microscope (Olympus, Japan).
Statistical analysis
Data are expressed as the mean ± SD. Data analysis
was performed using GraphPad Prism 6.0 software (GraphPad Software,
San Diego, CA, USA). Student's t-test or one-way ANOVA was used to
analyze the significance of differences among groups depending on
the experimental conditions. Statistical significance was
considered at P<0.05.
Results
Expression level of CerS6 is low in the
melanoma cell lines
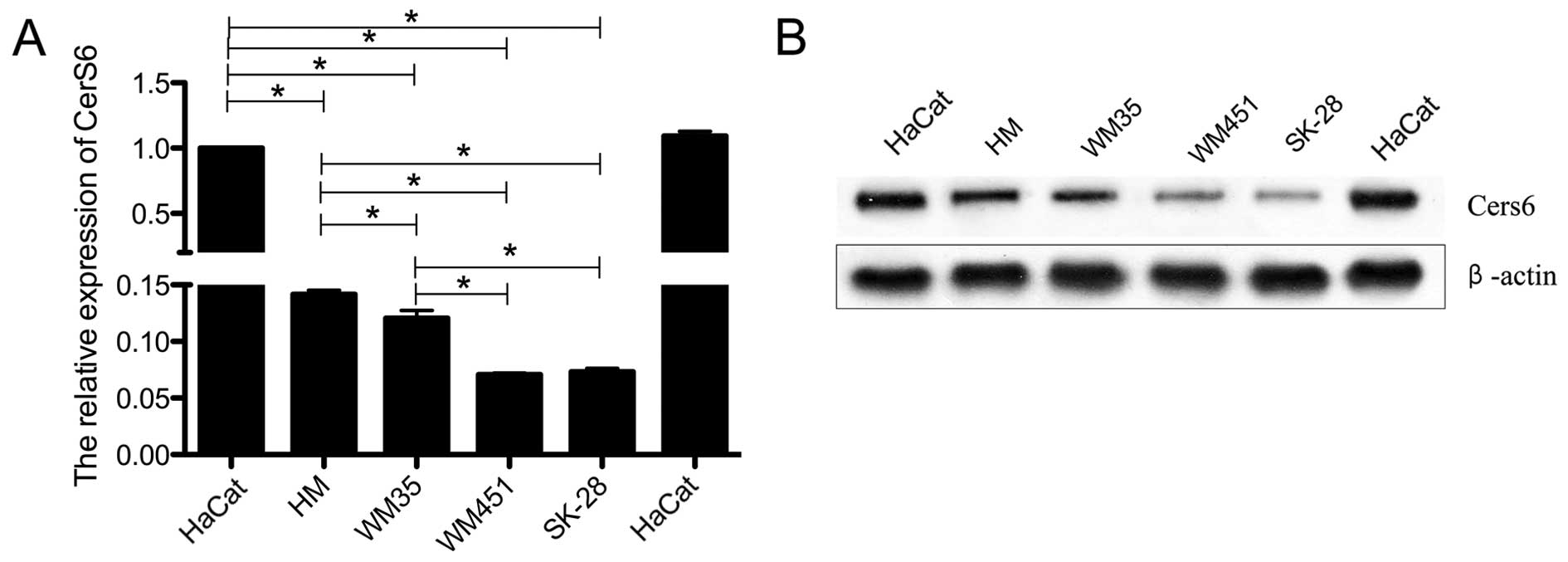
We detected the expression of CerS6 in human
immortalized epidermal cell line HaCaT, human normal melanophore
HM, and three melanoma cell lines with different grades of
malignancy, including WM35, WM451 and SK-28. The mRNA expression of
CerS6 in the three melanoma cell lines was significantly reduced
when compared with that in the HaCaT cells (P<0.05; Fig. 1A). Additionally, CerS6 was
significantly highly expressed in the WM35 cell line when compared
to its level in the WM451 (P<0.05) and SK-28 cells (P<0.05).
This was further verified by western blotting at the protein level
of CerS6 (Fig. 1B). This indicated
that the expression level of CerS6 may be closely related to the
malignancy of melanoma cells.
Decreased CerS6 expression promotes the
ability of invasion and proliferation in melanoma cell lines
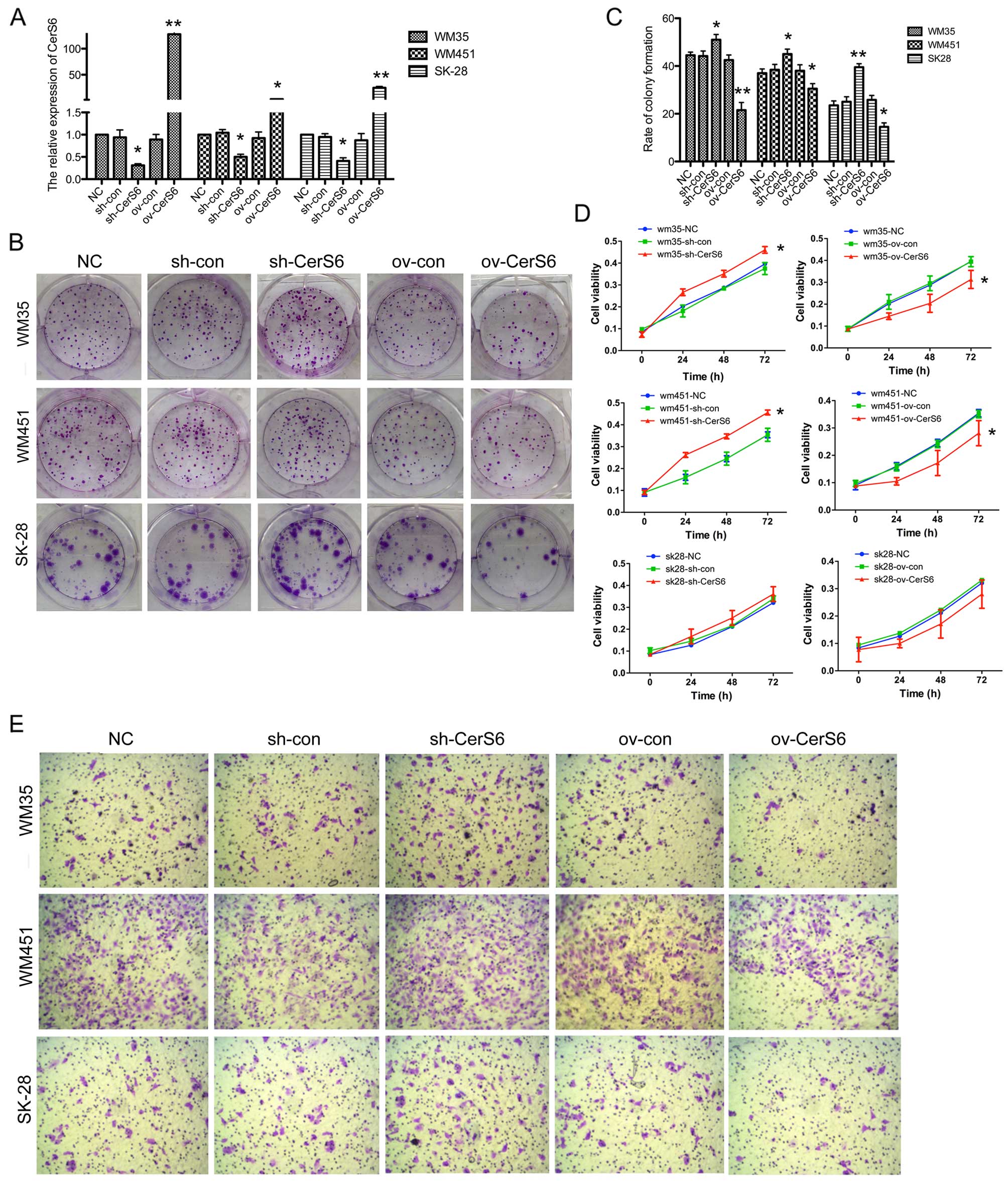
In order to detect the effects of CerS6 on melanoma,
we carried out transfection in the WM35, WM451 and SK-28 cell
lines. The mRNA expression of CerS6 was significantly upregulated
after transfection with the CerS6-overexpressing plasmid
(P<0.01), and was decreased when shRNA was transfected into the
cell lines (P<0.05) (Fig. 2A).
In the colony formation assay, the CerS6-overexpressing cell lines
formed fewer colonies (WM35, P<0.01; WM451, P<0.05; SK-28,
P<0.05), and the number of colonies was increased after CerS6
silencing (WM35, P<0.05; WM451, P<0.05; SK-28, P<0.01)
(Fig. 2B and C). Additionally, cell
proliferation was measured by MTT assay in the WM35, WM451 and
SK-28 cells. CerS6-overexpressing cells showed significantly
reduced cell viability, which was increased in the CerS6-silenced
WM35 (P<0.05) and WM451 cells (P<0.05), while no changes were
observed in the SK-28 cells (Fig.
2D). Furthermore, we detected the invasive ability of the cell
lines by Transwell assay (Fig. 2E).
Overexpression of CerS6 reduced the invasion capability of the
melanoma cells. In contrast, the silencing of CerS6 expression led
to a significantly increased number of cells migrating through the
Transwell membrane. These collectively indicated that silencing of
CerS6 promoted the invasion and proliferation ability of the
melanoma cell lines.
Decreased CerS6 expression upregulates
glycolysis in the melanoma cell lines
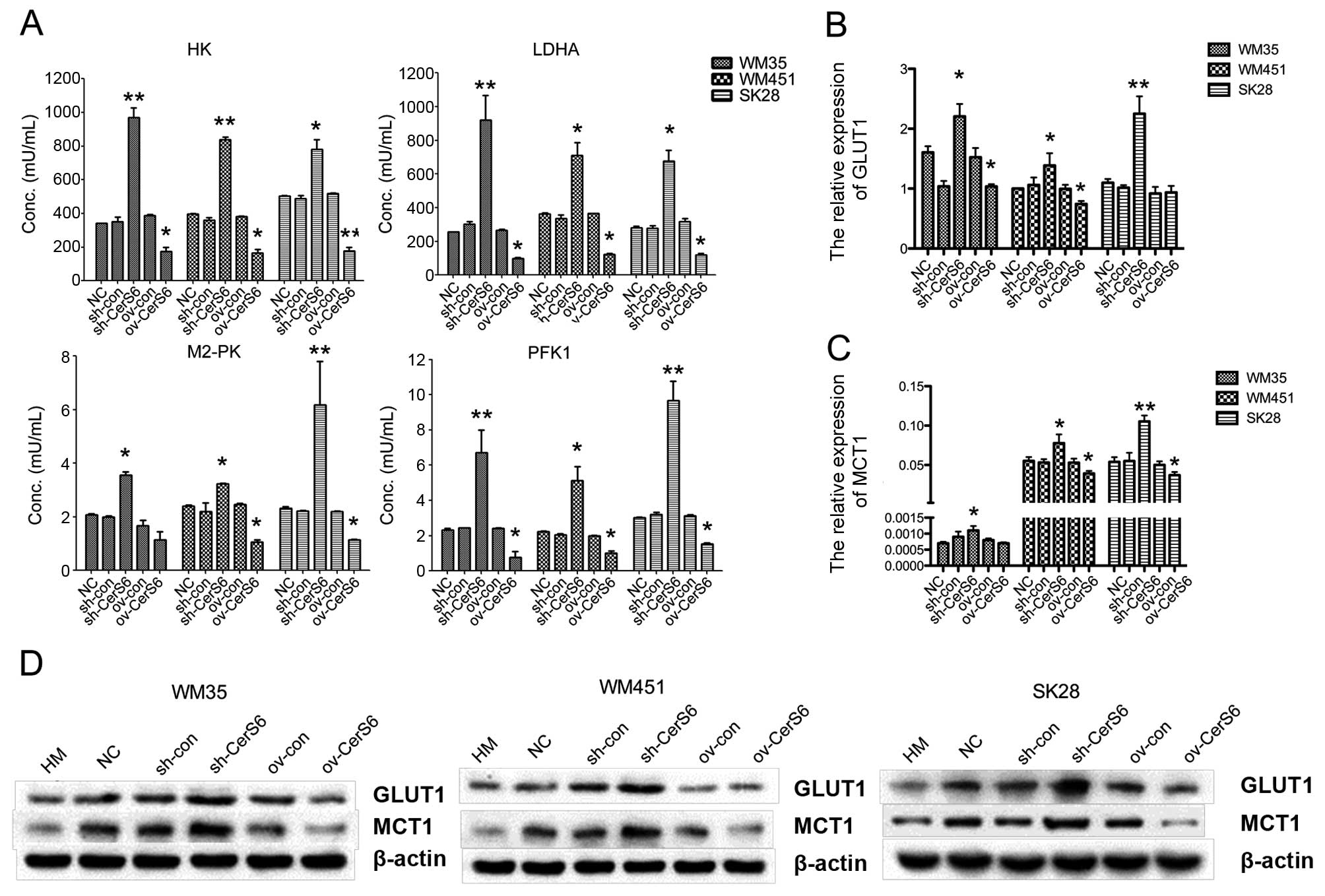
To analyze the effect of CerS6 on the glycolysis of
melanoma cell lines, we firstly detected the activity of several
key enzymes involved in glycolysis (HK, M2-PK, PFK1 and LDHA). As
depicted in Fig. 3A, overexpression
of CerS6 significantly downregulated the enzyme activity of HK,
M2-PK, PFK1 and LDHA in the WM35 cells (HK, P<0.01; M2-PK,
P<0.05; PFK1, P<0.01; LDHA, P<0.05), in the WM451 cells
(HK, P<0.01; M2-PK, P<0.01; PFK1, P<0.01; LDHA, P<0.05)
and in the SK-28 cells (HK, P<0.01; M2-PK, P<0.05; PFK1,
P<0.01; LDHA, P<0.05), which was significantly increased
following knockdown of CerS6. Additionally, the expression levels
of glycolysis-related genes, GLUT1 and MCT1, in melanoma cells
after transfection, were assessed by qPCR and western blotting,
respectively. The expression of GLUT1 and MCT1 at the mRNA and
protein levels was downregulated in the CerS6-overexpressing cells,
and was increased after CerS6 silencing (Fig. 3B and C). Collectively, silencing of
CerS6 upregulated the glycolysis in the melanoma cell lines.
Silencing of CerS6 in melanoma cell lines
leads to the upregulation of GLUT1 and downregulation of WNT5A
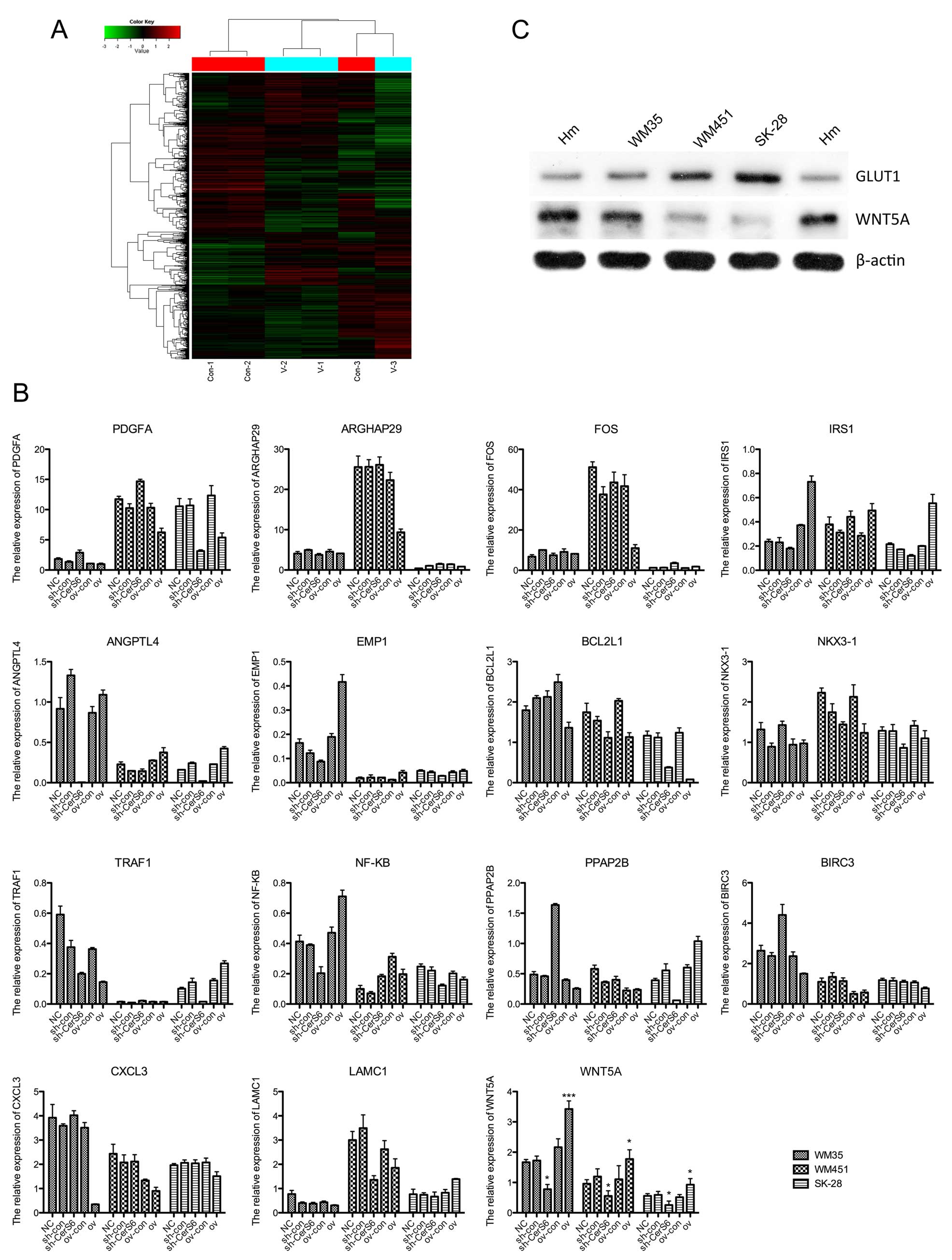
We detected the differences in the gene expression
profile between CerS6 silenced and control cells using gene chip,
and found that PDGFA, ARGHAP29, FOS, IRS1, ANGPTL4, EMP1 and BCL2L1
were upregulated in the CerS6-silenced cells. The expression of
SLC2A1, which encoded the important human glucose transporter
GLUT1, was also upregulated after silencing of CerS6. The
downregulated genes included 8 genes, such as NKX3-1, TRAF1, NF-KB,
PPAP2B, BIRC3, CXCL3, LAMC1 and WNT5A (Fig. 4A). In order to confirm the
expression changes of these genes, we performed qPCR for all of
these genes. As shown in Fig. 4B,
no uniform change was found in three melanoma cell lines with CerS6
overexpression or silencing in 14 genes, except SLC2A1 (also known
as GLUT1) and WNT5A. WNT5A was significantly downregulated in the
CerS6-silenced melanoma cells, including WM35 (P<0.05), WM451
(P<0.05) and SK-28 cell lines (P<0.05), and the expression of
WNT5A was increased when CerS6 was overexpressed in the WM35
(P<0.001), WM451 (P<0.05) and SK-28 cell lines (P<0.05).
Additionally, in the melanoma cell lines without any treatment, the
protein expression of GLUT1 was higher compared with the Hm cells,
while the expression of WNT5A was lower than that of the Hm cells
(Fig. 4C). Collectively, in three
CerS6-silenced melanoma cell lines, the expression of GLUT1 was
upregulated while WNT5A was downregulated.
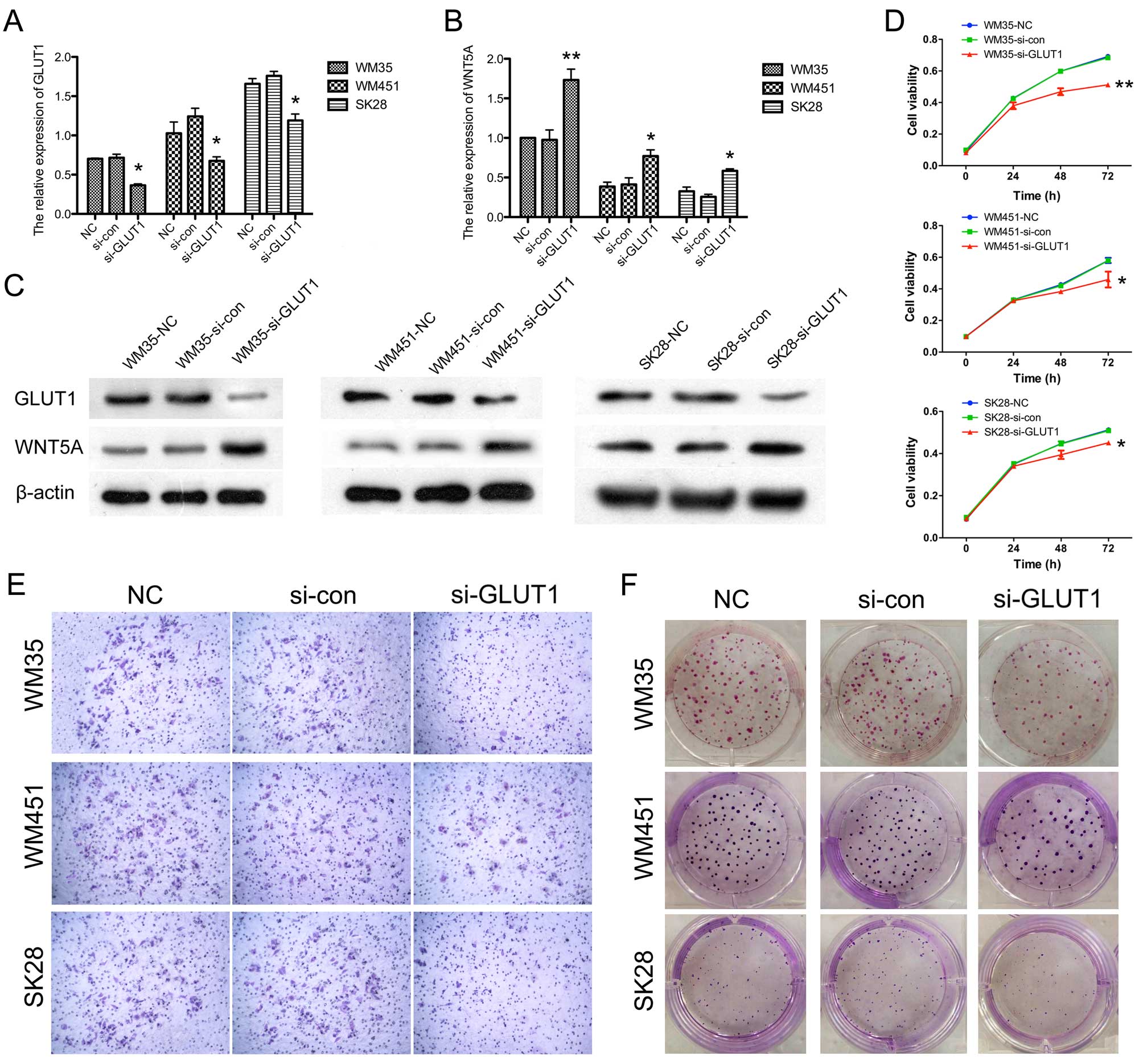
Silencing of GLUT1 promotes the
expression of WNT5A and inhibits the proliferation and invasion in
the melanoma cell lines
In order to confirm the role of GLUT1 in regulating
the invasion and proliferation of melanoma cells, siRNA-induced
silencing of GLUT1 expression was carried out. The expression of
GLUT1 was significantly downregulated after siRNA transfection
(Fig. 5A). WNT5A signaling was
found to increase aerobic glycolysis in the melanoma cells.
Accordingly, we measured the expression of WNT5A after silencing of
GLUT1. Our data showed that silencing of GLUT1 led to the
upregulated expression of WNT5A at the mRNA and protein level
(Fig. 5B and C). Cell proliferation
was measured in the WM35, WM451 and SK-28 cells by MTT assay.
GLUT1-silenced cells showed statistically reduced cell viability
(Fig. 5D). As shown in Fig. 5E, silencing of GLUT1 significantly
decreased the number of cells that migrated through the Transwell
membrane. In the colony formation assay, the number of colonies was
also decreased in the GLUT1-silenced cells (Fig. 5F). These collectively indicated that
silencing of GLUT1 promoted the upregulation of expression of WNT5A
and inhibited the invasion and proliferation of the melanoma cell
lines.
Discussion
Melanomas are prone to invasion and metastasis, and
are associated with a high mortality rate (28). Accordingly, further research on the
treatment of melanoma is greatly needed. The ʻWarburg effect'
states that cancer cells take up increased glucose compared to
their non-transformed counterparts and preferentially utilize
glycolytic metabolism to produce ATP, even in the presence of
oxygen, a phenomenon termed aerobic glycolysis (5). Accordingly, glycolysis is a potential
target of cancer therapy. CerSs, which consists of six members,
CerS1 to CerS6, regulates sphingolipid synthesis and numerous
aspects of cell biology, including cell growth, apoptosis,
autophagy and particularly cancer development (18–21).
In the present study, we analyzed the effect and mechanism of CerS6
in the regulation of the invasion and glycolysis of melanoma. CerS6
was found to be lowly expressed in the melanoma cell lines when
compared with the normal human epithelial cells. Silencing of CerS6
promoted the invasion and proliferation of the melanoma cell lines.
Additionally, CerS6 upregulated the activity of glycolysis-related
enzymes and the expression of glycolysis-related genes including
GLUT1 and MCT1. Furthermore, using gene chip and qPCR confirmation,
we found the expression of glycolysis-related gene SLC2A1 which
encoded protein GLUT1 was upregulated in the CerS6-silenced
melanoma cells, and notably WNT5A was downregulated. Silencing of
GLUT1 resulted in the increased expression of WNT5A in the melanoma
cells, and inhibited the invasion and proliferation capability of
the melanoma cells.
Recent data revealed that CerSs play important roles
in cancer regulation. CerS1-dependent generation of C18-ceramide
plays important roles in the pathogenesis of head and neck squamous
cell carcinoma (HNSCC) (29,30).
Additionally, as an important member of the CerS family, various
studies also suggest that CerS6 is involved in cancer etiology.
Knockdown of CerS6 resulted in the decreased expression of
C16-ceramide, and reduced TRAIL-induced apoptosis in colon
adenocarcinoma cells, while upregulated expression of CerS6
sensitized the cells to TRAIL (22). Moreover, downregulation of CerS6 in
HNSCC induced ER stress, and increased HNSCC tumor development and
growth were observed in CerS6-overexpressing cells (24). Furthermore, a microarray study
indicated CerS6 as an important participant in cancer
differentiation and early embryonic development (31). In the present study, we found that
CerS6 regulated the ability of proliferation, invasion and
glycolysis in the melanoma cell lines. Its suppressed expression in
melanoma cells may play essential roles in the development of
melanoma, and further research on targeting CerS6 for melanoma
treatment is warranted.
The role of CerS6 in cell apoptosis is distinctively
reported in different tissues. Senkal et al showed the
anti-apoptotic role of CerS6 in HNSCC (23). However, developmentally regulated
CerS6 was found to increase mitochondrial Ca2+ loading
capacity and promote apoptosis (32). Similarly, CerS6 knockdown inhibited
TRAIL-induced apoptosis (22). In
the present study, we detected the cell viability and colony
formation capability of melanoma cell lines after CerS6
overexpression and silencing. We found that the decreased
expression of CerS6 in melanoma cell lines led to the promotion of
cell viability and colony formation capability.
After silencing of CerS6, we found that the activity
of glycolysis-related enzymes was upregulated, as well as the
expression of glycolysis-related genes GLUT1 and MCT1. The
upregulated expression of GLUT1 decreased the expression of WNT5A,
the signaling of which in malignant melanoma cells alters cellular
energy metabolism and specifically increases aerobic glycolysis
(33). In a recent study, WNT5A was
suggested to promote melanoma cell migration/invasion via an
FZD-4-β-catenin-dependent mechanism (34). This is not in accordance with our
findings. In the present study, the upregulation of WNT5A induced
by the silencing of GLUT1 was accompanied by decreased invasion
capability in the melanoma cells. The reason for the descrepant
results between our results and the previous study may be
associated with different signaling pathways, or the downregulated
invasion in our study may not be directly regulated by WNT5A.
There is a limitation to our study. We investigated
the role of CerS6 which relied on overexpression and silencing of
single CerS6 protein as a gain-of-function model. However, little
is known about the regulation and interregulation of other CerS
proteins with regard to maintaining overall sphingolipid
homeostasis. Mullen et al demonstrated that the ability of
CerS knockdown to cause increases in sphingolipids was exemplified
by their attempted simultaneous knockdown of CerS2/5/6 (35). Unlike individual knockdown of CerS2
and CerS6, the resulting increases in sphingolipids imply that
there is a significant response of cells to the reduced CerS
expression or activity that involves the accumulation of these
lipids. Accordingly, the regulatory role of a single CerS still
needs further confirmation in a more systemic circumstance.
Collectively, CerS6 promoted the invasion and
glycolysis in melanoma cells. This provides a new mechanism of
CerS6 action in melanoma cells and should further establish its
future use as a valid chemopreventive and chemotherapeutic agent
against melanoma.
Acknowledgments
The present study was supported by the National
Natural Science Foundation of China (nos. 81572689, 81372140,
81301688, 81272192 and 81171882), the Ph.D. Programs Foundation of
the Ministry of Education of China (nos. 20130162110050 and
20130162120093), the Natural Science Foundation of Hunan Province
(no. 2015JJ4053), the Project of Science and Technology of Hunan
Province (nos. 2014SK2018 and 2015SF2066-2), the Program for New
Century Excellent Talents in University (NCET-11-0527), the
Postdoctoral Foundation of Central South University (no. 131425),
and the ʻ125 Talent Project' and ʻNew Xiangya Talent Project' of
the Third Xiangya Hospital of Central South University.
References
|
1
|
Xu D, Tan J, Zhou M, Jiang B, Xie H, Nie
X, Xia K and Zhou J: Let-7b and microRNA-199a inhibit the
proliferation of B16F10 melanoma cells. Oncol Lett. 4:941–946.
2012.PubMed/NCBI
|
|
2
|
Thompson JF, Scolyer RA and Kefford RF:
Cutaneous melanoma. Lancet. 365:687–701. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Stathem M, Marimuthu S, O'Neal J, Rathmell
JC, Chesney JA, Beverly LJ and Siskind LJ: Glucose availability and
glycolytic metabolism dictate glycosphingolipid levels. J Cell
Biochem. 116:67–80. 2015. View Article : Google Scholar
|
|
5
|
Vander Heiden MG, Cantley LC and Thompson
CB: Understanding the Warburg effect: The metabolic requirements of
cell proliferation. Science. 324:1029–1033. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Warburg O: On the origin of cancer cells.
Science. 123:309–314. 1956. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hannun YA and Obeid LM: Principles of
bioactive lipid signalling: Lessons from sphingolipids. Nat Rev Mol
Cell Biol. 9:139–150. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ogretmen B: Sphingolipids in cancer:
Regulation of pathogenesis and therapy. FEBS Lett. 580:5467–5476.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ogretmen B and Hannun YA: Biologically
active sphingolipids in cancer pathogenesis and treatment. Nat Rev
Cancer. 4:604–616. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mathias S, Peña LA and Kolesnick RN:
Signal transduction of stress via ceramide. Biochem J. 335:465–480.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Knapp P, Baranowski M, Knapp M, Zabielski
P, Błachnio-Zabielska AU and Górski J: Altered sphingolipid
metabolism in human endometrial cancer. Prostaglandins Other Lipid
Mediat. 92:62–66. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wu J, Cheng Y, Nilsson A and Duan RD:
Identification of one exon deletion of intestinal alkaline
sphingomyelinase in colon cancer HT-29 cells and a
differentiation-related expression of the wild-type enzyme in
Caco-2 cells. Carcinogenesis. 25:1327–1333. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bektas M, Jolly PS, Müller C, Eberle J,
Spiegel S and Geilen CC: Sphingosine kinase activity counteracts
ceramide-mediated cell death in human melanoma cells: Role of Bcl-2
expression. Oncogene. 24:178–187. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Samsel L, Zaidel G, Drumgoole HM, Jelovac
D, Drachenberg C, Rhee JG, Brodie AM, Bielawska A and Smyth MJ: The
ceramide analog, B13, induces apoptosis in prostate cancer cell
lines and inhibits tumor growth in prostate cancer xenografts.
Prostate. 58:382–393. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gouazé V, Liu YY, Prickett CS, Yu JY,
Giuliano AE and Cabot MC: Glucosylceramide synthase blockade
down-regulates P-glycoprotein and resensitizes multidrug-resistant
breast cancer cells to anticancer drugs. Cancer Res. 65:3861–3867.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Levy M and Futerman AH: Mammalian ceramide
synthases. IUBMB Life. 62:347–356. 2010.PubMed/NCBI
|
|
17
|
Pewzner-Jung Y, Ben-Dor S and Futerman AH:
When do Lasses (longevity assurance genes) become CerS (ceramide
synthases)?: Insights into the regulation of ceramide synthesis. J
Biol Chem. 281:25001–25005. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Teufel A, Maass T, Galle PR and Malik N:
The longevity assurance homologue of yeast lag1 (Lass) gene family
(Review). Int J Mol Med. 23:135–140. 2009.PubMed/NCBI
|
|
19
|
Harel R and Futerman AH: Inhibition of
sphingolipid synthesis affects axonal outgrowth in cultured
hippocampal neurons. J Biol Chem. 268:14476–14481. 1993.PubMed/NCBI
|
|
20
|
Bose R, Verheij M, Haimovitz-Friedman A,
Scotto K, Fuks Z and Kolesnick R: Ceramide synthase mediates
daunorubicin-induced apoptosis: An alternative mechanism for
generating death signals. Cell. 82:405–414. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Spassieva SD, Mullen TD, Townsend DM and
Obeid LM: Disruption of ceramide synthesis by CerS2 down-regulation
leads to autophagy and the unfolded protein response. Biochem J.
424:273–283. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
White-Gilbertson S, Mullen T, Senkal C, Lu
P, Ogretmen B, Obeid L and Voelkel-Johnson C: Ceramide synthase 6
modulates TRAIL sensitivity and nuclear translocation of active
caspase-3 in colon cancer cells. Oncogene. 28:1132–1141. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Senkal CE, Ponnusamy S, Rossi MJ,
Bialewski J, Sinha D, Jiang JC, Jazwinski SM, Hannun YA and
Ogretmen B: Role of human longevity assurance gene 1 and
C18-ceramide in chemotherapy-induced cell death in human
head and neck squamous cell carcinomas. Mol Cancer Ther. 6:712–722.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Senkal CE, Ponnusamy S, Bielawski J,
Hannun YA and Ogretmen B: Antiapoptotic roles of
ceramide-synthase-6-generated C16-ceramide via selective
regulation of the ATF6/CHOP arm of ER-stress-response pathways.
FASEB J. 24:296–308. 2010. View Article : Google Scholar :
|
|
25
|
Zhou J, Liu R, Wang Y, Tang J, Tang S,
Chen X, Xia K, Xiong W, Xu D, Wang S, et al: miR-199a-5p regulates
the expression of metastasis-associated genes in B16F10 melanoma
cells. Int J Clin Exp Pathol. 7:7182–7190. 2014.PubMed/NCBI
|
|
26
|
Zhou J, Xu D, Xie H, Tang J, Liu R, Li J,
Wang S, Chen X, Su J, Zhou X, Xia K, He Q, Chen J, Xiong W, Cao P
and Cao K: miR-33a functions as a tumor suppressor in melanoma by
targeting HIF-1α. Cancer Biol Ther. 16:846–855. 2015. View Article : Google Scholar
|
|
27
|
Zhang Y, Liu H, Jin J, Zhu X, Lu L and
Jiang H: The role of endogenous reactive oxygen species in
oxymatrine-induced caspase-3-dependent apoptosis in human melanoma
A375 cells. Anticancer Drugs. 21:494–501. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sun J, Han J, Zhu Q, Li Z and Hu J:
Camptothecin fails to induce apoptosis in tumor necrosis
factor-alpha-treated HaCaT cells. Pharmacology. 89:58–63. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Koybasi S, Senkal CE, Sundararaj K,
Spassieva S, Bielawski J, Osta W, Day TA, Jiang JC, Jazwinski SM,
Hannun YA, et al: Defects in cell growth regulation by
C18:0-ceramide and longevity assurance gene 1 in human
head and neck squamous cell carcinomas. J Biol Chem.
279:44311–44319. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Karahatay S, Thomas K, Koybasi S, Senkal
CE, Elojeimy S, Liu X, Bielawski J, Day TA, Gillespie MB, Sinha D,
et al: Clinical relevance of ceramide metabolism in the
pathogenesis of human head and neck squamous cell carcinoma
(HNSCC): Attenuation of C18-ceramide in HNSCC tumors
correlates with lymphovascular invasion and nodal metastasis.
Cancer Lett. 256:101–111. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Weinmann A, Galle PR and Teufel A: LASS6,
an additional member of the longevity assurance gene family. Int J
Mol Med. 16:905–910. 2005.PubMed/NCBI
|
|
32
|
Novgorodov SA, Chudakova DA, Wheeler BW,
Bielawski J, Kindy MS, Obeid LM and Gudz TI: Developmentally
regulated ceramide synthase 6 increases mitochondrial
Ca2+ loading capacity and promotes apoptosis. J Biol
Chem. 286:4644–4658. 2011. View Article : Google Scholar :
|
|
33
|
Sherwood V, Chaurasiya SK, Ekström EJ,
Guilmain W, Liu Q, Koeck T, Brown K, Hansson K, Agnarsdóttir M,
Bergqvist M, et al: WNT5A-mediated β-catenin-independent signalling
is a novel regulator of cancer cell metabolism. Carcinogenesis.
35:784–794. 2014. View Article : Google Scholar :
|
|
34
|
Grossmann AH, Yoo JH, Clancy J, Sorensen
LK, Sedgwick A, Tong Z, Ostanin K, Rogers A, Grossmann KF, Tripp
SR, et al: The small GTPase ARF6 stimulates β-catenin
transcriptional activity during WNT5A-mediated melanoma invasion
and metastasis. Sci Signal. 6:ra142013. View Article : Google Scholar
|
|
35
|
Mullen TD, Spassieva S, Jenkins RW,
Kitatani K, Bielawski J, Hannun YA and Obeid LM: Selective
knockdown of ceramide synthases reveals complex interregulation of
sphingolipid metabolism. J Lipid Res. 52:68–77. 2011. View Article : Google Scholar :
|