Introduction
Pituitary adenomas are relatively common neoplasms
that account for 10–15% of all intracranial tumors. More than
two-thirds of pituitary adenomas are characterized by excessive
hormonal secretion, which manifests in the form of distinctive
clinical syndromes (1,2). Based on the clinical presentation,
pituitary adenomas are classified as growth hormone- (GH),
adrenocorticotropic hormone- (ACTH), prolactin- (PRL), and
thyroid-stimulating hormone (TSH)-secreting pituitary adenomas, or
clinically non-functional adenomas. The most common
hormone-secreting adenomas are prolactinomas, followed by growth
hormone (GH) secreting and mixed GH and prolactin-producing
adenomas (3).
Surgical resection is the primary therapeutic
modality, although there are medical treatments available for
GH-secreting tumors (4). Indeed, in
patients with intrasellar microadenomas, microsurgical removal of
the tumor alone is sufficient for biochemical control as the
procedure is associated with normalization of IGF-I in 75–95% of
patients. However, in macroadenomas or aggressive adenomas, surgery
alone is not expected to achieve disease control or hormonal
remission in most cases (5,6). Surgical treatment of aggressive
GH-secreting pituitary adenomas carries a high risk of
recurrence/progression. These patients almost always need further
therapy to achieve biochemical control of the disease. Currently,
there is neither an effective methodology to distinguish
non-aggressive GH-secreting pituitary adenomas from aggressive
adenomas, nor is there an efficient way to predict the
recurrence/progression after surgery. Hence, research on
bio-markers associated with aggressive pituitary adenomas and
prognosis is a key imperative.
The Wnt signaling pathway is involved in several
developmental processes, including, tissue differentiation,
proliferation and apoptosis (7,8).
Aberrant regulation of the Wnt signaling pathway is thought to play
a role in tumorigenesis (9),
especially in pituitary adenomas (10,11).
The secreted frizzled related protein (sFRP) family, coded by a
class of pro-apoptotic genes, is thought to play an important role
in tumorigenesis by inhibiting Wnt signaling pathway (12). Upregulation of sFRPs expression, and
in particular that of sFRP4, has been shown to correlate with
apoptosis in various tissues (13–17).
Further, the restoration or upregulation of sFRPs expression in
cancer cells has been shown to attenuate grades and invasive growth
characteristics of tumors such as, prostate, ovarian, and cervical
cancer, which suggests that activities of sFRPs are fundamental for
tissue homeostasis (18–23).
Elston et al (24) reported significant downregulation of
sFRP4 in pituitary adenomas. Our previous study on gene microarrays
also demonstrated significant downregulation of sFRP4 in aggressive
NFPAs, as compared to that in non-aggressive NFPAs and normal
pituitary tissues (25). Similar
observations were forthcoming from yet another study conducted by
our group, where we demonstrated a negative correlation of sFRP4
with aggressiveness of NFPAs (26).
Promoter hypermethylation often accounts for the
loss of expression of tumor suppressor genes (27). Dense CpG islands flank the first
exons of sFRP1, sFRP2, sFRP4 and sFRP5, but not
sFRP3. These sequences have been reported to be
hypermethylated in several types of carcinoma, and particularly in
colorectal, gastric, mammary, prostate, renal cell, and chronic
lymphocytic leukemia (22,28–35).
In this study, we investigated the difference in
sFRP4 expression between the aggressive GH pituitary adenoma and
the non-aggressive GH pituitary adenoma through quantitative
real-time polymerase chain reaction (qRT-PCR), western blot
analysis, and tissue microarrays. The objective was to investigate
the potential role of sFRP4 as a prognostic biomarker for
GH-secreting pituitary adenomas, in terms of predicting
aggressiveness and the risk of recurrence. Furthermore, methylation
analysis of the sFRP4 promoter region was performed to figure out
the underlying mechanism for abnormal expression of sFRP4.
Materials and methods
Ethical approval
All procedures performed in studies involving human
participants were in accordance with the ethical standards of the
Ethics Committee of Beijing Tiantan Hospital, Beijing, China, and
with the 1964 Helsinki declaration and its later amendments or
comparable ethical standards.
Sample collection
A total of 52 patients with GH-secreting pituitary
adenoma (age range, 13–75 years) were enrolled at the Beijing
Tiantan Hospital (2008–2012). None of the patients received
somatostatin analog therapy in the preoperative period due to
economic reasons.
The inclusion criteria were: i) availability of
sufficient pituitary tissue specimen to allow for analysis by
tissue microarrays; ii) no history of pre-operative radiation; iii)
availability of clinical data, including endocrinological
evaluation results and imaging results; iv) minimum follow-up
duration of 3 years.
The diagnosis of biological behavior of the tumor
was made on the basis of preoperative magnetic resonance imaging
(MRI) and computed tomography (CT). Pituitary adenomas that were
classified as grade III or IV according to Hardy or Knops
classification were defined as aggressive.
Ten specimens of normal pituitary glands were
obtained from a donation program. The donors included 6 men and 4
women aged 21–45 years (mean age, 35 years). All of the donors had
died of non-neurological diseases. Written informed consent was
obtained from all donors prior to their enrollment; the study
protocol was approved by the Ethics Committee at the Beijing
Tiantan Hospital, Beijing, China.
Specimens were categorized into three groups: the
normal pituitary control group, non-aggressive group and aggressive
group. Pituitary tumors were removed by trans-sphenoidal surgery
and immediately 'flash-frozen' in liquid nitrogen until further
analyses. Suitable parts of each sample were embedded in
paraffin.
All patients were diagnosed based on clinical
symptoms, preoperative sellar MRI and postoperative
histopathological examination. Postoperative sella MRI scans were
performed within 72 h after surgery, to evaluate the residual mass.
Two neuroradiologists, and one neurosurgeon blinded to the
patients' characteristics conducted the evaluation. The same were
repeated at 6-month intervals in the first 2 postoperative years.
To investigate for tumor recurrence, serial sella MRI scans were
performed at 1-year intervals. However, in the presence of clinical
symptoms, sella MRI scan was performed immediately.
Recurrence/progression was defined on the basis of 1 or more of the
following parameters: i) presence of a new tumor in patients with a
total resection, based on the first post-operative MRI scan; ii)
evidence of new growth of an incompletely resected tumor on serial
postoperative MRI scans versus the immediate postoperative MRI
scan; iii) clinical deterioration or recurrence of symptoms after
surgery, and associated with elevated serum hormone levels.
Preoperative serum GH levels were recorded to
estimate the association of serum GH level with aggressiveness and
recurrence/progression of tumors. The results were categorized as
high level (≥ median level) and low level (< median level).
Quantitative real-time polymerase chain
reaction
Total RNA was isolated from frozen pituitary
adenomas and normal pituitaries (100–150 mg) using TRIzol reagent
(Invitrogen Life Technologies, 15596-026). The housekeeping gene
coding for glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was
used as an internal control. Relative quantification of gene
expression was determined using the 2−∆∆CT method as
described by Livak and Scmittgen (36). The primers used in the Qrt-PCR assay
are listed as follows: sFRP4 (forward, TGGCAACGTATCTCAGCAAA;
reverse, GGATGGGTGATGAGGACTTG), GAPDH (forward,
ACCACAGTCCATGCCATCACT; reverse, GTCCACCACCCTGTTGCTGTA). The
specificity of Qrt-PCR products was verified by performing
dissociation reaction plots.
Protein extraction and western blot
analysis
Protein extraction and western blot analysis (WB)
were performed as described elsewhere (37), using antibodies for anti-sFRP4
(1:5,000, Abcam, Cambridge, UK). Rabbit anti-GAPDH (1:1,000, Sigma,
St. Louis, MO, USA) was used as an internal control. Horseradish
peroxisdase-conjugated secondary antibodies (1:5,000, Sigma) were
used followed by enhanced chemiluminescence development (Amersham
Pharmacia Biotech, Piscataway, NJ, USA). The final data were
subjected to grayscale scanning and semi-quantitative analysis
using Quantity One Software (Bio-Rad, Hercules, CA, USA).
TMA construction and
immunohistochemistry
Formalin-fixed paraffin-embedded tissue blocks were
sliced and eosin-stained (H&E) slides were prepared. Three core
biopsies, 2.0-mm diameter, were selected from the paraffin-embedded
tissue. The cores were transferred to tissue microarrays using the
Leica Bond-III fully automated arrayer from Leica Biosystems
(Aperio, CA, USA). The locations of the core samples were in random
order, and the pathologist were blinded to the identity of the TMA
slides. The tissue microarrays were cut into 4-µm sections
using a serial microtome and placed in a water bath at 50°C,
followed by its application onto positively-charged glass slides.
Slides were dewaxed and then rehydrated through graded alcohols
into water. After mounting, the slides were dried at room
temperature for 24–48 h and stored at 4°C until further testing. To
minimize loss of antigenicity, the microarray slide was processed
within 1 week of cutting.
All TMA slides were evaluated in advance using an
H&E stain to assess tumor content and quality. The TMAs were
placed in the Leica BOND-III instrument, which is a fully
automated, random and continuous access slide-staining system that
processes IHC tests simultaneously. IHC protocol F was selected in
the machine, and 3 min with ER1 (epitope retrieval) was set for
heat-induced epitope retrieval (HIER) parameter. Bond™ Ploymer
Refine Detection (Leica Biosystems, DS9800) was used for detection
of primary antibodies. The slides were scanned into digital
pictures and expression was examined using Aperio AT2 (Leica
Biosystems). Primary antibodies, anti-sFRP4 (1:5,000, Abcam) were
used. The optimal titer of the primary Abs for the remainder was
determined based on pre-experiment results.
Evaluation of immunohistochemical
staining
Staining for sFRP4 was present in the cytoplasm and
nucleus. The results were calculated using Aperio AT2 (Leica
Biosystems) with digital slide viewing software. The staining
intensity of sFRP4 was scored as follows: 0, no; 1, weak; 2,
moderate; and 3, high intensity. The distribution of positively
stained cells was scored on a scale of 0–5 as follows: 0, no
staining; 1, <20%; 2, 20–40%; 3, 40–60%; 4, 60–80%; and 5,
80–100%. The total score was calculated as staining intensity ×
distribution (score ≤6: weak expression; score >6, strong
expression).
Methylation and sequencing analysis
DNA was extracted from 37 fresh-frozen
non-aggressive pituitary adenomas (15–50 mg), using TRIzol reagent
according to the manufacturer's protocol (Invitrogen). DNA
concentration and purity were measured by UV absorbance at 260/280
nm (Nanodrop ND-1000, USA). Before carrying out the methylation
analysis, we quantified the yield of extracted DNA from the
samples. The extracted DNA was of sufficient quality to allow
successful amplification. Bisulfite treatment of DNA was performed
using The EZ-96 DNA Methylation kit (Zymo Research, Orange, CA,
USA). Two overlapping PCRs were performed to amplify a 1,924-bp
area (−820 to +1,104, relative to the translation start site) of
the sFRP4 promoter containing 134 CpG dinucleotides. The RNase-A
enzyme (Sequenom, USA) was added to cleave the in vitro
transcripts (T-cleavage assay). For every cleaved CpG site, the
mass spectra were collected using MassARRAY Compact MALDI-TOF
(Sequenom) and the spectra methylation ratios were generated by
EpiTYPER software v1.0 (Sequenom).
Cell culture, DAC treatment and western
blot analysis of cell lines
GH3 pituitary adenoma cell lines were cultured in
Dulbecco's modified Eagle's medium (DMEM; Gibco-BRL, Gaithersburg,
MD, USA) supplemented with 10% fetal bovine serum, at 37°C in a
humidified atmosphere containing 5% CO2. The
demethylating agent, DAC (5-aza-2-deoxycytidine; Sigma), was
freshly prepared in ddH2O. GH3 cells (3×105
cells/well) in exponential growth phase were seeded in 6-well
plates. After 24 h of culture, cells were treated with DAC at
concentration of 0, 10, 20, 30 and 40 nM for 72 h. The culture
medium was replaced every 24 h with fresh media containing DAC.
Total proteins were extracted for WB analysis, which was performed
using the standard procedure, and the proteins were identified
using anti-sFRP4 (1:5,000, Abcam), rabbit anti-GAPDH (1:1,000,
Sigma) antibodies.
Cell viability assay (MTS)
Cell viability was assessed by
3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium
(MTS) assay using MTS reagent (CellTiter 96 AQueous One Solution
Cell Proliferation assay, Promega). Briefly, 2×104
exponentially growing GH3 cells were seeded in 96-well culture
plates with DAC at concentration of 0, 10, 20, 40 and 80 nM. After
24, 48, 72 of incubation at 37°C, 20 µl of MTS was added to
each well, and the samples incubated for a further 3 h at 37°C.
Plates were analyzed on a Tecan M200Pro multimode microplate reader
at 492 nm. The cell inhibition rate was calculated as follows:
Inhibition rate (%) = (OD of control group - OD of drug group)/OD
of control group × 100%.
Statistical analysis
Differences between subgroups were analyzed using
the Student's t-test for normally distributed continuous values,
and Mann-Whitney U test for non-normally distributed continuous
values. The χ2 test was used to analyze categorical
variables. Variables that showed association with aggressiveness
and recurrence of GH-secreting pituitary adenomas were subjected to
univariate and multivariate analyses. Two-sided P-values of
<0.05 were considered statistically significant. SPSS software
version 17.0 (IBM Corp., Armonk, NY, USA) was used for statistical
analyses.
Results
Clinical and pathological features
Details of the GH pituitary adenoma specimens
included in the study are shown in Table I. Fifty-two GH-secreting pituitary
adenomas met the inclusion criteria. Patient age ranged from 13 to
75 years (mean, 40.3 years; median, 39.5 years) at the time of the
first surgical treatment. Out of the 52 patients, 24 patients
(46.2%) were female, and 28 (53.8%) were male. There were 4 (26.7%)
females and 11 (73.3%) males in the aggressive group (N=23), and 20
(54.1%) females and 17 (45.9%) males in the non-aggressive group
(N=25). Gross total resection was found in 35 (67.3%) out of the 52
patients. The preoperative serum GH results ranged from 0.13 to 48
ng/ml (mean, 22.82 ng/ml, median, 21.35 ng/ml).
 | Table IClinico-pathological characteristics
of 52 patients with GH-secreting pituitary adenomas. |
Table I
Clinico-pathological characteristics
of 52 patients with GH-secreting pituitary adenomas.
| Variables | N | Percentage (%) |
|---|
| Gender | | |
| Male | 28 | 53.8 |
| Female | 24 | 46.2 |
| Age (years) | | |
| Mean | 40.3 | |
| Median | 39.5 | |
| Aggressiveness | | |
| Aggressive | 15 | 28.8 |
|
Non-aggressive | 37 | 71.2 |
| Preoperative serum
GH level (ng/ml) | | |
| Mean | 22.82 | |
| Median | 21.35 | |
| Surgical
extent | | |
| Gross total
resection | 35 | 67.3 |
| Residual | 17 | 32.7 |
| Recurrence (within
42 months) | | |
| Yes | 11 | 21.2 |
| No | 41 | 78.8 |
Quantitative-PCR and western blot
analysis
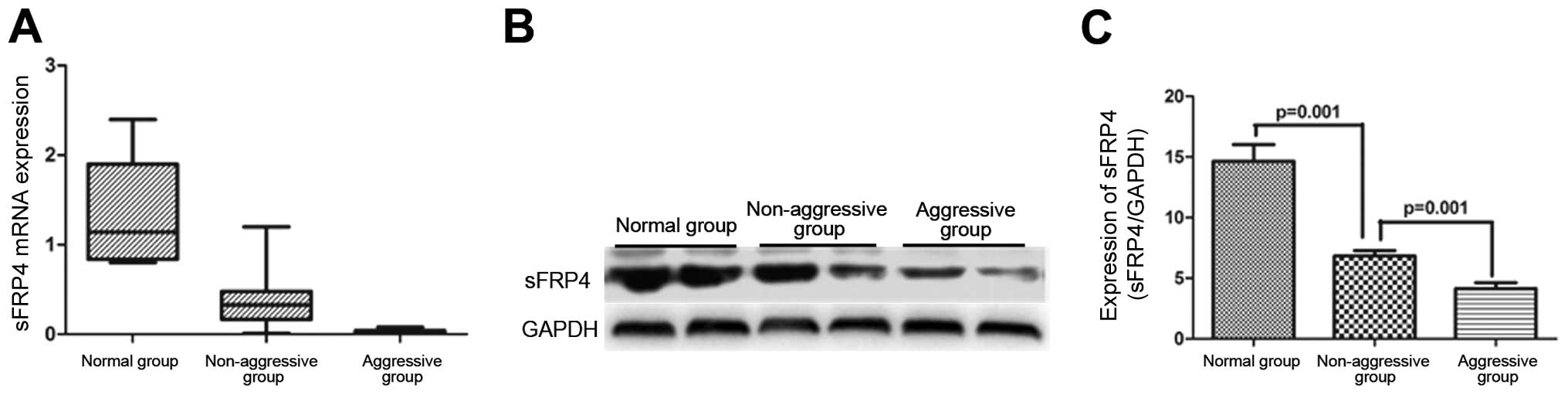
qRT-PCR revealed a significant downregulation of
sFRP4 mRNA level in both aggressive- as well as non-aggressive
GH-secreting pituitary adenomas, as compared to that in the normal
controls (0.031±0.006 vs. 1.35±0.19, P<0.001, N=15 vs. N=10;
0.981±0.111 vs. 1.35±0.19, P<0.001, N=37 vs. N=10) (Fig. 1A). The sFRP4 mRNA level was
significantly lower in aggressive group as compared to that in the
non-aggressive group (0.031±0.006 vs. 0.981±0.111, P=0.001, N=15
vs. N=37) (Fig. 1A).
The sFRP4 protein expression in the three groups was
assessed by WB (Fig. 1B). There was
a significantly lower sFRP4 protein level in the non-aggressive
group as compared to that in the normal pituitary tissues
(6.82±0.45 vs. 14.65±1.38, P<0.001, N=37 vs. N=10); the
expression was even lower in the aggressive group as compared to
that in the non-aggressive group (4.13±0.49 vs. 6.82±0.45, P=0.004,
N=15 vs. N=37) (Fig. 1C).
Tissue microarrays analysis
sFRP4 expression was detected in all specimens by
TMA (Fig. 2). On univariate
analysis, sFRP4 expression showed no association with age, gender,
and preoperative serum GH levels. However, a significant
relationship was found between aggressive behavior and sFRP4
expression (P=0.024) (Table
II).
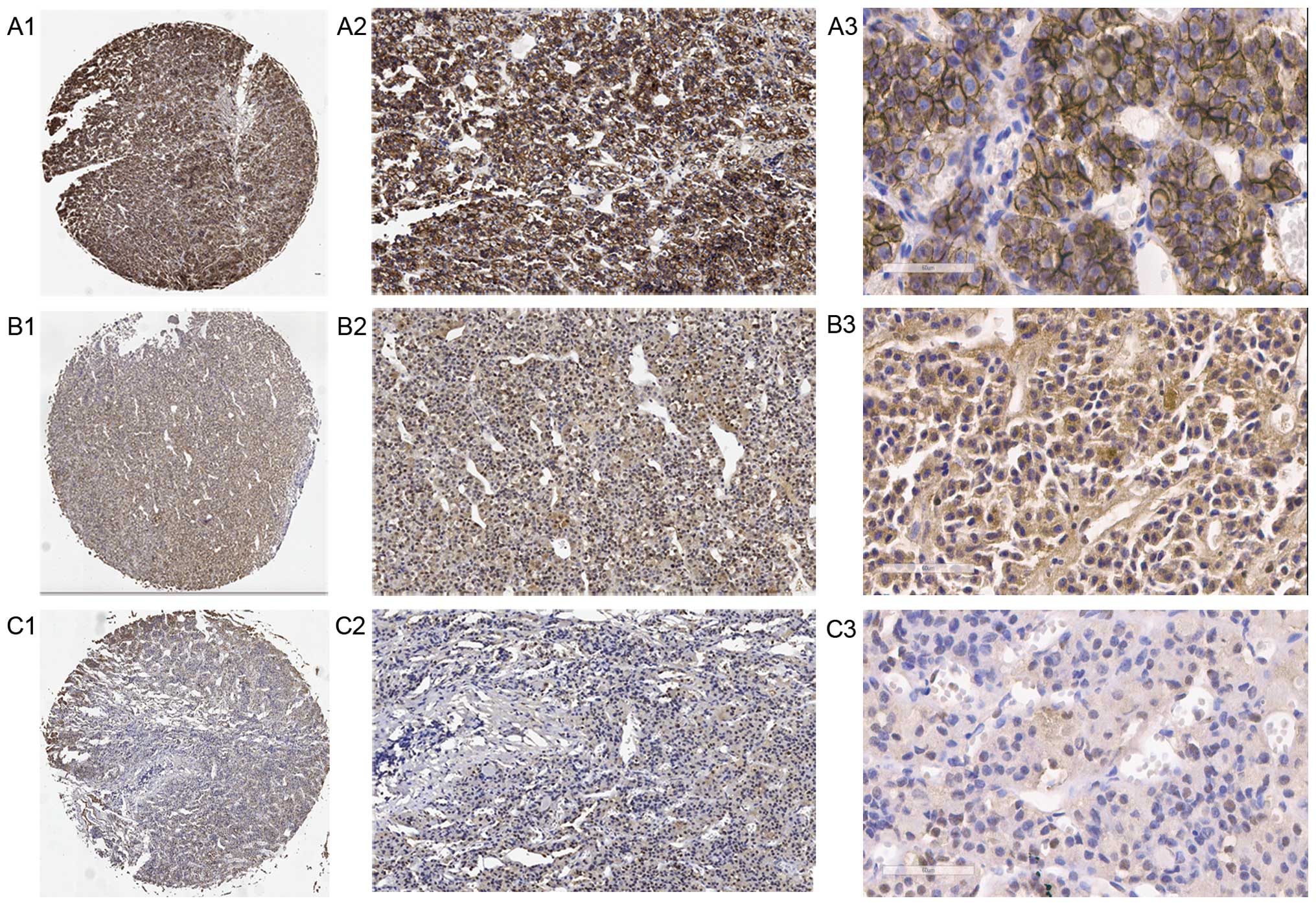 | Figure 2Expression of sFRP4 was assessed by
TMA. (A) Weak sFRP4-positive cells from normal pituitary tissues
(A1, ×40, A2, × and A3, ×400 magnification). (B) Weak
sFRP4-positive cells from non-aggressive adenoma (B1, ×40, B2, ×
and B3, ×400 magnification). (C) Strong sFRP4-positive cells from
aggressive pituitary adenomas (C1, ×, C2, ×100 and C3, ×400
magnification). TMA, tissue microarray. |
 | Table IIAssociation between sFRP4 (TMA)
expression and clinico-pathological characteristics. |
Table II
Association between sFRP4 (TMA)
expression and clinico-pathological characteristics.
| Variables | sFRP4 expression
| Univariate analysis
|
|---|
Weak
(N=22) | Strong
(N=30) | χ2 | P-value |
|---|
| Gender | | | | |
| Male | 14 (63.6%) | 14 (46.7%) | 1.471 | 0.225 |
| Female | 8 (36.4%) | 16 (53.3%) | | |
| Age | | | | |
| ≤39 | 11 (53.8%) | 15 (46.2%) | 0 | 1 |
| >39 | 11 (46.2%) | 15 (53.8%) | | |
| Aggressiveness | | | | |
| Yes | 10 (45.5%) | 5 (16.7%) | 5.125 | 0.024 |
| No | 12 (54.5%) | 25 (83.3%) | | |
| Preoperative serum
GH level | | | | |
| High | 10 (45.5%) | 13 (43.3%) | 0.023 | 0.879 |
| Low | 12 (54.5%) | 17 (56.7%) | | |
On comparing the total score for sFRP4 staining with
the aggressive behavior, the sFRP4 expression was downregulated in
most of the aggressive GH-secreting pituitary adenomas (10/15,
66.7%) (Table III). Only 12
(32.4%) pituitary adenomas expressed weak sFRP4 expression among
the 37 non-aggressive GH-secreting pituitary adenomas. On
univariate analysis a significant association was found between
sFRP4 expression and aggressiveness of tumors (P=0.024) (Table III). However, age, gender and
preoperative serum GH level were not found to be associated with
aggressiveness (Table III).
 | Table IIIUnivariate and multivariate analyses
for the clinico-pathological correlates of aggressive GH secreting
pituitary adenomas. |
Table III
Univariate and multivariate analyses
for the clinico-pathological correlates of aggressive GH secreting
pituitary adenomas.
| Variables | Aggressiveness N
(%)
| Univariate analysis
|
|---|
| No (N=37) | Yes (N=15) | χ2 | P-value |
|---|
| Gender | | | | |
| Male | 17 (45.9%) | 11 (73.3%) | 3.221 | 0.073 |
| Female | 20 (54.1%) | 4 (26.7%) | | |
| Age | | | | |
| ≤39 | 17 (45.9%) | 9 (60%) | 0.843 | 0.358 |
| >39 | 20 (54.1%) | 6 (40%) | | |
| sFRP4 | | | | |
| Yes | 25 (67.6%) | 5 (33.3%) | 5.125 | 0.024 |
| No | 12 (32.4%) | 10 (66.7%) | | |
| Preoperative serum
GH level | | | | |
| Low | 23 (62.2%) | 6 (40%) | 2.125 | 0.145 |
| High | 14 (37.8%) | 9 (60%) | | |
Follow-up data were available for all 52 patients.
Patients were followed up for 42 months. During follow-up, 11
patients (21.2%) experienced recurrence (Table I). Out of the 11 recurrent adenomas,
10 (90.9%) had weak sFRP4 expression (Table IV). Weak sFRP4 expression was found
only in 16 of 41 patients (39%) with non-recurrent GH-secreting
pituitary adenomas. On univariate analysis, weak sFRP4 expression
(P=0.002), increased aggressiveness (P=0.034), and surgical extent
(P= 0.014) were significantly associated with
recurrence/progression (Table IV).
Gender, age, and preoperative serum GH levels were not associated
with recurrence (Table IV).
 | Table IVUnivariate and multivariate analyses
for the clinico-pathological correlates of
recurrence/progression-free survival. |
Table IV
Univariate and multivariate analyses
for the clinico-pathological correlates of
recurrence/progression-free survival.
| Variables |
Recurrence
(within 42 months)
| Univariate analysis
| Multivariate
analysis
|
|---|
| Yes (N=11) | No (N=41) | χ2 | P-value | Odds ratio (95%
CI) | P-value |
|---|
| Gender | | | | | | |
| Male | 6 (54.5%) | 22 (53.7%) | 0.003 | 0.958 | | |
| Female | 5 (45.5%) | 19 (46.3%) | | | | |
| Age | | | | | | |
| ≤39 | 5 (45.5%) | 21 (51.2%) | 0.115 | 0.734 | | |
| >39 | 6 (54.5%) | 20 (48.8%) | | | | |
| sFRP4 (TMA) | | | | | | |
| Strong | 1 (9.1%) | 25 (61%) | 9.339 | 0.002 | 0.063
(0.06–0.722) | 0.026 |
| Weak | 10 (90.9%) | 16 (39%) | | | | |
| Aggressiveness | | | | | | |
| Yes | 6 (54.5%) | 9 (22%) | 4.489 | 0.034 | 2.367
(0.14–39.82) | 0.55 |
| No | 5 (45.5%) | 32 (78%) | | | | |
| Preoperative serum
GH level | | | | | | |
| Low | 5 (45.5%) | 24 (58.5%) | 0.602 | 0.438 | | |
| High | 6 (54.5%) | 17 (41.5%) | | | | |
| Surgical
extent | | | | | | |
| Gross total
resection | 4 (36.4%) | 31 (75.6%) | 6.071 | 0.014 | 0.139
(0.009–2.15) | 0.155 |
| Partial
resection | 7 (63.6%) | 10 (24.4%) | | | | |
On multivariate analysis, only decreased sFRP4
expression was found to be independently associated with increased
aggressiveness [odds ratio (OR): 0.063, P=0.026] (Table IV).
Methylation analysis of the sFRP4
promoter region
Methylation analysis was performed to investigate
the contribution of hypermethylation to the loss of sFRP4
expression. The 52 samples were classified into two groups: weak
sFRP4 staining and strong sFRP4 staining groups, according to the
total TMA score. One CpG site (+351 bp) was found to be
hypermethylated in pituitary adenomas. The methylation ratio of the
CpG site in the weak sFRP4 staining group was higher than that in
strong sFRP4 staining group (11.83±1.12 vs. 5.53±0.58%, P<0.001,
N=22 vs. N=30).
Western blot and MTS analysis of cell
lines
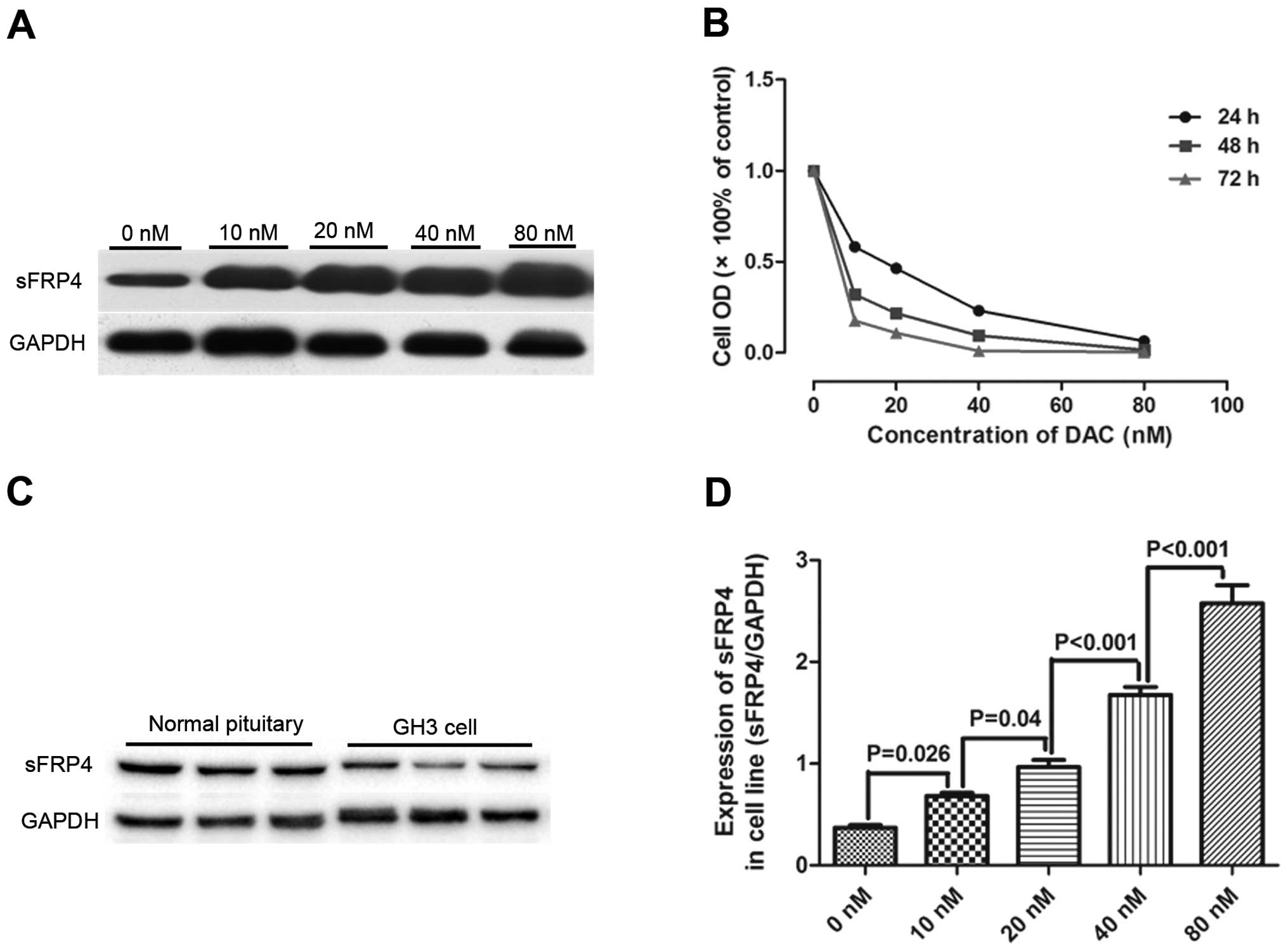
The sFRP4 protein expressions in pituitary adenoma
cell lines (GH3), as well as in normal human pituitary tissue were
assessed by western blotting. As shown in Fig. 3A, sFRP4 expression was significantly
downregulated in pituitary adenoma cell lines (GH3) as compared to
that in the normal human pituitary (0.32±0.03 vs. 0.7±0.05,
P=0.023, N=9 vs. N=9). MTS analysis showed a dose- and
time-dependent inhibition of GH3 cell proliferation induced by DAC.
A maximal inhibition of 87.6±2.2% at a concentration of 80 nM after
72 h was observed. The cells were significantly inhibited at all
concentrations of DAC used in this study (0, 10, 20, 40 and 80 nM)
after 3-day exposure. Growth curves for GH3 cells for various DAC
concentrations are shown in (Fig.
3B), indicating that DAC suppressed cell growth in a
dose-dependent and time-dependent manner. We evaluated the level of
sFRP4 protein in GH3 cells after DAC treatment by WB analysis
(Fig. 3C). GH3 cells after DAC
treatment at the concentration of 10 nM exhibited upregulation of
sFRP4 protein compared with GH3 cells without DAC treatment
(0.32±0.03 vs. 0.7±0.05, P=0.023, N=8 vs. N=8). There was a
progressive increase in sFRP4 expression with the increase in DAC
concentration (Fig. 3D). Both MTS
and WB analysis revealed that DAC inhibits the growth of GH3 cells
possibly by upregulating the expression of sFRP4 in GH3 cells.
Discussion
In this study, we compared the expression of sFRP4,
both at the mRNA and protein level, in normal pituitary tissue, and
in non-aggressive and aggressive GH-secreting pituitary adenomas.
We documented a significant downregulation of sFRP4 mRNA levels in
aggressive GH-secreting pituitary adenomas as compared to that in
both normal controls and in the non-aggressive GH-secreting
pituitary adenomas. This was further confirmed by WB and tissue
microarray analyses. Expression of sFRP4 was negatively linked to
aggressiveness and recurrence/progression of tumors, indicating
that sFRP4 may be used as biomarker for predicting aggressiveness
and recurrence/progression of GH-secreting pituitary adenomas.
However, no significant association of sFRP4 expression with
gender, age and preoperative serum GH level, was observed.
Methylation and sequencing analysis of the sFRP4 promoter region
showed it to be densely methylated in the weak sFRP4 staining
group, whereas there was significantly decreased methylation in
strong sFRP4 staining group. In addition, treatment with
demethylation agent (5-Aza-dc) and histone deacetylase inhibitor
(TSA) restored sFRP4 mRNA expression in pituitary adenoma cell
lines (GH3). Our results indicate that downregulation of sFRP4
expression in the weak sFRP4 staining group correlate with CpG
methylation of the sFRP4 promoter.
To the best of our knowledge, this study is the
first to demonstrate correlation of sFRP4 expression with
aggressiveness of GH-secreting pituitary adenomas. Similar results
have been reported elsewhere in other cancers (21,38).
Even though we showed a negative relationship between weak sFRP4
expression and aggressiveness, we did not find any relationship
between gender, age, preoperative serum GH level and aggressiveness
of the tumors. So weak sFRP4 maybe serve as an independent factor
of aggressiveness in GH-secreting pituitary adenomas.
Accumulated evidence indicates an association of low
sFRP4 expression with recurrence/progression in several cancers
(39,40). In this study, 52 patients were
followed up for a period of 42 months with clinical and imaging
data, and is one of the few studies to report recurrence rates for
GH-secreting pituitary adenomas after surgery. In our study, the
univariate analysis showed a significant association between low
sFRP4 expression, surgical extent, tumor aggressiveness and
recurrence/progression of tumors. However, on multivariate
analysis, only low sFRP4 expression was an independent predictor of
recurrence/progression of tumor, and tumor aggressiveness and
surgical extent were not significantly associated with the
recurrence/progression. This observation is in contrast to findings
of some other reports that tumor resection and tumor aggressiveness
were predictive factors for recurrence of pituitary adenomas
(41,42). The relatively short duration of
follow-up may, at least partially, explain this difference.
Further, we used logistic regression analysis
instead of survival analysis in consideration of potential
censoring caused by low recurrence rate, so survival time was not
included in our study. All of these factors could have led to the
contradictory results. These differences highlight the continued
need for further research with a larger sample size and longer
duration of clinical follow-up.
Hypermethylation of the sFRP4 gene has been reported
in various cancers, and is associated with tumor progression and
malignancy (22,43). In our study, silencing of SFRP4
expression correlated with the promoter methylation. At cellular
level, we observed a restoration of SFRP4 expression in pituitary
adenoma cells (GH3) after treatment with the demethylating agent,
5-aza-2 V-deoxycytidine. These findings are consistent with studies
conducted on various other malignancies, including gastric,
cervical, hepatocellular, pancreatic, oral squamous cell, breast,
colon, and bladder cancers (44–49).
In conclusion, in this study weak sFRP4 expression
appeared to be a predictive factor for aggressive behavior of
GH-secreting pituitary adenomas. Furthermore, weak sFRP4 expression
was also associated with post-operative tumor
recurrence/progression. We believe that methylation of the sFRP4
promoter could account for the decreased sFRP4 expression.
Acknowledgments
This study was supported by the National High-tech
Research and Development Program (2014AA020610), National Health
and Famliy Planning Commission for Public Welfare Industry Research
Project (201402008), National Natural Science Foundation of China
(3157060076).
Abbreviations:
|
sFRPs
|
secreted frizzled-related proteins
|
|
GH
|
growth hormone
|
|
CT
|
computed tomography
|
|
MRI
|
magnetic resonance imaging
|
|
NFPs
|
non-functioning pituitary adenomas
|
|
PCR
|
polymerase chain reaction
|
|
TMA
|
tissue microarray
|
|
IHC
|
immunohistochemistry
|
|
GAPDH
|
glyceraldehyde-3-phosphate
dehydrogenase
|
References
|
1
|
Ezzat S, Asa SL, Couldwell WT, Barr CE,
Dodge WE, Vance ML and McCutcheon IE: The prevalence of pituitary
adenomas: A systematic review. Cancer. 101:613–619. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Melmed S: Pathogenesis of pituitary
tumors. Nat Rev Endocrinol. 7:257–266. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Horn K, Erhardt F, Fahlbusch R, Pickardt
CR, Werder KV and Scriba PC: Recurrent goiter, hyperthyroidism,
galactorrhea and amenorrhea due to a thyrotropin and
prolactin-producing pituitary tumor. J Clin Endocrinol Metab.
43:137–143. 1976. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dusek T, Kastelan D, Melada A, Baretic M,
Skoric Polovina T, Perkovic Z, Giljevic Z, Jelcic J, Paladino J,
Aganovic I, et al: Clinical features and therapeutic outcomes of
patients with acromegaly: Single-center experience. J Endocrinol
Invest. 34:e382–e385. 2011.PubMed/NCBI
|
|
5
|
Hofstetter CP, Mannaa RH, Mubita L, Anand
VK, Kennedy JW, Dehdashti AR and Schwartz TH: Endoscopic endonasal
transsphenoidal surgery for growth hormone-secreting pituitary
adenomas. Neurosurg Focus. 29:E62010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tabaee A, Anand VK, Barrón Y, Hiltzik DH,
Brown SM, Kacker A, Mazumdar M and Schwartz TH: Endoscopic
pituitary surgery: A systematic review and meta-analysis. J
Neurosurg. 111:545–554. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Clevers H and Nusse R: Wnt/β-catenin
signaling and disease. Cell. 149:1192–1205. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
MacDonald BT, Tamai K and He X:
Wnt/beta-catenin signaling: Components, mechanisms, and diseases.
Dev Cell. 17:9–26. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Herr P, Hausmann G and Basler K: WNT
secretion and signalling in human disease. Trends Mol Med.
18:483–493. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gueorguiev M and Grossman AB: Pituitary
gland and beta-catenin signaling: From ontogeny to oncogenesis.
Pituitary. 12:245–255. 2009. View Article : Google Scholar
|
|
11
|
Gueorguiev M and Grossman AB: Pituitary
tumors in 2010: A new therapeutic era for pituitary tumors. Nat Rev
Endocrinol. 7:71–73. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cruciat CM and Niehrs C: Secreted and
transmembrane wnt inhibitors and activators. Cold Spring Harb
Perspect Biol. 5:a0150812013. View Article : Google Scholar
|
|
13
|
Constantinou T, Baumann F, Lacher MD,
Saurer S, Friis R and Dharmarajan A: SFRP-4 abrogates
Wnt-3a-induced beta-catenin and Akt/PKB signalling and reverses a
Wnt-3a-imposed inhibition of in vitro mammary differentiation. J
Mol Signal. 3:102008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Longman D, Arfuso F, Viola HM, Hool LC and
Dharmarajan AM: The role of the cysteine-rich domain and
netrin-like domain of secreted frizzled-related protein 4 in
angiogenesis inhibition in vitro. Oncol Res. 20:1–6. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Froeling FE, Feig C, Chelala C, Dobson R,
Mein CE, Tuveson DA, Clevers H, Hart IR and Kocher HM: Retinoic
acid-induced pancreatic stellate cell quiescence reduces paracrine
Wnt-beta-catenin signaling to slow tumor progression.
Gastroenterology. 141:1486–1497. 2011. View Article : Google Scholar
|
|
16
|
Granados-Principal S, Quiles JL,
Ramirez-Tortosa C, Camacho-Corencia P, Sanchez-Rovira P,
Vera-Ramirez L and Ramirez-Tortosa MC: Hydroxytyrosol inhibits
growth and cell proliferation and promotes high expression of sfrp4
in rat mammary tumours. Mol Nutr Food Res. 55(Suppl 1): S117–S126.
2011. View Article : Google Scholar
|
|
17
|
Frank B, Hoffmeister M, Klopp N, Illig T,
Chang-Claude J and Brenner H: Single nucleotide polymorphisms in
Wnt signaling and cell death pathway genes and susceptibility to
colorectal cancer. Carcinogenesis. 31:1381–1386. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hrzenjak A, Tippl M, Kremser ML,
Strohmeier B, Guelly C, Neumeister D, Lax S, Moinfar F, Tabrizi AD,
Isadi-Moud N, et al: Inverse correlation of secreted
frizzled-related protein 4 and beta-catenin expression in
endometrial stromal sarcomas. J Pathol. 204:19–27. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
O'Hurley G, Perry AS, O'Grady A, Loftus B,
Smyth P, O'Leary JJ, Sheils O, Fitzpatrick JM, Hewitt SM, Lawler M,
et al: The role of secreted frizzled-related protein 2 expression
in prostate cancer. Histopathology. 59:1240–1248. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Saran U, Arfuso F, Zeps N and Dharmarajan
A: Secreted frizzled-related protein 4 expression is positively
associated with responsiveness to cisplatin of ovarian cancer cell
lines in vitro and with lower tumour grade in mucinous ovarian
cancers. BMC Cell Biol. 13:252012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Marsit CJ, Karagas MR, Andrew A, Liu M,
Danaee H, Schned AR, Nelson HH and Kelsey KT: Epigenetic
inactivation of SFRP genes and TP53 alteration act jointly as
markers of invasive bladder cancer. Cancer Res. 65:7081–7085. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Qi J, Zhu YQ, Luo J and Tao WH:
Hypermethylation and expression regulation of secreted
frizzled-related protein genes in colorectal tumor. World J
Gastroenterol. 12:7113–7117. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chung MT, Lai HC, Sytwu HK, Yan MD, Shih
YL, Chang CC, Yu MH, Liu HS, Chu DW and Lin YW: SFRP1 and SFRP2
suppress the transformation and invasion abilities of cervical
cancer cells through Wnt signal pathway. Gynecol Oncol.
112:646–653. 2009. View Article : Google Scholar
|
|
24
|
Elston MS, Gill AJ, Conaglen JV, Clarkson
A, Shaw JM, Law AJ, Cook RJ, Little NS, Clifton-Bligh RJ, Robinson
BG, et al: Wnt pathway inhibitors are strongly down-regulated in
pituitary tumors. Endocrinology. 149:1235–1242. 2008. View Article : Google Scholar
|
|
25
|
Hong L, Wu Y, Feng J, Yu S, Li C, Wu Y, Li
Z, Cao L, Wang F and Zhang Y: Overexpression of the cell adhesion
molecule claudin-9 is associated with invasion in pituitary
oncocytomas. Hum Pathol. 45:2423–2429. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wu Y, Bai J, Li Z, Wang F, Cao L, Liu C,
Yu S, Yu G and Zhang Y: Low expression of secreted frizzled-related
protein 4 in aggressive pituitary adenoma. Pituitary. 18:335–342.
2015. View Article : Google Scholar
|
|
27
|
Choi JD and Lee JS: Interplay between
epigenetics and genetics in Cancer. Genomics Inform. 11:164–173.
2013. View Article : Google Scholar
|
|
28
|
Kinoshita T, Nomoto S, Kodera Y, Koike M,
Fujiwara M and Nakao A: Decreased expression and aberrant
hypermethylation of the SFRP genes in human gastric cancer.
Hepatogastroenterology. 58:1051–1056. 2011.PubMed/NCBI
|
|
29
|
Urakami S, Shiina H, Enokida H, Kawakami
T, Kawamoto K, Hirata H, Tanaka Y, Kikuno N, Nakagawa M, Igawa M,
et al: Combination analysis of hypermethylated Wnt-antagonist
family genes as a novel epigenetic biomarker panel for bladder
cancer detection. Clin Cancer Res. 12:2109–2116. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liu TH, Raval A, Chen SS, Matkovic JJ,
Byrd JC and Plass C: CpG island methylation and expression of the
secreted frizzled-related protein gene family in chronic
lymphocytic leukemia. Cancer Res. 66:653–658. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Perry AS, O'Hurley G, Raheem OA, Brennan
K, Wong S, O'Grady A, Kennedy AM, Marignol L, Murphy TM, Sullivan
L, et al: Gene expression and epigenetic discovery screen reveal
methylation of SFRP2 in prostate cancer. Int J Cancer.
132:1771–1780. 2013. View Article : Google Scholar
|
|
32
|
Kawakami K, Yamamura S, Hirata H, Ueno K,
Saini S, Majid S, Tanaka Y, Kawamoto K, Enokida H, Nakagawa M, et
al: Secreted frizzled-related protein-5 is epigenetically
downregulated and functions as a tumor suppressor in kidney cancer.
Int J Cancer. 128:541–550. 2011. View Article : Google Scholar
|
|
33
|
Zhang YW, Miao YF, Yi J, Geng J, Wang R
and Chen LB: Transcriptional inactivation of secreted
frizzled-related protein 1 by promoter hypermethylation as a
potential biomarker for non-small cell lung cancer. Neoplasma.
57:228–233. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Su HY, Lai HC, Lin YW, Liu CY, Chen CK,
Chou YC, Lin SP, Lin WC, Lee HY and Yu MH: Epigenetic silencing of
SFRP5 is related to malignant phenotype and chemoresistance of
ovarian cancer through Wnt signaling pathway. Int J Cancer.
127:555–567. 2010. View Article : Google Scholar
|
|
35
|
Suzuki H, Watkins DN, Jair KW, Schuebel
KE, Markowitz SD, Chen WD, Pretlow TP, Yang B, Akiyama Y, Van
Engeland M, et al: Epigenetic inactivation of SFRP genes allows
constitutive WNT signaling in colorectal cancer. Nat Genet.
36:417–422. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
36
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
37
|
Zhao P, Wang H, Gao H, Li C and Zhang Y:
Reversal of multidrug resistance by magnetic
chitosan-Fe3O4 nanoparticle-encapsulated
MDR1 siRNA in glioblastoma cell line. Neurol Res.
35:821–828. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Horvath LG, Lelliott JE, Kench JG, Lee CS,
Williams ED, Saunders DN, Grygiel JJ, Sutherland RL and Henshall
SM: Secreted frizzled-related protein 4 inhibits proliferation and
metastatic potential in prostate cancer. Prostate. 67:1081–1090.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Páez D, Gerger A, Zhang W, Yang D, Labonte
MJ, Benhanim L, Kahn M, Lenz F, Lenz C, Ning Y, et al: Association
of common gene variants in the WNT/β-catenin pathway with colon
cancer recurrence. Pharmacogenomics J. 14:142–150. 2014. View Article : Google Scholar
|
|
40
|
Yip PY, Kench JG, Rasiah KK, Benito RP,
Lee CS, Stricker PD, Henshall SM, Sutherland RL and Horvath LG: Low
AZGP1 expression predicts for recurrence in margin-positive,
localized prostate cancer. Prostate. 71:1638–1645. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Shimon I, Cohen ZR, Ram Z and Hadani M:
Transsphenoidal surgery for acromegaly: endocrinological follow-up
of 98 patients. Neurosurgery. 48:1239–1243; discussion 1244–1235.
2001.PubMed/NCBI
|
|
42
|
Shirvani M and Motiei-Langroudi R:
Transsphenoidal surgery for growth hormone-secreting pituitary
adenomas in 130 patients. World Neurosurg. 81:125–130. 2014.
View Article : Google Scholar
|
|
43
|
Nojima M, Suzuki H, Toyota M, Watanabe Y,
Maruyama R, Sasaki S, Sasaki Y, Mita H, Nishikawa N, Yamaguchi K,
et al: Frequent epigenetic inactivation of SFRP genes and
constitutive activation of Wnt signaling in gastric cancer.
Oncogene. 26:4699–4713. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Lin YW, Chung MT, Lai HC, De Yan M, Shih
YL, Chang CC and Yu MH: Methylation analysis of SFRP genes family
in cervical adenocarcinoma. J Cancer Res Clin Oncol. 135:1665–1674.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Shih YL, Hsieh CB, Yan MD, Tsao CM, Hsieh
TY, Liu CH and Lin YW: Frequent concomitant epigenetic silencing of
SOX1 and secreted frizzled-related proteins (SFRPs) in human
hepatocellular carcinoma. J Gastroenterol Hepatol. 28:551–559.
2013. View Article : Google Scholar
|
|
46
|
Botla SK, Gholami AM, Malekpour M,
Moskalev EA, Fallah M, Jandaghi P, Aghajani A, Bondar IS,
Omranipour R, Malekpour F, et al: Diagnostic values of GHSR DNA
methylation pattern in breast cancer. Breast Cancer Res Treat.
135:705–713. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Bu XM, Zhao CH, Zhang N, Gao F, Lin S and
Dai XW: Hypermethylation and aberrant expression of secreted
frizzled-related protein genes in pancreatic cancer. World J
Gastroenterol. 14:3421–3424. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Kohno H, Amatya VJ, Takeshima Y, Kushitani
K, Hattori N, Kohno N and Inai K: Aberrant promoter methylation of
WIF-1 and SFRP1, 2, 4 genes in mesothelioma. Oncol Rep. 24:423–431.
2010.PubMed/NCBI
|
|
49
|
Griffiths EA, Gore SD, Hooker C, McDevitt
MA, Karp JE, Smith BD, Mohammad HP, Ye Y, Herman JG and Carraway
HE: Acute myeloid leukemia is characterized by Wnt pathway
inhibitor promoter hypermethylation. Leuk Lymphoma. 51:1711–1719.
2010. View Article : Google Scholar : PubMed/NCBI
|