Introduction
Hyperthermia has a long history and is widely used
in various medical fields (1).
Radiofrequency (RF) hyperthermia (HT) has been performed in Japan
and is associated with two major issues: i) this modality has not
been approved as a standardized treatment in oncology, and ii)
there is a risk of a fatal complication, the hot spot phenomenon,
which is induced by RF thermal therapy itself (2,3). Many
randomized trials of HT have demonstrated a significant improvement
in clinical outcome for several tumor types (4–6).
However, due to the lack of standardization parameters, and absence
of a reference point for this therapy, clinical studies have had
contradictory outcomes, thereby raising doubts about efficacy.
Conversely, rectal cancer shows higher local
recurrence rates than colon cancer after surgery (7–9). Since
the National Comprehensive Cancer Network Practice Guidelines for
treatment of primary rectal cancer were specified in 2009,
neoadjuvant chemoradiation (NACR) has been accepted as the standard
therapy worldwide, except in Japan. Many studies have demonstrated
that NACR increases local control but exerts no influence on
overall survival (10–12). New strategies that incorporate
neoadjuvant therapy are required for rectal cancer.
We reported that hyperthermo-chemoradiotherapy
(HCRT) for rectal cancer is performed safely (13). The main endpoint of this study was
the evaluation of the pathological and clinical responses after
HCRT using the Hidaka RF output classification (HROC: termed by
us).
Materials and methods
Between December 2011 and January 2014, 51
consecutive patients with primary rectal cancers were included in
this study. Patients received pre-treatment and post-treatment
diagnostic examinations, including computed tomography (CT),
positron emission tomography/CT (PET/CT), and magnetic resonance
imaging (MRI), at Hidaka Hospital. The extent and location of the
tumor were classified according to the tumor-node-metastasis TNM
staging (14). Patients underwent
HCRT at Hidaka Hospital. Operations were performed at the
Department of General Surgical Science, Gunma University, or at the
Division of Surgery, Hidaka Hospital. Each resected specimen was
evaluated histologically at the Department of Pathology, Gunma
University. The study was approved by the Ethics Committees of the
Hidaka Hospital and Gunma University. Each patient gave written
informed consent prior to enrollment in the study.
Chemoradiotherapy
Intensity-modulated radiotherapy was administered
conventionally once daily 5 times/week using
TomoTherapy® (Hi-Art® treatment system;
Accuray). Neoadjuvant radiotherapy (NART) consisted of 50 Gy
delivered to the posterior pelvis in 25 fractions of 2 Gy each.
Concurrent neoadjuvant chemotherapy was delivered in 5-day courses
during the first to fifth weeks of NART. Capecitabine was
administered orally at a dose of 1,700 mg/m2/day.
Hyperthermia
RF thermal treatment was performed using the
Thermotron-RF 8 (Yamamoto Vinita Co., Ltd., Japan) and administered
once a week for 5 weeks with a 50-min irradiation. From December
2011 to November 2012, 19 patients underwent abdominal hyperthermia
treatment and the RF output was retrospectively evaluated and from
November 2012 to January 2014, 32 patients prospectively received a
standardized increasing output method (which we termed neothermia)
based on retrospective data. Details of the method for increasing
output have been reported previously (15). Briefly, group A included patients
with a thickness of fat of the abdominal wall <16 mm, visceral
fat area <100 cm2 and total fat area <190
cm2, and group B included patients with either one of
the aforementioned factors. For patients in group A, the output was
increased to 50 W/min, whereas patients in group B received 25
W/min. The operator started the output from 200 W and increased to
1,200 W until output limiting symptoms occurred and then decreased
the output by 100 W. Most patients did not complain and continued
the first RF thermal treatment. Subtracting 100 W output was judged
as the optimal energy output dose without output limiting symptoms.
From the second to fifth RF thermal treatment, this output was
applied for 50 min. These principles were maintained in patients
with neothermia in this prospective study.
Thermal output
A sensor catheter with 4 temperature points was
placed in the rectum of 12 patients while it was attached to the
skin on the lateral abdominal side, as well as in 39 patients who
received neothermia and in 7 who did not. The accumulated thermal
output was calculated from the estimated internal temperature of
patients during the 50-min duration of each irradiation. An
increased thermometric scale of the skin and the rectum was added
to the pretreatment axillary temperature of the patients to obtain
a hypothetical internal body temperature. Temperature and output
curves were recorded at 1-min intervals from treatment initiation
to completion (50 min).
RF output
Details of the HROC have been reported previously
(15). Briefly, the total
accumulated irradiation output (W/min) was classified into four
groups: ≤26,000, 260,001–32,600, 32,601–39,500, and ≥39,501, as 1
point, 2 points, 3 points, and 4 points, respectively. The HROC was
further classified into three groups: ≤9, 10–16, and ≥17 points,
which were the sum of the five treatments.
Evaluation of objective response
All patients were evaluated according to the
Response Evaluation Criteria in Solid Tumors using MRI and PET/CT
(16). Each resected specimen was
examined for histological changes based on the histological
criteria of the Japanese Classification of Colorectal Carcinoma.
The CRPD group included patients in whom local tumors showed a
complete response (CR), although new distant metastasis appeared.
For the response assessment 8 weeks after HCRT, we evaluated CR as
disappearance of the tumor on PET/CT and MRI and a positive to
negative change in PET/CT. Adverse effects of these treatments were
evaluated based on the criteria defined by the Common Terminology
Criteria for Adverse Events (17).
Statistical analysis
SPSS Statistics (IBM, Armonk, NY, USA) version 21
was used to analyze all data. Mean values were compared using the
Student's t-test. All reported p-values are two-tailed and were
considered significant at P<0.05.
Results
Table I shows the
patient characteristics. One patient had grade 3 perianal
dermatitis. Only 2 patients with grade 2 disease wanted to decrease
the dose of capecitabine (complete treatment, 96.1%). No output
limiting symptoms were observed in 63.5% of the patients, whereas
30.2% suffered pain, and 2.0% had subcutaneous induration.
 | Table IPatient characteristics. |
Table I
Patient characteristics.
|
Characteristics | Data |
|---|
| Total no. of
patients | 51 |
| Age (years) | |
| Median | 62 |
| Range | 33–89 |
| Gender, n (%) | |
| Female | 13 (25.5) |
| Male | 38 (74.5) |
| Stoma, n (%) | |
| (−) | 41 (83.7) |
| (+) | 8 (16.3) |
| Tumor location, n
(%) | |
| Ra | 5 (9.8) |
| Rb | 30 (58.8) |
| RbP | 15 (29.4) |
| P | 1 (2.0) |
| Primary tumor, n
(%) | |
| T2 | 9 (17.6) |
| T3 | 36 (70.6) |
| T4 | 6 (11.8) |
| Regional lymph node
status, n (%) | |
| N(−) | 30 (58.8) |
| N(+) | 21 (41.2) |
| Distant metastasis,
n (%) | |
| M0 | 46 (90.2) |
| M1 | 5 (9.8) |
| TNM stagea, n (%) | |
| Stage 1 | 7 (13.7) |
| Stage 2 | 21 (41.2) |
| Stage 3 | 18 (35.3) |
| Stage 4 | 5 (9.8) |
| Tumor
differentiation, n (%) | |
| Well
differentiated | 27 (52.9) |
| Moderately
different | 21 (41.2) |
| Poorly
differentiated | 3 (5.9) |
| A–V distance
(cm) | |
| Median | 3.0 |
| Average (±
SE) | 2.70 (0.33) |
Good local control (ypCR + CR + CRPD) was observed
in 32.7% of the patients in this study. Pathological complete
response (ypCR) was observed in 15.7% of the total 51 patients and
in 24.2% of the 33 patients who underwent surgery. Patients
underwent surgery 8 weeks after HCRT. Abdominoperitoneal resection,
lower anterior resection, intersphincteric resection, and partial
resection were performed in 25, 43.7, 21.9, and 9.4% of the
patients, respectively. One patient could not undergo resection of
the primary tumor, and 5 patients could not undergo surgery due to
progressive disease (PD); 13 (3 CR and 10 PR, SD) patients refused
surgery mainly due to a permanent colostomy. Complete pathological
response (ypCR), grade 2, grade 1b, and grade 1a were observed in
25.0, 31.3, 21.9, and 18.8% of 32 patients, whose tumors were
resected, respectively. Two patients with grade 1b showed PD. A
change from T2 to T0 was observed in 66.7%, T3 to T2 and T0 in
69.4%, T4 to T2 and T3 in 50.0%, N(+) to yN(−) in 66.7%, and M0 to
M1 in 8.7% of the patients.
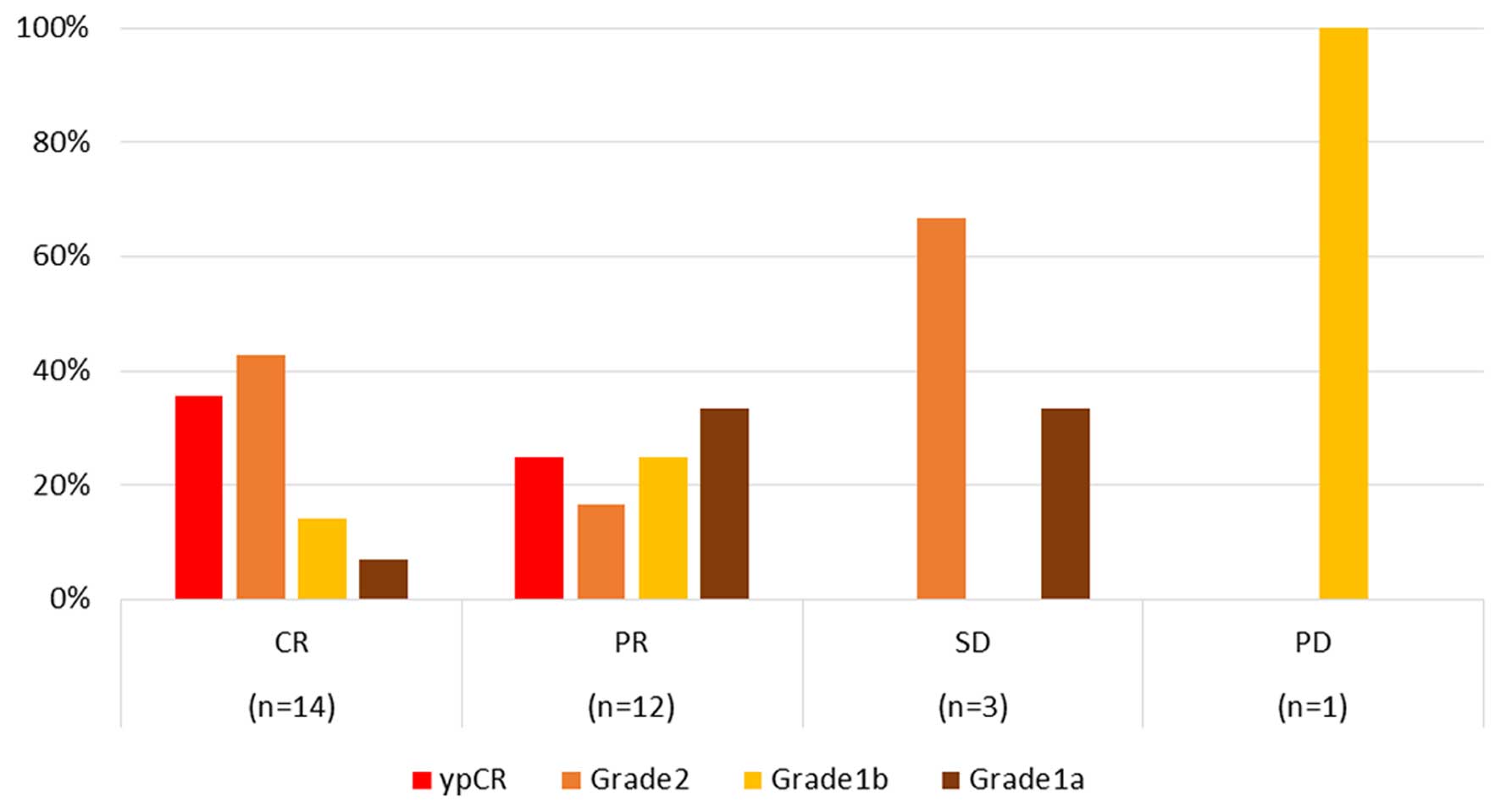
Fig. 1 illustrates
the discrepancies between clinical and histological responses; CR
and partial response (PR) were observed in 35.7 and 25.0% of
patients showing ypCR, respectively, whereas no ypCR was observed
in patients with both stable disease (SD) and PD.
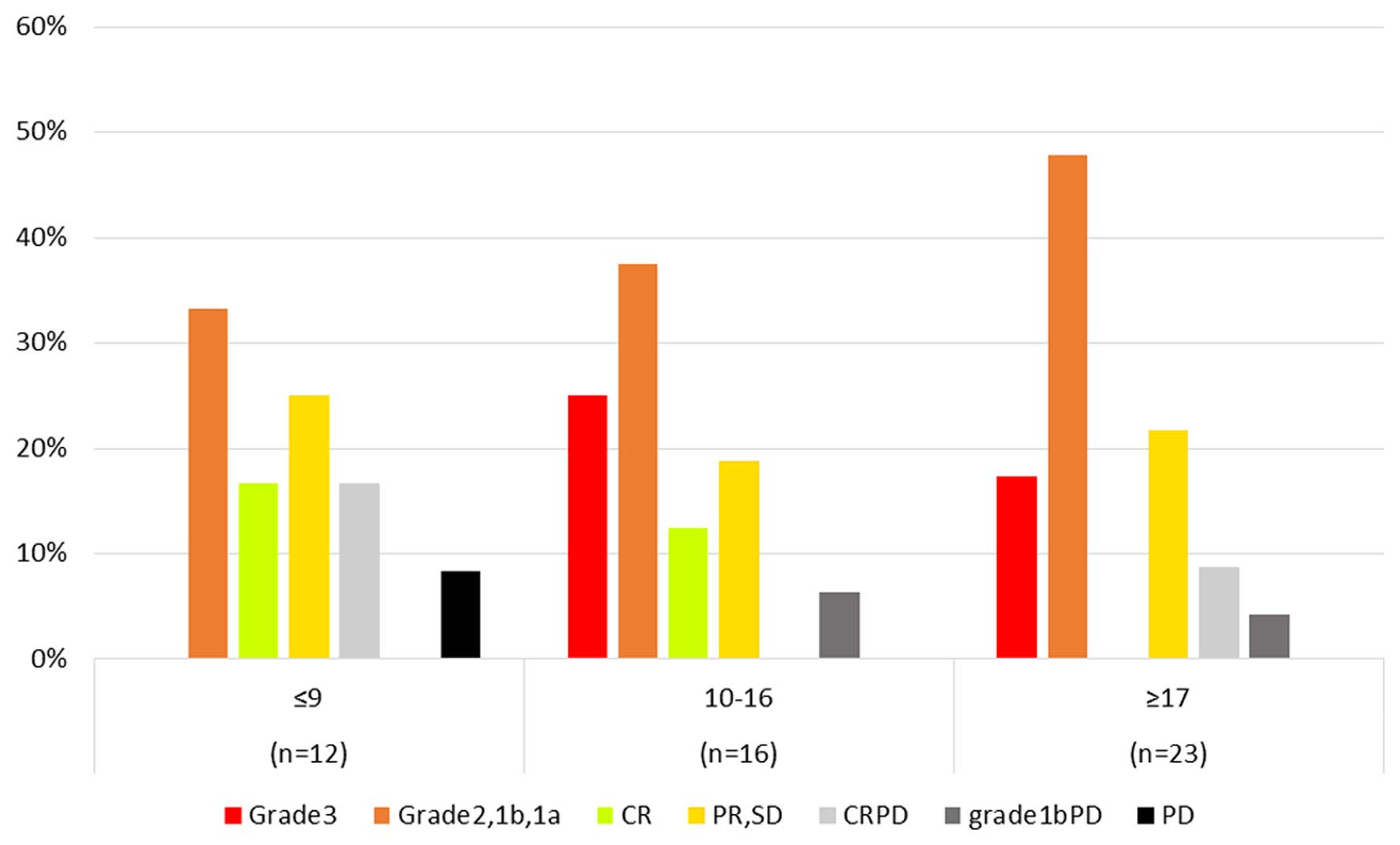
Fig. 2 illustrates
the results of the correlation between objective response and the
HROC. Eight patients with ypCR presented with ≥10 points, whereas 4
patients with PD also presented with ≥10 points. There was no
patients with ypCR among those with ≤9 points in the HROC.
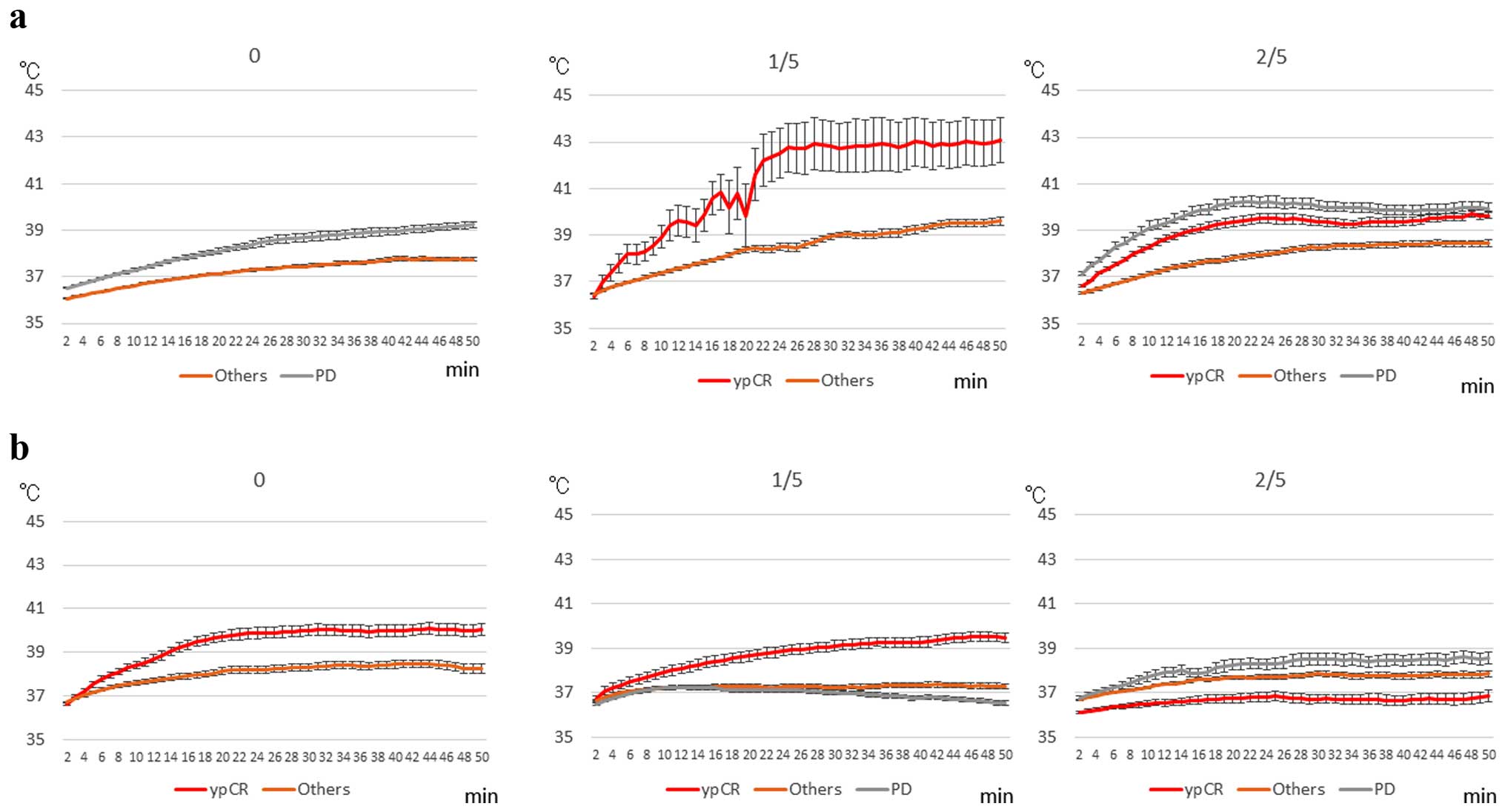
Fig. 3 shows the
results of the correlation between objective response, the HROC,
and the incidence of output limiting symptoms. Patients with ypCR
either experienced output limiting symptoms or were free of output
limiting symptoms. PD was not observed in patients with ≥17 points
without output limiting symptoms, whereas ypCR was not observed in
patients with ≤9 points. Three patients with PD (CRPD+grade 1bPD)
and output limiting symptoms presented with ≥17 points.
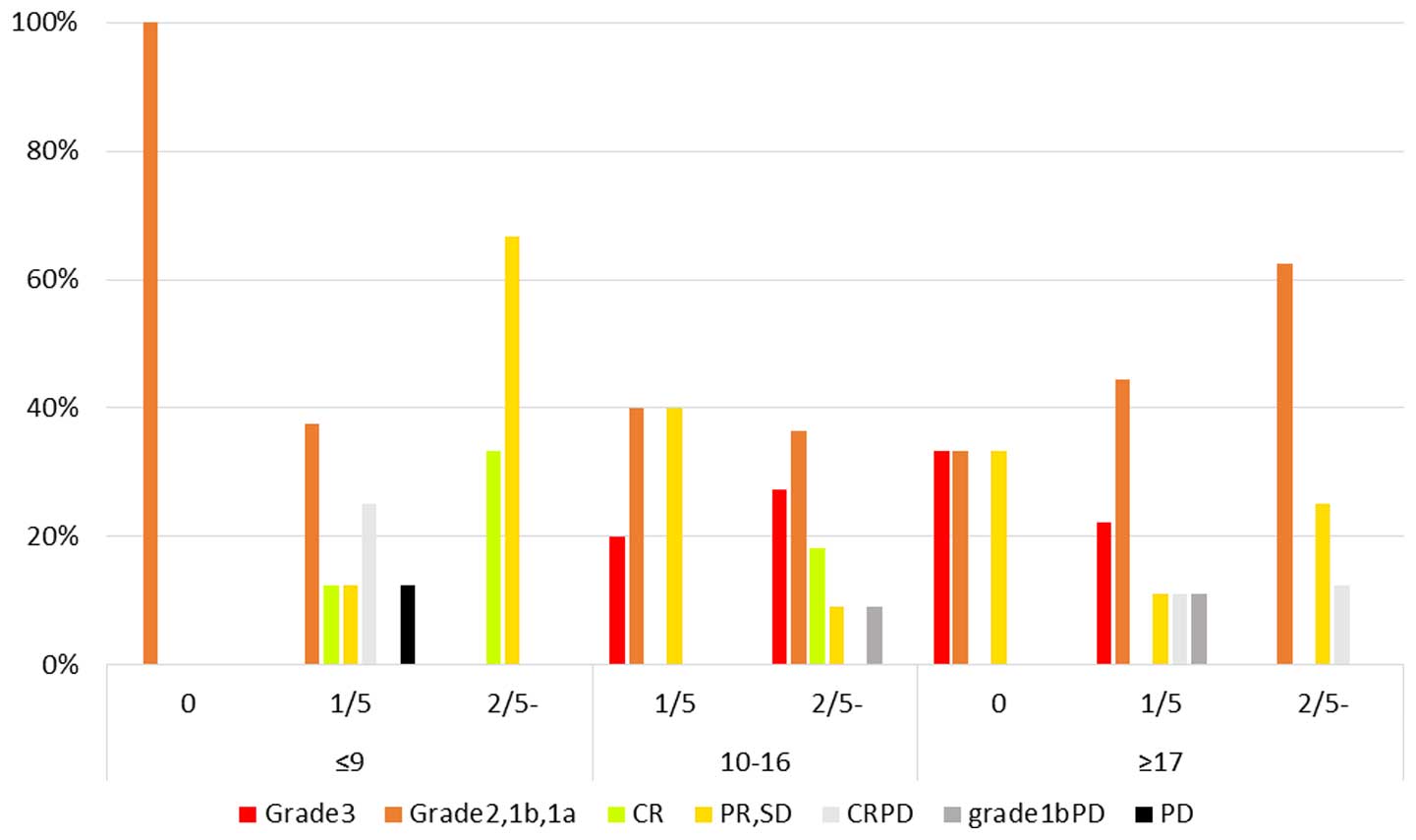 | Figure 3Results of the correlation among the
objective response, the Hidaka RF output classification (HROC), and
the incidence of output limiting symptoms. No output limiting
symptoms, 1 output limiting symptoms, and ≥2 output limiting
symptoms during the 5 thermal treatments are represented as 0, 1/5
and 2/5, respectively. CR, complete response; PR, partial response;
SD, stable disease; CRPD, local CR but distant PD; PD, progressive
disease. Grade; pathological complete response (pCR), grade 1bPD;
local grade 1b but PD. |
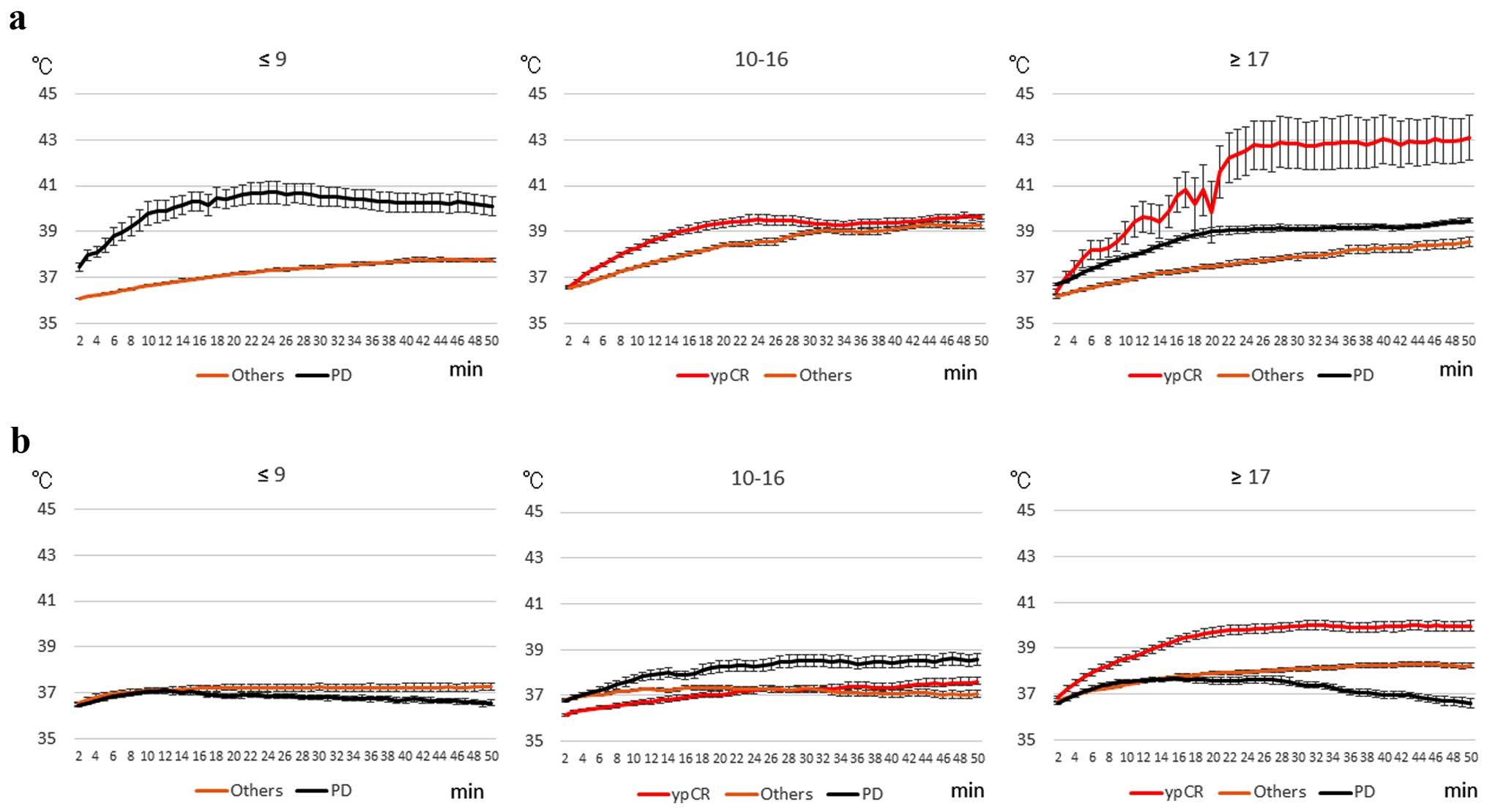
Fig. 4 illustrates
the changes in rectal (Fig. 4a) and
skin (Fig. 4b) temperatures during
RF thermal treatment for 50 min, based on the HROC and objective
response. For ≤9 points, the rectal temperature of PD patients was
increased significantly when compared with the rectal temperature
of the others, while, skin temperature of the others was slightly
increased. For 10–16 points, the rectal temperature of the ypCR
patients was significantly increased when compared with the rectal
temperature of the others, while, skin temperatures of the PD
patients was significantly increased when compared with skin
temperatures of patients with pyCR and others (P<0.05). In
regards to ≥17 points, rectal and skin temperatures of the ypCR
patients were significantly increased when compared with these
temperatures of others and PD (P<0.05).
Fig. 5 shows the
changes in rectal (Fig. 5a) and
skin (Fig. 5b) temperatures during
RF treatment for 50 min, based on the incidence of output limiting
symptoms and objective response. In patients without output
limiting symptoms, rectal temperature of the PD patients was
significantly increased than those of others, while skin
temperature of the ypCR patients was significantly increased when
compared with the skin temperature of the others (P<0.05). In
patients who suffered output limiting symptoms once during the 5
treatments, both rectal and skin temperatures of the ypCR patients
were significantly increased when compared with those of the other
responses (P<0.05). However, in patients who experienced output
limiting symptoms ≥2 times, both rectal and skin temperatures of
the PD patients showed significantly higher temperature increases
than those with others and ypCR (P<0.05).
Based on the results of Figs. 4 and 5, two types of patients were identified:
patients with or without increased temperatures, and consequently,
those who benefited or those who did not; and patients with or
without increased temperatures in both the ypCR and PD groups, even
though they received similar RF outputs.
Discussion
In this retrospective and prospective study, we
aimed to establish a standardized protocol for RF hyperthermia
safety, and 15.7, 7.8, and 7.8% of patients experienced ypCR, CR,
and CRPD, respectively; 31.4 and 13.7% of patients showed good
local control (ypCR + CR + CRPD) and PD (CRPD + grade 1b PD + PD),
respectively. All ypCR cases had ≥10 points, while no ypCR patients
presented with ≤9 points according to the HROC. We also
demonstrated that there were two types of patients: patients with
or without increased temperatures and who consequently received a
benefit or not from treatment, even though they received similar RF
outputs. Previously, we had reported that all patients with
clinical CR showed significantly higher increases in temperatures
than those with other responses, whereas in PR + SD patients the
increase of temperature or not depended on whether the patients
experienced any output limiting symptoms or not, and consequently,
had good or poor outcomes (15).
Our results indicate that increased temperatures correlate with the
clinical response but not the histological response; increased
temperatures served to control tumors but not kill tumor cells.
Randomized NART for rectal cancer showed a ypCR rate
ranging from 13 to 20%, with grade 3 toxicity ranging from 6 to 25%
(18). Oxaliplatin-based
neoadjuvant chemotherapy resulted in an increase in ypCR rates and
grade 3 toxicity (19–21). For rectal cancers, NART plus
capecitabine showed a ypCR rate ranging from 6.7 to 31%, with grade
3 toxicity ranging from 5 to 15% (22). Capecitabine plus IRMT showed a ypCR
ranging from 14.1 to 30.6%, with grade 3 toxicity ranging from 11.1
to ×17.6% (23). Lu et al
reported a ypCR rate of 20%, grade 3 toxicity of 22%, and PD rates
of 17% (24). Whereas NACR showed
superior local tumor control and higher rates of side effects than
our results, most studies failed to report PD cases.
The correlation between the efficacy of hyperthermia
and temperature has been reported (25). Based on our results and other
reports of NART, the following two questions were raised: i) no
ypCR was observed among patients with ≤9 points, and ii) ypCR
patients did not have increased temperature, but had a good
outcome. These questions may be pivotal in predicting the response
to hyperthermia based on the control mechanism of a set point of
core temperatures and thermoregulation in individual patients.
In this study, we analyzed skin temperature as a
simple reproducible marker. Thermal control of skin temperature
depended on a fundamental homeostatic function. Therefore, skin
thermoregulation depends on the thermoregulatory center and
thermoreceptors on the skin (26).
Recently an association was observed between thermoregulation and
the transient receptor potential (TRP) family; TRP vaniloid-1 was
one of the important factors for thermoregulation and was activated
at a noxious heat range (>43°C) or at temperatures above 32°C,
and it was correlated with pain threshold (27–29).
The correlation between the TRP family and thermal treatment will
be considered in the future.
In conclusion, we proposed a standardization of RF
thermal treatment safety. Neothermia with chemoradiation is a
potential new treatment for rectal cancer; further studies on
preventing output limiting symptoms and evaluating thermoregulatory
control mechanisms in individual patients are needed in the
future.
Acknowledgments
We would like to thank all participating patients
and the radiological technicians Mr S. Suda, Mr K. Sugawara, and Mr
K. Jinbo for their assistance.
References
|
1
|
Roussakow S: The History of Hyperthermia
Rise and Decline. Conference Papers in Medicine; 2013. article ID
428027. 2013, View Article : Google Scholar
|
|
2
|
Lee CK, Song CW, Rhee JG, Foy JA and
Levitt SH: Clinical experience using 8 MHz radiofrequency
capacitive hyperthermia in combination with radiotherapy: Results
of a phase I/II study. Int J Radiat Oncol Biol Phys. 32:733–745.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wiersma J, van Wieringen N, Crezee H and
van Dijk JD: Delineation of potential hot spots for hyperthermia
treatment planning optimisation. Int J Hyperthermia. 23:287–301.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Vasanthan A, Mitsumori M, Park JH, Zhi-Fan
Z, Yu-Bin Z, Oliynychenko P, Tatsuzaki H, Tanaka Y and Hiraoka M:
Regional hyperthermia combined with radiotherapy for uterine
cervical cancers: A multi-institutional prospective randomized
trial of the international atomic energy agency. Int J Radiat Oncol
Biol Phys. 61:145–153. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Roussakow S: Critical analysis of
randomized trials on hyperthermia: dubious effect and multiple
biases. Conference Papers in Medicine; 2013. article ID 412186.
2013, View Article : Google Scholar : 2013
|
|
6
|
van der Zee J, González González D, van
Rhoon GC, van Dijk JD, van Putten WL and Hart AA; Dutch Deep
Hyperthermia Group: Comparison of radiotherapy alone with
radiotherapy plus hyperthermia in locally advanced pelvic tumours:
A prospective, randomised, multicentre trial. Lancet.
355:1119–1125. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gérard A, Buyse M, Nordlinger B, Loygue J,
Pène F, Kempf P, Bosset JF, Gignoux M, Arnaud JP, Desaive C, et al:
Preoperative radiotherapy as adjuvant treatment in rectal cancer.
Final results of a randomized study of the European Organization
for Research and Treatment of Cancer (EORTC). Ann Surg.
208:606–614. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Påhlman L and Glimelius B: Pre- or
postoperative radiotherapy in rectal and rectosigmoid carcinoma.
Report from a randomized multicenter trial. Ann Surg. 211:187–195.
1990. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
No authors listed. Preoperative short-term
radiation therapy in operable rectal carcinoma. A prospective
randomized trial. Stockholm Rectal Cancer Study Group. Cancer.
66:49–55. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bosset JF, Collette L, Calais G, Mineur L,
Maingon P, Radosevic-Jelic L, Daban A, Bardet E, Beny A and Ollier
JC; EORTC Radiotherapy Group Trial 22921: Chemotherapy with
preoperative radiotherapy in rectal cancer. N Engl J Med.
355:1114–1123. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gérard JP, Conroy T, Bonnetain F, Bouché
O, Chapet O, Closon-Dejardin MT, Untereiner M, Leduc B, Francois E,
Maurel J, et al: Preoperative radiotherapy with or without
concurrent fluorouracil and leucovorin in T3-4 rectal cancers:
Results of FFCD 9203. J Clin Oncol. 24:4620–4625. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bosset JF, Calais G, Mineur L, Maingon P,
Stojanovic-Rundic S, Bensadoun RJ, Bardet E, Beny A, Ollier JC,
Bolla M, et al EORTC Radiation Oncology Group: Fluorouracil-based
adjuvant chemotherapy after preoperative chemoradiotherapy in
rectal cancer: Long-term results of the EORTC 22921 randomised
study. Lancet Oncol. 15:184–190. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Asao T, Sakurai H, Harashima K, Yamaguchi
S, Tsutsumi S, Nonaka T, Shioya M, Nakano T and Kuwano H: The
synchronization of chemotherapy to circadian rhythms and
irradiation in pre-operative chemoradiation therapy with
hyperthermia for local advanced rectal cancer. Int J Hyperthermia.
22:399–406. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Japanese Colon Cancer Association:
Japanese Classification of Colon Carcinoma. 8th edition. Kanehara.
Tokyo: 2013
|
|
15
|
Shoji H, Motegi M, Osawa K, Okonogi N,
Okazaki A, Andou Y, Asao T, Kuwano H, Takahashi T and Ogoshi K:
Does standardization of radiofrequency hyperthermia benefit
patients with malignancies? Ann Cancer Res Ther. 22:28–35. 2014.
View Article : Google Scholar
|
|
16
|
Therasse P, Arbuck SG, Eisenhauer EA,
Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van
Oosterom AT, Christian MC, et al: New guidelines to evaluate the
response to treatment in solid tumors European Organization for
Research and Treatment of Cancer, National Cancer Institute of the
United States, National Cancer Institute of Canada. J Natl Cancer
Inst. 92:205–216. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Common Terminology Criteria for Adverse
Events (CTCAE): U.S. National Cancer Institute, Cancer Therapy
Evaluation Program. http://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm.
Accessed December 2, 2014.
|
|
18
|
Gerard JP, Rostom Y, Gal J, Benchimol D,
Ortholan C, Aschele C and Levi JM: Can we increase the chance of
sphincter saving surgery in rectal cancer with neoadjuvant
treatments: Lessons from a systematic review of recent randomized
trials. Crit Rev Oncol Hematol. 81:21–28. 2012. View Article : Google Scholar
|
|
19
|
Ricardi U, Racca P, Franco P, Munoz F,
Fanchini L, Rondi N, Dongiovanni V, Gabriele P, Cassoni P,
Ciuffreda L, et al: Prospective phase II trial of neoadjuvant
chemo-radiotherapy with oxaliplatin and capecitabine in locally
advanced rectal cancer (XELOXART). Med Oncol. 30:5812013.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Passoni P, Fiorino C, Slim N, Ronzoni M,
Ricci V, Di Palo S, De Nardi P, Orsenigo E, Tamburini A, De Cobelli
F, et al: Feasibility of an adaptive strategy in preoperative
radiochemotherapy for rectal cancer with image-guided tomotherapy:
Boosting the dose to the shrinking tumor. Int J Radiat Oncol Biol
Phys. 87:67–72. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Landry JC, Feng Y, Cohen SJ, Staley CA
III, Whittington R, Sigurdson ER, Nimeiri H, Verma U, Prabhu RS and
Benson AB: Phase 2 study of preoperative radiation with concurrent
capecitabine, oxaliplatin, and bevacizumab followed by surgery and
postoperative 5-fluorouracil, leucovorin, oxaliplatin (FOLFOX), and
bevacizumab in patients with locally advanced rectal cancer: ECOG
3204. Cancer. 119:1521–1527. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Huang MY, Chen CF, Huang CM, Tsai HL, Yeh
YS, Ma CJ, Wu CH, Lu CY, Chai CY, Huang CJ, et al: Helical
tomotherapy combined with capecitabine in the preoperative
treatment of locally advanced rectal cancer. Biomed Res Int.
2014:3520832014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hernando-Requejo O, López M, Cubillo A,
Rodriguez A, Ciervide R, Valero J, Sánchez E, Garcia-Aranda M,
Rodriguez J, Potdevin G, et al: Complete pathological responses in
locally advanced rectal cancer after preoperative IMRT and
integrated-boost chemoradiation. Strahlenther Onkol. 190:515–520.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lu JY, Xiao Y, Qiu HZ, Wu B, Lin GL, Xu L,
Zhang GN and Hu K: Clinical outcome of neoadjuvant chemoradiation
therapy with oxaliplatin and capecitabine or 5-fluorouracil for
locally advanced rectal cancer. J Surg Oncol. 108:213–219. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kapp DS and Cox RS: Thermal treatment
parameters are most predictive of outcome in patients with single
tumor nodules per treatment field in recurrent adenocarcinoma of
the breast. Int J Radiat Oncol Biol Phys. 33:887–899. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Romanovsky AA: Thermoregulation: Some
concepts have changed. Functional architecture of the
thermoregulatory system. Am J Physiol Regul Integr Comp Physiol.
292:R37–R46. 2007. View Article : Google Scholar
|
|
27
|
Caterina MJ, Schumacher MA, Tominaga M,
Rosen TA, Levine JD and Julius D: The capsaicin receptor: A
heat-activated ion channel in the pain pathway. Nature.
389:816–824. 1997. View
Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tominaga M, Caterina MJ, Malmberg AB,
Rosen TA, Gilbert H, Skinner K, Raumann BE, Basbaum AI and Julius
D: The cloned capsaicin receptor integrates multiple pain-producing
stimuli. Neuron. 21:531–543. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yao J, Liu B and Qin F: Kinetic and
energetic analysis of thermally activated TRPV1 channels. Biophys
J. 99:1743–1753. 2010. View Article : Google Scholar : PubMed/NCBI
|