Introduction
Hepatocellular carcinoma (HCC) is the sixth most
common cancer and the third most frequent cause of cancer-related
mortality worldwide (1,2). Although patients with early-stage HCC
can receive curable treatment, the prognosis of patients with
advanced disease is relatively poor. Drug resistance contributes to
the poor prognosis in patients with advanced HCC, and elucidation
of the molecular mechanisms underlying drug resistance would
facilitate the development of more effective therapeutic strategies
(3,4).
Drug efflux is a major molecular mechanism thought
to affect drug resistance in patients with HCC. Increased drug
efflux can decrease the accumulation of anticancer drugs (5). Multidrug resistance proteins (MRPs)
are members of the ATP-binding cassette (ABC) transporter
superfamily and are involved in drug efflux (6), contributing to the drug resistance
observed in patients with HCC (5).
Notably, MRPs are upregulated in 5-fluorouracil (5-FU)-resistant
cancer cells, and MRP5 expression has been shown to influence 5-FU
resistance in pancreatic carcinoma cells (7,8).
Furthermore, various studies have shown that there is a significant
association between MRPs and 5-FU sensitivity (9–12).
Drug resistance to cisplatin (CDDP), another common anticancer
agent used for HCC chemotherapy (13–16),
is also mediated by MRPs (17–20).
Human Runt-related transcription factor 3 (RUNX3), a
tumor suppressor expressed in gastric cancers (21), regulates cell growth and apoptosis
as a downstream effector of transforming growth factor-β (TGF-β)
signaling (22). RUNX3 was
originally reported as a tumor suppressor in gastric cancer
(21) and has been shown to
function as a tumor suppressor in HCC as well (23,24).
We and other researchers have reported that the protein and mRNA
expression of RUNX3 are decreased in HCC and that loss of RUNX3
expression induces various effects in HCC, including prevention of
apoptosis, induction of cancer stem cell-like changes, and
promotion of the epithelial-mesenchymal transition (EMT) (24,25).
Moreover, RUNX3 has been shown to regulate MRPs in pancreatic
cancer (26). However, the
mechanisms through which RUNX3 and MRPs may mediate drug resistance
in HCC are not completely understood.
Therefore, in the present study, we assessed the
relationship between RUNX3 and MRP expression in HCC. We also
evaluated the effects of RUNX3 re-expression on drug resistance to
5-FU and CDDP in HCC.
Materials and methods
Cell lines and cell culture
The human HCC cell lines Hep3B, Huh7 and HLF were
obtained from the American Type Culture Collection (ATCC; Manassas,
VA, USA). The cells were maintained in Dulbecco's modified Eagle's
medium (DMEM; Invitrogen, Carlsbad, CA, USA), supplemented with 10%
heat-inactivated fetal bovine serum (FBS), 1% non-essential amino
acids, 1% sodium pyruvate and 1% penicillin/streptomycin solution
(all from Sigma, St. Louis, MO, USA). The cells were cultured at
37°C in an atmosphere containing 5% CO2 and 95% air.
Cell growth was halted at subconfluency under restricted serum
conditions with 0.1% dialyzed FBS for 36 h before the experiments,
if needed.
Ectopic RUNX3 expression
A human RUNX3 construct was obtained by reverse
transcription-polymerase chain reaction (RT-PCR)-based cloning of
the gene from normal human hepatocytes (Sanko Junyaku, Co., Ltd.,
Tokyo, Japan). Human RUNX3 and/or chloramphenicol acetyltransferase
(CAT; mock) constructs were transfected into Hep3B, Huh7 and HLF
cells using FuGENE 6 transfection reagent (Roche Diagnostics,
Basel, Switzerland). At least two independent transfections were
performed for each cell line. The cells were incubated under
serum-starved conditions for 24 h and used for the following
experiments.
Immunoblot analysis
Cells were plated in 6-well plastic tissue culture
dishes and grown to confluency. The cells were washed twice with
cold phosphate-buffered saline (PBS) and lysed in 150 µl of
sample buffer [100 mNM Tris-HCl (pH 6.8), 10% glycerol, 4% sodium
dodecyl sulfate (SDS), 1% bromophenol blue and 10%
β-mercaptoethanol]. The samples were resolved by sodium dodecyl
sulfate (SDS)-polyacrylamide gel electrophoresis and transferred to
Immobilon-P polyvinylidene difluoride membranes (Millipore
Corporation, Bedford, MA, USA). The membranes were blocked using
Tris-buffered saline with Tween-20 (TBS-T; Sigma) containing 5%
bovine serum albumin for 1 h. The membranes were then incubated
with antibodies against RUNX3, MRP1 and MRP2 (all from Abcam,
Cambridge, MA), MRP3 (Sigma), MRP5 (Abcam), and β-actin (Sigma)
overnight at 4°C. The membranes were washed 3 times with TBS-T and
probed with horseradish peroxidase-conjugated secondary antibodies
before being developed with an enhanced chemiluminescence western
blotting detection system (Amersham Biosciences, Piscataway, NJ,
USA).
MTT assay
Cell proliferation was assessed using
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT)
assays. Briefly, cells were grown in 96-well plastic tissue culture
dishes at a density of 1×104 cells/ml. After 24 h of
quiescence, cells were cultured for the indicated period with or
without 10% FBS. Then, 10 µl of MTT (5 mg/ml in PBS) was
added to each well, and the cells were incubated for an additional
4 h at 37°C. The purple-blue formazan precipitate was dissolved in
100 µl of dimethyl sulfoxide (DMSO). Mitochondrial activity,
which reflected cell viability, was evaluated by measuring the
optical density at 570 nm on a microplate reader (Bio-Rad,
Hercules, CA, USA).
To evaluate 5-FU and CDDP resistance, the cells were
treated with various concentrations of 5-FU and/or CDDP for 72 h,
and MTT assays were then performed as described above.
Gene silencing of RUNX3 with small
interfering RNA (siRNA)
RUNX3-expressing Hep3B and Huh7 cells were
transfected with either scrambled negative control siRNA or RUNX3
siRNA (Applied Biosystems, Foster City, CA, USA) using RNAiFect
transfection reagent (Qiagen, Hilden, Germany). The cells were
incubated for 24 h, and then serum starved for 48 h. MTT assays
were then performed.
HCC tissues and immunohistochemistry
A group of 23 patients [18 men (age range, 52–78
years; average age 65.1 years) and five women (age range, 55–74
years; average age, 65.8 years)] were included in the present
study. Resected HCC tissues were obtained after receiving written
informed consent that adhered to the stringent ethical criteria of
the Okayama University Graduate School of Medicine, Dentistry and
Pharmaceutical Sciences. Immunohistochemistry was performed on
formalin-fixed paraffin-embedded sections prepared from the
resected tissue. Sections were dewaxed and dehydrated; after
rehydration, endogenous peroxidase activity was blocked for 30 min
in a methanol solution containing 0.3% hydrogen peroxide. Following
antigen retrieval in citrate buffer, the sections were blocked
again overnight at 4°C. The sections were probed with anti-RUNX3
mouse monoclonal antibodies (ab40278), anti-MRP1 monoclonal
antibodies (ab32574), anti-MRP2 monoclonal antibodies (ab15603)
(all from Abcam), anti-MRP3 monoclonal antibodies (M6567; Sigma)
and anti-MRP5 monoclonal antibodies (ab24107; Abcam). The primary
antibody was detected using a biotinylated anti-rabbit antibody or
a biotinylated anti-mouse antibody (both from Dako Japan). The
signal was amplified by avidinbiotin complex formation and was
developed with diaminobenzidine, followed by dehydration in alcohol
and xylene. The sections were then mounted and scored for RUNX3,
MRP1, MRP2, MRP3 and MRP5 using a four-point scale: 0, negative; 1,
weak signal; 2, intermediate signal; and 3, strong signal. All
sections were scored independently by two observers without prior
knowledge of the clinical background. All discrepancies in scoring
were reviewed, and a consensus was reached. Statistical analyses
were performed using JMP software (SAS Institute, Inc., Cary, NC,
USA).
Gene expression profiling analysis in
human HCC
To further investigate correlations between RUNX3
and MRP expression in HCC, publicly available HCC data sets were
evaluated using Oncomine (http://www.oncomine.org). In brief, mRNA expression
profiles for RUNX3, MRP1, MRP2, MRP3 and MRP5 were evaluated using
RUNX3/106_at, RUNX3/204197_s_at, RUNX3/204198_s_at, MRP1/34384_at,
MRP2/206155_at, MRP3/209641_s_at, and MRP5/209380_s_at,
respectively, in HCCs from the human liver data sets. Statistical
analyses were performed using JMP software.
Results
Ectopic RUNX3 expression reduces MRP
expression in HCC cell lines
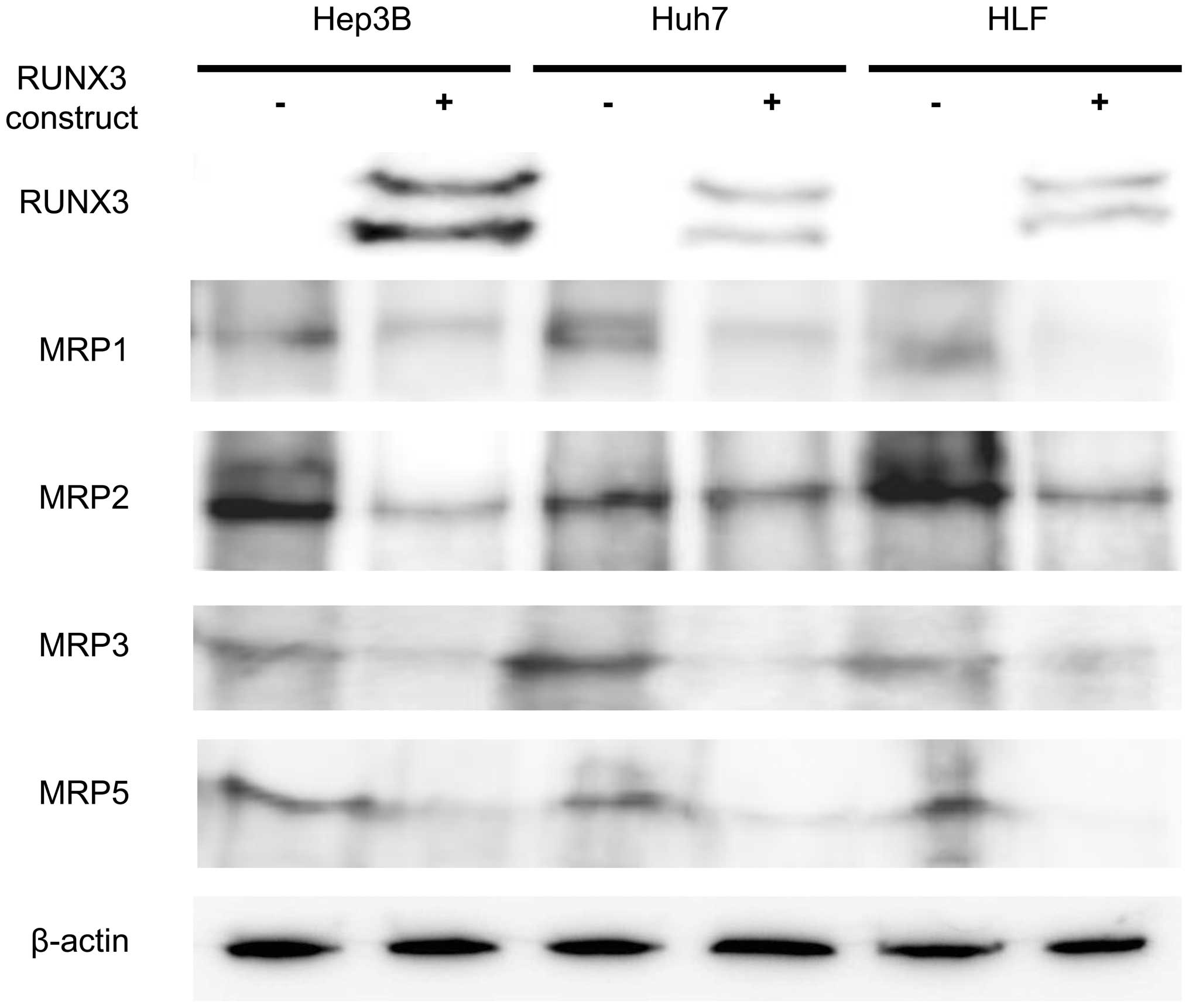
Ectopic RUNX3 protein expression was regulated in 3
HCC cell lines exhibiting positive or negative endogenous RUNX3
expression (Hep3B, Huh7 and HLF cells) (24). RUNX3 protein expression was detected
by immunoblot analysis after transfection with the RUNX3 plasmid
(Fig. 1). Hep3B, Huh7 and HLF cells
expressed MRP1, MRP2, MRP3 and MRP5. Ectopic RUNX3 protein
expression generally decreased MRP expression levels in all 3 cell
lines. Expression of MRP1, MRP2, MRP3 and MRP5 in the
RUNX3-expressing Hep3B, Huh7 and HLF cells was weaker than that in
the control CAT-expressing cells (Fig.
1).
Ectopic RUNX3 protein expression
suppresses cell growth and increases 5-FU and CDDP sensitivity
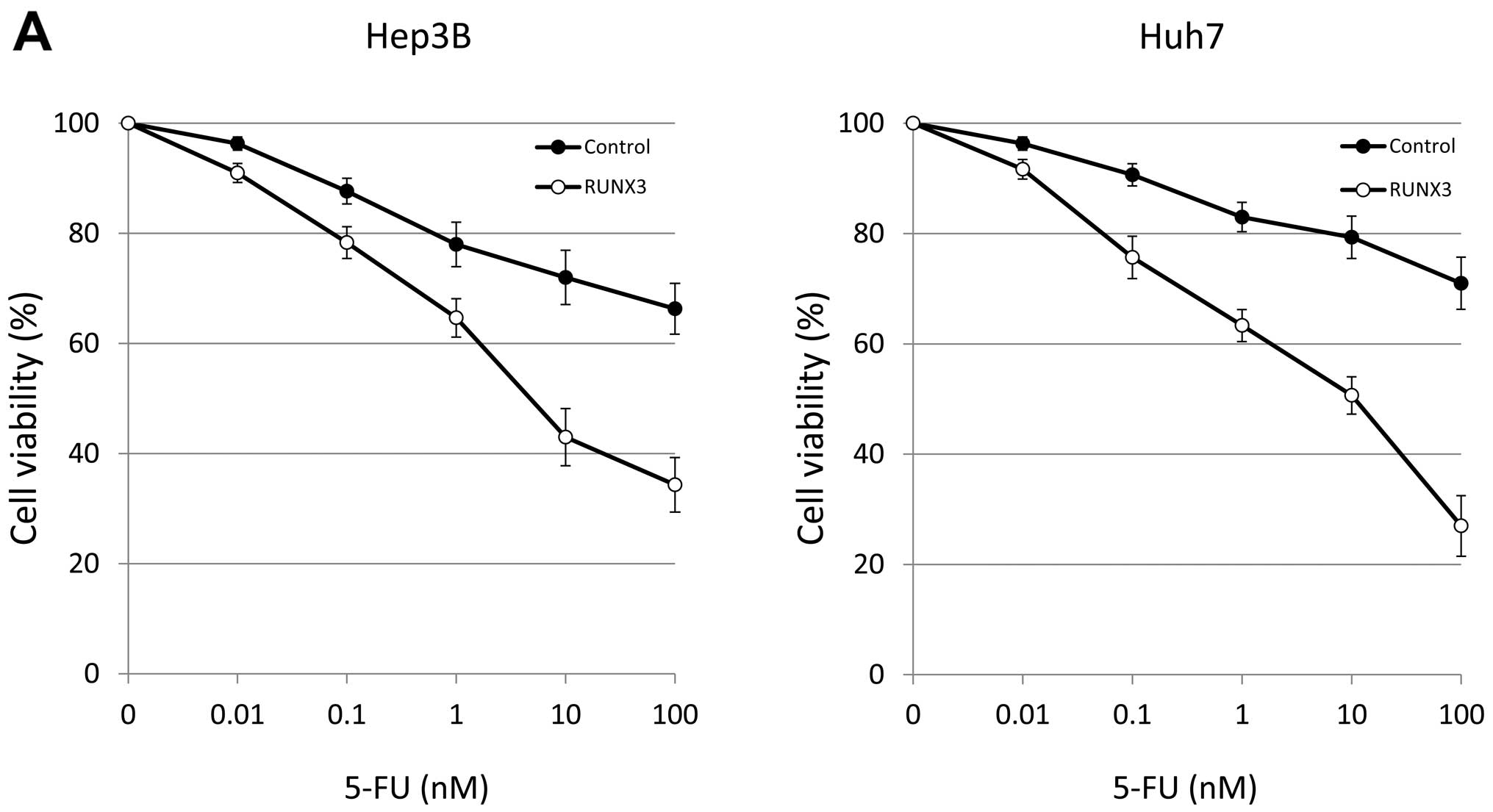
Ectopic RUNX3 expression suppressed cell growth in
the Hep3B and Huh7 cells compared with that in the control Hep3B
and Huh7 cells at 3 days after transfection (Fig. 2). Next, we analyzed the effects of
RUNX3 on chemosensitivity in the RUNX3- or CAT (mock)-transfected
Hep3B and Huh7 cells. RUNX3 expression enhanced 5-FU sensitivity in
both cell lines; the 50% inhibitory concentration (IC50)
of 5-FU decreased from 8.16 to 4.84 nM and from 9.81 to 4.76 nM in
the Hep3B and Huh7 cells, respectively (Fig. 2A). RUNX3 expression also enhanced
CDDP sensitivity in both cell lines; the IC50 of CDDP
decreased from 6.76 to 4.58 nM and from 9.28 to 4.57 nM in the
Hep3B and Huh7 cells, respectively (Fig. 2B).
MRP expression is inversely correlated
with RUNX3 expression in human HCC tissues
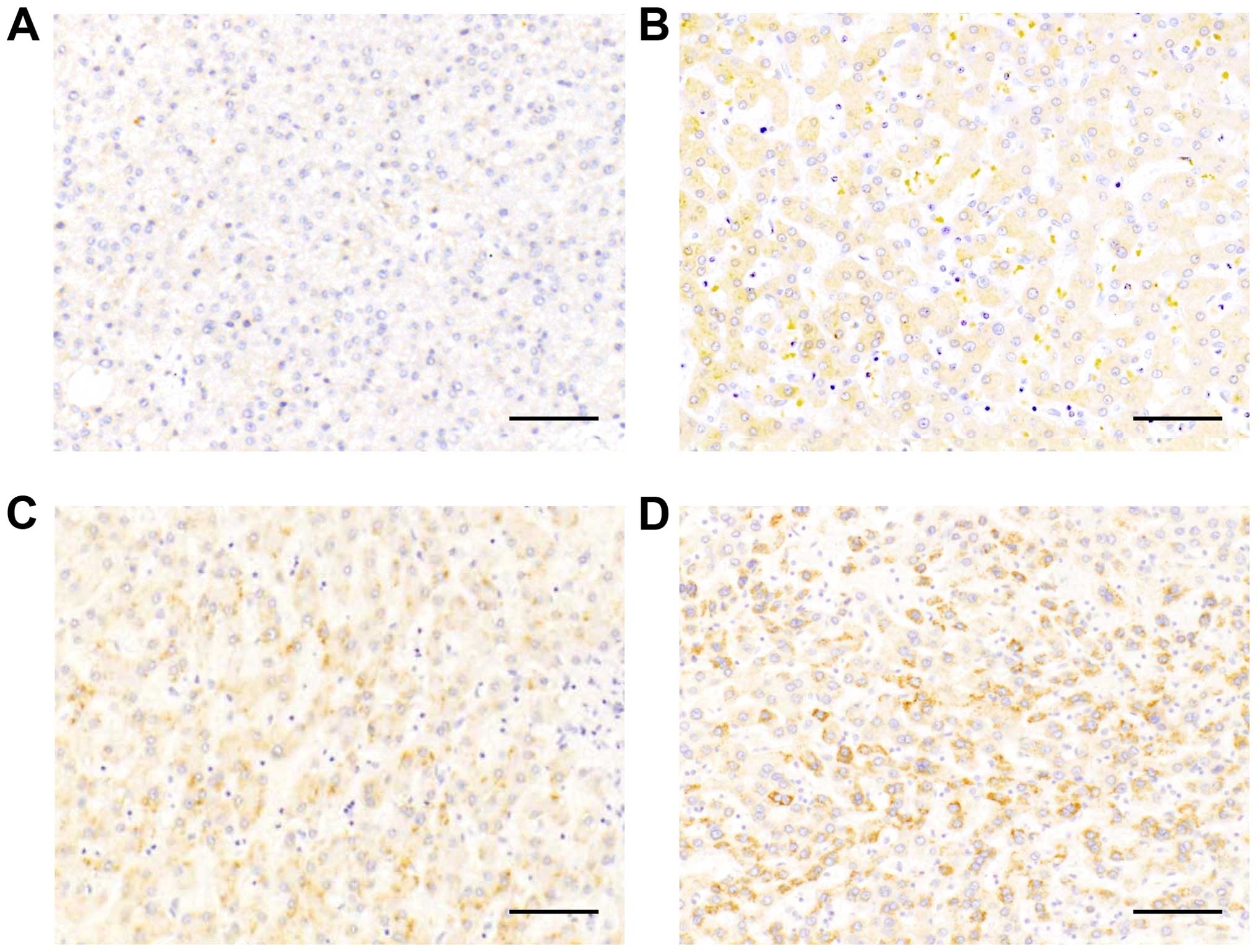
Twenty-three HCC tissue samples were available for
comparison of MRP and RUNX3 protein expression by
immunohistochemistry. Representative images of tissues with
different levels of MRP1 expression, i.e., negative (score 0), weak
signal (score 1), intermediate signal (score 2) and strong signal
(score 3), are shown in Fig. 3A–D.
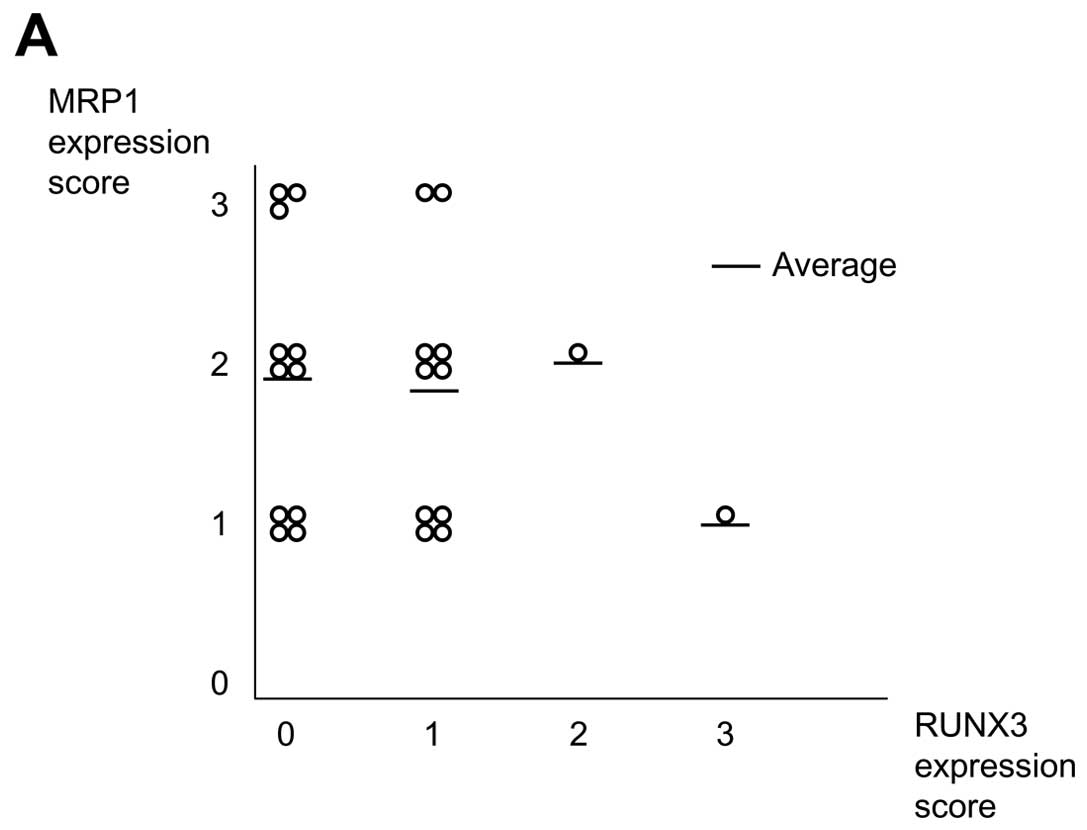
Correlation analysis showed that high MRP expression scores were
generally observed in tissues with low RUNX3 expression (Fig. 4).
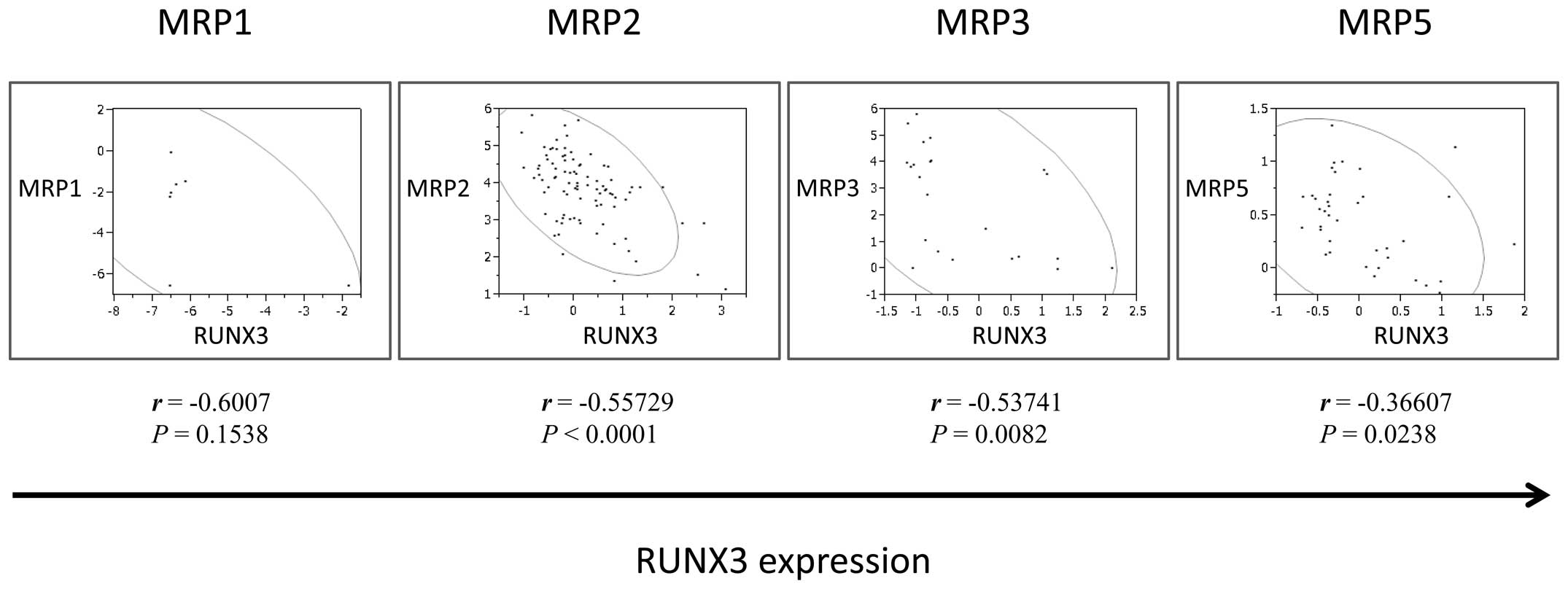
Gene expression profiling analysis in
human HCC
Lastly, we evaluated the effects of ectopic RUNX3
expression on MRPs in order to elucidate the mechanisms underlying
RUNX3 expression-induced chemosensitivity. We analyzed Oncomine
data sets to examine the correlation between the expression of
RUNX3 and MRPs. The results revealed that RUNX3 mRNA expression was
inversely correlated with MRP1, MRP2, MRP3 and MRP5 mRNA expression
(MRP1, r=−0.6007, P=0.1538; MRP2, r=−0.55729, P<0.05; MRP3,
r=−0.53741, P<0.05; MRP5, r=−0.36607, P<0.05; Fig. 5).
Discussion
Chemoresistance is a major challenge encountered
during the therapeutic treatment of HCC. In the present study, we
evaluated the relationship between RUNX3 expression and
chemosensitivity in HCC. RUNX3 was found to act as a tumor
suppressor in HCC, consistent with previous studies showing that
RUNX3 is frequently lost in HCC (23,27–29)
and that loss of RUNX3 contributes to the malignant transformation
of HCC (23–25).
In a previous study of pancreatic cancer, we
reported that the RUNX3 expression status affects gemcitabine
sensitivity by attenuating MRP expression (26). Therefore, we hypothesized that RUNX3
expression may influence chemosensitivity in HCC. Drug resistance
can be induced by several cellular processes. For example, MRPs
mediate drug efflux, which decreases the accumulation of drugs
within cancer cells. In the present study, we found that MRP
expression generally decreased following ectopic expression of
RUNX3 in all 3 HCC cell lines examined. Moreover, these cells
exhibited higher chemosensitivity to 5-FU and CDDP than control
cells, further supporting the role of MRPs in drug resistance in
these cells.
Consistent with a previous study in pancreatic
cancer, we found that RUNX3 blocked MRP expression in HCC cells.
Moreover, MRP expression varied in HCC tissues and was positively
correlated with RUNX3 protein expression. Since the number of HCC
tissues in the present study was relatively small, we also assessed
the relationship between MRPs and RUNX3 expression using the
Oncomine database; this analysis further supported the positive
correlation between RUNX3 and MRP expression. These results
indicated that RUNX3 expression may regulate MRP expression.
Notably, RUNX3 expression has been shown to sensitize gastric
cancer cells to chemotherapeutic drugs by downregulating MRP1
through inhibition of MRP1 promoter activity (30). Thus, our findings suggest that RUNX3
may regulate MRP expression by inhibiting promoter activity in HCC
cells and that targeting of RUNX3 and related signaling molecules
may be a potential treatment modality for HCC.
Although we assessed the relationship between MRPs
and RUNX3 expression in the present study, other factors have also
been implicated in 5-FU and CDDP resistance. Multidrug resistance 1
(MDR1/ABCB1) and other ABCB transporters are also involved in the
resistance against 5-FU (10,31,32).
CDDP resistance is established via a multifactorial mechanism
including drug efflux and drug inactivation (33–35).
In the present study, we did not evaluate the effects of RUNX3
protein expression on these mechanisms; thus, further studies are
necessary.
In conclusion, the loss of RUNX3 expression may
upregulate MRP expression, thereby contributing to 5-FU and CDDP
resistance in patients with HCC. Moreover, re-expression of RUNX3
downregulated MRP expression and resensitized HCC cells to 5-FU and
CDDP.
Acknowledgments
The authors thank Shin-ichi Nishina and Minoru
Matsubara for their valuable suggestions.
Abbreviations:
|
ABC
|
ATP-binding cassette
|
|
ABCC
|
ATP-binding cassette subfamily C
|
|
CAT
|
chloramphenicol acetyltransferase
|
|
cDNA
|
complementary DNA
|
|
CDDP
|
cisplatin
|
|
EMT
|
epithelial-mesenchymal transition
|
|
FBS
|
fetal bovine serum
|
|
5-FU
|
5-fluorouracil
|
|
HCC
|
hepatocellular carcinoma
|
|
MRP
|
multidrug resistance-associated
protein
|
|
MTT
|
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
|
|
RUNX3
|
Runt-related transcription factor
3
|
|
SDS
|
sodium dodecyl sulfate
|
|
siRNA
|
small interfering RNA
|
|
TBS-T
|
Tris-buffered saline with Tween-20
|
|
TGF-β
|
transforming growth factor-β
|
References
|
1
|
El-Serag HB and Rudolph KL: Hepatocellular
carcinoma: Epidemiology and molecular carcinogenesis.
Gastroenterology. 132:2557–2576. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
El-Serag HB: Hepatocellular carcinoma. N
Engl J Med. 365:1118–1127. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Arumugam T, Ramachandran V, Fournier KF,
Wang H, Marquis L, Abbruzzese JL, Gallick GE, Logsdon CD, McConkey
DJ and Choi W: Epithelial to mesenchymal transition contributes to
drug resistance in pancreatic cancer. Cancer Res. 69:5820–5828.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cheng L, Luo S, Jin C, Ma H, Zhou H and
Jia L: FUT family mediates the multidrug resistance of human
hepatocellular carcinoma via the PI3K/Akt signaling pathway. Cell
Death Dis. 4:e9232013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Marin JJ, Monte MJ, Blazquez AG, Macias
RI, Serrano MA and Briz O: The role of reduced intracellular
concentrations of active drugs in the lack of response to
anticancer chemotherapy. Acta Pharmacol Sin. 35:1–10. 2014.
View Article : Google Scholar :
|
|
6
|
Dean M, Hamon Y and Chimini G: The human
ATP-binding cassette (ABC) transporter superfamily. J Lipid Res.
42:1007–1017. 2001.PubMed/NCBI
|
|
7
|
Hagmann W, Jesnowski R, Faissner R, Guo C
and Löhr JM: ATP-binding cassette C transporters in human
pancreatic carcinoma cell lines. Upregulation in
5-fluorouracil-resistant cells. Pancreatology. 9:136–144. 2009.
View Article : Google Scholar
|
|
8
|
Nambaru PK, Hübner T, Köck K, Mews S,
Grube M, Payen L, Guitton J, Sendler M, Jedlitschky G, Rimmbach C,
et al: Drug efflux transporter multidrug resistance-associated
protein 5 affects sensitivity of pancreatic cancer cell lines to
the nucleoside anticancer drug 5-fluorouracil. Drug Metab Dispos.
39:132–139. 2011. View Article : Google Scholar
|
|
9
|
Hagmann W, Faissner R, Schnölzer M, Löhr M
and Jesnowski R: Membrane drug transporters and chemoresistance in
human pancreatic carcinoma. Cancers. 3:106–125. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hlavata I, Mohelnikova-Duchonova B,
Vaclavikova R, Liska V, Pitule P, Novak P, Bruha J, Vycital O,
Holubec L, Treska V, et al: The role of ABC transporters in
progression and clinical outcome of colorectal cancer. Mutagenesis.
27:187–196. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Long J, Zhang Y, Yu X, Yang J, LeBrun DG,
Chen C, Yao Q and Li M: Overcoming drug resistance in pancreatic
cancer. Expert Opin Ther Targets. 15:817–828. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang WB, Yang Y, Zhao YP, Zhang TP, Liao Q
and Shu H: Recent studies of 5-fluorouracil resistance in
pancreatic cancer. World J Gastroenterol. 20:15682–15690. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Park JY, Ahn SH, Yoon YJ, Kim JK, Lee HW,
Lee Y, Chon CY, Moon YM and Han KH: Repetitive short-course hepatic
arterial infusion chemotherapy with high-dose 5-fluorouracil and
cisplatin in patients with advanced hepatocellular carcinoma.
Cancer. 110:129–137. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ma MC, Chen YY, Li SH, Cheng YF, Wang CC,
Chiu TJ, Pei SN, Liu CT, Huang TL, Huang CH, et al: Intra-arterial
chemotherapy with doxorubicin and cisplatin is effective for
advanced hepatocellular cell carcinoma. Scientific World Journal.
2014:1601382014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Johnson PJ: Are there indications for
chemotherapy in hepatocellular carcinoma? Surg Oncol Clin N Am.
12:127–134. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nishimura M: A successful treatment by
hepatic arterial infusion therapy for advanced, unresectable
biliary tract cancer. World J Hepatol. 2:192–197. 2010.PubMed/NCBI
|
|
17
|
Shen DW, Pouliot LM, Hall MD and Gottesman
MM: Cisplatin resistance: A cellular self-defense mechanism
resulting from multiple epigenetic and genetic changes. Pharmacol
Rev. 64:706–721. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kowalski P, Surowiak P and Lage H:
Reversal of different drug-resistant phenotypes by an autocatalytic
multitarget multiribozyme directed against the transcripts of the
ABC transporters MDR1/P-gp, MRP2, and BCRP. Mol Ther. 11:508–522.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kuwano M, Toh S, Uchiumi T, Takano H,
Kohno K and Wada M: Multidrug resistance-associated protein
subfamily transporters and drug resistance. Anticancer Drug Des.
14:123–131. 1999.PubMed/NCBI
|
|
20
|
Materna V, Holm PS, Dietel M and Lage H:
Kinetic characterization of ribozymes directed against the
cisplatin resistance-associated ABC transporter cMOAT/MRP2/ABCC2.
Cancer Gene Ther. 8:176–184. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li QL, Ito K, Sakakura C, Fukamachi H,
Inoue K, Chi XZ, Lee KY, Nomura S, Lee CW, Han SB, et al: Causal
relationship between the loss of RUNX3 expression and gastric
cancer. Cell. 109:113–124. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Subramaniam MM, Chan JY, Yeoh KG, Quek T,
Ito K and Salto-Tellez M: Molecular pathology of RUNX3 in human
carcinogenesis. Biochim Biophys Acta. 1796:315–331. 2009.PubMed/NCBI
|
|
23
|
Nakanishi Y, Shiraha H, Nishina S, Tanaka
S, Matsubara M, Horiguchi S, Iwamuro M, Takaoka N, Uemura M, Kuwaki
K, et al: Loss of runt-related transcription factor 3 expression
leads hepatocellular carcinoma cells to escape apoptosis. BMC
Cancer. 11:32011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nishina S, Shiraha H, Nakanishi Y, Tanaka
S, Matsubara M, Takaoka N, Uemura M, Horiguchi S, Kataoka J,
Iwamuro M, et al: Restored expression of the tumor suppressor gene
RUNX3 reduces cancer stem cells in hepatocellular carcinoma by
suppressing Jagged1-Notch signaling. Oncol Rep. 26:523–531.
2011.PubMed/NCBI
|
|
25
|
Tanaka S, Shiraha H, Nakanishi Y, Nishina
S, Matsubara M, Horiguchi S, Takaoka N, Iwamuro M, Kataoka J,
Kuwaki K, et al: Runt-related transcription factor 3 reverses
epithelial-mesenchymal transition in hepatocellular carcinoma. Int
J Cancer. 131:2537–2546. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Horiguchi S, Shiraha H, Nagahara T,
Kataoka J, Iwamuro M, Matsubara M, Nishina S, Kato H, Takaki A,
Nouso K, et al: Loss of runt-related transcription factor 3 induces
gemcitabine resistance in pancreatic cancer. Mol Oncol. 7:840–849.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mori T, Nomoto S, Koshikawa K, Fujii T,
Sakai M, Nishikawa Y, Inoue S, Takeda S, Kaneko T and Nakao A:
Decreased expression and frequent allelic inactivation of the RUNX3
gene at 1p36 in human hepatocellular carcinoma. Liver Int.
25:380–388. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Xiao WH and Liu WW: Hemizygous deletion
and hypermethylation of RUNX3 gene in hepatocellular carcinoma.
World J Gastroenterol. 10:376–380. 2004.PubMed/NCBI
|
|
29
|
Li X, Zhang Y, Zhang Y, Qiao T, Wu K, Ding
J, Liu J and Fan D: RUNX3 inhibits growth of HCC cells and HCC
xenografts in mice in combination with adriamycin. Cancer Biol
Ther. 7:669–676. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Guo C, Ding J, Yao L, Sun L, Lin T, Song
Y, Sun L and Fan D: Tumor suppressor gene Runx3 sensitizes gastric
cancer cells to chemotherapeutic drugs by downregulating Bcl-2,
MDR-1 and MRP-1. Int J Cancer. 116:155–160. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lu F, Hou YQ, Song Y and Yuan ZJ: TFPI-2
downregulates multidrug resistance protein in 5-FU-resistant human
hepatocellular carcinoma BEL-7402/5-FU cells. Anat Rec. 296:56–63.
2013. View
Article : Google Scholar
|
|
32
|
Gu W, Fang FF, Li B, Cheng BB and Ling CQ:
Characterization and resistance mechanisms of a
5-fluorouracil-resistant hepatocellular carcinoma cell line. Asian
Pac J Cancer Prev. 13:4807–4814. 2012. View Article : Google Scholar
|
|
33
|
Zisowsky J, Koegel S, Leyers S,
Devarakonda K, Kassack MU, Osmak M and Jaehde U: Relevance of drug
uptake and efflux for cisplatin sensitivity of tumor cells. Biochem
Pharmacol. 73:298–307. 2007. View Article : Google Scholar
|
|
34
|
Siddik ZH: Cisplatin: Mode of cytotoxic
action and molecular basis of resistance. Oncogene. 22:7265–7279.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Samimi G, Katano K, Holzer AK, Safaei R
and Howell SB: Modulation of the cellular pharmacology of cisplatin
and its analogs by the copper exporters ATP7A and ATP7B. Mol
Pharmacol. 66:25–32. 2004. View Article : Google Scholar : PubMed/NCBI
|