Introduction
Hepatocellular carcinoma (HCC) is one of the major
causes of cancer deaths throughout the globe and every year around
600,000 new cases are detected (1,2). The
rate of prognosis in HCC patients is very poor and depends on the
stage of diagnosis. The commonly used treatment strategies for HCC
at present include, chemotherapy, radiation therapy and surgery,
however, none of the strategies are sufficiently efficient
(3). Metastasis of HCC to other
organs including lungs, lymph nodes, kidneys and brain is commonly
observed in the patients with liver cancer (4). Thus, the discovery of new molecules
for the treatment of hepatocellular carcinoma is required. A small
population of cancer cells bestowed with the ability to initiate,
promote and sustain tumor growth is known as cancer stem cells
(CSCs) (5,6). These cells are capable of undergoing
self-renewal and proliferation at an enormous rate (7). CSCs are resistant to radiotherapy as
well as chemotherapy and facilitate the metastasis of cancer cells
to various other organs. Thus, it is believed that targeting the
CSCs can be a promising strategy for the treatment of cancer.
Natural products isolated from plant and animal
sources have been the source of drugs for various diseases
(8). Cantharidin is obtained by the
phytochemical analysis of Blister Beetles and belongs to the
class of terpenoids (8).
Cantharidin has a long traditional medicinal importance in Chinese
system of medicine (9). The
mechanism of action of cantharidin has been found to involve arrest
of cell cycle and induction of apoptosis in the cancer cells
(10,11). Cantharidin treatment exhibits
inhibitory effect on various types of cancer cells including,
colon, liver, breast, bladder oral buccal and leukemia (11–15).
Treatment of T24 cells with cantharidin induces expression of COX2,
PGE2 and causes cell cycle arrest in G2/M phase (13). However, the effect of cantharidin on
cell proliferation and self-renewal, cell cycle arrest and
induction of apoptosis in HCSCs have not been reported. Therefore,
the effect of cantharidin on the hepatocellular carcinoma stem
cells was investigated. It was observed that cantharidin treatment
inhibited cell viability and self-renewal capacity, arrested the
cell cycle and induced apoptosis in HepG2 CD133+
HCSCs.
Materials and methods
Chemical and reagents
Cantharidin and dimethyl sulphoxide (DMSO) were
purchased from Sigma-Chemical Co. (St. Louis, MO, USA). Trypsin,
MTT reagent and lithium chloride were purchased from Gibco-BRL
(Grand Island, NY, USA). The antibodies for human β-catenin, cyclin
D1 and β-actin were obtained from the Health Science Research
Resources Bank (Osaka, Japan).
Cell lines and culture
The HepG2 hepatocellular carcinoma cell line was
purchased from the American Type Culture Collection (ATCC;
Manassas, VA, USA). The cells were maintained in RPMI-1640 medium
(RPMI:ECM=4:1) supplemented with 10% fetal bovine serum in a
humidified atmosphere of 5% CO2 and 95% air at 37°C.
Cell separation and culture of
spheres
HepG2 hepatocellular carcinoma cells were sorted
using magnetic activated cell sorting (MACS) separation column (BD
Biosciences, Mountain View, CA, USA) based on the presence of
surface marker, CD133+. Briefly, the cells after
phosphate-buffered saline (PBS) washing were treated with 0.5%
bovine serum albumin (BSA). To the 200 µl of anti-CD133
antibody were added 200 µl of CD133-conjugated MicroBeads,
2×107 cells and the sample. After incubation for 1 h,
the cells were rinsed in PBS and then CD133+ and
CD133− cells were separated. The CD133+ and
parental cells were rinsed twice in PBS followed by incubation in
serum-free RPMI-1640 containing antibiotics, penicillin and
streptomycin. The cells at a density of 2×105 cells/ml
were distributed onto 6-well ultralow attachment plates (BD
Biosciences) in stem cell media. Following incubation for 5 days
the cultures were passaged to determine the number of colonies with
more than 50 cells per colony using a microscope (IX71; Olympus,
Tokyo, Japan). All the calculations were performed in
triplicate.
Analysis of colony formation
The effect of cantharidin treatment on formation of
colonies in HepG2 CD133+ cells was also determined.
Briefly, the cells at a density of 2×105 cells/ml were
seeded onto 96-well plates. The cells were incubated with various
concentrations of cantharidin dissolved in DMSO or with DMSO alone
as negative control. Following incubation for a period of 5 days,
the ability of the cells to form colonies was determined by
counting the number of colonies using an Olympus CX22 microscope
(Olympus).
The sphere forming ability of the cells was analyzed
after 2.5×105 cells/ml were seeded onto 6-well ultralow
attachment plates. In another experiment, 1×105 cells/ml
were distributed on to the 6-well ultralow attachment plates and
then treated with different doses of cantharidin. HepG2
CD133+ single cell suspensions were exposed to
cantharidin (5 µM), lithium chloride (2 µM),
casticin-LiCl (5 or 5 µM) or control to DMSO, respectively.
After 24 h, the effect of cantharidin on the ability of cells to
form a carcinoma mass was analyzed.
In vivo tumorigenicity assay
Twenty pathogen-free male Balb/c nu mice (56 weeks
of age) were purchased from the Animal Institute of the Chinese
Academy of Medical Science. The animal studies were performed in
accordance with the standard protocols approved by the Ethics
Committee of Hunan National University and the Committee of
experimental Animal feeding and Management (Changsha, China). The
study was approved by the Ethics Committee of Hunan National
University and the Committee of experimental Animal Feeding and
Management (Changsha, China) under the reference number
007/2013-HNU. The mice were randomly divided into five groups (n=4
per group) and maintained under standard conditions, according to
typical protocols. The cells were suspended in a serum-free
DMEM/Matrigel (BD Biosciences, Franklin Lakes, NJ, USA) mixture
(1:1 volume). The mice were inoculated with different quantities of
CD133+ SFCs (5×102, 1×103,
5×103, 1×104 and 5×104 cells) in
one flank, and unsorted MHCC97 cells (5×104,
1×105, 2×105, 5×105 and
1×106 cells) in the other. Tumorigenicity experiments
were terminated two months after cell inoculation. Tumor size was
measured using a caliper and the volume was calculated as follows:
V (mm3) = L × W2 × 0.5, where L denotes length and W the
width. The harvested tumors were photographed and weighed
immediately. Specimens from tumor tissue samples were fixed in 10%
neutral buffered formalin, processed in paraffin blocks and
sectioned. The sections were stained with hematoxylin and eosin
(H&E) and examined under an inverted microscope (IX71;
Olympus).
MTT assay
On to the 96-well tissue culture plates
2.5×105 CD133+ or parental HepG2 cells were
dispersed and cultured in RPMI-1640 supplemented with 10% fetal
bovine serum along with 2 mM L-glutamine. The cells after
attachment for 12 h were treated with various concentrations of
cantharidin for 36 h. To each well of the plate, 150 µl
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
and incubated for 4 h. DMSO (150 µl) was added to each well
of the plate to dissolve the formed formazan crystals. A microplate
reader (SpectraMax Plus; Molecular Devices) was used to measure
absorbance for each well at 465 nm three times independently. All
the experiments were performed three times.
Analysis is apoptosis
CD133+ or parental HepG2 cells were
seeded at a density of 3×105 cells into the 10-cm
culture dishes to attain confluence overnight. The cells were
exposed to cantharidin in DMSO or DMSO alone as the control for 48
h. FACSCalibur flow cytometer (BD Biosciences, San Jose, CA, USA)
and the fluorescein isothiocyanate (FITC) Annexin V apoptosis
detection kit (BD Biosciences, San Diego, CA, USA) were used to
analyze the percentage of apoptotic cells according to the
manufacturer's instruction.
Cell cycle analysis
CD133+ or parental HepG2 cells were
seeded at a density of 3×106 cells into the tissue
culture flasks (T75 flask; Nunc A/S). The flasks were supplemented
with RPMI-1640 medium containing 2% FBS and incubated for 24 h with
various doses of cantharidin. Following incubation, the cells were
rinsed in PBS buffer and fixed in 70% ethanol overnight at 40°C.
Cell contents were subjected to centrifugation at 15000 × g for
half an hour and then treated with 200 µl PBS supplemented
with 1 mM RNase A (Calbiochem, San Diego, CA, USA) for 40 min.
Propidium iodide (Sigma-Aldrich) at the concentration of 50
µg/ml was added to the cell cultures and incubation was
continued for half an hour. DNA content of the cells was examined
using FACSVantage Se flow cytometry system and CellQuest program
(BD Biosciences).
Western blot analysis
The cells deprived of serum were treated with
cantharidin or only DMSO as control in 6-well plates for 24 h.
Following incubation, the cells were washed with PBS and then
treated with lysis buffer (50 µM Tris-HCl pH 7.4, 10%
glycerol, 137 µM NaCl, 1 µM PMSF, 100 µM
sodium vanadate, 10 mg/ml leupeptin, 10 mg/ml aprotinin, 1% NP-40
and 5 µM cocktail). Concentration of proteins in the cell
lysates was determined by bicinchoninic acid assay (BCA) method.
The proteins were separated on to 10% polyacrylamide gel and then
transferred to a PVDF membrane. The membrane was blocked overnight
using 5% non-fat dry milk followed by TBST washing. The membrane
was then incubated for 12 h with primary antibodies for β-catenin
or cyclin D1, washed with TBST followed by incubation with
secondary antibodies for 1 h. The X-ray autoradiography was
performed and the gray scale images were analysed.
Statistical analysis
The data expressed are the mean ± SD. The
differences between the groups were analyzed using Student's t-test
and SPSS software, version 15.0 (SPSS, Inc., Chicago, IL, USA). The
differences at P<0.05 were considered statistically
significant.
Results
Separation of hepatocellular carcinoma
stem cells from HepG2 cell line
The CD133+ cells were separated from
HepG2 cell cultures by examination using FCM and then cultured to
obtain stem cell rich cultures, and at 5 days, the spheroids formed
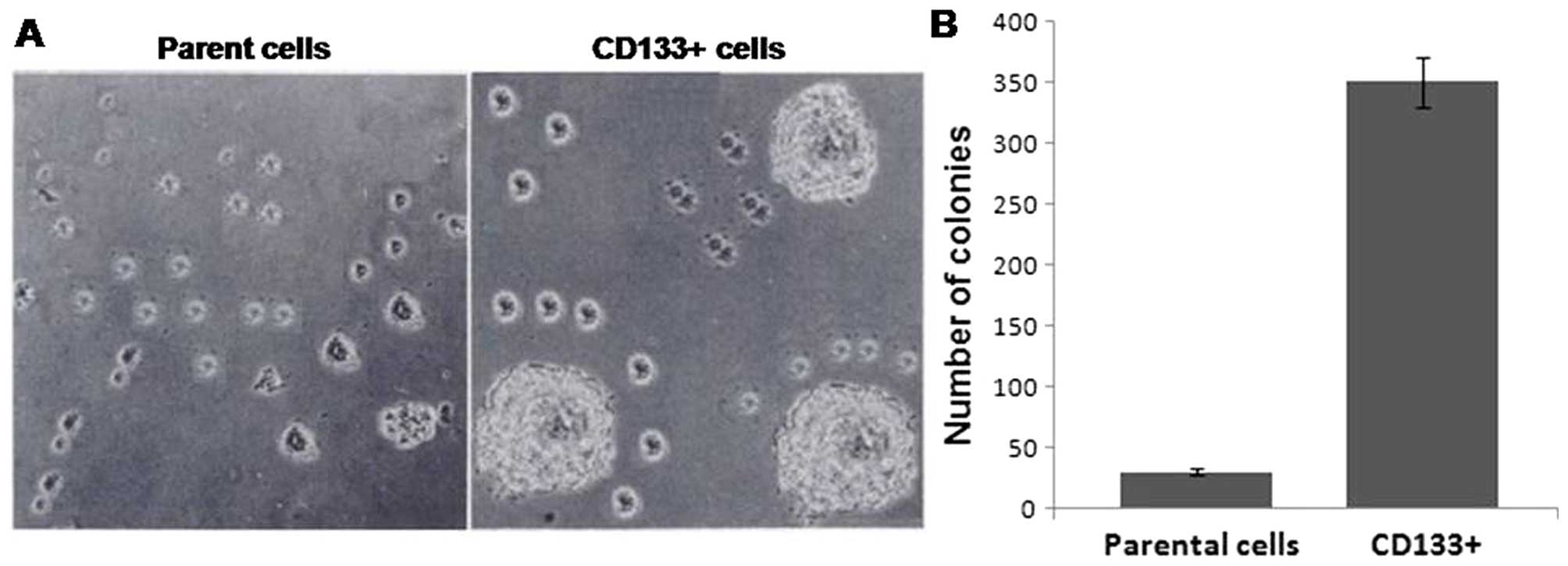
by CD133+ and parental cells were collected. It was
observed that compared to CD133+ cells, the parental
cells formed larger sized and more number of tumorspheres (Fig. 1A). Analysis of the capacity of
selfrenewal revealed that CD133+ cells formed spheroidal
mass of undifferentiated CD133+ cells within 5 days
(Fig. 1B). Thus, CD133+
cells possess the capacity of self-renewal.
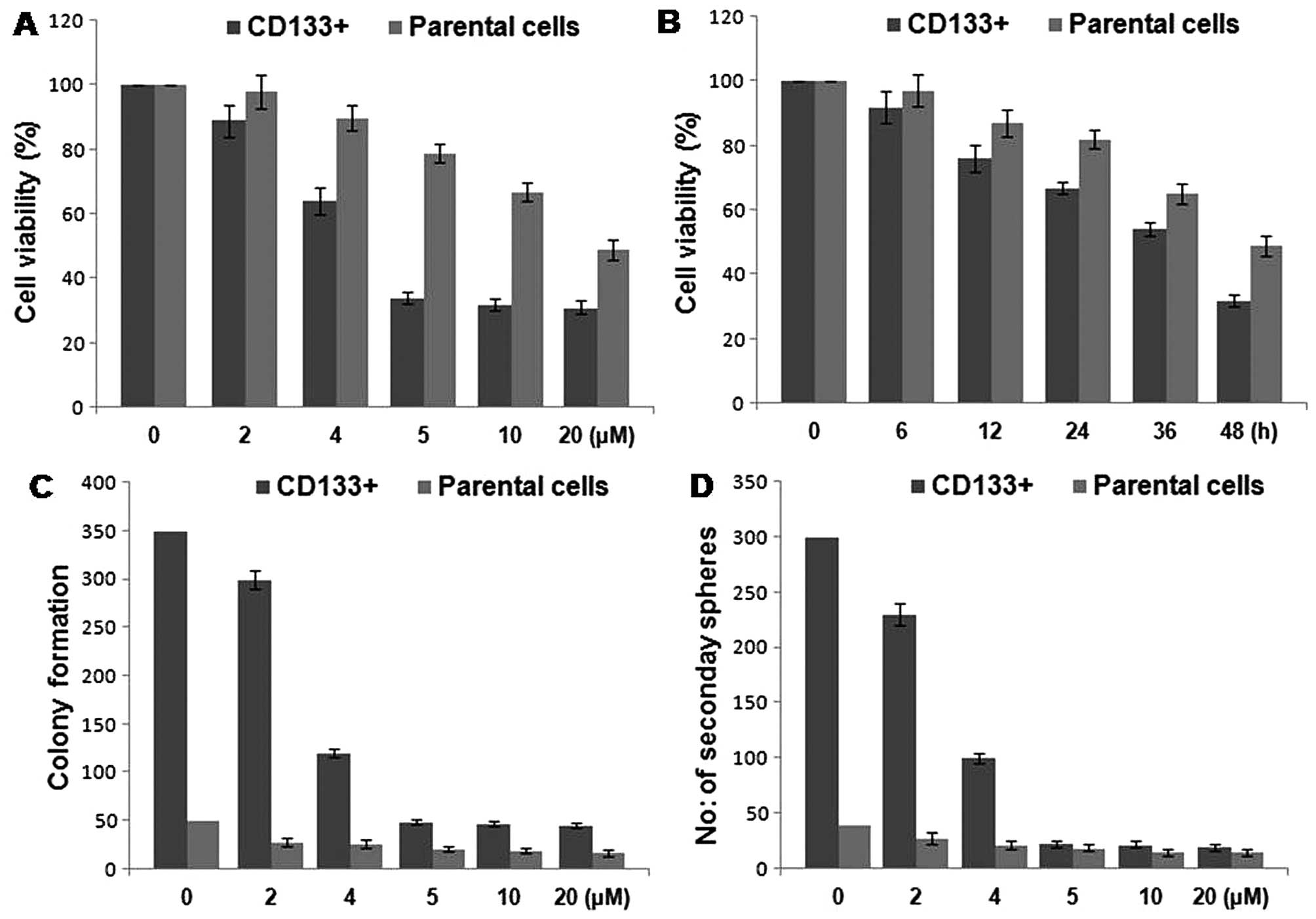
Inhibition of proliferation and
selfrenewal of HCSCs from the HepG2 cell line by cantharidin
The cells were exposed to various concentrations of
cantharidin from 0 to 20 µM for various time periods. The
results revealed a concentration-dependent inhibition of cell
viability of CD133+ HCSCs by cantharidin after 48-h
treatment. The reduction in cell viability of CD133+
HCSCs was significant at 5 µM concentration of cantharidin.
However, in parental cells the inhibition in viability was
significant at 20 µM concentration after 48 h (Fig. 2A and B). Cantharidin treatment at a
concentration of 5 µM significantly inhibited the tendency
of colony formation in HCSCs after 48 h. Compared to the primary
tumorspheres of HCSCs of untreated group, the number of primary as
well as secondary tumorspheres in the cantharidin treated cultures
were significantly reduced (Fig. 2C and
D).
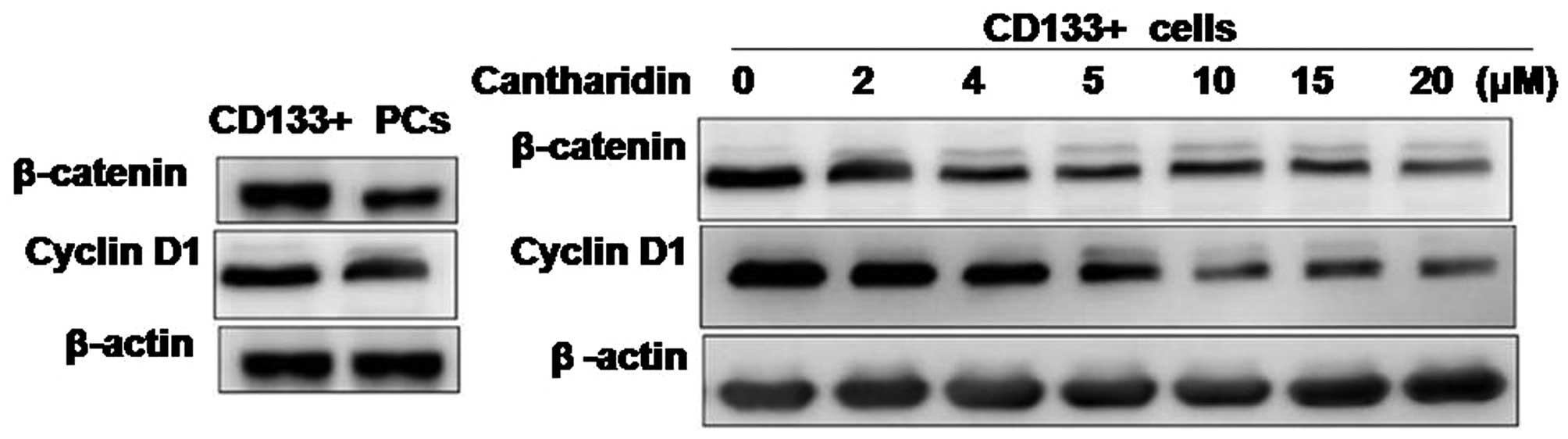
Inhibition of selfrenewal in HCSCs by
cantharidin involves alteration in β-catenin expression
Effect of cantharidin treatment on the expression of
β-catenin and cyclin D1 in CD133+ HCSCs and parental
cells was analyzed by western blot analysis. Results showed a
significantly higher expression of β-catenin and cyclin D1 in the
CD133+ HCSCs compared to the parental cells. However,
cantharidin treatment induced a concentration-dependent inhibition
of β-catenin and cyclin D1 expression. The reduction in expression
of β-catenin and cyclin D1 was significant at the concentration of
5 µM of cantharidin after 48 h in HCSCs (Fig. 3).
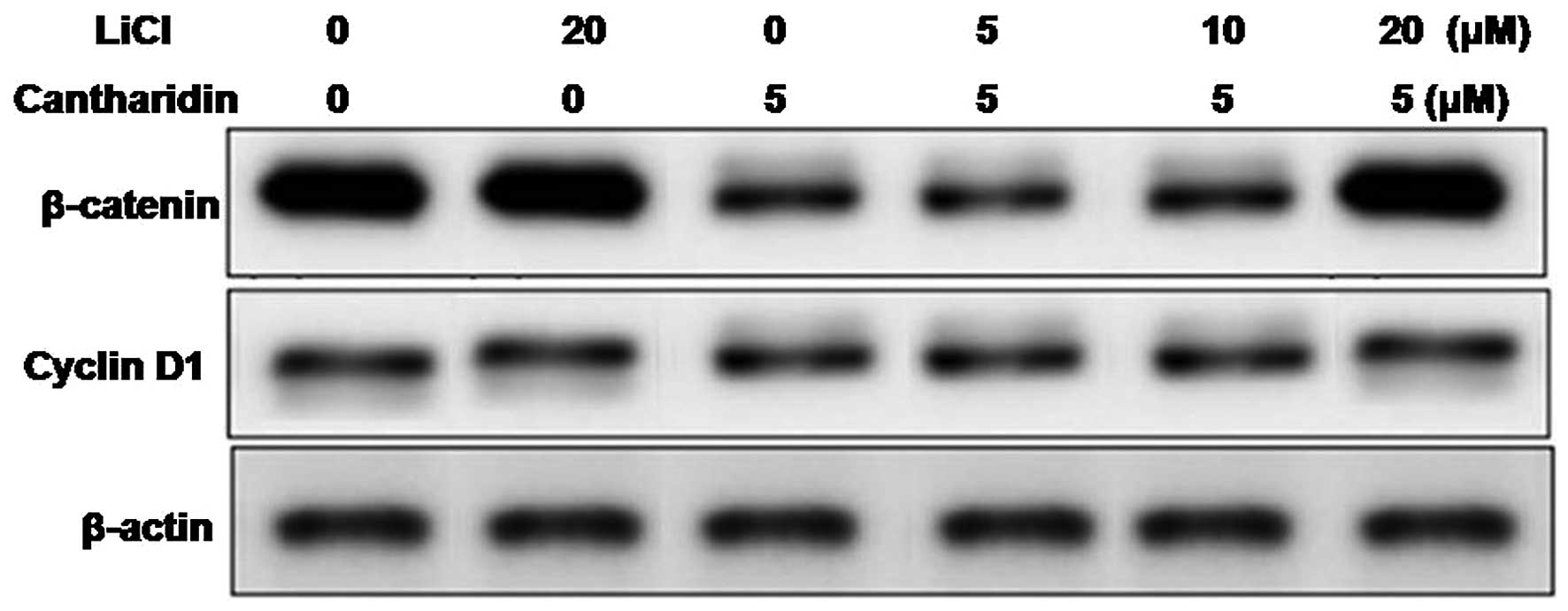
Treatment of the HCSCs with lithium chloride, a
factor known for activation of Wnt/β-catenin pathway led to the
enhancement in expression of β-catenin and cyclin D1. When the
lithium chloride pretreated HCSCs were exposed to cantharidin the
inhibition in expression of β-catenin and cyclin D1 caused by
cantharidin was suppressed (Fig.
4). Since β-catenin pathway plays an important role in the
self-renewal of cancer stem cells, cantharidin treatment in HCSCs
inhibits self-renewal by inhibiting the expression of β-catenin and
cyclin D1.
Cantharidin treatment arrests cell cycle
in G2/M phase in the HCSCs
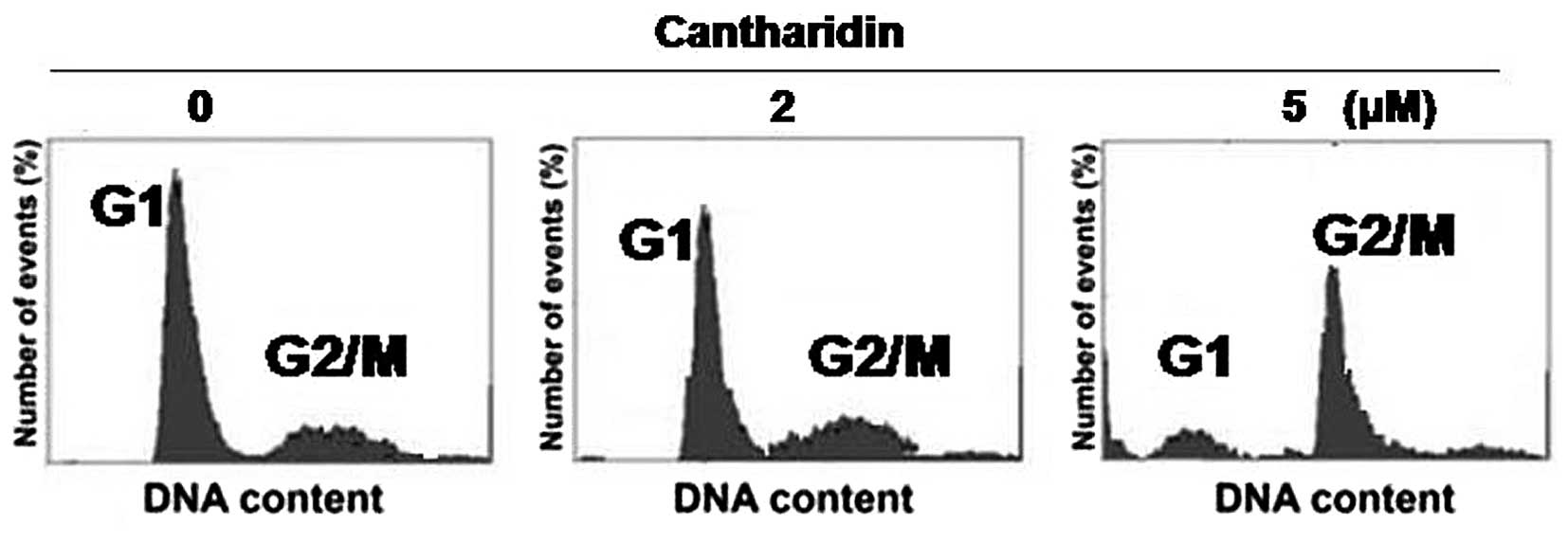
Flow cytometry was used to analyze the effect of
cantharidin on cell cycle in the HCSCs. It was observed that
cantharidin treatment at 5 µM concentration significantly
enhanced the cell population in G2/M phase and decreased the
population in the G1 phase. The population of CD133+
HCSCs in the G2/M phase increased from 19.5±1.8 to 73.4±4.2% with
the increase in treatment time from 24 to 48 h. In G1 phase the
population of cells decreased from 17±2.0 to 9.6±1.8% with the
increase in treatment time from 24 to 48 h. However, in parental
cells the proportion of cells in G2/M phase were 7.5±1.2 and
11.2±2.1%, respectively after 24 and 48 h (Fig. 5).
Alteration in cell cycle regulatory
proteins by cantharidin treatment in the HCSCs
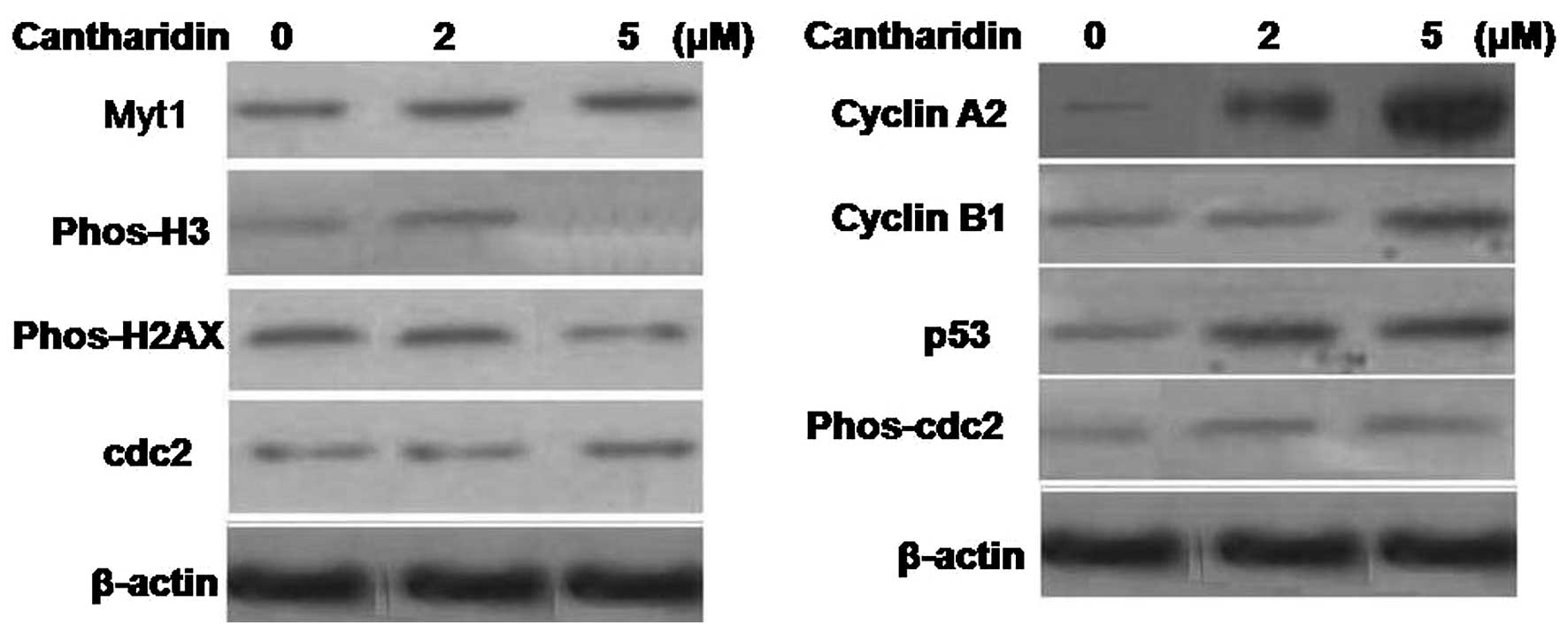
Cantharidin treatment in the HCSCs for 48 h induced
a significant increase in histone H2AX expression compared to the
parental cells. It also promoted the Myt1 protein expression and
cdc2 (Tyr15) phosphorylation in HCSCs (Fig. 6). Analysis of cyclin A2, cyclin B1
and p53 revealed a significant increase in the expression of all
the three proteins in the cantharidin treated HCSCs. However, the
histone H3 expression was inhibited significantly in HCSCs on
treatment with cantharidin for 48 h.
Induction of apoptosis in HCSCs by
cantharidin treatment
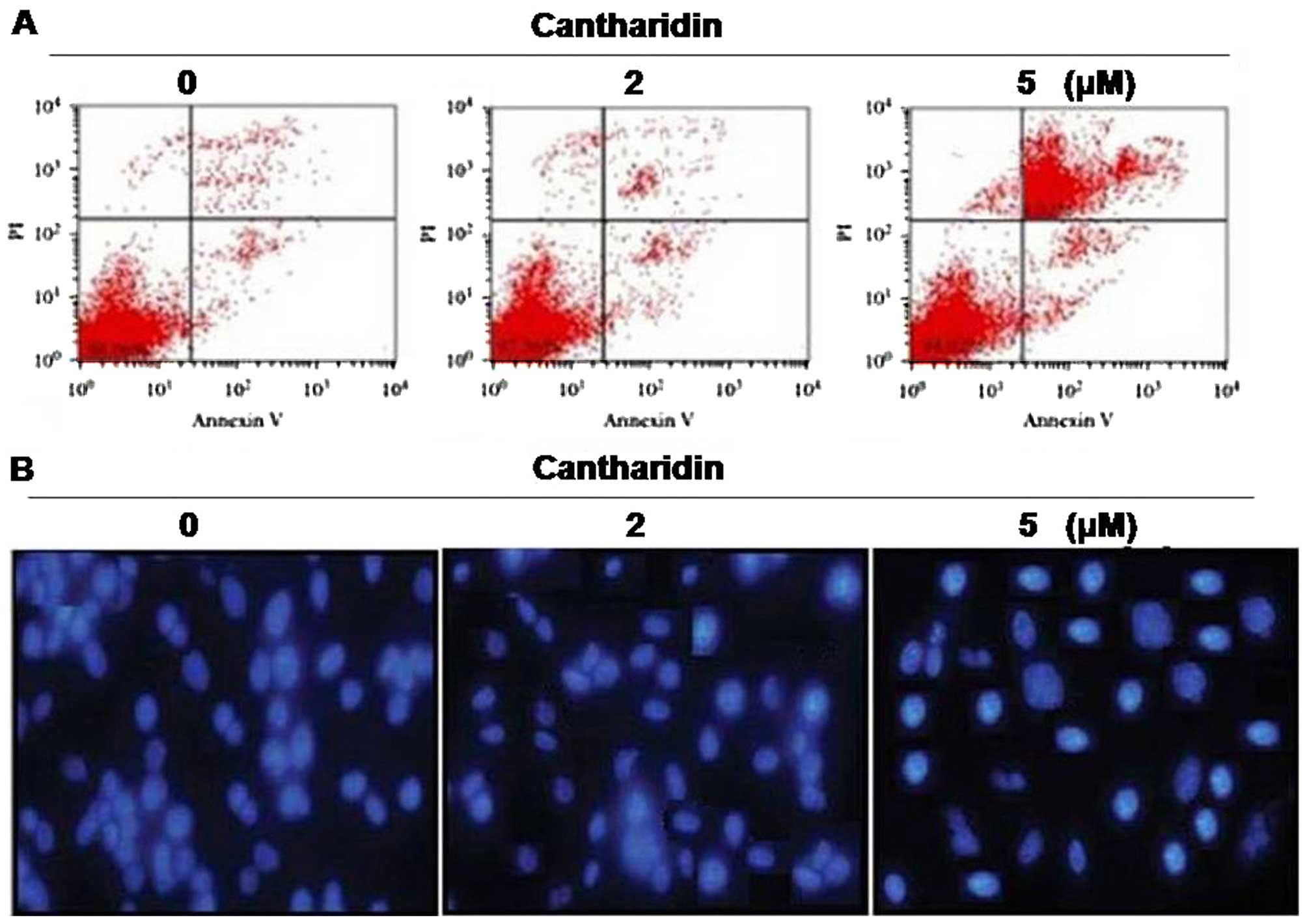
Exposure of the HCSCs to cantharidin for 48 h at a
concentration of 5 µM caused a significant increase in the
proportion of apoptotic cells. Annexin V/FITC staining showed that
cantharidin treatment significantly increased the Annexin
V/FITCstained HCSCs cells compared to the untreated cells (Fig. 7A). HCSCs cells treated with
cantharidin for 48 h showed significant morphological alterations
by immunofluorescence (Fig.
7B).
Discussion
Carcinoma stem cells (CSCs) play a vital role in the
progression of cancer and its resistance to chemotherapeutic
agents. Therefore, inhibition of CSC proliferation by arresting
cell cycle and induction of apoptosis is of promising importance
for the treatment of cancer (16).
It is reported that CSC proliferation can be inhibited by targeting
a number of factors including hedgehog, Wnt/β-catenin, Notch and
EGFR pathways (17,18). The present study demonstrates the
effect of cantharidin on inhibition of cell proliferation,
self-renewal, cell cycle arrest and induction of apoptosis in
CD133+ hepatocellular carcinoma cell line, derived from
HepG2 cell line. It was observed that cantharidin treatment
inhibited cell viability and tendency of self-renewal, arrested
cell cycle in G2/M phase and induced apoptosis in CD133+
HCSCs. CSCs are present in various types of cancers and are
identified on the basis of presence of CD133, surface marker
(19). In the present study,
CD133+ HCSCs were separated from HepG2 cell lines and
cultured in media conditioned for stem cell to form tumorspheres.
It was observed that CD133+ HCSCs formed larger sized
and greater number of tumorspheres compared to the parent cells.
Exposure of the HCSCs to cantharidin significantly inhibited the
cell proliferation and tendency of self-renewal after 48 h of
treatment compared to the parental cells. Cantharidin treatment
also led to inhibition of the tendency to form spheres in
HCSCs.
Wnt/β-catenin signaling pathway plays an important
role in the self-renewal of the cancer stem cells and hence
progression and invasion of carcinoma (20). Therefore, the effect of cantharidin
on the β-catenin and cyclin D1 expression was also investigated. It
was observed that cantharidin treatment significantly inhibited the
β-catenin and cyclin D1 expression in CD133+ HCSCs
compared to the parental cells. However, lithium chloride, a factor
known for activation of Wnt/β-catenin pathway exposure to
cantharidin pretreated cells prevented the cantharidin-induced
inhibition of β-catenin and cyclin D1 expression. These findings
suggest that inhibition of self-renewal in CD133+ HCSCs
by cantharidin treatment is associated with β-catenin and cyclin D1
expression.
Progression of cell cycle is regulated by various
factors including, cyclins and cyclin-dependent kinases and
activated cdc2-linked cyclin A and cyclin B regulate the
progression of G2/M phase. Activation of cdc2 Tyr15 and Thr14 by
Myt1 kinases results in inactivation of the cdc2/cyclin B complex
(21–23). In the present study cantharidin
treatment significantly increased the expression of Myt1 protein in
CD133+ HCSCs. The phospho-cdc2 (Tyr15) and cyclin A2
protein expression was also promoted in CD133+ HCSCs
compared to the parental cells. The phosphorylation of histone H2AX
proteins was increased and the cell cycle was arrested in the G2/M
phase. The process of apoptosis involving the removal of damaged
cell is inhibited in the carcinoma cells (24). In the present study cantharidin
treatment induced apoptosis in CD133+ HCSCs
significantly compared to the parental cells after 48 h.
In conclusion, cantharidin treatment inhibits
proliferation and self-renewal, arrests cell cycle in G2/M phase
and induces apoptosis in CD133+ HCSCs derived from HepG2
cell line. Therefore, cantharidin is a potent agent for the
inhibition of hepatocellular carcinoma.
Acknowledgments
The study was supported by the Jiangxi Major
Technology Project (no. 20144BBG70001).
References
|
1
|
Ma S, Chan KW and Guan XY: In search of
liver cancer stem cells. Stem Cell Rev. 4:179–192. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tomuleasa C, Soritau O, Rus-Ciuca D, Pop
T, Todea D, Mosteanu O, Pintea B, Foris V, Susman S, Kacsó G, et
al: Isolation and characterization of hepatic cancer cells with
stem-like properties from hepatocellular carcinoma. J
Gastrointestin Liver Dis. 19:61–67. 2010.PubMed/NCBI
|
|
3
|
Ricci-Vitiani L, Lombardi DG, Pilozzi E,
Biffoni M, Todaro M, Peschle C and De Maria R: Identification and
expansion of human colon-cancer-initiating cells. Nature.
445:1111152007. View Article : Google Scholar
|
|
4
|
Lee TK, Castilho A, Ma S and Ng IO: Liver
cancer stem cells: Implications for a new therapeutic target. Liver
Int. 29:955–965. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mackillop WJ, Ciampi A, Till JE and Buick
RN: A stem cell model of human tumor growth: Implications for tumor
cell clonogenic assays. J Natl Cancer Inst. 70:9–16.
1983.PubMed/NCBI
|
|
6
|
Gupta PB, Chaffer CL and Weinberg RA:
Cancer stem cells: Mirage or reality? Nat Med. 15:1010–1012. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chiba T, Kita K, Zheng YW, Yokosuka O,
Saisho H, Iwama A, Nakauchi H and Taniguchi H: Side population
purified from hepatocellular carcinoma cells harbors cancer stem
cell-like properties. Hepatology. 44:240–251. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Curry SC, Carlton MW and Raschke RA:
Prevention of fetal and maternal cyanide toxicity from
nitroprusside with coinfusion of sodium thiosulfate in gravid ewes.
Anesth Analg. 84:1121–1126. 1997.PubMed/NCBI
|
|
9
|
Honkanen RE: Cantharidin, another natural
toxin that inhibits the activity of serine/threonine protein
phosphatases types 1 and 2A. FEBS Lett. 330:283–286. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Clarke PR, Hoffmann I, Draetta G and
Karsenti E: Dephosphorylation of cdc25-C by a type-2A protein
phosphatase: Specific regulation during the cell cycle in Xenopus
egg extracts. Mol Biol Cell. 4:397–411. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen YN, Chen JC, Yin SC, Wang GS, Tsauer
W, Hsu SF and Hsu SL: Effector mechanisms of norcantharidin-induced
mitotic arrest and apoptosis in human hepatoma cells. Int J Cancer.
100:158–165. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kok SH, Cheng SJ, Hong CY, Lee JJ, Lin SK,
Kuo YS, Chiang CP and Kuo MY: Norcantharidin-induced apoptosis in
oral cancer cells is associated with an increase of proapoptotic to
antiapoptotic protein ratio. Cancer Lett. 217:43–52. 2005.
View Article : Google Scholar
|
|
13
|
Huan SK, Lee HH, Liu DZ, Wu CC and Wang
CC: Cantharidin-induced cytotoxicity and cyclooxygenase 2
expression in human bladder carcinoma cell line. Toxicology.
223:136–143. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Williams LA, Möller W, Merisor E, Kraus W
and Rösner H: In vitro anti-proliferation/cytotoxic activity of
cantharidin (Spanish Fly) and related derivatives. West Indian Med
J. 52:10–13. 2003.PubMed/NCBI
|
|
15
|
Yi SN, Wass J, Vincent P and Iland H:
Inhibitory effect of norcantharidin on K562 human myeloid leukemia
cells in vitro. Leuk Res. 15:883–886. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Naujokat C and Steinhart R: Salinomycin as
a drug for targeting human cancer stem cells. J Biomed Biotechnol.
2012:9506582012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang Z, Li Y, Ahmad A, Azmi AS, Kong D,
Banerjee S and Sarkar FH: Targeting miRNAs involved in cancer stem
cell and EMT regulation: An emerging concept in overcoming drug
resistance. Drug Resist Updat. 13:109–118. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu J, Kopecková P, Bühler P, Wolf P, Pan
H, Bauer H, Elsässer-Beile U and Kopeček J: Biorecognition and
subcellular trafficking of HPMA copolymeranti PSMA antibody
conjugates by prostate cancer cells. Mol Pharm. 6:9599702009.
View Article : Google Scholar
|
|
19
|
Mizrak D, Brittan M and Alison M: CD133:
Molecule of the moment. J Pathol. 214:3–9. 2008. View Article : Google Scholar
|
|
20
|
Li L, Ying J, Li H, Zhang Y, Shu X, Fan Y,
Tan J, Cao Y, Tsao SW, Srivastava G, et al: The human cadherin 11
is a proapoptotic tumor suppressor modulating cell stemness through
Wnt/betacatenin signaling and silenced in common carcinomas.
Oncogene. 31:390139122012. View Article : Google Scholar
|
|
21
|
Booher RN, Holman PS and Fattaey A: Human
Myt1 is a cell cycle-regulated kinase that inhibits Cdc2 but not
Cdk2 activity. J Biol Chem. 272:22300–22306. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu F, Stanton JJ, Wu Z and PiwnicaWorms
H: The human Myt1 kinase preferentially phosphorylates Cdc2 on
threonine 14 and localizes to the endoplasmic reticulum and Golgi
complex. Mol Cell Biol. 17:5715831997. View Article : Google Scholar
|
|
23
|
Parker LL and Piwnica-Worms H:
Inactivation of the p34cdc2-cyclin B complex by the human WEE1
tyrosine kinase. Science. 257:195519571992. View Article : Google Scholar
|
|
24
|
Raff MC, Barres BA, Burne JF, Coles HS,
Ishizaki Y and Jacobson MD: Programmed cell death and the control
of cell survival: Lessons from the nervous system. Science.
257:1955–1957. 1992.
|