Introduction
Colon carcinoma is one of the most common and
aggressive malignant tumors worldwide (1). Currently, treatments for colon
carcinoma are mainly surgery and chemotherapy, but the curative
effect of existing chemotherapeutic drugs is not good, and they
have numerous side-effects, including myelosuppression, neutropenia
and thrombocytopenia (2).
Therefore, the exploration of a new approach, such as novel drugs
with specific effects on colon carcinoma treatment is urgently
needed.
Harmine, a β-carboline alkaloid isolated from the
seeds of Peganum harmala (Fig.
1), has been traditionally used for ritual and medicinal
preparations in the Middle East, Central Asia and South America
(3). Previous research has shown
that harmine plays roles in anticancer treatments (4–6) and
possesses anti-leishmanial properties (7) and antiviral effects (8) via inhibition of DYRK1A substrate
phosphorylation. This compound was also found to interfere with
neuritogenesis in cultured hippocampal neurons (9) and inhibit angiogenesis (10), telomerase activity (5) and mitochondrial signaling pathways
(6,11), in addition to inducing DNA single-
or double-strand breaks (12). It
has been reported that harmine activates both the intrinsic and
extrinsic pathways of apoptosis and regulates various transcription
factors and pro-inflammatory cytokines in B16F-10 cells (6). Furthermore, the in vivo
anti-angiogenic activity of harmine was studied using B16F-10
melanoma cells in C57BL/6 mice. The results showed that harmine
decreased tumor capillary formation and inhibited angiogenesis
(4). Harmine was also shown to
induce apoptosis and suppress tumor cell proliferation through the
downregulation of cyclooxygenase-2 expression in gastric cancer
(13). Additionally, harmine can
upregulate p21 and p27, enhance the formation of complexes with the
G1-S phase CDKs and cyclins, and induce G1 arrest to stall cancer
progression in human breast cancer MCF7 (p53 wild-type), MCF7 (p53
knockdown) and MDA-MB-468 (p53 mutant) cells (14). In cytotoxicity assays, harmine
exhibited a strong inhibitory effect on the growth and
proliferation of carcinoma cells, whereas it had no significant
effects on quiescent fibroblasts (15). However, no detailed data are
available in regards to the growth inhibition of human colon
carcinoma cells. In the present study, we investigated the effect
of harmine on the growth of human SW620 cells.
Materials and methods
Materials
Harmine was purchased from the Xi'an Feida Bio-Tech
Co., Ltd. (Xi'an, China). Fetal bovine serum (FBS), RPMI-1640
medium, trypsin and EDTA were purchased from Gibco-BRL
(Gaithersburg, MD, USA); Cell Counting Kit-8 (CCK-8/WST-8 kit) was
purchased from Dojindo, Japan. Dimethyl sulfoxide (DMSO), Annexin
V-fluorescent isothiocyanate (FITC) and propidium iodide (PI) were
purchased from Sigma-Aldrich (St. Louis, MO, USA). Antibodies
against Bax, Bcl-2, Bcl-xL, Mcl-1, caspase-9 and -3,
poly(ADP-ribose) polymerase (PARP), cleaved PARP, cyclin A, B1, D1
and E2, CDK4, cdc2, p-cdc2 (Tyr15), Myt-1, Akt, p-Akt (Ser473),
p-Akt (Thr308), GSK3α/β, p-GSK3β (Ser9), FoxO3a, p-FoxO3a
(Ser318/321) and GAPDH as well as all secondary antibodies were
purchased from Cell Signaling Technology Ltd. (Danvers, MA,
USA).
Cell culture and treatment
Cells were cultured in RPMI-1640 medium supplemented
with 10% FBS at 37°C in a humidified atmosphere containing 5%
CO2. Harmine was dissolved in DMSO and diluted to
appropriate concentrations with culture medium. The final
concentration of DMSO in the culture medium did not exceed
0.1%.
CCK-8 assay
CCK-8 testing was used to monitor cell
proliferation. Cells were plated at a density of 5,000 cells/well
in 96-well plates. After 24 h in culture, cells were treated with
harmine at the final concentrations of 0, 0.625, 1.25, 2.50, 5.00,
10.00 and 20.00 μg/ml for 48 h. Control cells were treated
with DMSO. After cells were incubated with harmine for 48 h, 10
μl CCK-8 was added to each well. Absorbance was detected
with an enzyme calibrator at 570 nm and then optical density (OD)
values were measured. Inhibition of cell growth was computed as the
percentage of viable cells compared with the control: Percentage
(%) = (ODcontrol −
ODtreatment)/ODcontrol × 100%. Experiments
were performed in triplicate.
Clone formation assay
A clone formation assay was used to evaluate the
effects of harmine on the proliferation of SW620 cells. Cells were
first cultured in 12-well microplates (300 cells/well) in 2.0 ml of
complete RPMI-1640 for 24 h. Then, the cells were treated with the
indicated concentrations of harmine for 7 days. Finally, the cells
were stained with crystal violet for 20 min. A digital camera
captured images of the colonies as previously described (16).
Fluorescence microscopy assay
Harmine-induced apoptosis in SW620 cells was
assessed by Hoechst 33258 staining. Following treatment with the
indicated concentrations of harmine for 48 h, the cells were
harvested and smeared on slides. The slides were air-dried, fixed
in methanol-acetone (3:1, v/v) and stained with Hoechst 33258 (5
μg/ml) at 37°C in the dark for 20 min. Nuclear morphology
was examined using fluorescence microscopy (DFC480; Leica
Microsystems, Wetzlar, Germany) to identify cells undergoing
apoptosis.
Flow cytometry
SW620 cells at a density of 20,000 cells/well were
incubated in 6-well plates for 48 h with the indicated
concentrations of harmine. After incubation, the cells were
harvested, washed with phosphate-buffered solution (PBS), and fixed
in 70% ice-cold ethanol overnight. Then, the fixed cells were
incubated with 20 U/ml RNase I and 50 μg/ml PI for 30 min.
The DNA content was determined by flow cytometry (FCM; Beckman
Coulter, Fullerton, CA, USA). Apoptotic cells were identified by
the sub-G1 phase in the cell cycle distribution. For assessment of
the apoptotic rate, Annexin V-FITC/PI staining was performed
according to the manufacturer's protocol. The cells that were
Annexin V-positive and PI-negative were defined as early apoptotic
cells, and cells that were both Annexin V and PI-positive were
defined as late apoptotic cells. The apoptotic rate was measured by
FCM (Becton-Dickinson, USA) using CellQuest software.
Membrane potential of the mitochondria
(ΔΨm)
Changes in the membrane potential of mitochondria
were analyzed with JC-1 staining. SW620 cells were treated with
2.50 μg/ml harmine for 48 h, and then the cells were
harvested, washed with PBS, fixed in JC-1 at 37°C in the dark for
30 min, then harvested and smeared on slides. Changes in ΔΨm were
measured with a fluorescence microscope according to the
manufacturer's protocol.
Western blot analysis
Total protein was extracted by incubation of cell
pellets with lysis buffer. The protein concentration was determined
using the BCA assay (Sigma) according to the manufacturer's
instructions. Cell lysates were electrophoresed on 10–15% sodium
dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gels.
The protein bands were then transferred to polyvinylidene
difluoride (PVDF) membranes. After blocking with 5% dried skimmed
milk, the membranes were incubated overnight at 4°C with the
appropriate primary antibody. Then, the membranes were washed three
times in Tris-buffered saline and Tween-20 (TBST) and incubated for
1 h at room temperature with a secondary antibody conjugated to
horseradish peroxidase. After washing in TBST, the bound antibody
complex was detected using an ECL chemiluminescence reagent and
X-ray film (Kodak, Rochester, NY, USA).
Statistical analysis
Data were analyzed by ANOVA and Student's t-tests.
These analyses were performed using SPSS 13.0 software. All results
are expressed as the means ± standard error (SE) of three
independent experiments. Differences with P<0.05 were considered
to be statistically significant.
Results
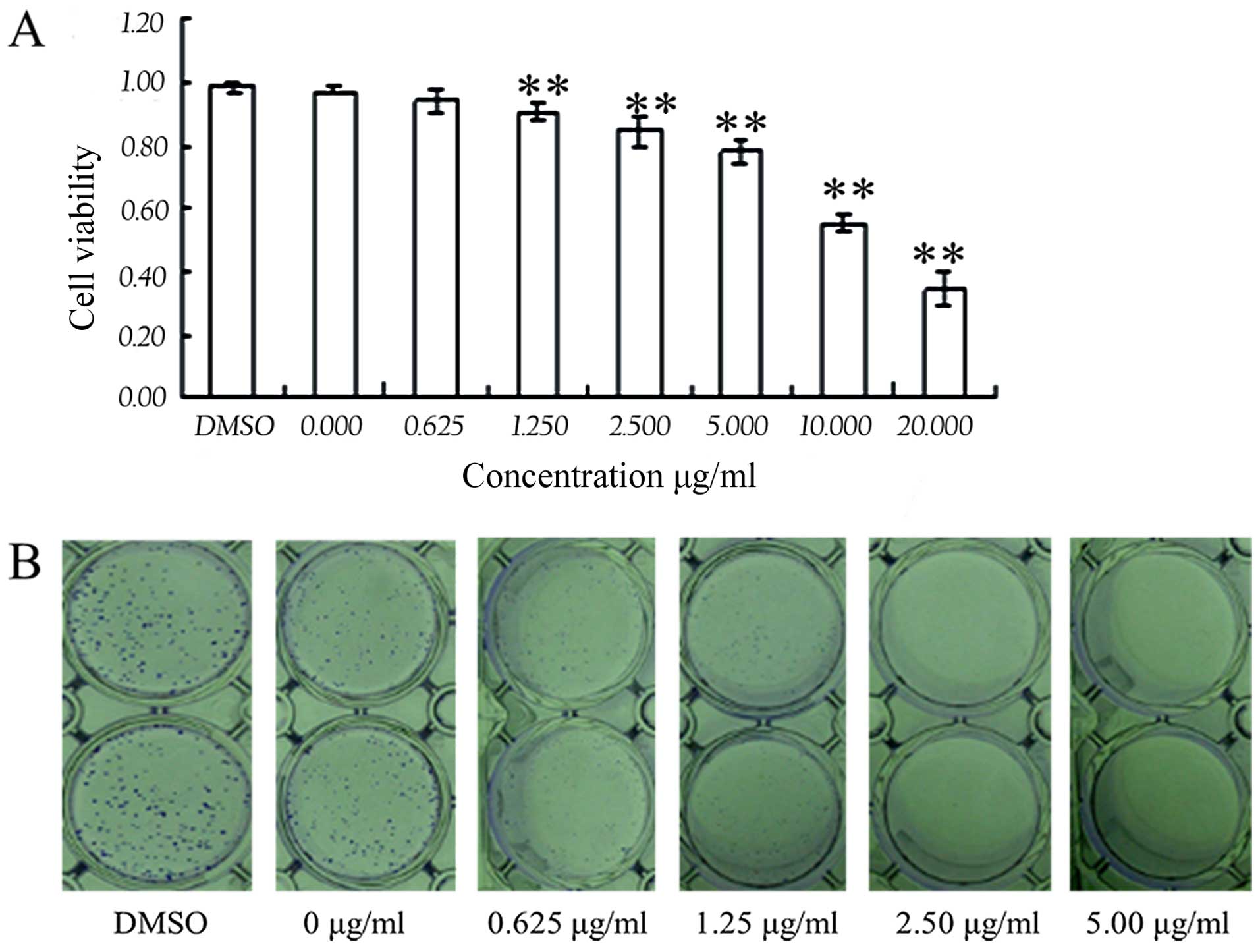
Harmine inhibits SW620 cell
proliferation
To study the effects of harmine on cell
proliferation, we used a CCK-8/WST-8 assay to analyze the
proliferation of the SW620 cells. After cells were incubated with
the indicated concentrations of harmine for 48 h, cell viability
was markedly decreased (Fig. 2A).
Harmine inhibited the growth of SW620 cells in a dose-dependent
manner (P<0.05 vs. control), with a half-maximal inhibitory
concentration (IC50) value of 5.13 μg/ml. We
further assessed the effect of harmine on the proliferation of
SW620 cells using a clone formation assay. The results showed that
harmine strongly inhibited SW620 cell proliferation in a
dose-dependent manner after treatment with the indicated
concentrations of harmine for 7 days (Fig. 2B).
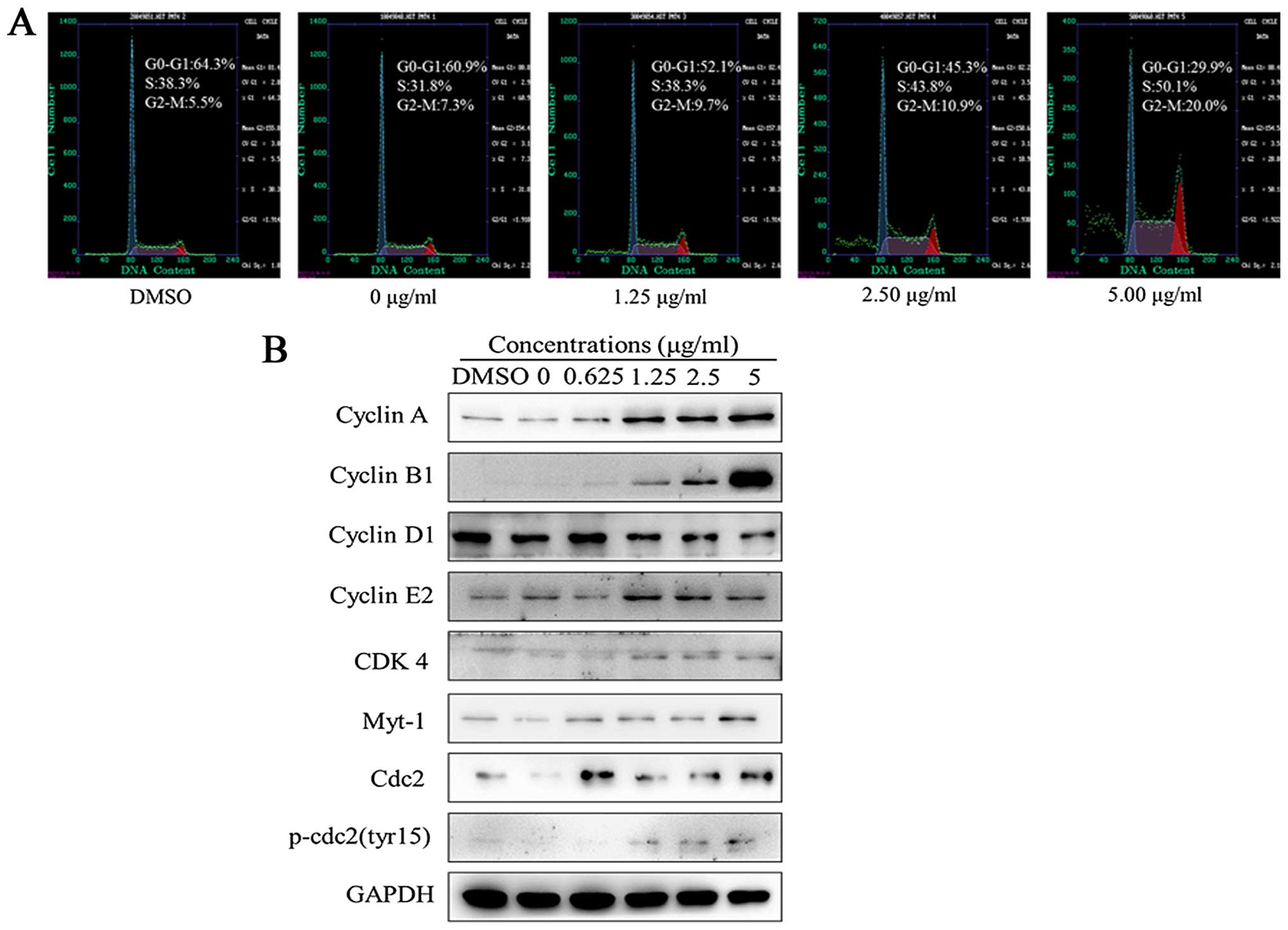
Harmine alters the cell cycle and
modulates the expression of cell cycle regulatory proteins in SW620
cells
Flow cytometry with a PI staining assay was used to
analyze the effects of harmine on the cell cycle distribution. As
shown in Fig. 3A, a significant
population of cells was arrested in the S phase (38.3–50.1%),
compared to 38.3% in the DMSO-treated controls and the G2/M phase
(9.7–20.0%), compared to 5.5% in the DMSO-treated controls after
treatment with harmine at the indicated concentrations for 48 h.
The population of cells in the S and G2-M phase increased, and the
population of cells in the G1 phase decreased with increasing
concentrations of harmine. These results indicate that cell cycle
distribution was significantly arrested in the S and G2/M phases
upon harmine treatment.
As significant cell cycle arrest was observed with
harmine treatment, we next assessed the effect of harmine on cell
cycle regulatory proteins by western blot analysis. As shown in
Fig. 3B, the expression of the G1
phase-related protein as cyclin D1, was significantly reduced after
treatment with harmine for 48 h. However, the expression levels of
S- and G2/M phase-related proteins, such as cyclin A, B1 and E2,
increased, and those of G2/M phase-related proteins, such as cdc2,
Myt-1 and p-cdc2 (Tyr15), were also increased.
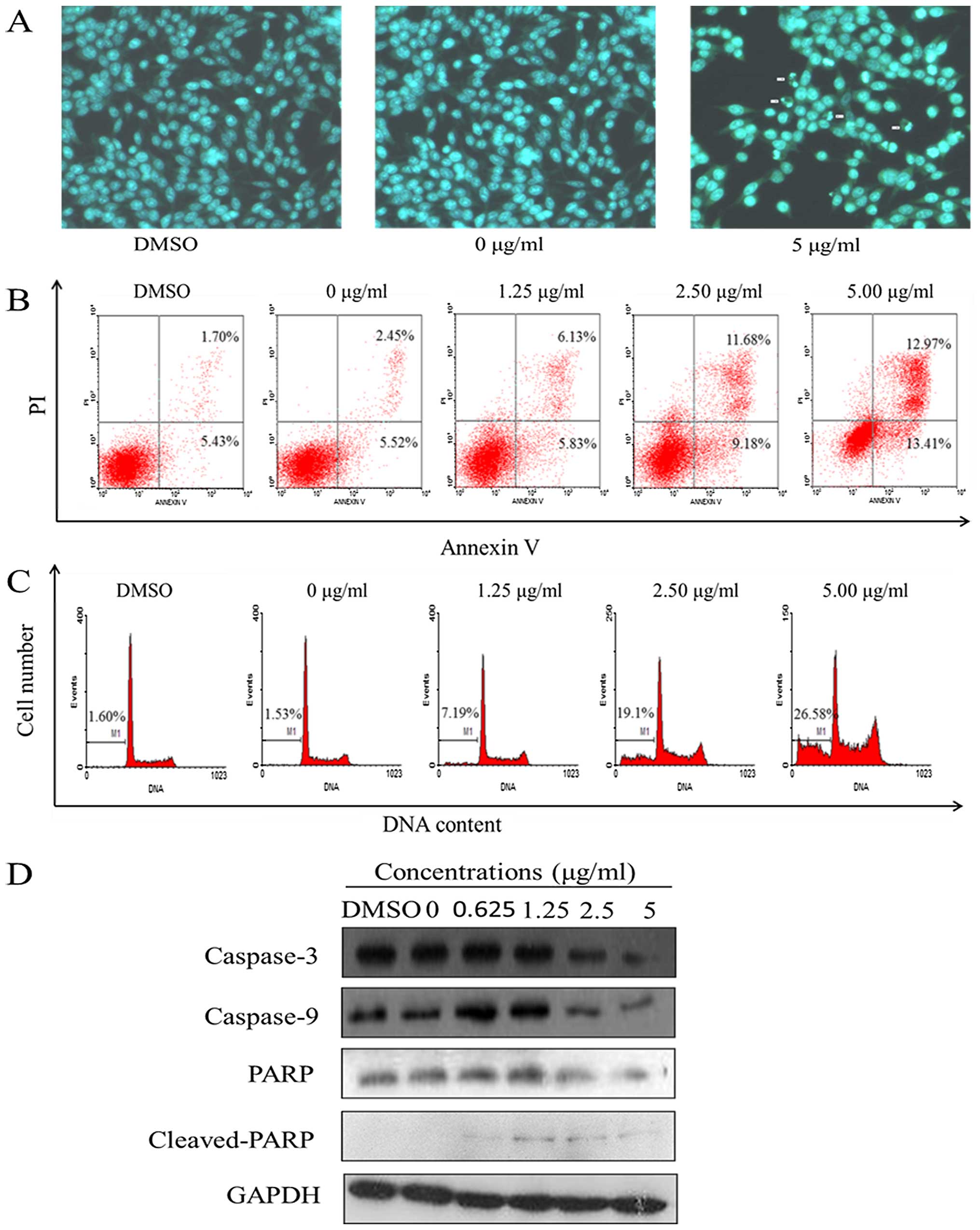
Harmine induces apoptosis in SW620
cells
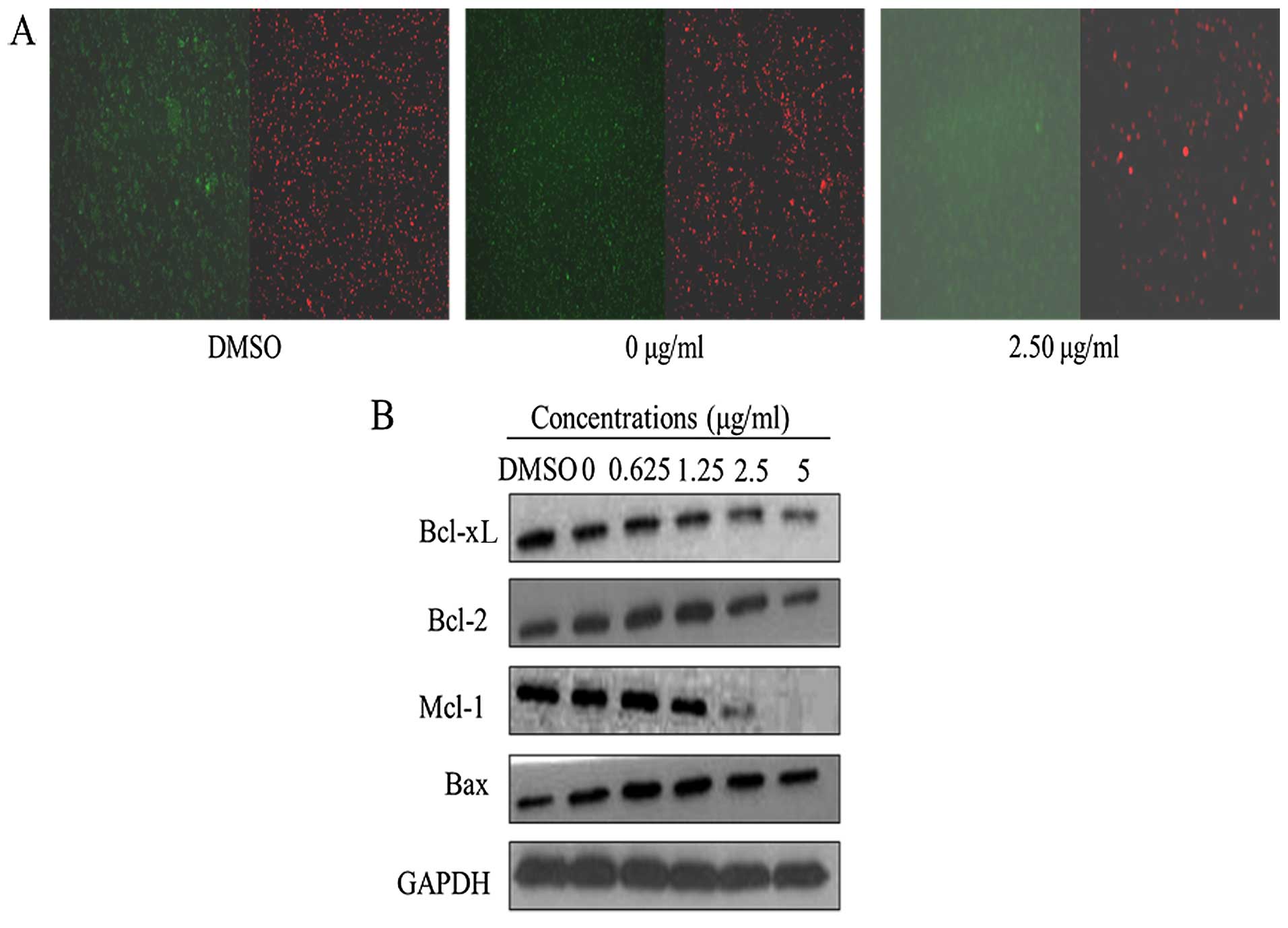
Apoptotic nuclear morphology was observed after
Hoechst 33258 staining using fluorescence microscopy. After
treatment with 5.00 μg/ml harmine for 48 h, the SW620 cells
began to exhibit apoptotic characteristics, such as cell shrinkage,
nuclear condensation and fragmentation. In the control group, the
cells were regular in morphology, grew fully in patches and were
confluent, rarely sloughing off (Fig.
4A).
Apoptosis was also detected through a fluorescein
Annexin V-FITC/PI double staining assay. The staining of cells with
Annexin V-FITC and PI was used to distinguish and quantitatively
determine the percentage of apoptotic cells. The SW620 cells
underwent apoptosis after exposure to harmine at 1.25, 2.50 and
5.00 μg/ml for 48 h, and the percentage of apoptotic cells
stained by Annexin V-FITC is shown in Fig. 4B. The early and late apoptotic cells
increased from 11.96 to 26.38% when incubated with the indicated
concentrations of harmine for 48 h, while in the control group,
<8% of cells were apoptotic.
PI staining and flow cytometric assays were used to
investigate the percentage of sub-G1 cells, which indicates late
apoptotic cells. The SW620 cells underwent apoptosis after being
exposed to harmine at 1.25, 2.50 and 5.00 μg/ml for 48 h,
and the percentage of apoptotic cells as sub-G1 cells increased
from 7.19 to 26.58%, while in the control group, sub-G1 cells
increased from 1.53 to 1.60% (Fig.
4C). The percentage of the sub-G1 fractions increased in a
dose-dependent manner.
The caspases, a family of cysteine acid proteases,
are known to act as important mediators of apoptosis and can cleave
various cellular substrates. To study the mechanism by which
harmine induces apoptosis, western blot analysis was conducted.
Exposure of the SW620 cells to harmine resulted in cleavage of
caspase-3 and -9, and PARP (Fig.
4D). Increased harmine concentrations resulted in the
disappearance of the intact proteins and the appearance of
proteolytic cleavage bands in a concentration-dependent manner,
which indicates that the cells were undergoing apoptosis.
Effect of harmine on mitochondrial
membrane potential (ΔΨm) and protein expression levels of the Bcl-2
family
Loss of ΔΨm is a crucial step in the apoptotic
process and is lethal to cells since it leads to the release of
diverse pro-apoptotic factors, such as Smac and cytochrome
c, from the mitochondria into the cytoplasm (17,18).
In the present study, we used the cationic dye JC-1 to determine
the status of the mitochondria in SW620 cells. In non-apoptotic
cells, JC-1 enters the negatively charged mitochondria, where it
aggregates and turns red. However, in cells undergoing apoptosis
where the ΔΨm has collapsed, JC-1 exists as monomers in the cytosol
and turns green. Our results showed that harmine induced a
depletion of ΔΨm in the SW620 cells (Fig. 5A).
Bcl-2 family proteins are key regulators of
mitochondrial permeability (19).
Therefore, we investigated whether the mitochondrial-mediated
apoptosis in SW620 cells induced by harmine occurred as a result of
the modulation of Bcl-2 family members. Harmine suppressed the
expression of anti-apoptotic Bcl-2, Mcl-1 and Bcl-xL and increased
the expression of pro-apoptotic Bax (Fig. 5B). As a result of these changes, the
ratios of Bcl-2/Bax, Mcl-1/Bax and Bcl-xL/Bax were significantly
reduced during apoptosis.
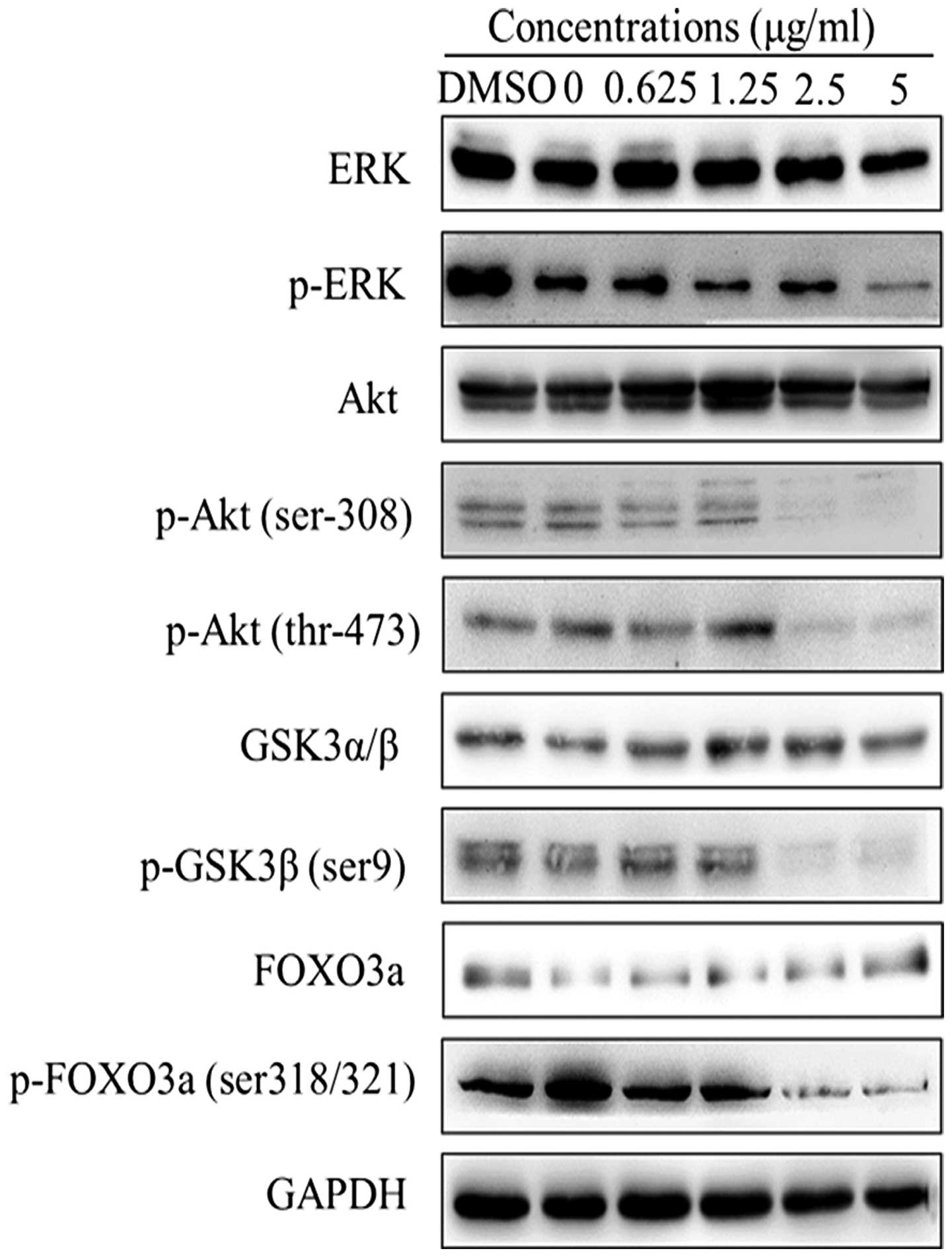
Effects of harmine on the phosphorylation
of Akt and its downstream targets in SW620 cells
To explore the mechanism of cellular apoptosis
induced by harmine in SW620 cells, we examined the Akt signaling
pathway. The results showed that increased harmine concentrations
downregulated phosphory-lation of Ser473-Akt and Thr308-Akt in a
dose-dependent manner without affecting the total amount of Akt
(Fig. 6). Therefore, we examined
whether harmine could inhibit the phosphorylation of downstream
targets of Akt. As expected, the phosphorylation levels of FoxO3a
and GSK-3β were partially attenuated by harmine in a dose-dependent
manner without affecting the amount of total protein in the SW620
cells. Meanwhile, the phosphorylation levels of ERK were also
inhibited without affecting the amount of total protein.
Discussion
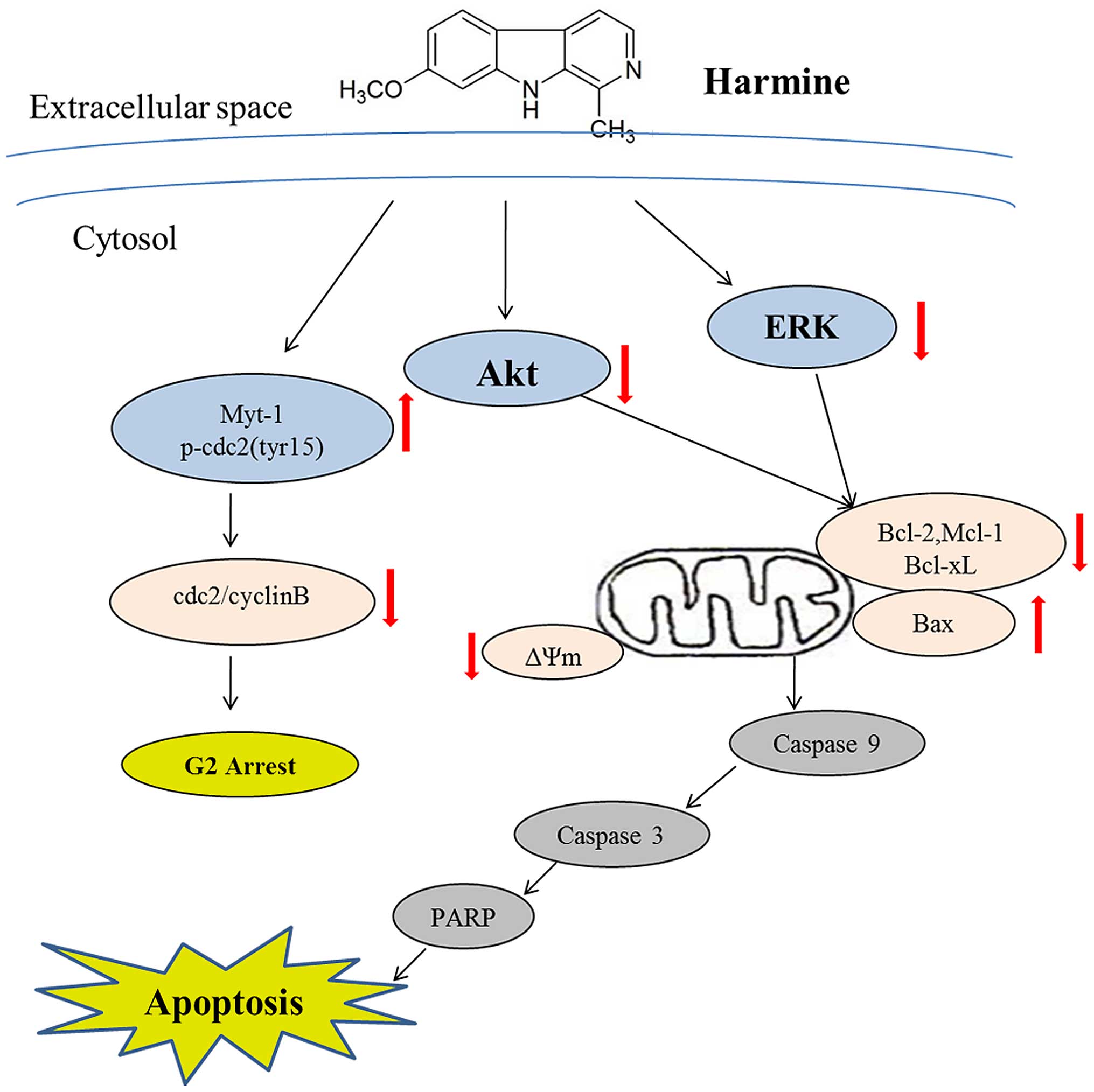
In the present study, we demonstrated that harmine
dose-dependently inhibited the growth of SW620 cells, induced
mitochondrial-dependent apoptosis, and arrested the cell cycle in
the S and G2/M phases. More significantly, we demonstrated that
inhibition of the ERK and Akt signaling pathways was involved in
the antitumor activity of harmine (Fig.
7).
The cell cycle is a common phenomenon of eukaryotic
cell division. In terms of the effects on the cell cycle, there are
four key checkpoints in cell cycle progression: G1/S, S, G2/M and
spindle assembly checkpoints (20).
Cyclin-dependent kinase inhibitors (CKIs), p53, Wee1 and Myt1 play
important roles in the regulation of the cell cycle (21). At the G2/M phase checkpoint, Akt
downstream proteins, Wee1 and Myt1 can inhibit the progression of
the cell cycle by phosphorylating cyclin-dependent kinases (CDKs)
at specific sites, such as the Tyr15 and Thr14 in the ATP binding
loop of cdc2 (22,23). In these experiments, the proportions
of cells in the S and G2/M phases were increased in a
dose-dependent manner, as shown by flow cytometric analysis.
Western blot analysis showed that harmine downregulated cyclin D1
but upregulated cyclin A, E2 and B1, in accordance with the flow
cytometric analysis results. Harmine changes the cell cycle
distribution, and this differs among cell types. Yang et al
indicated that harmine treatment resulted in G1 cell cycle arrest
in a dose-dependent manner in human breast cancer cells (14), and harmine induced human umbilical
vein endothelial cells (HUVECs) to arrest at the S and G2/M phases
(10). Our previous study showed
that harmine also blocked the cell cycle at the S and G2/M phases
in HepG2 cells, similar to our results in SW620 cells (11). Our results also showed that Myt1 and
p-cdc2 (Tyr15) were upregulated, and p-Akt was downregulated
following treatment with harmine. The upregulation of Myt1 reduced
the activity of cdc2 through phosphorylation of Tyr15, which can
induce inhibition of the cdc2/cyclin B complex and cell cycle
arrest in the S and G2/M phases in SW620 cells.
Four experiments, including Hoechst 33258 staining
and fluorescence microscopy; PI staining and flow cytometry;
Annexin V-FITC and PI staining and flow cytometry; and western blot
analysis for the detection of caspase-9 and -3 and PARP, revealed
that harmine treatment resulted in SW620 cells undergoing
apoptosis, and all of the results suggest that the growth
inhibition of SW620 cells by harmine is due to its ability to
induce apoptosis.
The Bcl-2 family proteins play important roles in
the regulation of cellular apoptosis (19). When levels of anti-apoptotic
proteins on the mitochondrial membrane, such as Bcl-2, Bcl-xL and
Mcl-1, decrease and levels of pro-apoptotic proteins (Bax and Bak)
increase or remain unchanged, the ratios of Bcl-2/Bax, Bcl-xL/Bax
and Mcl-1/Bax decrease, leading to a relative increase in Bax. Bax
proteins change their configurations to form oligomers and insert
into the mitochondrial membrane as pores, which leads to the
release of cytochrome c from the mitochondria and the
initiation of the caspase cascade and subsequently to apoptosis
(24,25). In our experiments, harmine caused
disruption of ΔΨm and increased the expression of pro-apoptotic Bax
but decreased the expression levels of anti-apoptotic Bcl-2, Bcl-xL
and Mcl-1, leading to the upregulation of the ratios of Bax/Bcl-2,
Bax/Bcl-xL and Bax/Mcl-1. These results demonstrated that harmine
induced apoptosis through the mitochondrial pathway in SW620
cells.
The PI3K-Akt signaling pathway is an important
intracellular signal transduction pathway. It plays important roles
in cell proliferation, cell growth and metabolism, cell survival
and angiogenesis by affecting the activity of downstream molecules,
and it is closely associated with the development and progression
of human tumors. This pathway can directly phosphorylate Bad on
Ser136, and this creates a binding site for 14-3-3 proteins, which
triggers the release of Bad from its target proteins. Akt can
phosphorylate Ser196 on human pro-caspase-9, and this
phosphorylation correlates with a decrease in the protease activity
of caspase-9 in vitro. Akt also phosphorylates MDM2 on
Ser166 and Ser186, and this promotes the translocation of MDM2 to
the nucleus, where it negatively regulates p53. Other substrates of
Akt include FoxO, GSK3, ASK1, TSC2, RAF1, Chk1, IKKα,
p21cip1 and p27kip1, which affect cell
survival and cell cycle progression (26–28).
In the present study, we demonstrated that harmine inhibits Akt
kinase activity by decreasing the phosphorylation of Akt on Thr308
and Ser473, and its downstream targets p-FoxO3a and p-GSK3β were
markedly decreased in the presence of harmine in vitro.
The targets of FoxO transcription factors include
cyclin A, cyclin B, cyclin D, cyclin E, cyclin G2; p15, p19, p21,
p27, p130 (cell cycle); and Bim, BNIP3, Fas-ligand, PUMA, PTEN,
TRAIL and ATG12 (apoptosis and autophagy). Phosphorylation by Akt
inactivated FoxOs, resulting in its accumulation in the cytoplasm
and inhibiting its transcriptional function. Through this
mechanism, Akt blocks the FoxO-mediated transcription of target
genes that promote apoptosis, cell cycle arrest, and metabolic
processes (28). Akt also
phosphorylates GSK3 isoforms at a highly conserved N-terminal
regulatory site (GSK3α-Ser21 and GSK3β-Ser9), and this
phosphorylation inactivates the kinase. GSK3-mediated
phosphorylation of the G1 phase cyclins such as cyclin D and E, and
the transcription factors c-jun and c-myc, which all play central
roles in the cell cycle transition, targets them for proteasomal
degradation, and GSK3 can also target and inhibit Mcl-1 (29,30).
In the present study, we found that harmine inhibits the Akt
signaling pathway by inhibiting the expression level of p-Akt and
its downstream proteins, such as p-FoxO3 and p-GSK3β, thus inducing
cell cycle arrest and mitochondrial apoptosis.
The activation of ERK1/2 has been shown to inhibit
apoptosis in response to a wide range of stimuli. The activation of
ERK1/2, induced by different initiating signals, results in the
phosphorylation of different substrates; thus far, more than 150
substrates have been identified. ERK phosphorylates and inhibits
the pro-apoptotic proteins such as caspase-8 and -9, Bad, Bim, and
STAT3/5, and phosphorylates and activates anti-apoptotic proteins,
such as Mcl-1, Bcl-xL, c-Flip, IEX-1, and CBP. Therefore, such as
PKB/Akt, activated ERK regulates a number of cellular events,
including cell proliferation and survival (31,32).
In the present study, we found that harmine downregulated the
expression of phosphorylated ERK and induced cell cycle arrest and
mitochondrial apoptosis.
In conclusion, the present study demonstrated that
harmine induced cell death and growth inhibition in human
colorectal carcinoma SW620 cells. Harmine altered the cell cycle
distribution by decreasing the proportion of cells in the G0–G1
phase and increasing the proportion in the S and G2-M phase, partly
through upregulation of Myt-1 and p-cdc2 (Tyr15). Harmine induced
mitochondrial-related cellular apoptosis by modulating the
expression of Bcl-2 family proteins and decreasing mitochondrial
transmembrane potential (ΔΨm). We also found that harmine decreased
the levels of p-Akt and p-ERK, and this inhibition of the PI3K/Akt
and ERK signaling pathways may be involved in harmine-induced cell
cycle arrest and apoptosis in SW620 cells.
Acknowledgments
The present study was supported by grants from the
Cultivation Fund of the First Affiliated Hospital of Jinan
University (2014203), the Sci-Tech Project Foundation of Guangdong
Province in China (2011B031800012), and the Natural Science
Foundation of Guangdong Province in China (2014A030313356).
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kuebler JP, Wieand HS, O'Connell MJ, Smith
RE, Colangelo LH, Yothers G, Petrelli NJ, Findlay MP, Seay TE,
Atkins JN, et al: Oxaliplatin combined with weekly bolus
fluorouracil and leucovorin as surgical adjuvant chemotherapy for
stage II and III colon cancer: Results from NSABP C-07. J Clin
Oncol. 25:2198–2204. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Patel K, Gadewar M, Tripathi R, Prasad SK
and Patel DK: A review on medicinal importance, pharmacological
activity and bioanalytical aspects of beta-carboline alkaloid
ʻHarmineʼ. Asian Pac J Trop Biomed. 2:660–664. 2012. View Article : Google Scholar
|
|
4
|
Hamsa TP and Kuttan G: Harmine inhibits
tumour specific neo-vessel formation by regulating VEGF, MMP, TIMP
and pro-inflammatory mediators both in vivo and in vitro. Eur J
Pharmacol. 649:64–73. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhao L and Wink M: The β-carboline
alkaloid harmine inhibits telomerase activity of MCF-7 cells by
down-regulating hTERT mRNA expression accompanied by an accelerated
senescent phenotype. PeerJ. 1:e1742013. View Article : Google Scholar
|
|
6
|
Hamsa TP and Kuttan G: Harmine activates
intrinsic and extrinsic pathways of apoptosis in B16F-10 melanoma.
Chin Med. 6:112011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lala S, Pramanick S, Mukhopadhyay S,
Bandyopadhyay S and Basu MK: Harmine: Evaluation of its
antileishmanial properties in various vesicular delivery systems. J
Drug Target. 12:165–175. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hudson JB, Graham EA and Towers GH:
Antiviral effect of harmine, a photoactive beta-carboline alkaloid.
Photochem Photobiol. 43:21–26. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Göckler N, Jofre G, Papadopoulos C, Soppa
U, Tejedor FJ and Becker W: Harmine specifically inhibits protein
kinase DYRK1A and interferes with neurite formation. FEBS J.
276:6324–6337. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dai F, Chen Y, Song Y, Huang L, Zhai D,
Dong Y, Lai L, Zhang T, Li D, Pang X, et al: A natural small
molecule harmine inhibits angiogenesis and suppresses tumour growth
through activation of p53 in endothelial cells. PLoS One.
7:e521622012. View Article : Google Scholar
|
|
11
|
Cao MR, Li Q, Liu ZL, Liu HH, Wang W, Liao
XL, Pan YL and Jiang JW: Harmine induces apoptosis in HepG2 cells
via mitochondrial signaling pathway. Hepatobiliary Pancreat Dis
Int. 10:599–604. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Boeira JM, Viana AF, Picada JN and
Henriques JA: Genotoxic and recombinogenic activities of the two
beta-carboline alkaloids harman and harmine in Saccharomyces
cerevisiae. Mutat Res. 500:39–48. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang H, Sun K, Ding J, Xu H, Zhu L, Zhang
K, Li X and Sun W: Harmine induces apoptosis and inhibits tumor
cell proliferation, migration and invasion through down-regulation
of cyclooxy-genase-2 expression in gastric cancer. Phytomedicine.
21:348–355. 2014. View Article : Google Scholar
|
|
14
|
Yang X, Wang W, Qin JJ, Wang MH, Sharma H,
Buolamwini JK, Wang H and Zhang R: JKA97, a novel benzylidene
analog of harmine, exerts anti-cancer effects by inducing G1
arrest, apoptosis, and p53-independent up-regulation of p21. PLoS
One. 7:e343032012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Song Y, Kesuma D, Wang J, Deng Y, Duan J,
Wang JH and Qi RZ: Specific inhibition of cyclin-dependent kinases
and cell proliferation by harmine. Biochem Biophys Res Commun.
317:128–132. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang W, Liao XL, Chen JH, Li DD, Lin CL,
Yan YX, Tang YH and Jiang JW: Sodium valproate induces
mitochondria-dependent apoptosis in human hepatoblastoma cells.
Chin Med J. 124:2167–2172. 2011.PubMed/NCBI
|
|
17
|
Tan ML, Ooi JP, Ismail N, Moad AI and
Muhammad TS: Programmed cell death pathways and current antitumor
targets. Pharm Res. 26:1547–1560. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Qin S, Yang C, Li S, Xu C, Zhao Y and Ren
H: Smac: Its role in apoptosis induction and use in lung cancer
diagnosis and treatment. Cancer Lett. 318:9–13. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhou F, Yang Y and Xing D: Bcl-2 and
Bcl-xL play important roles in the crosstalk between autophagy and
apoptosis. FEBS J. 278:403–413. 2011. View Article : Google Scholar
|
|
20
|
Sánchez I and Dynlacht BD: New insights
into cyclins, CDKs, and cell cycle control. Semin Cell Dev Biol.
16:311–321. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Besson A, Dowdy SF and Roberts JM: CDK
inhibitors: Cell cycle regulators and beyond. Dev Cell. 14:159–169.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Berry LD and Gould KL: Regulation of Cdc2
activity by phosphorylation at T14/Y15. Prog Cell Cycle Res.
2:99–105. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fattaey A and Booher RN: Myt1: A Wee1-type
kinase that phosphorylates Cdc2 on residue Thr14. Prog Cell Cycle
Res. 3:233–240. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tait SW and Green DR: Mitochondria and
cell death: Outer membrane permeabilization and beyond. Nat Rev Mol
Cell Biol. 11:621–632. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Adams JM and Cory S: The Bcl-2 apoptotic
switch in cancer development and therapy. Oncogene. 26:1324–1337.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Manning BD and Cantley LC: AKT/PKB
signaling: Navigating downstream. Cell. 129:1261–1274. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Faes S and Dormond O: PI3K and AKT:
Unfaithful partners in cancer. Int J Mol Sci. 16:21138–21152. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang X, Tang N, Hadden TJ and Rishi AK:
Akt, FoxO and regulation of apoptosis. Biochim Biophys Acta.
1813:1978–1986. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Luo J: Glycogen synthase kinase 3beta
(GSK3beta) in tumorigenesis and cancer chemotherapy. Cancer Lett.
273:194–200. 2009. View Article : Google Scholar
|
|
30
|
Phukan S, Babu VS, Kannoji A, Hariharan R
and Balaji VN: GSK3beta: Role in therapeutic landscape and
development of modulators. Br J Pharmacol. 160:1–19. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Samatar AA and Poulikakos PI: Targeting
RAS-ERK signalling in cancer: Promises and challenges. Nat Rev Drug
Discov. 13:928–942. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
32
|
Peng S, Zhang Y, Zhang J, Wang H and Ren
B: ERK in learning and memory: A review of recent research. Int J
Mol Sci. 11:222–232. 2010. View Article : Google Scholar : PubMed/NCBI
|