Introduction
Chemotherapeutic agents such as cisplatin are an
important means of adjuvant therapy for the treatment of patients
with ovarian cancer. The major drawback, however, is the
development of drug resistance. Tumor necrosis factor (TNF)-related
apoptosis-inducing ligand (TRAIL) is a type of cytokine produced
and secreted by many tissues and cells including ovarian tissue
(1). It is widely thought to have
strong antitumor activity in a variety of tumor cell types
(1–3). Therefore, we hypothesized that
cisplatin-resistant ovarian cancer cells are able to escape from
TRAIL-mediated apoptosis. According to this, TRAIL tolerance would
be a potential limitation in the treatment of cisplatin-resistant
ovarian cancer, and the elucidation of the mechanism leading to
TRAIL tolerance may be an important therapeutic target for the
treatment of cisplatin-resistant epithelial ovarian cancer.
Solid cancer tumors can develop cisplatin
resistance, which may be mainly linked to the fact that only a low
dose of cisplatin is able to penetrate into the depth of the tumor
(4–7). To prove that TRAIL tolerance is linked
to cisplatin resistance, it needs to be ascertained whether TRAIL
tolerance can be induced by a low dose of cisplatin. Furthermore,
the functional role of TRAIL and the mechanisms of TRAIL tolerance
require elucidation. Recently, cisplatin has been proven to produce
oxidative stress in vivo and in vitro (8–10).
Some research groups have demonstrated that different levels of
oxidative stress can determine cancer cell fate (11–14).
According to these reports, mild oxidative stress promotes cell
viability, whereas high oxidative stress induces cell death in a
variety of tumor cell types. Therefore, we hypothesized that TRAIL
tolerance may be associated with mild oxidative stress mediated by
a low dose of cisplatin, which is already supported by evidence.
For example, Xia et al detected high levels of reactive
oxygen species (ROS) and much higher levels of NADPH oxidase Nox4
in ovarian cancer cells (15). Some
authors have suggested that oxidative stress production during
ovarian cancer progression may contribute to tumorigenicity and
chemoresistance of human ovarian cancer cells (16,17).
Furthermore, early transformative changes in normal ovarian surface
epithelium are possibly induced by oxidative stress (18). Recently, it has been demonstrated
that oncogene-transformed epithelial cancer cells show a
significant increase in mitochondrial mass, which is strictly
dependent on oxidative stress (19). In addition, the generation of ROS
has been reported to contribute to resistance to TRAIL-mediated
apoptosis in human astrocytoma cells (20). However, increasing oxidative stress
has been identified to improve TRAIL sensitivity in ovarian cancer
cell lines (21). Some groups have
furthermore confirmed that oxidative stress promotes the apoptosis
of cancer cells (22,23). A variety of natural and synthetic
compounds such as LY35001, wogonin, and diallyl polysulfides were
found to be capable of increasing the intracellular hydrogen
peroxide level and potentiating TRAIL cytotoxicity toward different
human malignant cells (24–27). Thus, remaining questions are:
whether cisplatin resistant-ovarian cancer cell lines are tolerant
to TRAIL exposure, and which functions different levels of
cisplatin-mediated oxidative stress exert on TRAIL chemotherapy in
epithelial ovarian cancer cells.
In the present study, we demonstrated that the
cisplatin-resistant ovarian cancer cell line SKOV3/DDP is tolerant
to TRAIL, in contrast to the cisplatin-sensitive ovarian cancer
cell line SKOV3. However, a low dose of cisplatin resulted in TRAIL
tolerance in SKOV3 cells, which was ascribed to the production of
mild oxidative stress. This was confirmed by the observation that
SKOV3/DDP cells exhibited much higher levels of oxidative stress
than SKOV3 cells, and that the oxidative stress scavenger
N-acetyl-cysteine (NAC) could reverse the cisplatin-induced
tolerance of TRAIL in the SKOV3 and SKOV3/DDP cells. Taken
together, these results showed that mild oxidative stress resulting
from exposure to a low dose of cisplatin contributes to the escape
of TRAIL-mediated apoptosis in the SKOV3 cell line.
Materials and methods
Cells and reagents
SKOV-3 and SKOV-3/DDP cells were purchased from the
Shanghai Cellular Research Institute (Shanghai, China) and
maintained in RPMI-1640 medium supplemented with 10% fetal bovine
serum (both from Hyclone, USA), 100 U/ml penicillin and 100
µg/ml streptomycin at 37°C in a humidified atmosphere of 5%
CO2. The cells were trypsinized at 80% confluency with
0.25% trypsin/0.02% EDTA in Hank's solution for 1–3 min and
resuspended in a complete culture medium. Recombinant human
TRAIL/Apo2L was purchased from PeproTech (London, UK).
cis-Diammineplatinum(II) (cisplatin),
N-acetyl-cysteine (NAC) and hydrogen peroxide
(H2O2) were obtained from Sigma (St. Louis,
MO, USA). CM-H2DCFDA was purchased from Invivogen (San
Diego, CA, USA). Annexin V-FITC apoptosis kits were obtained from
BD Pharmingen (San Diego, CA, USA).
3-(4,5-Dimethyl-2-thiazolyl)-2,5-diphenyl-2-H-tetrazolium bromide
(MTT) was purchased from Sigma.
MTT assay
Cell viability was determined using the MTT assay.
Near-confluent SKOV3 and SKOV3/DDP cells were seeded on a 96-well
plate in 100 µl of RPMI-1640 medium supplemented with
cisplatin (1, 10 µM; Sigma) or H2O2
(0.1, 1 mM) for the indicated time. Cisplatin (1, 10 µM) or
H2O2 (0.1, 1 mM) treatment was chosen in the
present study since 1 µM cisplatin and 0.1 mM
H2O2 have no effects on apoptosis which are
different from 10 µM cisplatin and 1 mM
H2O2 (data not shown). Next, the medium was
removed and replaced with 10 µl reconstituted MTT in the
RPMI-1640 medium. The plate was then incubated under standard
incubation conditions for 2 h. After the incubation period, the
culture fluid was removed. This was followed by the addition of 100
µl MTT solubilization solution, and the plate was placed on
a mini-shaker for 10 min to assist in dissolving the crystals. The
absorbance was read at a wavelength of 570 nm and the background at
655 nm.
Analysis of apoptosis
Apoptosis was analyzed using Fluorescence-Activated
Cell Sorting (FACS). After 1×105 cells/well of SKOV3 and
SKOV3/DDP cells were seeded on a 6-well plate for 24 h, the cells
were treated with or without cisplatin (1, 10 µM) and/or
TRAIL (500 ng/ml) for the indicated time. The current treatment of
TRAIL was used in the present study according to a previous study
(20). Then, the cells were
harvested, washed with PBS, and stained with FITC-Annexin V (Sigma)
and propidium iodide (PI). Cell apoptosis was analyzed by flow
cytometry using CellQuest™ software.
Measurement of reactive oxygen species
generation
5-(and-6)-Chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate,
acetylester (CM-H2DCFDA; Invivogen) was deacetylated by
intracellular esterase to the non-fluorescent DCFH, which can be
oxidized by ROS to the fluorescent compound
2′,7′-dichlorofluorescein (DCF). The fluorescence intensity of DCF
is proportional to the amount of ROS produced by cells. After
1×105 cells/well of SKOV3 and SKOV3/DDP cells were
seeded on a 6-well plate for 24 h, the cells were treated with
cisplatin (1, 10 µM) or H2O2 (0.1, 1
mM) for 1 h. In brief, the cells were pre-treated with cisplatin or
H2O2 for 1 h for the production of ROS. Data
analysis was performed with CellQuest™ software and the mean
fluorescence intensity was used to quantify the results.
Statistical analysis
Data are expressed as the mean ± standard deviation
(SD). Data were analyzed with the SPSS 17.0 software and tested by
the one-way analysis of variance (ANOVA). P-values of P<0.05
were defined as indicating a statistically significant result.
Results
SKOV3/DDP cells display tolerance to
TRAIL exposure
To study the TRAIL tolerance in ovarian cancer
cells, the cell viability of the ovarian cancer cell lines was
examined after treatment with TRAIL at different concentrations (0,
10, 50, 100, 250, 500, and 1,000 ng/ml) for 48 h, respectively.
TRAIL exposure was found to significantly inhibit the viability of
the SKOV3 cells, which successively decreased with an increasing
TRAIL concentration from 1.00±0.00 down to 0.55±0.15. The cell
viability of the SKOV3/DDP cells, in contrast, successively
increased upon treatment with increasing concentrations of TRAIL
from 1.00±0.00 to 1.91±0.22 (Fig.
1A). The viability of the SKOV3/DDP cells was significantly
higher compared with the SKOV3 cells upon treatment with the same
concentration of TRAIL (P<0.01, P<0.001, n=3).
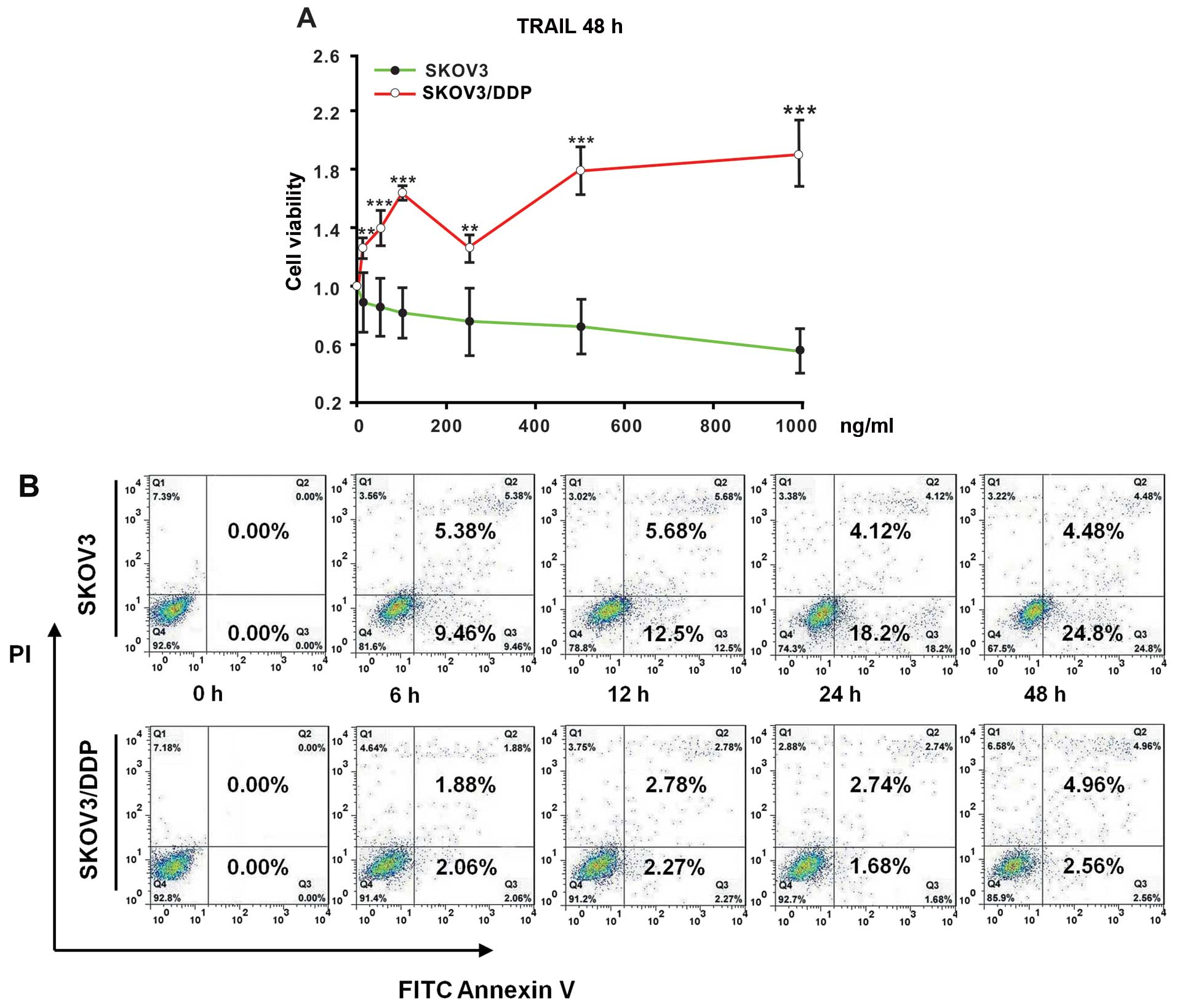 | Figure 1SKOV3/DDP cells display tolerance
under TRAIL exposure. (A) Measurements of the cell viability with
the MTT assay. SKOV3 and SKOV3/DDP cells were treated for 48 h with
increasing concentrations of TRAIL (0, 10, 50, 100, 250, 500 and
1,000 ng/ml). All assays were performed in triplicate and repeated
three times. The data are expressed as the mean ± SEM of triplicate
samples (**P<0.01, ***P<0.001, compared
with the SKOV3 cells under treatment with the same concentration of
TRAIL). The percentage of cell viability was defined as the
relative absorbance of treated vs. untreated cells. (B) Flow
cytometric analysis of cell apoptosis. SKOV3 and SKOV3/DDP cells
were incubated with 500 ng/ml of TRAIL for the indicated times (0,
6, 12, 24, and 48 h). Next, the cells were harvested, washed with
PBS, and stained with FITC-Annexin V and propidium iodide (PI).
Data were analyzed using CellQuest™ software. |
Furthermore, the cell apoptosis levels were analyzed
after treatment with TRAIL (500 ng/ml) in a time-dependent manner.
The SKOV3/DDP cells exhibited a lower apoptosis level after TRAIL
treatment than the SKOV3 cells (Fig.
1B). This finding agrees with the TRAIL tolerance of SKOV3/DDP
cells, which was absent in the SKOV3 cells.
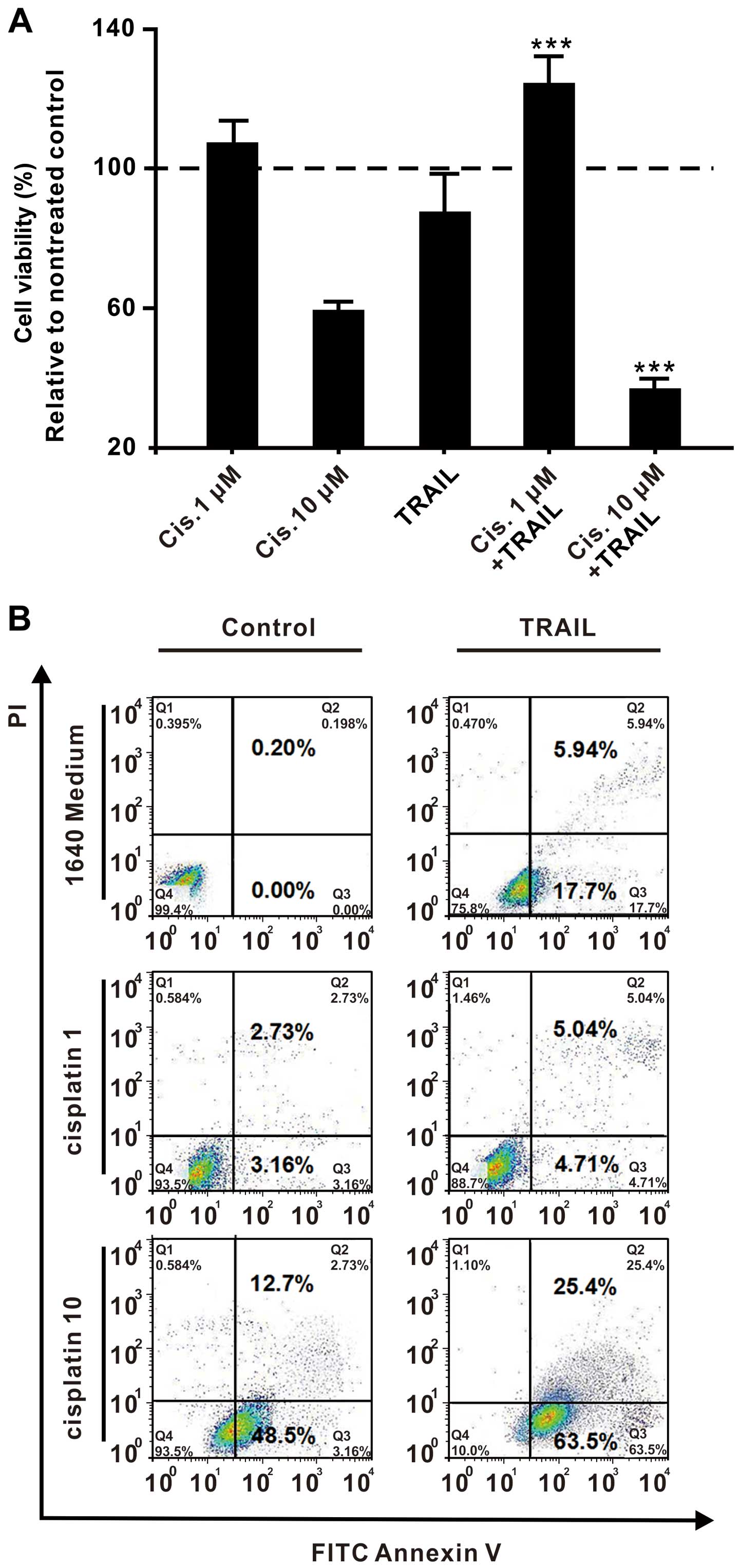
Low dose of cisplatin leads to the
development of TRAIL tolerance in SKOV3 cells
To ascertain whether TRAIL tolerance is induced by
cisplatin due to chemotherapeutic failure, we pretreated SKOV3
cells with two different concentrations of cisplatin (1 or 10
µM) for 1 h before the cells were treated with (500 ng/ml)
TRAIL or without for another 24 h. Measuring the cell viability
revealed that pretreatment with the higher concentration of
cisplatin (10 µM) inhibited, but pretreatment with the lower
cisplatin concentration (1 µM) promoted the growth of SKOV3
cells upon TRAIL treatment. For example, the percentage of cell
viability (defined as the relative absorbance of the treated vs.
the untreated cells) was 97.40±6.09 in the cells that were treated
with a low cisplatin concentration of 1 µM, whereas it was
59.26±2.75 in the cells that were treated with a high cisplatin
concentration of 10 µM. The same tendency was observed in
the SKOV3 cells that were treated with TRAIL. Cells that were
treated with TRAIL only revealed a cell viability of 87.15±11.20%.
Pretreatment with a high cisplatin concentration of 10 µM
reduced the cell viability to 37.67±1.18%, whereas pretreatment
with a low cisplatin concentration of 1 µM increased the
cell viability to 125.11±6.29% (Fig.
2A). Comparing the cell apoptosis levels under the same culture
conditions demonstrated that a high dose of cisplatin promoted
TRAIL-induced apoptosis, while a low dose of cisplatin nearly
completely inhibits TRAIL-mediated apoptosis (Fig. 2B). These results indicate that a
high dose of cisplatin possibly cooperates with TRAIL to kill the
SKOV3 cells, while a low dose of cisplatin renders the SKOV3 cells
tolerant to TRAIL.
Lower levels of oxidative stress promote
TRAIL tolerance in SKOV3 cells
To study the impact of oxidative stress on the
cisplatin-mediated TRAIL tolerance in SKOV3 cells, we first
examined whether cisplatin induces an increase in the intracellular
ROS levels. The same amounts of ovarian cancer cells were treated
for 1 h with cisplatin (1 or 10 µM), or alternatively with
H2O2 (0.1 or 1 mM) as a positive control.
Intracellular ROS levels were measured by CM-H2DCFDA
staining and FACS analysis. Interestingly, ROS levels in the
SKOV3/DDP cells were generally much higher than levels in the SKOV3
cells. We determined a relative fluorescence intensity of ROS in
the SKOV3/DDP control group of 193.6±8.2, whereas the SKOV3 control
group only exhibited a value of 23.7±3.2 (P<0.001, n=3).
Furthermore, we observed a dose-dependent increase in the
intracellular ROS levels upon cisplatin and
H2O2 treatment in both the SKOV3 and
SKOV3/DDP cells. The relative fluorescence intensity of ROS in the
SKOV3 cells that had been treated with 1 µM of cisplatin was
48.3±4.7, whereas treatment with a higher concentration of
cisplatin (10 µM) resulted in a 3-fold higher value of
156.3±5.6. The same effect was observed for
H2O2, which yielded a value of only 55.7±7.7
upon treatment with 0.1 mM H2O2, whereas
treatment with the higher H2O2 concentration
of 1 mM led to a nearly 3-fold higher relative fluorescence
intensity of ROS of 147.6±5.2. The same tendency was observed for
the SKOV3/DDP cells, which revealed lower relative fluorescence
intensities of ROS of 212.0±2.6 or 220.0±5.1 upon treatment with a
low concentration of cisplatin (1 µM) or
H2O2 (0.1 mM), respectively. Treatment with a
higher concentration of cisplatin (10 µM) or
H2O2 (1 mM) increased the values to
256.3±10.3 or 288.3±9.1, respectively, although this increase was
not as marked as the increase observed for the SKOV3 cells
(Fig. 3A).
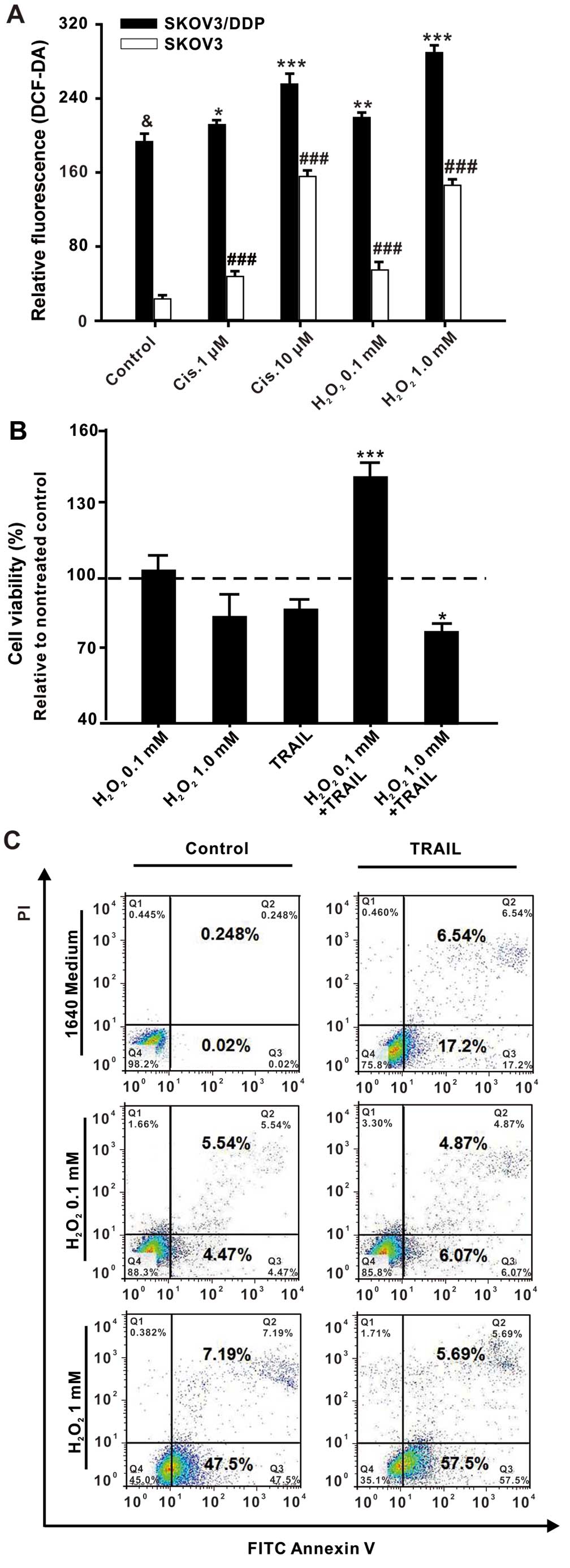 | Figure 3Cisplatin-induced mild oxidative
stress promotes TRAIL tolerance in SKOV3 cells. (A)
CM-H2DCFDA staining and FACS analysis of the
intracellular ROS levels in SKOV3 or SKOV3/DDP cells. The same
amounts of SKOV3 or SKOV3/DDP cells (1×105) were treated
with different concentrations of cisplatin (1 or 10 µM) or
H2O2 (0.1 or 1 mM) for 1 h. All assays were
performed in triplicate and repeated three times. The data are
expressed as the mean ± SEM of triplicate samples
(*P<0.05, **P<0.01,
***P<0.001, compared with the control SKOV3/DDP
cells; &P<0.001, ###P<0.001,
compared with the control SKOV3 cells). (B) Measurement of the cell
viability with the MTT assay. SKOV3 cells were pretreated with
H2O2 (0.1 or 1 mM) for 1 h and then treated
without or with TRAIL (500 ng/ml) for 24 h. All assays were
performed in triplicate and repeated three times. The data are
expressed as the mean ± SEM of triplicate samples
(*P<0.05, ***P<0.001, compared with the
TRAIL group). The percentage of cell viability was defined as the
relative absorbance of treated vs. untreated cells. (C) Flow
cytometric analysis of cell apoptosis. SKOV3 cells were pretreated
with H2O2 (0.1 or 1 mM) for 1 h and then
treated without or with TRAIL (500 ng/ml) for 24 h. Next, the cells
were harvested, washed with PBS, and stained with FITC-Annexin V
and propidium iodide (PI). Data were analyzed using CellQuest™
software. |
Subsequently, the cell viability was measured with
the MTT assay and the cell apoptosis was analyzed by the FACS
method. Therefore, the SKOV3 cells were pretreated with
H2O2 (0.1 or 1 mM) for 1 h and then treated
with or without TRAIL (500 ng/ml) for 24 h. As shown in Fig. 3B, the percentage of cell viability
was similar for the cells treated with TRAIL (87.15±11.20) and
those that had been treated with a high concentration of
H2O2 (1 mM) plus TRAIL (81.35±5.11), whereas
cells that had been treated with a low concentration of
H2O2 (0.1 mM) plus TRAIL revealed a
significantly higher cell viability of 142.72±5.33% (Fig. 3B). In agreement with this, a high
dose of H2O2 was found to promote the
apoptosis caused by TRAIL, while a low dose of
H2O2 inhibited TRAIL-induced apoptosis
(Fig. 3C). These results revealed
that the TRAIL tolerance of SKOV3 cells developed upon treatment
with a low dose of H2O2 (0.1 mM), whereas
treatment with a high dose of H2O2 (1 mM)
improved the TRAIL sensitivity in the SKOV3 cells. This suggests
that lower levels of oxidative stress are associated with TRAIL
tolerance in SKOV3 cells.
NAC reverses the cisplatin-induced TRAIL
tolerance in SKOV3 cells
Next, we aimed to investigate whether the
cisplatin-induced oxidative stress can modulate TRAIL-induced
apoptosis. To this goal, we studied the impact of the oxidative
stress scavenger N-acetyl-cysteine (NAC), which is expected
to inhibit the ROS production in the SKOV3 cells. Therefore, the
cells were pretreated with NAC in addition to cisplatin (1
µM) or H2O2 (0.1 mM), respectively,
and the relative fluorescence intensity of ROS as well as the cell
viability were analyzed. The results were compared with the
respective control cells pretreated with cisplatin (1 µM) or
H2O2 (0.1 mM), but without the addition of
NAC. Cells that had been treated with NAC,
H2O2 and TRAIL exhibited a relative
fluorescence intensity of ROS of 27.6±5.8, which was much lower
than the value of 87.5±6.5 of the control group that had not been
treated with NAC. The additional NAC pretreatment of the cisplatin
and TRAIL-treated cells also decreased the relative fluorescence
intensity of ROS with respect to the control group. The cisplatin
and TRAIL-treated control group exhibited a value of 89.6±5.3,
whereas the cells that had been additionally pretreated with NAC
exhibited a significantly lower value of 26.3±5.3 (Fig. 4A). In conclusion, pretreatment with
NAC significantly inhibited the ROS increase induced by cisplatin
(1 µM) or H2O2 (0.1 mM).
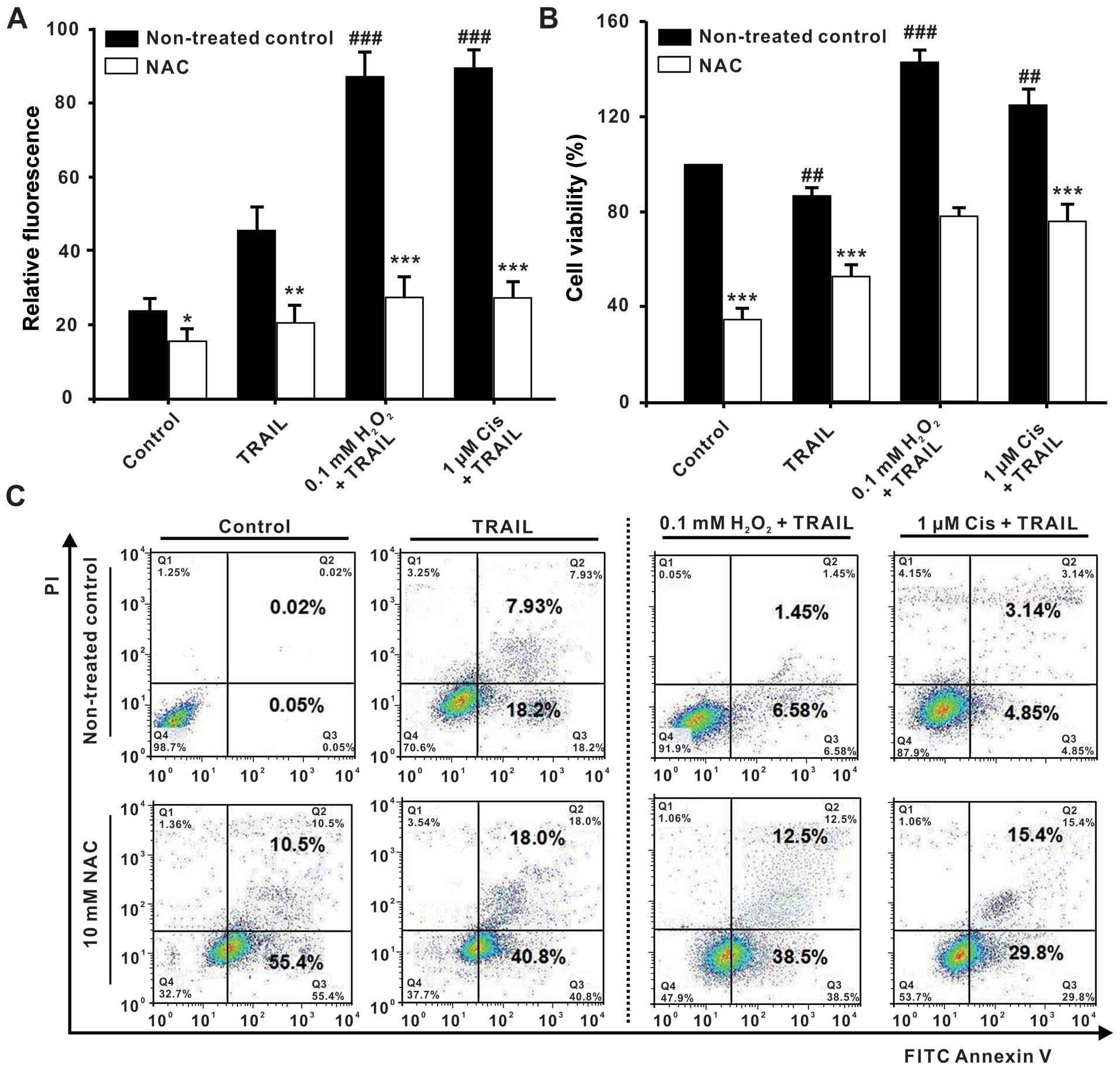 | Figure 4Oxidative stress regulation reverses
the TRAIL tolerance in SKOV3 cells. After pretreatment without or
with N-acetyl-cysteine (NAC) (0, 10 mM) for 1 h, the SKOV3
cells were treated with cisplatin (1 µM) or
H2O2 (0.1 mM). One hour later, the cells were
treated together with TRAIL (500 ng/ml) for another 24 h. (A)
CM-H2DCFDA staining and FACS analysis of the
intracellular ROS levels in the SKOV3/DDP cells. (B) Measurement of
the cell viability with the MTT assay. The percentage of cell
viability was defined as the relative absorbance of treated vs.
untreated cells. (C) Flow cytometric analysis of cell apoptosis.
SKOV3 cells were harvested, washed with PBS, and stained with
FITC-Annexin V and propidium iodide (PI). Data were analyzed using
CellQuest™ software. All assays were performed in triplicate and
repeated three times. The data are expressed as the mean ± SEM of
triplicate samples (#P<0.05,
###P<0.001, compared with the control groups;
**P<0.05, **P<0.01,
***P<0.001, compared with the 1640 medium groups,
respectively). |
Consistent with these findings, NAC pretreatment
furthermore inhibited the viability of the SKOV3 cells. Control
cells that had been treated with H2O2 and
TRAIL revealed a cell viability of 142.72±5.33%, whereas cells that
had additionally been treated with NAC exhibited a lower cell
viability of 78.56±3.21%. Analogously, the cell viability of cells
that had been treated with NAC, cisplatin, and TRAIL was lower
(76.25±7.24%) than that of the corresponding control group that had
been treated with cisplatin and TRAIL only, which exhibited a cell
viability of 125.18±6.63% (Fig.
4B). These results were corroborated by flow cytometric
analysis of cell apoptosis (Fig.
4C), which revealed that NAC abrogated the inhibition of the
TRAIL-induced apoptosis mediated by a low dose of cisplatin or
H2O2 by acting as a scavenger of ROS that had
been produced by cisplatin or H2O2.
Therefore, these results confirm the cisplatin-induced generation
of low levels of intracellular ROS as the causative mechanism of
TRAIL tolerance in SKOV3 cells.
The tolerance of TRAIL in SKOV3/DDP cells
is dependent on oxidative stress
As mentioned above, the ROS levels of SKOV3/DDP
cells were generally much higher than those of the SKOV3 cells.
Whether the regulation of oxidative stress could reverse the TRAIL
tolerance in SKOV3/DDP cells was investigated. NAC was used to
inhibit the ROS production in the SKOV3/DDP cells. When NAC was
used at a concentration of 10 mM, the relative fluorescence
intensity of ROS decreased from 190.5±7.2 in the untreated control
group to 170.0±5.6 in the cells that were additionally treated with
NAC, and the relative fluorescence intensity of ROS decreased from
203.0±3.2 in the TRAIL-treated control group to 184.0±4.5 in the
cells that were additionally treated with NAC (Fig. 5A). Contrary to our expectations, NAC
(10 mM) only slightly increased the TRAIL-induced apoptosis of the
SKOV3/DDP cells (Fig. 5B).
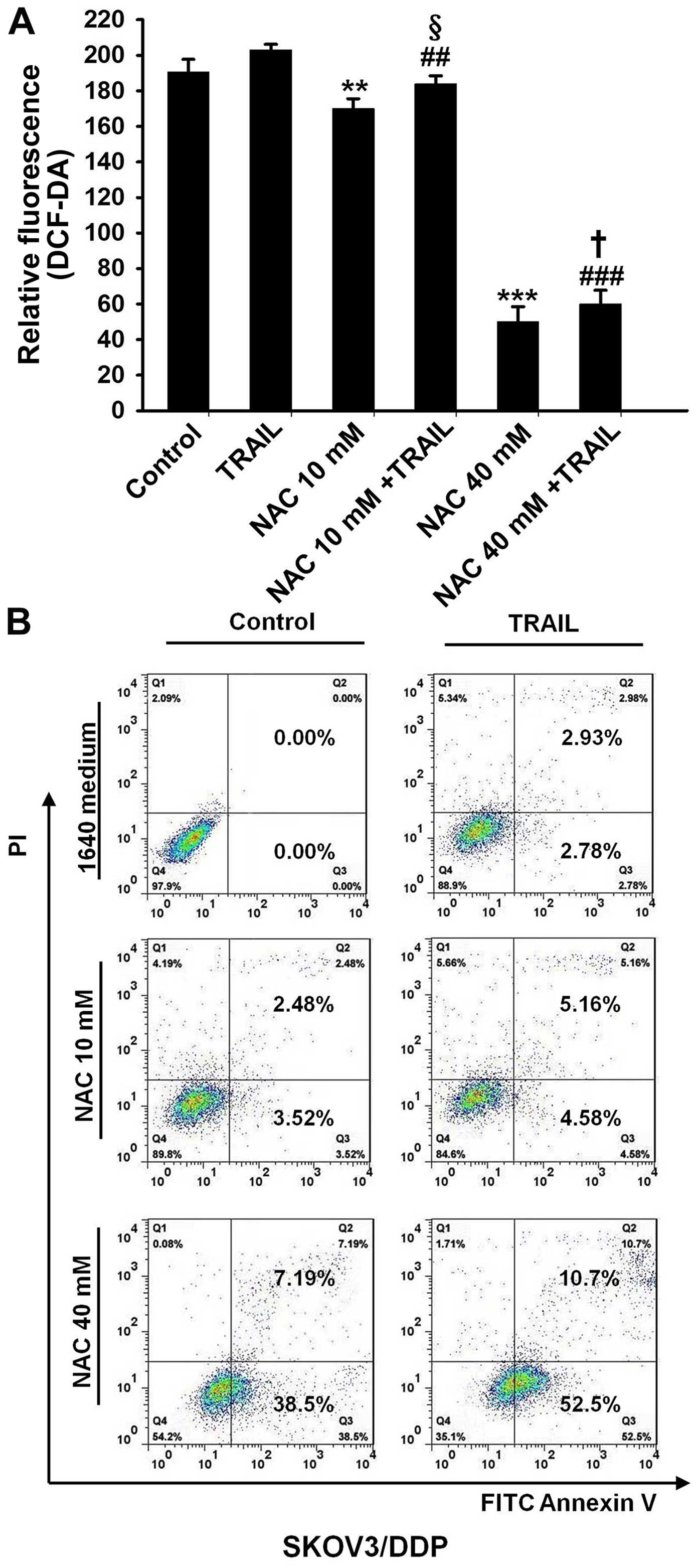 | Figure 5The development of TRAIL tolerance in
SKOV3/DDP cells is dependent on oxidative stress. (A)
CM-H2DCFDA staining and FACS analysis of the
intracellular ROS levels in SKOV3/DDP cells. SKOV3-DDP cells were
pretreated with the oxidative stress scavenger
N-acetyl-cysteine (NAC) (0, 10, and 40 mM) for 1 h and then
the cells were treated without or with TRAIL (500 ng/ml) for 24 h.
All assays were performed in triplicate and repeated three times.
The data are expressed as the mean ± SEM of triplicate samples
[**P<0.01, ***P<0.001, compared with
the control groups; ##P<0.01,
###P<0.001, compared with the TRAIL groups;
§P<0.05, compared with the NAC (10 mM) groups;
†P<0.05, compared with the NAC (40 mM) groups]. (B)
Flow cytometric analysis of cell apoptosis. SKOV3-DDP cells were
pretreated with the oxidative stress scavenger NAC (10, and 40 mM)
for 1 h and then treated without or with TRAIL (500 ng/ml) for
another 24 h. Then, the cells were harvested, washed with PBS, and
stained with FITC-Annexin V and propidium iodide (PI). Data were
analyzed using CellQuest™ software. |
When NAC was increased to 40 mM to further decrease
ROS in the SKOV3/DDP cells, the relative fluorescence intensity of
ROS in the cells that were additionally treated with NAC was
inhibited by 74.8±4.5% compared with that in the untreated control
group, and the relative fluorescence intensity of ROS in the cells
that were additionally treated with NAC was inhibited 70.5±3.8%
compared with that in the TRAIL-treated control group (Fig. 5A). Ultimately, NAC (40 mM)
significantly increased the TRAIL-induced apoptosis of the
SKOV3/DDP cells (Fig. 5B). Taken
together, we conclude that the development of TRAIL tolerance in
SKOV3/DDP cells was at least partially dependent on oxidative
stress.
Discussion
TRAIL belongs to a class of cytokines and displays
specific anticancer activity against a wide range of cancer cells
without showing significant side effects (1–3).
However, TRAIL tolerance is still a potential limitation in the
treatment of cisplatin-resistant ovarian cancer. In the present
study, we confirmed that SKOV3/DDP cells are tolerant to TRAIL,
that a low dose of cisplatin produces TRAIL tolerance in SKOV3
cells, and that cisplatin-induced oxidative stress modulates
TRAIL-induced apoptosis. These observations suggest that the
regulation of cisplatin-induced oxidative stress may be a promising
new treatment strategy for improving TRAIL tolerance in
cisplatin-resistant epithelial ovarian cancer.
Chemotherapeutic agents are an important means of
adjuvant therapy for the treatment of patients with ovarian cancer.
For example, combination therapy using cisplatin and paclitaxel is
considered to be the gold standard of chemotherapy (7). The antitumor effect of cisplatin has
been ascribed to its binding to the DNA double-helix at the N7
atoms of guanine bases. This binding prevents the strands from
uncoiling and separating and results ultimately in cellular
apoptosis (28–30). A major drawback, however, is the
development of drug resistance, where a low dose of cisplatin may
be one of the important reasons. For example, cisplatin can bind to
plasma proteins, such as human serum albumin, and has poor
solubility (31,32). In addition, high interstitial fluid
pressure in solid tumors including epithelial ovarian cancer
provokes vascular tortuosity and leakage, which makes it difficult
for chemotherapeutic agents to penetrate into the depth of the
tumor (33,34). These undesirable effects may limit
the availability of cisplatin at lower concentrations. In addition,
cisplatin-resistant cell lines of human ovarian cancer have been
derived from cisplatin-sensitive cell lines by repeated exposure to
low but continuously increasing concentrations of cisplatin
(35). In the present study, a low
dose of cisplatin (1 µM), but not a high dose of cisplatin
(10 µM) caused TRAIL tolerance in the SKOV3 cells. These
results explain why a low dose of cisplatin produces cisplatin
resistance in ovarian cancer, where TRAIL tolerance in cisplatin
may be one of the reasons.
In general, it is difficult to give a general
definition for the levels of oxidative stress. In the present
study, low doses of cisplatin (1 µM) or
H2O2 (0.1 mM) produced a lower level of ROS,
which we regarded as mild oxidative stress, while higher doses of
cisplatin (10 µM) or H2O2 (1 mM)
induced a higher level of ROS, which we considered as high
oxidative stress. Thereby, the two different levels of oxidative
stress provoked contrary effects, as mild oxidative stress promotes
cell proliferation, whereas serious oxidative stress induces cell
apoptosis. For example, mild oxidative stress induced by cisplatin
(1 µM) or H2O2 (1 mM) increased the
viability of the SKOV3 cells and inhibited cell apoptosis. In
contrast, serious oxidative stress induced by higher concentrations
of cisplatin (1 µM) or H2O2 (1 mM)
decreased SKOV3 cell viability and significantly promoted cell
apoptosis. In addition, high doses of cisplatin (10 µM) or
H2O2 (1 mM) enhanced the chemotherapeutic
effect of TRAIL in SKOV3 cells, while a combination of TRAIL and a
low dose of cisplatin (1 µM) or H2O2
(0.1 mM) enhanced the proliferation of SKOV3 cells much more than
treatment only with a low dose of cisplatin (1 µM) or
H2O2 (0.1 mM). Our results corroborate
previous studies that showed that different levels of oxidative
stress determine cancer cell fate (11–14).
In addition, the impact of TRAIL on cells is associated with
differences in the expression of TRAIL receptors, such as DR4, DR5,
DcR1, Dcr2 and OPG, on the surface of ovarian cancer cells. A
recent review demonstrated that following ligand binding, the TRAIL
death receptors DR4 and DR5 are able to induce cell death
(apoptosis or necroptosis). Alternatively, these receptors may also
enhance cell proliferation, inflammation, migration and invasion
via activation of multiple 'non-death-inducing' signal transduction
pathways (36). The mechanisms of
TRAIL tolerance production may be complex and require further
investigation. However, these findings suggest that mild oxidative
stress induced by a low dose of cisplatin may be one of the
mechanisms producing cisplatin resistance including TRAIL
tolerance, and TRAIL tolerance in cisplatin-resistant ovarian
cancer cells may be associated with mild oxidative stress.
Furthermore, to identify whether mild oxidative
stress modulates TRAIL-induced apoptosis, cells were pretreated
with oxidative stress scavenger NAC to inhibit ROS production in
the SKOV3 and SKOV3/DDP cells. This revealed that NAC improved the
sensitivity of TRAIL in the SKOV3 cells following treatment with a
low dose of cisplatin (1 µM) or H2O2
(0.1 mM). Contrary to our expectations, NAC (10 mM) only slightly
increased the TRAIL-induced apoptosis of SKOV3/DDP cells and could
not completely combat the TRAIL tolerance induced by mild oxidative
stress. Next, the treatment of NAC was increased to 40 mM to
further decrease ROS in the SKOV3/DDP cells. As a result, NAC (40
mM) significantly increased the TRAIL-induced apoptosis of
SKOV3/DDP cells. As the ROS levels of the SKOV3/DDP cells were
generally much higher than those of the SKOV3 cells, it was more
difficult to combat apoptosis in the SKOV3/DDP cells than that in
the SKOV3 cells by oxidative stress regulation. Interesting, the
ROS levels in the SKOV3/DDP cell groups treated with NAC together
with TRAIL were higher compared with the NAC groups, while the
apoptosis of the former group was much higher than the latter
group. According to our knowledge, there are at least three types
of sources for the production of oxidative stress such as the
mitochondrium, NADPH oxidase, and the endoplasmic reticulum
(37,38). NAC is a thiol-containing compound
which reduces the reactive oxygen intermediate
H2O2 and protects against toxic effects.
Moreover, other unknown mechanism may be involved in the
cisplatin-induced TRAIL tolerance of SKOV3/DDP cells besides
oxidative stress.
In summary, we demonstrated that cisplatin evokes
oxidative stress in SKOV3 cells, that the SKOV3/DDP cells are
tolerant to TRAIL, and that the mild oxidative stress induced by a
low dose of cisplatin contributes to the tolerance of TRAIL in
SKOV3 cells. Thus, regulation of oxidative stress in order to
improve the therapeutic efficacy of TRAIL may be a new treatment
strategy for patients with cisplatin-resistant epithelial ovarian
cancer.
Acknowledgments
This study was supported by the Natural Science
Foundation of China (grant no. 8117245/H1621), the Outstanding
Medical Academic Leader Program of Hubei Province, and Hubei
Province Health and Family Planning Scientific Research Project
(WJ2015Q044).
References
|
1
|
Kimberley FC and Screaton GR: Following a
TRAIL: Update on a ligand and its five receptors. Cell Res.
14:359–372. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bernard D, Quatannens B, Vandenbunder B
and Abbadie C: Rel/NF-kappaB transcription factors protect against
tumor necrosis factor (TNF)-related apoptosis-inducing ligand
(TRAIL)-induced apoptosis by up-regulating the TRAIL decoy receptor
DcR1. J Biol Chem. 276:27322–27328. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wiley SR, Schooley K, Smolak PJ, Din WS,
Huang CP, Nicholl JK, Sutherland GR, Smith TD, Rauch C, Smith CA,
et al: Identification and characterization of a new member of the
TNF family that induces apoptosis. Immunity. 3:673–682. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Li X, Ling V and Li PC: Same-single-cell
analysis for the study of drug efflux modulation of multidrug
resistant cells using a microfluidic chip. Anal Chem. 80:4095–4102.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mundhenke C, Weigel MT, Sturner KH, Roesel
F, Meinhold-Heerlein I, Bauerschlag DO, Schem C, Hilpert F, Jonat W
and Maass N: Novel treatment of ovarian cancer cell lines with
imatinib mesylate combined with paclitaxel and carboplatin leads to
receptor-mediated antiproliferative effects. J Cancer Res Clin
Oncol. 134:1397–1405. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Seeber LM and van Diest PJ: Epigenetics in
ovarian cancer. Methods Mol Biol. 863:253–269. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yi C, Zhang L, Li L, Liu X, Ling S, Zhang
F and Liang W: Establishment of an orthotopic transplantation tumor
model in nude mice using a drug-resistant human ovarian cancer cell
line with a high expression of c-Kit. Oncol Lett. 8:2611–2615.
2014.PubMed/NCBI
|
|
8
|
Belotte J, Fletcher NM, Awonuga AO, Alexis
M, Abu-Soud HM, Saed MG, Diamond MP and Saed GM: The role of
oxidative stress in the development of cisplatin resistance in
epithelial ovarian cancer. Reprod Sci. 21:503–508. 2014. View Article : Google Scholar :
|
|
9
|
Fatima S, Al-Mohaimeed N, Arjumand S, Banu
N, Al-Jameil N and Al-Shaikh Y: Effect of pre- and post-combined
multidoses of epigallocatechin gallate and coenzyme Q10 on
cisplatin-induced oxidative stress in rat kidney. J Biochem Mol
Toxicol. 29:91–97. 2015. View Article : Google Scholar
|
|
10
|
Lin L, Zheng J, Zhu W and Jia N:
Nephroprotective effect of gelsemine against cisplatin-induced
toxicity is mediated via attenuation of oxidative stress. Cell
Biochem Biophys. 71:535–541. 2015. View Article : Google Scholar
|
|
11
|
Huang C, Han Y, Wang Y, Sun X, Yan S, Yeh
ET, Chen Y, Cang H, Li H, Shi G, et al: SENP3 is responsible for
HIF-1 transactivation under mild oxidative stress via p300
de-SUMOylation. EMBO J. 28:2748–2762. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
López-Lázaro M: Dual role of hydrogen
peroxide in cancer: Possible relevance to cancer chemoprevention
and therapy. Cancer Lett. 252:1–8. 2007. View Article : Google Scholar
|
|
13
|
Suzuki-Karasaki Y, Suzuki-Karasaki M,
Uchida M and Ochiai T: Depolarization controls TRAIL-sensitization
and tumor-selective killing of cancer cells: Crosstalk with ROS.
Front Oncol. 4:1282014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang J and Yi J: Cancer cell killing via
ROS: To increase or decrease, that is the question. Cancer Biol
Ther. 7:1875–1884. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xia C, Meng Q, Liu LZ, Rojanasakul Y, Wang
XR and Jiang BH: Reactive oxygen species regulate angiogenesis and
tumor growth through vascular endothelial growth factor. Cancer
Res. 67:10823–10830. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chan DW, Liu VW, Tsao GS, Yao KM, Furukawa
T, Chan KK and Ngan HY: Loss of MKP3 mediated by oxidative stress
enhances tumorigenicity and chemoresistance of ovarian cancer
cells. Carcinogenesis. 29:1742–1750. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shigetomi H, Tsunemi T, Haruta S, Kajihara
H, Yoshizawa Y, Tanase Y, Furukawa N, Yoshida S, Sado T and
Kobayashi H: Molecular mechanisms linking endometriosis under
oxidative stress with ovarian tumorigenesis and therapeutic
modalities. Cancer Invest. 30:473–480. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
King SM, Quartuccio SM, Vanderhyden BC and
Burdette JE: Early transformative changes in normal ovarian surface
epithelium induced by oxidative stress require Akt upregulation,
DNA damage and epithelial-stromal interaction. Carcinogenesis.
34:1125–1133. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Martinez-Outschoorn UE, Curry JM, Ko YH,
Lin Z, Tuluc M, Cognetti D, Birbe RC, Pribitkin E, Bombonati A,
Pestell RG, et al: Oncogenes and inflammation rewire host energy
metabolism in the tumor microenvironment: RAS and NFκB target
stromal MCT4. Cell Cycle. 12:2580–2597. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Choi K, Ryu SW, Song S, Choi H, Kang SW
and Choi C: Caspase-dependent generation of reactive oxygen species
in human astrocytoma cells contributes to resistance to
TRAIL-mediated apoptosis. Cell Death Differ. 17:833–845. 2010.
View Article : Google Scholar
|
|
21
|
Yi L, Zongyuan Y, Cheng G, Lingyun Z,
Guilian Y and Wei G: Quercetin enhances apoptotic effect of tumor
necrosis factor-related apoptosis-inducing ligand (TRAIL) in
ovarian cancer cells through reactive oxygen species (ROS) mediated
CCAAT enhancer-binding protein homologous protein (CHOP)-death
receptor 5 pathway. Cancer Sci. 105:520–527. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lee MW, Park SC, Kim JH, Kim IK, Han KS,
Kim KY, Lee WB, Jung YK and Kim SS: The involvement of oxidative
stress in tumor necrosis factor (TNF)-related apoptosis-inducing
ligand (TRAIL)-induced apoptosis in HeLa cells. Cancer Lett.
182:75–82. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang J, Jin L, Li X and Deng H, Chen Y,
Lian Q, Ge R and Deng H: Gossypol induces apoptosis in ovarian
cancer cells through oxidative stress. Mol Biosyst. 9:1489–1497.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Das A, Banik NL and Ray SK: Garlic
compounds generate reactive oxygen species leading to activation of
stress kinases and cysteine proteases for apoptosis in human
glioblastoma T98G and U87MG cells. Cancer. 110:1083–1095. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lee DH, Rhee JG and Lee YJ: Reactive
oxygen species up-regulate p53 and Puma; a possible mechanism for
apoptosis during combined treatment with TRAIL and wogonin. Br J
Pharmacol. 157:1189–1202. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Powolny AA and Singh SV: Multitargeted
prevention and therapy of cancer by diallyl trisulfide and related
Allium vegetable-derived organosulfur compounds. Cancer Lett.
269:305–314. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shenoy K, Wu Y and Pervaiz S: LY303511
enhances TRAIL sensitivity of SHEP-1 neuroblastoma cells via
hydrogen peroxide-mediated mitogen-activated protein kinase
activation and up-regulation of death receptors. Cancer Res.
69:1941–1950. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Pasini A and Zunino F: New cisplatin
analogues - on the way to better antitumor agents. Angew Chem Int
Ed Engl. 26:615–624. 1987. View Article : Google Scholar
|
|
29
|
Poirier MC, Lippard SJ, Zwelling LA, Ushay
HM, Kerrigan D, Thill CC, Santella RM, Grunberger D and Yuspa SH:
Antibodies elicited against
cis-diamminedichloroplatinum(II)-modified DNA are specific for
cis-diamminedichloroplatinum(II)-DNA adducts formed in vivo and in
vitro. Proc Natl Acad Sci USA. 79:6443–6447. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wong E and Giandomenico CM: Current status
of platinum-based antitumor drugs. Chem Rev. 99:2451–2466. 1999.
View Article : Google Scholar
|
|
31
|
Calderone V, Casini A, Mangani S, Messori
L and Orioli PL: Structural investigation of cisplatin-protein
interactions: Selective platination of His19 in a cuprozinc
superoxide dismutase. Angew Chem Int Ed Engl. 45:1267–1269. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
DeConti RC, Toftness BR, Lange RC and
Creasey WA: Clinical and pharmacological studies with
cis-diamminedichloroplatinum (II). Cancer Res. 33:1310–1315.
1973.PubMed/NCBI
|
|
33
|
Hagendoorn J, Tong R, Fukumura D, Lin Q,
Lobo J, Padera TP, Xu L, Kucherlapati R and Jain RK: Onset of
abnormal blood and lymphatic vessel function and interstitial
hypertension in early stages of carcinogenesis. Cancer Res.
66:3360–3364. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Jain RK: Normalizing tumor vasculature
with anti-angiogenic therapy: A new paradigm for combination
therapy. Nat Med. 7:987–989. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Behrens BC, Hamilton TC, Masuda H,
Grotzinger KR, Whang-Peng J, Louie KG, Knutsen T, McKoy WM, Young
RC and Ozols RF: Characterization of a
cis-diammine-dichloroplatinum(II)-resistant human ovarian cancer
cell line and its use in evaluation of platinum analogues. Cancer
Res. 47:414–418. 1987.PubMed/NCBI
|
|
36
|
Bertsch U, Röder C, Kalthoff H and
Trauzold A: Compartmentalization of TNF-related apoptosis-inducing
ligand (TRAIL) death receptor functions: Emerging role of nuclear
TRAIL-R2. Cell Death Dis. 5:e13902014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ashraf NU and Sheikh TA: Endoplasmic
reticulum stress and oxidative stress in the pathogenesis of
non-alcoholic fatty liver disease. Free Radic Res. 49:1405–1418.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Afanas'ev I: Reactive oxygen species
signaling in cancer: Comparison with aging. Aging Dis. 2:219–230.
2011.
|