Introduction
Acute myeloid leukemia (AML) is the most common type
among adults, accounting for the largest number of annual deaths
due to leukemia. Despite key advances in diagnosis and treatment
having made the disease more treatable and curable, 5-year overall
survival rate of patients with AML is still approximately 30%
(1–3). The standard induction therapy for AML
has changed little over the decades and includes cytarabine in
combination with an anthracycline as primary therapy, which can
acquire a high rate of complete remission, however, most patients
eventually relapse (4).
Furthermore, these chemotherapy drugs kill cancer cells as well
injure normal cells. Therefore, the development of effective drugs
against AML with relatively low cytotoxicity is highly desirable.
Targeting metabolic processes has been revealed as a promising
approach for cancer therapy (5,6).
Momordica anti-human immunodeficiency virus protein
of 30 kDa (MAP30) is extracted from Momordica charantia,
which is a vegetable widely distributed in Asia and Africa. MAP30
inactivates ribosiome through depurination of the adenine base at
position 2543 of 28S ribosomal RNA and the resulting inhibition of
ribosomal protein synthesis in cells (7,8). As a
ribosome-inactivating protein, MAP30 has been reported to exert
potent inhibitory effects and induce apoptosis on several solid
tumor cells (9–12). Whether MAP30 exerts similar effects
on malignant hematological cells remains unknown.
Except for apoptosis, autophagy also plays an
important role in tissue homeostasis, development, and disease.
Autophagy is an adaptive and protective cellular response to
nutrient deprivation, growth factor withdrawal, or metabolic stress
to sustain cellular homeostasis and recycle damaged cytoplasmic
organelles (13). This dynamic
process is characterized by the formation of double-membrane
vesicles called autophagosomes, which sequester cytoplasmic
organelles or long-lived proteins targeted for destruction and fuse
with lysosome for succeeding degradation (14). However, the role of autophagy in
cancer is complicated and depends upon tumor subtypes, stages of
tumor progression, cellular context or drugs that cause this
process (6,15). Autophagy can exhibit tumor
suppressive activities through the annihilation of oncogenic
protein substrates and toxic unfolded proteins (16,17),
and alternatively, it may exhibit tumor-promoting activities in
established cancers by maintaining cellular metabolism through
intracellular recycling when nutrients are limiting (15,18,19).
In addition, autophagy is implicated in other important aspects of
hematological malignancies as it improves immune competence and
antitumor immunity, and may even help to heighten patients'
tolerance to standard treatments (20). Mounting evidence has demonstrated
that autophagy can be regulated by several epigenetic modifications
including acetylation, phosphorylation, and ubiquitylation, and
importantly, an increase of acetylated level can inhibit the
autophagic flux and turnover of long-lived proteins (21–26).
Therefore, a detailed investigation is needed for the role of
autophagy and the underlying mechanisms according to cancer
type.
Here, we present data demonstrating that MAP30
effectively inhibits and kills the AML cells HL-60 and THP-1 as
well as primary AML cells. We provide compelling evidence that
MAP30 induces apoptosis in a caspase-dependent manner, and
attenuates cytoprotective autophagy by increasing the level of
histone transacetylase p300.
Materials and methods
Reagents and antibodies
MAP30 was a gift from School of Medical Laboratory
Science, Chengdu Medical College.
N-benzyloxycarbonyl-Val-Ala-Asp-fluoromethyl ketone (z-VAD-fmk),
rapamycin and bafilomycin A1 (baf A1) were purchased from Selleck
Chemicals (Houston, TX, USA), and C646 was purchased from
Sigma-Aldrich (St. Louis, MO, USA), which were dissolved in
dimethyl sulfoxide (DMSO). The final concentration of DMSO used did
not exceed 0.1%, which had no adverse effect on the cell cultures.
The antibodies against LC3, p62, beclin 1, caspase-3, caspase-8,
caspase-9, PARP, survivin, Bcl-2, Bax, GAPDH, acetylated-histone
H3, or histone H3 were provided by Cell Signaling Technology
(Beverly, MA, USA).
Cell culture and patient samples
HL-60 and THP-1 cells were cultured in RPMI-1640
medium supplemented with 10% fetal bovine serum (both from Gibco,
Grand Island, NY, USA) at 37°C in 5% CO2. Primary AML
cells were isolated from bone marrow aspirates of five newly
diagnosed and untreated patients with AML [M3, 1; M4, 4; the
diagnosis and classification was established according to the world
health organization criteria (27)]
using Ficoll-hypaque, and cultured in RPMI-1640 medium supplemented
with 20% fetal bovine serum at 37°C in 5% CO2. All
protocols and experiments were approved by The First Affiliated
Hospital of Wenzhou Medical university Institutional Review Board
for clinical experiments and use of human samples; written consents
were obtained from all subjects participated in this study in
accordance with the Declaration of Helsinki protocol.
Cytotoxicity assay
A Cell Counting Kit-8 (CCK-8) was used to assess the
cytotoxicity of MAP30 on AML cells according to the manufacturer's
protocol (Dojindo, Kumamoto, Japan). Briefly, AML cells were
diluted and seeded at a density of 4×103/well in 96-well
plates. The cells were subsequently exposed to MAP30 at different
concentrations for 48 and 72 h. Then, the CCK-8 was added, and
absorbance (A) was measured at 450 nm using an ELISA reader
(ELx800; Bio-Tek Instruments, Winooski, VT, USA). Cell viability
rate (%) = A450, MAP30/A450, control ×
100%.
Apoptosis assay
Apoptosis was determined using the Annexin
V-FITC/propidium iodide (PI) Detection kit (Beyotime Institute of
Biotechnology, Haimen, Jiangsu, China) according to the
manufacturer's instruction. Briefly, after treatment with MAP30 for
48 h, the cells were collected rapidly and washed by cold PBS and
subsequently incubated with FITC-labeled Annexin V and PI for 15
min at room temperature in the dark and then analyzed with
CellQuest software on a flow cytometry (FACSCalibur; BD
Biosciences, Mountain View, CA, USA).
Western blot analysis
After treatment with or without MAP30 at different
concentrations, the cells were collected and lysed immediately
using RIPA lysis buffer (Beyotime Institute of Biotechnology)
supplemented with PMSF and Halt protease and phosphatase inhibitor
cocktail (Pierce, Rockford, IL, USA). The protein was boiled for 5
min in 1× loading buffer and subjected to western blot analysis
according to our previously described method (28). Bands were visualized by enhanced
chemiluminescence reagents (Thermo Fisher, Fremont, CA, USA) and
the optical densities of the bands were analyzed using ImageJ
software (NIH, Bethesda, MD, USA).
Quantitative real-time PCR for gene
expression analysis
Quantitative real-time PCR was used to evaluate the
expression of p300 and a reference gene GAPDH according to our
previously described method (29).
The sequences of specific primers see Table I.
 | Table IThe sequences of the primers used for
real-time qPCR. |
Table I
The sequences of the primers used for
real-time qPCR.
| Primer |
|---|
| p300 | F:
5′-AGGCTGTATCAGAGCGTAT-3′
R:5′-TGCTTTCATTGCTGGTGT-3′ |
| GAPDH |
F:5′-ATCATCAGCAATGCCTCC-3′
R:5′-CATCACGCCACAGTTTCC-3′ |
Statistical analysis
The data are presented as mean ± SEM and analyzed by
one-way ANOVA followed by a post hoc Turkey's test to determine the
differences between the groups. Differences were considered
significant at P<0.05.
Results
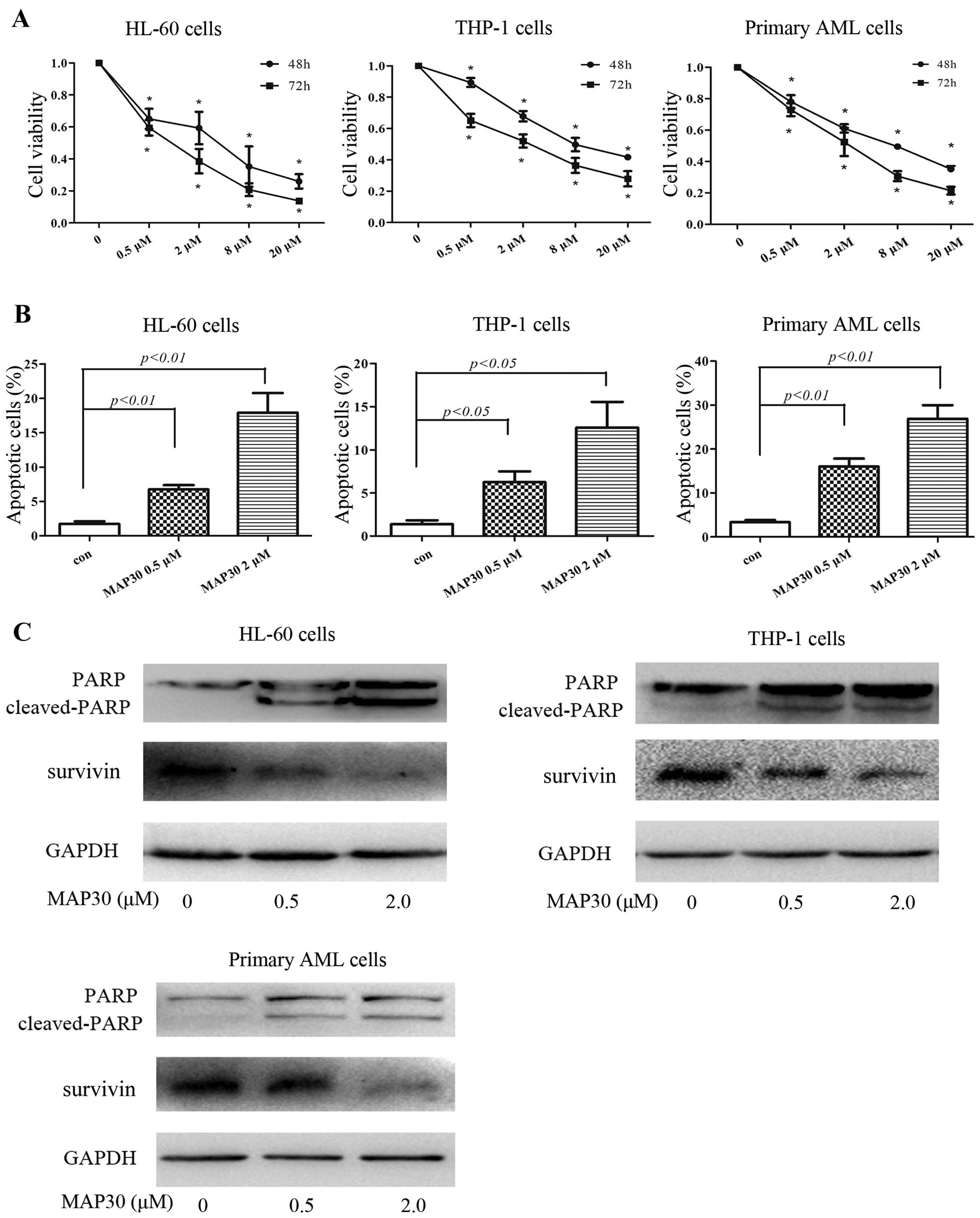
MAP30 inhibits proliferation and induces
apoptosis in AML cells
We first examined the effects of MAP30 on the
proliferation of AML cell lines HL-60 and THP-1, and primary AML
cells. As shown in Fig. 1A, MAP30
inhibited the proliferation of both HL-60 and THP-1 cells in a
dose- and time-dependent manner, with IC50 at 48 h of
2.6 and 9.2 µM, respectively. Furthermore, MAP30 also
inhibited the proliferation of primary AML cells, with
IC50 at 48 h of 4.7 to 8.1 µM (Fig. 1A). Since the induction of apoptosis
is a leading cause for MAP30-induced cytotoxicity against liver
cancer cell line HepG2 (11), we
next investigate whether apoptosis also occurs when AML cells are
inhibited by MAP30. As shown in Fig.
1B, MAP30 (0–2 µM) dose-dependently induced apoptosis of
AML HL-60 and THP-1 cells as well as primary AML cells by flow
cytometric analysis using Annexin V/PI staining. As expected, high
concentrations of MAP30 (8 µM, 20 µM) strongly
induced apoptosis in all AML cells tested (data not shown).
MAP30-induced apoptosis was further confirmed using western blot
analysis of two apoptosis-related proteins PARP and survivin. After
treatment with MAP30 for 48 h, cleaved-PARP was markedly increased
and conversely, survivin was decreased (Fig. 1C). Taken together, these data
suggested that MAP30 exhibits cytotoxicity in AML cells through
inducing apoptosis.
Both extrinsic and intrinsic pathways are
involved in MAP30-induced apoptosis
We further investigated the molecular mechanisms
involved in MAP30-induced apoptosis. As shown in Fig. 2A, MAP30 treatment resulted in the
cleavage of caspases, including the initiator caspase-8 and -9 and
the effector caspase-3 in AML cell lines HL-60 and THP-1 and
primary AML cells (Fig. 2A). These
data suggested that MAP30 induces apoptosis of AML cells in a
caspase-dependent manner, and both extrinsic and intrinsic
apoptotic pathways are involved. Cleaved caspase-3 subsequently
induced the cleavage of PARP, which ultimately led to apoptosis.
Additionally, the expression of anti-apoptotic protein Bcl-2 was
decreased and conversely, the expression of pro-apoptotic protein
Bax was significantly increased in HL-60 cells treated by MAP30
(Fig. 2B), indicating that some
Bcl-2 family members are also involved in caspase-dependent
apoptosis induced by MAP30. Finally, a pan-caspase inhibitor
z-VAD-fmk was employed to verify whether MAP30-induced apoptosis
depends on caspases. As shown in Fig.
2C and D, z-VAD-fmk significantly reduced MAP30-induced
apoptosis in HL-60 cells. Moreover, the growth inhibition of HL-60
cells induced by MAP30 was also partially rescued by z-VAD-fmk
(Fig. 2E), further confirming MAP30
induces apoptosis in a caspase-dependent manner.
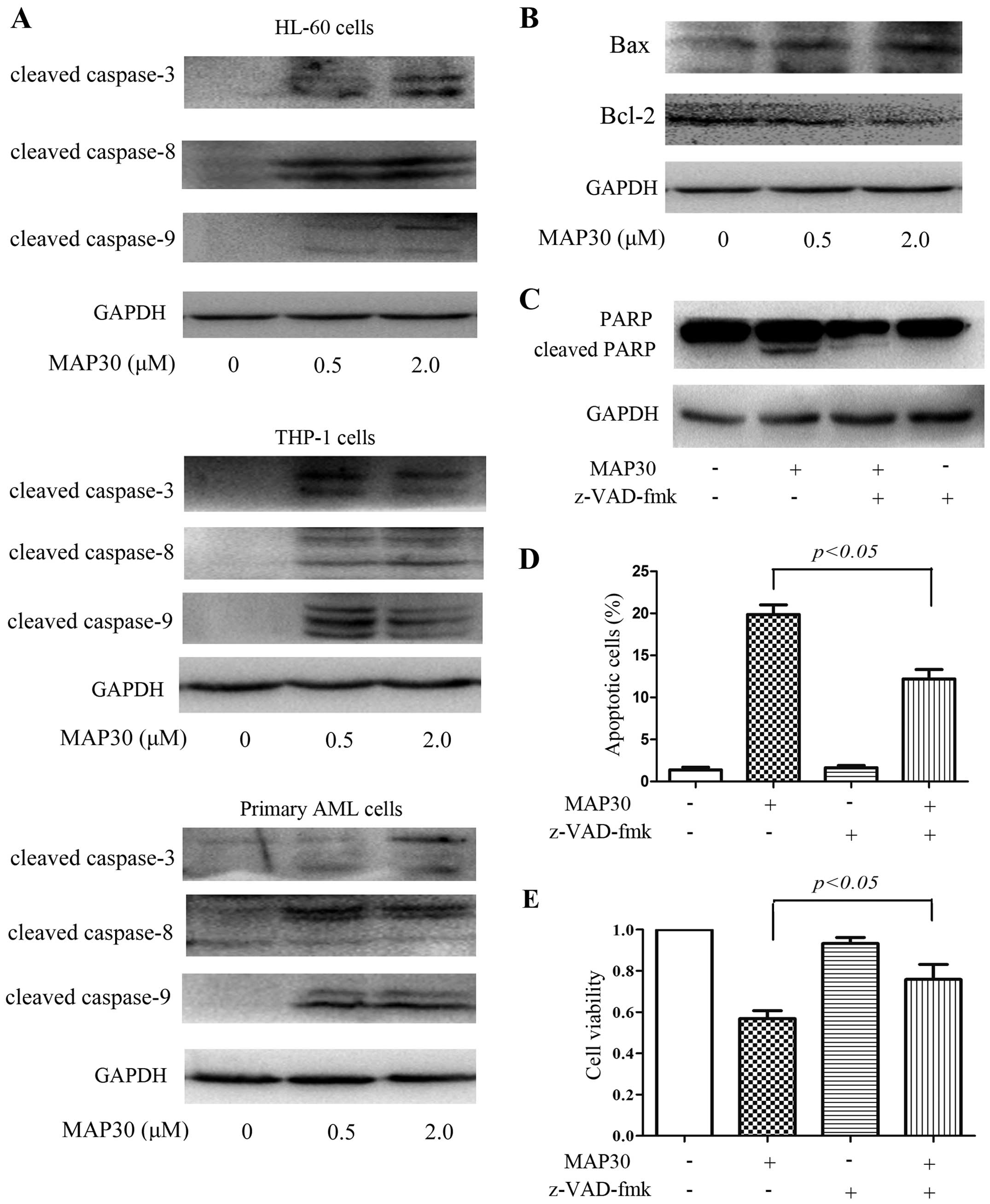 | Figure 2Both extrinsic and intrinsic caspase
pathways are involved in MAP30-induced apoptosis. (A) Two AML cell
lines, HL-60 and THP-1, and primary AML cells were treated with 0.5
and 2 µM of MAP30 for 48 h, and then western blot analysis
was performed to assess the expression levels of caspase-3,
caspase-8, and caspase-9. (B) HL-60 cells were treated with 0.5 and
2 µM of MAP30 for 48 h, and then western blot analysis was
performed to assess the expression levels of Bax and Bcl-2. (C–E)
HL-60 cells were incubated with 2 µM MAP30 in the presence
or absence of 20 µM z-VAD-fmk for 48 h, and then, western
blot analysis was performed to assess the levels of PARP and
cleaved-PARP, and Annexin V/PI staining was used to determine
apoptosis, and cell viability was determined by CCK-8 assay. Images
shown and statistical data are expressed as mean ± SEM of at least
three independent experiments. |
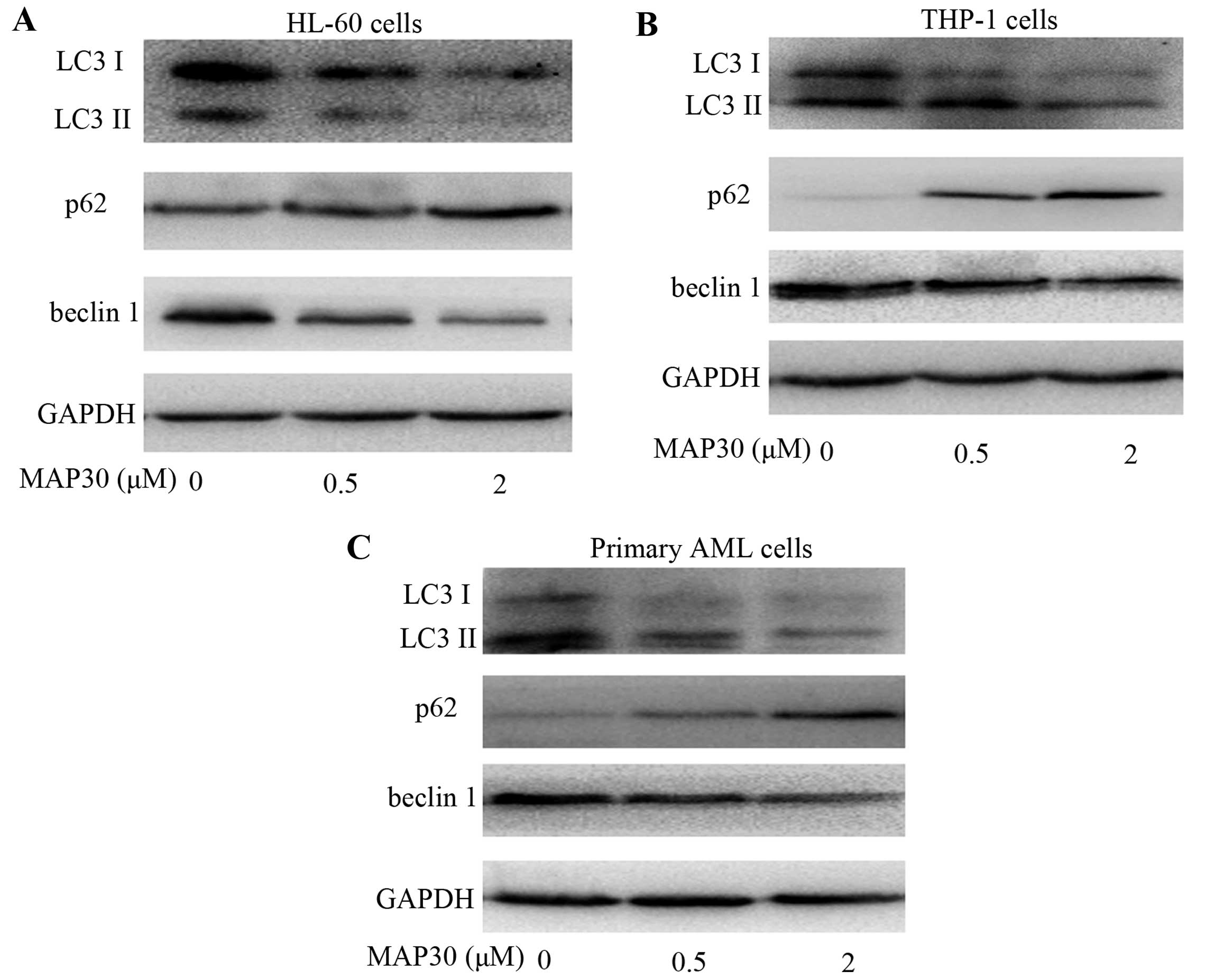
MAP30 inhibits autophagy in AML
cells
Low concentration of MAP30 (2 µM) exhibited
strong cytotoxicities against AML cells (Fig. 1A), whereas only modest apoptosis was
observed in AML cells induced by this concentration of MAP30
(Fig. 1B), suggesting that there
are other mechanisms involved in MAP30-induced cytotoxicity besides
the induction of apoptosis. Histone deacetylase inhibitors (HDACi)
have been shown to kill leukemia cells while sparing normal cells
by inhibiting autophagy (30,31).
Accumulating evidence shows that one key aspect of the pro-survival
function of autophagy is achieved through its ability to block
necrotic cell death (32). To
investigate whether MAP30 influences the autophagic flux of AML
cells, the expression of autophage-related proteins, including LC3,
p62, and beclin 1, were determined. As shown in Fig. 3, the expression of LC3II and beclin
1 were significantly decreased, whereas, p62 was increased in the
two AML cell lines HL-60 and THP-1 as well as primary AML cells
exposed to MAP30. These findings further supported the notion that
MAP30 treatment inhibits the autophagic flux in AML cells.
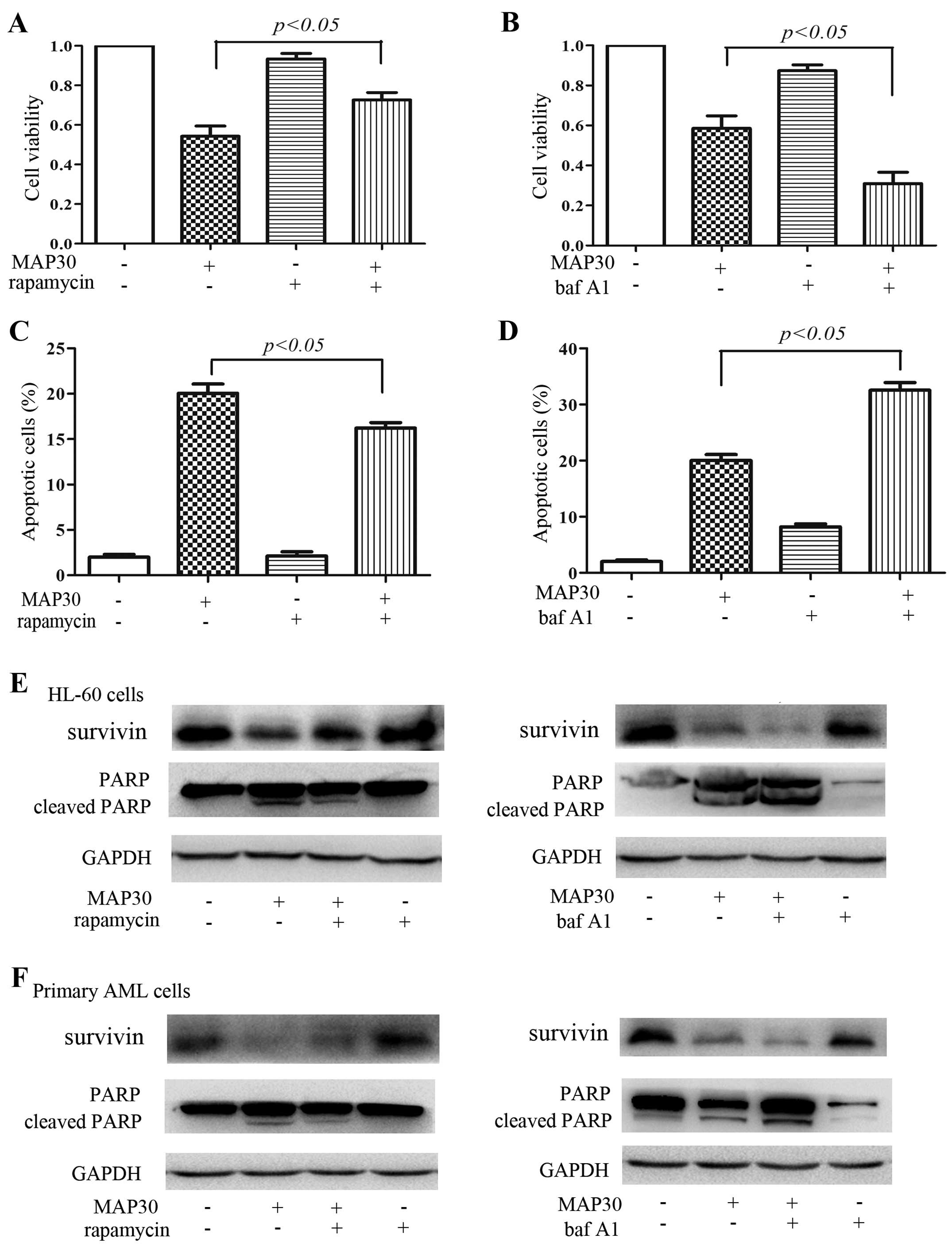
Inhibition of autophagy is implicated in
the cytotoxicity and induction of apoptosis of MAP30 in AML
cells
Undoubtedly, MAP30 can inhibit autophagy, which
prompted us to clarify whether the inhibition of autophagy plays an
important role in antileukemic effects of MAP30. We used autophagy
activator rapamycin, and another classical autophagy inhibitor baf
A1, which inhibits the vacuolar ATPase required for the fusion
between autophagosomes and lysosomes. Both rapamycin and baf A1
almost did not influence the cell viability and induce apoptosis of
HL-60 cells and primary AML cells (Fig.
4), but at these same concentrations of rapamycin and baf A1
can activate and inhibit the autophagic flux of HL-60 cells,
respectively (data not shown). As expected, rapamycin reduced the
cytotoxicity and apoptosis in HL-60 cells induced by MAP30
(Fig. 4A, C and E), and conversely,
baf A1 exacerbated the cytotoxicity and apoptosis in HL-60 cells
induced by MAP30 (Fig. 4B, D and
E). Similarly, rapamycin attenuated the cleavage of PARP and
the inhibition of survivin, and baf A1 had a synergistic effect on
the cleavage of PARP and the inhibition of survivin in primary AML
cells induced by MAP30 (Fig. 4F).
Taken together, these data further indicated that the inhibition of
autophagy is implicated in MAP30-induced cytotoxicity and induction
of apoptosis in AML cells.
MAP30 inhibits autophagy by enhancing
acetyltransferase p300
Since MCP30, a mixture of MAP30 and α-MMC, was shown
to promote histone H3 and H4 protein acetylation in our previous
report (33), we next investigate
whether the promotion of acetylation of MCP30 are attributed to its
main ingredient MAP30. As shown in Fig.
5A and B, MAP30 treatment increased the mRNA and protein
expression levels of p300 in HL-60 cells, which consequently
promoted the acetylation of histone H3 (Fig. 5C). Similar results have been
observed in primary AML cells treated by MAP30 (Fig. 5B and C). Accumulating evidence has
shown that p300 is a major endogenous repressor of autophagy
(23). To investigate whether the
inhibition of autophagy of MAP30 is attributed to p300, C646, a
pharmacological inhibitor of p300, was used. As shown in Fig. 5D and E, C646 almost completely
reversed the acetylation of histone H3 and promoted the autophagic
flux in the presence of MAP30 in HL-60 cells. Therefore, C646
partially rescued the cytotoxicity and apoptosis caused by MAP30 in
HL-60 cells and primary AML cells (Fig.
5F and G). Taken together, these findings suggested that MAP30
inhibits the autophagic flux of AML cells by enhancing the
acetyltransferase activity of p300.
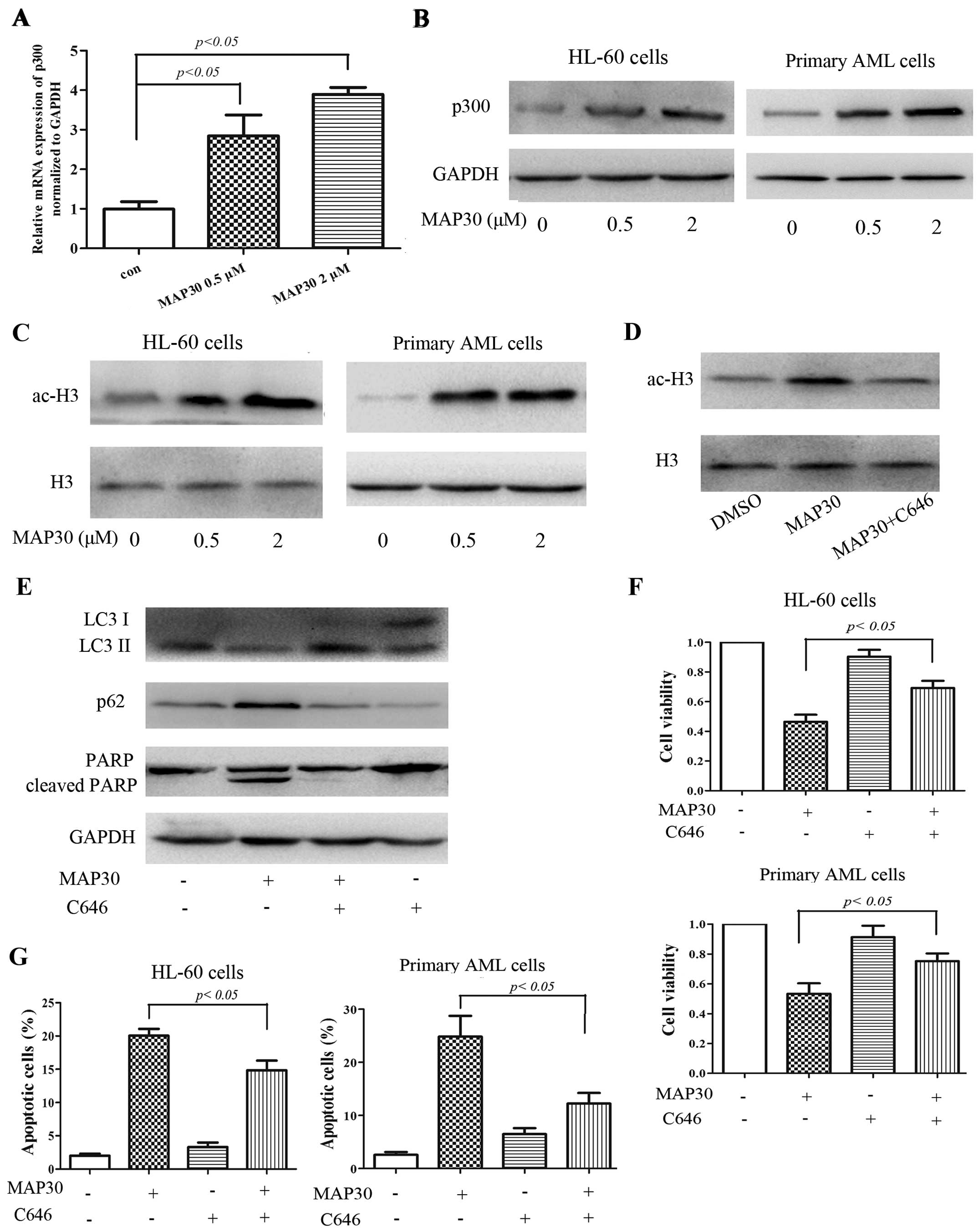 | Figure 5MAP30 inhibits autophagy by enhancing
acetyltransferase p300. (A–C) HL-60 cells and primary AML cells
were treated with 0.5 and 2 µM of MAP30 for 48 h, and then,
real-time PCR was performed to evaluate the mRNA level of p300, and
western blot analysis was performed to assess the levels of p300,
GAPDH, ac-histone H3 (ac-H3), and histone H3 (H3). (D and E) HL-60
cells were treated with 2 µM MAP30 for 48 h in the absence
or presence of C646, then western blot analysis was performed to
assess the levels of ac-H3, LC3I/II, p62 and PARP. (F and G) HL-60
cells and primary AML cells were treated with 2 µM MAP30 for
48 h in the absence or presence of C646, and then, cell viability
and apoptosis were determined by CCK-8 assay and Annexin V/PI
staining, respectively. Statistical data and images representing at
least three independent experiments are shown. |
Discussion
Increasing evidence has shown that MAP30 induces
cells death in several solid tumor cells, mostly resulting from
apoptosis. Here we have identified MAP30 as a potent antileukemic
agent, in which autophagy inhibition is a critical step in
mediating the antileukemic effects of MAP30 by increasing the p300,
an endogenous repressor of autophagy (23,34,35),
and subsequently potentiates the induction of apoptosis. To our
knowledge, this is the first report on MAP30 against hematological
malignancies.
Apoptosis has been deeply studied in the past two
decades and is widely accepted as a major mechanism of regulated
cell death. Therefore, measurement of apoptosis is frequently used
to evaluate the antitumor effects of cytotoxic agents besides cell
viability assessment. Our results demonstrated that MAP30 potently
inhibited the proliferation of HL-60 and THP-1 cells as well as
primary AML cells isolated from AML patients, and the induction of
apoptosis might be a major mechanism, similar to two previous
studies (9,11). Further molecular studies unraveled
the contribution of both the extrinsic pathway regulated by
caspase-8 cleavage and intrinsic pathway regulated by caspase-9
cleavage in MAP30-induced cell apoptosis of AML cells. Consistent
with our results, MAP30 also induces apoptosis in hepatocellular
carcinoma Hepg2 cells through both extrinsic and intrinsic pathways
(11).
The precise role of autophagy in cancer development
and treatment is still controversial. In AML, recent evidence
suggests that autophagy plays a pro-survival role in t(8;21) AML
cells (36), but the exact role of
this process in different subtypes of this hematologic tumor is
still undefined. As autophagy is an important catabolic process for
degrading bulky cytosolic contents, its inhibition is associated
with the production of reactive oxygen species (ROS), metabolic
insufficiency and increased proteotoxicity, and thus, promotes
cellular damage, reduces stress tolerance and compromises survival
(37). Many anticancer agents or
radiotherapy can trigger cell autophagy as a pro-survival activity,
which protects cancer cells against their killing action (38). In addition, autophagy is also needed
for leukemia cells due to high metabolic demand. In our present
study, except the induction of apoptosis, MAP30 also significantly
inhibited autophagy in two AML cell lines tested and all primary
AML cells. Based on the above, we speculate that MAP30 can break
the equilibrium of metabolism by suppressing basal autophagy,
resulting in accumulation of dysfunctional mitochondria,
subsequently triggering ROS production and DNA damage, and
ultimately inducing apoptosis and necrotic cell death. To confirm
our speculation, accompanied with MAP30 treatment, the autophagy
activator rapamycin was adopted to rescue and further induce
autophagy, and the autophagy inhibitor baf A1 was used to aggravate
the inhibition of autophagy in HL-60 cells and primary AML cells.
We found that MAP30-induced cell death significantly aggravated due
to autophagy inhibition when MAP30 combined with baf A1. Moreover,
the MAP30-induced cell death was partially rescued by rapamycin.
Thus, based on these findings, we think that MAP30-induced
autophagy inhibition contributes to the induction of apoptosis and
the resulting cell death in AML cells. Further investigation is
needed to clarify the relationship between autophagy and necrotic
cell death in MAP30-induced AML cell death.
MAP30 has been reported to increase the acetylation
level, while the underlying mechanisms remain unclear. We found
that p300 is a target of MAP30 action, and it increased the
expression of p300 at both the mRNA and protein levels in AML
cells. Moreover, the acetylation level of histone H3, an indicator
of p300 activity, was also increased by MAP30 treatment. It has
been well identified that p300 is a potent autophagy inhibitor
(35). To further identify the role
of p300 in MAP30-induced cell death, C646, a selective
pharmacological inhibitor of p300 activity, was used and partially
rescued the MAP30-induced cell death. Further research is needed to
investigate how MAP30 increased the p300 in AML cells.
In conclusion, although autophagy and apoptosis are
two independent cell death pathways, our findings offer specific
insight by which the pathways acted collaboratively to elicit
MAP30-induced cell death in AML cells. Our findings identify MAP30
as a potent anti-AML agent and provide molecular insight into the
anticancer potential of MAP30 by showing that both autophagic and
apoptotic signaling can work together in the induction of cell
death.
Acknowledgments
This study was supported by the National Natural
Science Foundation of China (no. 81100355, 81172613, 81300430),
Zhejiang Provincial Natural Science Foundation of China (no.
LQ12H08002, Y2111000, LY16H080007), and the grant of Wenzhou
Municipal Science and Technology Bureau (no. Y20150006, Y20150031,
Y20150034).
References
|
1
|
Dores GM, Devesa SS, Curtis RE, Linet MS
and Morton LM: Acute leukemia incidence and patient survival among
children and adults in the United States, 2001–2007. Blood.
119:34–43. 2012. View Article : Google Scholar :
|
|
2
|
Schiller GJ: Evolving treatment strategies
in patients with high-risk acute myeloid leukemia. Leuk Lymphoma.
55:2438–2448. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Liu N, Ning HM, Hu LD, Jiang M, Xu C, Hu
JW, Wang J, Li YH, Li BT, Lou X, et al: Outcome of myeloablative
allogeneic peripheral blood hematopoietic stem cell transplantation
for refractory/relapsed AML patients in NR status. Leuk Res.
39:1375–1381. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Döhner H, Weisdorf DJ and Bloomfield CD:
Acute myeloid leukemia. N Engl J Med. 373:1136–1152. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Xia HG, Najafov A, Geng J, Galan-Acosta L,
Han X, Guo Y, Shan B, Zhang Y, Norberg E, Zhang T, et al:
Degradation of HK2 by chaperone-mediated autophagy promotes
metabolic catastrophe and cell death. J Cell Biol. 210:705–716.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhao G, Han X, Zheng S, Li Z, Sha Y, Ni J,
Sun Z, Qiao S and Song Z: Curcumin induces autophagy, inhibits
proliferation and invasion by downregulating AKT/mTOR signaling
pathway in human melanoma cells. Oncol Rep. 35:1065–1074. 2016.
|
|
7
|
Lee-Huang S, Huang PL, Huang PL,
Bourinbaiar AS, Chen HC and Kung HF: Inhibition of the integrase of
human immunodeficiency virus (HIV) type 1 by anti-HIV plant
proteins MAP30 and gAP31. Proc Natl Acad Sci USA. 92:8818–8822.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang YX, Neamati N, Jacob J, Palmer I,
Stahl SJ, Kaufman JD, Huang PL, Huang PL, Winslow HE, Pommier Y, et
al: Solution structure of anti-HIV-1 and anti-tumor protein MAP30:
Structural insights into its multiple functions. Cell. 99:433–442.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fan JM, Luo J, Xu J, Zhu S, Zhang Q, Gao
DF, Xu YB and Zhang GP: Effects of recombinant MAP30 on cell
proliferation and apoptosis of human colorectal carcinoma LoVo
cells. Mol Biotechnol. 39:79–86. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fan X, He L and Meng Y, Li G, Li L and
Meng Y: A-MMC and MAP30, two ribosome-inactivating proteins
extracted from Momordica charantia, induce cell cycle arrest and
apoptosis in A549 human lung carcinoma cells. Mol Med Rep.
11:3553–3558. 2015.PubMed/NCBI
|
|
11
|
Fang EF, Zhang CZ, Wong JH, Shen JY, Li CH
and Ng TB: The MAP30 protein from bitter gourd (Momordica
charantia) seeds promotes apoptosis in liver cancer cells in vitro
and in vivo. Cancer Lett. 324:66–74. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hao L, Zhang ZG, Han CH, Zhao Y, Liang Q,
Jiang B, He HG, Zhang JJ and Zhang P: Expression of Momordica
charantia MAP30 and its anti-tumor effect on bladder cancer cells.
Minerva Urol Nefrol. Dec 17–2014.Epub ahead of print. PubMed/NCBI
|
|
13
|
Choi AM, Ryter SW and Levine B: Autophagy
in human health and disease. N Engl J Med. 368:651–662. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Boya P, Reggiori F and Codogno P: Emerging
regulation and functions of autophagy. Nat Cell Biol. 15:713–720.
2013. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
White E: Deconvoluting the
context-dependent role for autophagy in cancer. Nat Rev Cancer.
12:401–410. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Larrue C, Saland E, Boutzen H, Vergez F,
David M, Joffre C, Hospital MA, Tamburini J, Delabesse E, Manenti
S, et al: Proteasome inhibitors induce FLT3-ITD degradation through
autophagy in AML cells. Blood. 127:882–892. 2016. View Article : Google Scholar
|
|
17
|
Kumar S, Guru SK, Pathania AS, Manda S,
Kumar A, Bharate SB, Vishwakarma RA, Malik F and Bhushan S:
Fascaplysin induces caspase mediated crosstalk between apoptosis
and autophagy through the inhibition of PI3K/AKT/mTOR signaling
cascade in human leukemia HL-60 cells. J Cell Biochem. 116:985–997.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wei MF, Chen MW, Chen KC, Lou PJ, Lin SY,
Hung SC, Hsiao M, Yao CJ and Shieh MJ: Autophagy promotes
resistance to photodynamic therapy-induced apoptosis selectively in
colorectal cancer stem-like cells. Autophagy. 10:1179–1192. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yuan X, Du J, Hua S, Zhang H, Gu C, Wang
J, Yang L, Huang J, Yu J and Liu F: Suppression of autophagy
augments the radiosensitizing effects of STAT3 inhibition on human
glioma cells. Exp Cell Res. 330:267–276. 2015. View Article : Google Scholar
|
|
20
|
Nencioni A, Cea M, Montecucco F, Longo VD,
Patrone F, Carella AM, Holyoake TL and Helgason GV: Autophagy in
blood cancers: Biological role and therapeutic implications.
Haematologica. 98:1335–1343. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Huang R, Xu Y, Wan W, Shou X, Qian J, You
Z, Liu B, Chang C, Zhou T, Lippincott-Schwartz J, et al:
Deacetylation of nuclear LC3 drives autophagy initiation under
starvation. Mol Cell. 57:456–466. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lee IH, Cao L, Mostoslavsky R, Lombard DB,
Liu J, Bruns NE, Tsokos M, Alt FW and Finkel T: A role for the
NAD-dependent deacetylase Sirt1 in the regulation of autophagy.
Proc Natl Acad Sci USA. 105:3374–3379. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lee IH and Finkel T: Regulation of
autophagy by the p300 acetyltransferase. J Biol Chem.
284:6322–6328. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mariño G, Pietrocola F, Eisenberg T, Kong
Y, Malik SA, Andryushkova A, Schroeder S, Pendl T, Harger A,
Niso-Santano M, et al: Regulation of autophagy by cytosolic
acetyl-coenzyme A. Mol Cell. 53:710–725. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Xie Y, Kang R, Sun X, Zhong M, Huang J,
Klionsky DJ and Tang D: Posttranslational modification of
autophagy-related proteins in macroautophagy. Autophagy. 11:28–45.
2015. View Article : Google Scholar :
|
|
26
|
Yi C, Ma M, Ran L, Zheng J, Tong J, Zhu J,
Ma C, Sun Y, Zhang S, Feng W, et al: Function and molecular
mechanism of acetylation in autophagy regulation. Science.
336:474–477. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Vardiman JW, Thiele J, Arber DA, Brunning
RD, Borowitz MJ, Porwit A, Harris NL, Le Beau MM,
Hellström-Lindberg E, Tefferi A, et al: The 2008 revision of the
World Health Organization (WHO) classification of myeloid neoplasms
and acute leukemia: Rationale and important changes. Blood.
114:937–951. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang SH, Zhang Y, Shen J, Zhang S, Chen
L, Gu J, Mruk JS, Cheng G, Zhu L, Kunapuli SP, et al: Tumor
vascular disrupting agent 5,6-dimethylxanthenone-4-acetic acid
inhibits platelet activation and thrombosis via inhibition of
thromboxane A2 signaling and phosphodiesterase. J Thromb Haemost.
11:1855–1866. 2013.PubMed/NCBI
|
|
29
|
Han Y, Ye A, Bi L, Wu J, Yu K and Zhang S:
Th17 cells and interleukin-17 increase with poor prognosis in
patients with acute myeloid leukemia. Cancer Sci. 105:933–942.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
El-Khoury V, Pierson S, Szwarcbart E,
Brons NH, Roland O, Cherrier-De Wilde S, Plawny L, Van Dyck E and
Berchem G: Disruption of autophagy by the histone deacetylase
inhibitor MGCD0103 and its therapeutic implication in B-cell
chronic lymphocytic leukemia. Leukemia. 28:1636–1646. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Stankov MV, El Khatib M, Kumar Thakur B,
Heitmann K, Panayotova-Dimitrova D, Schoening J, Bourquin JP,
Schweitzer N, Leverkus M, Welte K, et al: Histone deacetylase
inhibitors induce apoptosis in myeloid leukemia by suppressing
autophagy. Leukemia. 28:577–588. 2014. View Article : Google Scholar :
|
|
32
|
Shen HM and Codogno P: Autophagy is a
survival force via suppression of necrotic cell death. Exp Cell
Res. 318:1304–1308. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Xiong SD, Yu K, Liu XH, Yin LH,
Kirschenbaum A, Yao S, Narla G, DiFeo A, Wu JB, Yuan Y, et al:
Ribosome-inactivating proteins isolated from dietary bitter melon
induce apoptosis and inhibit histone deacetylase-1 selectively in
premalignant and malignant prostate cancer cells. Int J Cancer.
125:774–782. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Dancy BM and Cole PA: Protein lysine
acetylation by p300/CBP. Chem Rev. 115:2419–2452. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Pietrocola F, Lachkar S, Enot DP,
Niso-Santano M, Bravo-San Pedro JM, Sica V, Izzo V, Maiuri MC,
Madeo F, Mariño G, et al: Spermidine induces autophagy by
inhibiting the acetyltransferase EP300. Cell Death Differ.
22:509–516. 2015. View Article : Google Scholar :
|
|
36
|
Torgersen ML, Engedal N, Bøe SO, Hokland P
and Simonsen A: Targeting autophagy potentiates the apoptotic
effect of histone deacetylase inhibitors in t(8;21) AML cells.
Blood. 122:2467–2476. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Galluzzi L, Pietrocola F, Levine B and
Kroemer G: Metabolic control of autophagy. Cell. 159:1263–1276.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Gammoh N, Lam D, Puente C, Ganley I, Marks
PA and Jiang X: Role of autophagy in histone deacetylase
inhibitor-induced apoptotic and nonapoptotic cell death. Proc Natl
Acad Sci USA. 109:6561–6565. 2012. View Article : Google Scholar : PubMed/NCBI
|