Introduction
Laryngeal cancer is a common malignant tumor
appearing in head and neck. More than 90% of laryngeal cancer is
laryngeal squamous cell carcinoma which accounts for 14% of the
squamous cell carcinoma of head and neck (1,2).
Despite advances in therapies, the incidence of laryngeal cancer is
high with ~160,000 new cases each year and the mortality of
laryngeal cancer is still high with a 64% 5-year survival rate
(3). Tumorigenesis of laryngeal
cancer is a complex process involved in genetic dysregulation.
Understanding fully the underlying mechanism of laryngeal cancer
tumorigenesis is imperative to the therapy of laryngeal cancer.
Nin one binding protein (NOB1) gene is located on
human chromosome 16q22.1 (4) and
NOB1 protein is mainly expressed in liver, lung and spleen. NOB1
protein is a nuclear protein composed of the PIN domain and the C
terminal zinc ribbon domain. NOB1 regulates the maturation process
of 18S rRNA through the PIN domain, and influences the formation of
ribosome, thus having impact on the synthesis of proteins (5–7). NOB1
also promotes the maturation of 20S proteasome as well as the
formation of 26S proteasome, mediating the ubiquitin-mediated
protein degradation (8,9). NOB1 plays crucial roles in the
synthesis and degradation of proteins, which indicates the
importance of NOB1 in multiple physiologic activities of cells.
Recent studies show that NOB1 has higher expression
in various tumors, such as breast (10), prostate (11) and lung cancer (12), than the adjacent non-tumor tissues.
Inhibition of NOB1 is shown to perform anticancer function in
breast (13), prostate (11), colon (14) and ovarian cancer (15). However, no data show the expression
and function of NOB1 in laryngeal cancer. In the present study, we
detected the expression level of NOB1 in laryngeal cancer patients
and explored the effect of NOB1 silence on the proliferation,
apoptosis and migration of laryngeal cancer cells. Results of the
present study showed that NOB1 acted as an oncogene in laryngeal
cancer cells and silence of NOB1 may become a promising therapeutic
method in the treatment of laryngeal cancer.
Materials and methods
Clinical exploration
Paired laryngeal cancers and adjacent normal tissues
were obtained from 28 patients who underwent primary surgical
resection of laryngeal cancer in Shengjing Hospital of China
Medical University from April, 2014 to April, 2015. Following
surgical removal, these tissue samples were cut into small pieces
immediately and frozen in liquid nitrogen. These tissue samples
were subjected to quantitative real-time PCR (RT-qPCR) and western
blotting as described below. The relative mRNA level of NOB1 in
laryngeal cancer patients was calculated using the
2−∆∆Ct method and the relative protein level of NOB1 was
normalized to β-actin. The study protocol was approved by the Human
Research Ethics Committee of China Medical University.
Cell culture
Human laryngeal cancer cell line Hep2 was obtained
from Type Culture Collection Center of Chinese Academy of Science
(Shanghai, China). Cells were cultured in RPMI-1640 medium (Gibco,
Grand Island, NY, USA) supplemented with 10% fetal bovine serum
(FBS; HyClone, Logan, UT, USA) and maintained in a humid atmosphere
at 37°C with 5% CO2.
Infection
Sequence containing NOB1 shRNA or its corresponding
negative control (NC) (Table I) was
synthesized by Sangon Biotech (Shanghai, China) and inserted into
pRNA-H1.1 plasmid. After sequencing confirmation, these two
plasmids were named as NOB1 shRNA and NC, respectively. Cells were
seeded into 6-well plates and infected with NOB1 shRNA or NC using
Lipofectamine 2000 reagent (Invitrogen, Carlsbad, CA, USA)
according to the manufacturer's protocol. Then, cells were cultured
in RPMI-1640 medium supplemented with 10% FBS and 100 µg/ml
G418 (Invitrogen) for positive colony selection.
 | Table ISequence of shRNA. |
Table I
Sequence of shRNA.
| Sense
(5′−>3′) | Antisense
(5′−>3′) |
|---|
| NOB1 shRNA |
GATCCCCGTGAGGACGTTCCAAGTGATTCA |
AGCTAAAAAGTGAGGACGTTCCAAGTGATC |
|
AGAGATCACTTGGAACGTCCTCACTTTTT |
TCTTGAATCACTTGGAACGTCCTCACGGG |
| NC |
GATCCCCTTCTCCGAACGTGTCACGTTTCAA |
AGCTAAAAATTCTCCGAACGTGTCACGTTCT |
|
GAGAACGTGACACGTTCGGAGAATTTTT |
CTTGAAACGTGACACGTTCGGAGAAGGG |
MTT assay
Cells were seeded into 96-well plates
(3×103 cells/well) in quintuplicate, and MTT was added
into each well at 0, 24, 48, 72 and 96 h. After incubation for
additional 4 h, the supernatant was removed gently and 200
µl dimethyl sulfoxide (DMSO) was added into each well. The
absorbance at 490 nm was measured using a microplate reader.
Colony formation
Cells were seeded into cell culture dishes (500
cells/dish). The dishes were maintained at 37°C for 7 days to allow
the colonies to form. The culture medium was changed every 2–3
days. After the colonies were formed, they were washed with
phosphate-buffered saline (PBS), fixed with 4% paraformaldehyde and
stained with Wright-Giemsa dye (Jiancheng Bio, Nanjing, China) for
5 min. Images of the stained colonies were captured and the ratio
of colony formation (>50 cells/colony) was calculated.
Cell cycle assay
The cell cycle assay was performed by flow
cytometry. Cells were harvested, washed with PBS and fixed with
ice-cold 70% ethanol. The fixed cells were stained with Cell Cycle
Detection kit (Beyotime, Shanghai, China), and incubated at 37°C
for 30 min in the dark. Then, the cell cycle distribution was
analyzed by flow cytometer (Becton-Dickinson, Franklin Lakes, NJ,
USA).
Apoptosis assay
The apoptosis of cells in each group was evaluated
by flow cytometry using a Cell Apoptosis Detection kit (Wanleibio,
Shenyang, China). Cells were harvested and resuspended in 500
µl binding buffer. Then, 5 µl Annexin V-FITC and 5
µl propidium iodide was added into the suspension. The cell
suspension was then incubated at room temperature for 15 min in the
dark. Then, apoptosis level of cells in each group was detected
using flow cytometry.
Hoechst staining
For Hoechst staining, cells were seeded in a 12-well
plate (1×105 cells/well). Cells were fixed after culture
at 37°C for 24 h. After washing with PBS, cells were stained with
Hoechst staining kit (Beyotime), then cells were observed under
fluorescence microscope with a magnification of ×400, and the
images were captured.
Wound-healing assay
Cells were seeded into 6-well plates. When cell
confluence was 80–90%, cells were treated with 1 µg/ml
mitomycin C for 1 h, and then 200 µl pipette tips were used
to make scratches on the cell monolayer to generate a wound. After
the scratches were made, cells were cultured in serum-free medium
and images of the wounds were captured at 0, 24 and 48 h. The
migration rate was calculated by measuring the gap size of the
wounds. Migration rate = (1 − gap size at 24 or 48 h/gap size at 0
h) × 100%.
Transwell assay
After infection with NOB1 shRNA, cells were made
into cell suspension and 200 µl cell suspension of each
group was added into the upper chamber of Transwell plate (Corning,
Tewksbury, MA, USA) pre-coated with Matrigel (Becton-Dickinson) at
a density of 2×104 cells/well. Medium (800 µl)
with 20% FBS were added into the lower chambers. After incubation
at 37°C for 24 h, cells above the microporous membrane were removed
by cotton swabs and cells at the bottom of the microporous membrane
were fixed with 4% paraformaldehyde for 20 min at room temperature,
and stained with 0.5% crystal violet for 5 min. Images of cells in
five random fields were captured using light microscopy with a
magnification of ×200.
RT-qPCR
Total RNA was extracted using total RNA extraction
kit (BioTeke, Beijing, China) according to the manufacturer's
protocol and reverse transcribed to cDNA with super M-MLV reverse
transcriptase (BioTeke) and oligo(dT)15. The mRNA level
of NOB1 was detected by SYBR-Green quantitative real-time PCR with
primers as in Table II. The
relative mRNA level of NOB1 was calculated using the
2−∆∆Ct method (16).
 | Table IISequence of primers used in
RT-qPCR. |
Table II
Sequence of primers used in
RT-qPCR.
| Forward primer
(5′−>3′) | Reverse primer
(5′−>3′) |
|---|
| NOB1 |
GCTTGTGAGCCTGAGAACCTG |
TTATCCAGCCACCCCCGTC |
| β-actin |
CTTAGTTGCGTTACACCCTTTCTTG |
CTGTCACCTTCACCGTTCCAGTTT |
Western blotting
Cells were harvested and lysed in RIPA lysis buffer
(Beyotime) with 1% phenylmethanesulfonyl fluoride, and protein was
extracted by centrifugation. After measurement of protein
concentration with a BCA protein assay kit (Beyotime), equal amount
of protein from each group was injected into SDS-PAGE for
electrophoresis. Then, protein was transferred to polyvinylidene
fluoride (PVDF) membranes. After blockade with 5% skim milk or 1%
BSA, the membranes were incubated with the primary antibodies
against NOB1 (1:1,000; Proteintech, Chicago, IL, USA),
cleaved-caspase-3, cleaved-PARP (1:1,000; Abcam, Cambridge, UK),
matrix metalloproteinases (MMPs)-2, MMP-9, B-cell lymphoma-2
(Bcl-2), Bcl-2-associated X protein (Bax) (1:400; Boster, Wuhan,
China), p-JNK, JNK (1:500; Bioss, Beijing, China) and β-actin
(1:1,000; Santa Cruz Biotechnology, Inc., Dallas, TX, USA). After
washing with TBST, the membranes were incubated with corresponding
horseradish peroxidase-conjugated secondary antibodies (1:5,000;
Beyotime). Then, the membranes were visualized using an enhanced
chemiluminescence (ECL) detection system and the gray scale
analysis was carried out with Gel-Pro-Analyzer.
Statistical analysis
All experiments were repeated three times and the
results are presented as means ± standard deviation (SD). One-way
analysis of variance (ANOVA) and Bonferroni's multiple comparison
were used to analyze the difference between each group. p<0.05
was considered to be significant.
Results
NOB1 level in laryngeal cancer
patients
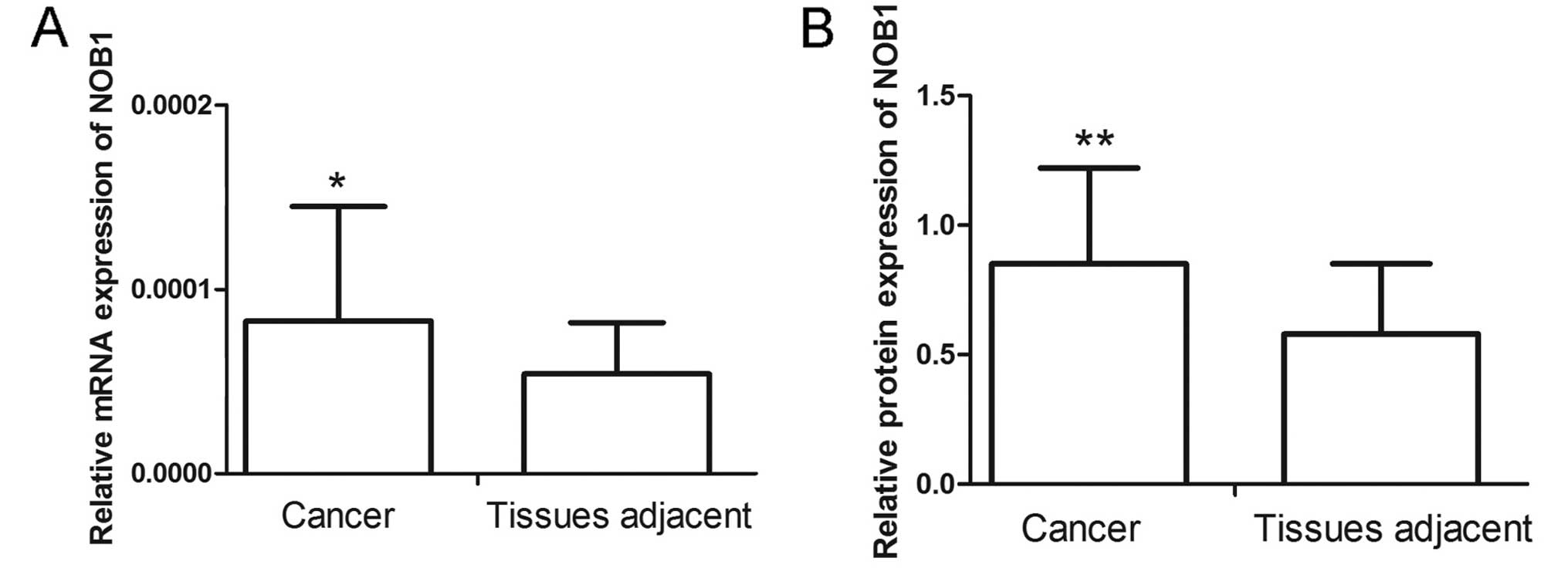
The NOB1 level in laryngeal cancer patients was
detected by RT-qPCR and western blotting. Results of RT-qPCR showed
that laryngeal cancer tissues had a higher NOB1 level than those of
the adjacent tissues (Fig. 1A).
Similar to the results of RT-qPCR, western blotting also showed
that NOB1 had a higher level in laryngeal cancer (Fig. 1B). These results demonstrated that
NOB1 expressed at high level in laryngeal cancer.
NOB1 shRNA decreases the level of
NOB1
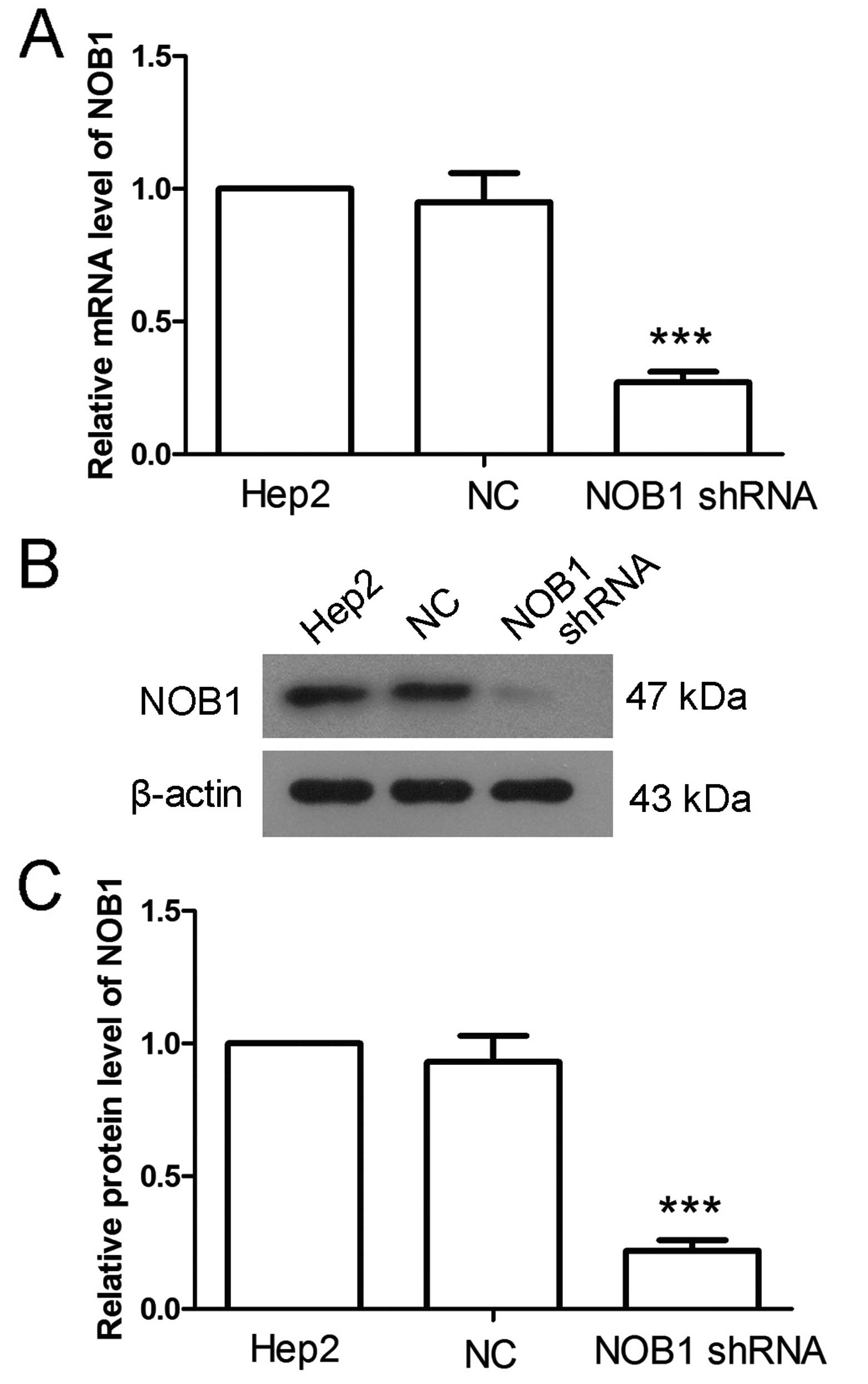
NOB1 shRNA was used to explore the function of NOB1.
RT-qPCR and western blotting were employed to detect the effect of
NOB1 shRNA. Results of RT-qPCR showed that, in cells infected with
NOB1 shRNA, the NOB1 level was decreased to 27±4% (Fig. 2A), but there was no significant
change in cells infected with NC. Similar altered patterns were
also found in the results of western blotting, after infected with
NOB1 shRNA, the protein level of NOB1 was decreased to 22±4%
(Fig. 2B and C). These results
demonstrate that NOB1 shRNA downregulates NOB1 level
effectively.
NOB1 shRNA inhibits the proliferation of
laryngeal cancer cells
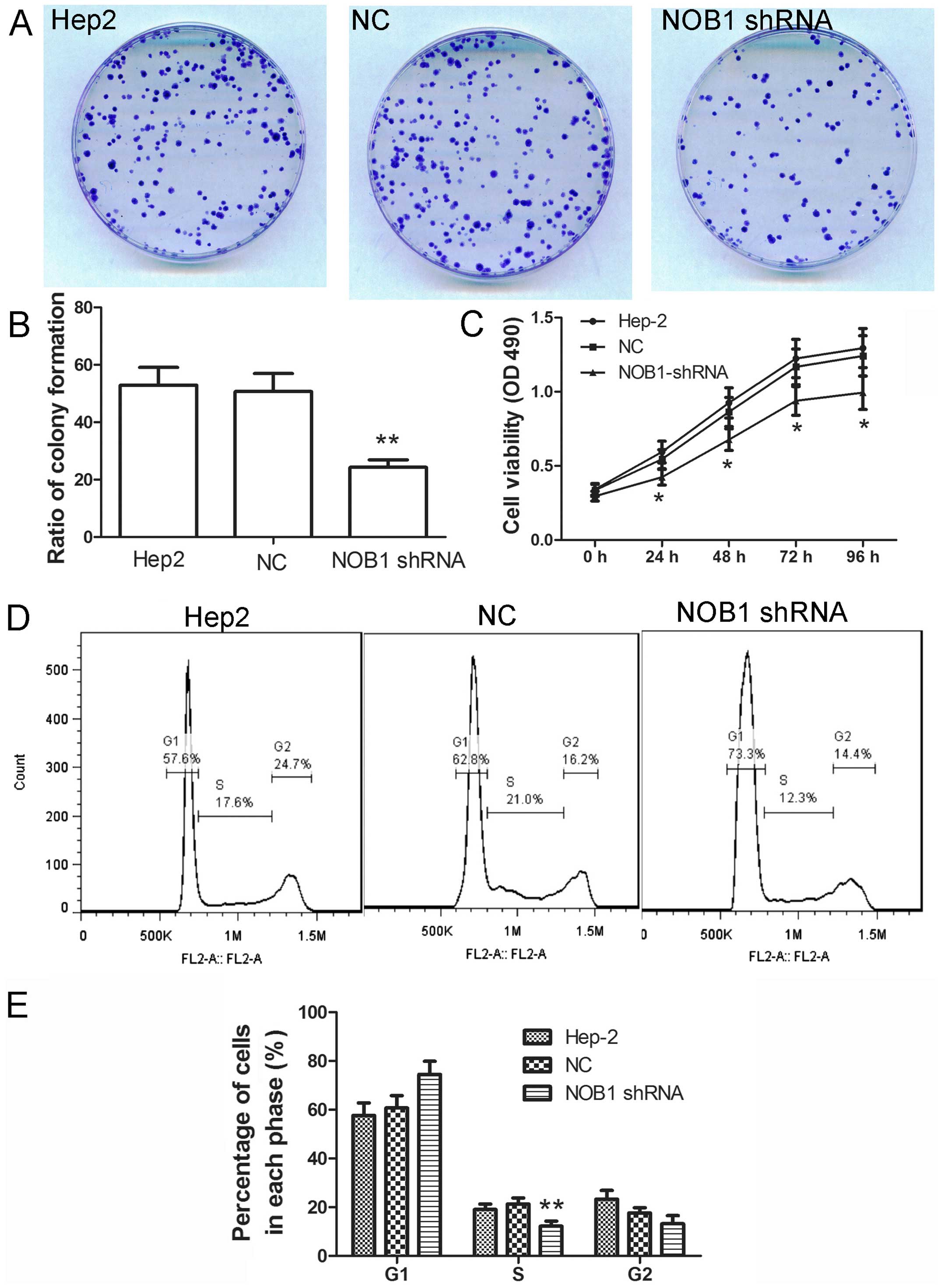
To explore the effect of NOB1 on the proliferation
of laryngeal cancer cells, colony formation assay was carried out
to explore the effect of NOB1. After infection with NOB1 shRNA, the
ratio of colony formation was significantly decreased comparing
with that of cells infected with NC (Fig. 3A and B; p<0.01). Then, MTT assay
was also carried out. As shown in Fig.
3C, cells infected with NOB1 shRNA showed a slow growth
comparing to cells infected with NC. These results suggest that
NOB1 shRNA inhibits growth of laryngeal cancer cells.
Cell cycle is a crucial event that influences the
growth of cells. The effect of NOB1 shRNA on cell cycle was
detected in the present study. As shown in Fig. 3D and E, after infection with NOB1
shRNA, the percentage of cells in G1 phase was increased from
60.67±5.14 to 74.47±5.44%, and the percentage of cells in S phase
was decreased from 21.30±2.46 to 12.23±3.36%. The distribution of
cell cycle was changed after infection with NOB1 shRNA, which
indicates the important role of NOB1 in the cell cycle.
NOB1 shRNA induces laryngeal cancer cell
apoptosis
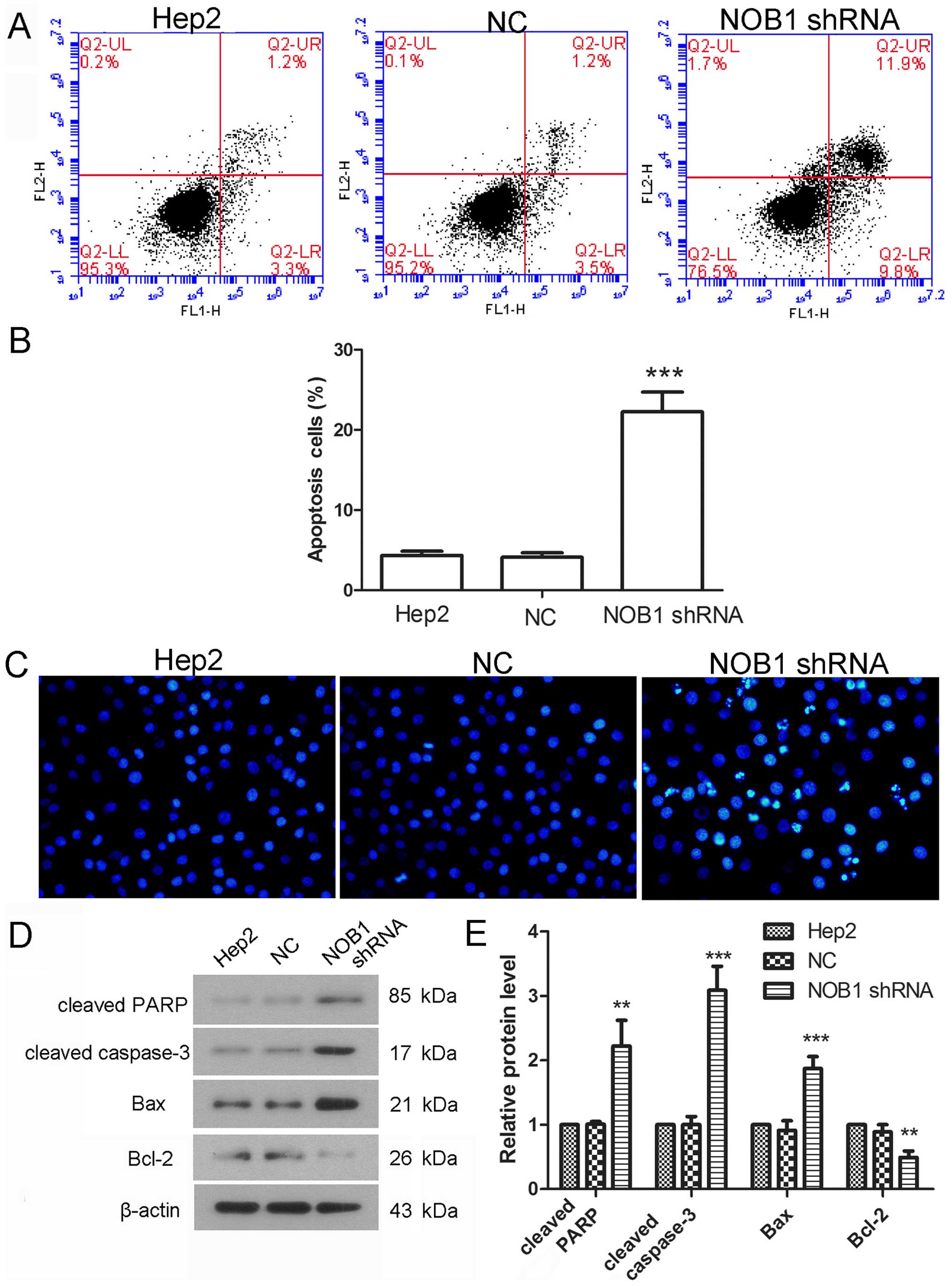
Cell apoptosis is also an important event that
influences cell growth. Effect of NOB1 shRNA on cell apoptosis was
detected using flow cytometry. Results showed that the percentage
of apoptotic cells was 4.12±0.54% in cells infected with NC, but
the percentage of apoptosis was increased to 22.25±2.46% after
infection with NOB1 shRNA (Fig. 4A and
B). Hoechst staining was also employed to detect cell
apoptosis. As shown in Fig. 4C,
there was obvious pyknosis of chromatin in cells infected with NOB1
shRNA. The protein levels of cleaved caspase-3, cleaved PARP, Bcl-2
and Bax were also detected in the present study. Results of western
blotting showed that the protein level of cleaved caspase-3 was
increased to 3.09±0.37-fold, the protein level of cleaved PARP was
increased to 2.2±0.4-fold, the protein level of Bax was increased
to 1.87±0.19-fold, but the protein level of Bcl-2 was decreased to
49±10% (Fig. 4D and E). These
results demonstrate that NOB1 shRNA induces apoptosis of laryngeal
cancer cells.
NOB1 shRNA inhibits migration and
invasion of laryngeal cancer cells
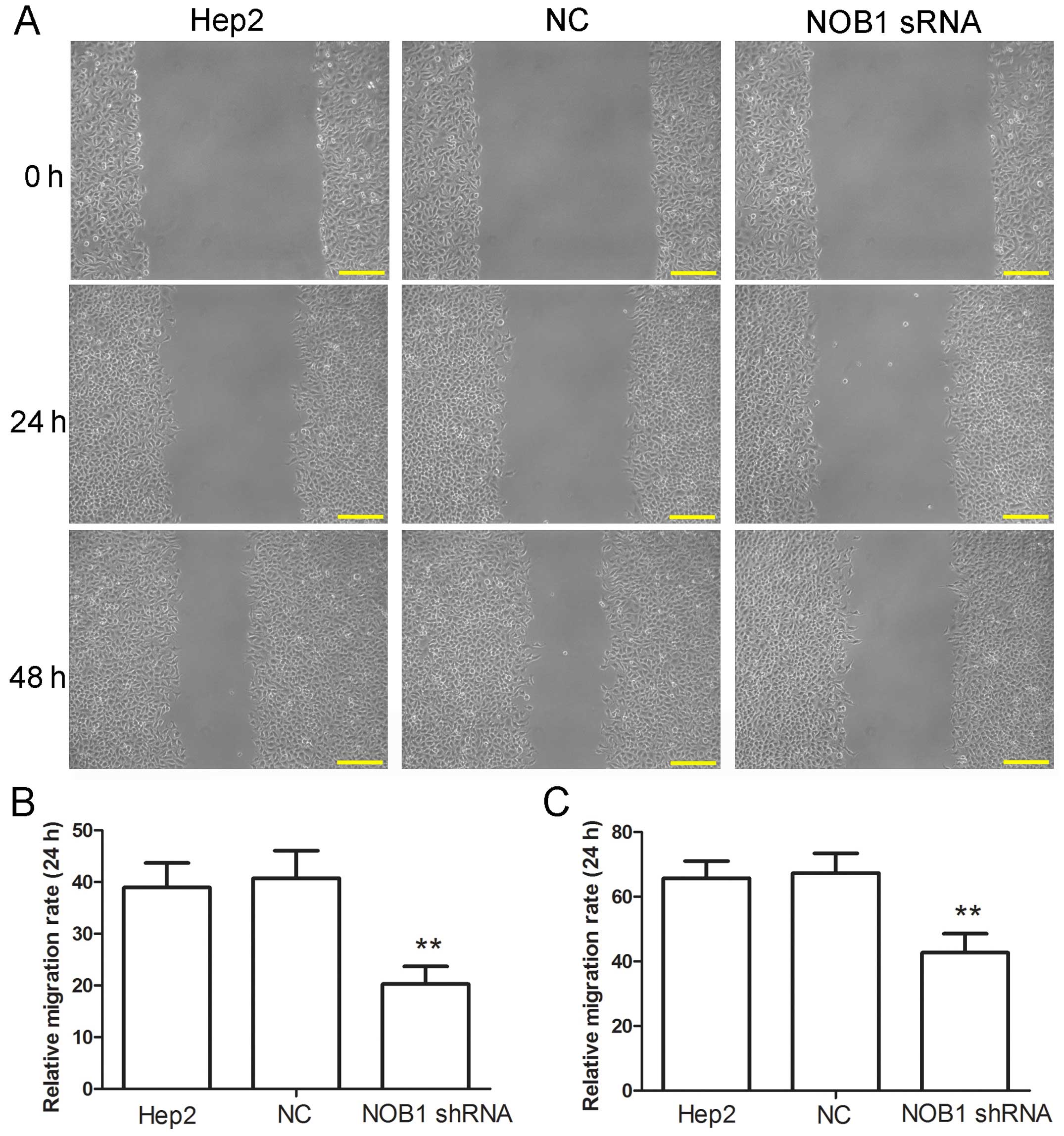
The effect of NOB1 shRNA on migration of laryngeal
cancer cells was also explored in the present study. Wound-healing
and Transwell assays were performed to detect the cell migration
capability. Results of wound-healing assay showed that the
migration capability of cells infected with NOB1 shRNA was
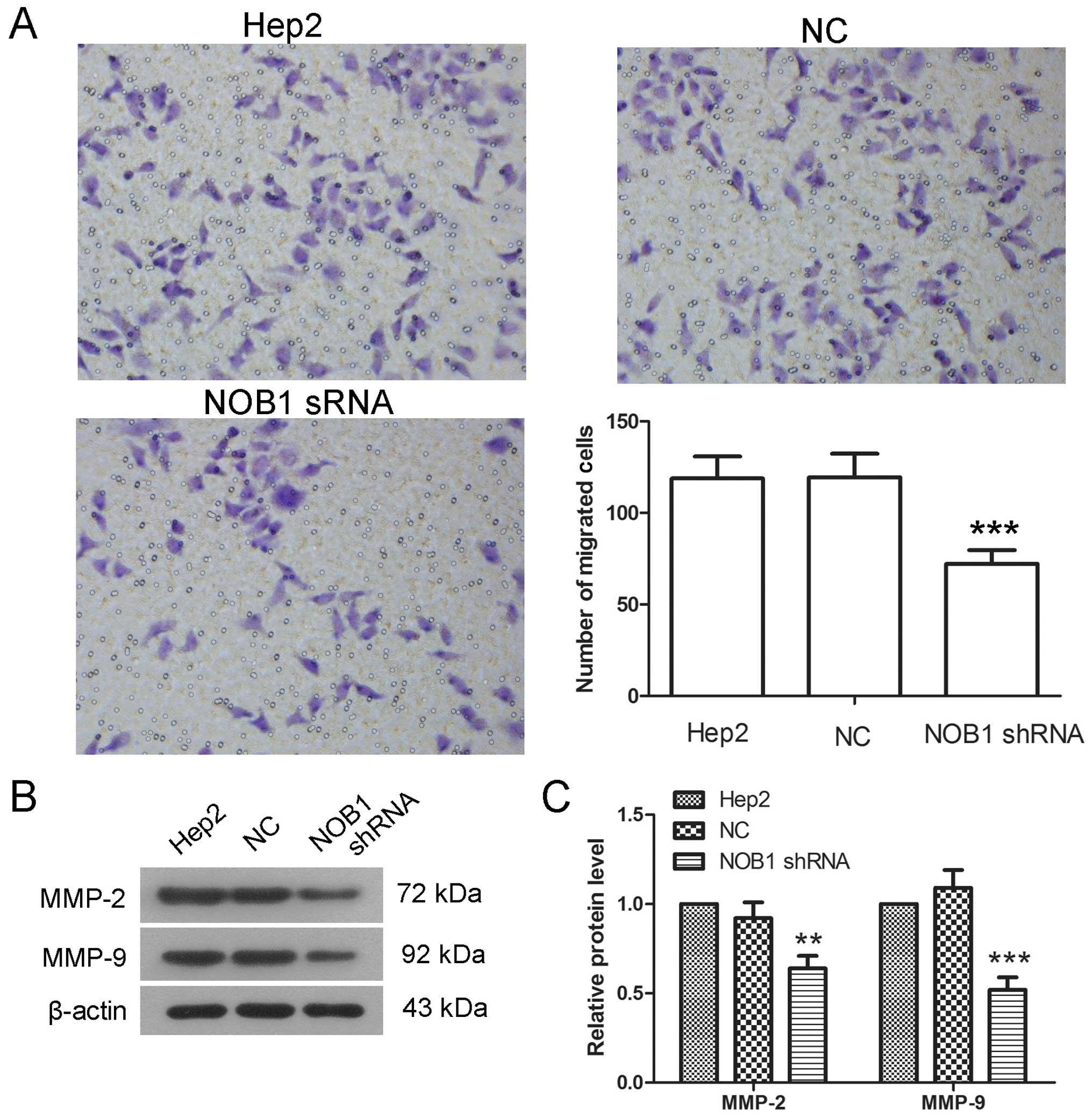
significantly decreased at both 24 and 48 h (Fig. 5). In the results of Transwell assay,
the number of cells passing through the micropore membrane in the
NOB1 shRNA group was 72.2±7.46, significantly lower than of the NC
group (119.4±12.9) (Fig. 6A). MMP-2
and MMP-9 play important roles in the process of cell migration and
were detected by western blotting in the present study. The protein
levels of MMP-2 and MMP-9 were decreased to 64±7 and 52±7%,
respectively, in cells infected with NOB1 shRNA, significantly
lower than that of cells infected with NC (Fig. 6B and C). These results suggest that
downregulation of NOB1 inhibits the migration of laryngeal cancer
cells.
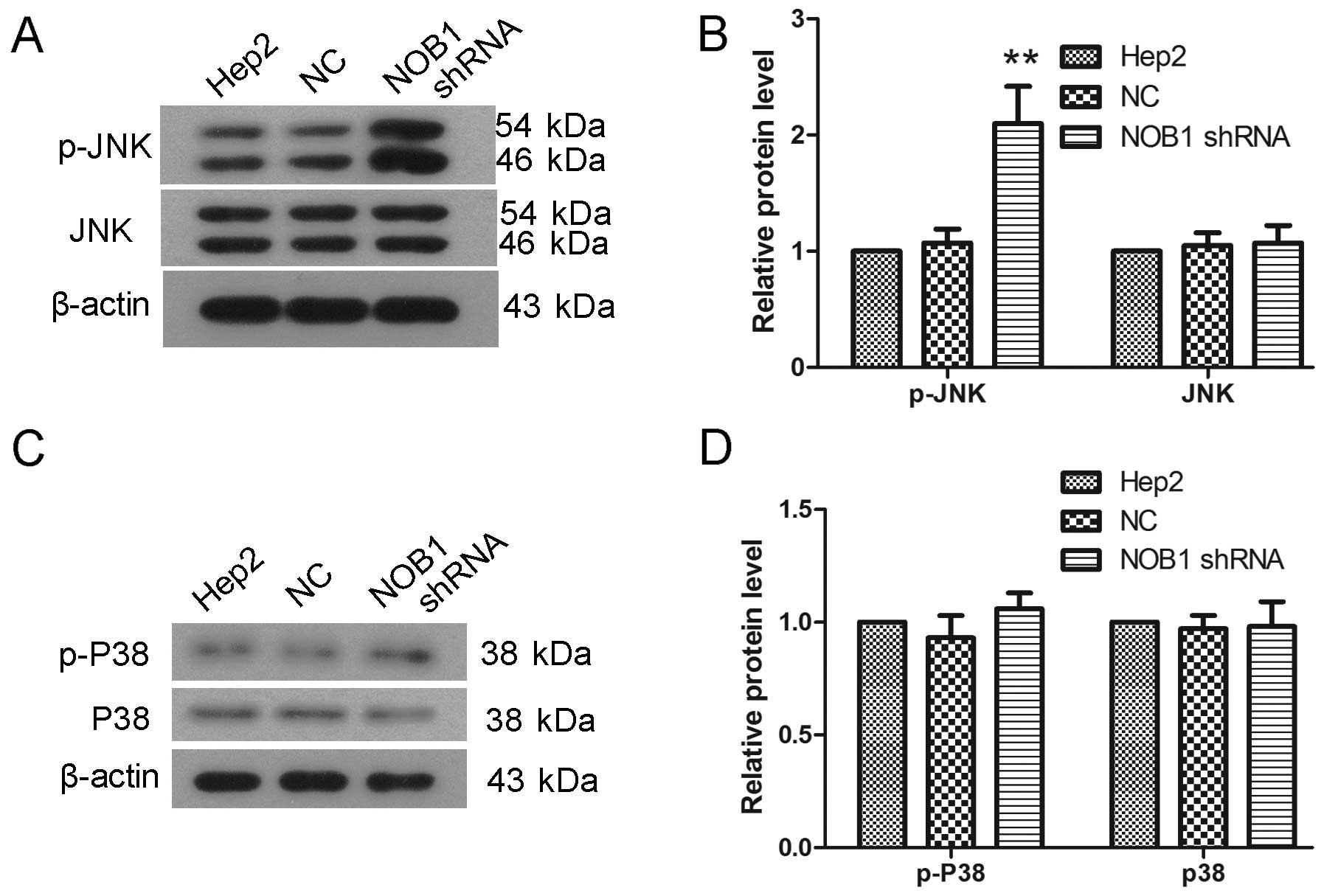
JNK signaling pathway is involved in the
function of NOB1
JNK and P38 signaling pathways play important roles
in various events, such as cell growth and migration. In the
present study, the protein levels JNK, P38, phosphorylated JNK
(p-JNK) and phosphorylated P38 (p-P38) were detected by western
blotting. As shown in Fig. 7, there
was a significant increase in the JNK phosphorylation level after
infection with NOB1 shRNA, leaving no significant changes in
protein level of JNK (Fig. 7A and
B; p<0.01). After infection with NOB1 shRNA, the P38
phosphorylation level showed a slight increase, but not
significantly (Fig. 7C and D).
These results indicate that JNK signaling pathway may be involved
in function of NOB1.
Discussion
In the present study, we found the expression level
of NOB1 in laryngeal cancer patients was high and we further
explored the function of NOB1 on proliferation, apoptosis and
migration of laryngeal cancer cells. Silence of NOB1 was found to
inhibit the proliferation of laryngeal cancer cells, arrest cell
cycle process and induce apoptosis. NOB1 silence also inhibited the
migration and invasion of laryngeal cancer cells. Further mechanism
study showed that the JNK was activated after infection with NOB1
shRNA, which indicates that the function of NOB1 on proliferation
and migration of laryngeal cancer cells may be associated with JNK
signaling pathway.
In the process of tumorigenesis, the expression of
multiple genes has been shown out of control. NOB1 shows high level
in breast (10) and prostate cancer
(11), and is associate with these
cancers (11,13). In the present study, clinical
detection showed that the expression level of NOB1 in laryngeal
cancer was high. This indicates that NOB1 may be associated with
tumorigenesis of laryngeal cancer.
Dysregulation of cell growth is a characteristic of
cancer. In the present study, we found that silencing of NOB1
inhibited the growth and colony formation capability of laryngeal
cancer cells. NOB1 was reported to influence the growth of breast
(13), ovarian (15), colon (14), prostate (11) and renal cancer (17), which was consistent with the results
of the present study. Cell cycle is an important event in the
process of cell growth, and in the present study, NOB1 showed a
regulatory role in the cell cycle process of laryngeal cancer
cells. Knockdown of NOB1 was also reported to have influence on the
expression of cell cycle-related genes, such as cyclin and CDKs
(18), and induce cell cycle arrest
at G0/G1 phase (11,13–15,17–19).
Apoptosis is also an important event that has
influence on cell growth. Results of the present study showed that
NOB1 had an anti-apoptosis function in laryngeal cancer cells.
Consistent with the present study, downregulation of NOB1 was
reported to induce cell apoptosis in colon (20) and lung cancer (21). The ratio of Bax and Bcl-2 is
associated with the opening of mitochondrion permeability
transition pore and the release of cytochrome c which
activates caspase-9 and induces cell apoptosis. In the present
study, the expression of Bax and Bcl-2 was also found to be
regulated by NOB1 silence. These results prompted us to clarify
whether the effect of NOB1 silencing on apoptosis is associated
with the mitochondria-mediated apoptosis. Cell apoptosis is an
important way through which radiotherapy and chemotherapy kill
tumor cells. Meng et al reported that silence of NOB1
enhanced the sensitivity of tumor cells to radiotherapy (22) and Liu et al also showed that
silence of NOB1 boosted the anticancer activity of
chemotherapeutics drugs (23). The
effects of NOB1 silencing on the proliferation and apoptosis of
cancer cells suggested that NOB1 may be a promising therapeutic
target in the treatment of laryngeal cancer.
Metastasis is a crucial cause of tumor
deterioration. The expression level of NOB1 was reported to be
associated with the mortality of cancer cells (24). Downregulation of NOB1 inhibits the
migration and invasion of glioma (19), prostate cancer (11) and osteosarcoma (25). Consistent with these reports, in the
present study, silencing of NOB1 was found to inhibit the migration
and invasion of laryngeal cancer cells. MMPs are crucial enzymes
that degrade extracellular matrix and contribute to the migration
and invasion of cells. Downregulation of MMP-2 and MMP-9 by NOB1
shRNA was also observed in the present study. Silence of NOB1 was
reported to increase the level of E-cadherin (25), which suggests the potential
regulatory role of NOB1 in EMT process.
JNK is a member of the mitogen-activated protein
kinases (MAPKs) which play important roles in cell proliferation,
migration and differentiation. JNK is activated by various stimuli
leading to contradictory responses (26), JNK phosphorylates the anti-apoptosis
Bcl-2 to promote apoptosis of cells (27) or phosphorylates the pro-apoptotic
BAD to inhibit apoptosis (28). In
laryngeal cancer cells, JNK plays a pro-apoptotic role (29). In the present study, we found that
the NOB1 silence induced the activation of JNK and this indicated
that JNK signaling may be involved in the function of NOB1. The
activation of P38 was also detected in the present study, but no
significant change was found after infection with NOB1 shRNA. These
results indicate that P38 signaling may be not involved in the
function of NOB1, at least in laryngeal cancer cells. However, Che
et al showed that the expression level of NOB1 was
associated with the activation of P38, and NOB1 silencing inhibited
the expression of P38 in prostate carcinoma (24).
In the present study, silence of NOB1 was found to
inhibit the proliferation and migration of laryngeal cancer cells,
arrest cell cycle and induce apoptosis, and these functions of NOB1
may be associated with the JNK signaling pathway. NOB1, which is
associated with the proliferation, apoptosis and migration of
laryngeal cancer cells, may be a promising therapeutic target in
the treatment of laryngeal cancer, and it may also show potential
as a clinical marker of laryngeal cancer.
Acknowledgments
The present study was supported by grants from the
Natural Science Foundation of Liaoning Province (no. 20092135).
Ethical approval was given by the Medical Ethics Committee of
Shengjing Hospital of China Medical University with the reference
no. 2014PS17K.
References
|
1
|
Morshed K, Polz-Dacewicz M, Szymański M
and Polz D: Short-fragment PCR assay for highly sensitive
broad-spectrum detection of human papillomaviruses in laryngeal
squamous cell carcinoma and normal mucosa: Clinico-pathological
evaluation. Eur Arch Otorhinolaryngol. 265(Suppl 1): S89–S96. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jemal A, Siegel R, Ward E, Hao Y, Xu J and
Thun MJ: Cancer statistics, 2009. CA Cancer J Clin. 59:225–249.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ramroth H, Schoeps A, Rudolph E, Dyckhoff
G, Plinkert P, Lippert B, Feist K, Delank KW, Scheuermann K, Baier
G, et al: Factors predicting survival after diagnosis of laryngeal
cancer. Oral Oncol. 47:1154–1158. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhang Y, Ni J, Zhou G, Yuan J, Ren W, Shan
Y, Tang W, Yu L and Zhao S: Cloning, expression and
characterization of the human NOB1 gene. Mol Biol Rep. 32:185–189.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fatica A, Oeffinger M, Dlakić M and
Tollervey D: Nob1p is required for cleavage of the 3′ end of 18S
rRNA. Mol Cell Biol. 23:1798–1807. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fatica A, Tollervey D and Dlakić M: PIN
domain of Nob1p is required for D-site cleavage in 20S pre-rRNA.
RNA. 10:1698–1701. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lamanna AC and Karbstein K: Nob1 binds the
single-stranded cleavage site D at the 3′-end of 18S rRNA with its
PIN domain. Proc Natl Acad Sci USA. 106:14259–14264. 2009.
View Article : Google Scholar
|
|
8
|
Tone Y and Toh-E A: Nob1p is required for
biogenesis of the 26S proteasome and degraded upon its maturation
in Saccharomyces cerevisiae. Genes Dev. 16:3142–3157. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Veith T, Martin R, Wurm JP, Weis BL,
Duchardt-Ferner E, Safferthal C, Hennig R, Mirus O, Bohnsack MT,
Wöhnert J, et al: Structural and functional analysis of the
archaeal endonuclease Nob1. Nucleic Acids Res. 40:3259–3274. 2012.
View Article : Google Scholar :
|
|
10
|
Li XY, Luo QF, Li J, Wei CK, Kong XJ,
Zhang JF and Fang L: Clinical significance of NOB1 expression in
breast infiltrating ductal carcinoma. Int J Clin Exp Pathol.
6:2137–2144. 2013.PubMed/NCBI
|
|
11
|
Zhang X, Zhang D, Qu F, Hong Y, Cao J, Pan
X, Li L, Huang Y, Huang H, Yin L, et al: Knockdown of NOB1
expression inhibits the malignant transformation of human prostate
cancer cells. Mol Cell Biochem. 396:1–8. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu K, Gu MM, Chen HL and You QS: NOB1 in
non-small-cell lung cancer: Expression profile and clinical
significance. Pathol Oncol Res. 20:461–466. 2014. View Article : Google Scholar
|
|
13
|
Huang WY, Chen DH, Ning L and Wang LW:
siRNA mediated silencing of NIN1/RPN12 binding protein 1 homolog
inhibits proliferation and growth of breast cancer cells. Asian Pac
J Cancer Prev. 13:1823–1827. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu Y, Huang H, Yuan B, Zhuang LY, Luo TP
and Zhang Q: Lentivirus-mediated knockdown of NOB1 suppresses the
proliferation of colon cancer cells. Z Gastroenterol. 52:429–435.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lin Y, Peng S, Yu H, Teng H and Cui M:
RNAi-mediated down-regulation of NOB1 suppresses the growth and
colony-formation ability of human ovarian cancer cells. Med Oncol.
29:311–317. 2012. View Article : Google Scholar
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2−ΔΔCt method. Methods.
25:402–408. 2001. View Article : Google Scholar
|
|
17
|
Jia JW, Liu AQ, Wang Y, Zhao F, Jiao LL
and Tan J: Evaluation of NIN/RPN12 binding protein inhibits
proliferation and growth in human renal cancer cells. Tumour Biol.
36:1803–1810. 2015. View Article : Google Scholar
|
|
18
|
Lu Z, Guo Q, Shi A, Xie F and Lu Q:
Downregulation of NIN/RPN12 binding protein inhibit the growth of
human hepatocellular carcinoma cells. Mol Biol Rep. 39:501–507.
2012. View Article : Google Scholar
|
|
19
|
Wang H, Li P and Zhao B: Knockdown of NOB1
expression by RNAi inhibits cellular proliferation and migration in
human gliomas. Gene. 528:146–153. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
He XW, Feng T, Yin QL, Jian YW and Liu T:
NOB1 is essential for the survival of RKO colorectal cancer cells.
World J Gastroenterol. 21:868–877. 2015.PubMed/NCBI
|
|
21
|
Li Y, Ma C, Qian M, Wen Z, Jing H and Qian
D: Downregulation of NOB1 suppresses the proliferation and tumor
growth of non-small cell lung cancer in vitro and in vivo. Oncol
Rep. 31:1271–1276. 2014.PubMed/NCBI
|
|
22
|
Meng W, Wang PS, Liu J, Xue S, Wang GM,
Meng XY and Chen G: Adenovirus-mediated siRNA targeting NOB1
inhibits tumor growth and enhances radiosensitivity of human
papillary thyroid carcinoma in vitro and in vivo. Oncol Rep.
32:2411–2420. 2014.PubMed/NCBI
|
|
23
|
Liu J, Dong BF, Wang PS, Ren PY, Xue S,
Zhang XN, Han Z and Chen G: Silencing NOB1 enhances doxorubicin
antitumor activity of the papillary thyroid carcinoma in vitro and
in vivo. Oncol Rep. 33:1551–1559. 2015.PubMed/NCBI
|
|
24
|
Che JP, Li W, Yan Y, Liu M, Wang GC, Li
QY, Yang B, Yao XD and Zheng JH: Expression and clinical
significance of the nin one binding protein and p38 MAPK in
prostate carcinoma. Int J Clin Exp Pathol. 6:2300–2311.
2013.PubMed/NCBI
|
|
25
|
Chen B, Liu J, Wu D, Qin Y, Peng C, Li C
and Wang J: Gene silencing of NOB1 by lentivirus suppresses growth
and migration of human osteosarcoma cells. Mol Med Rep.
9:2173–2179. 2014.PubMed/NCBI
|
|
26
|
Bode AM and Dong Z: The functional
contrariety of JNK. Mol Carcinog. 46:591–598. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Maundrell K, Antonsson B, Magnenat E,
Camps M, Muda M, Chabert C, Gillieron C, Boschert U, Vial-Knecht E,
Martinou JC, et al: Bcl-2 undergoes phosphorylation by c-Jun
N-terminal kinase/stress-activated protein kinases in the presence
of the constitutively active GTP-binding protein Rac1. J Biol Chem.
272:25238–25242. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yu C, Minemoto Y, Zhang J, Liu J, Tang F,
Bui TN, Xiang J and Lin A: JNK suppresses apoptosis via
phosphorylation of the proapoptotic Bcl-2 family protein BAD. Mol
Cell. 13:329–340. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Brahim S, Aroui S, Abid K and Kenani A:
Involvement of C-jun NH2-terminal kinase and apoptosis
induced factor in apoptosis induced by deglycosylated bleomycin in
laryngeal carcinoma cells. Cell Biol Int. 33:964–970. 2009.
View Article : Google Scholar : PubMed/NCBI
|