Introduction
Lung cancer is one of the most common malignancies
globally, with 1.6 million new cases being diagnosed annually and
is also the leading cause of cancer deaths worldwide (1). Non-small cell lung cancer (NSCLC)
accounts for more than 80% of all lung cancers accompanied by poor
prognosis (2). Despite recent
treatment advances, immunotherapy in particular, offers promising
treatment alternatives that could help fight disease mortality with
minimal impact on normal tissues (3), the chances of survival of NSCLC remain
bleak, and novel therapeutic approaches are required.
The human homolog of filamentous fungus
Aspergillus nidulans NUDC (nuclear distribution C), called
hNUDC, is structurally based on the similarity of its C-terminus to
that of the fungal NUDC from Aspergillus nidulans forms
(4). NUDC is necessary for nuclei
movement following mitosis as well as normal colony growth, which
is highly conserved structurally and functionally throughout most
evolution (5). The hNUDC has been
reported to be involved in mitosis, cytokinesis (6), neuronal migration (7) and hematopoietic cell growth in humans
(5). However, the hNUDC function in
NSCLC cells has not yet been investigated.
Thrombopoietin receptor (Mpl) is a class I cytokine
receptor, belonging to the hematopoietic super family of receptors.
hNUDC is confirmed to be the second natural ligand for Mpl (after
thrombopoietin) and binds to the extracellular domain of the Mpl
(8), thus, inducing a sustained
activation of the extracellular signal-regulated protein kinases-1
and -2 (ERK1/2), resulting in megakaryocytic proliferation and
differentiation (9). The
extracellular signal regulated kinase (ERK1/2), also called the
mitogen-activated protein kinase (MAPK), reportedly promotes cell
survival and chemotherapeutic resistance in NSCLC cell lines
(10). ERK signaling pathway was
suggested to play a role in the hNUDC overexpression-induced
apoptosis (11). Other reports
recorded that nuclear and cytoplasmic ERK1/2 activation positively
correlated with advanced and aggressive NSCLC tumors (12). These reports suggested that hNUDC
may affect the ERK1/2 pathway and regulate the processing of NSCLC
cells.
miRNAs have been identified as classical oncogenes
or tumor suppressor genes (13,14).
In lung cancer, miR-let-7c (15),
miR-506 (16) and miR-34a (17) have been identified as tumor
suppressors, whereas miR-21 (18),
miR-155 (19) and miR-31 (20) were found to be carcinogenesis
promoters. Mature miR-194 is involved in both pri-miR-194-2/192 and
pri-miR-194-1/215 clusters (14),
and has been suggested to be a putative tumor suppressor in liver
(21) and ovarian tissues (22). The miR-194 overexpression in these
cancer cells suppresses cell migration, invasion and metastasis.
The overexpression of the miR-194 in lung cancer cell lines has
also been reported to suppress metastasis of lung cancer cells
(14).
This study helped to identify miR-194 action in the
context of non-small cell lung cancer. We first found hNUDC
overexpression in NSCLC cell lines and NSCLC patients when compared
to healthy controls. Besides, miR-194 was predicted to target
hNUDC, which regulated the ERK1/2 pathway. Taken together, our
results suggest that miR-194 may provide novel insight into the
process of NSCLC via targeting hNUDC.
Materials and methods
Patients
Twenty-six patients (12 males and 14 females) with
non-small cell lung cancer and paired non-tumor lung tissues were
consecutively included in this study. Both tumor and non-tumor
samples were confirmed by the pathological examinations. Patients
were recruited from the Respiratory Department of Cangzhou Central
Hospital. The study was approved by the ethics committee of our
institution. Informed consent was signed by the participants.
Cell culture
First, 95C and 95D cells were subcloned from the
PLA-801 human giant-cell lung carcinoma cell line, but they had
different metastatic potentials (23). Human non-small cell lung cancer
cells HCC827, A549, NCI-H460 and human lung fibroblast (NHLF) cell
lines obtained from the American Type Culture Collection (ATCC;
Manassas, VA, USA), were cultivated in modified Eagle's medium
(MEM; Invitrogen, Carlsbad, CA, USA), supplemented with 20% fetal
bovine serum (FBS; Invitrogen) and 1% antibiotic-antimycotic
(Invitrogen), while the NSCLC cell lines 95C and 95D were grown in
MEM supplemented with 10% FBS and 1% antibiotic-antimycotic. All
the cells were incubated at 37°C in a humidified 21% O2,
5% CO2 atmosphere.
qRT-PCR
The total RNA was extracted from TRIzol reagent
(Invitrogen) following the manufacturer's instructions. For miR-194
detection, reverse transcription was performed using One Step
PrimeScript miRNA cDNA Synthesis kit (Takara, Dalian, China),
following the primers: 5′-UGUAACAGCA ACUCCAUGUGGA-3′ (sense);
common antisense primer, 5′-GACTGTTCCTCTCTTCCTC-3′. For mRNA
detection of hNUDC, the cDNA was generated using M-MLV reverse
transcriptase (Clontech Laboratories, Palo Alto, CA, USA), and
amplified following the primers: 5′-AGACCTGCCCAA TTACCGC-3′
(sense); 5′-GCTCCCCATCAATGATCGCT-3′ (antisense). To analyze the
gene expression, the qRT-PCR mixture system containing the cDNA
templates, primers and SYBR-Green qPCR Master Mix were subjected to
qRT-PCR quantification according to the standard methods. β-actin
and U6 SnRNA were used as the internal control of the mRNA or
miRNA, respectively. The human β-actin primers: 5′-GAT
CATGTTTGAGACCTTC-3′ (sense); 5′-GGCATACCCCTCG TAGATG-3′
(antisense), the U6 primers: 5′-GCTTCGGCAG CACATATACTAAAAT-3′
(sense); 5′-CGCTTCACGAATTT GCGTGTCAT-3′ (antisense). Relative gene
expression was quantified by 2−ΔΔCt method.
Western blot analysis
A total of 25 µg proteins were loaded and
separated via sodium dodecyl sulfate-polyacrylamide gel
electrophoresis and then electrotransferred to nitrocellulose
membranes (Amersham, Little Chalfont, UK). The membranes were then
blocked in 2.5% non-fat milk for 1 h at 37°C. After washing with
Tris-buffered saline with Tween, the membranes were incubated with
primary antibodies against Mpl, ERK1/2, c-myc, β-actin (Santa Cruz
Biotechnology, Santa Cruz, CA, USA) and p-CRBE (Cell Signaling
Technology, Danvers, MA, USA) at 4°C overnight. Then, the
peroxidase-conjugated secondary antibody (Wuhan Boster Biological
Technology, Ltd., Wuhan, China) diluted in 1:1,000 was added and
incubated for 1 h at room temperature. The immunoreactive protein
bands were then visualized using an enhanced chemiluminescence
detection system (Amersham).
Dual-luciferase reporter assay
The target gene was predicted by TargetScan
(http://www.targetscan.org/). The 3′-UTR
fragment of hNUDC mRNA containing the target sequence (CUGUUAC) of
miR-194 was amplified by RT-PCR. The fragment, designated hNUDC
3′-UTR, was inserted into the pMIR-REPORT™ luciferase reporter
vector (MluI and HindIII restriction enzyme sites;
Ambion, Austin, TX, USA). Another expressing vector was also
constructed by the insertion of a mutated hNUDC 3′-UTR in which the
target sequence of miR-194 was mutated into CUGUAAC using the
QuickChange Site-Directed Mutagenesis kit (Stratagene, Santa Clara,
CA, USA). The recombinant reporter vectors with normal and mutated
hNUDC 3′-UTR were co-transfected with miR-194 into 95D cells using
the TransMessenger™ Transfection reagent (Qiagen, Hilden, Germany).
The luciferase assay was performed according to the manufacturer′s
protocol. The relative luciferase activities were normalized to
that of the control cells.
Transfection assay
miRNA mimics and inhibitors, specific for miR-194
(Invitrogen), were used to increase and silence miR-194 expression
in 95C and 95D cell lines, respectively. Two hundred pmoles of
miR-194 mimics, miR-194 mimic control, miR-194 inhibitor, and
inhibitor control (Ambion) were transfected into 3×106
95C and 95D cells for 48 h by electroporation using a Nucleofector
instrument, respectively. After transfection, the cells were
allowed to recover by incubating them for 4 h at 37°C. The
experiment was replicated thrice for data calculations.
Giemsa staining
For Giemsa staining, incubation of the
1×104 95C and 95D cells was done on 35 mm of the cell
petri dishes with a coverslip in each dish. After 48 h, the
coverslips were immobilized using 100% methyl alcohol and then air
dried. The cells were then treated with Giemsa staining solution
and the cellular morphology was microscopically observed (Leica
AF6000; Leica Microsystems, Wetzlar, Germany).
MTT assay
Cell viability was assessed using
3-(4,5-dimeth-ylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay. Shortly afterwards, cells were transfected according to the
above description and were seeded in 96-well plates at
6×103 cells/well. The surviving fractions were
determined at 0, 24, 48, 72, 96 and 120 h. Thereafter, the old
medium was discarded and fresh medium containing MTT (5 mg/ml MTT
in PBS; Shanghai Sangon Biological Engineering Technology,
Shanghai, China) was added and incubated for an additional 4 h.
Then, cell viability was measured with a spectrophotometer (Bio-Rad
Laboratories, Hercules, CA, USA) at 470 nm. Each experiment was
performed in triplicate.
Cell cycle analysis
Cell cycle analysis was determined by flow cytometry
(BD Biosciences, San Jose, CA, USA). In short, 95C and 95D cells at
1×106 cells/well were cultured in 6-well plates and
transfected with 50 nM of the miR-194 mimics, miR-194 inhibitor or
their respective control RNA for 48 h. The cells were then
harvested and fixed in 70% ice-cold ethanol for 24 h, followed by
staining with propidium iodide (PI). The different cell cycle
phases were analyzed with the FACSCalibur instrument using
CellQuest software (Becton-Dickinson, Mountain View, CA, USA).
hNUDC overexpression
The hNUDC overexpression was achieved by PCR
amplification using hNUDC cDNA as a template, and the hNUDC
expressing vector was constructed by inserting the hNUDC cDNA into
pcDNA 3.1 vector. The recombinant plasmid and other agents were
co-transfected into 3×106 95D cells using a Nucleofector
instrument. Forty-eight hours later, subsequent experiments were
performed on the cells. The experiment was replicated thrice for
data calculations.
NSCLC xenografts
Nine NOD/SCID mice (Jackson Laboratory, Bar Harbor,
ME, USA) (male; body weight, 20–22 g; age, 8 weeks old) were
purchased from the Institute of Zoology, Chinese Academy of Medical
Sciences. 95D cells were transfected with miR-194 or negative
control miRNA (NC pre-miR™; Ambion) following same transfection
conditions. Cells (5×106) 95D transfected with miR-194
were injected subcutaneously into the right flank of NOD/SCID mice
(n=9). Cells transfected with negative control miRNA were injected
into the left flank of NOD/SCID mice (n=9). The tumor volumes were
measured daily after the injection, and all the rats were assigned
to euthanasia at the end of measurements (on day 27). All animal
experiments were performed in accordance with current prescribed
guidelines and under a protocol approved by the Institutional
Animal Care and Use Committee.
Statistical analysis
All results are presented as mean ± SD. Statistical
analysis was carried out using one-way analysis of variance (ANOVA)
followed by Bonferroni test. The difference was considered
statistically significant at P<0.05.
Results
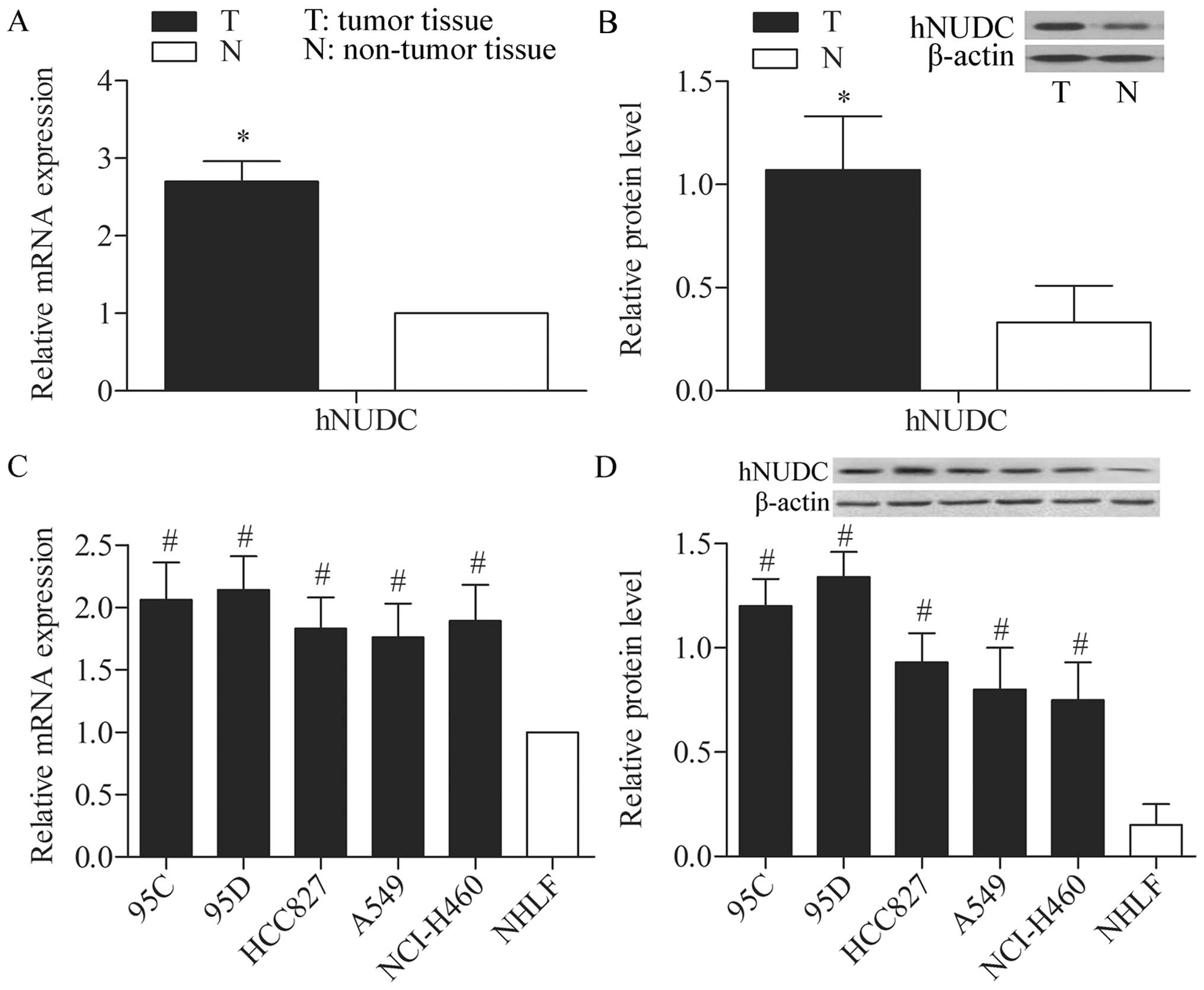
Inverse level of hNUDC in NSCLC and
adjacent non-tumor lung tissues
The level of hNUDC expression was detected in NSCLC
and adjacent non-tumor lung tissues via qPCR and western blot
analysis. The results indicated that the level of hNUDC expression
was significantly higher in tumor tissues compared with matched
non-tumor tissues (P<0.05) (Fig. 1A
and B). The level of hNUDC expression was also detected in 95C,
95D, HCC827, A549, NCI-H460 and NHLF cell lines by qPCR. The
results indicated that the mRNA expression level of the hNUDC was
significantly higher in the NSCLC cells (95C, 95D, HCC827, A549 and
NCI-H460) compared with NHLF cells (P<0.05) (Fig. 1C). The levels of the hNUDC protein
expression in the three cell lines examined by western blot
analysis were consistent with the mRNA expression levels (Fig. 1D). The results suggested that the
levels of hNUDC expression were significantly elevated in the NSCLC
patients and NSCLC cells compared with normal control.
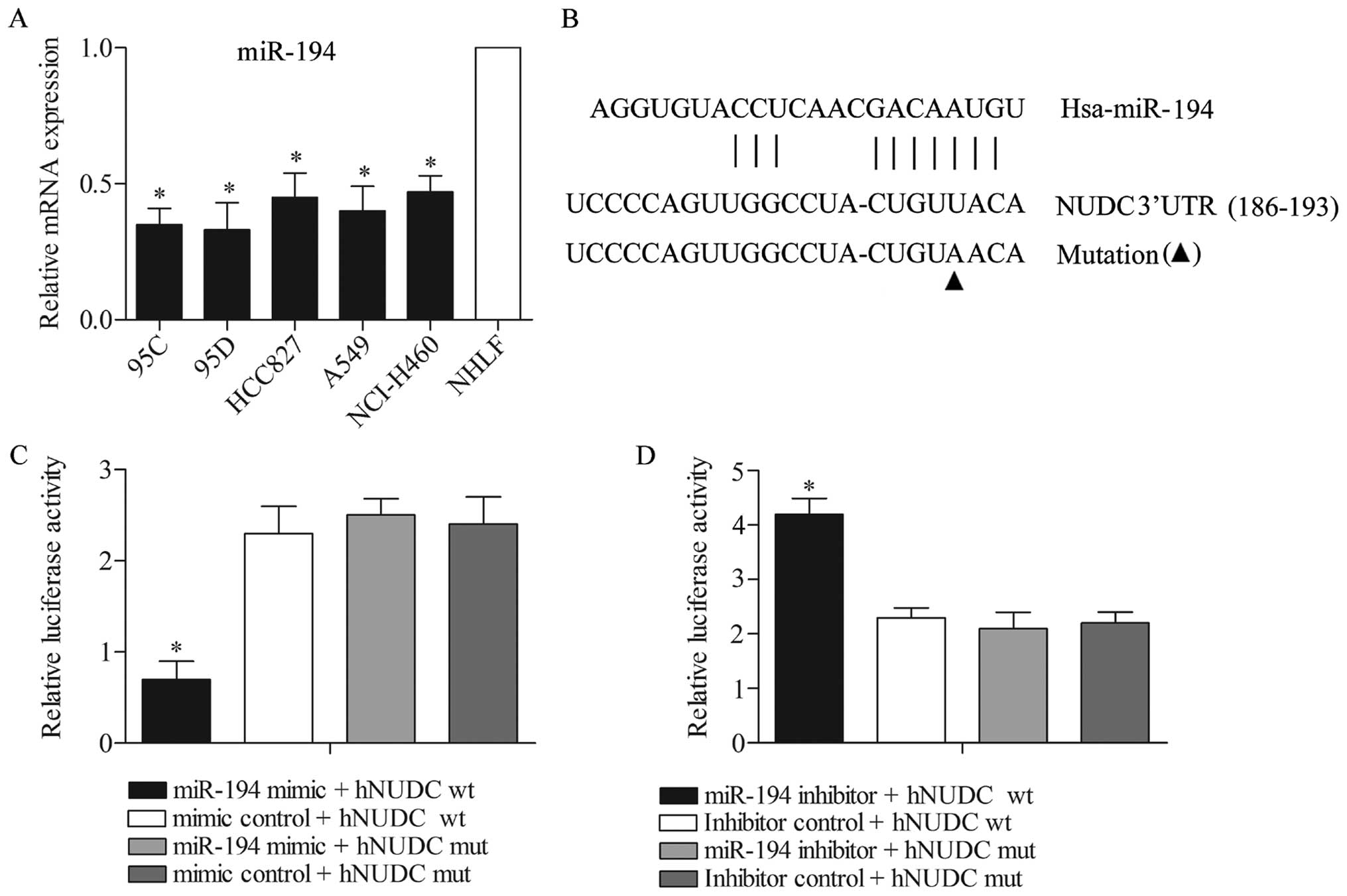
hNUDC is targeted by miR-194
Assuming that the hNUDC level was increased in the
NSCLC cell lines (especially in 95D), in order to obtain the
biological role of hNUDC in the NSCLC cells, it was of interest to
identify the microRNA that could target hNUDC. Predicted by the
bioinformatic software programs TargetScan, we found that hNUDC was
targeted by miR-194. The level of miR-194 expression was detected
in 95C, 95D, HCC827, A549, NCI-H460 and NHLF cell lines by qPCR.
The results indicated that the mRNA level of miR-194 expression was
significantly lower in NSCLC cells (95C, 95D, HCC827, A549 and
NCI-H460) compared with NHLF cells (P<0.05) (Fig. 2A). The potential binding target
sites of miR-194 were found in the 3′-UTR of hNUDC gene (Fig. 2B). To experimentally confirm that
hNUDC was an authentic target of miR-194 in the 95D cells, the
plasmid pMIR-REPORT-hNUDC-wt or pMIR-REPORT-hNUDC-mut was
transfected into 95D cells together with miR-194 mimics or mimic
control. After 48 h of transfection, the results showed that the
luciferase activity in the hNUDC-wt with miR-194 mimics
transfection group was significantly reduced compared with the
other three groups (Fig. 2C).
Consistently, the luciferase reporter vectors of the hNUDC-wt and
hNUDC-mut were co-transfected with the miR-194 inhibitors or
inhibitor controls into 95D cells. The results showed that the
luciferase activity in the hNUDC-wt with miR-194 inhibitor
co-transfection group was increased significantly compared with the
other three groups (Fig. 2D). The
data mentioned above demonstrated that hNUDC is a genuine target of
miR-194.
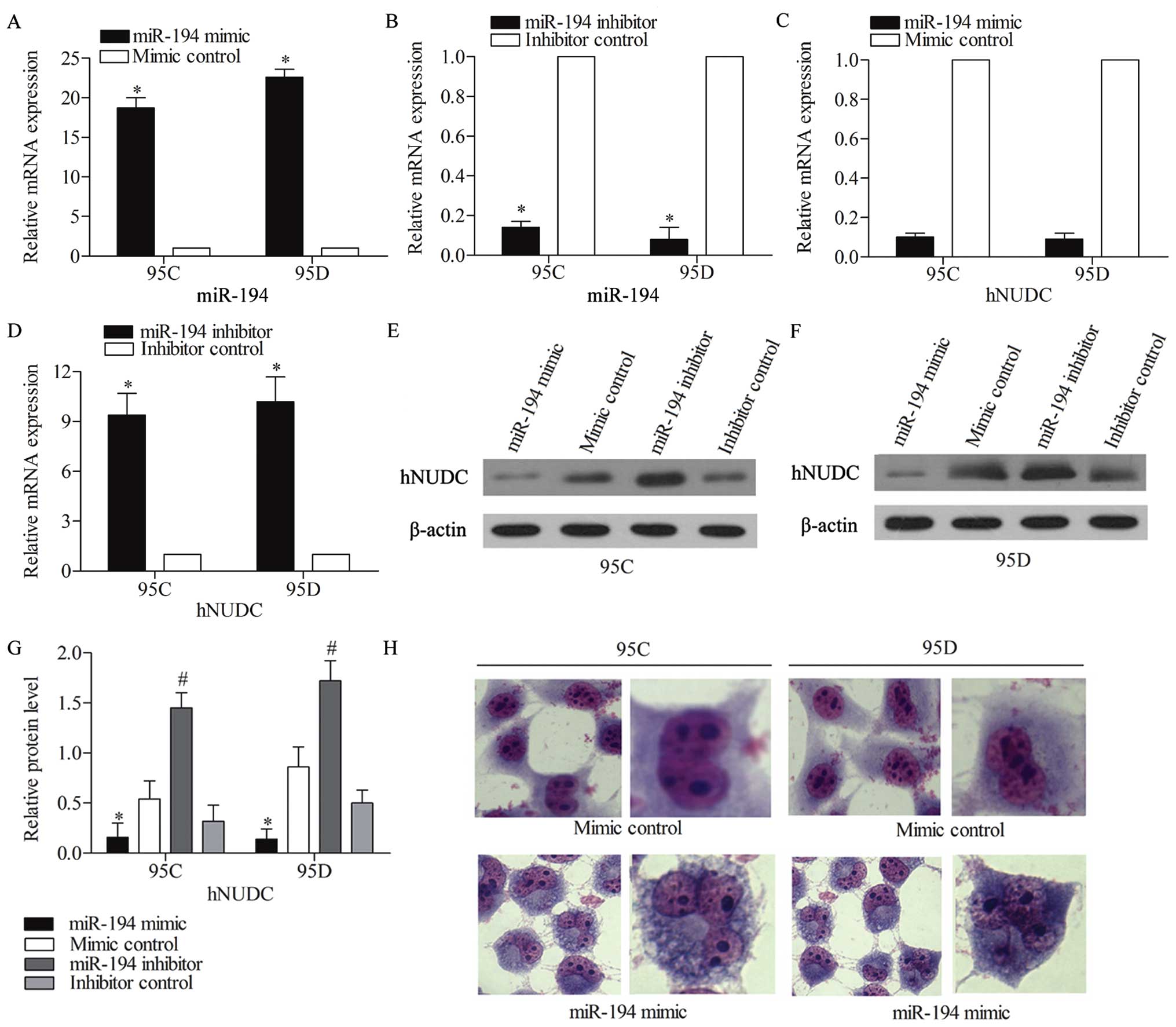
miR-194 causes abnormal mitosis of NSCLC
cells via targeting Hnudc
As hNUDC is associated with human nuclear migration,
we examined whether the overexpression or inhibition of miR-194 was
capable of affecting cell mitosis by targeting hNUDC. miR-194 mimic
was used to amplify the miR-194 expression, whereas a synthetic
inhibitor specific for miR-194 was employed to suppress the
expression of endogenous miR-194 in NSCLC cell lines. The
efficiency of this miR-194 mimic or inhibitor was confirmed by qPCR
assay (Fig. 3A and B), and 95C and
95D cells were transfected with miR-194 mimic, mimic control,
miR-194 inhibitor and inhibitor control, separately, while the mRNA
and protein level of hNUDC expression were examined by qRT-PCR
(Fig. 3C and D) and western blot
analysis (Fig. 3E and F),
respectively. The quantified relative protein expression is
summarized in Fig. 3G. The results
showed that both the mRNA and protein levels of hNUDC were
significantly downregulated when treated with miR-194 mimic, and
increased when treated with miR-194 inhibitor compared with control
groups. The Giemsa staining assay showed the nucleus was divided
into two in the mimic control group in 95C and 95D cells. However,
the nucleus exhibited abnormal division due to the overexpression
of miR-194, three nuclei were detected within one cell in 95C
cells, and sometimes even four (Fig.
3H). These results indicated that miR-194 affected the NSCLC
cell mitosis via regulating the expression of hNUDC.
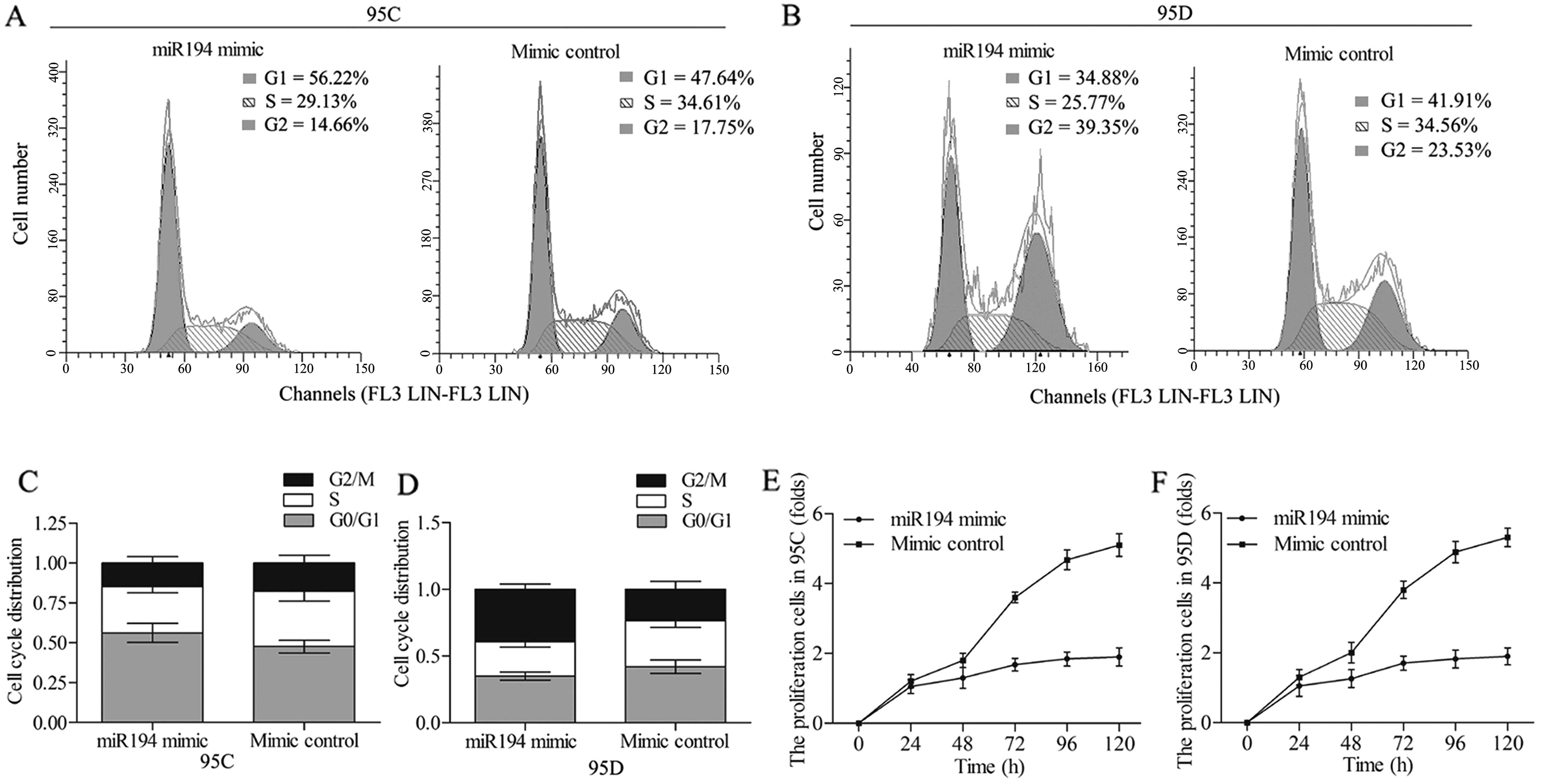
The effect of miR-194 on the cell cycle
and proliferation of 95C and 95D cells
To further investigate the impact of miR-194 on the
NSCLC cells, we studied whether the miR-194 overexpression was
capable of affecting cell cycle and proliferation. The 95C and 95D
cells were transfected with miR-194 mimic and mimic control
separately. The analysis of the cell cycle indicated that miR-194
overexpression induced an accumulation of 95C cells in G0/G1 phase
(Fig. 4A), and an accumulation of
95D cells in G2/M phase compared with mimic control (Fig. 4B), implying a cell cycle arrest in
95C and 95D cells with the change in the miR-194 levels. The cell
cycle distribution in each group in 95C and 95D cells is summarized
in Fig. 4C and D. The cell
proliferation assay was performed in the cell lines, and miR-194
mimic was observed to strongly suppress the 95C cell growth
compared with mimic control group (Fig.
4E). Similar MTT results were obtained in 95D cells, in which
the cell proliferation rate was decreased under the treatment of
miR-194 mimic (Fig. 4F) compared
with mimic control. These findings suggest that miR-194 suppresses
the cell cycle by decelerating the G0/G1 phase in 95C cells and the
G2/M phase in 95D cells, and inhibits cell proliferation in the
NSCLC cells.
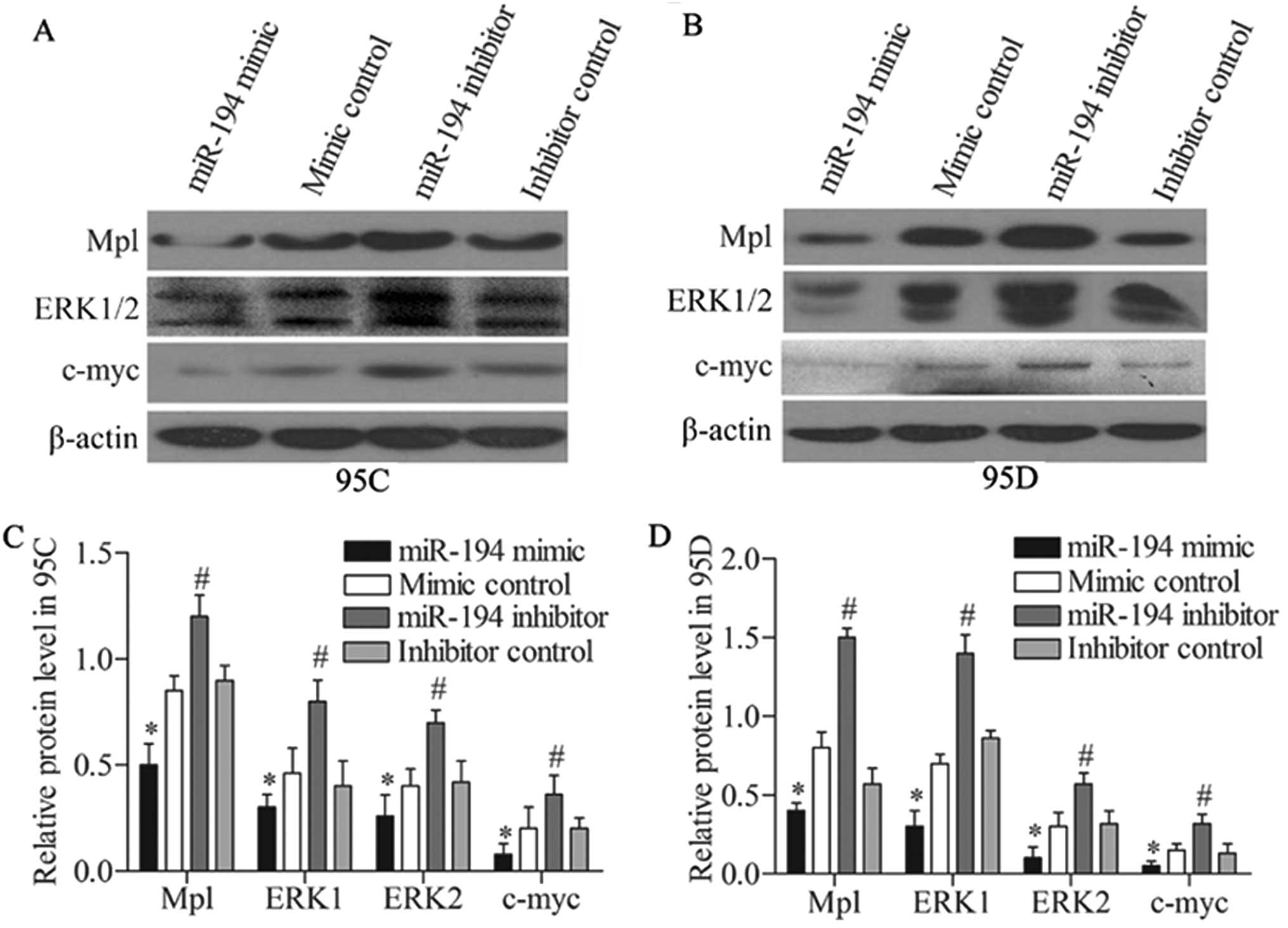
miR-194 inhibits the Mpl/ERK pathway by
targeting hNUDC
hNUDC has been reported to promote cell
proliferation and differentiation via activation of the
thrombopoietin receptor (Mpl) and the extracellular
signal-regulated protein kinases-1 and -2 (ERK1/2) pathway
(9). Thus, we detected the effect
of miR-194 overexpression and the suppression on the expression of
Mpl/ERK pathway, which included Mpl, ERK1/2 and c-myc genes. The
protein expression was detected via western blot analysis. The
results revealed that miR-194 mimic effectively decreased the
expressions of Mpl, ERK1/2 and c-mycin 95C cells compared with
mimic control. Whereas, miR-194 inhibitor promoted the expression
of these genes compared with mimic control (Fig. 5A). Western blotting assay was also
performed in 95D cells, miR-194 mimic intensively restrained the
expressions of Mpl, ERK1/2 and c-myc compared with the mimic
control group. Stronger expression of these genes was detected when
they were subjected to the miR-194 inhibitor treatment compared
with inhibitor control (Fig. 5B).
The quantified relative protein expressions of Mpl/ERK pathway in
95C and 95D cells is summarized in Fig.
5C and D. The data mentioned above suggested that miR-194
restrained the Mpl/ERK pathway in the NSCLC cells.
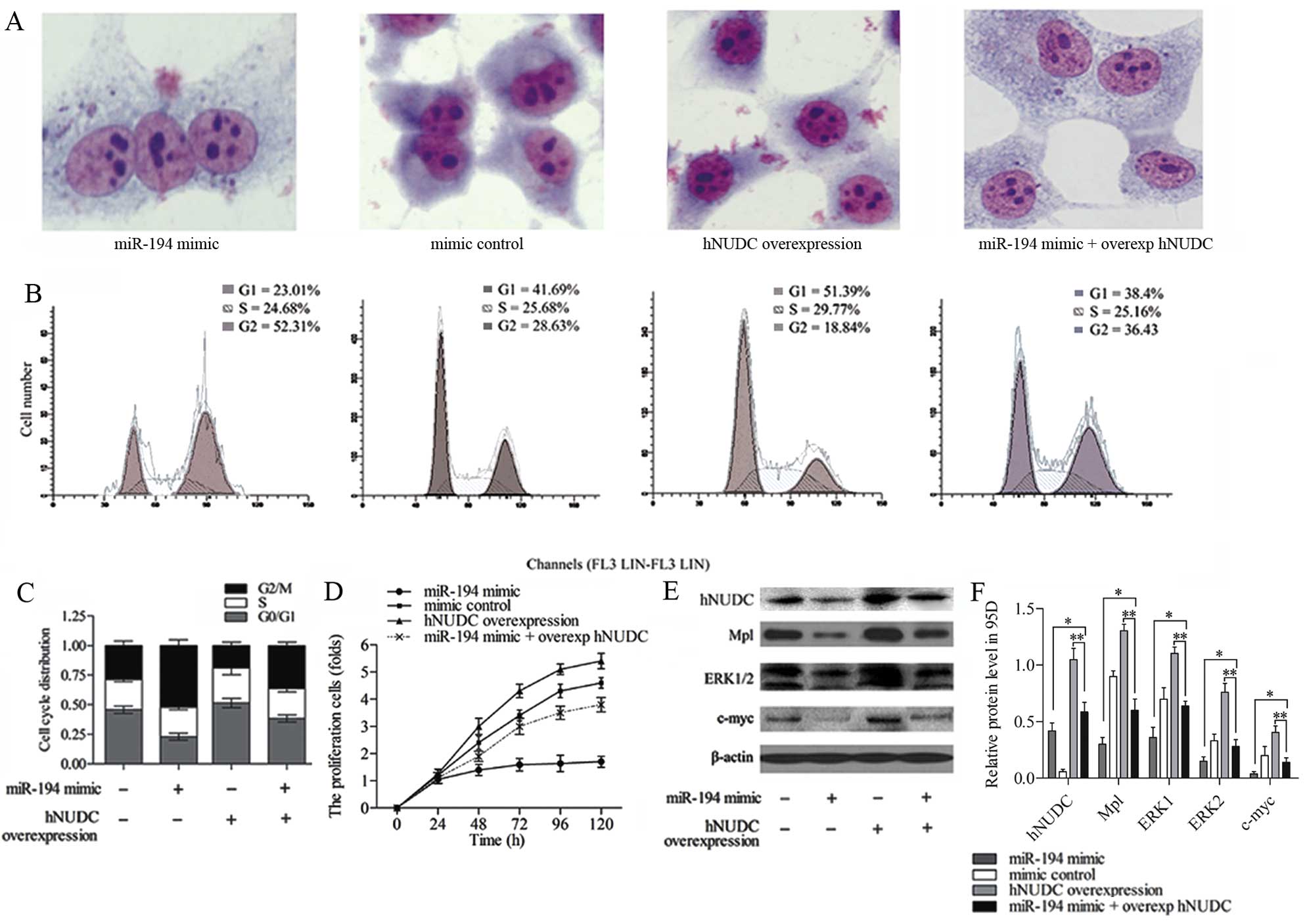
miR-194 suppression of hNUDC is necessary
to influence NSCLC cells and ERK pathway
Given that hNUDC is the target of miR-194, it was of
interest to study whether the hNUDC mediated the effect of miR-194
on the process of NSCLC cells and Mpl/ERK pathway. To determine
whether the overexpression of hNUDC counteracted the effect of
miR-194 in 95D cells, miR-194 mimic or mimic control with or
without the hNUDC overexpression vector were co-transfected into
95D cells. Giemsa staining assay showed the presence of three
nuclei within one cell in the miR-194 mimic group, however, cell
division was found to be normal after hNUDC was overexpressed. This
was because hNUDC is related to nucleus distribution, and the hNUDC
overexpression resulted in an increase in migration ability of the
nucleus. Cells co-transfected with miR-20b mimic plus
overexpression hNUDC vector exhibited relatively abnormal mitosis
compared with overexpression hNUDC group (Fig. 6A). Flow cytometric analysis of the
cell cycle progression demonstrated that the G2/M arrest in 95D
cells could be obviously detected in the miR-194 mimic group. After
co-transfecting the miR-194 mimic and overexpression of hNUDC in
95D cells, the G2/M phase was extended compared with overexpression
hNUDC group (Fig. 6B). The cell
cycle distribution in each group in 95D cells is summarized in
Fig. 6C. The results indicated that
the inhibition effect of the cell cycle by miR-194 mimic could be
reversed by the hNUDC overexpression.
To further confirm the enhanced expression of hNUDC
counteracting the effect of miR-194 mimic in 95D cells, MTT assay
indicated that the increasing hNUDC and miR-194 levels could
suppress the proliferation rates of 95D cells treated with
overexpression of hNUDC alone (Fig.
6D). Western blotting was used to measure the expression of
hNUDC, Mpl, ERK1/2 and c-myc (Fig.
6E), and the brands were quantified in Fig. 6F. In the miR-194 mimic plus
overexpression hNUDC group, the protein expression of hNUDC, Mpl,
ERK1/2 and c-myc was strongly decreased compared with the
overexpression hNUDC group (P<0.01), and increased when compared
with the miR-194 mimic group (P<0.05). The results illustrated
that the hNUDC overexpression was able to offset the effect of
miR-194 inhibition on Mpl/ERK pathway.
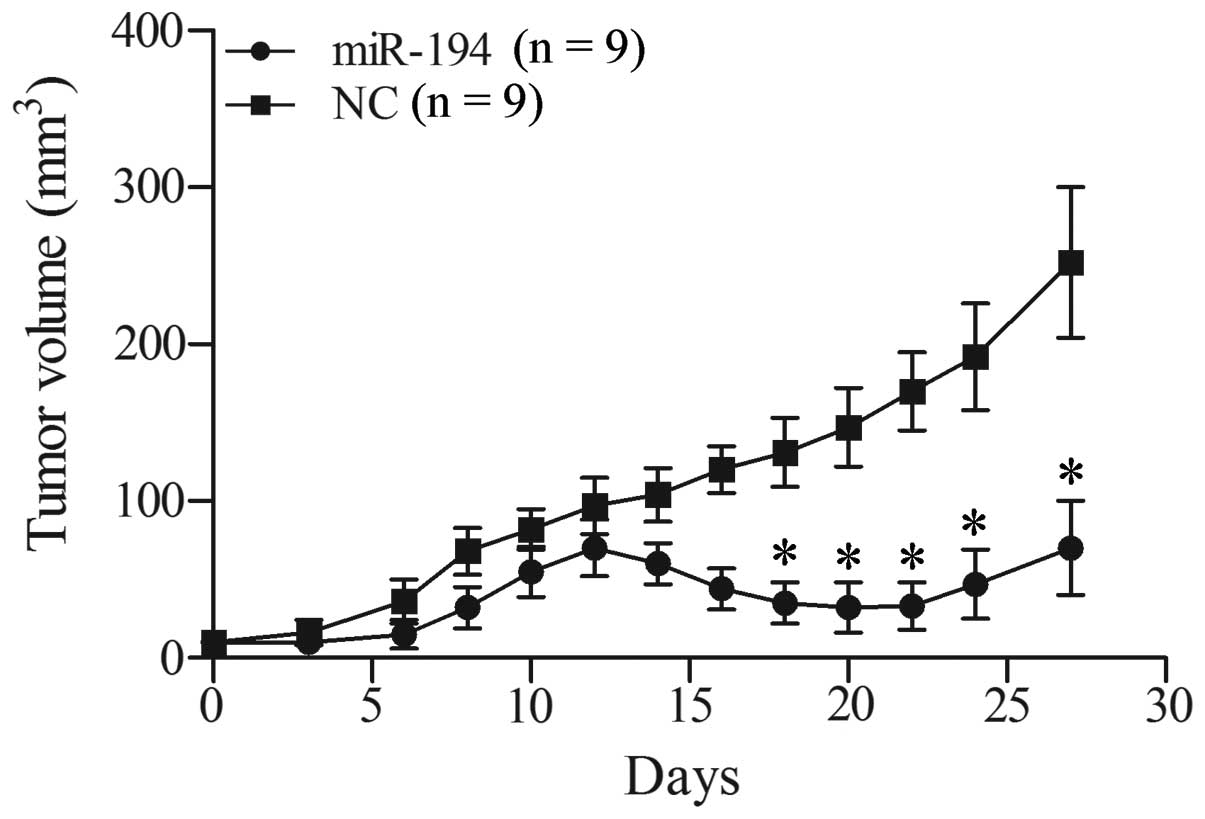
miR-194 reduces tumor growth in xenograft
models of lung cancer
To further explore the tumor suppressor effect of
miR-195, we assessed tumor growth of xenografts that were
transfected with miR-194 or negative control miRNA was subcutaneous
injected into NOD/SCID mice. As shown in Fig. 7, miR-194 inhibited tumor growth of
the 95D xenograft. At early time-points, tumors that developed from
miR-194-treated cells were smaller compared with their control
tumors. At later time-points, tumor growth was resumed but still
significantly reduced compared with the control group. The results
in vivo demonstrated the inhibition effect of miR-194 acting
towards cell growth of NSCLC.
Discussion
NSCLC is the leading cause of cancer death. The
hNUDC overexpression has been reported to induce differentiation in
megakaryocytes (11). In the
present study, the hNUDC expression is significantly increased in
the NSCLC tumor tissues and NSCLC cell lines compared with the
matched non-tumor tissues and NHLF cells. To investigate the role
of hNUDC in NSCLC, the non-coding region, 3′-UTR of hNUDC was
studied. miRNAs are non-coding RNAs that can suppress the
expression of the protein-coding genes by binding to the target
sequence at the 3′-UTR region of the target gene (24). In the present study, we predicted
miR-194 is the targeted miRNA for the hNUDC, which may be involved
in the NSCLC pathogenesis.
The strikingly decreased level of miR-194 expression
has been reported in many cancer cell lines. In gastric cancer
(GC), miR-194 was significantly downregulated and RING box protein1
(RBX1) was upregulated in GC tissues, the upregulation of miR-194
can inhibit proliferation and invasion of GC cells, possibly by
targeting RBX1 (25). Moreover, the
overexpression of miR-194 regulates osteoblast differentiation
through modulating STAT1-mediated Runx2 nuclear translocation
(26). Additionally, miR-194
expression was also found to be in a strongly negative association
with metastasis in the clinical specimens of NSCLC, as it
suppressed the metastasis of NSCLC through regulation of the
expression of BMP1 and p27kip1 (14). In the present study, the level of
hNUDC was strongly increased in NSCLC cells compared with NHLF
cells, and the target connection between hNUDC and miR-194 was
predicted by bioinformatic software program TargetScan. Therefore,
we chose miR-194 to be the targeted miRNA for hNUDC. The results
showed that miR-194 decreased significantly in the NSCLC cells
compared with the healthy controls, which was consistent with a
prior study by Wu et al (14). To verify the targeting reaction
between miR-194 and hNUDC, luciferase reporter vectors of the
wild-type and mutant hNUDC were constructed. The results confirmed
that hNUDC is a target gene for miR-194.
Nuclear migration is essential for growth,
development, and cellular function of eukaryotes (27). hNUDC protein plays an important role
in nuclear migration (28). The
increased hNUDC expression was found to be closely associated with
cell malignant hyperplasia in nasopharyngeal carcinoma. A report
indicated that anti-NUDC antibody could inhibit the growth of
nasopharyngeal carcinoma cell lines (27). From the perspective mentioned above,
targeting hNUDC with certain small RNAs offers a novel strategy to
prevent the abnormal growth of the NSCLC cells. Therefore, to
investigate the role of miR-194 in NSCLC cell process via targeting
hNUDC, we detected the effect of miR-194 overexpression on the
mitosis, cell proliferation and cell cycle ability of 95C and 95D
cells. The results showed that the miR-194 mimic strongly inhibited
the hNUDC expression, inducing abnormal nuclear division and
decreased cell proliferation rate. Multiple evidence confirms that
95C and 95D cells have relatively low and high metastasis potential
(23,29,30).
Consistent with these studies, the relatively low level of
malignancy of 95C cells renders them more sensitive to the external
factors compared with 95D cells. The cell growth was withheld in G1
phase during the treatment of miR-194 mimic compared with mimic
control. However, as 95D cells were characterized by rapid rate of
DNA synthesis and cell growth, they became accumulated in G2 phase
in the treatment with miR-194 mimic. It is reported that cells
arrested in G2/M achieved nuclear enlargement and polyplodization,
possibly through failure to complete both mitosis and cytokinesis
(31). These results confirmed that
miR-194 acted as a negative control in the cell processing in NSCLC
cells via targeting hNUDC.
hNUDC has been found to act as a secondary ligand
for Mpl involved in regulating proliferation and differentiation of
different types of megakaryocytes (32,33).
hNUDC plays a key role in the megakaryocytes undergoing endomitosis
through the interaction with Mpl (34). Native hNUDC and Mpl were seen around
the nuclei and in cytoplasm extensions at all stages of
megakaryocytic development (8). A
report also indicated that the overexpression of hNUDC activated
the EKR1/2 pathway (11), and this
activation was profound and prolonged in an Mpl-dependent manner
(34). The present study revealed
that the miR-194 mimic inhibited the expressions of Mpl/ERK/c-myc
pathway proteins by a decrease in hNUDC expression. The
downregulation of c-myc is an important event that has been
connected to the terminal differentiation and growth arrest of
several cell types (35). In order
to further confirm the inhibitory effect of miR-194 on the
expression of Mpl/ERK pathway proteins and on the cell process of
NSCLC cells via targeting hNUDC. hNUDC was overexpressed in 95D
cells and the results revealed that the overexpression of hNUDC
restored the inhibitory effect of the miR-194 on the mitosis, cell
growth and protein expression of Mpl/ERK pathway in 95D cells.
These results suggest that miR-194 is a newly identified miRNA that
suppresses the expression of hNUDC, at least in NSCLC cells
(possibly in other types of human cells). This finding can
contribute to a clearer understanding of the regulatory network of
Mpl/ERK pathway in human cancers. hNUDC is revealed as a new
NSCLC-associated tumor-promoting gene in this study. Notably, this
could facilitate the development of a therapeutic strategy
targeting hNUDC for lung cancer treatment in studies in the
future.
It has been reported that the overexpression of
miR-194 suppressed invasion and migration of liver cancer cells in
mice (36), indicating the
tumor-suppressing role of miR-194 in vivo. In this study,
the overexpression of miR-194 inhibited tumor growth of the 95D
xenograft in NOD/SCID mice at early time-points, however, miR-194
delayed the onset of 95D tumor growth and did not reduce growth of
the resulting tumors from day 21 to 27. We speculate that miR-194
induces a growth delay of the transplanted cells that is overcome
with time. Multiple administrations or stable expression systems
might be necessary to suppress tumor growth more efficiently.
In conclusion, our results demonstrate that the
miR-194 overexpression affects the hNUDC expression, resulting in
abnormal nuclear division, suppresses cell growth by cell cycle
arrest in G1 or G2 phase and downregulates Mpl/ERK pathway proteins
in NSCLC cells. The present study is an important step towards
understanding the pathogenesis of NSCLC and implicates miR-194 as a
potential therapeutic target for NSCLC.
Acknowledgments
The authors would like to thank the members of the
Respiratory Department, Cangzhou Central Hospital, for providing
helpful discussions concerning the present study.
Abbreviations:
|
hNUDC
|
human nuclear distribution C
|
|
miR-194
|
microRNA-194
|
|
Mpl
|
thrombopoietin receptor
|
|
ERK1/2
|
extracellular signal-regulated protein
kinases-1 and -2
|
|
3′-UTR
|
3′-untranslated region
|
|
miRNAs
|
microRNAs
|
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jemal A, Siegel R, Ward E, Hao Y, Xu J,
Murray T and Thun MJ: Cancer statistics, 2008. CA Cancer J Clin.
58:71–96. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Marschner JP, Quaratino S and Forssmann U:
Non-small cell lung cancer, NSCLC. Cancer Immunotherapy Meets
Oncology. Springer; pp. 193–201. 2014, View Article : Google Scholar
|
|
4
|
Miller BA, Zhang M-Y, Gocke CD, De Souza
C, Osmani AH, Lynch C, Davies J, Bell L and Osmani SA: A homolog of
the fungal nuclear migration gene nudC is involved in normal and
malignant human hematopoiesis. Exp Hematol. 27:742–750. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gocke CD, Reaman GH, Stine C, Zhang MY,
Osmani SA and Miller BA: The nuclear migration gene NudC and human
hematopoiesis. Leuk Lymphoma. 39:447–454. 2000. View Article : Google Scholar
|
|
6
|
Aumais JP, Williams SN, Luo W, Nishino M,
Caldwell KA, Caldwell GA, Lin SH and Yu-Lee LY: Role for NudC, a
dynein-associated nuclear movement protein, in mitosis and
cytokinesis. J Cell Sci. 116:1991–2003. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Aumais JP, Tunstead JR, McNeil RS, Schaar
BT, McConnell SK, Lin SH, Clark GD and Yu-Lee LY: NudC associates
with Lis1 and the dynein motor at the leading pole of neurons. J
Neurosci. 21:RC1872001.PubMed/NCBI
|
|
8
|
Pan RM, Yang Y, Wei MX, Yu XB, Ge YC and
Xu P: A microtubule associated protein (hNUDC) binds to the
extracellular domain of thrombopoietin receptor (Mpl). J Cell
Biochem. 96:741–750. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tang YS, Zhang YP and Xu P: hNUDC promotes
the cell proliferation and differentiation in a leukemic cell line
via activation of the thrombopoietin receptor (Mpl). Leukemia.
22:1018–1025. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Brognard J and Dennis PA: Variable
apoptotic response of NSCLC cells to inhibition of the MEK/ERK
pathway by small molecules or dominant negative mutants. Cell Death
Differ. 9:893–904. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xiao Y, Zheng Y, Tan P, Xu P and Zhang Q:
Overexpression of nuclear distribution protein (hNUDC) causes
pro-apoptosis and differentiation in Dami megakaryocytes. Cell
Prolif. 46:576–585. 2013.PubMed/NCBI
|
|
12
|
Vicent S, López-Picazo JM, Toledo G,
Lozano MD, Torre W, Garcia-Corchón C, Quero C, Soria JC,
Martín-Algarra S, Manzano RG, et al: ERK1/2 is activated in
non-small-cell lung cancer and associated with advanced tumours. Br
J Cancer. 90:1047–1052. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yang CH, Pfeffer SR, Sims M, Yue J, Wang
Y, Linga VG, Paulus E, Davidoff AM and Pfeffer LM: The oncogenic
microRNA-21 inhibits the tumor suppressive activity of FBXO11 to
promote tumorigenesis. J Biol Chem. 290:6037–6046. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wu X, Liu T, Fang O, Leach LJ, Hu X and
Luo Z: miR-194 suppresses metastasis of non-small cell lung cancer
through regulating expression of BMP1 and p27kip1.
Oncogene. 33:1506–1514. 2014. View Article : Google Scholar
|
|
15
|
Zhao B, Han H, Chen J, Zhang Z, Li S, Fang
F, Zheng Q, Ma Y, Zhang J, Wu N, et al: MicroRNA let-7c inhibits
migration and invasion of human non-small cell lung cancer by
targeting ITGB3 and MAP4K3. Cancer Lett. 342:43–51. 2014.
View Article : Google Scholar
|
|
16
|
Yin M, Ren X, Zhang X, Luo Y, Wang G,
Huang K, Feng S, Bao X, Huang K, He X, et al: Selective killing of
lung cancer cells by miRNA-506 molecule through inhibiting NF-κB
p65 to evoke reactive oxygen species generation and p53 activation.
Oncogene. 34:691–703. 2015. View Article : Google Scholar
|
|
17
|
Shi Y, Liu C, Liu X, Tang DG and Wang J:
The microRNA miR-34a inhibits non-small cell lung cancer (NSCLC)
growth and the CD44hi stem-like NSCLC cells. PLoS One.
9:e900222014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ma Y, Xia H, Liu Y and Li M: Silencing
miR-21 sensitizes non-small cell lung cancer A549 cells to ionizing
radiation through inhibition of PI3K/Akt. BioMed Res Int 2014.
Article ID 617868. 2014. View Article : Google Scholar
|
|
19
|
Gao F, Chang J, Wang H and Zhang G:
Potential diagnostic value of miR-155 in serum from lung
adenocarcinoma patients. Oncol Rep. 31:351–357. 2014.
|
|
20
|
Dong Z, Zhong Z, Yang L, Wang S and Gong
Z: MicroRNA-31 inhibits cisplatin-induced apoptosis in non-small
cell lung cancer cells by regulating the drug transporter ABCB9.
Cancer Lett. 343:249–257. 2014. View Article : Google Scholar
|
|
21
|
Bao C, Li Y, Huan L, Zhang Y, Zhao F, Wang
Q, Liang L, Ding J, Liu L, Chen T, et al: NF-κB signaling relieves
negative regulation by miR-194 in hepatocellular carcinoma by
suppressing the transcription factor HNF-1α. Sci Signal. 8:ra75.
2015. View Article : Google Scholar
|
|
22
|
Nakamura K, Sawada K, Kinose Y, Hashimoto
K, Mabuchi S and Kimura T: Identification of microRNA which
regulates paclitaxel resistance of ovarian cancer cells – a
potential of miR-194 by attenuating paclitaxel resistance through
the down-regulation of oncogene BMI-1. Cancer Res. 74(19 Suppl):
43862014. View Article : Google Scholar
|
|
23
|
Lu YL: Spontaneous metastasis of clonal
cell subpopulations of human lung giant cell carcinoma after
subcutaneous inoculation in nude mice. Zhonghua Zhong Liu Za Zhi.
11:1–7. 1989.In Chinese. PubMed/NCBI
|
|
24
|
Ambros V, Lee RC, Lavanway A, Williams PT
and Jewell D: MicroRNAs and other tiny endogenous RNAs in C.
elegans. Curr Biol. 13:807–818. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chen X, Wang Y, Zang W, Du Y, Li M and
Zhao G: miR-194 targets RBX1 gene to modulate proliferation and
migration of gastric cancer cells. Tumour Biol. 36:2393–2401. 2015.
View Article : Google Scholar
|
|
26
|
Li J, He X, Wei W and Zhou X: MicroRNA-194
promotes osteoblast differentiation via downregulating STAT1.
Biochem Biophys Res Commun. 460:482–488. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chen Y, Li T, Qu S and Tang X: Expression
and effects of nuclear distribution C (NUDC) protein in
nasopharyngeal carcinoma cell lines. Ai Zheng. 25:708–712. 2006.In
Chinese. PubMed/NCBI
|
|
28
|
Zhang MY, Huang NN, Clawson GA, Osmani SA,
Pan W, Xin P, Razzaque MS and Miller BA: Involvement of the fungal
nuclear migration gene nudC human homolog in cell proliferation and
mitotic spindle formation. Exp Cell Res. 273:73–84. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hou M, Tan L, Wang X and Zhu YS: Antisense
Tiam1 downregulates the invasiveness of 95D cells in vitro. Acta
Biochim Biophys Sin (Shanghai). 36:537–540. 2004. View Article : Google Scholar
|
|
30
|
Ren T, Wen ZK, Liu ZM, Liang YJ, Guo ZL
and Xu L: Functional expression of TLR9 is associated to the
metastatic potential of human lung cancer cell: Functional active
role of TLR9 on tumor metastasis. Cancer Biol Ther. 6:1704–1709.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lin S-H, Nishino M, Luo W, Aumais JP,
Galfione M, Kuang J and Yu-Lee LY: Inhibition of prostate tumor
growth by overexpression of NudC, a microtubule motor-associated
protein. Oncogene. 23:2499–2506. 2004. View Article : Google Scholar
|
|
32
|
Pang SF, Li XK, Zhang Q, Yang F and Xu P:
Interference RNA (RNAi)-based silencing of endogenous
thrombopoietin receptor (Mpl) in Dami cells resulted in decreased
hNUDC-mediated megakaryocyte proliferation and differentiation. Exp
Cell Res. 315:3563–3573. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wei MX, Yang Y, Ge YC and Xu P: Functional
characterization of hNUDC as a novel accumulator that specifically
acts on in vitro megakaryocytopoiesis and in vivo platelet
production. J Cell Biochem. 98:429–439. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhang YP, Tang YS, Chen XS and Xu P:
Regulation of cell differentiation by hNUDC via a Mpl-dependent
mechanism in NIH 3T3 cells. Exp Cell Res. 313:3210–3221. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Gartel AL and Shchors K: Mechanisms of
c-myc-mediated transcriptional repression of growth arrest genes.
Exp Cell Res. 283:17–21. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Meng Z, Fu X, Chen X, Zeng S, Tian Y, Jove
R, Xu R and Huang W: miR-194 is a marker of hepatic epithelial
cells and suppresses metastasis of liver cancer cells in mice.
Hepatology. 52:2148–2157. 2010. View Article : Google Scholar : PubMed/NCBI
|