Introduction
Pancreatic cancer is one of the most aggressive
malignant diseases with a median survival time of less than 6
months and a 5-year survival rate of <5% (1). In 2015, it was estimated that 48,960
subjects will be newly diagnosed with pancreatic cancer and will
account for 40,560 cancer-related deaths in the United States
(1). Surgical resection remains the
only way to cure this severe disease, however, due to the high
metastatic rate, the majority of patients are diagnosed at an
advanced inoperable stage, and less than 20% of patients are
amenable for surgery (2). Even
those patients with seemingly resectable pancreatic tumor are not
always cured by surgery due to the microscopic systemic spread of
cancer cells before the operation intervention (3). Currently, there are very limited
therapeutic options for pancreatic cancer, therefore, more
constructive and effective interventions for targeting cancer
metastasis are urgently needed.
Hypoxia (low oxygen tension) is commonly found in
solid tumors more than a few millimeters in size and is associated
with a poor prognosis (4,5). Tumor hypoxia is strongly associated
with enhanced tumor invasiveness, angiogenesis and distant
metastasis (4). Hypoxia-inducible
factor-1 (HIF-1), which belongs to the basic
helix-loop-helix-periodic acid-Schiff domain transcription factor
family, is a key mediator of the cellular response to hypoxia and
is overexpressed in a wide variety of solid tumors, including
pancreatic cancer (5). Our previous
study identified that the Hedgehog (Hh) signaling modulated hypoxia
induced pancreatic cancer epithelial to mesenchymal transition
(EMT) and invasion (5).
EMT has been recognized both as a physiological
mechanism for development and tissue remodeling, and as a
pathological mechanism in cancer progression, during which cells
lose their polarized epithelial traits and acquire mesenchymal
characteristics (6,7). The initiation of cancer metastasis
requires migration and invasion of cells, which is enabled by EMT
(8). EMT cells are a resource for
cancer stem-like cells which are more resistant to therapies,
because of the survival advantage with increased anti-apoptotic
activities (9). A typical
characteristic of EMT is the loss of the cell-cell adhesion
molecule E-cadherin expression and gain of mesenchymal markers,
such as vimentin, N-cadherin and others (7). Our recent study showed that hypoxic
condition was able to induce EMT in HepG2 hepatocellular carcinoma
cells (6).
Curcumin is a natural polyphenol present in turmeric
that possess many biological activities, including anti-infectious,
anti-inflammatory, anti-oxidant and chemopreventive effects
(10). Recent studies have shown
that curcumin is able to inhibit the proliferation, invasion and
metastasis of a variety of tumor cells (10,11).
Multiple cellular signaling pathways have been proven to be
regulated by curcumin in cancer treatment including
mitogen-activated protein kinase (MAPK), nuclear factor-κB (NF-κB),
Akt, Wnt/β-catenin and others (10). Sun et al (12) recently indicated that curcumin could
reverse pancreatic cancer progression by inhibiting the Hh
signaling pathway. The Hh signaling pathway, which is considered to
play an important role in tumorigenesis, is normally quiescent in
adult pancreas and has been shown to be very active in pancreatic
cancer (5). Our previous study
showed that hypoxia-induced invasion and EMT process is intimate
related with the Hh signaling pathway (5).
In the present study, we tested the hypothesis that
curcumin is able to inhibit hypoxia-induced proliferation, invasion
and migration as well as EMT progression of pancreatic cancer
cells. We also investigated the effect of curcumin on
hypoxia-induced activation of Hh pathway. Results from this study
suggest that curcumin treatment may be a novel option for therapy
of pancreatic cancer via the inhibition of the Hh signaling
pathway.
Materials and methods
Cell culture and reagents
The human pancreatic cancer cell line, Panc-1, was
obtained from the American Type Culture Collection (ATCC; Manassas,
VA, USA). The cells were cultured in DMEM medium containing 10%
dialyzed heat-inactivated fetal bovine serum (FBS), 100 U/ml
penicillin, and 100 µg/ml streptomycin in a humidified
atmosphere of 5% CO2 at 37°C. In experiments designed to
assess the role of hypoxia, cells were first cultured in normoxic
conditions to obtain the desired subconfluence level (65–70%) and
then were incubated in strictly controlled hypoxic conditions (1%
O2). Exponentially growing cells in complete medium were
pretreated for 1 h with 20 µM curcumin, followed by
continual incubation in normal culturing conditions or hypoxic
conditions for indicated time intervals according to the purpose of
the experiment. Dulbecco's modified Eagle's medium (DMEM) and FBS
were from Gibco (Grand Island, NY, USA). Curcumin was purchased
from Sigma-Aldrich (St. Louis, MO, USA). Millicell Transwells for
the invasion assays were obtained from Millipore (Billerica, MA,
USA). Matrigel was from BD Biosciences (Bedford, MA, USA). Primary
antibodies against E-cadherin, N-cadherin, vimentin, SHH, SMO and
GLI1 were procured from Santa Cruz Biotechnology (Santa Cruz, CA,
USA). Nitrocellulose membranes were from Millipore. The BCA assay
kit and the chemiluminescence kit were from Pierce (Rockford, IL,
USA). Other reagents were purchased from common commercial sources.
All drug solutions were freshly prepared on the day of testing.
MTT proliferation assays
Panc-1 cells were seeded in 96-well plates at the
density of 1×104 cells/well and incubated overnight in
10% FBS medium. The cells were then treated with curcumin in
normoxic or hypoxic condition. After incubation for 24, 48 and 72 h
at 37°C, 15 µl of MTT solution (5 mg/ml in
phosphate-buffered saline (PBS) was added to each well, and then
the cells were incubated for 4 h at 37°C. DMSO (100 µl) was
then added to each well. The optical density (OD) value at 490 nm
was determined using a spectrophotometer (Bio-Rad Laboratories,
Hercules, CA, USA).
Wound healing assay
Cell migratory ability was detected by a
wound-healing assay. Panc-1 cells were seeded in 24-well plates
(1.0×105 cells/500 µl). After the cells grew to
90–100% confluence, a sterile pipette tip was used to produce a
wound line between the cells. Cellular debris was removed by
washing with PBS and then allowed to migrate for 24 h. Images were
taken at time 0 and 24 h post-wounding under a Nikon Diaphot TMD
inverted microscope (×10). The relative distance traveled by the
leading edge from 0 to 24 h was assessed using Photoshop software
(n=5).
Transwell Matrigel invasion assay
The invasion of pancreatic cancer cells was
performed in Transwell chambers. The 8.0 µm pore inserts
were coated with 25 µl Matrigel. The cell suspensions
(5×104) were added to the upper chambers in DMEM
containing 1% FBS. Simultaneously, 500 ml of DMEM containing 20%
FBS was placed in the lower chambers. The cells were allowed to
migrate for 48 h at 37°C. The non-invading cells were removed from
the upper surface by scraping with a wet cotton swab. After rinsing
with PBS, the filter was fixed and stained with crystal violet. The
invasion ability was determined by counting the stained cells on
the bottom surface. Three random fields were captured at ×20
magnification (n=3).
Real-time quantitative PCR (QT-PCR)
Total RNA was extracted from the pancreatic cancer
cells using the Fastgen200 RNA isolation system (Fastgen, Shanghai,
China) according to the manufacturer's protocol. Total RNA was
reverse-transcribed into cDNA using the Fermentas RevertAid™ kit
(MBI Fermentas, Burlington, Canada). The primer sequences were as
follows: E-cadherin: forward, 5′-ATTCTGATTCTGCTGCTCTTG-3′ and
reverse, 5′-AGTCCTGGTCCTCTTCTCC-3′; N-cadherin: forward,
5′-TGTTTGACTATGAAGGCAGTGG-3′ and reverse,
5′-TCAGTCATCACCTCCACCAT-3′; vimentin: forward,
5′-AATGACCGCTTCGCCAAC-3′ and reverse, 5′-CCGCATCTCCTCCTCGTAG-3′;
β-actin: forward, 5′-GACTTAGTTGCGTTACACCCTTTCT-3′ and reverse,
5′-GAACGGTGAAGGTGACAGCAGT-3′.
The PCR reactions consisted of 30 sec at 95°C,
followed by 40 cycles of 95°C for 5 sec, 60°C for 30 sec and 72°C
for 30 sec. After each QT-PCR experiment, a dissociation curve
analysis was conducted. The relative gene expression was calculated
using the previously described 2−ΔΔCt method (13).
Western blotting
Proteins were electrophoretically resolved on a
denaturing SDS-polyacrylamide gel and electrotransferred onto
nitrocellulose membranes. The membranes were initially blocked with
5% non-fat dry milk in Tris-buffered saline (TBS) for 2 h and then
probed with antibodies against E-cadherin, N-cadherin, vimentin,
SHH, SMO, GLI1 or β-actin (loading control). After co-incubation
with the primary antibodies at 4°C overnight, membranes were
blotted with the secondary antibody for 2 h at 37°C. The results
were visualized using the ECL Western blotting substrate and
photographed by GeneBox (SynGene).
Immunofluorescence microscopy
Panc-1 cells were fixed with 4% paraformaldehyde for
10 min at room temperature, permeabilized in 0.5% Triton X-100 for
10 min, and blocked in 1% BSA for 1 h. Fixed cells were then
incubated with primary antibodies against E-cadherin (1:100),
N-cadherin (1:100) and vimentin (1:200) at 4°C overnight. Cells
were washed and incubated with fluorescein isothiocyanate
(FITC)-conjugated goat anti-rabbit IgG for 1 h in the dark. The
cells were visualized by a fluorescent microscope (Nikon, Tokyo,
Japan) using appropriate excitation and emission spectra at ×400
magnification.
Statistical analysis
Statistical analysis was performed using SPSS
software (version 17.0; SPSS, Inc., Chicago, IL, USA). Data are
presented as the means ± SEM of three replicate assays. Differences
between the groups were analyzed by analysis of variance (ANOVA).
Statistical significance was set at P<0.05. All experiments were
repeated independently at least three times.
Results
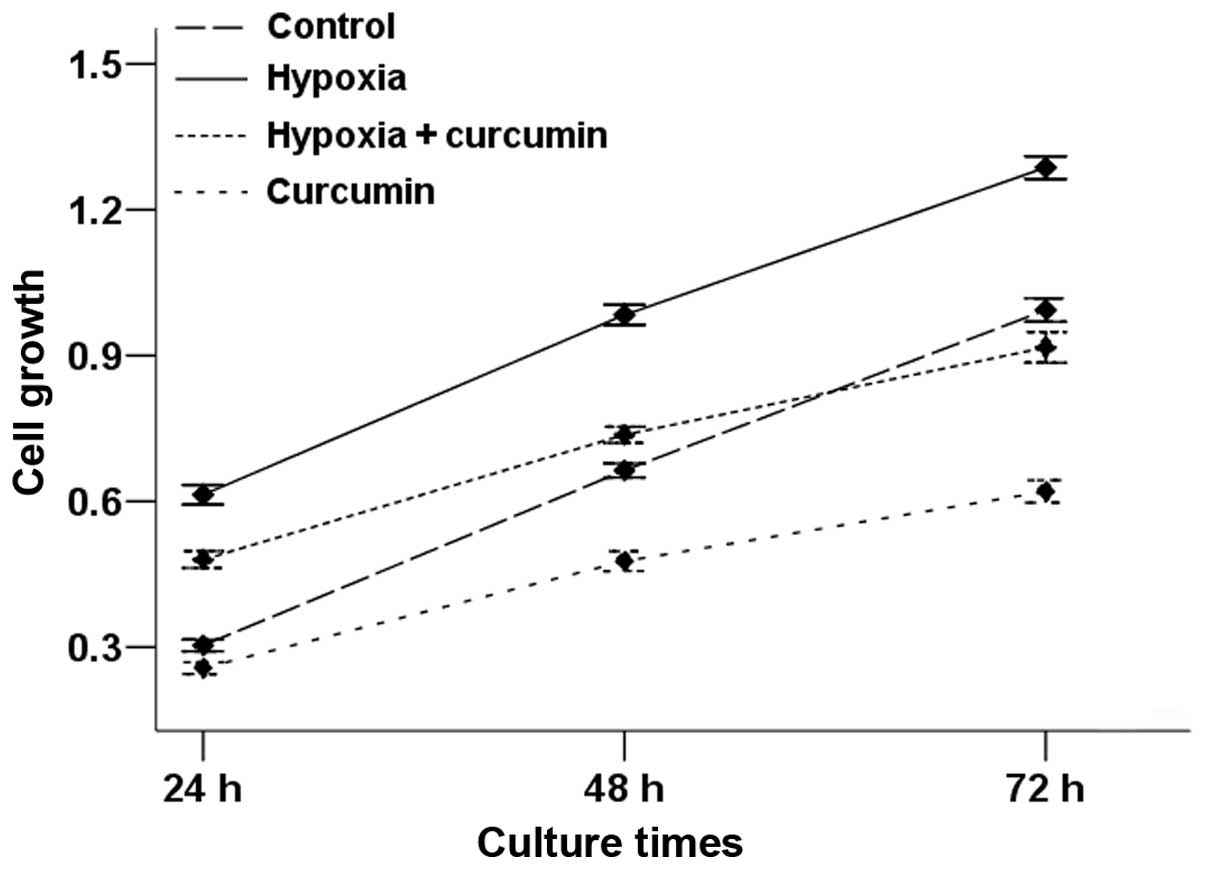
Curcumin inhibits the proliferation of
Panc-1 cells
Our previous study showed that curcumin is able to
inhibit the proliferation of HepG2 cells (6). In order to explore the role of
curcumin on the proliferative ability of pancreatic cancer cell,
Panc-1 cells were treated with hypoxic condition and curcumin alone
or in combination. At the time-points indicated in Fig. 1, the proliferative rate of Panc-1
cells was determined by the MTT assay. The results demonstrated
that the proliferation of Panc-1 cells increased in hypoxic
condition compared with the control group and the increased rate of
cell proliferation induced by hypoxic condition was reduced in the
presence of curcumin. Curcumin alone was also able to inhibit the
proliferative ability of Panc-1 cells.
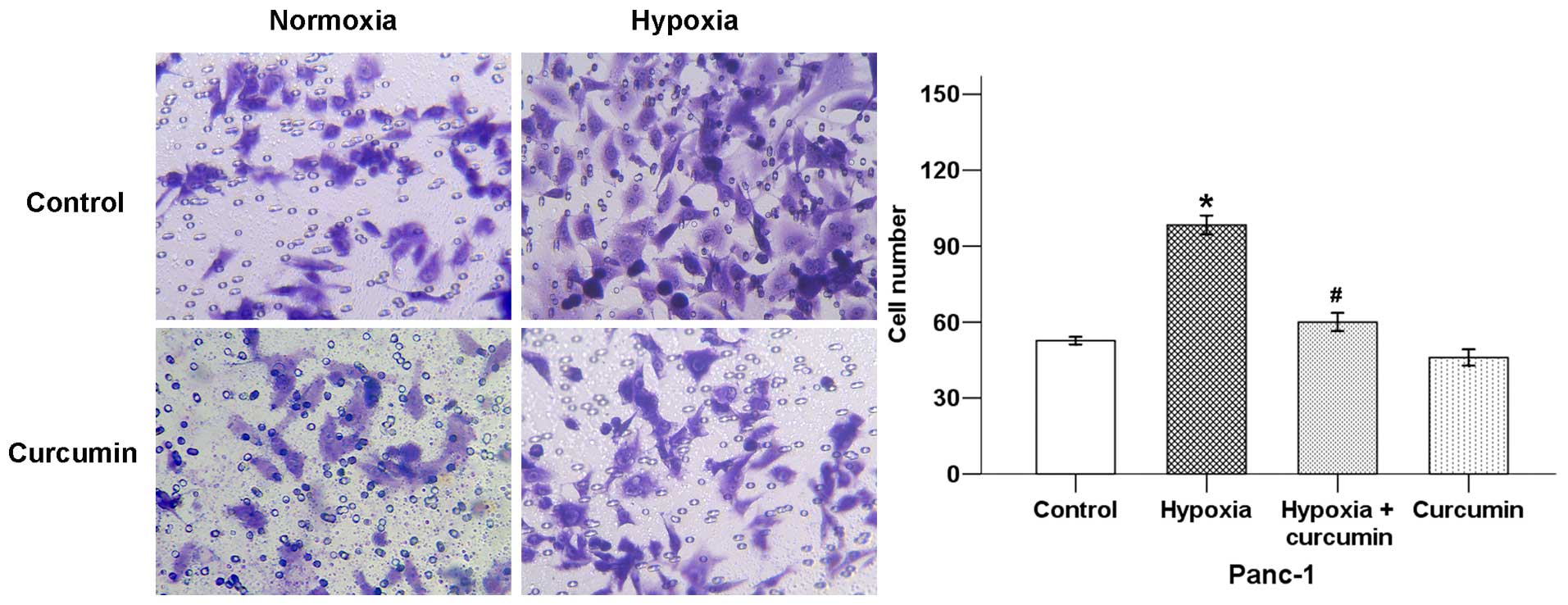
Curcumin inhibits hypoxia-induced
invasive ability of pancreatic cancer cells
The initiation of cancer metastasis requires
migration and invasion of cells, which is enabled by EMT (14). In order to confirm whether curcumin
could influence hypoxia-induced cancer cell invasive ability, we
used a Transwell invasion assay. As shown in Fig. 2, hypoxia exposure significantly
increased pancreatic cancer invasive ability, while curcumin
decreased the average cell number that invaded into the lower
chamber.
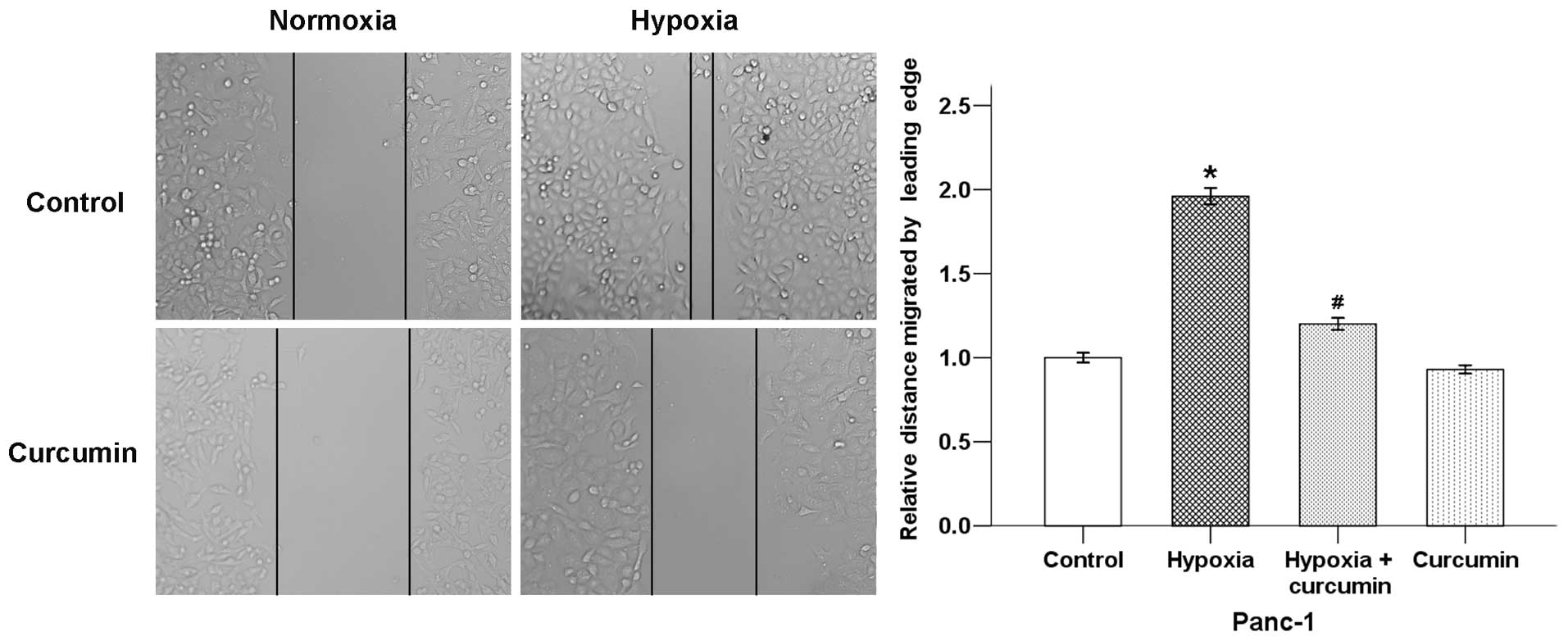
Curcumin inhibits hypoxia-induced wound
closure of pancreatic cancer cells
Migration is an important aspect that leads to the
ability of cancer cells to form metastasis. A wound-healing assay
was then used to test the effect of curcumin on hypoxia-induced
pancreatic cancer cell motility. Results showed that hypoxic
condition significantly increased the migratory ability of Panc-1
cells after incubation for 24 h. Curcumin counter-balanced this
effect of hypoxia (Fig. 3). These
results indicate that curcumin inhibits migration and invasion of
pancreatic cancer cells under hypoxic conditions.
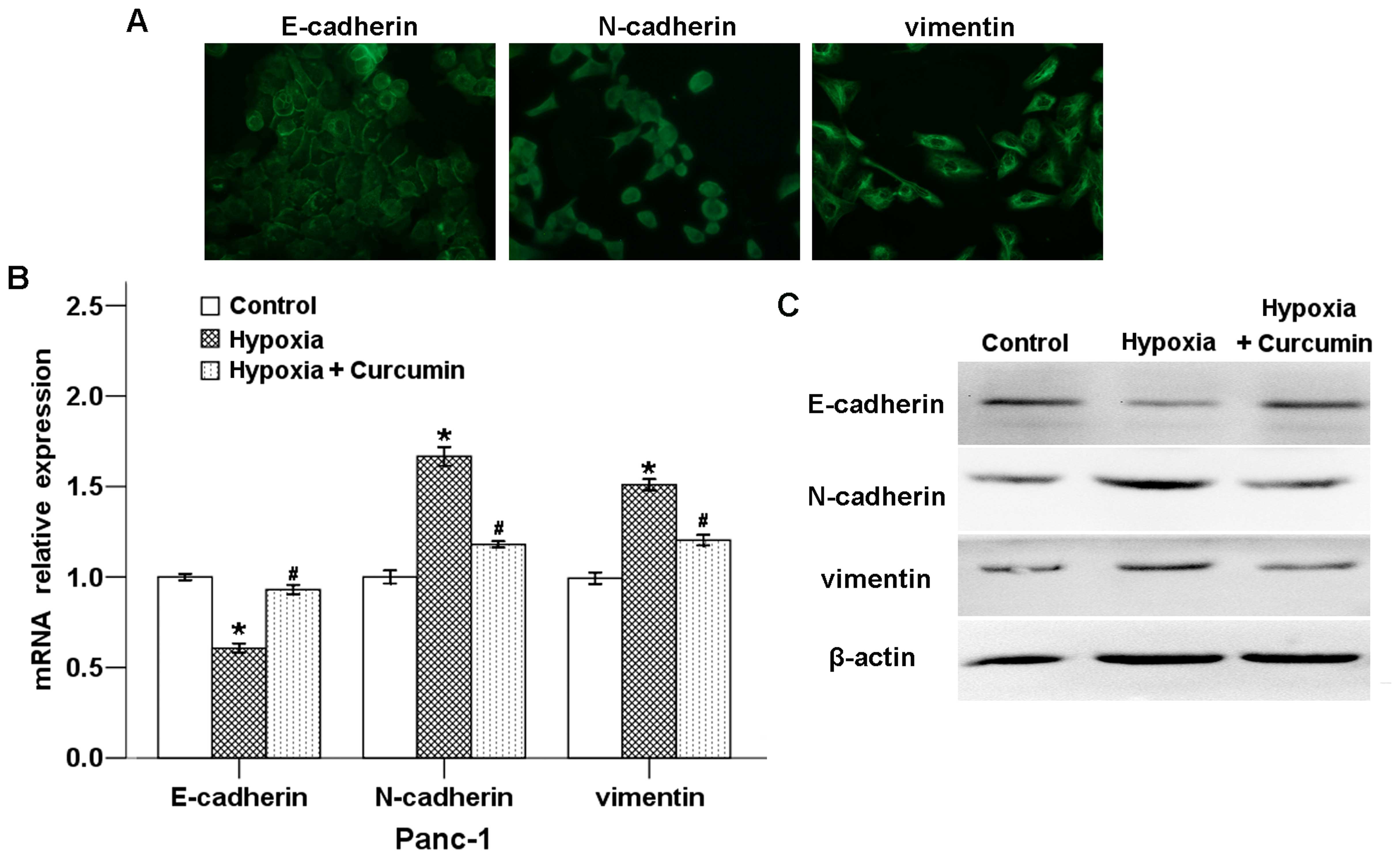
Effects of curcumin on the expression of
hypoxia-modulated EMT-related factors in pancreatic cancer
cells
EMT contains four important steps: loss of
epithelial cell adhesion, gain of mesenchymal proteins and
acquisition of a mesenchymal-like state, degradation of basement
membranes and enhanced cell invasive ability that facilitate tumor
cell invasion into stroma and in turn entrance to the circulation
(7). To confirm the effect of
curcumin on hypoxia-induced EMT, we determined the expression
levels of EMT-related genes after the cells were treated in hypoxic
condition with or without curcumin. As illustrated in Fig. 4A, the expression of E-cadherin was
located in cell membrane, whereas N-cadherin and vimentin were
localized in both cell membrane and cytoplasm. As shown in Fig. 4B, hypoxic condition downregulated
the mRNA level of the epithelial marker E-cadherin, while the
expression of mesenchymal markers N-cadherin and vimentin were
strongly increased. Curcumin could significantly reverse all of
these hypoxia-induced effects.
To evaluate the effects of hypoxia and curcumin on
the expression of E-cadherin, N-cadherin and vimentin at protein
level, we determined these proteins in Panc-1 cells using western
blotting. As shown in Fig. 4C,
curcumin counter-balanced the hypoxia-induced EMT-related factors
at the protein level, and the trend was consistent with the mRNA
results. These results indicate that hypoxic condition-induced EMT
progression could be inhibited by curcumin.
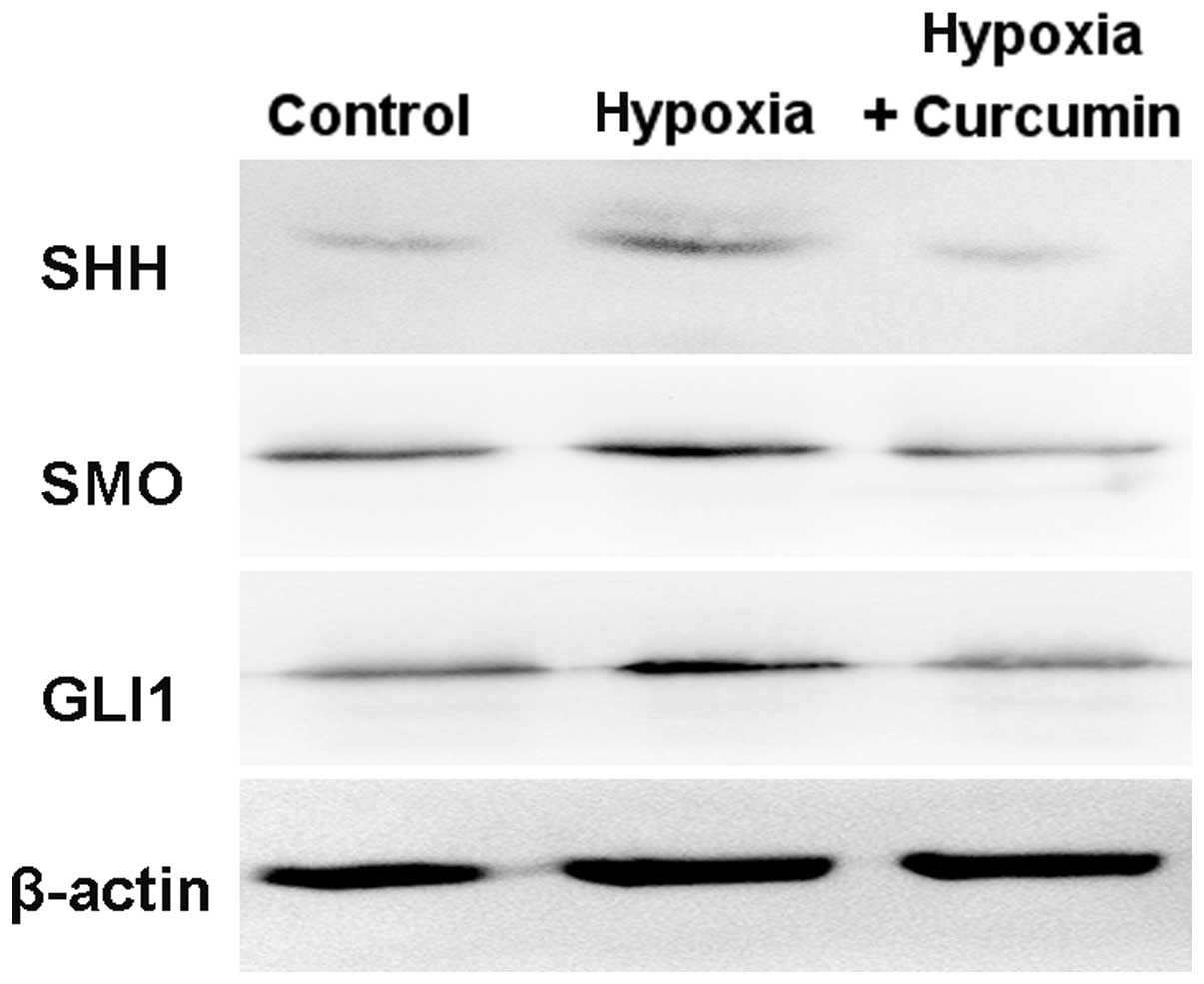
Curcumin downregulates hypoxia-activated
Hh signaling pathway
Hh signaling plays an important role in the
initiation and progression of pancreatic cancer (15). As shown in Fig. 5, the protein levels of SHH, SMO and
GLI1 were increased in Panc-1 cells under hypoxic condition, which
indicated that Hh signaling was activated by hypoxia. Curcumin
significantly decreased hypoxia-induced expression levels of SHH,
SMO and GLI1. Taken together, our results demonstrate that curcumin
inhibits hypoxia-induced EMT in Panc-1 cells via suppression of the
Hh signaling pathway.
Discussion
Due to both the inherently aggressive biology of the
disease and its late diagnosis in most cases, pancreatic cancer is
one of the most aggressive malignant digestive carcinoma with an
extremely high mortality rate (16). A number of studies have indicated
that hypoxia and hypoxia-induced signaling pathways are highly
associated with poor clinical outcome of patients diagnosed with
pancreatic cancer, because of the enhanced cancer cell progression
(17,18). Hypoxia-mediated target gene
expression has been shown to stimulate proliferation, angiogenesis,
metastasis, chemo-resistance and radio-resistance of tumor cells
(19–21). In recent years, many active
compounds with anti-invasive and anti-metastatic properties, such
as curcumin, α-mangostin and resveratrol, have been defined as new
chemotherapeutic agents (6,22–23).
Our previous study showed that curcumin inhibited hypoxia-induced
HIF-1α accumulation in a hypoxia model induced by CoCl2
and suppressed proliferation, migration, invasion and EMT of HepG2
cells in this environment (6). In
the present study, we focused on the underlying mechanisms through
which curcumin inhibits hypoxia-induced EMT in pancreatic cancer
cell line Panc-1.
Our data showed that hypoxic condition could
significantly modulate the expression of EMT-related factors,
E-cadherin, vimentin and N-cadherin in Panc-1 cells, which further
enhanced the capacity of the pancreatic cancer cells to migrate and
invade the extracellular matrix. Curcumin was able to terminate
these effects of hypoxic condition. In addition, we also tested the
effects of hypoxic condition and curcumin on the activation of SHH,
SMO and GLI1. Data showed that hypoxic condition significantly
increased the expression levels of SHH, SMO and GLI1 in pancreatic
cancer cells, whereas the addition of curcumin to the cell culture
resulted in a decrease of these Hh pathway related factors.
Curcumin (diferuloylmethane) is a bioactive natural
compound and a large number of experimental studies have shown that
curcumin is able to suppress initiation, progression and metastasis
of a variety of tumors, including pancreatic cancer (10,24).
Youns et al (24) reported
that curcumin could inhibit pancreatic cancer cell proliferation
and upregulate the extrinsic apoptotic pathway through activation
of caspase-3, caspase-8, Bid, Bax and downregulation of NF-κB and
Bcl-2 genes. Curcumin and its analogues (UBS109 and EF31) could
inhibit multiple angiogenic pathways and suppress tumor
angiogenesis (25). In animal
models of pancreatic cancer, the combination of curcumin and
gemcitabine is much more effective than gemcitabine alone in the
inhibition of tumor growth and anti-angiogenesis (26). A recent study showed that a novel
synthetic analog of curcumin referred to as difluorinated-curcumin
could inhibit cell survival, clonogenicity, migration, invasion,
angiogenesis and the cancer stem cell (CSC) self-renewal capacity
in human pancreatic cancer cells in vitro under hypoxic
conditions, consistent with the inhibition of miR-21, miR-210,
HIF-1α and CSC signature gene markers (27).
Accumulating evidence indicates that curcumin could
inhibit tumor progression via multiple cellular signaling pathways,
including MAPK, NF-κB, Akt, Wnt/β-catenin and Hh signaling pathway
(10,12). The Hh signaling pathway, initiated
through the binding of secreted Hh ligands to the membrane receptor
patched1 (PTCH1), results in smoothened (SMO) dissociating, nuclear
translocation and activation of the transcription factors of the
GLI family. The expression of SMO and GLI1 is presumed to be the
markers of the Hh pathway activation (5). Elamin et al (28) observed that curcumin caused
inhibition of medulloblastoma cell growth and induction of
apoptotic cell death by downregulating Hh pathway proteins,
including SHH, PTCH1 and GLI1. In addition, curcumin also enhanced
the anti-tumor effects of cisplatin and γ-rays by targeting
pathways that are crucial for tumor survival. Slusarz et al
(29) reported that curcumin caused
major reductions in GLI1 mRNA concentrations in transgenic prostate
carcinoma mice. A recent study also revealed that resveratrol and
curcumin synergistically caused apoptosis in cigarette smoke
induced breast cancer cells through p21 (Waf/Cip1) mediated
inhibition of Hedgehog-GLI cascade (30). In the present study, we showed that
curcumin remarkably inhibited hypoxia-mediated activation of Hh
signaling pathway.
Cancer metastasis is a process of dissemination of
tumor cells from a primary tumor mass to a different site through
blood vessels and lymphatic vessels. EMT is a characteristic
feature of most metastatic cells and has been regarded as the
possible first step in the complex process of metastasis (31). In this process, epithelial cells are
transformed from highly differentiated, polarized and organized
cells into undifferentiated, isolated mesenchymal-like cells with
migratory and invasive properties (7). A typical characteristic of EMT is the
loss of the cell-cell adhesion molecule E-cadherin expression and
gain of mesenchymal markers, such as vimentin, N-cadherin, which in
turn lead to reorganization of the cytoskeleton to acquire a more
spindle-like morphology, and increased motility that involves
dynamic actin microfilament networks (7,32). Our
previous study showed that superoxide dismutase-induced hydrogen
peroxide production can promote EMT in pancreatic cancer, leading
to increased motility and invasion via activation of ERK signaling
pathway (33). We have also
vertified that resveratrol plays an important role in suppressing
the proliferation, migration and invasion of pancreatic cancer
cells in vitro by modulating EMT-related factors via the
PI-3K/Akt/NF-κB signaling pathway. Resveratrol was also able to
suppress the migration and invasion of pancreatic cancer cells by
inhibiting TGF-β-mediated EMT (23). In this study, we showed that
curcumin is able to suppress hypoxia-induced activation of Hh
pathway and, thus, inhibits pancreatic cancer cell invasive and
migratory ability.
In conclusion, the present study demonstrates that
curcumin plays an important role in suppressing hypoxia-induced
proliferation, migration, invasion and EMT of pancreatic cancer
cells in vitro by inhibiting the Hh signaling pathway. These
results suggest that curcumin might be a potential anticancer agent
for the treatment of pancreatic cancer.
Acknowledgments
The present study is supported by the National
Natural Science Foundation of China (Grant serial nos. 81502840 and
81301846).
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sarkar FH, Banerjee S and Li Y: Pancreatic
cancer: Pathogenesis, prevention and treatment. Toxicol Appl
Pharmacol. 224:326–336. 2007. View Article : Google Scholar
|
|
3
|
Castellanos EH, Cardin DB and Berlin JD:
Treatment of early-stage pancreatic cancer. Oncology (Williston
Park). 25:182–189. 2011.
|
|
4
|
Chang J and Erler J: Hypoxia-mediated
metastasis. Adv Exp Med Biol. 772:55–81. 2014. View Article : Google Scholar
|
|
5
|
Lei J, Ma J, Ma Q, Li X, Liu H, Xu Q, Duan
W, Sun Q, Xu J, Wu Z, et al: Hedgehog signaling regulates hypoxia
induced epithelial to mesenchymal transition and invasion in
pancreatic cancer cells via a ligand-independent manner. Mol
Cancer. 12:662013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Duan W, Chang Y, Li R, Xu Q, Lei J, Yin C,
Li T, Wu Y, Ma Q and Li X: Curcumin inhibits hypoxia inducible
factor-1α-induced epithelial-mesenchymal transition in HepG2
hepatocellular carcinoma cells. Mol Med Rep. 10:2505–2510.
2014.PubMed/NCBI
|
|
7
|
Li W, Ma Q, Liu J, Han L, Ma G, Liu H,
Shan T, Xie K and Wu E: Hyperglycemia as a mechanism of pancreatic
cancer metastasis. Front Biosci (Landmark Ed). 17:1761–1774. 2012.
View Article : Google Scholar
|
|
8
|
Thiery JP, Acloque H, Huang RY and Nieto
MA: Epithelial-mesenchymal transitions in development and disease.
Cell. 139:871–890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pirozzi G, Tirino V, Camerlingo R, Franco
R, La Rocca A, Liguori E, Martucci N, Paino F, Normanno N and Rocco
G: Epithelial to mesenchymal transition by TGFβ-1 induction
increases stemness characteristics in primary non small cell lung
cancer cell line. PLoS One. 6:e215482011. View Article : Google Scholar
|
|
10
|
Shanmugam MK, Rane G, Kanchi MM, Arfuso F,
Chinnathambi A, Zayed ME, Alharbi SA, Tan BK, Kumar AP and Sethi G:
The multifaceted role of curcumin in cancer prevention and
treatment. Molecules. 20:2728–2769. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kocaadam B and Sanlier N: Curcumin, an
active component of turmeric (Curcuma longa), and its effects on
health. Crit Rev Food Sci Nutr. 0:Nov 3–2015.Epub ahead of
print.
|
|
12
|
Sun XD, Liu XE and Huang DS: Curcumin
reverses the epithelial-mesenchymal transition of pancreatic cancer
cells by inhibiting the Hedgehog signaling pathway. Oncol Rep.
29:2401–2407. 2013.PubMed/NCBI
|
|
13
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
14
|
Nieto MA: Epithelial-mesenchymal
transitions in development and disease: Old views and new
perspectives. Int J Dev Biol. 53:1541–1547. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Spivak-Kroizman TR, Hostetter G, Posner R,
Aziz M, Hu C, Demeure MJ, Von Hoff D, Hingorani SR, Palculict TB,
Izzo J, et al: Hypoxia triggers hedgehog-mediated tumor-stromal
interactions in pancreatic cancer. Cancer Res. 73:3235–3247. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Vincent A, Herman J, Schulick R, Hruban RH
and Goggins M: Pancreatic cancer. Lancet. 378:607–620. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu H, Ma Q, Xu Q, Lei J, Li X, Wang Z and
Wu E: Therapeutic potential of perineural invasion, hypoxia and
desmoplasia in pancreatic cancer. Curr Pharm Des. 18:2395–2403.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Vasseur S, Tomasini R, Tournaire R and
Iovanna JL: Hypoxia induced tumor metabolic switch contributes to
pancreatic cancer aggressiveness. Cancers (Basel). 2:2138–2152.
2010. View Article : Google Scholar
|
|
19
|
Ghattass K, Assah R, El-Sabban M and
Gali-Muhtasib H: Targeting hypoxia for sensitization of tumors to
radio- and chemotherapy. Curr Cancer Drug Targets. 13:670–685.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liao D and Johnson RS: Hypoxia: A key
regulator of angiogenesis in cancer. Cancer Metastasis Rev.
26:281–290. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nagaraju GP, Bramhachari PV, Raghu G and
El-Rayes BF: Hypoxia inducible factor-1α: Its role in colorectal
carcinogenesis and metastasis. Cancer Lett. 366:11–18. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lei J, Huo X, Duan W, Xu Q, Li R, Ma J, Li
X, Han L, Li W, Sun H, et al: α-Mangostin inhibits hypoxia-driven
ROS-induced PSC activation and pancreatic cancer cell invasion.
Cancer Lett. 347:129–138. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li W, Ma J, Ma Q, Li B, Han L, Liu J, Xu
Q, Duan W, Yu S, Wang F, et al: Resveratrol inhibits the
epithelial-mesenchymal transition of pancreatic cancer cells via
suppression of the PI-3K/Akt/NF-κB pathway. Curr Med Chem.
20:4185–4194. 2013. View Article : Google Scholar
|
|
24
|
Youns M and Fathy GM: Upregulation of
extrinsic apoptotic pathway in curcumin-mediated antiproliferative
effect on human pancreatic carcinogenesis. J Cell Biochem.
114:2654–2665. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nagaraju GP, Zhu S, Ko JE, Ashritha N,
Kandimalla R, Snyder JP, Shoji M and El-Rayes BF: Antiangiogenic
effects of a novel synthetic curcumin analogue in pancreatic
cancer. Cancer Lett. 357:557–565. 2015. View Article : Google Scholar
|
|
26
|
Kunnumakkara AB, Guha S, Krishnan S,
Diagaradjane P, Gelovani J and Aggarwal BB: Curcumin potentiates
antitumor activity of gemcitabine in an orthotopic model of
pancreatic cancer through suppression of proliferation,
angiogenesis, and inhibition of nuclear factor-kappaB-regulated
gene products. Cancer Res. 67:3853–3861. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bao B, Ali S, Ahmad A, Azmi AS, Li Y,
Banerjee S, Kong D, Sethi S, Aboukameel A, Padhye SB, et al:
Hypoxia-induced aggressiveness of pancreatic cancer cells is due to
increased expression of VEGF, IL-6 and miR-21, which can be
attenuated by CDF treatment. PLoS One. 7:e501652012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Elamin MH, Shinwari Z, Hendrayani SF,
Al-Hindi H, Al-Shail E, Khafaga Y, Al-Kofide A and Aboussekhra A:
Curcumin inhibits the Sonic Hedgehog signaling pathway and triggers
apoptosis in medulloblastoma cells. Mol Carcinog. 49:302–314.
2010.
|
|
29
|
Slusarz A, Shenouda NS, Sakla MS,
Drenkhahn SK, Narula AS, MacDonald RS, Besch-Williford CL and
Lubahn DB: Common botanical compounds inhibit the hedgehog
signaling pathway in prostate cancer. Cancer Res. 70:3382–3390.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mohapatra P, Satapathy SR, Siddharth S,
Das D, Nayak A and Kundu CN: Resveratrol and curcumin
synergistically induces apoptosis in cigarette smoke condensate
transformed breast epithelial cells through a
p21Waf1/Cip1 mediated inhibition of Hh-Gli signaling.
Int J Biochem Cell Biol. 66:75–84. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Guan X: Cancer metastases: Challenges and
opportunities. Acta Pharm Sin B. 5:402–418. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Liu ZJ, Semenza GL and Zhang HF:
Hypoxia-inducible factor 1 and breast cancer metastasis. J Zhejiang
Univ Sci B. 16:32–43. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Li W, Cao L, Han L, Xu Q and Ma Q:
Superoxide dismutase promotes the epithelial-mesenchymal transition
of pancreatic cancer cells via activation of the
H2O2/ERK/NF-κB axis. Int J Oncol.
46:2613–2620. 2015.
|