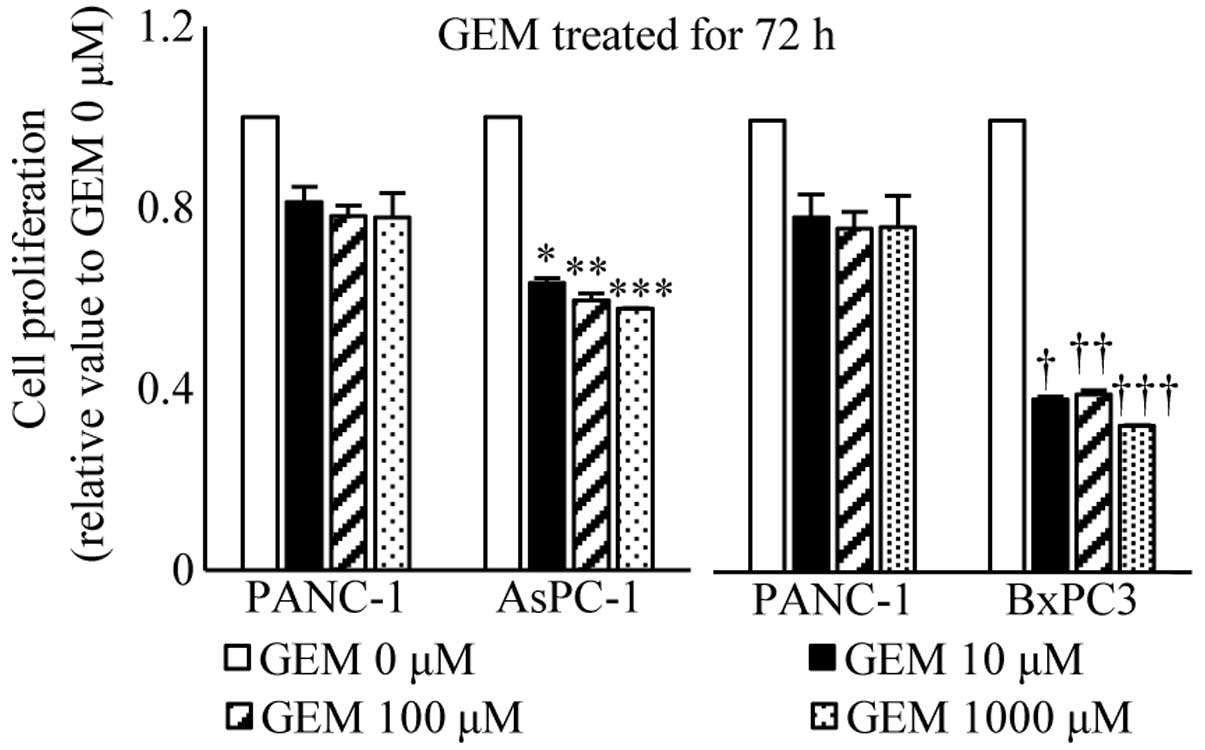
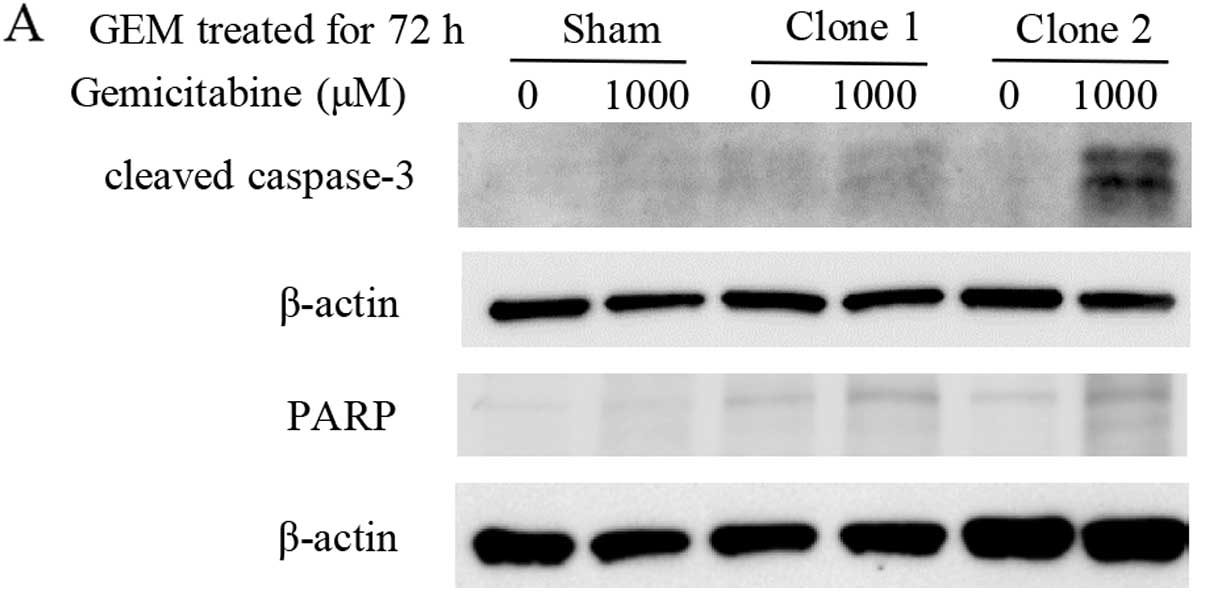
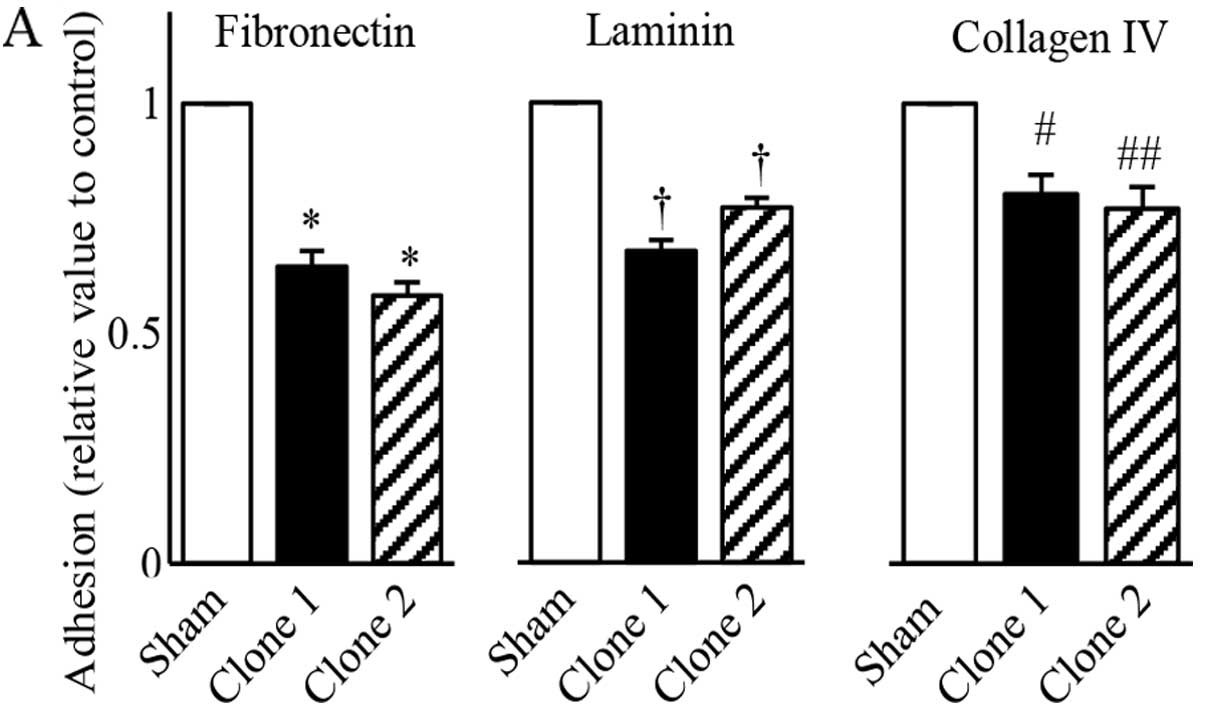
|
1
|
Siegel R, Ma J, Zou Z and Jemal A: Cancer
statistics, 2014. CA Cancer J Clin. 64:9–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hidalgo M: Pancreatic cancer. N Engl J
Med. 362:1605–1617. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Stathis A and Moore MJ: Advanced
pancreatic carcinoma: Current treatment and future challenges. Nat
Rev Clin Oncol. 7:163–172. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Burris HA III, Moore MJ, Andersen J, Green
MR, Rothenberg ML, Modiano MR, Cripps MC, Portenoy RK, Storniolo
AM, Tarassoff P, et al: Improvements in survival and clinical
benefit with gemcitabine as first-line therapy for patients with
advanced pancreas cancer: A randomized trial. J Clin Oncol.
15:2403–2413. 1997.PubMed/NCBI
|
|
5
|
Mini E, Nobili S, Caciagli B, Landini I
and Mazzei T: Cellular pharmacology of gemcitabine. Ann Oncol.
17(Suppl 5): v7–v12. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bowman T, Garcia R, Turkson J and Jove R:
STATs in oncogenesis. Oncogene. 19:2474–2488. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yu H and Jove R: The STATs of cancer - new
molecular targets come of age. Nat Rev Cancer. 4:97–105. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Darnell JE Jr: STATs and gene regulation.
Science. 277:1630–1635. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ferbeyre G and Moriggl R: The role of
Stat5 transcription factors as tumor suppressors or oncogenes.
Biochim Biophys Acta. 1815:104–114. 2011.
|
|
10
|
Levine RL, Pardanani A, Tefferi A and
Gilliland DG: Role of JAK2 in the pathogenesis and therapy of
myeloproliferative disorders. Nat Rev Cancer. 7:673–683. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Scholz A, Heinze S, Detjen KM, Peters M,
Welzel M, Hauff P, Schirner M, Wiedenmann B and Rosewicz S:
Activated signal transducer and activator of transcription 3
(STAT3) supports the malignant phenotype of human pancreatic
cancer. Gastroenterology. 125:891–905. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sahu RP and Srivastava SK: The role of
STAT-3 in the induction of apoptosis in pancreatic cancer cells by
benzyl isothiocyanate. J Natl Cancer Inst. 101:176–193. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wakao H, Gouilleux F and Groner B: Mammary
gland factor (MGF) is a novel member of the cytokine regulated
transcription factor gene family and confers the prolactin
response. EMBO J. 13:2182–2191. 1994.PubMed/NCBI
|
|
14
|
Ren S, Cai HR, Li M and Furth PA: Loss of
Stat5a delays mammary cancer progression in a mouse model.
Oncogene. 21:4335–4339. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Vafaizadeh V, Klemmt P, Brendel C, Weber
K, Doebele C, Britt K, Grez M, Fehse B, Desriviéres S and Groner B:
Mammary epithelial reconstitution with gene-modified stem cells
assigns roles to Stat5 in luminal alveolar cell fate decisions,
differentiation, involution, and mammary tumor formation. Stem
Cells. 28:928–938. 2010.PubMed/NCBI
|
|
16
|
Ahonen TJ, Xie J, LeBaron MJ, Zhu J, Nurmi
M, Alanen K, Rui H and Nevalainen MT: Inhibition of transcription
factor Stat5 induces cell death of human prostate cancer cells. J
Biol Chem. 278:27287–27292. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kazansky AV, Spencer DM and Greenberg NM:
Activation of signal transducer and activator of transcription 5 is
required for progression of autochthonous prostate cancer: Evidence
from the transgenic adenocarcinoma of the mouse prostate system.
Cancer Res. 63:8757–8762. 2003.PubMed/NCBI
|
|
18
|
Lee TK, Man K, Poon RT, Lo CM, Yuen AP, Ng
IO, Ng KT, Leonard W and Fan ST: Signal transducers and activators
of transcription 5b activation enhances hepatocellular carcinoma
aggressiveness through induction of epithelial-mesenchymal
transition. Cancer Res. 66:9948–9956. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pastuszak-Lewandoska D, Domańska D,
Czarnecka KH, Kordiak J, Migdalska-Sęk M, Nawrot E, Kiszałkiewicz
J, Antczak A, Górski P and Brzeziańska E: Expression of STAT5,
COX-2 and PIAS3 in correlation with NSCLC histhopathological
features. PLoS One. 9:e1042652014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Du W, Wang YC, Hong J, Su WY, Lin YW, Lu
R, Xiong H and Fang JY: STAT5 isoforms regulate colorectal cancer
cell apoptosis via reduction of mitochondrial membrane potential
and generation of reactive oxygen species. J Cell Physiol.
227:2421–2429. 2012. View Article : Google Scholar
|
|
21
|
Liang QC, Xiong H, Zhao ZW, Jia D, Li WX,
Qin HZ, Deng JP, Gao L, Zhang H and Gao GD: Inhibition of
transcription factor STAT5b suppresses proliferation, induces G1
cell cycle arrest and reduces tumor cell invasion in human
glioblastoma multiforme cells. Cancer Lett. 273:164–171. 2009.
View Article : Google Scholar
|
|
22
|
Jackerott M, Møldrup A, Thams P, Galsgaard
ED, Knudsen J, Lee YC and Nielsen JH: STAT5 activity in pancreatic
beta-cells influences the severity of diabetes in animal models of
type 1 and 2 diabetes. Diabetes. 55:2705–2712. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kataoka TR, Ioka T, Tsukamoto Y, Matsumura
M, Ishiguro S and Nishizawa Y: Nuclear expression of STAT5 in
intraductal papillary mucinous neoplasms of the pancreas. Int J
Surg Pathol. 15:277–281. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Canales NA, Marina VM, Castro JS, Jiménez
AA, Mendoza-Hernández G, McCARRON EL, Roman MB and Castro-Romero
JI: A1BG and C3 are overexpressed in patients with cervical
intraepithelial neoplasia III. Oncol Lett. 8:939–947.
2014.PubMed/NCBI
|
|
25
|
Matsushita A, Götze T and Korc M:
Hepatocyte growth factor-mediated cell invasion in pancreatic
cancer cells is dependent on neuropilin-1. Cancer Res.
67:10309–10316. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fukasawa M, Matsushita A and Korc M:
Neuropilin-1 interacts with integrin beta1 and modulates pancreatic
cancer cell growth, survival and invasion. Cancer Biol Ther.
6:1173–1180. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Rowland-Goldsmith MA, Maruyama H, Kusama
T, Ralli S and Korc M: Soluble type II transforming growth
factor-beta (TGF-beta) receptor inhibits TGF-beta signaling in
COLO-357 pancreatic cancer cells in vitro and attenuates tumor
formation. Clin Cancer Res. 7:2931–2940. 2001.PubMed/NCBI
|
|
28
|
Kawamoto M, Ishiwata T, Cho K, Uchida E,
Korc M, Naito Z and Tajiri T: Nestin expression correlates with
nerve and retroperitoneal tissue invasion in pancreatic cancer. Hum
Pathol. 40:189–198. 2009. View Article : Google Scholar :
|
|
29
|
Kang N, Zhang JH, Qiu F, Tashiro S,
Onodera S and Ikejima T: Inhibition of EGFR signaling augments
oridonin-induced apoptosis in human laryngeal cancer cells via
enhancing oxidative stress coincident with activation of both the
intrinsic and extrinsic apoptotic pathways. Cancer Lett.
294:147–158. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Strasser A, Cory S and Adams JM:
Deciphering the rules of programmed cell death to improve therapy
of cancer and other diseases. EMBO J. 30:3667–3683. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Schimmer AD, Hedley DW, Penn LZ and Minden
MD: Receptor-and mitochondrial-mediated apoptosis in acute
leukemia: A translational view. Blood. 98:3541–3553. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Socolovsky M, Fallon AE, Wang S, Brugnara
C and Lodish HF: Fetal anemia and apoptosis of red cell progenitors
in Stat5a−/−5b−/− mice: A direct role for Stat5 in Bcl-X(L)
induction. Cell. 98:181–191. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Liotta LA, Steeg PS and Stetler-Stevenson
WG: Cancer metastasis and angiogenesis: An imbalance of positive
and negative regulation. Cell. 64:327–336. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hood JD and Cheresh DA: Role of integrins
in cell invasion and migration. Nat Rev Cancer. 2:91–100. 2002.
View Article : Google Scholar
|
|
35
|
Thoennissen NH, Iwanski GB, Doan NB,
Okamoto R, Lin P, Abbassi S, Song JH, Yin D, Toh M, Xie WD, et al:
Cucurbitacin B induces apoptosis by inhibition of the JAK/STAT
pathway and potentiates antiproliferative effects of gemcitabine on
pancreatic cancer cells. Cancer Res. 69:5876–5884. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ghaneh P, Kawesha A, Evans JD and
Neoptolemos JP: Molecular prognostic markers in pancreatic cancer.
J Hepatobiliary Pancreat Surg. 9:1–11. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Takahashi H, Chen MC, Pham H, Matsuo Y,
Ishiguro H, Reber HA, Takeyama H, Hines OJ and Eibl G: Simultaneous
knock-down of Bcl-xL and Mcl-1 induces apoptosis through Bax
activation in pancreatic cancer cells. Biochim Biophys Acta.
1833:2980–2987. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kloth MT, Catling AD and Silva CM: Novel
activation of STAT5b in response to epidermal growth factor. J Biol
Chem. 277:8693–8701. 2002. View Article : Google Scholar
|
|
39
|
Paukku K, Valgeirsdóttir S, Saharinen P,
Bergman M, Heldin CH and Silvennoinen O: Platelet-derived growth
factor (PDGF)-induced activation of signal transducer and activator
of transcription (Stat) 5 is mediated by PDGF beta-receptor and is
not dependent on c-src, fyn, jak1 or jak2 kinases. Biochem J.
345:759–766. 2000. View Article : Google Scholar : PubMed/NCBI
|