Introduction
Hepatocellular carcinoma (HCC) is the fifth most
prevalent malignancy diagnosed worldwide. However, the etiology and
progression events intrinsic to the malignant properties of HCC
remain obscure.
Fibulin-3, also called epidermal growth
factor-containing fibulin-like extracellular matrix protein 1
(EFEMP1), is a member of the fibulin family of extracellular
glycoproteins distributed in various human tissues (1). They modulate cell growth, morphology,
adhesion and motility (1). The
fibulin-3 encodes a 493-amino acid protein with a molecular mass of
54 kD, and its gene, located at chromosome 2p16, contains 11 exons
(1). Whether EFEMP1 promotes or
inhibits cancer development remains obscure in some tumors
(2–4). EFEMP1 was decreased due to methylation
in HCC cases (5), and it was
regarded as an oncogene and promotes HCC development (6). However, the underlying mechanism that
EFEMP1 promotes HCC development has not yet been explored.
Here we found that down-regulation of EFEMP1
significantly promoted HCC cell migration as determined in
vitro. Moreover, downregulation of EFEMP1 enhanced the
expression of MMP2 and MMP9. Importantly, downregulation of EFEMP1
attenuated the expression of MMP2 and MMP9 at least partially via
ERK1/2 activity. In clinical samples, MMP2 and MMP9 expression were
greatly higher in low EFEMP1 expression samples. EFEMP1 was
downregulated in HCC tissues, and lower EFEMP1 expression was
significantly associated with patients with ascites (P=0.050),
vascular invasion (P=0.044), poorer differentiation (P=0.002) and
higher clinical stage (P=0.003).
Materials and methods
Cell lines
Five cell lines including SMMC-7721, Bel-7402, H2P,
Huh7 and HepG2 were obtained from our laboratory cell bank
(Guangzhou, China). All cells were grown in Dulbecco's modified
Eagle's minimal essential medium (DMEM; Gibco, Carlsbad, CA, USA).
All media were supplemented with 8% fetal bovine serum (FBS;
Hyclone, Tauranga, New Zealand), 100 µg/µl
streptomycin and 100 µg/µl penicillin in a 37°C
incubator containing 5% CO2.
siRNA
Cells were seeded in 6-well plates at a density of
1×105 cells/well. Cationic lipid complex was prepared by
incubating 50 nM siRNA with 5 µl of
Lipofectamine® RNAiMAX Transfection reagent (Invitrogen,
Carlsbad, CA, USA) in 500 µl of Opti-MEM® I
Reduced Serum Medium (Invitrogen) for at least 20 min and added to
the cell medium. After 6-h incubation, the medium was replaced with
fresh medium. The cells were harvested at 24–72 h after
transfection for analysis. The EFEMP1 siRNA-1 (#1),
5′-GCAAUGCACUGACGGAU-AUdTdT-3′ and 3′-dTdTCGUUACGUGACUGCCUAUA-5′
and the EFEMP1 siRNA-2 (#2), 5′-GAGUUCUACCUACGACAAAdTdT-3′ and
3′-dTdTCUCAAGAUGGAUGCUGUUU-5′ were synthesized by Ribobio
(Guangzhou, China).
HCC cell line RNA extraction and
qRT-PCR
Total RNA was extracted using TRIzol reagent
(Invitrogen) according to the manufacturer's instruction. One
microgram of RNA from each sample was used for cDNA synthesis and
quantitative real-time PCR analysis (both from Roche, Basel,
Switzerland).
Western blotting
Standard western blotting was conducted for the
protein expression analyses. The protein contents of cleared
lysates were determined using a BCA Protein Quantitative Analysis
kit (CoWin Biotech Co., Ltd., China). The membranes were incubated
with primary antibodies overnight at 4°C and then with the
appropriate secondary antibody. The following primary antibodies
were used: EFEMP1 antibody (AP9095a), MMP-2 antibody (AM1844a),
MMP-9 (AP6214a) (all from Abgent, San Diego, CA, USA),
phospho-NF-κB p65 antibody (3033; Cell Signaling Technology),
anti-p44/42 MAPK (ERK1/2, 1:1,000), anti-phospho-p44/42 MAPK
(ERK1/2, 1:1,000), and anti-GAPDH (1:2,000; Cell Signaling
Technology, Danvers, MA, USA).
Migration assays
Migration was measured using Transwell cell culture
chambers according to the manufacturer's manual. We used 24-well
BioCoat cell culture inserts (BD) with a polyethylene terephthalate
membrane (8-µm porosity). Cells (0.5–2×105) were
placed into the top chamber of each insert and incubated at 37°C
for 24–48 h, according to the migration ability of different cell
lines.
Clinical samples and characteristics
Paraffin-embedded HCC specimens (n=215) were
analyzed. All cases had been clinically and histopathologically
diagnosed at The First Affiliated Hospital of Sun Yat-Sen
University (Guangzhou, China) from January 2014 to January 2015.
The histology of the disease was determined according to the
criteria of the World Health Organization. Tumor stage was defined
according to the tumor-node-metastasis (TNM) classification of the
American joint Committe on International Union against Cancer. For
research purposes, prior patient (or guardian) consent and approval
of the Institutional Research Ethics Committee were obtained. These
215 cases had full detailed clinical data, including age, gender,
ascites, cirrhosis, macroscopy, vascular invasion, differenciation,
tumor size, AFP, CA125 and clinical stage. This study was approved
by the Research Ethics Committee of The First Affiliated Hospital
at Sun Yat-sen University.
Immunohistochemistry
Four-micrometer-thick sections were deparaffinized,
rehydrated in serially graded ethanol, heated in citric buffer (pH
6.0) once for 20 min in a microwave oven for antigen retrieval, and
blocked with 3% hydrogen peroxide for 15 min. The samples were then
labeled with EFEMP1 antibody (AP9095a, 1:100), MMP-2 antibody
(AM1844a, 1:50), MMP-9 (AP6214a, 1:50) (all from Abgent) at 4°C
overnight. The next day, after washing with phosphate-buffered
saline (PBS), the sections were incubated with EnVision-HRP
secondary antibody (Dako, Carpinteria, CA, USA) for 30 min at 37°C
in a water bath, washed with PBS, stained with 0.5%
diaminobenzidine and counterstained with Mayer's hematoxylin, then
air dried, and mounted with resin.
Evaluation of immunohistochemistry
The immunohistochemical staining in HCC and non HCC
liver samples were subjected to microscopy and image analysis
(Nanozoomer, Hamamatsu, Japan). Briefly, after IHC staining, if a
cell or tissue was stained from light yellow to brown, it would be
recorded as positive immunostaining. The areas from cancer were
selected for analysis. The intensity of the staining signal was
measured and documented using the Image-Pro Plus 6.0 image analysis
software (Media Cybernetics, Inc., Silver Spring, MD USA). The mean
densitometry of the digital image (×400) is designated as
representative IHC staining intensity. The signal density of tissue
areas from three randomly selected visions were counted blindly and
subjected for statistical analysis.
Statistical analysis
Data between two groups were evaluated using a
two-tailed Student's t-test. Data among four groups were evaluated
using One-way Anova. For all comparisons, a p-value <0.05 was
considered statistically significant unless otherwise
indicated.
Results
EFEMP1 knock-down increases HCC cell
migration
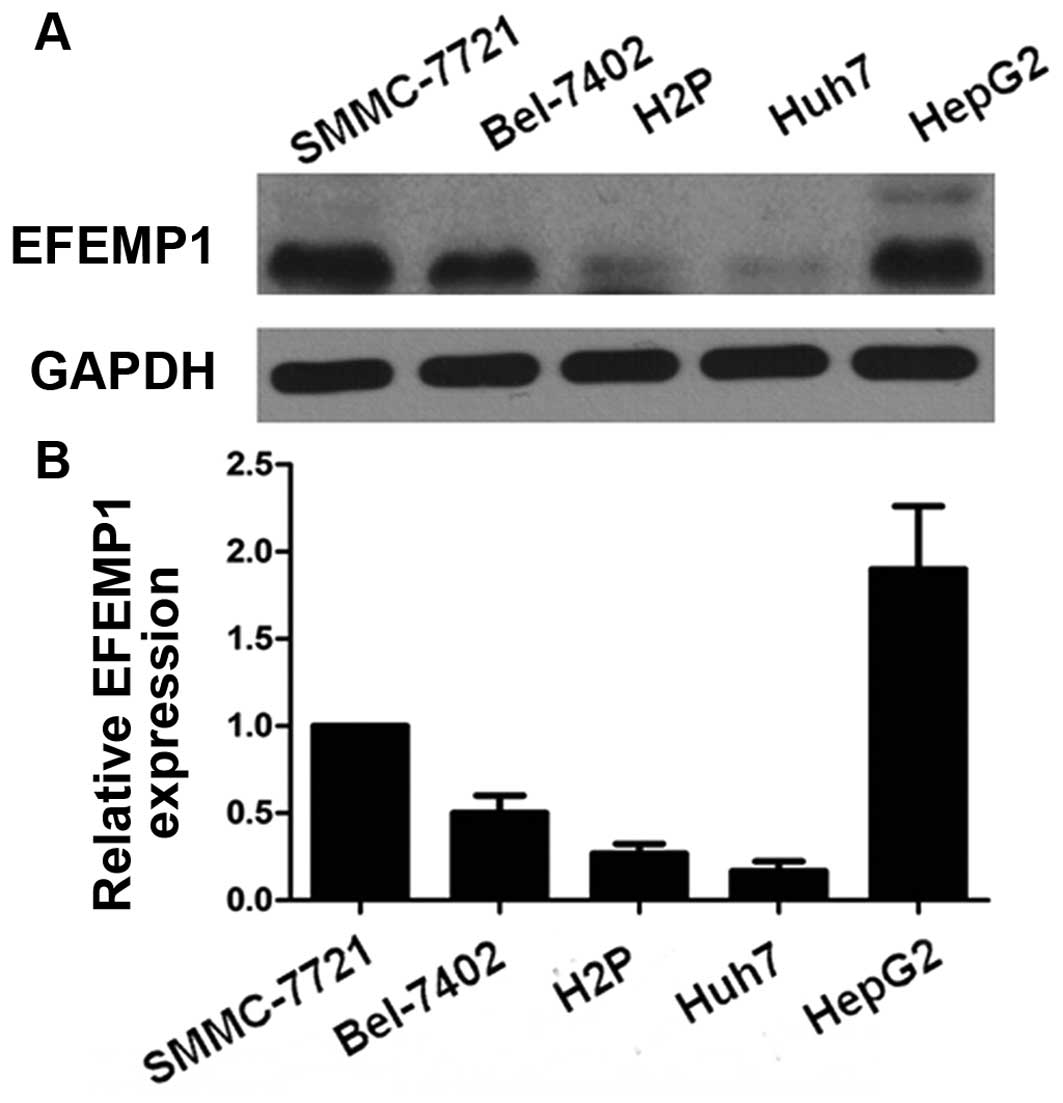
We first determined the expression level of EFEMP1
in HCC cell lines using qRT-PCR and western blotting. SMMC-7721,
HepG2 and Bel-7402 expressed noticeable EFEMP1 level while HuH7
expressed the lowest EFEMP1 level (Fig.
1).
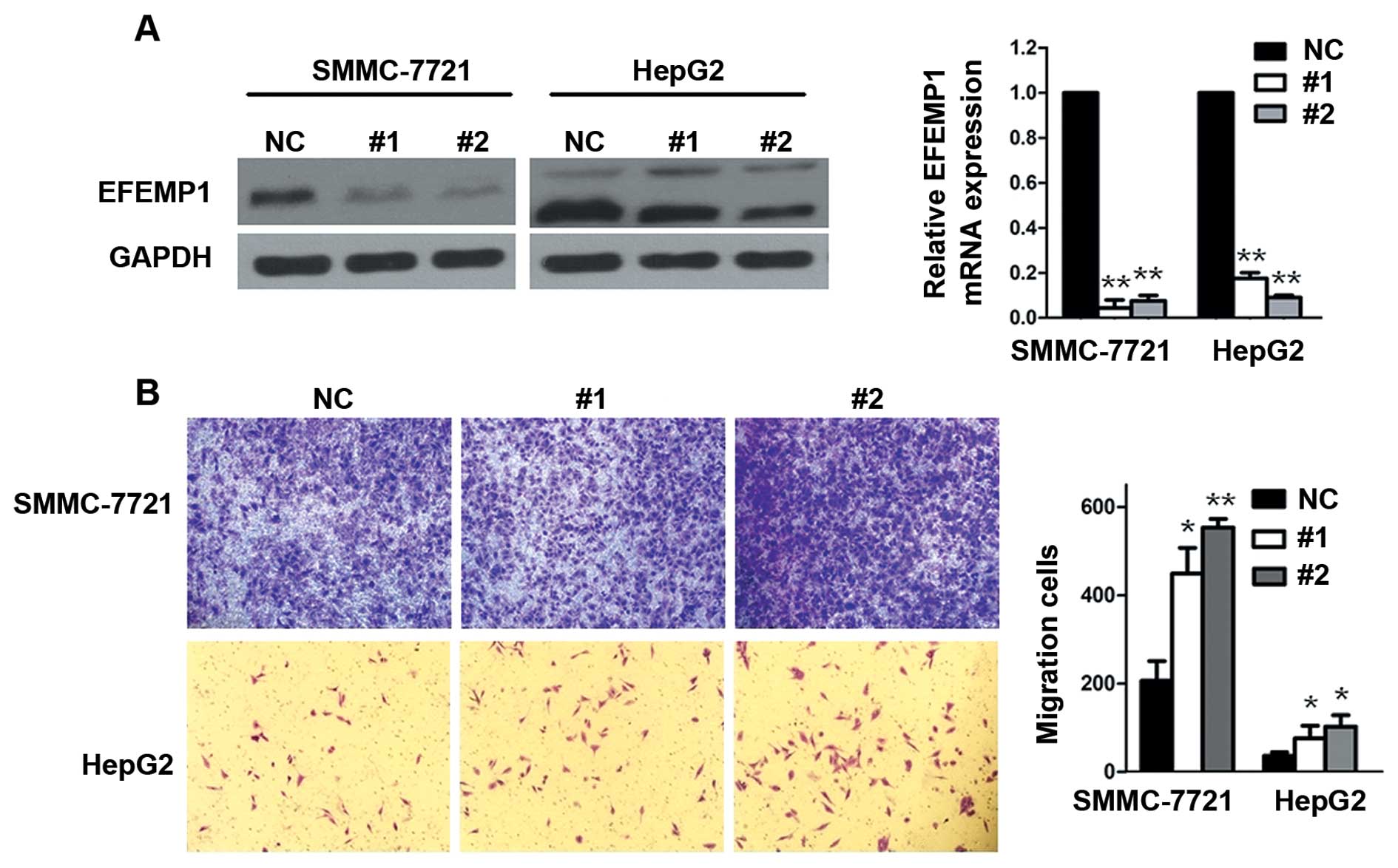
To confirm the effect of EFEMP1 on HCC cell
migration, siRNA techniques were used to inhibit the endogenous
expression of EFEMP1 (Fig. 2A). We
chose relatively higher EFEMP1 expression HCC cell lines (SMMC-7721
and HepG2). Results showed that EFEMP1 inhibition significantly
increased serum-induced transwell migration in these cells
(Fig. 2B).
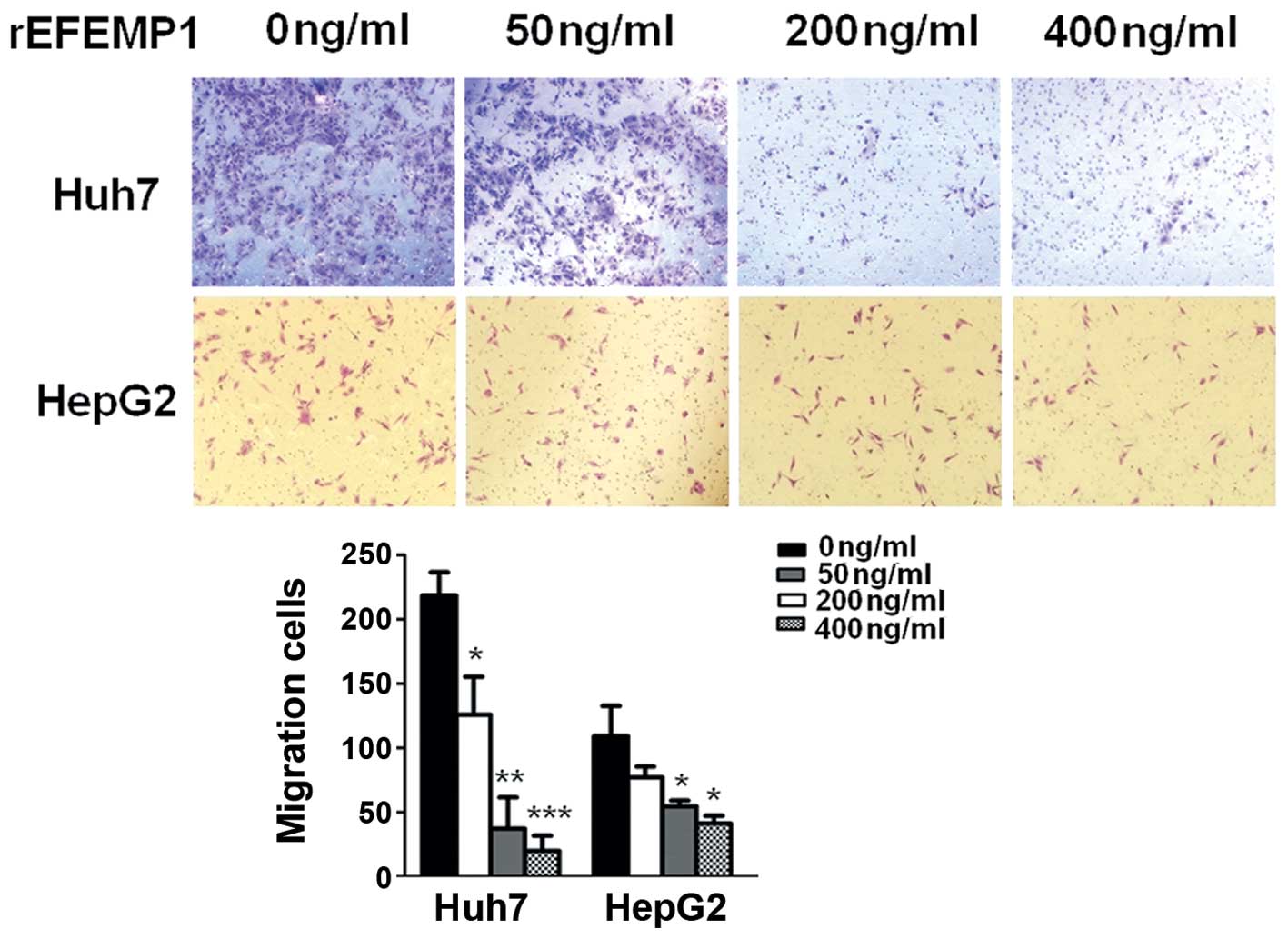
Exogenous EFEMP1 attenuated HCC cells
migration
EFEMP1 is an extracellular matrix protein. HCC cells
were treated with purified EFEMP1 protein to induce exogenous
overexpression of EFEMP1 in tumor microenvironment. The migratory
ability of HCC cells after treatment with EFEMP1 protein (50, 200
and 400 ng/ml) were strongly reduced compared to that of the
negative controls in Huh7 cells (Fig.
3). However, in HepG2 cells, significant changes were found
only in very high concentration (200 and 400 ng/ml) of EFEMP1
protein (Fig. 3).
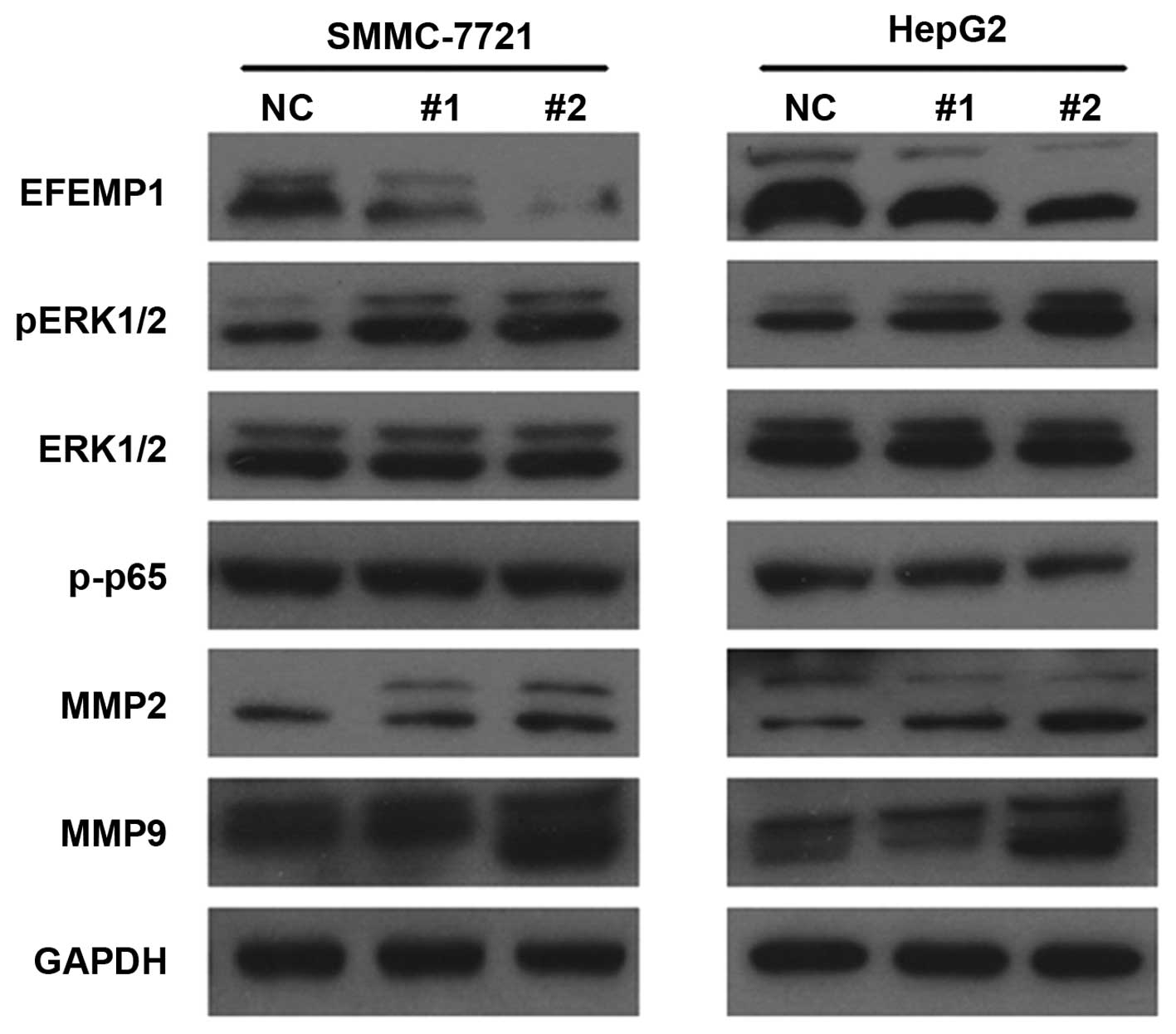
Downregulation of EFEMP1 increased the
expression of pERK1/2, MMP2 and MMP9
To investigate the molecular mechanism of
EFEMP1-mediated HCC cell migration, we found that MMP2 and MMP9
levels increased after down-regulating EFEMP1 (Fig. 4). In addition, pERK1/2 increased
without changing ERK1/2 expression (Fig. 4).
We further used U0126, a highly selective and potent
inhibitor of pERK1/2, to investigate the modulating role of EFEMP1.
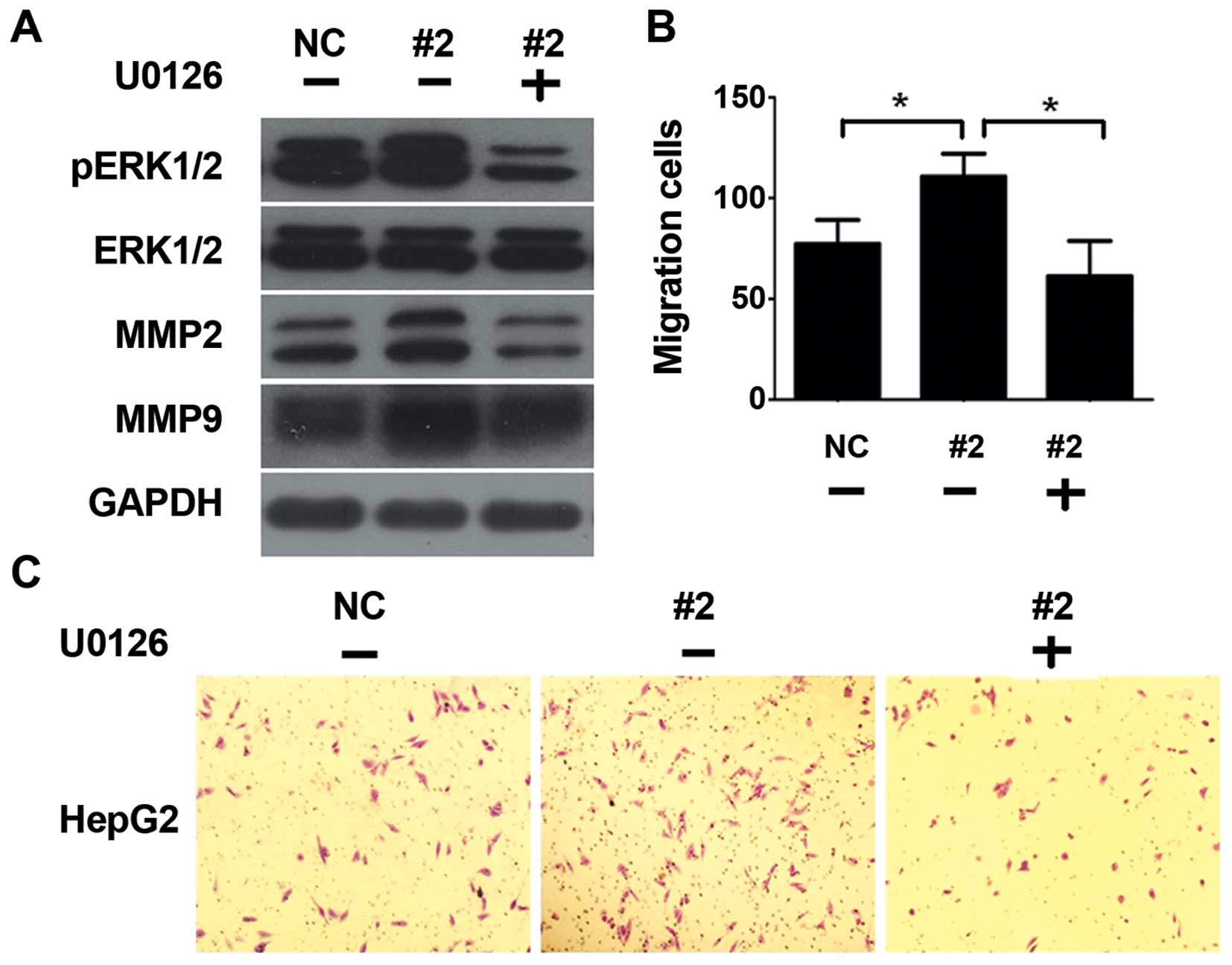
As was shown in Fig. 5A, the
expression levels of pERK1/2 were significantly reduced after using
U0126, accompanied by the decreasing of MMP2 and MMP9 levels. U0126
abrogate the migration ability enhanced by siRNA (Fig. 5B and C). These results suggest that
down-regulation of EFEMP1 enhanced the expression of MMP2 and MMP9
at least partially via ERK1/2 activity.
EFEMP1 expression in HCC tissues is
inversely associated with MMP2 and MMP9 levels
We next detected EFEMP1 expression in clinical
samples by immunohistochemistry and found that EFEMP1 mainly
located in the cytoplasm with minor nuclei distribution. In eight
pairs of HCC tumor tissues and non-tumor liver samples (data not
shown), EFEMP1 expression was much lower in HCC tissues than in
non-tumor liver tissues.
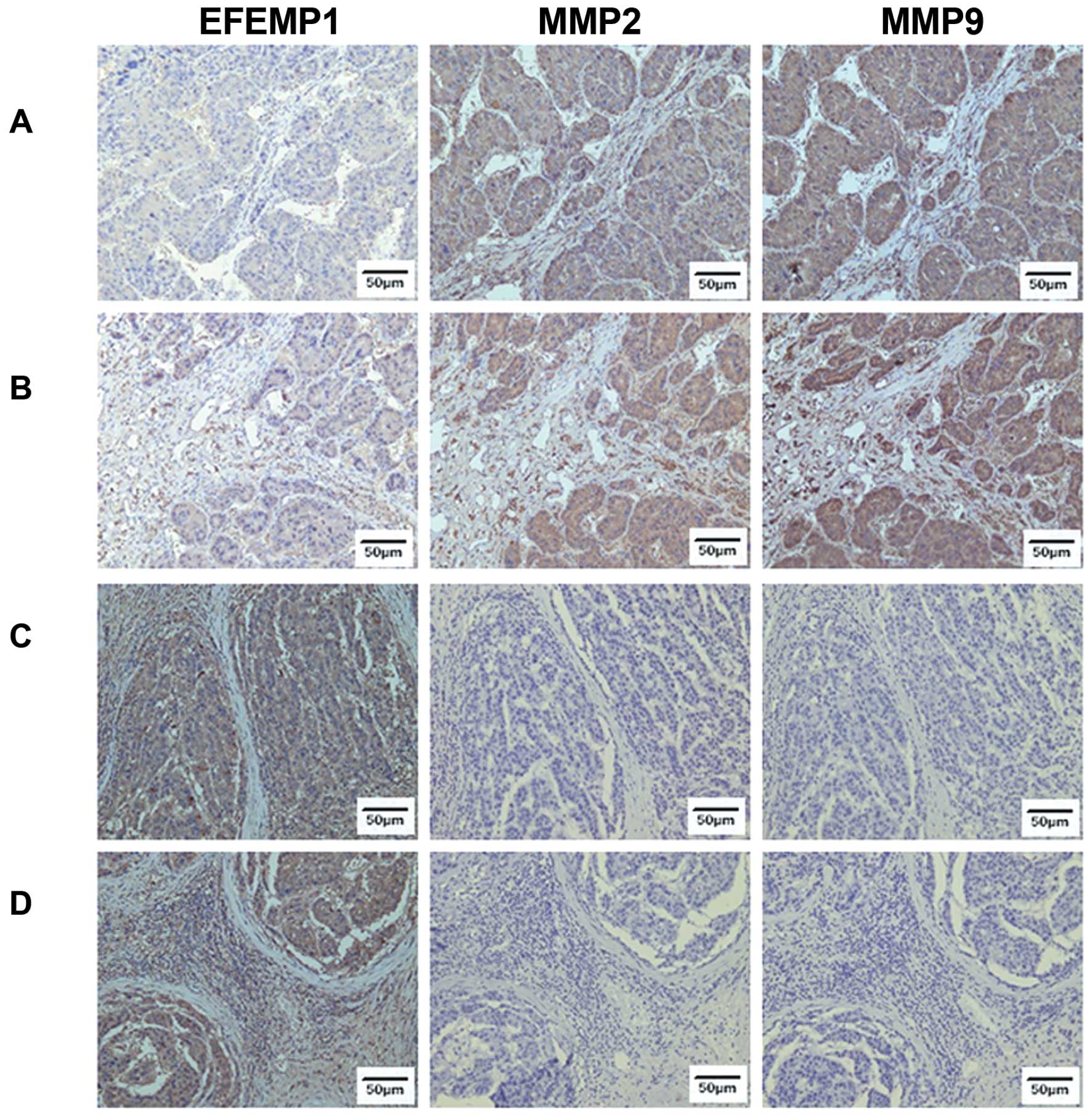
We then detect EFEMP1, MMP2 and MMP9 in both high
EFEMP1 expression and low EFEMP1 expression HCC samples. We found
that low EFEMP1 expression samples expressed high MMP2 and MMP9
levels (Fig. 6A and B), while high
EFEMP1 expression samples expressed low MMP2 and MMP9 levels
(Fig. 6C and D).
Association between EFEMP1 expression and
clinicopathological features
We also investigated the relationship between EFEMP1
expression and clinical pathological features of HCC patients. A
total of 215 patients had EFEMP1 expression of 0.232±0.162. The
statistical analysis demonstrated that lower EFEMP1 expression was
significantly associated with patients who had ascites (P=0.050),
vascular invasion (P=0.044), poorer differentiation (P=0.002) and
higher clinical stage (P=0.003) (Table
I). However, patients who had higher AFP level showed no
significant difference compared to patients who had normal AFP
level in EFEMP1 expression.
 | Table ICorrelation between EFEMP1 expression
and clinicopathologic variables. |
Table I
Correlation between EFEMP1 expression
and clinicopathologic variables.
| Variable | Cases | EFEMP1
expression | P-value |
|---|
| Age (years) | | | 0.541 |
| ≤40 | 46 | 0.242±0.161 | |
| >40 | 169 | 0.230±0.163 | |
| Gender | | | 0.150 |
| Male | 191 | 0.226±0.161 | |
| Female | 24 | 0.282±0.165 | |
| Ascites | | | 0.050 |
| yes | 40 | 0.187±0.151 | |
| No | 175 | 0.243±0.163 | |
| Cirrhosis | | | 0.931 |
| No-mild | 165 | 0.232±0.165 | |
| Moderate-severe | 50 | 0.234±0.152 | |
| Tumor
multiplicity | | | 0.358 |
| Single | 157 | 0.239±0.167 | |
| Multiple | 58 | 0.216±0.147 | |
| Macroscopy | | | 0.128 |
| Nodular | 174 | 0.247±0.167 | |
| Nodular merged | 6 | 0.164±0.123 | |
| Massive | 5 | 0.161±0.075 | |
| Giant | 30 | 0.170±0.129 | |
| Vascular
invasion | | | |
| Yes | 27 | 0.174±0.121 | 0.044 |
| No | 188 | 0.241±0.166 | |
| Tumor size (cm) | | | |
| ≤5 | 104 | 0.248±0.162 | 0.159 |
| >5 | 111 | 0.217±0.161 | |
| AFP (ng/ml) | | | 0.069 |
| ≤20 | 96 | 0.255±0.161 | |
| >20 | 119 | 0.214±0.161 | |
| CA125 (U/ml) | | | 0.829 |
| ≤35 | 161 | 0.234±0.162 | |
| >35 | 54 | 0.228±0.163 | |
| Stage | | | 0.003 |
| I | 52 | 0.374±0.154 | |
| II | 50 | 0.248±0.137 | |
| III | 107 | 0.161±0.123 | |
| IV | 6 | 0.146±0.220 | |
| Differentiation | | | 0.002 |
| Well-moderate | 133 | 0.260±0.159 | |
|
Poor-undifferentiated | 82 | 0.188±0.158 | |
Discussion
EFEMP1 as an antitumor glycoprotein in HCC has been
confirmed in some studies (5,6).
EFEMP1 was decreased in HCC patients and was associated with
unfavorable prognosis, and it acts as an independent prognostic
biomarker in HCC. Downregulation of EFEMP1 increased cell viability
and promoted cell invasion in HCC cells (5). We found that knockdown of EFEMP1
promoted HCC cell migration. Adding purified EFEMP1 protein
inhibited HCC cell migration. This indicated that EFEMP1 inversely
correlated HCC cell migration. Only very high concentration (200
and 400 ng/ml) of EFEMP1 protein could inhibit HepG2 migration.
This might due to HepG2 expressing high EFEMP1, and therefore this
cell line was not sensitive to low EFEMP1 protein concentration
stimulation.
EFEMP1 (also called fibulin-3) is a member of the
fibulin family of extracellular glycoproteins which are distributed
in various human tissues (1). Luo
et al (6) demonstrated that
EFEMP1 was present both in the cytoplasm and the nucleus. However,
we found that this protein mainly distributed in cytoplasm. This
might due to the antibodies used from different companies. In
agreement with our data, EFEMP1 protein expression was decreased in
HCC patients. Lower EFEMP1 correlated with higher stage and poor
differentiation. In addition, we found that HCC patients with
ascites and vascular invasion had lower EFEMP1 expression. These
results further confirmed that EFEMP1 played a pivotal role in HCC
development. AFP assessment is comprehensively used in clinical
test for HCC patients. Our data found that AFP was not associated
with EFEMP1 expression in HCC patients. In addition, recent studies
revealed that AFP assessment lacks adequate sensitivity and
specificity for effective surveillance and diagnosis (7). Collectively, EFEMP1 might be of
clinical significance in predicting the prognosis of HCC
patients.
Effects of EFEMP1 on tumor progression have been
reported in two aspects. One aspect is its pro-tumor role. By
increasing the expression of VEGF, overexpression of EFEMP1 in HeLa
cells promotes angiogenesis, proliferation and invasion (8). In pancreatic adenocarcinomas, EFEMP1
binds EGFR (competitive to EGF) leading to autophosphorylation of
EGFR at Tyr-992 and Tyr-1068 and the subsequent phosphorylation of
AKT and ERK and, then, accelerates pancreatic adenocarcinoma growth
(9). By promoting Notch-1 cleavage
and upregulating the active Notch-1 intracellular domain (NICD),
EFEMP1 promoted glioma growth and reduce apoptosis (4). Another aspect is its antitumor role.
Overexpression of EFEMP1 inhibited malignant glioma proliferation
by suppressing EGFR-AKT signaling (3). Hwang et al reported that EFEMP1
inhibited nasopharyngeal carcinoma cell migration and invasion by
decreasing the phosphorylation of AKT at Ser-473 (10). In addition, EFEMP1 sensitized
pancreatic cancer cells to a PI3K/mTOR inhibitor by interacting
with p27Kip1 (11).
However, the mechanism of how EFEMP1 affects HCC cell migration
remained obscure. In the present study, we found that EFEMP1 could
negatively regulate the expression of pERK1/2 without changing
total ERK1/2, which might explain the negative regulation role of
EFEMP1 in HCC.
The antitumor and pro-tumor molecular mechanism of
EFEMP1 may occur via different ways. One of the mechanisms may
occur via an association with MMPs. Our previous study found that
EFEMP1 can increase the expression and activity of MMP-2 and MMP-9
in malignant gliomas (12).
However, another connection between EFEMP1 and MMPs is their
opposite expression levels. EFEMP1 abrogated angiogenic activities
and sprouting in MB114 cells by decreasing the expression of MMP-2
and MMP-3 (13). EFEMP1 is
associated with decreased MMP-2 and MMP-7 levels in lung cancer
(14) and MMP-2 and MMP-9 levels in
endometrial carcinoma (15), which
are somewhat similar to our data. We found that down-regulation of
EFEMP1 increased the expression of MMP2 and MMP9, and MMP2 and MMP9
level in clinical samples showed the same tendency: the lower the
EFEMP1 the higher the MMP2 and MMP9 expression was. Furthermore,
such regulating role of EFEMP1 was modulated through ERK1/2
activity.
In summary, our findings provide an understanding
that EFEMP1 negatively modulate the migration ability in HCC.
Reduction of EFEMP1 promotes the migration ability though
increasing MMP2 and MMP9 expression via ERK1/2 activity.
Acknowledgments
This study was supported by the National Natural
Science Foundation of China (grant nos. 81272636, 81201582 and
81172232), the Foundations of China National Science (no.
81402465), and the Foundation of China Guangdong Science and
Technology (no. 2013B021800128).
References
|
1
|
Timpl R, Sasaki T, Kostka G and Chu ML:
Fibulins: A versatile family of extracellular matrix proteins. Nat
Rev Mol Cell Biol. 4:479–489. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Obaya AJ, Rua S, Moncada-Pazos A and Cal
S: The dual role of fibulins in tumorigenesis. Cancer Lett.
325:132–138. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hu Y, Pioli PD, Siegel E, Zhang Q, Nelson
J, Chaturbedi A, Mathews MS, Ro DI, Alkafeef S, Hsu N, et al:
EFEMP1 suppresses malignant glioma growth and exerts its action
within the tumor extracellular compartment. Mol Cancer. 10:1232011.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hu B, Nandhu MS, Sim H, Agudelo-Garcia PA,
Saldivar JC, Dolan CE, Mora ME, Nuovo GJ, Cole SE and Viapiano MS:
Fibulin-3 promotes glioma growth and resistance through a novel
paracrine regulation of Notch signaling. Cancer Res. 72:3873–3885.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nomoto S, Kanda M, Okamura Y, Nishikawa Y,
Qiyong L, Fujii T, Sugimoto H, Takeda S and Nakao A: Epidermal
growth factor-containing fibulin-like extracellular matrix protein
1, EFEMP1, a novel tumor-suppressor gene detected in hepatocellular
carcinoma using double combination array analysis. Ann Surg Oncol.
17:923–932. 2010. View Article : Google Scholar
|
|
6
|
Luo R, Zhang M, Liu L, Lu S, Zhang CZ and
Yun J: Decrease of fibulin-3 in hepatocellular carcinoma indicates
poor prognosis. PLoS One. 8:e705112013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Attwa MH and El-Etreby SA: Guide for
diagnosis and treatment of hepatocellular carcinoma. World J
Hepatol. 7:1632–1651. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Song EL, Hou YP, Yu SP, Chen SG, Huang JT,
Luo T, Kong LP, Xu J and Wang HQ: EFEMP1 expression promotes
angiogenesis and accelerates the growth of cervical cancer in vivo.
Gynecol Oncol. 121:174–180. 2011. View Article : Google Scholar
|
|
9
|
Camaj P, Seeliger H, Ischenko I, Krebs S,
Blum H, De Toni EN, Faktorova D, Jauch KW and Bruns CJ: EFEMP1
binds the EGF receptor and activates MAPK and Akt pathways in
pancreatic carcinoma cells. Biol Chem. 390:1293–1302. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hwang CF, Chien CY, Huang SC, Yin YF,
Huang CC, Fang FM, Tsai HT, Su LJ and Chen CH: Fibulin-3 is
associated with tumour progression and a poor prognosis in
nasopharyngeal carcinomas and inhibits cell migration and invasion
via suppressed AKT activity. J Pathol. 222:367–379. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Diersch S, Wenzel P, Szameitat M, Eser P,
Paul MC, Seidler B, Eser S, Messer M, Reichert M, Pagel P, et al:
Efemp1 and p27(Kip1) modulate responsiveness of pancreatic cancer
cells towards a dual PI3K/mTOR inhibitor in preclinical models.
Oncotarget. 4:277–288. 2013.PubMed/NCBI
|
|
12
|
Wang Z, Cao CJ, Huang LL, Ke ZF, Luo CJ,
Lin ZW, Wang F, Zhang YQ and Wang LT: EFEMP1 promotes the migration
and invasion of osteosarcoma via MMP-2 with induction by AEG-1 via
NF-κB signaling pathway. Oncotarget. 6:14191–14208. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Albig AR, Neil JR and Schiemann WP:
Fibulins 3 and 5 antagonize tumor angiogenesis in vivo. Cancer Res.
66:2621–2629. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xu S, Yang Y, Sun YB, Wang HY, Sun CB and
Zhang X: Role of fibulin-3 in lung cancer: In vivo and in vitro
analyses. Oncol Rep. 31:79–86. 2014.
|
|
15
|
Yang T, Qiu H, Bao W, Li B, Lu C, Du G,
Luo X, Wang L and Wan X: Epigenetic inactivation of EFEMP1 is
associated with tumor suppressive function in endometrial
carcinoma. PLoS One. 8:e674582013. View Article : Google Scholar : PubMed/NCBI
|