Introduction
Tongue squamous cell carcinoma (TSCC) is one type of
head and neck squamous cell carcinoma and remains one of the top 10
leading cancers in the US (1).
Despite the largely improved surgical and medical management of
patients with TSCC, recurrence of the carcinoma remains one of the
crucial impediments in its treatment (2). The main reason for the high mortality
rate is the lack of diagnostic methods for early stage detection
and effective strategies for treatment. Therefore, understanding
the molecular pathogenesis and uncovering molecular biomarkers of
TSCC would facilitate early detection and improve the survival of
patients.
Karyopherin α2 (KPNA2), a member of the importin α
family, is thought to play an important role in nucleocytoplasmic
transport (3–5). Recently, clinical studies have
demonstrated that KPNA2 is upregulated in multiple malignancies and
is associated with an adverse outcome in affected patients
(6–12). The biological functions of KPNA2
have been examined in various cancer cell lines; for example,
overexpression of KPNA2 in a benign breast cell line increased cell
colony formation ability and migration activity in a manner similar
to that found in malignant cells (13). KPNA2 can also enhance cell migratory
ability and viability (13).
Additionally, knockdown of KPNA2 inhibited the proliferation of
cells derived from prostate (12)
and ovarian cancer (14). However,
the role of KPNA2 in TSCC disease progression remains unclear.
Although KPNA2 plays an important role in cancers, limited
information is available regarding factors that control its
expression. Therefore, the molecular mechanisms underlying
knockdown of KPNA2 remain to be further elucidated.
In the present study, we reported that KPNA2
knockdown inhibited the growth and migration, and induced apoptosis
of TSCC, and the molecular mechanisms were found to be associated
with the enhancement of intracellular caspase-3 and activation of
the p53 signaling pathway. In brief, the results of our study may
provide greater insight into improving the therapeutic efficacy of
TSCC.
Materials and methods
Reagents
Antibodies for Bax and Bcl-2 were obtained from
Sangong Biotech (Shanghai, China). KPNA2, Bad, p-Bad, cytochrome
c and p53 were purchased from Cell Signaling Technology
(Danvers, MA, USA), p21Cip1/Waf1 was purchased from BD
Pharmingen (Franklin Lakes, NJ, USA), and p16INK4a was
obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Other
chemicals were purchased from Sigma-Aldrich (St. Louis, MO,
USA).
CAL-27 cell culture
The TSCC CAL-27 cell line was provided by the
Department of Oral and Maxillofacial Surgery, The Second Affiliated
Hospital of Harbin Medical University (Heilongjiang, China), and
cultured in RPMI-1640 medium containing 10% (v/v) heat-inactivated
fetal bovine serum (FBS; Gibco, Grand Island, NY, USA) at 37°C in
an incubator containing humidified air with 5% (v/v)
CO2.
RNA interference
The cells were transfected using the Lipofectamine
2000 reagent (Life Technologies, Carlsbad, CA, USA) according to
the manufacturer's instructions. A pool of two sequence-validated
and knockdown-warranted KPNA2-siRNA were used: homo-1111,
5′-GACUCAGGUUGUGAUUGAUTT-3′ and 5′-AUCAAUCACAACCUGAGUCTT-3′;
homo-1400, 5′-CCGUUGAUGAACCUCUUAATT-3′ and
5′-UUAAGAGGUUCAUCAACGGTT-3′ (GenePharma, Shanghai, China).
Commercial FAM-tagged, negative control siRNAs (NC siRNAs),
5′-UCCUCCGAACGUGUCACGUTT-3′ and 5′-ACGUGACACGUUCGGAGAATT-3′
(GenePharma) were used as an efficiency control and as a control
for unspecific side-effects. Cell lysates were prepared for western
blotting 48 h after transfection to determine the efficiency of
gene expression ablation.
MTT cell proliferation and viability
assay
An MTT assay was carried out to evaluate the cell
viability as previously described (15). Cells (8×103) were seeded
onto 96-well plates 24 h before treatment with KPNA2-siRNA for 48 h
using Lipofectamine 2000. After 48 h, 15 µl (5 mg/ml) MTT
(Sigma-Aldrich) was added to each well, and the cells were
incubated for a further 4 h at 37°C. After incubation, 150
µl dimethyl sulfoxide (DMSO) was added to dissolve the
crystals. The mixtures were shaken for 10–15 min to fully dissolve
the crystals. Absobance (A570) was measured using a Tecan
microplate reader, and cell viability was calculated as the
percentage change in A570 between the control and treated
cells.
Acridine orange/ethidium bromide
fluorescence staining
The cells were incubated with acridine orange and
ethidium bromide (AO/EB) mixing solution for 5 min (Solarbio of
Biotechnology, Beijing, China; http://solarbio.en.alibaba.com) (16). Cellular morphological changes were
examined using fluorescence microscopy (3200) at a magnification of
×200. The percentage of apoptotic cells was calculated by the
following formula: Apoptotic rate (%) = number of apoptotic
cells/number of all cells counted (17,18).
Western blot analysis
Total protein was extracted from the CAL-27 cells
for immunoblotting analysis. Protein samples (80 µg of
protein) were separated with 12% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and blotted
to nitrocellulose membranes. After blocking, the membranes were
probed with Bcl-2 (1:1,000 dilution), Bax (1:1,000 dilution), Bad
(1:500 dilution), p-Bad (1:500 dilution), cytochrome c
(1:500 dilution), KPNA2 (1:1,000 dilution), XIAP (1:500 dilution),
p53 (1:500 dilution), p21Cip1/Waf1 (1:500 dilution),
p16INK4a (1:200 dilution) and glyceraldehyde-3-phosphate
dehydrogenase (GAPDH; 1:1,000 dilution) antibodies incubated
overnight at 4°C. Infrared (IR) fluorescent dye-labeled secondary
antibody (Alexa Fluor; Molecular Probes, Eugene, OR, USA;
http://probes.invitrogen.com) was
incubated with the membrane for 1 h. Western blotting bands were
collected using an IR Imaging System (LI-COR Biosciences, Lincoln,
NE, USA; http://www.licor.com), and the band
density was quantified using Odyssey 3.0 software for each group
and normalized to GAPDH.
Caspase-3 activity assay
A total of 5×103 cells were seeded in
96-well cell culture plates. After a 48 h siRNA treatment,
apoptosis rates were measured based on the activation of caspase-3
effectors using the caspase-3 activity assay kit (Beyotime, China)
according to the manufacturer's instructions. All samples were
performed in triplicate.
Wound healing assay
CAL-27 cells were cultured in a 6-well plate for 24
h. Wound healing assay was carried out by introducing a small
linear scratch with a pipette tip. Then, the cells in the each well
were exposed to serum-free Dulbecco's modified Eagle's medium
(DMEM) containing the indicated concentrations of KPNA2-siRNA for
48 h. The intervals after the scratch in the cultured cells were
photographed under a phase-contrast microscope (magnification,
x200) to monitor the cell migration ability.
Transwell assay
Transwell assay was performed using a Transwell
chamber with pore size of 8.0 µm (Millipore, Billerica, MA,
USA). The cells were resuspended in serum-free medium, and then
planted into the upper chamber with 5% CO2 at 37°C.
After treatment with KPNA2-siRNA for 48 h, the cells in the upper
chamber were removed, and the attached cells in the lower section
were stained with 0.1% crystal violet. The migration rate was
quantified by counting the migrating cells in 6 random fields under
a light microscope.
Data analysis
Data were obtained from 3 to 6 independent
experiments and are presented as the mean ± standard deviation.
Data were evaluated by the unpaired Student's t-test, and P<0.05
was considered to represent a significant difference.
Results
KPNA2 knockdown suppresses the viability
of CAL-27 cells
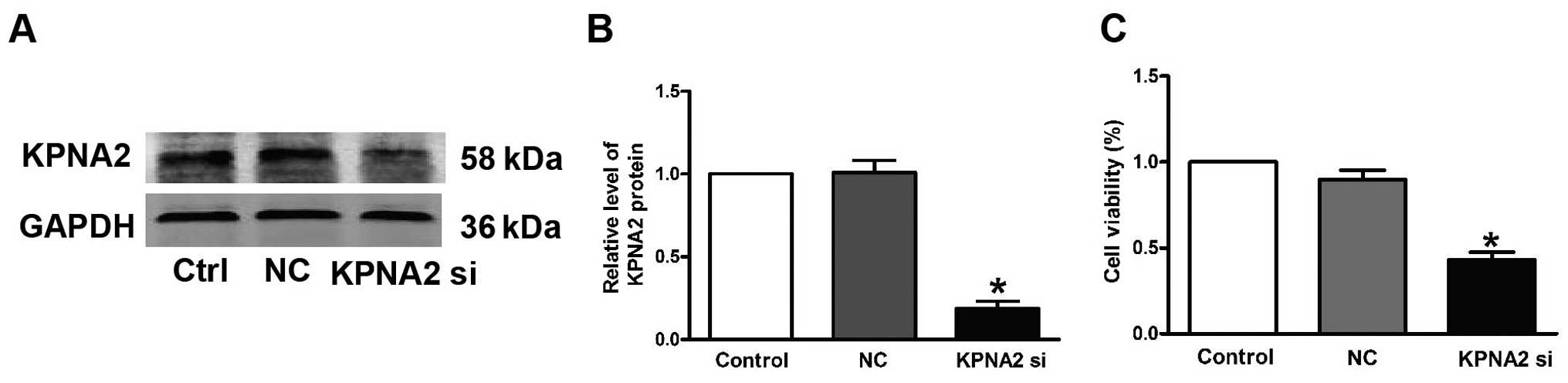
Successful transfection of KPNA2-siRNA was verified
by our data shown in Fig. 1A and B.
Western blotting showed that the KPNA2 protein level was decreased
(Fig. 1A and B). Fig. 1C shows that the viability of the
CAL-27 cells transfected with KPNA2-siRNA was reduced by
58.1±6.8%.
KPNA2 knockdown induces the apoptosis of
CAL-27 cells
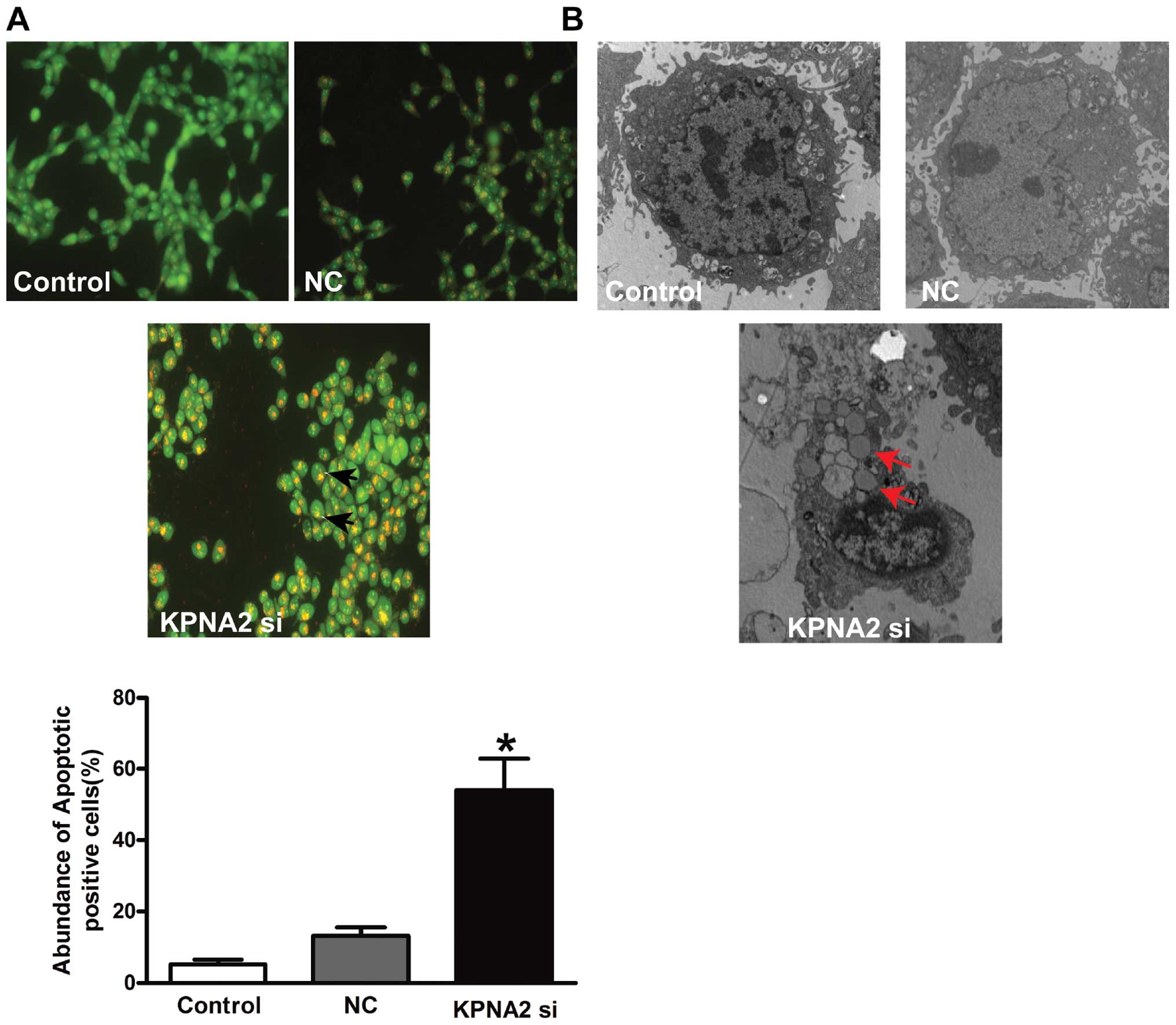
To assess whether increased apoptosis was involved
in the significant decrease in cell viability following KPNA2
knockdown, AO/EB staining and electron microscopy were used to
detect apoptotic cells. The results from our fluorescence
microscopic analysis are shown in Fig.
2A. Three types of cells were recognized under a fluorescence
microscope: live cells (green), apoptotic cells (yellow) and
necrotic cells (red). Forced expression following KPNA2 knockdown
induced substantial apoptotic cells (P<0.05), while the empty
vector failed to do so. Under an electron microscope, the cells
with KPNA2 knockdown exhibited robust changes in microstructure,
including cell surface microvilli reduction, nuclear chromatin
condensation, margination and membrane blistering (Fig. 2B).
KPNA2 knockdown inhibits the migration of
CAL-27 cells
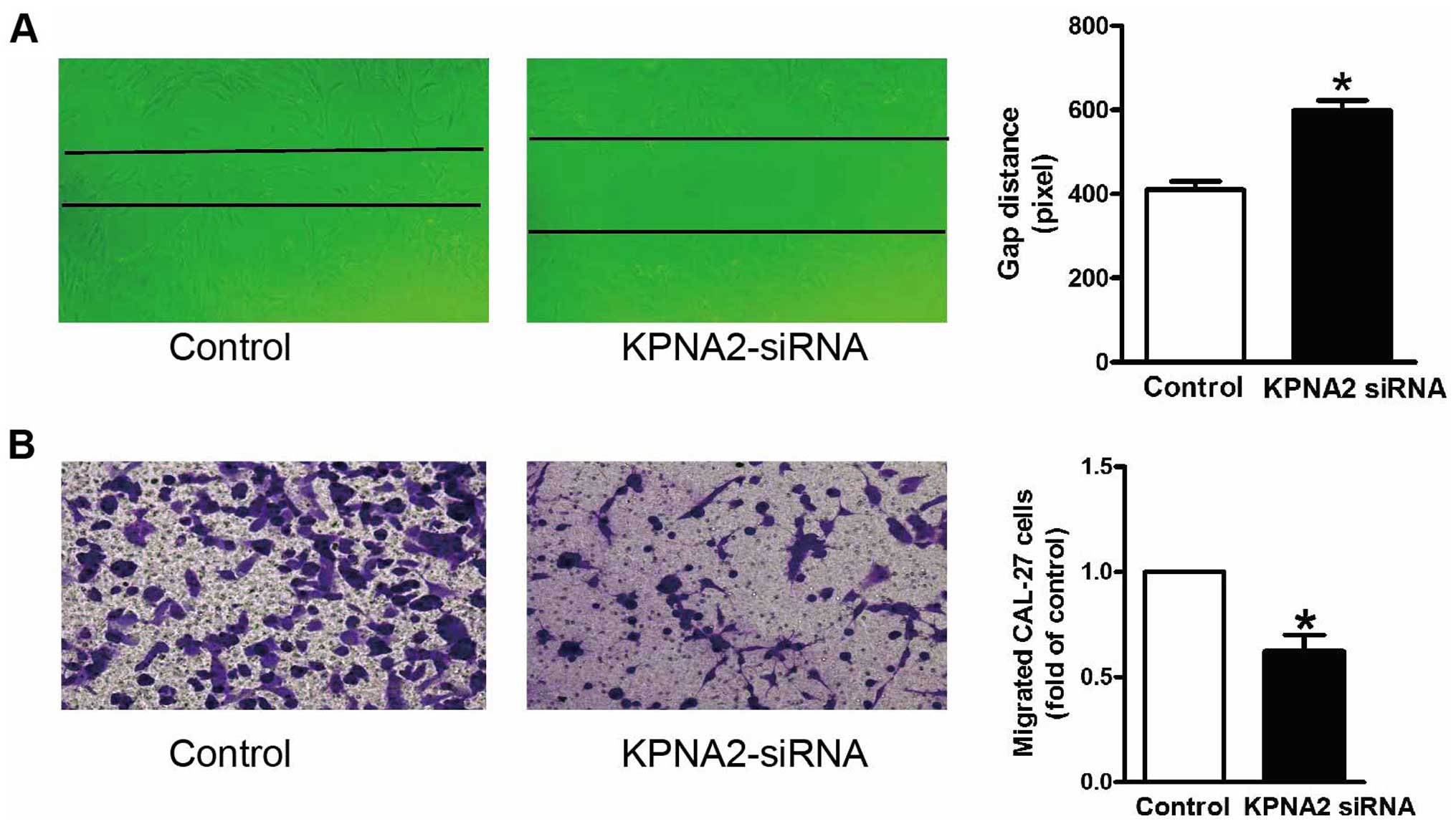
In addition to cellular proliferation, we also
examined the effect of KPNA2 on the migration activity of CAL-27
cells. The wound healing assay in vitro was carried out to
determine the cell migration ability of the CAL-27 cells in the
absence and presence of KPNA2 knockdown. Fig. 3A shows the quantified wound closure
in the CAL-27 cells after KPNA2-siRNA treatment. Wound closure was
reduced in the KPNA2-siRNA-treated CAL-27 cells at 48 h after
wounding, compared with the rate of closure of the control group.
Moreover, Transwell migration assay was also performed in the
CAL-27 cells after KPNA2-siRNA treatment. In agreement with the
wound healing assay, treatment with KPNA2-siRNA produced a
reduction in the number of migrated cells (Fig. 3B). These data demonstrated that
KPNA2 induced the suppression of migration of the CAL-27 cells.
KPNA2 knockdown activates
caspase-dependent proapoptotic signaling pathways
To explore the mechanisms by which KPNA2 knockdown
induces apoptosis in CAL-27 cells, we measured the downstream
proteins of the KPNA2 apoptotic pathway, including Bax, Bcl-2, Bad,
p-Bad cytochrome c and XIAP. Silencing of KPNA2 upregulated
Bad, cytochrome c and Bax, and downregulated p-Bad and Bcl-2
expression (Fig. 4A, B and D).
Knockdown of KPNA2 decreased XIAP protein expression, which acts as
a vital anti-apoptotic protein (Fig.
4E), while the empty vector did not affect the above proteins.
In addition, relative caspase-3 activity was significantly
increased by 2.2-fold due to KPNA2-siRNA transfection, but no
obvious change was noted with the NC (Fig. 4C).
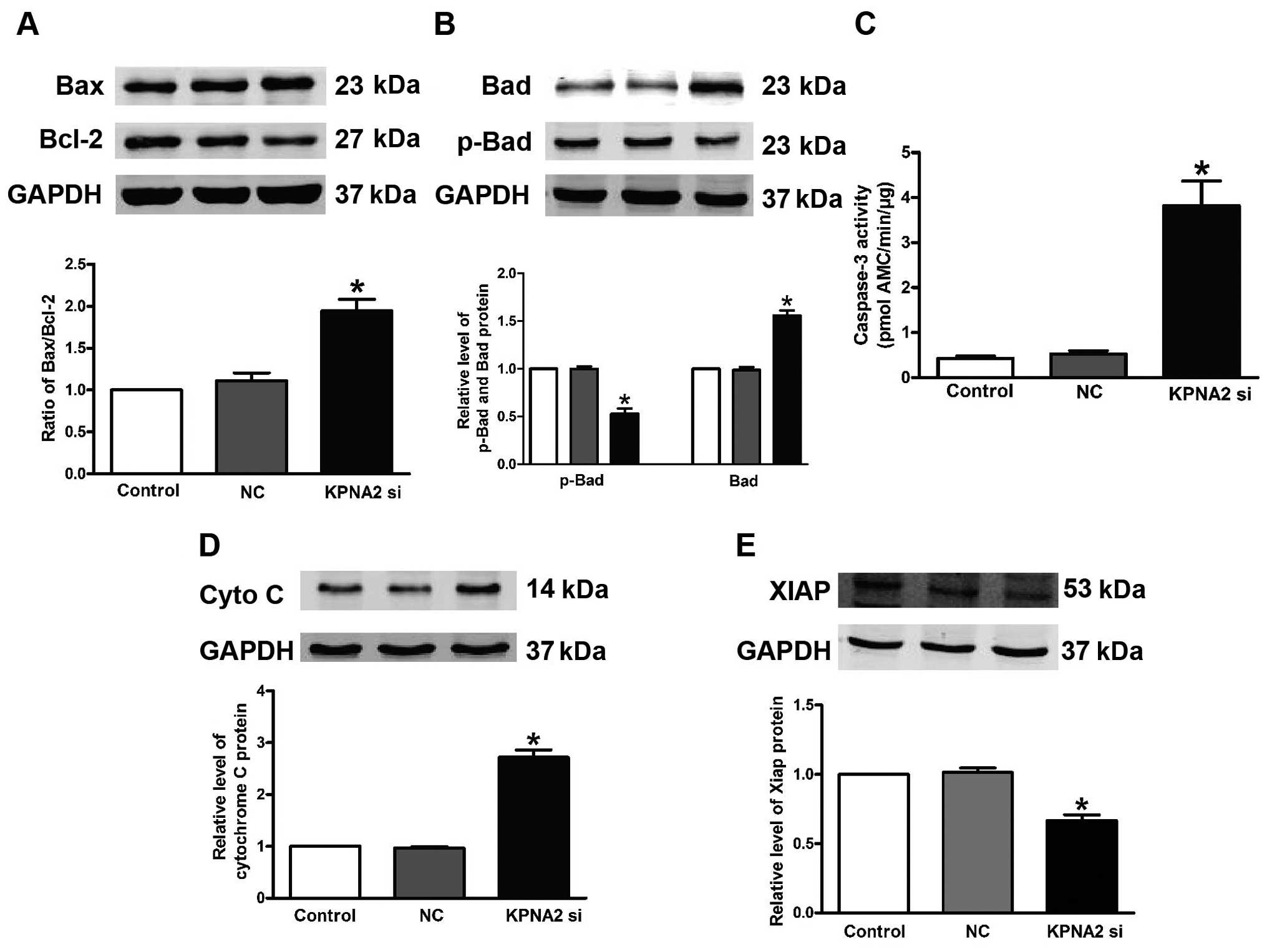 | Figure 4KPNA2 knockdown alters p-Bad, Bad,
Bax, Bcl-2 cytochrome c and XIAP expression, and promotes
caspase-3 activation. Western blotting was used to detect (A) Bax,
Bcl-2, (B) p-Bad, Bad, (D) cytochrome c and (E) XIAP
expression in the CAL-27 cells transfected with KPNA2. Relative
expression of Bax, Bcl-2, p-Bad, Bad and XIAP was normalized to
GAPDH. n=3 independent experiments for each group. (C) Activation
of caspase-3 by KPNA2-directed siRNA. The data are expressed as
mean ± SEM, similar results were observed from another three
experiments; *P<0.05 compared with the control. |
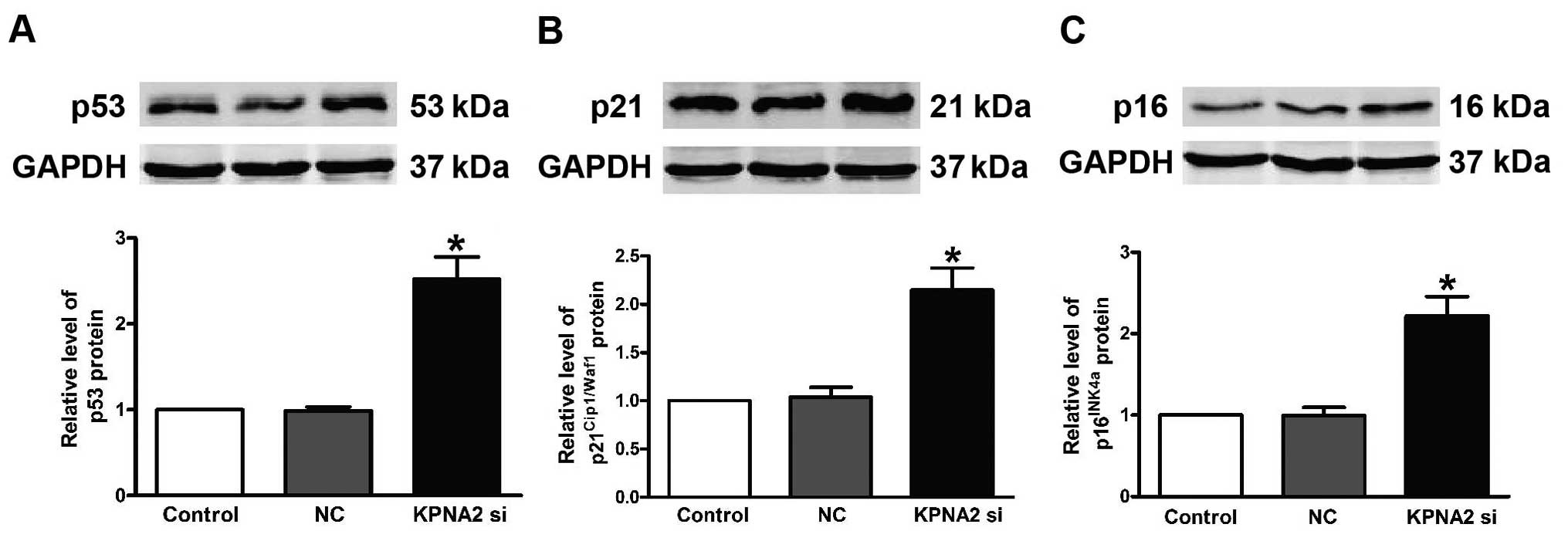
Knockdown of KPNA2 decreases expression
of p53, p21Cip1/Waf1 and p16INK4a in CAL-27
cells
To further explore the mechanisms associated with
KPNA2 knockdown, the expression of p53, p21Cip1/Waf1 and
p16INK4a at the protein level was demonstrated to be
markedly upregulated in the CAL-27 cells. Knockdown of KPNA2 caused
an aberrant upregulation of p53 (Fig.
5A), p21Cip1/Waf1 (Fig.
5B) and p16INK4a (Fig.
5C). These observations showed that
p53/p21Cip1/Waf1/p16INK4a has an important
role in KPNA2-induced apoptosis in CAL-27 cells.
Discussion
In the present study, we suggest that knockdown of
KPNA2 has a potent antiproliferative effect on CAL-27 cells by
inducing apoptosis in vitro. We further elucidated that
activation of both caspase-3-dependent and p53-dependent pathways
are important mechanisms associated with KPNA2-induced apoptosis.
Therefore, the present study may provide a new pathway into the
clinical role of KPNA2 in TSSC.
Previous studies have shown that KPNA2 takes part in
anticancer activities (6–12,19–21)
and its alteration is often associated with an adverse outcome for
breast carcinomas (7–9), esophageal (10), lung (11), prostate (12), brain (21) and ovarian cancer (19,20).
However, the impact of KPNA2 on the growth and apoptosis of TSSC
remains unknown.
The present study showed the antiproliferative,
proapoptotic and anti-migratory effects of KPNA2 on tongue squamous
cell carcinoma (TSCC) cells. We found that KPNA2-siRNA
significantly inhibited the growth of CAL-27 cells. AO/EB staining
displayed the apoptotic nuclear changes in CAL-27 cells. These
findings confirmed that the inhibition of KPNA2 was able to cause
apoptosis of TSCC cells. We further tested whether the KPNA2-siRNA
led to the inhibition of migration in CAL-27 cells. The results
confirmed that KPNA2-knockdown was able to inhibit the migration of
TSCC cells.
Then, several assays were carried out to understand
the molecular mechanism underlying the antitumor effects of KPNA2
knockdown on TSCC. Several studies have reported that Bax, Bcl-2
and caspase-3 are the key molecules participating in the apoptosis
in CAL-27 cells. Chloroform extracts of Solanum lyratum
induced apoptosis by upregulating Bax protein expression and
caspase-3 activity and downregulating Bcl-2 expression in CAL-27
cells (22). The AKT
serine/threonine protein kinase modulates bufalin-triggered
intrinsically induced apoptosis in CAL-27 cells by upregulating Bax
and caspase-3 expression and downregulating Bcl-2 (23). The above results are consistent with
our findings that KPNA2 induces apoptosis by regulating Bcl-2 and
Bax, and activates caspase-3 expression in CAL-27 cells. We
observed that the ratio of Bcl-2/Bax was significantly decreased,
indicating that KPNA2 knockdown induced apoptosis in the CAL-27
cells. In addition, Bad, a member of the Bcl-2 family, normally
binds to the Bcl-2/Bcl-X complex and triggers apoptosis (24). Bad was downregulated, while levels
of p-Bad and XIAP were elevated following KPNA2 knockdown. XIAP
interacts with caspase-3 to block its full activation, substrate
cleavage and cell death (25).
Furthermore, Bax and Bad activate caspase-3. These data confirm
that caspase-3-dependent apoptotic signaling plays a critical role
in the anticancer activity of KPNA2 knockdown.
The p53 signaling pathway is also pivotal in
apoptosis (26,27), such as the tissue dependent
interactions between p53 and bcl-2 in vivo (28). Adenoviral-mediated p53
overexpression diversely influences the cell cycle of HepG2 and
CAL-27 cell lines upon cisplatin and methotrexate treatment
(29). An indirubin derivative,
indirubin-3′-monoxime, induced apoptosis in oral cancer
tumorigenesis through caspase-3 activity and upregulation of p53
expression in CAL-27 cells (30).
Our research showed that caspase-3 activity was increased after
KPNA2 knockdown. We demonstrated that KPNA2 is able to induce
apoptosis in CAL-27 cells, and this effect appears to be mediated
by anti-p53/p21Cip1/Waf1/p16INK4a. In our
results, we observed that the expression of p53 protein in CAL-27
cells was significantly higher than that noted in the control group
(P<0.05), as was the expression of p21Cip1/Waf1 and
p16INK4a protein (P<0.05). We observed that KPNA2
resulted in the p53/p21Cip1/Waf1/p16INK4a
from the mitochondria to apoptosis, suggesting that the
p53/p21Cip1/Waf1/p16INK4a signaling pathway
is involved in apoptosis induced by KPNA2.
In conclusion, the present study demonstrated that
KPNA2 knockdown inhibited the proliferation and migration, and
promoted the apoptosis of TSCC cells, which is associated with
increased intracellular caspase-3 and activation of
p53/p21Cip1/Waf1/p16INK4a protein. However,
the present study does not rule out the potential participation of
other factors/pathways as mechanisms for the KPNA2-knockdown
apoptotic effects in CAL-27 cells. The present study suggests KPNA2
as a new therapeutic agent for TSCC.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Song B, Yang Y, Wang YL, Fan XH, Huang YM,
Ci HS and Zuo JH: Adenovirus expressing IFN-λ (Ad/hIFN-λ) produced
anti-tumor effects through inducing apoptosis in human tongue
squamous cell carcinoma cell. Int J Clin Exp Med. 8:12509–12518.
2015.
|
|
3
|
Tseng SF, Chang CY, Wu KJ and Teng SC:
Importin KPNA2 is required for proper nuclear localization and
multiple functions of NBS1. J Biol Chem. 280:39594–39600. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zannini L, Lecis D, Lisanti S, Benetti R,
Buscemi G, Schneider C and Delia D: Karyopherin-alpha2 protein
interacts with Chk2 and contributes to its nuclear import. J Biol
Chem. 278:42346–42351. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Teng SC, Wu KJ, Tseng SF, Wong CW and Kao
L: Importin KPNA2, NBS1, DNA repair and tumorigenesis. J Mol
Histol. 37:293–299. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dahl E, Kristiansen G, Gottlob K, Klaman
I, Ebner E, Hinzmann B, Hermann K, Pilarsky C, Dürst M,
Klinkhammer-Schalke M, et al: Molecular profiling of
laser-microdissected matched tumor and normal breast tissue
identifies karyopherin alpha2 as a potential novel prognostic
marker in breast cancer. Clin Cancer Res. 12:3950–3960. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sotiriou C, Wirapati P, Loi S, Harris A,
Fox S, Smeds J, Nordgren H, Farmer P, Praz V, Haibe-Kains B, et al:
Gene expression profiling in breast cancer: Understanding the
molecular basis of histologic grade to improve prognosis. J Natl
Cancer Inst. 98:262–272. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dankof A, Fritzsche FR, Dahl E, Pahl S,
Wild P, Dietel M, Hartmann A and Kristiansen G: KPNA2 protein
expression in invasive breast carcinoma and matched peritumoral
ductal carcinoma in situ. Virchows Arch. 451:877–881. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gluz O, Wild P, Meiler R, Diallo-Danebrock
R, Ting E, Mohrmann S, Schuett G, Dahl E, Fuchs T, Herr A, et al:
Nuclear karyopherin alpha2 expression predicts poor survival in
patients with advanced breast cancer irrespective of treatment
intensity. Int J Cancer. 123:1433–1438. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sakai M, Sohda M, Miyazaki T, Suzuki S,
Sano A, Tanaka N, Inose T, Nakajima M, Kato H and Kuwano H:
Significance of karyopherin-α 2 (KPNA2) expression in esophageal
squamous cell carcinoma. Anticancer Res. 30:851–856.
2010.PubMed/NCBI
|
|
11
|
Wang CI, Wang CL, Wang CW, Chen CD, Wu CC,
Liang Y, Tsai YH, Chang YS, Yu JS and Yu CJ: Importin subunit
alpha-2 is identified as a potential biomarker for non-small cell
lung cancer by integration of the cancer cell secretome and tissue
transcriptome. Int J Cancer. 128:2364–2372. 2011. View Article : Google Scholar
|
|
12
|
Mortezavi A, Hermanns T, Seifert HH,
Baumgartner MK, Provenzano M, Sulser T, Burger M, Montani M,
Ikenberg K, Hofstädter F, et al: KPNA2 expression is an independent
adverse predictor of biochemical recurrence after radical
prostatectomy. Clin Cancer Res. 17:1111–1121. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Noetzel E, Rose M, Bornemann J, Gajewski
M, Knüchel R and Dahl E: Nuclear transport receptor karyopherin-α2
promotes malignant breast cancer phenotypes in vitro. Oncogene.
31:2101–2114. 2012. View Article : Google Scholar
|
|
14
|
Huang L, Wang HY, Li JD, Wang JH, Zhou Y,
Luo RZ, Yun JP, Zhang Y, Jia WH and Zheng M: KPNA2 promotes cell
proliferation and tumorigenicity in epithelial ovarian carcinoma
through upregulation of c-Myc and downregulation of FOXO3a. Cell
Death Dis. 4:e7452013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Khan M, Yu B, Rasul A, Al Shawi A, Yi F,
Yang H and Ma T: Jaceosidin induces apoptosis in U87 glioblastoma
cells through G2/M phase arrest. Evid Based Complement Alternat
Med. 2012:7030342012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
McGahon AJ, Martin SJ, Bissonnette RP,
Mahboubi A, Shi Y, Mogil RJ, Nishioka WK and Green DR: The end of
the (cell) line: Methods for the study of apoptosis in vitro.
Methods Cell Biol. 46:153–185. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ni B, Bai FF, Wei Y, Liu MJ, Feng ZX,
Xiong QY, Hua LZ and Shao GQ: Apoptosis induced by lipid-associated
membrane proteins from Mycoplasma hyopneumoniae in a porcine lung
epithelial cell line with the involvement of caspase 3 and the MAPK
pathway. Genet Mol Res. 14:11429–11443. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
He Y, Chen W, Hu Y, Luo B, Wu L, Qiao Y,
Mo Q, Xu R, Zhou Y, Ren Z, et al: E. adenophorum induces cell cycle
and apoptosis of renal cells through mitochondrial pathway and
caspase activation in Saanen goat. PLoS One. 10:e01385042015.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zheng M, Tang L, Huang L, Ding H, Liao WT,
Zeng MS and Wang HY: Overexpression of karyopherin-2 in epithelial
ovarian cancer and correlation with poor prognosis. Obstet Gynecol.
116:884–891. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
He L, Ding H, Wang JH, Zhou Y, Li L, Yu
YH, Huang L, Jia WH, Zeng M, Yun JP, et al: Overexpression of
karyopherin 2 in human ovarian malignant germ cell tumor correlates
with poor prognosis. PLoS One. 7:e429922012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gousias K, Becker AJ, Simon M and
Niehusmann P: Nuclear karyopherin a2: A novel biomarker for
infiltrative astrocytomas. J Neurooncol. 109:545–553. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chiu CH, Chou YC, Lin JP, Kuo CL, Lu HF,
Huang YP, Yu CC, Lin ML and Chung JG: Chloroform extract of Solanum
lyratum induced G0/G1 arrest via p21/p16 and induced apoptosis via
reactive oxygen species, caspases and mitochondrial pathways in
human oral cancer cell lines. Am J Chin Med. 43:1453–1469. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tsai SC, Lu CC, Lee CY, Lin YC, Chung JG,
Kuo SC, Amagaya S, Chen FN, Chen MY, Chan SF, et al: AKT
serine/threonine protein kinase modulates bufalin-triggered
intrinsic pathway of apoptosis in CAL 27 human oral cancer cells.
Int J Oncol. 41:1683–1692. 2012.PubMed/NCBI
|
|
24
|
Huang C, Gu H, Zhang W, Herrmann JL and
Wang M: Testosterone-down-regulated Akt pathway during cardiac
ischemia/reperfusion: A mechanism involving BAD, Bcl-2 and FOXO3a.
J Surg Res. 164:e1–e11. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Paulsen M, Ussat S, Jakob M, Scherer G,
Lepenies I, Schütze S, Kabelitz D and Adam-Klages S: Interaction
with XIAP prevents full caspase-3/-7 activation in proliferating
human T lymphocytes. Eur J Immunol. 38:1979–1987. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Aroui S, Dardevet L, Ajmia WB, de
Boisvilliers M, Perrin F, Laajimi A, Boumendjel A, Kenani A, Muller
JM and De Waard M: A novel platinum-maurocalcine conjugate induces
apoptosis of human glioblastoma cells by acting through the
ROS-ERK/AKT-p53 pathway. Mol Pharm. 12:4336–4348. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ikenberg K, Valtcheva N, Brandt S, Zhong
Q, Wong CE, Noske A, Rechsteiner M, Rueschoff JH, Caduff R, Dellas
A, et al: KPNA2 is overexpressed in human and mouse endometrial
cancers and promotes cellular proliferation. J Pathol. 234:239–252.
2014.PubMed/NCBI
|
|
28
|
Li X, Miao X, Wang H, Xu Z and Li B: The
tissue dependent interactions between p53 and Bcl-2 in vivo.
Oncotarget. 6:35699–35709. 2015.PubMed/NCBI
|
|
29
|
Kraljević Pavelić S, Marjanović M, Poznić
M and Kralj M: Adenovirally mediated p53 overexpression diversely
influence the cell cycle of HEp-2 and CAL 27 cell lines upon
cisplatin and methotrexate treatment. J Cancer Res Clin Oncol.
135:1747–1761. 2009. View Article : Google Scholar
|
|
30
|
Lo WY and Chang NW: An indirubin
derivative, indirubin-3′-monoxime suppresses oral cancer
tumorigenesis through the downregulation of survivin. PLoS One.
8:e701982013. View Article : Google Scholar
|