Introduction
Hepatocellular carcinoma (HCC) ranks as the sixth
most common malignancy as well as the third most common cause of
cancer leading to death worldwide, and its incidence continues to
rise (1,2). Hepatitis virus infection (hepatitis B
or C viruses), alcohol-related liver cirrhosis, and nonalcoholic
steatohepatitis have been recognized as the main risk factors for
HCC (3). Despite improvements in
surgical and medical treatments in the past decade, the outcome of
patients with HCC remains unsatisfactory. Recurrence and metastasis
are the two leading causes of poor prognosis of HCC patients
(4). Therefore, there is an urgent
need to understand the cellular mediators that contribute to the
invasion and migration of HCC and explore new therapeutic
strategies targeting these mediators.
Cyclooxygenase-2 (COX-2) has been considered a
potential mediator of invasion and migration in a number of
malignant diseases (5–8). It has also been reported to be
associated with poor prognosis in many cancers (9,10).
Overexpression of COX-2 enhances the extent of prostaglandin E2
(PGE2) which is the main metabolite of COX-2 and a ligand of G
protein-coupled receptors including EP1, EP2, EP3, and EP4.
Recently, several studies reported that COX-2/PGE2 stimulates AKT,
NF-κB, and ERK1/2 signaling pathways to promote tumor angiogenesis,
apoptosis, and invasiveness (8,11–13).
Our previous studies demonstrated that meloxicam, a selective COX-2
inhibitor, suppresses HCC cell proliferation, migration, and
invasion via regulating expression of matrix metalloproteinase
(MMP)-2 and E-cadherin in a COX-2-dependent manner (14). However, the precise mechanisms of
migration and invasion of HCC are largely unknown.
Accumulating evidence has demonstrated that
COX-2/PGE2 is associated with the β-catenin signaling pathway
contributing to the growth of many cancers, such as skin (15), breast (16), neuroblastoma (17) and colorectal cancer (18). β-catenin is a 90 kD cytosolic
protein and an important component of the Wnt signaling pathway. In
the absence model of Wnt signaling, β-catenin is recruited to the
phosphorylation/destruction complex. Disturbance of the complex
promotes the phosphorylation of β-catenin by glycogen synthase
kinase-3β (GSK-3β) and casein kinase 1α (CK1α) leading to the
proteasomal degradation of β-catenin (19). β-catenin accumulation eventually
leads to its nuclear translocation and then it binds to members of
the TCF/LEF family of transcription factors, thus regulating
expression of various target genes which are associated with many
cellular processes including cell survival, proliferation, and
migration (20–22).
Meloxicam (Mel) is an NSAID that specifically
inhibits COX-2. This selective COX-2 inhibitor has been
demonstrated to exert an anti-invasion response in various tumors
(5,23,24)
including HCC cancer (14,25,26).
However, whether meloxicam inhibits HCC cell invasion/migration by
targeting COX-2/PGE2-regulated activation of the β-catenin
signaling pathway remains unclear. In this study, we investigated
the effects of meloxicam on the proliferation and migration
potential of HCC cells and explored whether the antitumor effect of
meloxicam is associated with the inactivation of the β-catenin
signaling pathway and whether COX-2/PGE2 plays any part in this
process.
Materials and methods
Cell culture
Of the 5 human HCC cell lines, HepG2, Bel-7402, and
Huh-7 were obtained from the American Type Culture Collection
(ATCC, Rockville, MD, USA), and SMMC-7721 and SMMC-7402 were
obtained from the Type Culture Collection Cell Bank, Chinese
Academy of Science (Shanghai, China). The cells were routinely
cultured in RPMI-1640 medium (Gibco)/DMEM (Hyclone) supplemented
with 10% fetal bovine serum (Gibco) and 1% antibiotics at 37°C in
95% air and 5% CO2.
Reagents and antibodies
Meloxicam was obtained from Merck Millipore
(Darmstadt, Germany). PGE2 and FH535 were obtained from
Sigma-Aldrich (San Diego, CA, USA). Primary antibodies to COX-2,
p21, p27, MMP-2, and MMP-9 were obtained from Cell Signaling
Technologies (Danvers, MA, USA). Antibodies to E-cadherin,
β-catenin, GSK-3β, p-GSK-3β, Histone H3, and GAPDH were obtained
from Abcam (Cambridge, UK).
Cell viability analysis
The effect of meloxicam on the cell viability of HCC
cells was determined using the Cell Counting Kit-8 (CCK-8, Dojindo
Molecular Technologies, Inc., Kumamoto, Japan) assay as previously
described (25).
Enzyme-linked immunosorbent assay
(ELISA)
The concentrations of PGE2 in supernatants of cell
cultures were measured using the PGE2 ELISA Assay kit (R&D
Systems, Inc., Minneapolis, MN, USA) according to the
manufacturer's instructions.
Cell cycle analysis
HepG2 and SMMC-7721 cells were grown in medium as
mentioned above. At 50% confluency, cells were treated with
meloxicam or not for 24 h. Cells were collected and processed for
cell cycle analysis. Briefly, 5×104 cells were suspended
in 0.5 ml of PI solution, and incubated for 30 min in the dark
according to the manufacturer's instruction. The cell cycle
distribution was analyzed by FACS flow cytometry.
Cell migration analysis
The methods were previously described (14,25).
In brief, 1×105 cells in 300 µl of RPMI-1640
medium/DMEM (with 1% FBS) containing meloxicam or PGE2 alone or in
combination were seeded into the upper chamber of a Transwell
chamber (Corning, New York, USA). The bottom wells of the chambers
were filled with 500 µl RPMI-1640 medium/DMEM containing 10%
fetal bovine serum. After 48 h incubation, the chambers were fixed
with 95% ethanol and then stained with 1% crystal violet. Images of
three different fields (×100 magnification) were captured from each
membrane, and the number of migrated cells counted.
Total RNA extraction and real-time
PCR
Total RNA was extracted from the cells using TRIzol
reagent (Invitrogen, Carlsbad, CA, USA), and cDNA was synthesized
by using a cDNA synthesis kit (Invitrogen). The reaction mixtures
for quantitative RT-PCR were prepared as previously described
(14). The primers targeting MMP-2
were (5′-TGACGGTAAGGACGGACTC-3′; 5′-ATACTTCACACGGACCACTTG-3′), MMP9
(5′-CCTCTGGAGGTTCGACGTGA-3′; 5′-TAGGCTTTCTCTCGGTACTGGAA-3′),
E-cadherin (5′-TGCCCAGAAAATGAAAAAGG-3′; 5′-GGATGACAG CGTGAGAGA-3′),
and GAPDH (5′-TTACTCCTTGGAGGCCATGTGGGC-3′;
5′-ACTGCCACCCAGAAGACTGTGGATGG-3′). Expression levels were
normalized to GAPDH. All protocols were carried out according to
the manufacturer's instructions. Real-time PCR was performed using
MX3000P Real-time PCR systems (Stratagene, Wilmington, DE, USA).
Experiments were performed in triplicate, and the data were
calculated by ΔΔCt methods.
Western blot analysis
The method was previously described (27). After different treatments, protein
concentrations in cell extracts were determined (Bio-Rad, Richmond,
CA, USA). Equal amounts of each sample were resolved in SDS-PAGE
gels, then transferred to a polyvinylidene fluoride (PVDF) membrane
(Millipore, Billerica, MA, USA), and probed with the primary
antibodies described in reagents and antibodies.
siRNA transfection
β-catenin and COX-2 siRNA were purchased from Santa
Cruz Biotechnology, Inc. (Santa Cruz, CA, USA) and transfection was
performed using Lipofectamine 2000 Transfection Reagent
(Invitrogen) according to the manufacturer's instructions.
Statistical analysis
Data are presented as the mean ± standard deviation
(SD) and analyzed by one-way ANOVA followed by Dunnett's test with
SPSS software (version 17.0, SPSS China, Shanghai, China), with
values of P<0.05 considered statically significant.
Results
Effects of meloxicam on the PGE2 level
and the proliferation potential of HCC cells in vitro
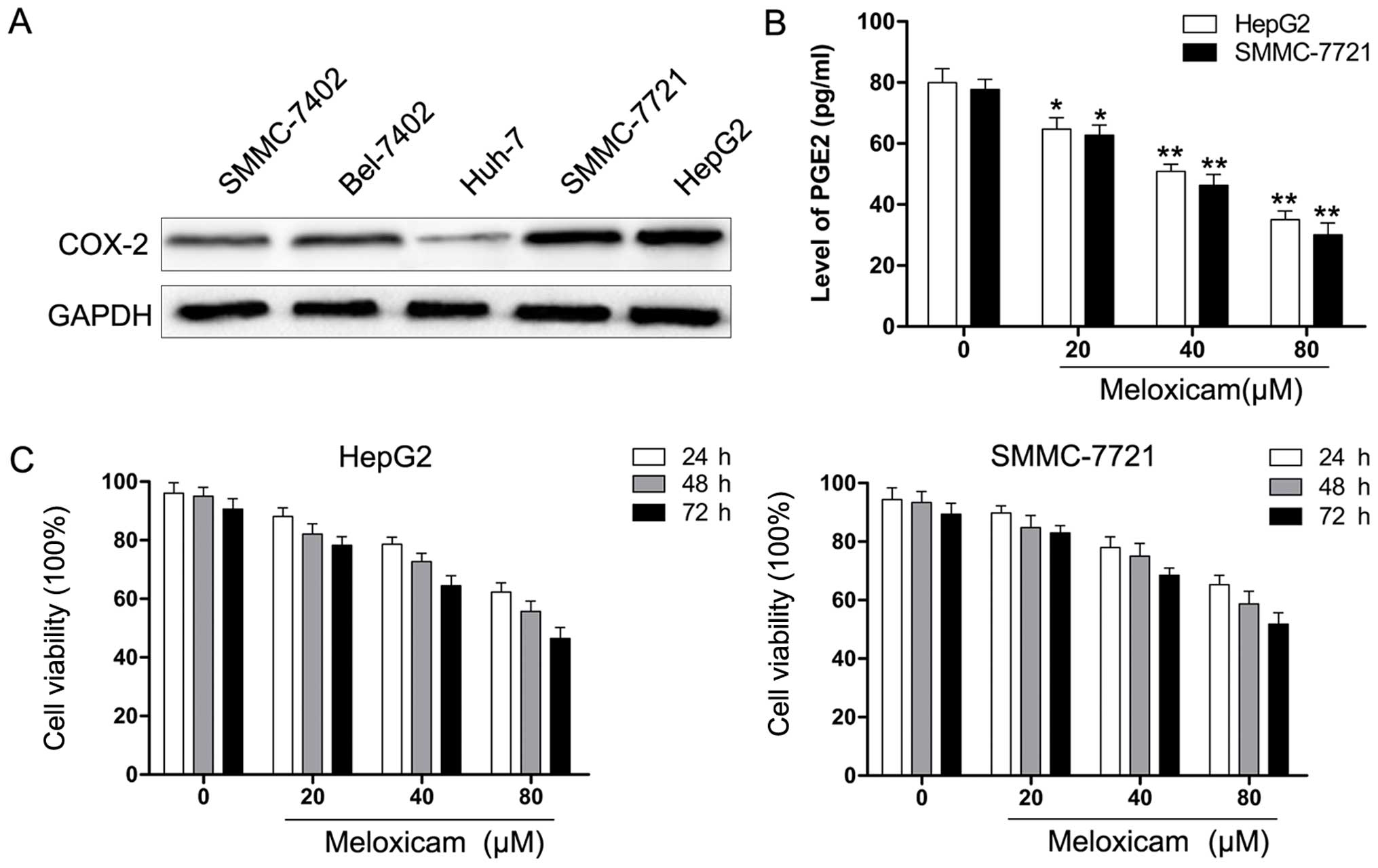
First, we examined expression of COX-2 protein in
HCC cells by western blot analysis. As shown in Fig. 1A, HCC cell lines exhibited different
levels of COX-2 and the results were consistent with our previous
data (14). Based on the data,
HepG2 and SMMC-7721 cells were chosen for the following
experiments. Next, the PGE2 level of HepG2 and SMMC-7721 cells was
determined by ELISA analysis. As shown in Fig. 1B, the level of PGE2 was
significantly decreased with meloxicam treatment in a
dose-dependent manner. HCC cells were exposed to various
concentrations of meloxicam (0–80 µM) for 24, 48, or 72 h
and cell viability was determined using the CCK-8 assay. As shown
in Fig. 1C, the viability of HCC
cells exposed to meloxicam was significantly reduced in a time- and
concentration-dependent manner. This result showed the efficacy of
meloxicam against HCC cell proliferation.
Effects of meloxicam on the cell cycle of
HCC cells in vitro
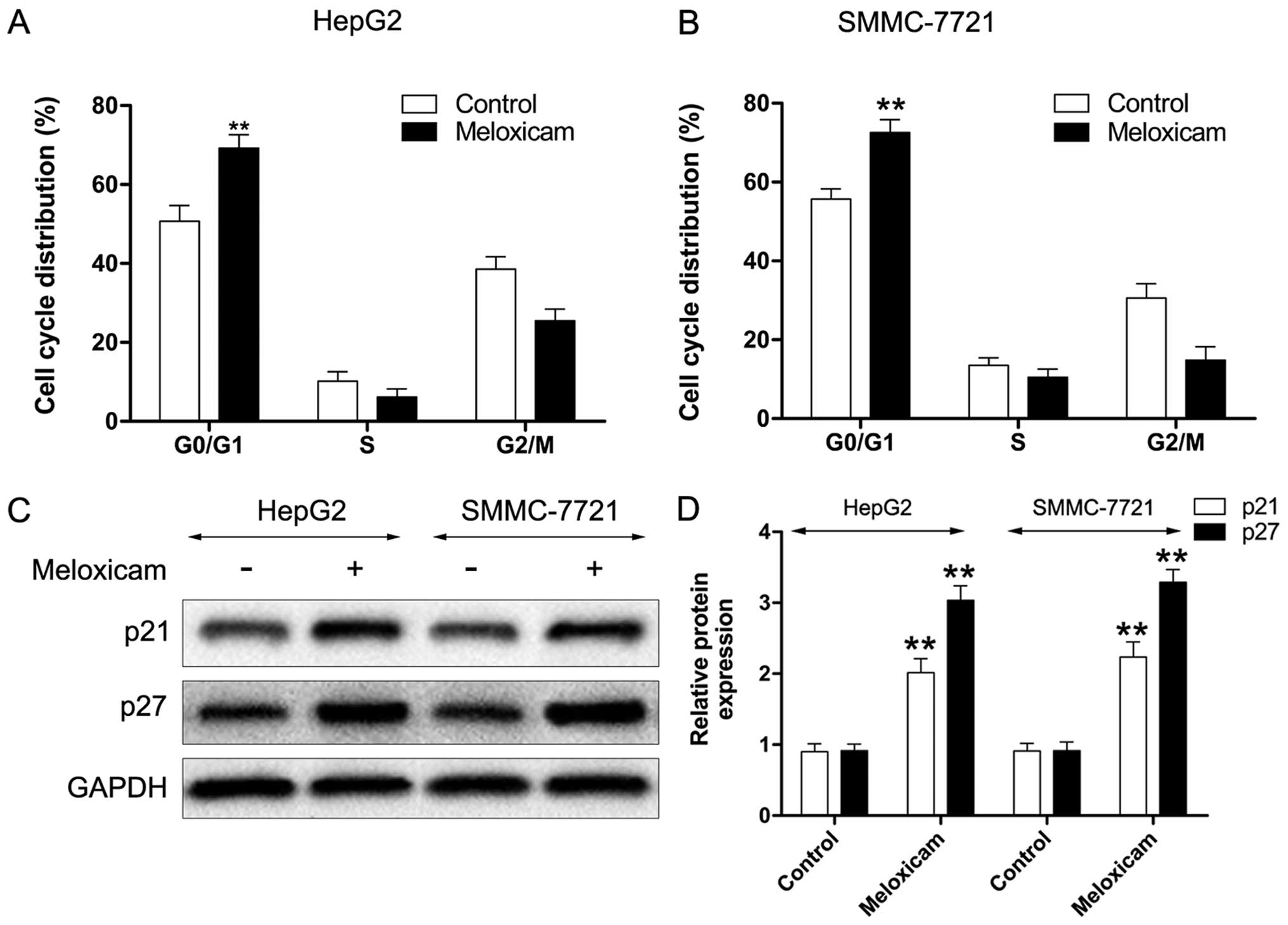
In our previous study, we demonstrated that
meloxicam has a cell cycle arrest effect in human HCC cells
(25). In the current work, we
further investigated the mechanism of meloxicam in regulating the
cell cycle in HepG2 and SMMC-7721 cells. As shown in Fig. 2A and B, with meloxicam treatment
both types of HCC cells were suppressed in the G1 phase after 24 h
treatment. Furthermore, we found that expression levels of
cyclin-dependent kinase (CDK) inhibitor proteins p21 and p27 in
HepG2 and SMMC-7721 cells were significantly enhanced after
treatment with meloxicam (Fig. 2C and
D).
Effects of PGE2 and meloxicam on HCC cell
migration in vitro
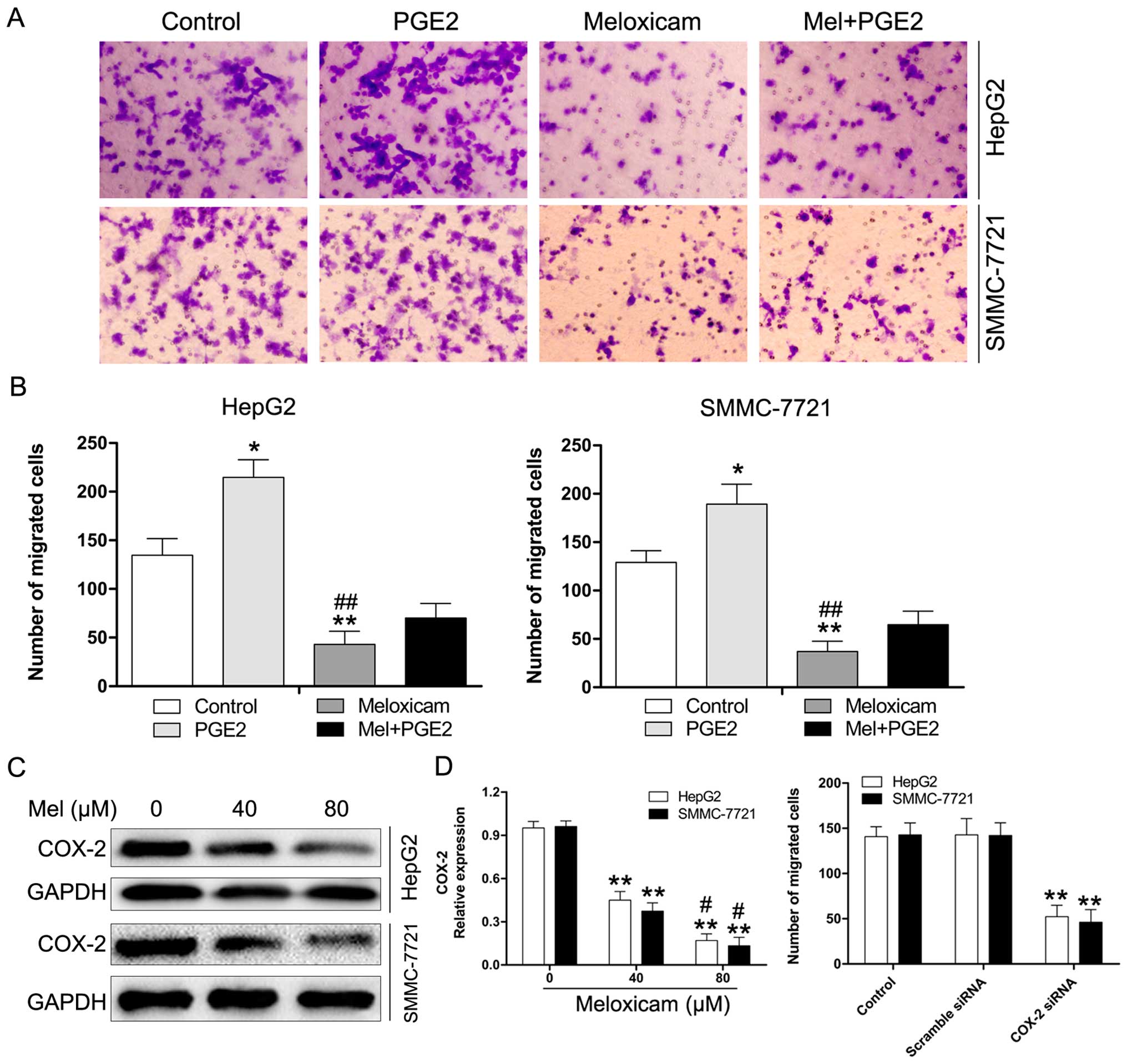
Previous studies reported that COX-2/PGE2 plays an
important role in exerting pro-invasion/migration effects in many
cancers (28–30). Here, we investigated the
anti-migration effects of meloxicam on HCC cells with or without
treatment with exogenous PGE2. As shown in Fig. 3A and B, treatment with PGE2
significantly enhanced the migration of HepG2 and SMMC-7721 cells.
However, this effect could be reversed by meloxicam. Moreover, we
examined expression of COX-2 in HepG2 and SMMC-7721 cells treated
with meloxicam or not using western blot analysis. As expected, we
found that treatment of HepG2 and SMMC-7721 cells with meloxicam
for 24 h induced a marked reduction of COX-2 expression in these
cells (Fig. 3C). The effect of
COX-2/PGE2 in HCC cell migration was further verified by
downregulation of COX-2 by siRNA. As shown in Fig. 3D, transfection with COX-2 siRNA
notably decreased the migration in HepG2 and SMMC-7721 cells. These
results revealed that the suppression of endogenous levels of
COX-2/PGE2 expression is associated with the inhibition of HCC cell
migration.
Effects of PGE2 and meloxicam on MMP-2/9
and E-cadherin expression in HCC cells
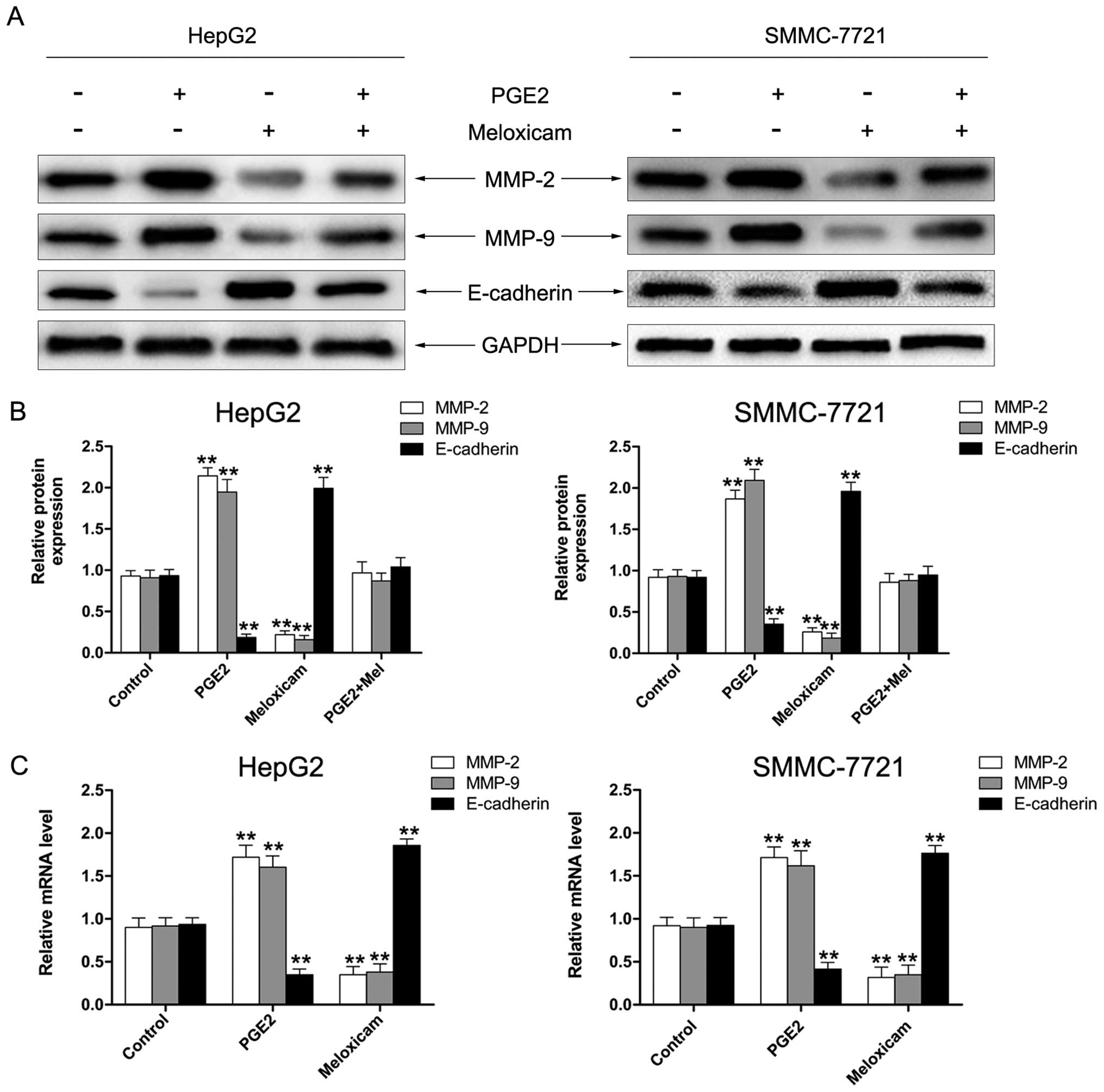
Since the downregulation of E-cadherin and
upregulation of expression of MMP-2/9 is associated with
enhancement in migration/invasion of cancer cells (31–34),
we investigated protein expression and mRNA of MMP-2/9 and
E-cadherin in HepG2 and SMMC-7721 cells. As shown in Fig. 4A and B, the level of MMP-2 and MMP-9
was significantly enhanced by PGE2 and reversed by meloxicam.
Expression of E-cadherin was decreased by treatment with PGE2
whereas it was increased after being exposed to meloxicam. The
results of RT-PCR showed similar effects in mRNA expression of
MMP-2/9 and E-cadherin (Fig. 4C).
These results suggested that meloxicam decreases MMP-2/9 activity
and enhances the level of E-cadherin to inhibit the migration of
HCC cells.
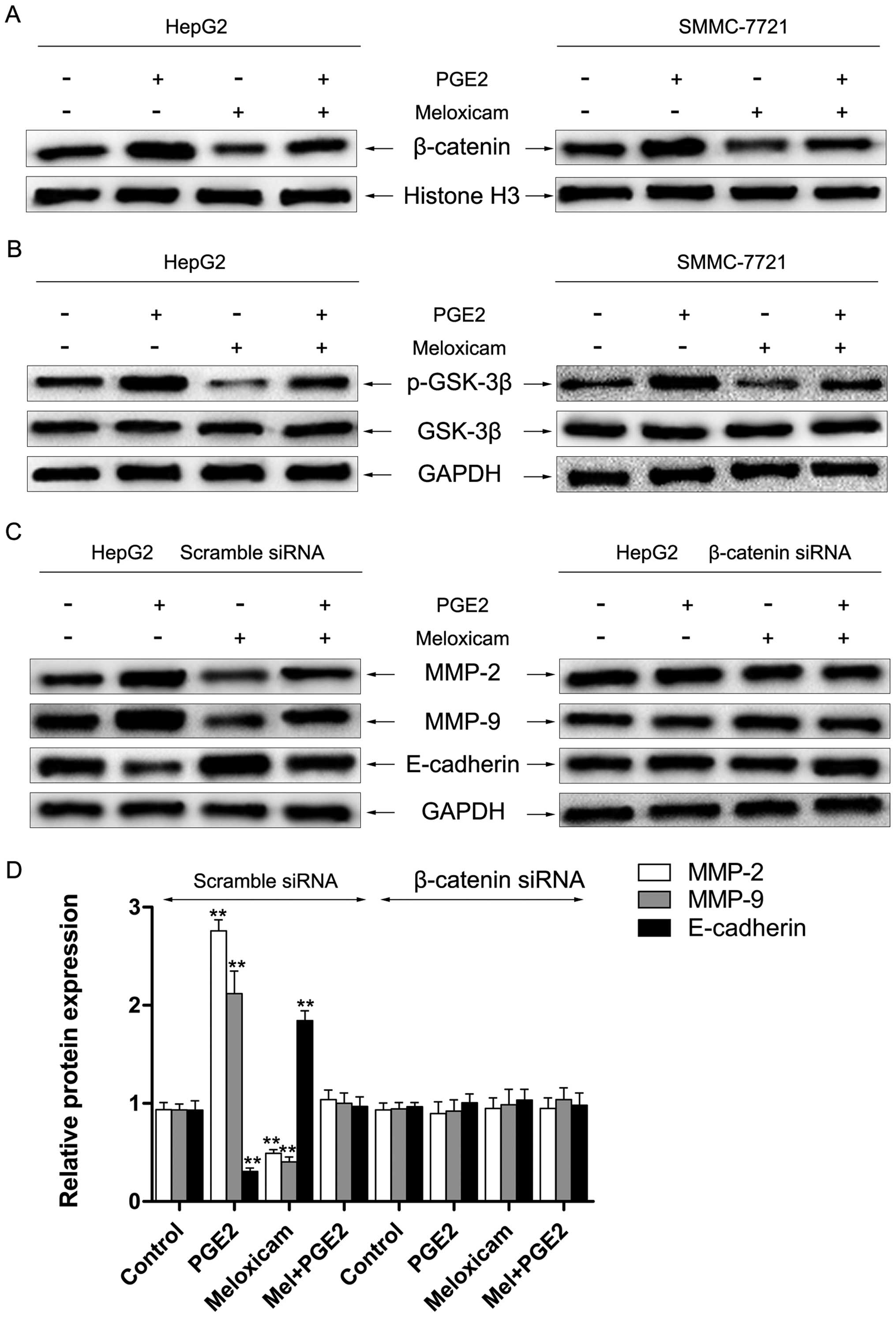
Effects of PGE2 and meloxicam on the
β-catenin signaling pathway in HCC cells in vitro
Accumulating evidence has demonstrated that PGE2
exerts a crucial role in promoting migration and regulating
expression of MMP-2/9 and E-cadherin via the β-catenin signaling
pathway (15,35). In this study, we investigated the
role of meloxicam on the β-catenin signaling pathway. As depicted
in Fig. 5A, treatment with PGE2
significantly enhanced nuclear accumulation of β-catenin which
could be reversed by meloxicam in HepG2 and SMMC-7721 cells.
Several studies reported that PGE2 can inactivate GSK3β and result
in a consequent intracellular accumulation of β-catenin (36). Thus, we investigated the role of
meloxicam on the level and activation of GSK3β. We found that
treatment with meloxicam significantly inhibited the
phosphorylation of GSK3β, however, expression of GSK3β was only
slightly changed (Fig. 5B).
Furthermore, knockdown of β-catenin by siRNA was utilized to
examine expression of MMP-2/9 and E-cadherin. As shown in Fig. 5C and D, downregulation of β-catenin
notably alleviated meloxicam-induced suppression of MMP-2/9
upregulation and E-cadherin downregulation by treatment of PGE2 in
HepG2 cells. A similar result was also found in SMMC-7721 cells
(data not shown).
FH535, an inhibitor of β-catenin,
suppresses PGE2-induced cell migration of HCC cells in vitro
To further investigate whether activation of
β-catenin and PGE2 has a role in migration of HCC cells, HepG2 and
SMMC-7721 cells were exposed to PGE2 with or without treatment with
FH535, an inhibitor of β-catenin. As shown in Fig. 6A, treatment with PGE2 significantly
increased the migration ability of HepG2 and SMMC-7721 cells.
However, treatment of cells with FH535 markedly suppressed
PGE2-enhanced migration of HCC cells. The results of western
blotting showed that the level of MMP-2/9 was reduced and
E-cadherin was enhanced by treatment with FH535 in HepG2 and
SMMC-7721 cells (Fig. 6B and C). We
also found similar effects in mRNA expression of MMP-2/9 and
E-cadherin by RT-PCR assay (Fig.
6D).
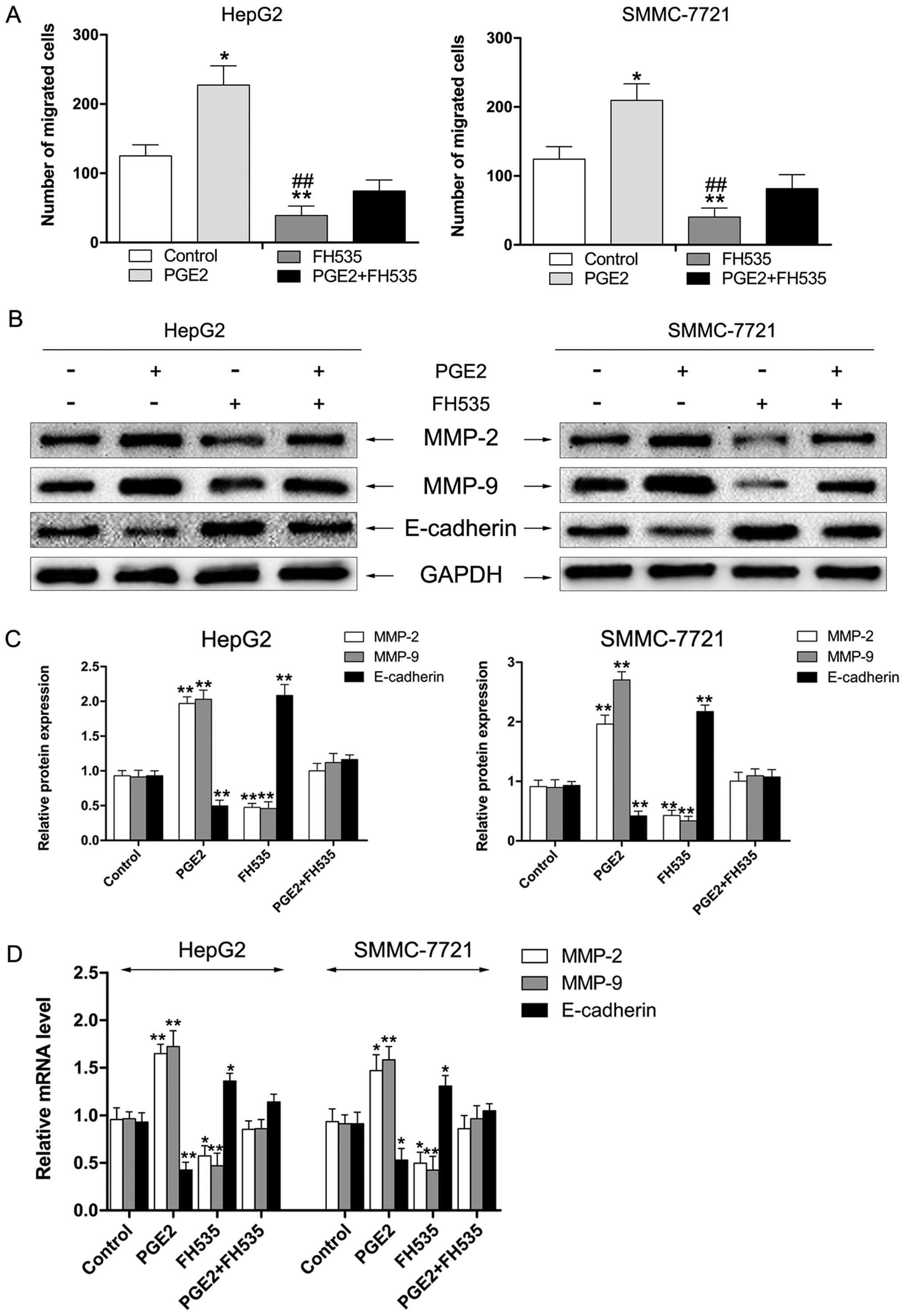 | Figure 6FH535 inhibits PGE2-enhanced cell
migration of HCC cells in vitro. (A) FH535 suppresses
PGE2-enhanced migration of HepG2 and SMMC-7721 cells.
*P<0.05, **P<0.01 vs. control,
##P<0.01 vs. PGE2. (B and C) HCC cells were treated
with PGE2 (10 µM), FH535 (5 µM), or a combination and
total protein was extracted. Western blotting was performed to
detect protein levels of MMP-2, MMP-9, and E-cadherin. GAPDH was
measured as the loading control. Data represent three independent
experiments. **P<0.01 vs. control. (D) HCC cells were
treated with PGE2 (10 µM), FH535 (5 µM), or a
combination and total RNA was extracted. mRNA expression of MMP-2,
MMP-9, and E-cadherin was determined by RT-PCR. GAPDH served as an
internal control. Data represent three independent experiments.
*P<0.05, **P<0.01 vs. control. |
Discussion
It was reported that COX-2 is overexpressed in
various cancers and is associated with cancer cell
migration/invasion. The selective COX-2 inhibitor has been
recognized as exerting antitumor effects through suppression of
PGE2 production. COX-2 overexpression has been considered to be
linked to cancer cell-derived PGE2 which promotes tumor cell
proliferation, invasion, and migration and reduces apoptosis
(37). Our previous studies also
demonstrated that meloxicam produces its antitumor effects against
hepatocellular carcinoma in COX-2-dependent and -independent
pathways (14,26). However, the exact anti-migration
mechanism of meloxicam on the downstream pathway of PGE2 in HCC
cells remains unknown. In this study, we found that HCC cell lines
expressed different levels of COX-2 protein and that meloxicam
exerted an anti-proliferation effect in HepG2 and SMMC-7721 cells
via blocking the cell cycle in the G1 phase through regulating
expression of CDK inhibitor proteins p21 and p27. Moreover, we
observed that treatment with PGE2 significantly potentiated the
migration potential whereas this effect was reversed after exposure
to meloxicam, which suggested that meloxicam inhibition of the
migration potential may be linked to the suppression of endogenous
expression of COX-2 and production of PGE2 in HepG2 and SMMC-7721
cells. This concept is also supported by the evidence that
transfection of HCC cells with COX-2 siRNA led to a marked
reduction of cell migration in HepG2 and SMMC-7721 cells as
compared to the migration of scramble siRNA-transfected HCC
cells.
A large number of studies revealed that MMP-2/9
promote tumor metastasis and invasion by degrading extracellular
matrix proteins (38,39). In addition, E-cadherin has been
reported as a suppressor of invasion and metastasis in many cancers
(40). In the present study, we
explored the protein and mRNA MMP-2/9 and E-cadherin expression
following exposure to meloxicam with or without PGE2. Our results
showed that expression of MMP-2 and MMP-9 was increased by PGE2 and
reversed by meloxicam and the extent of E-cadherin was decreased
following treatment with PGE2 whereas it was increased after
exposed to meloxicam. We also observed similar effects in mRNA
expression of MMP-2/9 and E-cadherin by RT-PCR assay. These data
demonstrated that meloxicam has an inhibitory role in the migration
and invasion of HCC cells through mediating the level of MMP-2/9
and E-cadherin.
Various studies reported the role of β-catenin in
cancer invasion/migration through regulating cell-to-cell adhesion
(41). The constitutively active
β-catenin signaling pathway results in disturbed cell-to-cell
adhesion and consequent upregulation of the migration potential of
tumor cells. Moreover, some studies demonstrated that PGE2 could
have pro-oncogenic actions including proliferation and metastasis
by stimulating β-catenin-mediated transcription in carcinogenesis
(22,42). The data presented in the present
study provide evidence that meloxicam exerts its anti-migration
effects through downregulation of nuclear accumulation of β-catenin
and inhibiting the phosphorylation of GSK-3β. Knockdown of
β-catenin by siRNA significantly reduced meloxicam-induced
suppression of MMP-2/9 upregulation and E-cadherin downregulation
by treatment of PGE2 in HepG2 and SMMC-7721 cells. To further
explore the link between COX-2/PGE2 and β-catenin in HCC cell
migration, we used FH535 (an inhibitor of β-catenin) to investigate
whether PGE2-enhanced migration was dependent on the β-catenin
signaling pathway. We observed that FH535 inhibited PGE2-enhanced
migration of HCC cells.
In conclusion, the present study showed that
meloxicam suppresses the migration of HCC cells by targeting
PGE2-regulated activation of the GSK-3β/β-catenin signaling
pathway. These findings suggest that meloxicam may be a potential
therapeutic option for preventing HCC invasion/migration.
Acknowledgments
This research was supported from the National
Natural Scientific Foundation of China (30972890 and 81172331),
Shandong Provincial Science and Technology Development Planning,
China (2015GGB14168) and Shandong Provincial Natural Science
Foundation, China (ZR2015HL080). Thanks to Dr Edward C. Mignot,
Shandong University, for linguistic advice.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Forner A, Llovet JM and Bruix J:
Hepatocellular carcinoma. Lancet. 379:1245–1255. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bruix J, Gores GJ and Mazzaferro V:
Hepatocellular carcinoma: Clinical frontiers and perspectives. Gut.
63:844–855. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang C, Jin G, Jin H, Wang N, Luo Q, Zhang
Y, Gao D, Jiang K, Gu D, Shen Q, et al: Clusterin facilitates
metastasis by EIF3I/Akt/MMP13 signaling in hepatocellular
carcinoma. Oncotarget. 6:2903–2916. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yusup G, Akutsu Y, Mutallip M, Qin W, Hu
X, Komatsu-Akimoto A, Hoshino I, Hanari N, Mori M, Akanuma N, et
al: A COX-2 inhibitor enhances the antitumor effects of
chemotherapy and radiotherapy for esophageal squamous cell
carcinoma. Int J Oncol. 44:1146–1152. 2014.PubMed/NCBI
|
|
6
|
Zheng CY, Xiao W, Zhu MX, Pan XJ, Yang ZH
and Zhou SY: Inhibition of cyclooxygenase-2 by tetramethylpyrazine
and its effects on A549 cell invasion and metastasis. Int J Oncol.
40:2029–2037. 2012.PubMed/NCBI
|
|
7
|
Qian M, Qian D, Jing H, Li Y, Ma C and
Zhou Y: Combined cetuximab and celecoxib treatment exhibits a
synergistic anticancer effect on human oral squamous cell carcinoma
in vitro and in vivo. Oncol Rep. 32:1681–1688. 2014.PubMed/NCBI
|
|
8
|
Chen Z, Liu M, Liu X, Huang S, Li L, Song
B, Li H, Ren Q, Hu Z, Zhou Y, et al: COX-2 regulates E-cadherin
expression through the NF-κB/Snail signaling pathway in gastric
cancer. Int J Mol Med. 32:93–100. 2013.PubMed/NCBI
|
|
9
|
Honjo S, Kase S, Osaki M, Ardyanto TD,
Kaibara N and Ito H: COX-2 correlates with F-box protein, Skp2
expression and prognosis in human gastric carcinoma. Int J Oncol.
26:353–360. 2005.PubMed/NCBI
|
|
10
|
Chen JY, Li CF, Kuo CC, Tsai KK, Hou MF
and Hung WC: Cancer/stroma interplay via cyclooxygenase-2 and
indoleamine 2,3-dioxygenase promotes breast cancer progression.
Breast Cancer Res. 16:4102014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu M, Li CM, Chen ZF, Ji R, Guo QH, Li Q,
Zhang HL and Zhou YN: Celecoxib regulates apoptosis and autophagy
via the PI3K/Akt signaling pathway in SGC-7901 gastric cancer
cells. Int J Mol Med. 33:1451–1458. 2014.PubMed/NCBI
|
|
12
|
Zhang H, Li Z and Wang K: Combining
sorafenib with celecoxib synergistically inhibits tumor growth of
non-small cell lung cancer cells in vitro and in vivo. Oncol Rep.
31:1954–1960. 2014.PubMed/NCBI
|
|
13
|
Xia Q, Hu Q, Wang H, Yang H, Gao F, Ren H,
Chen D, Fu C, Zheng L, Zhen X, et al: Induction of COX-2-PGE2
synthesis by activation of the MAPK/ERK pathway contributes to
neuronal death triggered by TDP-43-depleted microglia. Cell Death
Dis. 6:e17022015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dong X, Li R, Xiu P, Dong X, Xu Z, Zhai B,
Liu F, Jiang H, Sun X, Li J, et al: Meloxicam executes its
antitumor effects against hepatocellular carcinoma in
COX-2-dependent and -independent pathways. PLoS One. 9:e928642014.
View Article : Google Scholar
|
|
15
|
Prasad R and Katiyar SK: Ultraviolet
radiation-induced inflammation activates β-catenin signaling in
mouse skin and skin tumors. Int J Oncol. 44:1199–1206.
2014.PubMed/NCBI
|
|
16
|
Tu B, Ma TT, Peng XQ, Wang Q, Yang H and
Huang XL: Targeting of COX-2 expression by recombinant adenovirus
shRNA attenuates the malignant biological behavior of breast cancer
cells. Asian Pac J Cancer Prev. 15:8829–8836. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jansen SR, Holman R, Hedemann I, Frankes
E, Elzinga CR, Timens W, Gosens R, de Bont ES and Schmidt M:
Prostaglandin E2 promotes MYCN non-amplified neuroblastoma cell
survival via β-catenin stabilization. J Cell Mol Med. 19:210–226.
2015. View Article : Google Scholar :
|
|
18
|
Shafie NH, Mohd Esa N, Ithnin H, Md Akim
A, Saad N and Pandurangan AK: Preventive inositol hexaphosphate
extracted from rice bran inhibits colorectal cancer through
involvement of Wnt/β-catenin and COX-2 pathways. Biomed Res Int.
2013:6810272013. View Article : Google Scholar
|
|
19
|
Gavert N and Ben-Ze'ev A: beta-Catenin
signaling in biological control and cancer. J Cell Biochem.
102:820–828. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Klaus A and Birchmeier W: Wnt signalling
and its impact on development and cancer. Nat Rev Cancer.
8:387–398. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jiang S, Zhu L, Tang H, Zhang M, Chen Z,
Fei J, Han B and Zou GM: Ape1 regulates WNT/β-catenin signaling
through its redox functional domain in pancreatic cancer cells. Int
J Oncol. 47:610–620. 2015.PubMed/NCBI
|
|
22
|
Du M, Shi F, Zhang H, Xia S, Zhang M, Ma
J, Bai X, Zhang L, Wang Y, Cheng S, et al: Prostaglandin E2
promotes human cholangiocarcinoma cell proliferation, migration and
invasion through the upregulation of β-catenin expression via EP3–4
receptor. Oncol Rep. 34:715–726. 2015.PubMed/NCBI
|
|
23
|
Qiu X, Cheng JC, Chang HM and Leung PC:
COX2 and PGE2 mediate EGF-induced E-cadherin-independent human
ovarian cancer cell invasion. Endocr Relat Cancer. 21:533–543.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang S, Da L, Yang X, Feng D, Yin R, Li
M, Zhang Z, Jiang F and Xu L: Celecoxib potentially inhibits
metastasis of lung cancer promoted by surgery in mice, via
suppression of the PGE2-modulated β-catenin pathway. Toxicol Lett.
225:201–207. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhong J, Xiu P, Dong X, Wang F, Wei H,
Wang X, Xu Z, Liu F, Li T, Wang Y, et al: Meloxicam combined with
sorafenib synergistically inhibits tumor growth of human
hepatocellular carcinoma cells via ER stress-related apoptosis.
Oncol Rep. 34:2142–2150. 2015.PubMed/NCBI
|
|
26
|
Zhong J, Dong X, Xiu P, Wang F, Liu J, Wei
H, Xu Z, Liu F, Li T and Li J: Blocking autophagy enhances
meloxicam lethality to hepatocellular carcinoma by promotion of
endoplasmic reticulum stress. Cell Prolif. 48:691–704. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang F, Dong X, Xiu P, Zhong J, Wei H, Xu
Z, Li T, Liu F, Sun X and Li J: T7 peptide inhibits angiogenesis
via downregulation of angiopoietin-2 and autophagy. Oncol Rep.
33:675–684. 2015.
|
|
28
|
Wang L, Wang Z, Li J, Zhang W, Ren F and
Yue W: NFATc1 activation promotes the invasion of U251 human
glioblastoma multiforme cells through COX-2. Int J Mol Med.
35:1333–1340. 2015.PubMed/NCBI
|
|
29
|
Park SY, Jin ML, Kim YH, Lee SJ and Park
G: Sanguinarine inhibits invasiveness and the MMP-9 and COX-2
expression in TPA-induced breast cancer cells by inducing HO-1
expression. Oncol Rep. 31:497–504. 2014.
|
|
30
|
Liu X, Ji Q, Ye N, Sui H, Zhou L, Zhu H,
Fan Z, Cai J and Li Q: Berberine inhibits invasion and metastasis
of colorectal cancer cells via COX-2/PGE2 mediated JAK2/STAT3
signaling pathway. PLoS One. 10:e01234782015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lin X, Li HR, Lin XF, Yu ME, Tu XW, Hua
ZD, Lin M, Xu NL, Han LL and Chen YS: Silencing of Livin inhibits
tumorigenesis and metastasis via VEGF and MMPs pathway in lung
cancer. Int J Oncol. 47:657–667. 2015.PubMed/NCBI
|
|
32
|
Chou YC, Chang MY, Wang MJ, Yu FS, Liu HC,
Harnod T, Hung CH, Lee HT and Chung JG: PEITC inhibits human brain
glioblastoma GBM 8401 cell migration and invasion through the
inhibition of uPA, Rho A, and Ras with inhibition of MMP-2, -7 and
-9 gene expression. Oncol Rep. 34:2489–2496. 2015.PubMed/NCBI
|
|
33
|
Campbell K and Casanova J: A role for
E-cadherin in ensuring cohesive migration of a heterogeneous
population of non-epithelial cells. Nat Commun. 6:79982015.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Fang Y, Liang X, Jiang W, Li J, Xu J and
Cai X: Cyclin b1 suppresses colorectal cancer invasion and
metastasis by regulating e-cadherin. PLoS One. 10:e01268752015.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Singh T and Katiyar SK: Honokiol inhibits
non-small cell lung cancer cell migration by targeting
PGE2-mediated activation of β-catenin signaling. PLoS
One. 8:e607492013. View Article : Google Scholar
|
|
36
|
Yun SP, Ryu JM, Park JH, Kim MO, Lee JH
and Han HJ: Prostaglandin E2 maintains mouse ESC
undifferentiated state through regulation of connexin31, connexin43
and connexin45 expression: involvement of glycogen synthase kinase
3β/β-catenin. Biol Cell. 104:378–396. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ho MY, Hung SW, Liang CM and Liang SM:
Recombinant viral capsid protein VP1 suppresses lung cancer
metastasis by inhibiting COX-2/PGE2 and MIG-7. Oncotarget.
5:3931–3943. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lee CS, Cho HJ, Jeong YJ, Shin JM, Park
KK, Park YY, Bae YS, Chung IK, Kim M, Kim CH, et al:
Isothiocyanates inhibit the invasion and migration of C6 glioma
cells by blocking FAK/JNK-mediated MMP-9 expression. Oncol Rep.
34:2901–2908. 2015.PubMed/NCBI
|
|
39
|
Chen F, Deng J, Liu X, Li W and Zheng J:
HCRP-1 regulates cell migration and invasion via EGFR-ERK mediated
up-regulation of MMP-2 with prognostic significance in human renal
cell carcinoma. Sci Rep. 5:134702015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Peng X, Zhou Y, Tian H, Yang G, Li C, Geng
Y, Wu S and Wu W: Sulforaphane inhibits invasion by phosphorylating
ERK1/2 to regulate E-cadherin and CD44v6 in human prostate cancer
DU145 cells. Oncol Rep. 34:1565–1572. 2015.PubMed/NCBI
|
|
41
|
Tao H, Guo L, Chen L, et al: MSX1 inhibits
cell migration and invasion through regulating the Wnt/beta-catenin
pathway in glioblastoma. Tumour Biol. Aug 15–2015.Epub ahead of
print.
|
|
42
|
Yang C, Li C, Li M, Tong X, Hu X, Yang X,
Yan X, He L and Wan C: CYP2S1 depletion enhances colorectal cell
proliferation is associated with PGE2-mediated activation of
β-catenin signaling. Exp Cell Res. 331:377–386. 2015. View Article : Google Scholar : PubMed/NCBI
|