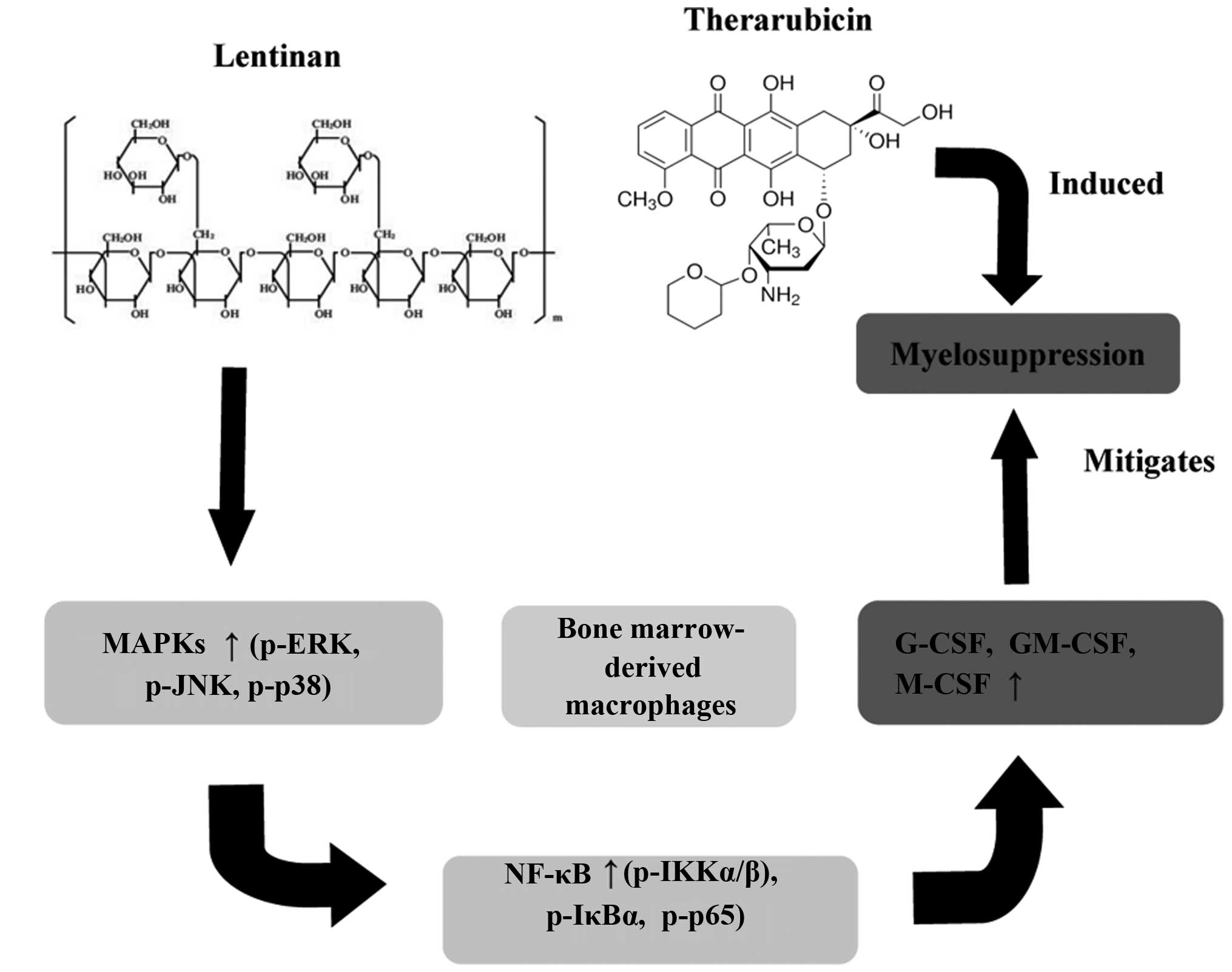
Introduction
Therarubicin (THP) is an anticancer agent that acts
on the chemical structure of DNA to inhibit the abnormal
proliferation of cancer cells. THP is primarily used for the
treatment of bladder and ureter cancer (1), malignant lymphoma (2), acute leukaemia (3), breast (4) and ovarian cancer (5). The most common side-effect of THP is
bone marrow (BM) suppression (as with most anticancer drugs), which
primarily manifests as granulocytopaenia (6). Recombinant human granulocyte
colony-stimulating factor (rhG-CSF) is often used to prevent
granulocytopaenia after radiotherapy and chemotherapy in the clinic
(7,8). rhG-CSF is associated with high
treatment costs and adverse reactions (9); therefore, the identification of an
effective, safe and inexpensive treatment regimen is imperative.
Lentinan (LNT) (Fig. 1A), a glucan
extracted from dried shiitake mushrooms (Lentinula edodes),
exhibits pharmacological effects such as tumour suppression, immune
regulation and antioxidation both in vivo and in
vitro (10). It was recently
reported that LNT protects against radiation-induced spleen cell
damage (11) and alleviates the BM
damage induced by paclitaxel (12).
However, no study has reported the role of LNT in THP-induced
myelosuppression.
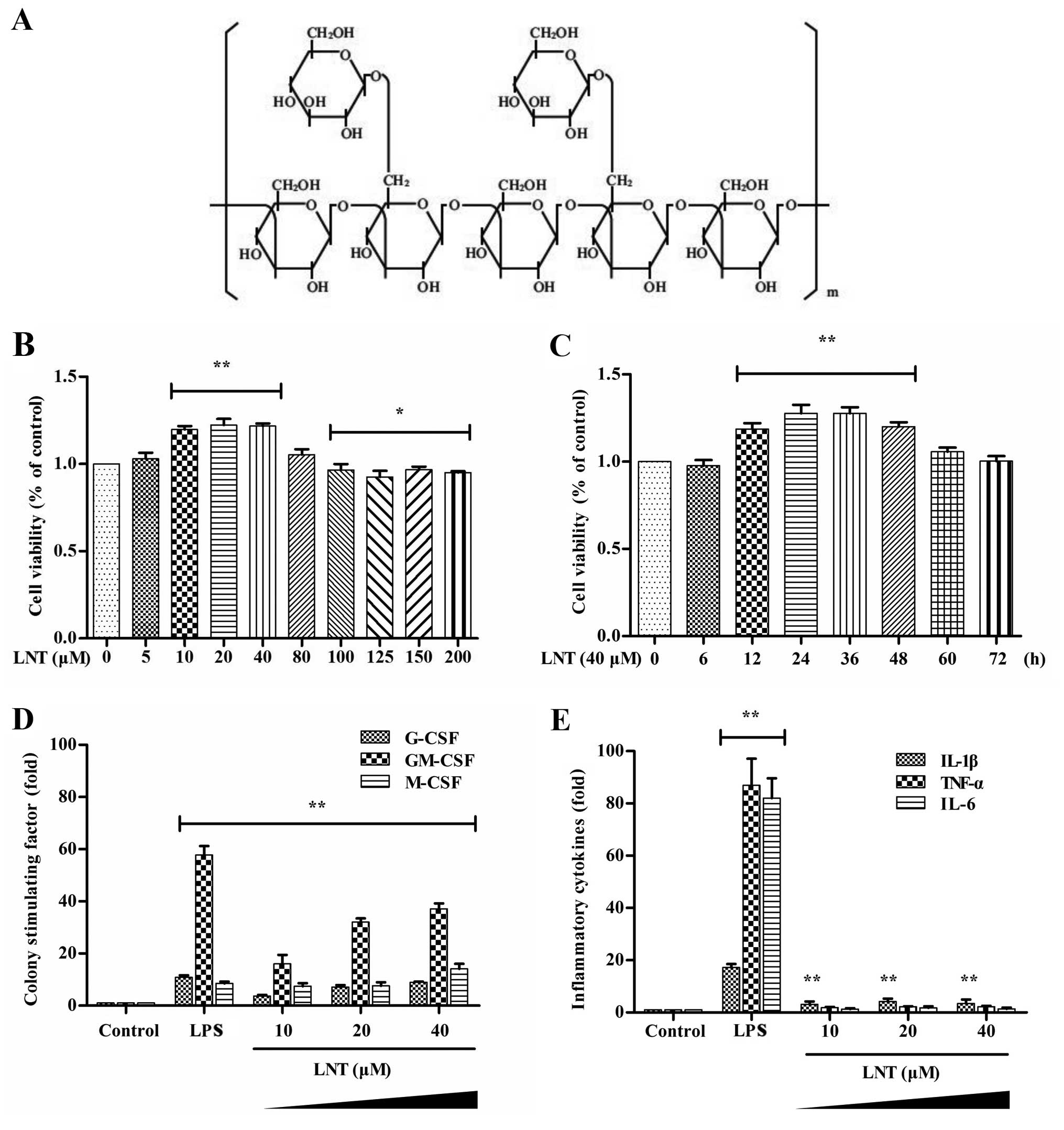 | Figure 1Effect of lentinan (LNT) on
colony-stimulating factor and pro-inflammatory cytokine production
in BM-derived macrophages (BMDMs). (A) Chemical structure of LNT.
(B) BMDMs were incubated with various concentrations of LNT (0, 5,
10, 20, 40, 80, 100, 125, 150 and 200 µM) for 24 h, and cell
viability was then determined by the CCK-8 assay. (C) BMDMs were
incubated with 40 µM LNT for 0, 6, 12, 24, 36, 48, 60 and 72
h, and cell viability was then determined by the CCK-8 assay. (D
and E) BMDMs were incubated with 10, 20 or 40 µM LNT or 200
ng/ml LPS for 48 h. The secretion of G-CSF, GM-CSF, M-CSF, TNF-α,
IL-6 and IL-1β was measured using an ELISA kit. Data are presented
as the mean ± SD of 5 independent experiments;
*P<0.05 and **P<0.01 vs. the control
groups. |
Human CSF is mainly secreted by BM cell lines
(particularly by BM macrophages) and includes granulocytic
colony-stimulating factor (G-CSF), granulocytic-macrophage
colony-stimulating factor (GM-CSF) and macrophage
colony-stimulating factor (M-CSF). The secretion of these factors
by the BM is inducible. As a common inducer, the strong
inflammatory agent lipopolysaccharides (LPSs) activate BM
macrophages through multiple signalling pathways, such as MAPK,
NF-κB (13) and PI3K-AKT (14), thereby inducing the production of
various inflammatory cytokines (13) and colony-stimulating factors
(15). CSF receptors (CSFRs) are
located on the surface of granulocyte-committed haematopoietic
progenitor, neutrophils and endothelial cells (16). When CSF binds to the receptor,
tyrosine phosphorylation of JAK family proteins occurs to recruit
STAT family proteins. Once phosphorylated by JAK, STAT enters the
nucleus and binds to the promoter region of target genes,
initiating the expression of effector proteins and inducing the
differentiation of haematopoietic progenitor cells into mature
granulocytes (17) to mediate the
immune and haematopoietic systems. However, the expression of a
variety of inflammatory factors induced by LPS may increase the
risk of immunosuppression after chemotherapy. Therefore,
researchers are actively searching for mild inflammatory
immunomodulatory agents to reduce this risk.
In the present study, Swiss mouse cells were
cultured in vitro and used as models to determine the effect
of LNT on the mitigation of THP-induced myelosuppression.
BM-derived macrophages (BMDMs) cultured in vitro were used
as a model to examine the mechanism of LNT-induced CSF
production.
Materials and methods
Reagents and animals
THP was purchased from Aladdin (Shanghai, China).
LNT was purchased from Kanghaipharm (Nanjing, China). All
antibodies were purchased from Cell Signaling Technology (Danvers,
MA, USA). Secondary antibodies were purchased from LI-COR
Biosciences (Lincoln, NE, USA). Inhibitors of ERK, p38, JNK and
NF-κB were purchased from Sigma-Aldrich (St. Louis, MO, USA). Swiss
mice were provided by Vital River (Beijing, China). Six-week-old
male and female mice weighing 20±2 g were used in the experiments.
Swiss mouse programs were implemented following a protocol approved
by the Institutional Animal Care Committee of Shanghai Institute of
Biochemistry and Cell Biology (Shanghai, China).
BMDMs
The hind femur of Swiss mice was collected. After
the muscle tissue was removed, BM was obtained by flushing the
femur with phosphate-buffered saline (PBS) containing 1%
penicillin/streptomycin. Following filtration and centrifugation,
an erythrocyte lysis buffer (Beyotime, Shanghai, China) was added,
and the BM was placed in an incubator. BM cells were collected by
centrifugation and resuspended in RPMI-1640 growth medium
containing 1% penicillin/streptomycin, 4% foetal bovine serum (FBS)
and 8% L929 cell conditioned medium (L929-CM). The medium was
exchanged every 3 days for the resuspended BM cells. Cells that
exhibited adherent growth were considered BMDMs. When all BM cells
were adherent, the medium was changed to BMDM medium containing 10%
FBS and 10% L929-CM for culture and experiments. Cells were counted
using a cell counter (18).
L929-CM: L929 cells were purchased from the American
Type Culture Collection (ATCC; Rockville, MD, USA) at 50%
confluency and were seeded in RPMi-1640 medium with 10% FBS for 5
days. The culture solution was collected and filtered through a
0.22-µm membrane filter. The filtrate was stored at −20°C
for future usage.
Cell viability assays
Cells were seeded into 96-well plates at
1×104 cells/well and cultured for 24 h. After 24 h of
incubation with LNT, the medium was exchanged and 10 µl of
Cell Counting Kit-8 (CCK-8) reagent (Dojindo, Kumamoto, Japan) was
added. The culture was incubated for another 2 h at 37°C before the
absorbance was measured at 450 nm using a microplate reader
(Thermo, Waltham, MA, USA).
May-Grünwald and Giemsa (MGG) staining of
blood and BM smears
The total number of cells in the blood and BM was
counted using a haemocytometer. Blood and BM samples were stained
with MGG reagents (Sigma-Aldrich). The slides were examined under
an inverted microscope at a magnification of ×100. The relative
number of cells was calculated (19).
Cytokine assays
Blood samples were collected by cardiac puncture.
Whole blood was allowed to stand overnight at 4°C and was
centrifuged at 12,000 rpm for 20 min to obtain the supernatant.
Following drug treatment or transfection, the cultured cells were
centrifuged at 12,000 rpm for 5 min. The levels of G-CSF, GM-CSF,
M-CSF, TNF-α, IL-6 and IL-1β were assayed using Quantikine ELISA
kits developed for the corresponding mouse cytokines (R&D
Systems, Minneapolis, MN, USA).
Confocal laser scanning microscopy
analysis of p65 nuclear import
Following treatment with LNT, BMDMs were fixed with
4% paraformaldehyde and incubated with an anti-p65 antibody
overnight. Cell nuclei were stained with
4′,6-diamidino-2-phenylindole (DAPI; Sigma-Aldrich) for 20 min.
After rinsing with PBS, the nuclear import of p65 was examined
using a confocal laser scanning microscope (magnification, ×2400)
(olympus, Tokyo, Japan).
MPO activity assays in blood and BM
cells
Blood samples were collected by cardiac puncture,
and BM samples were obtained by washing the tibiofemoral cavity
with PBS. Myeloperoxidase (MPO) activity was assayed using an MPO
Colorimetric Activity Assay kit (Sigma-Aldrich) and is expressed as
nmol/min/ml (milliunit/ml).
Haematoxylin and eosin (H&E)
staining
Mice were decapitated, and the complete femur was
immersed in 4% paraformaldehyde for 24 h, followed by
decalcification with 50% formic acid and 15% sodium citrate for 48
h. The standard histological technique of paraffin embedding was
used to create longitudinal 5-µm sections. The sections were
stained with H&E and examined under an inverted microscope
(magnification, ×40) (IX71; Olympus).
Western blotting
The cells were rinsed with PBS and lysed on ice
using a lysis buffer (Beyotime) for 40 min. The cell lysate was
boiled with 2X SDS loading buffer and loaded onto a gel for
SDS-PAGE. The gels were transferred to a nylon membrane and blocked
with 5% non-fat milk. The membrane was incubated with a primary
antibody at 4°C overnight, followed by incubation with a
fluorescent secondary antibody for 2 h. Images were collected and
analysed using the Odyssey imaging system (LI-COR Biosciences).
Statistical analysis
All data are shown as the mean ± standard deviation
(SD). The results were compared between groups using the t-test and
one-way analysis of variance (ANOVA). P<0.05 was considered to
indicate a statistically significant result. Statistical analyses
were performed with SPSS 19.0 software (SPSS, Inc., Chicago, IL,
USA).
Results
Effect of LNT on colony-stimulating
factor and pro-inflammatory cytokine production in the BMDMs
To confirm that LNT was not toxic to BMDMs, we
incubated BMDMs with various concentrations of LNT (0, 5, 10, 20,
40, 80, 100, 125, 150 and 200 µM) for 24 h and then
determined the cell viability by the CCK-8 assay (Fig. 1B). BMDM viability after different
periods of incubation (0, 6, 12, 24, 36, 48, 60 and 72 h) with 40
µM LNT is shown in Fig. 1C.
LNT exerted no toxic side-effects on BMDMs and increased the cell
viability over the concentration range of 5–40 µM for 6–48
h. We thus defined 10, 20 and 40 µM LNT as low, medium and
high doses at the cellular level. To evaluate the inflammatory and
immunomodulatory effect of LNT in the BMDMs, we incubated the cells
with 10, 20 and 40 µM LNT or 200 ng/ml LPS as a positive
control group for 24 h. Then, we collected the culture supernatant
and assessed the secretion of the CSFs G-CSF, GM-CSF and M-CSF
(Fig. 1D) and the pro-inflammatory
cytokines TNF-α, IL-6 and IL-1β (Fig.
1E) using an ELISA kit. We found that LNT significantly
stimulated production of G-CSF, GM-CSF, M-CSF and IL-1β without
affecting TNF-α and IL-6 secretion by the BMDMs. The results
indicated that LNT significantly stimulated CSF production and
induced mild inflammation in the BMDMs.
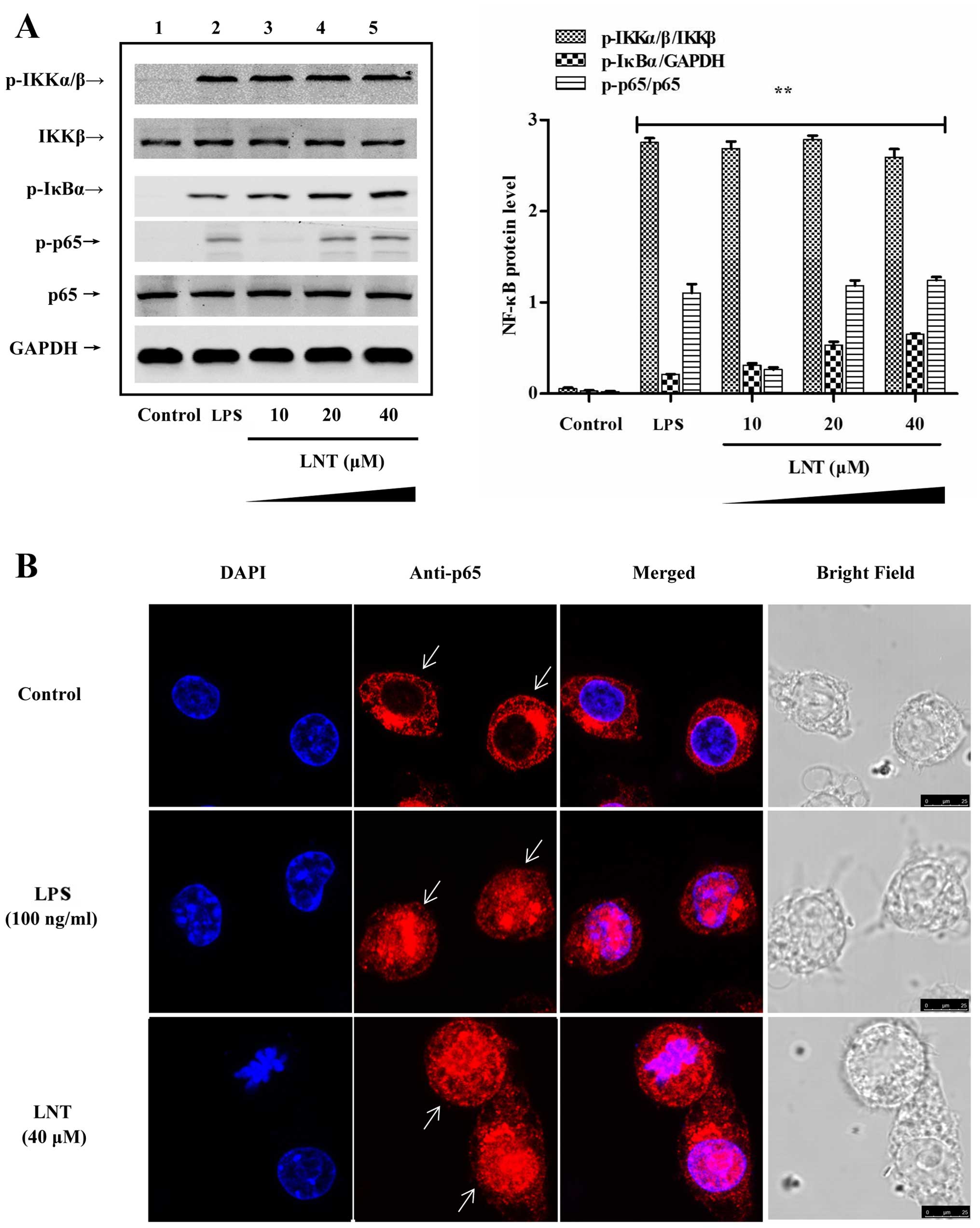
Effect of LNT on NF-κB signalling in the
BMDMs
To determine the signals upstream to the generation
of G-CSF, GM-CSF and M-CSF by LNT, we incubated cells with 10, 20
and 40 µM LNT for 1 h and determined the activation of
IKKα/β, IκBα and p65 by western blotting. Additionally, an anti-p65
antibody was labelled with red fluorescent protein, and the nuclear
import of p65-NF-κB was examined by laser confocal microscopy. We
found that LNT activated NF-κB (Fig.
2A) and induced the nuclear import of p65-NF-κB (Fig. 2B). To examine the relationship
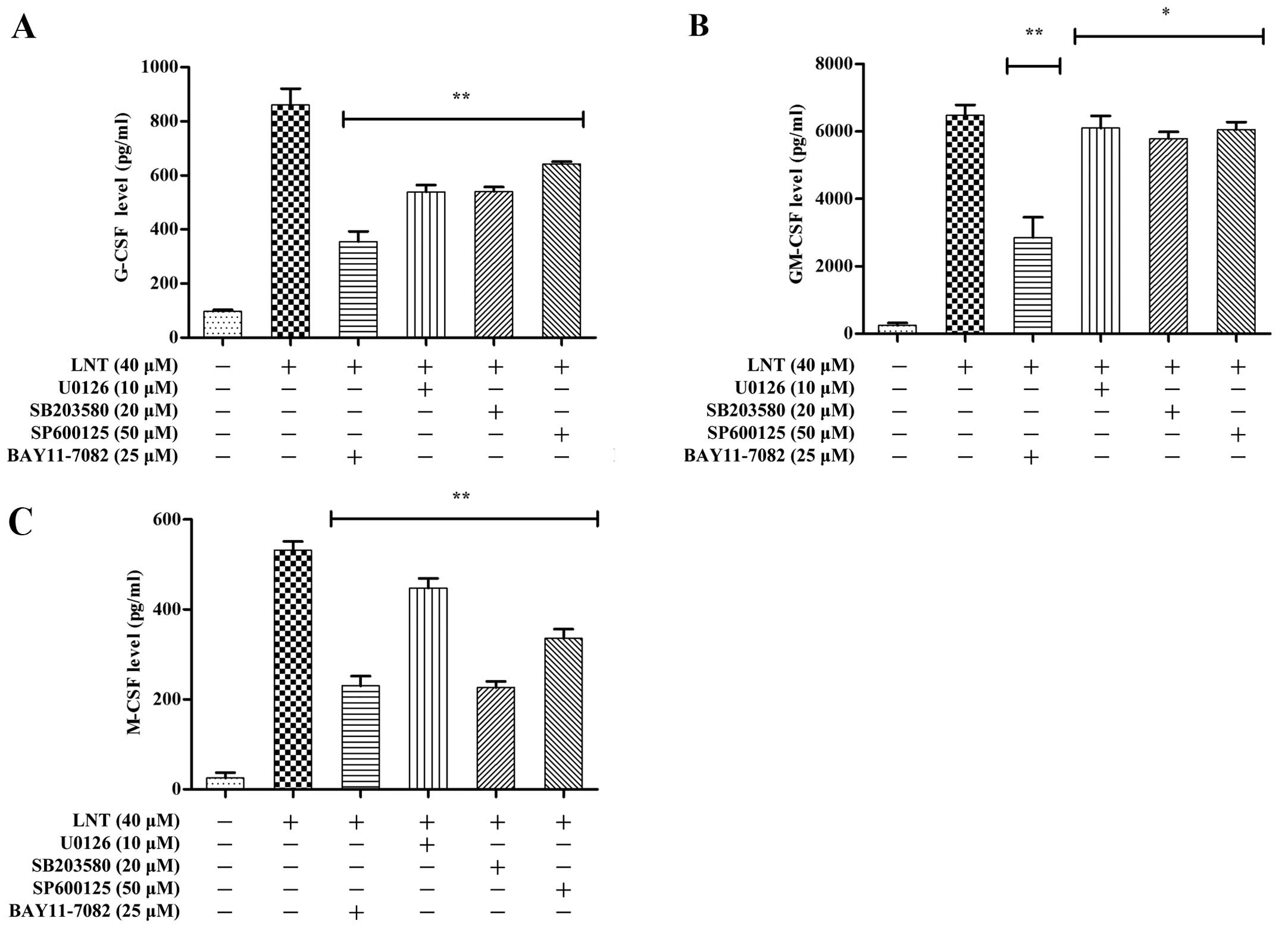
between NF-κB and G-CSF, GM-CSF, M-CSF in our experimental system,
we pre-incubated BMDMs for 2 h with the NF-κB-specific inhibitor
BAY 11-7082 (25 µM). After 24 h, ELISA assays were performed
to examine the effects of the BAY 11-7082 inhibitor on G-CSF,
GM-CSF and M-CSF secretion (Fig.
4A–C). The LNT-induced expression of G-CSF, GM-CSF and M-CSF
was mediated by the NF-κB signalling pathway.
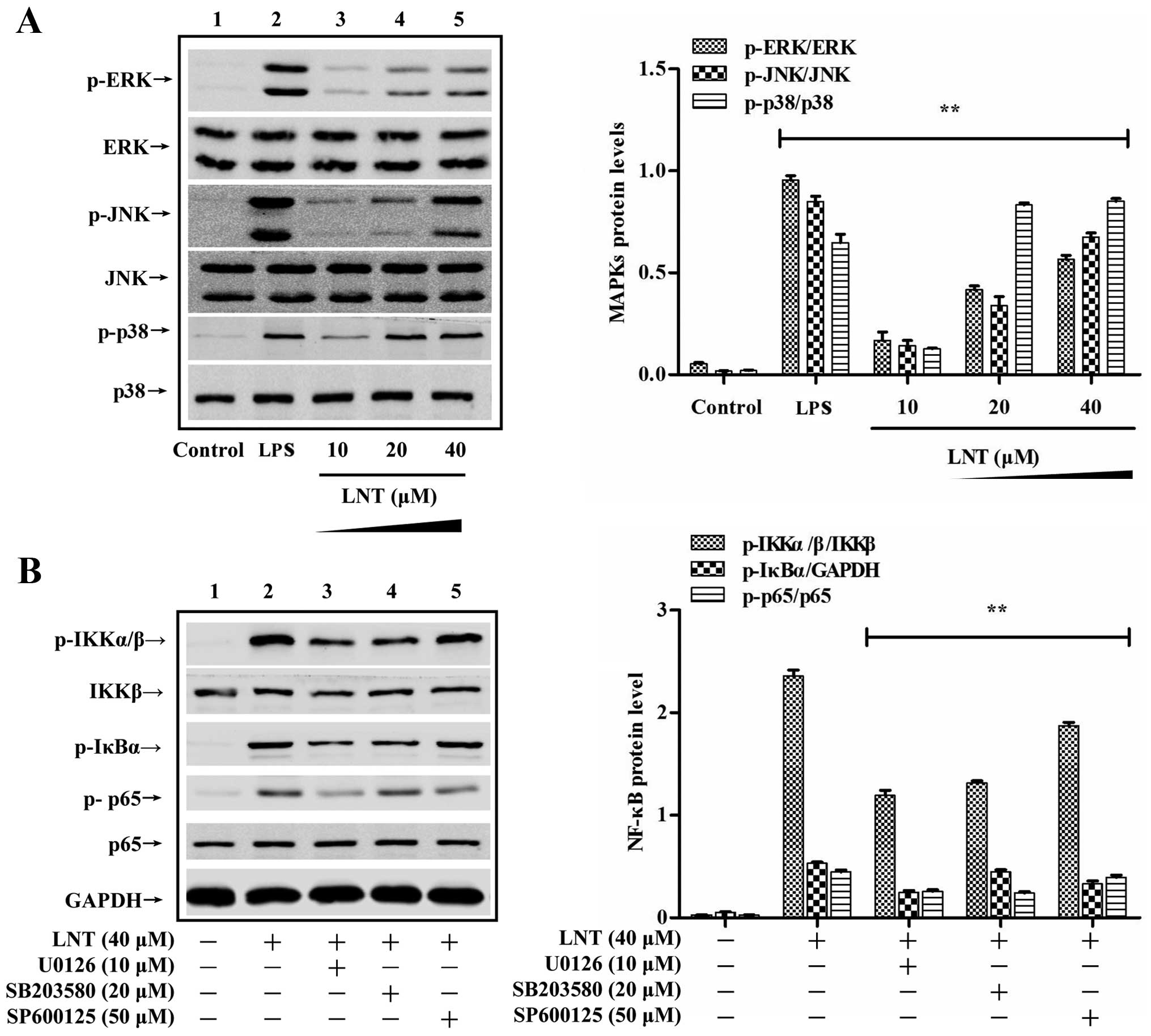
Effect of LNT on MAPK signalling in the
BMDMs
To determine the signals upstream of LNT-mediated
activation of NF-κB signalling, we incubated cells with 10, 20 and
40 µM LNT for 30 min and determined the activation of ERK,
JNK and p38 by western blotting. We found that LNT activated the
MAPKs ERK, JNK and p38 in a dose-dependent manner (Fig. 3A). To confirm that MAPKs signal
upstream of NF-κB-dependent production of G-CSF, GM-CSF and M-CSF
in our system, the levels of the signalling proteins IKKα/β, IκBα
and p65 were measured after a 1-h treatment with 40 µM LNT.
BMDMs were pre-treated with the specific ERK inhibitor u0126 (10
µM), the specific JNK inhibitor SP600125 (50 µM) and
the specific p38 inhibitor SB203580 (20 µM) for 2 h. As
shown in Fig. 3B, the suppression
of ERK, JNK and p38 phosphorylation also decreased the
phosphorylation of IKKα/β, IκBα and p65. We also measured the
effect of U0126, SP600125 and SB203580 on the production of G-CSF,
GM-CSF and M-CSF induced by LNT after 24 h. As shown in Fig. 4A–C, the suppression of MAPKs
decreased NF-κB-dependent G-CSF, GM-CSF and M-CSF production. These
results indicate that LNT increased NF-κB activation by activating
the ERK, JNK and p38 signalling proteins, which in turn upregulated
G-CSF, GM-CSF and M-CSF production.
Effect of LNT on serum G-CSF, GM-CSF and
M-CSF levels in mice treated with THP chemotherapy
MPo, a specific marker of myelocytes, is present in
the azurophilic granules of cells from the myeloid lineage (mainly
neutrophils and monocytes). To evaluate the effect of LNT on
THP-induced myelosuppression in Swiss mice, we administered various
concentrations of THP (single dose) by tail vein injection and LNT
(one dose every 12 h) by intraperitoneal injection after the
administration of THP. Seventy-two hours later, blood samples were
collected by cardiac puncture to measure serum MPO activity.
According to the experimental results (Fig. 5A and B), we selected 25 mg/kg THP as
the toxic concentration and 20 mg/kg LNT as the therapeutic
concentration at the animal level. Swiss mice (n=120) were then
randomly divided into a control group (10 mice), a THP treatment
group (55 mice) and an LNT + THP treatment group (55 mice).
Excluding the control group, all of the remaining mice were
administered a single tail vein injection of THP (25 mg/kg),
followed by an intraperitoneal injection of LNT (20 mg/kg/12 h).
The animals were decapitated to obtain bilateral femurs after blood
sampling by cardiac puncture at 1, 3, 5, 7, 9, 11, 13, 15, 17, 19
and 21 days. Similarly to the in vitro results, LNT
increased the levels of G-CSF, M-CSF and GM-CSF in the serum of the
mice on days 3, 9 and 3 after THP administration, respectively
(Fig. 5C–E). These data
demonstrated that LNT drove a marked increase in the serum levels
of G-CSF, GM-CSF and M-CSF in mice treated with THP
chemotherapy.
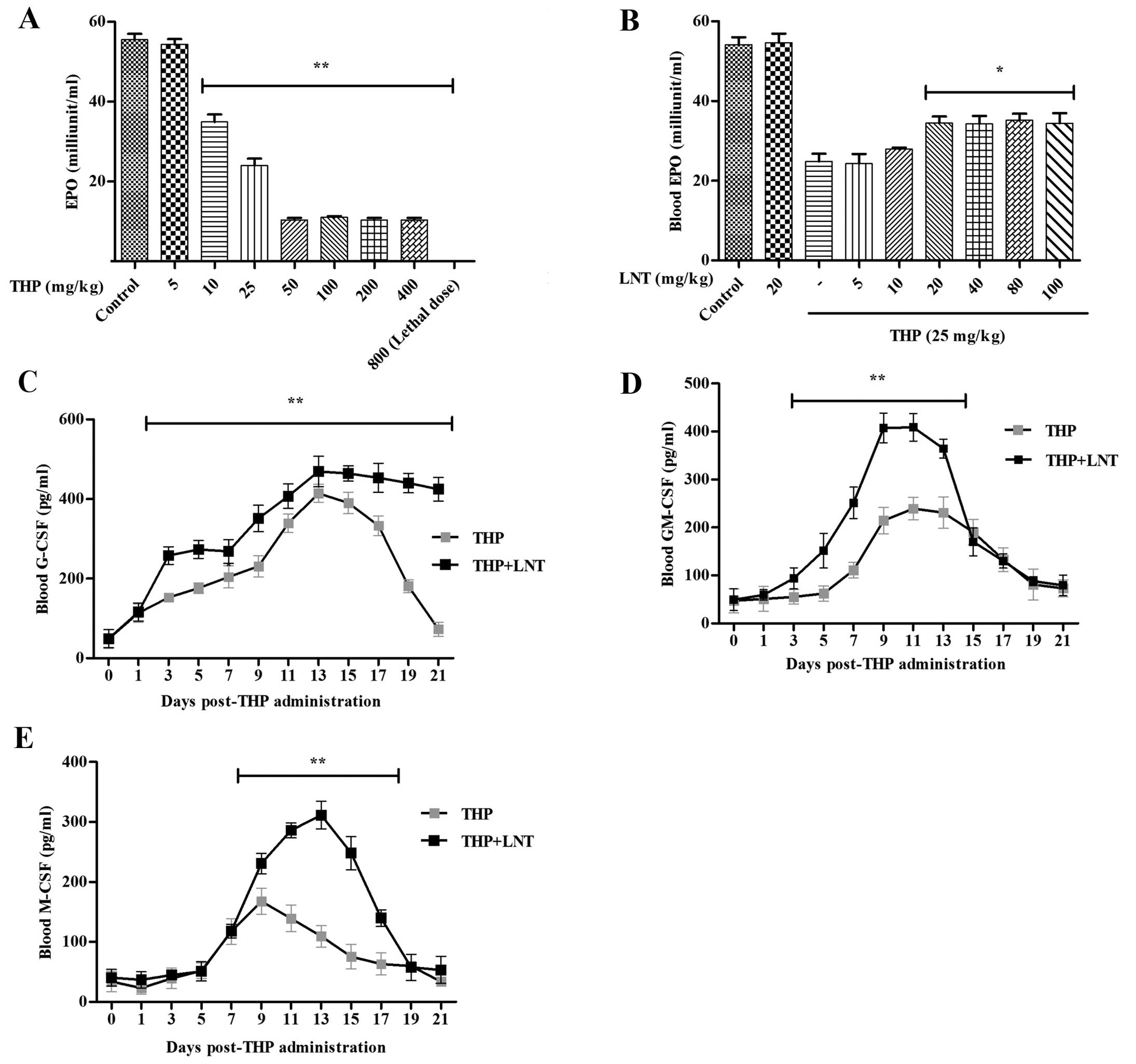 | Figure 5Effect of lentinan (LNT) on serum MPO
and G-CSF, GM-CSF, M-CSF levels in mice treated with therarubicin
(THP) chemotherapy. Swiss mice were treated with different
concentrations of THP by tail vein injection and LNT by
intraperitoneal injection after the administration of THP. (A and
B) Seventy-two hours later, blood samples were collected by cardiac
puncture to measure serum myeloperoxidase (MPO) activity. A total
of 120 Swiss mice were randomly divided into a control group, a THP
treatment group and an LNT treatment group. Excluding the control
group, all of the remaining mice were administered a single tail
vein injection of THP, followed by an intraperitoneal injection of
LNT. The animals were decapitated to obtain blood sampling by
cardiac puncture at 1, 3, 5, 7, 9, 11, 13, 15, 17, 19 and 21 days.
The levels of blood (C) G-CSF, (D) GM-CSF and (E) M-CSF were
measured using ELISA kits. Data are presented as the mean ± SD of 5
independent experiments; *P<0.05 and
**P<0.01 vs. the monotherapy THP group. |
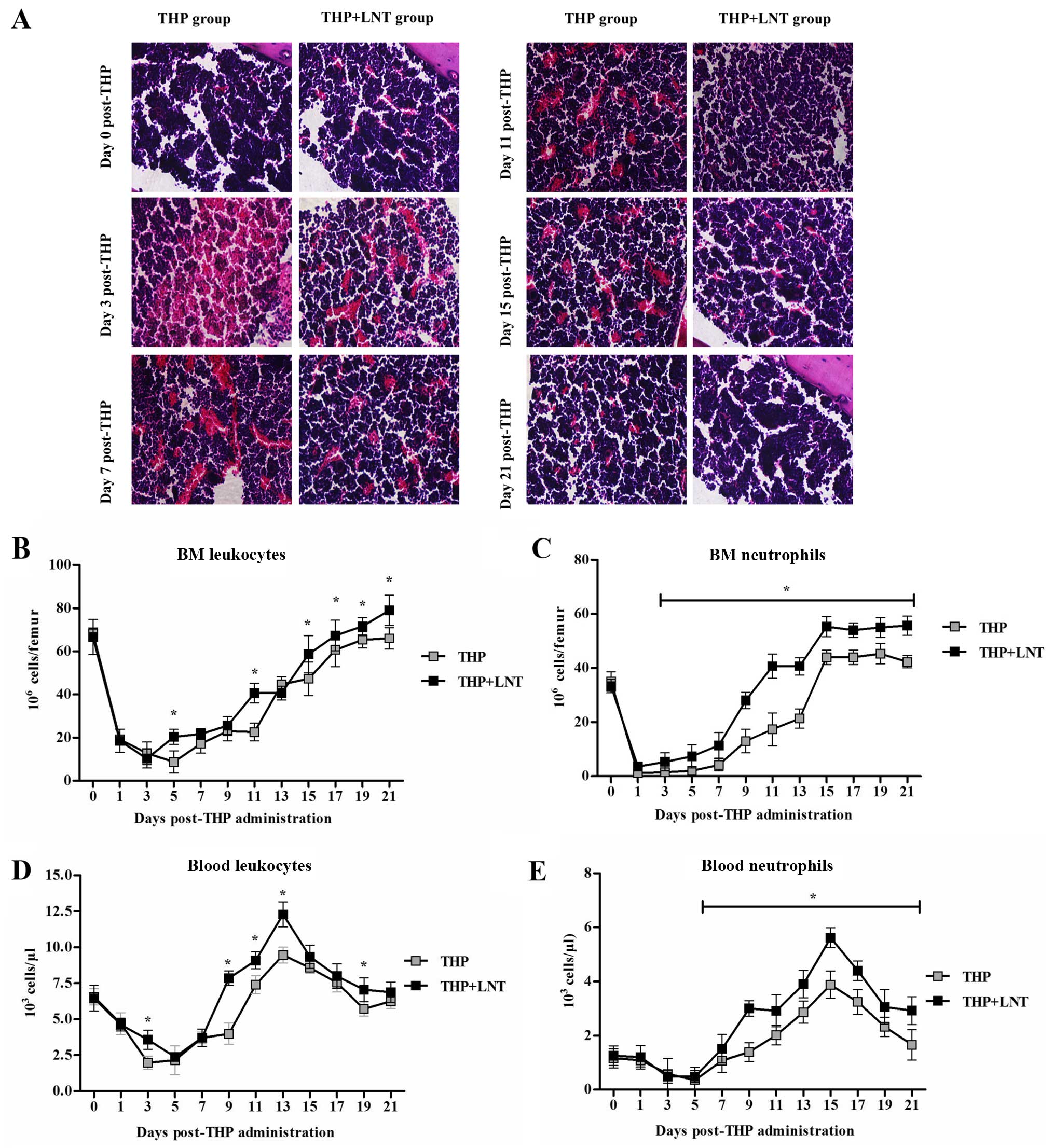
Effect of LNT on granulocyte and
leukopenia in mice treated with THP chemotherapy
As shown in Fig.
6B–E, we evaluated the numbers of leukocytes and granulocytes
in the blood and BM. In the THP treatment group, the numbers of
leukocytes and granulocytes were markedly reduced at the early
stage of chemotherapy but returned to normal at 21 days. Compared
with mice treated with THP, the LNT treatment significantly reduced
the recovery period and maintained a higher number of leukocytes
and granulocytes. Additionally, H&E staining of the BM
(Fig. 6A) revealed that in the THP
treatment group, the BM myeloid/erythroid ratio was markedly
reduced at the early stage of chemotherapy; acute BM injury began
to heal at 11 days, and complete recovery was achieved at 21 days.
The LNT treatment significantly mitigated THP-induced early acute
BM injury and reduced the healing period to 7 days, with complete
recovery at 15 days.
Discussion
Therarubicin (THP) is an anthraquinone anticancer
drug of the adriamycin family. This agent inserts into the DNA and
interacts with topoisomerase II, thereby blocking DNA replication
and arresting tumour cells in the G2 phase. As a consequence, the
tumour cells cannot enter the cell division phase, which leads to
cell death (20). THP inhibits
tumour cells with abnormal proliferation and neutrophils with rapid
cell division. Granulocytopaenia resulting from THP chemotherapy
often suppresses the immune system, increases the risk of
infection, extends the treatment period and may be fatal (21). Combination therapy with rhG-CSF is
commonly used to mitigate THP-induced granulocytopaenia in the
clinic. However, rhG-CSF combination therapy is expensive and
associated with multiple side-effects (22). Therefore, new strategies to prevent
or reduce the toxicity of chemotherapy at the level of
haematopoiesis are essential to improve therapeutic outcome and
prognosis for these patients (19).
LNT is a β-D-glucan extracted from shiitake mushrooms (Lentinus
edodes). A role for LNT in regulating the immune system has
been implicated both in vivo and in vitro by several
studies (13–22). Moreover, LNT has antitumour
(23), antioxidation (24) and DNA damage repair activities
(12). The present study is the
first to report that LNT significantly stimulates G-CSF, GM-CSF and
M-CSF production in BMDMs and protects against THP-induced
myelosuppression in vivo.
Early clinical assessments have demonstrated that
the major side-effects of THP include leucopaenia and
thrombocytopaenia (25,26); patients cannot undergo the second
round of THP treatment until after 21 days (27,28).
As expected, we found that 25 mg/kg THP substantially inhibited
blood MPO activity, reduced the numbers of granulocytes and
leukocytes in the blood and BM and injured the structure of the BM
(ruptured BM capillaries and reduced the myeloid/erythroid ratio)
in Swiss mice. This in vivo study demonstrated that LNT
significantly mitigated myelosuppression in mice induced by THP
injection by regulating the numbers of leukocytes and granulocytes
and by suppressing structural injury in the BM. Most importantly,
LNT reduced the recovery period of THP-induced myelosuppression to
2 weeks, which suggests that an LNT injection can simultaneously
reduce the radiotherapy cycle of THP. This finding has great
implications for the treatment of cancer.
After BM transplantation or chemotherapy, an
effective means to regulate the immune system is to induce mild
inflammation with polysaccharides (29,30).
The purpose of our experiments was to identify a polysaccharide
with immunomodulatory activity and decreased pro-inflammatory
activity to reduce the side-effects of chemotherapy. Many studies
have suggested that LNT can be used as an immune modulator to
activate the production of various cytokines by macrophages
(10,31). In the present study, we isolated
BMDMs from BM tissue to examine the mechanism of action of LNT in
regulating myelosuppression after chemotherapy. The experiments
revealed that non-toxic levels of LNT significantly induced the
production of G-CSF, GM-CSF and M-CSF in BMDMs. Further analysis
demonstrated that LNT activated the NF-κB signalling pathway in
BMDMs in a dose-dependent manner. After LNT-induced BMDMs were
treated with NF-κB-specific inhibitors, the production of G-CSF,
GM-CSF and M-CSF was significantly inhibited; thus, we believe that
the LNT-induced secretion of G-CSF, GM-CSF and M-CSF was due to
NF-κB activation. Additionally, the present study showed that LNT
stimulated the activation of the ERK, JNK and p38 MAPK signalling
pathways in a dose-dependent manner. To investigate whether MAPK
signalling pathway activation was upstream of NF-κB activation in
our experimental system, we used specific MAPK inhibitors that
significantly blocked LNT-induced activation of NF-κB signalling.
More importantly, MAPK inhibitors also blocked LNT-induced G-CSF,
GM-CSF and M-CSF production by BM cells. These results suggest that
MAPKs act upstream of NF-κB and mediate the production of G-CSF,
GM-CSF and M-CSF.
In conclusion, the present study is the first to
demonstrate that LNT mitigates myelosuppression caused by THP
chemotherapy in vivo. In vitro experiments revealed
that LNT stimulated MAPK and NF-κB signalling to produce G-CSF,
GM-CSF and M-CSF in BMDMs (Fig. 7).
These experiments provide conceptual and theoretical support for
the treatment of granulocytopaenia after clinical chemotherapy.
References
|
1
|
Ito A, Shintaku I, Satoh M, Ioritani N,
Aizawa M, Tochigi T, Kawamura S, Aoki H, Numata I, Takeda A, et al:
Prospective randomized phase II trial of a single early
intravesical instillation of pirarubicin (THP) in the prevention of
bladder recurrence after nephroureterectomy for upper urinary tract
urothelial carcinoma: The THP Monotherapy Study Group Trial. J Clin
Oncol. 31:1422–1427. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tomita N, Kodama F, Tsuyama N, Sakata S,
Takeuchi K, Ishibashi D, Koyama S, Ishii Y, Yamamoto W, Takasaki H,
et al: Biweekly THP-COP therapy for newly diagnosed peripheral
T-cell lymphoma patients. Hematol Oncol. 33:9–14. 2015. View Article : Google Scholar
|
|
3
|
Shimomura Y, Baba R, Watanabe A, Horikoshi
Y, Asami K, Hyakuna N, Iwai A, Matsushita T, Yamaji K, Hori T, et
al Japanese Childhood Cancer and Leukemia Study Group (JCCLSG):
Assessment of late cardiotoxicity of pirarubicin (THP) in children
with acute lymphoblastic leukemia. Pediatr Blood Cancer.
57:461–466. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Li Y, Tang JH, Huang XE and Li CG:
Clinical comparison on the safety and efficacy of
fluorouracil/pirarubicin/cyclophosphamide (FPC) with
fluorouracil/epirubicin/cyclophosphamide (FEC) as postoperative
adjuvant chemotherapy in breast cancer. Asian Pac J Cancer Prev.
12:1795–1798. 2011.
|
|
5
|
du Bois A, Meerpohl HG, Madjar H, Spinner
D, Dall P, Pfisterer J and Bauknecht T: phase II study of
pirarubicin combined with cisplatin in recurrent ovarian cancer. J
Cancer Res Clin Oncol. 120:173–178. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhao H, Yao Y, Wang Z, Lin F, Sun Y and
Chen P: Therapeutic effect of pirarubicin-based chemotherapy for
osteosarcoma patients with lung metastasis. J Chemother.
22:119–124. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hyun SY, Cheong JW, Kim SJ, Min YH, Yang
DH, Ahn JS, Lee WS, Ryoo HM, Do YR, Lee HS, et al: High-dose
etoposide plus granulocyte colony-stimulating factor as an
effective chemomobilization regimen for autologous stem cell
transplantation in patients with non-Hodgkin Lymphoma previously
treated with CHOP-based chemotherapy: A study from the Consortium
for Improving Survival of Lymphoma. Biol Blood Marrow Transplant.
20:73–79. 2014. View Article : Google Scholar
|
|
8
|
Asakuma M, Yamamoto M, Wada M, Ryuge S,
Katono K, Yokoba M, Fukui T, Takakura A, Otani S, Maki S, et al:
Phase I trial of irinotecan and amrubicin with granulocyte
colony-stimulating factor support in extensive-stage small-cell
lung cancer. Cancer Chemother Pharmacol. 69:1529–1536. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gudi R, Krishnamurthy M and Pachter BR:
Astemizole in the treatment of granulocyte colony-stimulating
factor-induced bone pain. Ann Intern Med. 123:236–237. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chihara G, Hamuro J, Maeda YY, Shiio T,
Suga T, Takasuka N and Sasaki T: Antitumor and
metastasis-inhibitory activities of lentinan as an immunomodulator:
An overview. Cancer Detect Prev Suppl. 1:423–443. 1987.PubMed/NCBI
|
|
11
|
Liu YH, Ma SD, Fu QJ, Zhao LY, Li Y, Wang
HQ and Li MC: Effect of lentinan on membrane-bound protein
expression in splenic lymphocytes under chronic low-dose radiation.
Int Immunopharmacol. 22:505–514. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Attia SM, Harisa GI, Abd-Allah AR, Ahmad
SF and Bakheet SA: The influence of lentinan on the capacity of
repair of DNA damage and apoptosis induced by paclitaxel in mouse
bone marrow cells. J Biochem Mol Toxicol. 27:370–377. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Joo SY, Song YA, Park YL, Myung E, Chung
CY, Park KJ, Cho SB, Lee WS, Kim HS, Rew JS, et al:
Epigallocatechin-3-gallate inhibits LPS-induced NF-κB and MAPK
signaling pathways in bone marrow-derived macrophages. Gut Liver.
6:188–196. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li X, Liu Y, Wang L, Li Z and Ma X:
Unfractionated heparin attenuates LPS-induced IL-8 secretion via
PI3K/Akt/NF-κB signaling pathway in human endothelial cells.
Immunobiology. 220:399–405. 2015. View Article : Google Scholar
|
|
15
|
Mannello F, Ligi D, Canale M and Raffetto
JD: Sulodexide down-regulates the release of cytokines, chemokines,
and leukocyte colony stimulating factors from human macrophages:
Role of glycosaminoglycans in inflammatory pathways of chronic
venous disease. Curr Vasc Pharmacol. 12:173–185. 2014. View Article : Google Scholar
|
|
16
|
Lee KY, Suh BG, Kim JW, Lee W, Kim SY, Kim
YY, Lee J, Lim J, Kim M, Kang CS, et al: Varying expression levels
of colony stimulating factor receptors in disease states and
different leukocytes. Exp Mol Med. 32:210–215. 2000. View Article : Google Scholar
|
|
17
|
Kamezaki K, Shimoda K, Numata A, Haro T,
Kakumitsu H, Yoshie M, Yamamoto M, Takeda K, Matsuda T, Akira S, et
al: Roles of Stat3 and ERK in G-CSF signaling. Stem Cells.
23:252–263. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Davis BK: Isolation, culture, and
functional evaluation of bone marrow-derived macrophages. Methods
Mol Biol. 1031:27–35. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Salva S, Marranzino G, Villena J, Agüero G
and Alvarez S: Probiotic Lactobacillus strains protect against
myelosuppression and immunosuppression in cyclophosphamide-treated
mice. Int Immunopharmacol. 22:209–221. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zheng SE, Xiong S, Lin F, Qiao GL, Feng T,
Shen Z, Min DL, Zhang CL and Yao Y: Pirarubicin inhibits
multidrug-resistant osteosarcoma cell proliferation through
induction of G2/M phase cell cycle arrest. Acta Pharmacol Sin.
33:832–838. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Munck JN, Rougier P, Chabot GG, Ramirez
LH, Bognel C, Lumbroso J, Herait P, Elias D, Lasser P and Gouyette
A: Phase I and pharmacological study of intra-arterial hepatic
administration of pirarubicin in patients with advanced hepatic
metastases. Eur J Cancer. 30A:289–294. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Baldo BA: Side effects of cytokines
approved for therapy. Drug Saf. 37:921–943. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ina K, Kataoka T and Ando T: The use of
lentinan for treating gastric cancer. Anticancer Agents Med Chem.
13:681–688. 2013. View Article : Google Scholar :
|
|
24
|
Fehér J, Chihara G, Vallent K, Deák G,
Blázovics A, Gergely P and Kaneko Y: Effect of lentinan on
superoxide dismutase enzyme activity in vitro. Immunopharmacol
Immunotoxicol. 11:55–61. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sridhar KS, Hussein AM, Benedetto P,
Ardalan B, Savaraj N and Richman SP: Phase II trial of
4′-0-tetrahydropyranyladriamycin (pirarubicin) in head and neck
carcinoma. Cancer. 70:1591–1597. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kleeberg UR, Reichel L, Wander HE, Beyer
JH, Essers U, Fiebig HH and Edler L: Phase II study of pirarubicin
in metastatic breast cancer. Onkologie. 13:175–179. 1990.PubMed/NCBI
|
|
27
|
Rapoport BL and Falkson G: phase II
clinical study of pirarubicin in hormone resistant prostate cancer.
Invest New Drugs. 10:119–121. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang H, Wang M, Chen J, Tang Y, Dou J, Yu
J, Xi T and Zhou C: A polysaccharide from Strongylocentrotus nudus
eggs protects against myelosuppression and immunosuppression in
cyclophosphamide-treated mice. Int Immunopharmacol. 11:1946–1953.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yu Q, Nie SP, Wang JQ, Liu XZ, Yin PF,
Huang DF, Li WJ, Gong DM and Xie MY: Chemoprotective effects of
Ganoderma atrum polysaccharide in cyclophosphamide-induced mice.
Int J Biol Macromol. 64:395–401. 2014. View Article : Google Scholar
|
|
30
|
Bhatia S, Rathee P, Sharma K, Chaugule BB,
Kar N and Bera T: Immuno-modulation effect of sulphated
polysaccharide (porphyran) from Porphyra vietnamensis. Int J Biol
Macromol. 57:50–56. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Xu X, Pan C, Zhang L and Ashida H:
Immunomodulatory beta-glucan from Lentinus edodes activates
mitogen-activated protein kinases and nuclear factor-kappaB in
murine RAW 264.7 macrophages. J Biol Chem. 286:31194–31198. 2011.
View Article : Google Scholar : PubMed/NCBI
|