Introduction
Colorectal cancer (CRC) is the third most common
cancer in men and the second in women worldwide, accounting for
roughly 1.36 million new cases and 694,000 deaths per year
[http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx
(accessed December 8, 2015)]. Surgery combined with
radiochemotherapy is the principle therapeutic strategy for CRC.
However, the recurrence of advanced CRC cannot be prevented by
current treatment. Twenty-five percent of CRC patients present with
distant metastases at the time of initial diagnosis, and up to 50%
of patients will develop metastatic disease (1). Due to the poor prognosis of CRC,
understanding the underlying mechanisms of the development and
progression of CRC is highly critical.
Glycoprotein WNTs have important functions in
carcinogenesis and embryogenesis (2,3). WNT
signals transduce through at least three distinct intracellular
signaling pathways including the canonical WNT/β-catenin pathway,
the WNT/Ca2+ pathway and the WNT/polarity pathway
(4). The canonical pathway has been
intensely studied. In this pathway, WNT1, 2, 3 and 8 bind to
members of the Frizzled (Fzd) gene family and members of the
LDL-receptor-related protein (LRP) family (5,6) and
inhibit the degradation of β-catenin. In the non-canonical pathway,
WNT5 and WNT11 bind to Frizzled and stimulate the
WNT/Ca2+ and WNT/c-jun NH2-terminal kinase (JNK)
pathways (7,8).
Wingless-type MMTV integration site family member 5B
(WNT5B) was first cloned by Tetsuroh Saitoh and Masaru Katoh in
2001. The WNT5b gene is a homologue of WNT5a, and the WNT5B protein
shows 80.2% total amino-acid identity to WNT5A (9). Studies have demonstrated that WNT5B is
expressed in esophageal, panereatic and breast cancer and embryonal
tumor cell lines (10), which
indicate a relationship between WNT5B expression and the malignant
phenotype of these cancers. In addition, WNT5B was found to be
over-expressed in leiomyoma cells compared with that in normal
myometrial smooth muscle cells (11), and WNT5B was shown to be involved in
the migratory ability of oral squamous cell carcinoma cells
(12). These data suggest that
WNT5B may be associated with tumorigenesis and metastasis.
Importantly, a recent study found that the minor allele of WNT5B
rs2010851 T>G was significantly associated with a shorter tumor
recurrence (13). Together with a
finding that WNT5B was high expressed in the colonic tissues from
ulcerative colitis patients, we hypothesized that WNT5B may be
involved in the development of CRC. A previous study demonstrated
that suppression of WNT5B inhibited cell growth, migration and
mammosphere formation in triple-negative breast cancer cells
(14), whereas its role in the
invasion and metastasis of CRC has not yet been reported.
In the present study, the role of WNT5B in the
proliferation, migration and invasion was evaluated in the COLO 205
cell line. In addition, we aimed to ascertain whether the
tumor-promoting effect of WNT5B is mediated by JNK.
Materials and methods
Cell culture
Human colon cancer cell lines HT-29, SW620, LoVo,
HCT 15 and COLO 205 were purchased from the Type Culture Collection
of the Chinese Academy of Sciences Cell Bank (Shanghai, China). The
HT-29 cells were cultured in McCOY's 5A medium (Sigma-Aldrich, St.
Louis, MO, USA). The SW620 cells were cultured in L15 medium (Gibco
Life Technologies, Carlsbad, CA, USA). The LoVo cells were cultured
in F12K medium (Sigma-Aldrich). The HCT 15 cells were cultured in
RPMI-1640 medium (Gibco). The COLO 205 cells were cultured in
RPMI-1640 medium. All the media were supplemented with 10% FBS
(Hyclone, Logan, UT, USA) and 1% antibiotics
(penicillin-streptomycin; Sigma-Aldrich). The cells were maintained
at 37°C in a 5% CO2 incubator.
Plasmid construction
The plasmid expressing WNT5B was obtained by cloning
the full coding sequences for the wild-type into the vector
pcDNA3.1 (Invitrogen). Briefly, the open-reading frame (ORF) of the
human WNT5B gene was amplified by PCR using the following primers:
WNT5B forward, 5′-TTAGGATCCATGCCCAGCCTGCTGCTGCT-3′ and reverse,
5′-CTCGAATTCCTATTTACAGATGTACTG GTCCACG-3′. The 5′ end of the
upstream primer pair and the 3′ end of the downstream primer pair
had restriction enzyme BglII cutting sites GGATCC, and
SalI cutting sites GAATTC, respectively. PCR products were
separated by electrophoresis using 1.0% polyacrylamide gels, and
the target fragment was purified and isolated using the Agarose Gel
DNA Recovery kit (BioTeke Corporation, Beijing, China). The
purified PCR fragment was ligated into the BamHI/XhoI
(Takara Bio, Dalian, China) digested pcDNA3.1 vector to yield the
pcDNA3.1-WNT5B construct. The code sequence of the plasmid was
confirmed by sequencing.
Stable transfection
COLO 205 cells were seeded into a 24-well plate at a
density of 1×105 cells/well without antibiotics. Cells
were transfected with the pcDNA3.1-WNT5B plasmid using
Lipofectamine 2000 (Invitrogen) and selected with 600 µg/ml
G418 (Invitrogen) for 2 weeks. Subsequently, individual colonies
were isolated, expanded and maintained in 300 µg/ml G418.
The overexpression of WNT5B in these clones was confirmed by
quantitative real-time PCR and western blotting.
Transfection with small interfering RNA
(siRNA) targeting JNK
JNK siRNA and scramble siRNA were purchased from
Genechem Co., Ltd. (Shanghai, China). The siRNA sequence for JNK
targeting was 5′-GCCCAGUAAUAUAGUAGUATT-3′. Scramble siRNA consisted
of a scrambled sequence that does not lead to the specific
degradation of any known cellular mRNA. siRNA transient
transfection was performed using Lipofectamine 2000 (Invitrogen)
according to the recommended instructions.
MTT assay
Cell proliferation was analyzed in vitro
using the tetrazolium salt
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
method. Cells were seeded into 96-well plates at the density of
5×103/well. MTT solution (100 µl, 0.5 mg/ml;
Sigma-Aldrich) was added into each well and incubated for 4 h at
37°C. The supernatant was then removed, and the resultant formazan
crystals were dissolved in dimethyl sulfoxide (DMSO;
Sigma-Aldrich). The absorbance value was read at 570 nm using a
microplate reader.
Wound healing assay
Cell mobility was assessed by a wound healing assay
in vitro. Approximately 5×105 cells were seeded
into 6-well plates until confluent. An incision was made in the
central area with a 200-µl pipette tip. After being washed
twice with serum-free medium, the cells were then allowed to
migrate into the cell-free area. The cells were photographed at 0
and 24 h using a microscope (Motic China Group Co., Ltd., Xiamen,
China) at a magnification of ×100. Cell migration was calculated as
the mean percentage of the cell migrated distance compared with the
initial wound distance. The experiment was performed in triplicate
with three independent repeats.
In vitro Matrigel invasion assay
Twenty-four-well Transwell chambers (Costar,
Cambridge, MA, USA) containing polycarbonate filters with
8-µm pores coated with Matrigel (1 mg/ml, BD Biosciences,
San Jose, CA, USA) were used for cell invasion capacity measurement
in vitro. Approximately 5×104 cells in 500
µl of serum-free medium were seeded into the upper chamber.
Medium (750 µl) containing 10% FBS was added to the lower
chamber to attract cells. After allowing the cells to invade for 24
h, the non-invasive cells were removed. The cells that penetrated
the lower surface of the membrane were fixed in methanol and
stained with hematoxylin. The numbers of the invasive cells were
determined from six random fields using a microscope (Motic China
Group Co., Ltd.) at a magnification of ×200.
RNA extraction and RT-PCR
Total RNA was extracted from the cells using the
RNAsimple Total RNA kit [Tiangen Biotech (Beijing) Co., Ltd,
Beijing, China] according to the manufacturer's introductions. cDNA
was synthesized using Super Moloney Murine Leukemia Virus Reverse
Transcriptase (BioTeke Corp., Beijing, China). cDNA was then
amplified by SYBR-Green (Solarbio) based real-time PCR on an
Exicycler™ 96 thermal block (Bioneer, Daejeon, Korea). The primers
used were as follows: WNT5B forward primer,
5′-CGTGGAGTACGGCTACCGCT-3′ and reverse primer,
5′-CAGGCTACGTCTGCCATCTTAT-3′; JNK forward primer,
5′-GGATATAGCTTTGAGAAACTCTTCC-3′, and reverse primer,
TCTAACTGCTTGTCAGGGATCTT-3′; β-actin forward primer,
5′-CTTAGTTGCGTTACACCCTTT CTTG-3′ and reverse primer,
5′-CTGTCACCTTCACCGTT CCAGTTT-3′. Relative mRNA expression
normalized to β-actin was calculated by 2−ΔCt. The
2−ΔΔCt method was used for fold change calculation.
Western blotting
The cells were dissolved in NP-40 lysis buffer
(Beyotime) containing 1% Triton X-100 with 1 mM
phenylmethanesulfonyl fluoride and quantified using a commercial
BCA protein assay kit (Beyotime). Samples containing 40 µg
proteins were heat-denatured and separated using sodium dodecyl
sulfate polyacrylamide gel electrophoresis (SDS-PAGE), and then
transferred onto polyvinylidene fluoride membranes (EDM Millipore,
Billerica, MA, USA). After a block stage using 5% non-fat milk, the
membranes were incubated with WNT5B (1:200, sc-109464; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA), MMP 2 (1:400, BA0569) and
MMP 9 (1:400, BA05730 (both from Boster, Wuhan, China), c-jun
(1:500, bs-0670R), p-c-jun (1:500, bs-3209R), JNK (1:500,
bs-10562R) and p-JNK (1:500, bs-1640R) (all from Bioss, Beijing,
China) primary anibodies at 4°C overnight. Subsequently, the
membranes were washed twice using TBST and incubated with goat
anti-rabbit immunoglobulin G (IgG)-HRP-conjugated secondary
antibodies (1:5000, Beyotime) for 45 min at 37°C. The blots were
visualized using enhanced chemiluminescence regent (Wanleibio) on
X-ray film (Fuji Photo Film Co., Ltd., Tokyo, Japan). The protein
levels were quantified by gray analysis using Gel-Pro-Analyzer
software (Media Cybernetics, Bethesda, MD, USA). The grey levels
are expressed as a percentage of β-actin levels (loading
control).
Gelatin zymography
Activities of MMP 2 and MMP 9 in the culture medium
of cells were analyzed using gelatin zymography as previously
reported (15). The medium was
collected and centrifuged at 1,000 ×g for 2 min to remove debris.
Equal amounts of protein were separated on a 10% SDS-PAGE gel
containing 1 mg/ml gelatin. The gels were washed in renaturing
buffer [50 mmol/l Tris (pH 7.6), 5 mmol/l CaCl2, 1
µmol/l ZnCl2 and 2.5% Triton X-100] twice and
then incubated overnight in developing buffer [50 mmol/l Tris-HCl
(pH 7.6), 200 mmol/l NaCl, 5 mmol/l CaCl2, 1
µmol/l ZnCl2 and 0.02% Brij] at 37°C for 40 h.
The gels were then stained using 0.05% Coomassie blue R-250 in 30%
methanol and 5% acetic acid and destained with destaining solution
A (30% methanol, 10% acetic acid) for 0.5 h, B (20% methanol, 10%
acetic acid) for 1 h, and C (10% methanol, 5% acetic acid) for 2 h.
The image was captured and analyzed using gel imaging and analysis
system (WD-9413B; Beijing Liuyi Biotechnology Co., Ltd., Beijing,
China).
Immunofluorescence
COLO 205 cells were cultured on glass coverslips and
fixed with 4% paraformaldehyde (Sinopharm Chemical Reagent Co.,
Ltd, Beijing, China) for 15 min. The cells were then treated with
0.5% Triton X-100 for 10 min and blocked with goat serum (Solarbio
Science & Technology, Co., Ltd., Beijing, China) for 15 min at
room temperature. Subsequently, the cells were incubated with
anti-p-c-jun primary antibody (1:200, bs-3209R; Bioss) at 4°C
overnight. After a washing stage with 1X PBS, the cells were
incubated in secondary antibody coupled to Cy3 (1:200; Beyotime) in
darkness for 1 h at room temperature. The cells were then
counterstained with 4′,6-diamidino-2-phenylindole (DAPI) (Biosharp,
Hefei, China) and observed using a fluorescence microscope (BX53;
Olympus, Tokyo, Japan).
Statistical analysis
Data are presented as the mean ± SD. The results
were assessed using a one-way analysis of variance (ANOVA) followed
by an LSD test for comparisons among groups. Statistical analyses
were conducted using SPSS 19 (IBM SPSS, Armonk, NY, USA). P-values
<0.05 were considered to be indicative of statistical
significance.
Results
Effect of WNT5B on cell proliferation,
migration and invasion
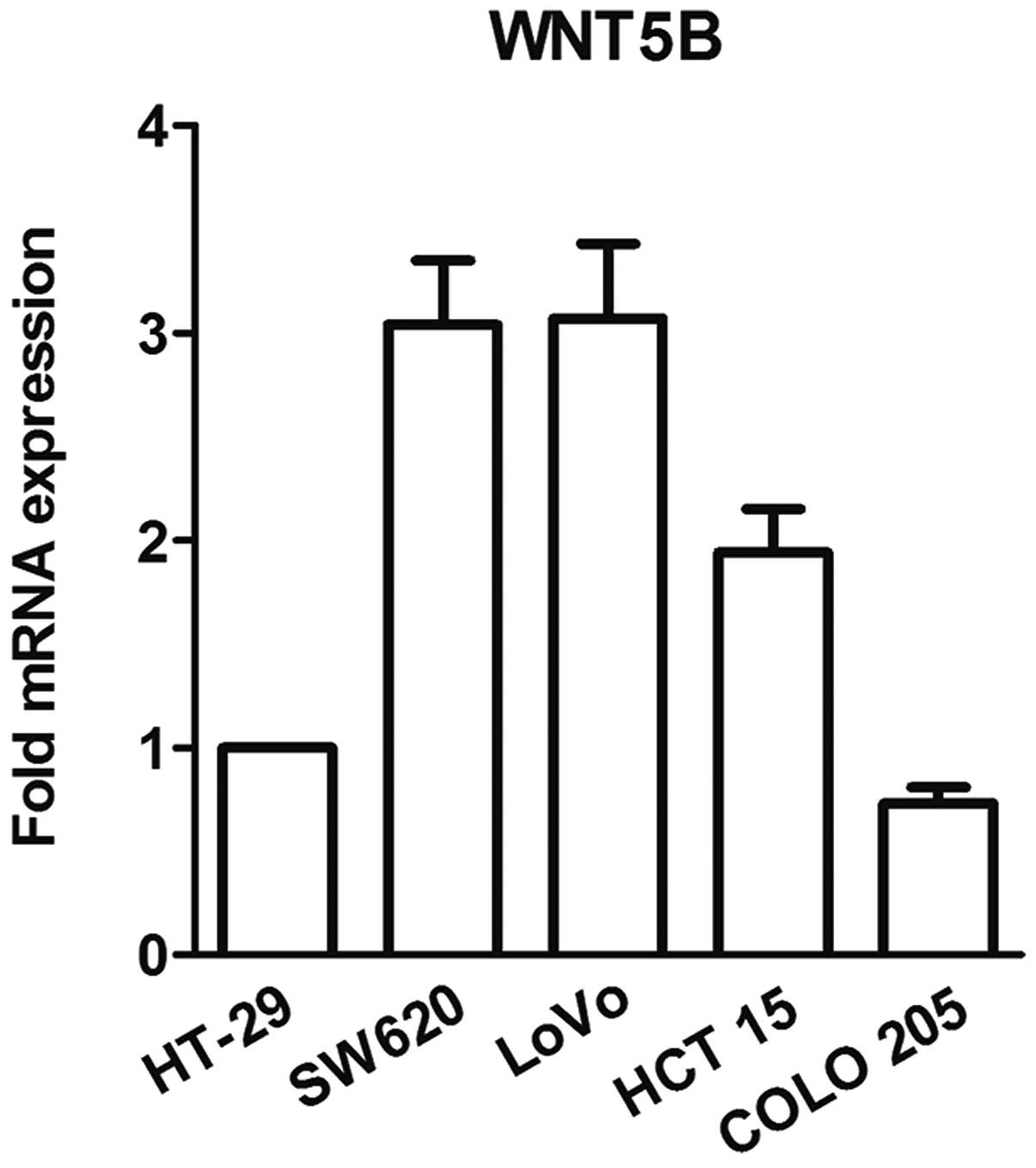
Prior to the studies, WNT5B expression in five cell
lines was tested using real-time PCR. COLO 205 cells, which
exhibited the lowest level of WNT5B expression (Fig. 1), were used in the following
studies.
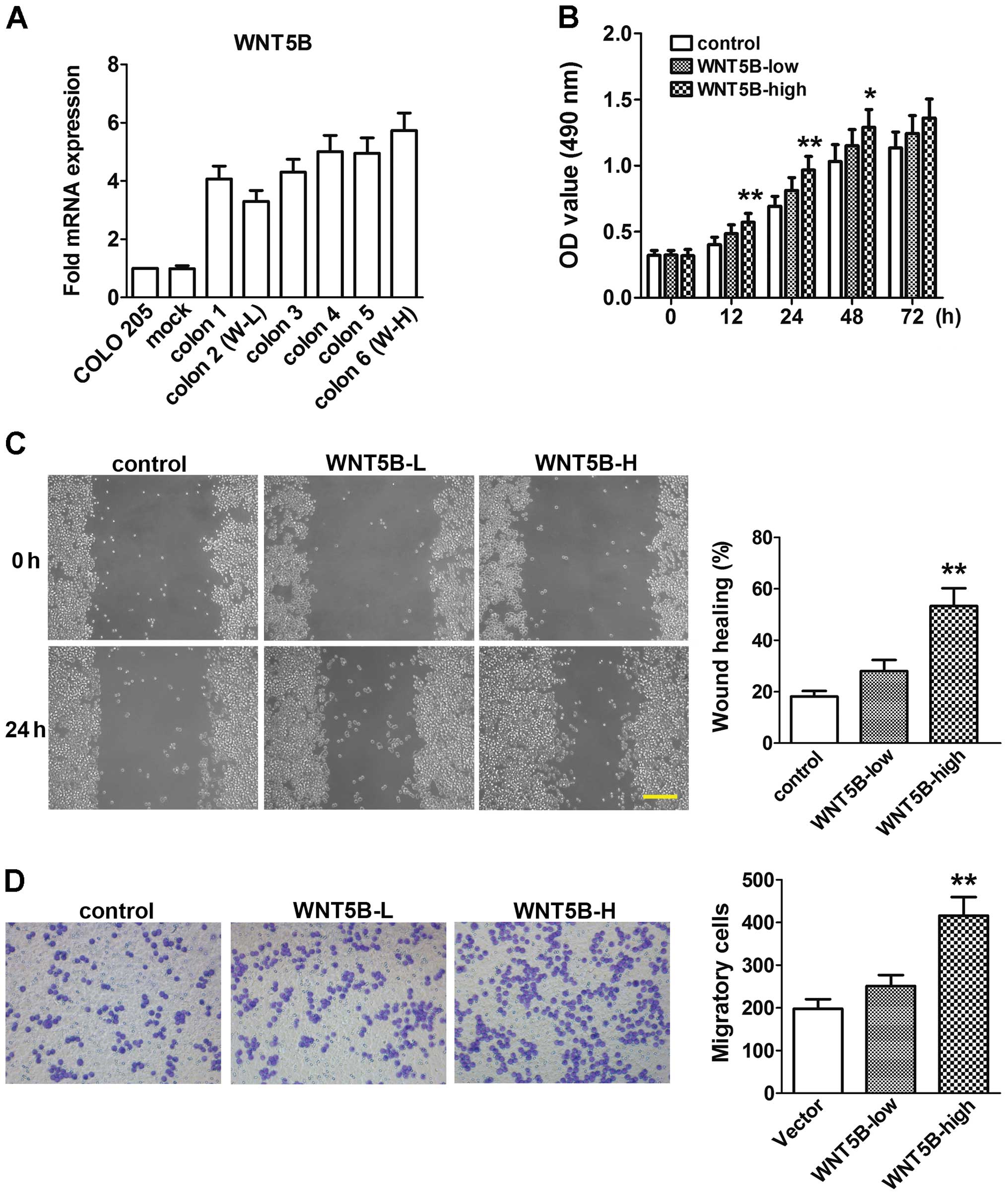
Expression of WNT5B mRNA in COLO 205 cells
transfected with the pcDNA3.1-WNT5B or empty vector is shown in
Fig. 2A. Six colons were chosen
randomly to be examined. WNT5B mRNA expression was significantly
increased in the six colons compared to the untreated COLO 205
cells or cells transfected with the empty vector (mock). Colon 2,
the lowest one (WNT5B-L), and colon 6, the highest one (WNT5B-H),
were used in the following experiments.
The proliferation of cells was assessed using MTT
assay. As shown in Fig. 2B, at 12,
24 and 48 h, cell proliferation was significantly increased in the
WNT5B-H cells. A modest increase in cell proliferation was also
found in the WNT5B-L cells, but did not achieve a significant
difference. Cell migration and invasion were assessed using wound
healing and Transwell assays. Migration and invasion activities
were also significantly increased in the WNT5B-H cells compared to
the mock, although the WNT5B-L cells did not show significant
changes (Fig. 2C and D).
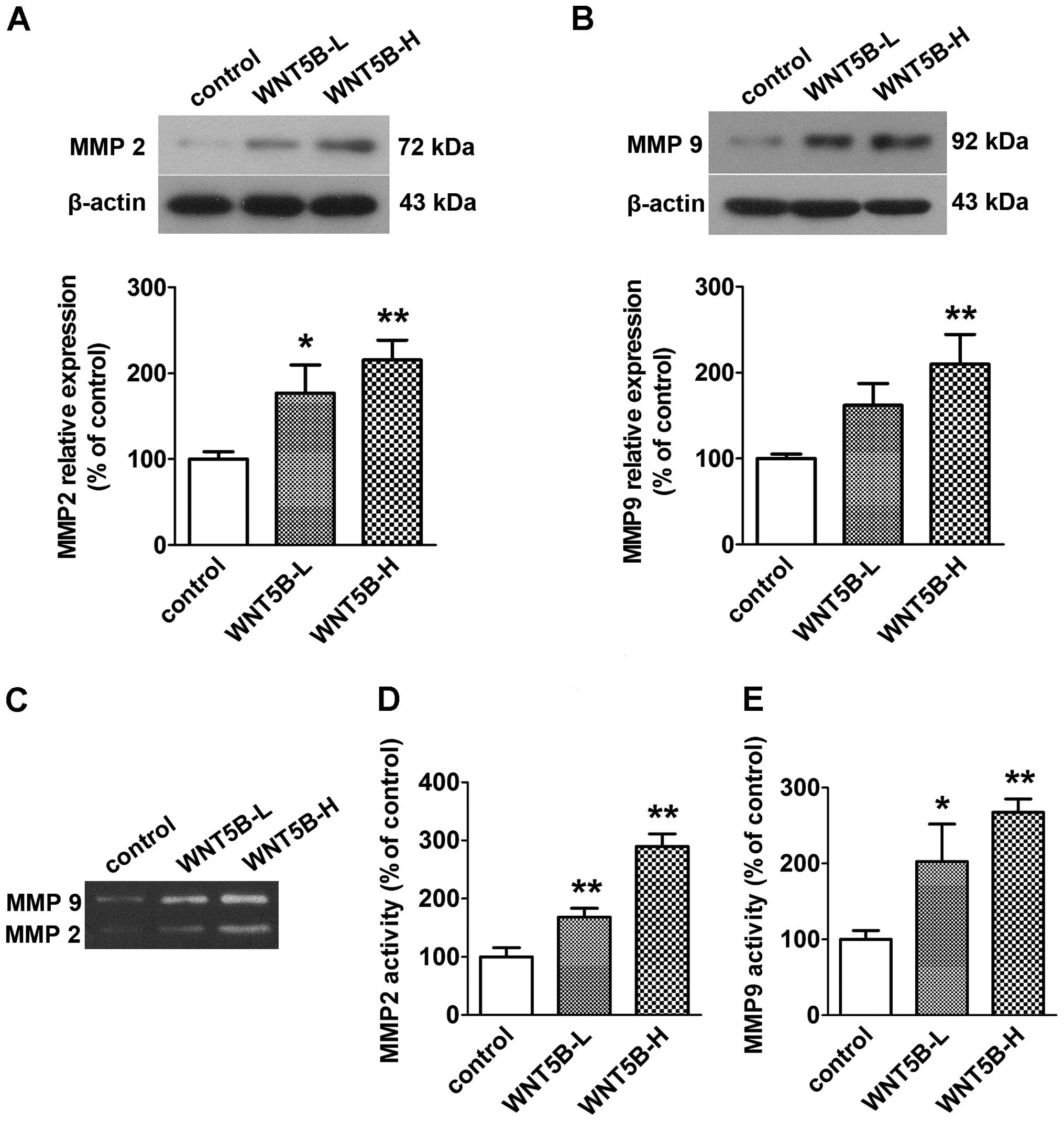
Effects of WNT5B on MMP 2 and MMP 9
expression and secretory activities
The protein expression of MMP 2 and MMP 9 was
examined using western blotting. Secretory activities of MMP 2 and
MMP 9 were examined using gelatin zymography. As illustrated in
Fig. 3, the protein expression and
secretory activities were significantly enhanced by WNT5B
transfection in a WNT5B expression-dependent manner.
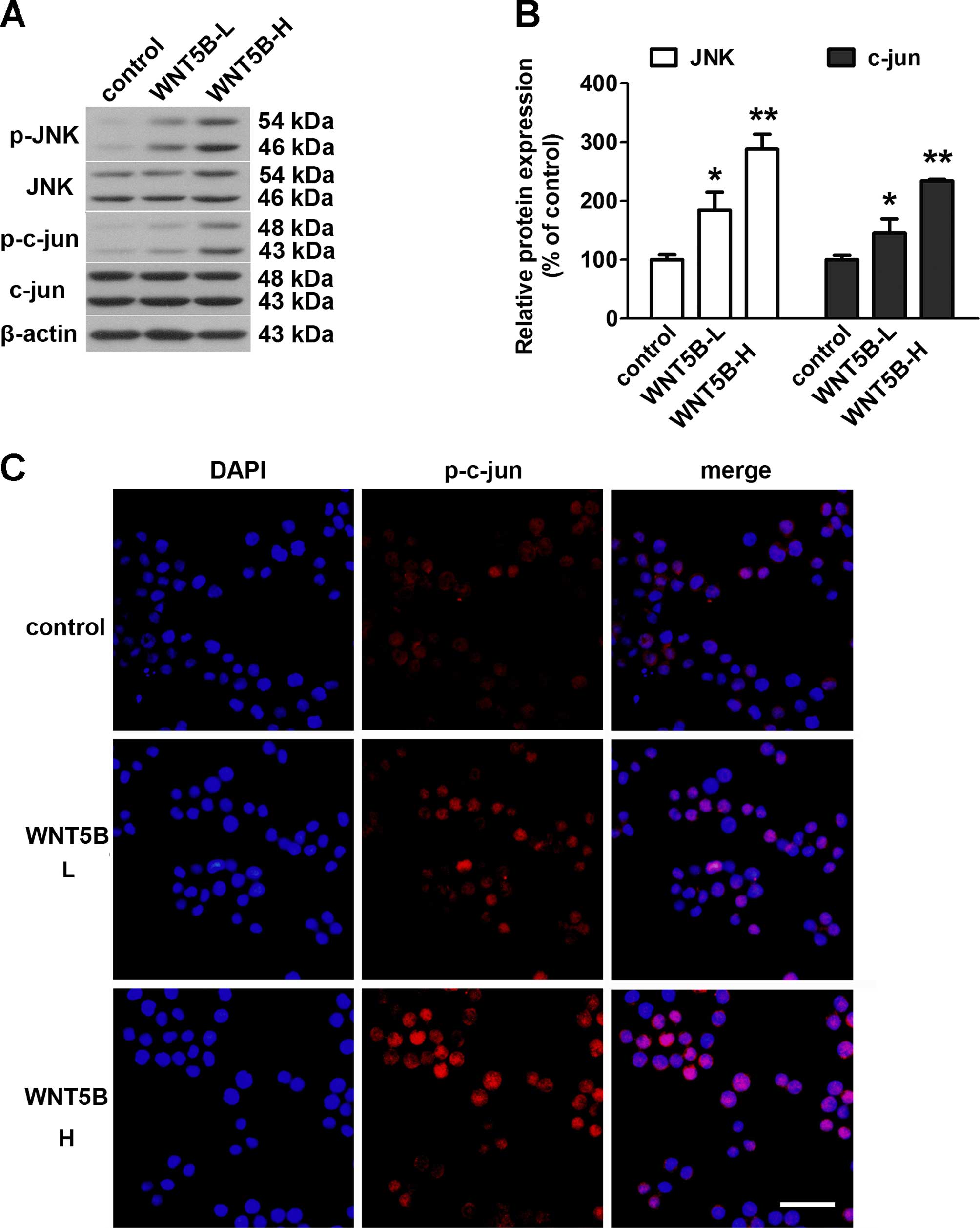
Effects of WNT5B on activity of the
WNT/JNK signaling pathway
The activation of the WNT/JNK signaling pathway was
evaluated using western blotting and immunofluorescence analysis.
As shown in the Fig. 4A and B,
WNT5B overexpression markedly induced phosphorylation of JNK and
c-jun in the COLO 205 cells, and the phospho-protein/total protein
ratios in the WNT5B-high group were higher than that in the
WNT5B-low group. The immunofluorescence analysis confirmed the
findings of the western blot analysis (Fig. 4C). The WNT5B-overexpressing cells
showed significantly higher levels of c-jun phosphorylation than
that noted in the control cells.
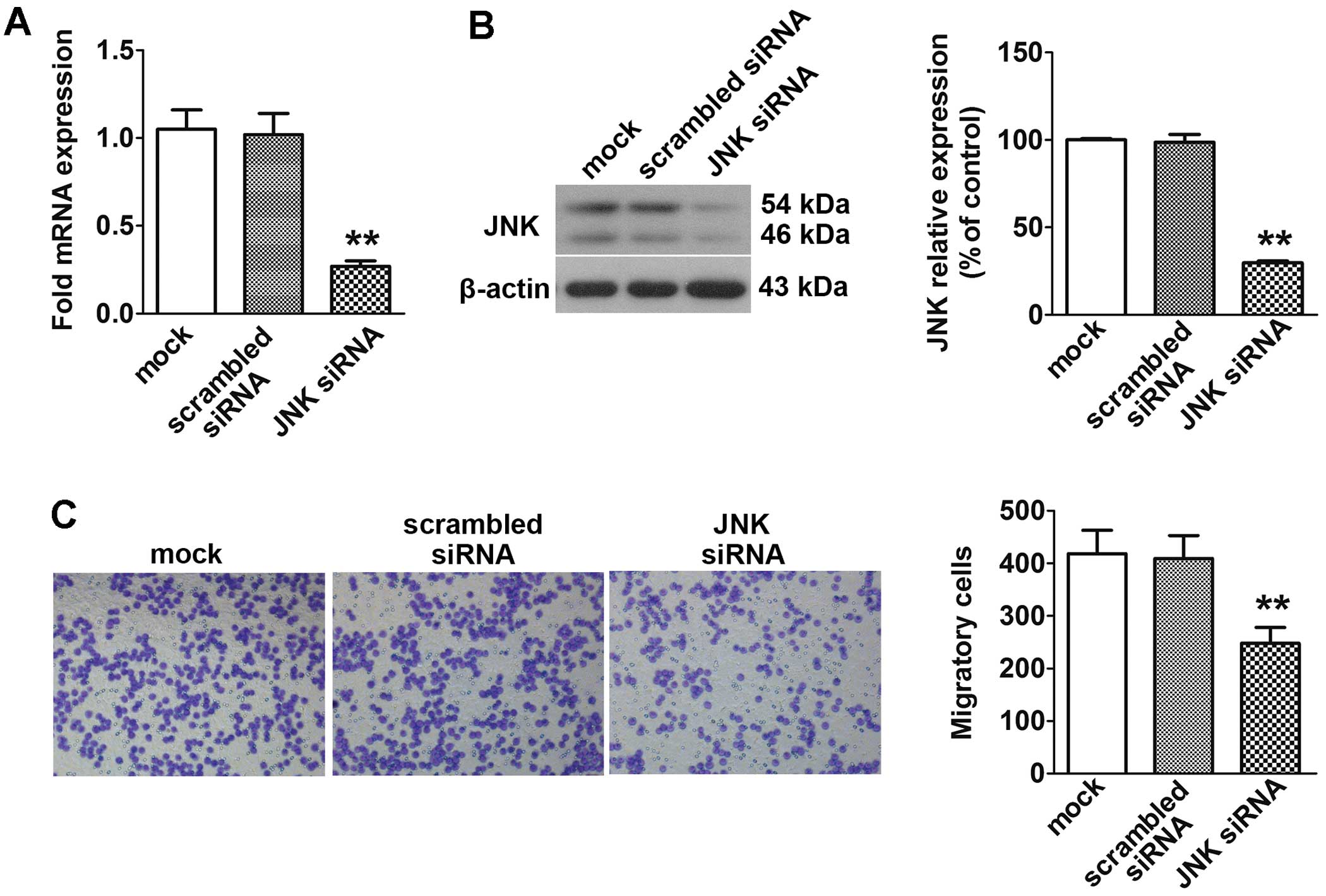
Effect of JNK siRNA transfer on the
migration activity of the WNT5B transfectants
To evaluate the involvement of JNK in the increased
migration activities of the WNT5B-transfected COLO 205 cells, JNK
siRNA was tranfected into the WNT5B-H cells. As illustrated in
Fig. 5A and B, the expression
levels of JNK mRNA and protein were markedly decreased by RNA
interference of JNK. Migration activity of the JNK
siRNA-transfected WNT5B-H cells was markedly decreased in
comparison with the mock cells (Fig.
5C).
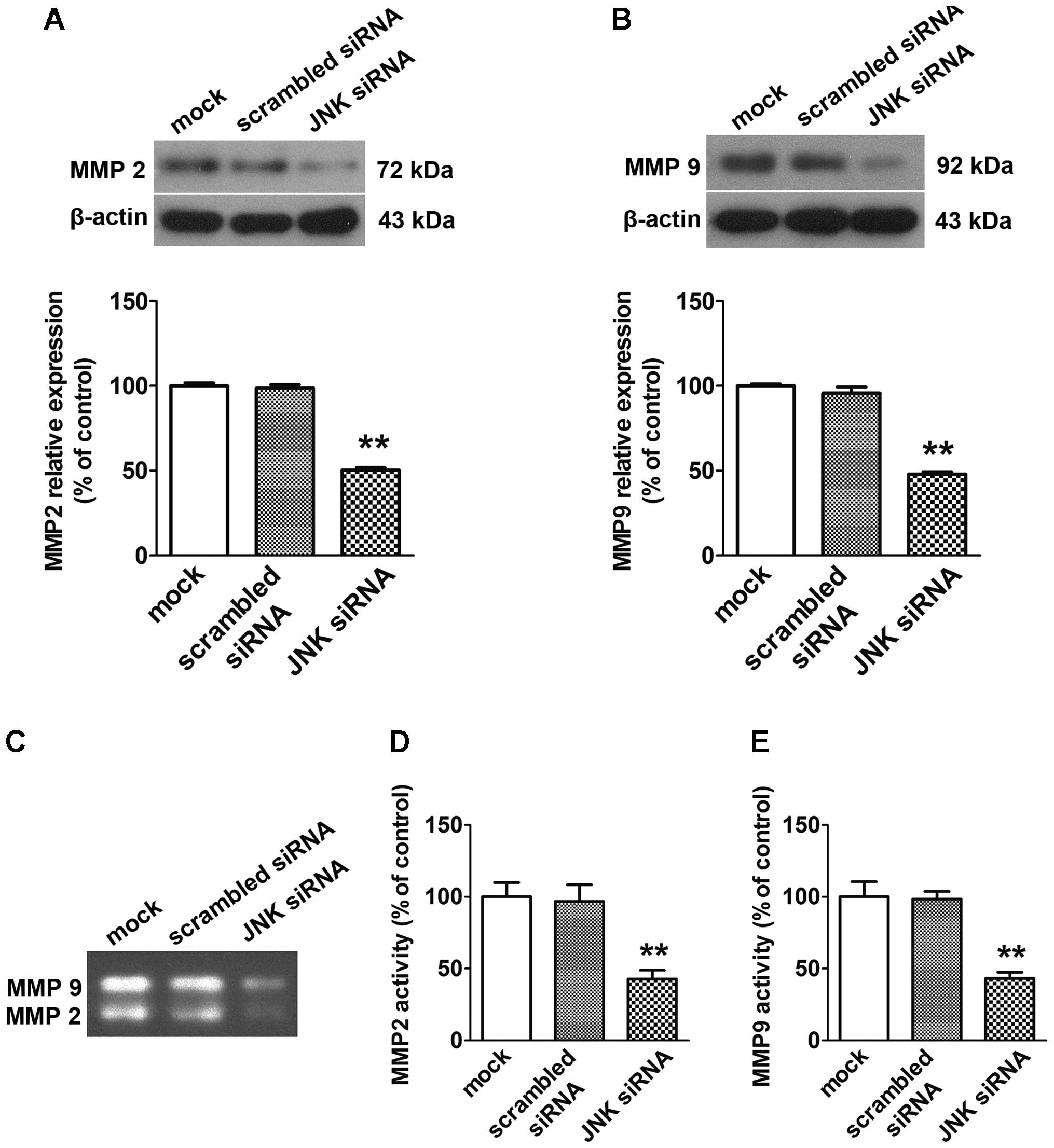
Effect of JNK siRNA transfer on MMP 2 and
MMP 9 expression and secretory activities of WNT5B
transfectants
As shown in Fig. 6,
JNK siRNA transfection significantly inhibited the secretory
activities and protein expression levels of MMP 2 and MMP 9.
Discussion
Our present findings showed, for the first time,
that WNT5B promoted cell proliferation, migration and invasion
abilities in human colon cancer cell line COLO 205 in vitro
through the JNK signaling pathway.
Migration and invasion are important prerequisites
of cancer progression and metastasis. In the present study, wound
healing and Transwell invasion assays were conducted to determine
the effects of WNT5B overexpression on the migration and invasion
abilities of COLO 205 cells. The results showed that overexpression
of WNT5B significantly increased the invasive and metastatic
ability of the COLO 205 cells in vitro. Matrix
metalloproteinases (MMPs), a family of zinc-dependent
endopeptidases (16,17), play an important role in proteolytic
degradation of the basement membrane and extracellular matrix
(ECM), which is the initial step of tumor invasion and metastasis.
In particular, MMP 2 and MMP 9 have been considered to be
associated with increased invasiveness in various cancer cells
(18). Invadopodia, actin-rich,
finger-like cellular membrane projections located at the ventral
side of the cell, are involved in ECM degradation through
disintegrating the basement membrane (19). MMP 2 and MMP 9 have been shown to be
enriched in invadopodia where they contribute to ECM degradation
in vitro and in vivo (19). The present study demonstrated a
marked increase in protein levels and secretory activities of MMP 2
and MMP 9 in the COLO 205 cells with WNT5B overexpression. Although
the present data do not provide direct evidence for the effect of
WNT5B on MMPs, overexpression of WNT5B may promote the metastatic
ability of COLO 205 cells by upregulating MMP 2 and MMP 9
expression and increasing their secretory activities.
JNK is a member of the mitogen activated protein
kinases (MAPK) and is involved in non-canonical WNT signaling
(20). In the present study, we
examined the expression and phosphorylation of JNK and c-jun. The
results revealed that the phosphorylation levels of the two
proteins were increased in the WNT5B transfectants compared to the
mock cells, and siRNA-mediated gene knockdown of JNK in the WNT5B
transfectants was shown to decrease their migratory activity. This
suggests that WNT5B may regulate cell migration activity at least
partly through regulation of the WNT/JNK signaling pathway in COLO
205 cells.
The role of JNK in tumorigenesis is ambiguous. On
the one hand, accumulating evidence suggests that JNK has
tumor-promoting function. Mice with JNK1 gene double knockout
exhibited a marked decrease in the development of
N-methyl-N-nitrosourea-induced gastric tumors compared with
wild-type mice (21). Moreover,
JNK1−/− mice were shown to be much less susceptible to
hepatocarcinogenesis caused by diethylnitrosamine, and the absence
of JNK1 resulted in reduced cell proliferation and tumor
neovascularization (22). In
addition, activation of JNK signaling has been found to accelerate
colitis-induced tumorigenesis (23). On the other hand, other findings
indicate that JNK has tumor-suppressing function. She and
colleagues demonstrated that JNK1−/− mice were more
susceptible to TPA-induced skin tumor development than wild-type
mice (24). Mice lacking the JNK1
gene were found to exhibit increased intestinal tumor formation
(25). Data from WNT family
member-associated studies showed discrepancies as well. WNT5A, the
homologous gene of WNT5B, has been demonstrated to enhance the
motility of malignant cells and tumor invasion in various types of
cancer (26,27). It was found to regulate cell
migration through JNK activation in pancreatic cancer and human
osteosarcoma cells (28,29), which suggests that the
pro-tumorigenic function of WNT5A is associated with JNK
activation. WNT11 gene overexpression was found to induce JNK and
c-jun phosphorylation and increase migration and invasion in CRC
HCT-116 cells (30), and
downregulation of Frizzled-7 with siRNA resulted in decreased
phosphorylation of JNK and migration in HCT-116 cells (31), which indicate the involvement of the
WNT/JNK signaling pathway in the tumor-promoting activity of
WNT11/Frizzled-7. However, WNT7a/Frizzled-9 signaling was found to
inhibit the growth of non-small cell lung cancer cells through
activation of the JNK pathway (32). In the present study, similar to its
homologous protein WNT5A, WNT5B promoted the migration and invasion
through activation of the JNK signaling pathway in COLO 205 cells,
and increased MMP 2 and MMP 9 expression and secretory activities
as well. This discrepancy can be attributed to the difference in
cell type, organ or stimuli, while the detailed mechanisms require
further investigation.
In conclusion, this is the first study to show that
WNT5B may play an important role in the regulation of migration and
invasion activities of CRC cells through the WNT/JNK signaling
pathway. These results suggest that WNT5B/JNK may be involved in
CRC progression.
References
|
1
|
Worni M, Shah KN and Clary BM: Colorectal
cancer with potentially resectable hepatic metastases: optimizing
treatment. Curr Oncol Rep. 16:4072014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Moon RT, Brown JD and Torres M: WNTs
modulate cell fate and behavior during vertebrate development.
Trends Genet. 13:157–162. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Peifer M and Polakis P: Wnt signaling in
oncogenesis and embryogenesis - a look outside the nucleus.
Science. 287:1606–1609. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Miller JR: The Wnts. Genome Biol.
3:REVIEWS3001. 2002.
|
|
5
|
Bejsovec A: Wnt signaling: an
embarrassment of receptors. Curr Biol. 10:R919–R922. 2000.
View Article : Google Scholar
|
|
6
|
Pandur P and Kühl M: An arrow for wingless
to take-off. BioEssays. 23:207–210. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kühl M, Sheldahl LC, Park M, Miller JR and
Moon RT: The Wnt/Ca2+ pathway: a new vertebrate Wnt
signaling pathway takes shape. Trends Genet. 16:279–283. 2000.
View Article : Google Scholar
|
|
8
|
Weston CR and Davis RJ: The JNK signal
transduction pathway. Curr Opin Genet Dev. 12:14–21. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Saitoh T and Katoh M: Molecular cloning
and characterization of human WNT5B on chromosome 12p13.3 region.
Int J Oncol. 19:347–351. 2001.PubMed/NCBI
|
|
10
|
Saitoh T and Katoh M: Expression and
regulation of WNT5A and WNT5B in human cancer: up-regulation of
WNT5A by TNFalpha in MKN45 cells and up-regulation of WNT5B by
beta-estradiol in MCF-7 cells. Int J Mol Med. 10:345–349.
2002.PubMed/NCBI
|
|
11
|
Mangioni S, Viganò P, Lattuada D, Abbiati
A, Vignali M and Di Blasio AM: Overexpression of the Wnt5b gene in
leiomyoma cells: implications for a role of the Wnt signaling
pathway in the uterine benign tumor. J Clin Endocrinol Metab.
90:5349–5355. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Takeshita A, Iwai S, Morita Y,
Niki-Yonekawa A, Hamada M and Yura Y: Wnt5b promotes the cell
motility essential for metastasis of oral squamous cell carcinoma
through active Cdc42 and RhoA. Int J Oncol. 44:59–68. 2014.
|
|
13
|
Páez D, Gerger A, Zhang W, Yang D, Labonte
MJ, Benhanim L, Kahn M, Lenz F, Lenz C, Ning Y, et al: Association
of common gene variants in the WNT/β-catenin pathway with colon
cancer recurrence. Pharmacogenomics J. 14:142–150. 2014. View Article : Google Scholar
|
|
14
|
Yang L, Perez AA, Fujie S, Warden C, Li J,
Wang Y, Yung B, Chen YR, Liu X, Zhang H, et al: Wnt modulates MCL1
to control cell survival in triple negative breast cancer. BMC
Cancer. 14:1242014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Huang Q, Shen HM and Ong CN: Inhibitory
effect of emodin on tumor invasion through suppression of activator
protein-1 and nuclear factor-kappaB. Biochem Pharmacol. 68:361–371.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Min KW, Kim DH, Do SI, Kim K, Lee HJ, Chae
SW, Sohn JH, Pyo JS, Oh YH, Kim WS, et al: Expression patterns of
stromal MMP-2 and tumoural MMP-2 and -9 are significant prognostic
factors in invasive ductal carcinoma of the breast. APMIS.
122:1196–1206. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Stellas D and Patsavoudi E: Inhibiting
matrix metalloproteinases, an old story with new potentials for
cancer treatment. Anticancer Agents Med Chem. 12:707–717. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bauvois B: New facets of matrix
metalloproteinases MMP-2 and MMP-9 as cell surface transducers:
outside-in signaling and relationship to tumor progression. Biochim
Biophys Acta. 1825:29–36. 2012.
|
|
19
|
Murphy DA and Courtneidge SA: The 'ins'
and 'outs' of podosomes and invadopodia: characteristics, formation
and function. Nat Rev Mol Cell Biol. 12:413–426. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Veeman MT, Axelrod JD and Moon RT: A
second canon. Functions and mechanisms of beta-catenin-independent
Wnt signaling. Dev Cell. 5:367–377. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shibata W, Maeda S, Hikiba Y, Yanai A,
Sakamoto K, Nakagawa H, Ogura K, Karin M and Omata M: c-Jun
NH2-terminal kinase 1 is a critical regulator for the
development of gastric cancer in mice. Cancer Res. 68:5031–5039.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sakurai T, Maeda S, Chang L and Karin M:
Loss of hepatic NF-kappa B activity enhances chemical
hepatocarcinogenesis through sustained c-Jun N-terminal kinase 1
activation. Proc Natl Acad Sci USA. 103:10544–10551. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sancho R, Nateri AS, de Vinuesa AG,
Aguilera C, Nye E, Spencer-Dene B and Behrens A: JNK signalling
modulates intestinal homeostasis and tumourigenesis in mice. EMBO
J. 28:1843–1854. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
She QB, Chen N, Bode AM, Flavell RA and
Dong Z: Deficiency of c-Jun-NH(2)-terminal kinase-1 in mice
enhances skin tumor development by
12-O-tetradecanoylphorbol-13-acetate. Cancer Res. 62:1343–1348.
2002.PubMed/NCBI
|
|
25
|
Tong C, Yin Z, Song Z, Dockendorff A,
Huang C, Mariadason J, Flavell RA, Davis RJ, Augenlicht LH and Yang
W: c-Jun NH2-terminal kinase 1 plays a critical role in
intestinal homeostasis and tumor suppression. Am J Pathol.
171:297–303. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kurayoshi M, Oue N, Yamamoto H, Kishida M,
Inoue A, Asahara T, Yasui W and Kikuchi A: Expression of Wnt-5a is
correlated with aggressiveness of gastric cancer by stimulating
cell migration and invasion. Cancer Res. 66:10439–10448. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yamanaka H, Moriguchi T, Masuyama N,
Kusakabe M, Hanafusa H, Takada R, Takada S and Nishida E: JNK
functions in the non-canonical Wnt pathway to regulate convergent
extension movements in vertebrates. EMBO Rep. 3:69–75. 2002.
View Article : Google Scholar
|
|
28
|
Nomachi A, Nishita M, Inaba D, Enomoto M,
Hamasaki M and Minami Y: Receptor tyrosine kinase Ror2 mediates
Wnt5a-induced polarized cell migration by activating c-Jun
N-terminal kinase via actin-binding protein filamin A. J Biol Chem.
283:27973–27981. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wei W, Li H, Li N, Sun H, Li Q and Shen X:
WNT5A/JNK signaling regulates pancreatic cancer cells migration by
phosphorylating paxillin. Pancreatology. 13:384–392. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Nishioka M, Ueno K, Hazama S, Okada T,
Sakai K, Suehiro Y, Okayama N, Hirata H, Oka M, Imai K, et al:
Possible involvement of WNT11 in colorectal cancer progression. Mol
Carcinog. 52:207–217. 2013. View
Article : Google Scholar
|
|
31
|
Ueno K, Hazama S, Mitomori S, Nishioka M,
Suehiro Y, Hirata H, Oka M, Imai K, Dahiya R and Hinoda Y:
Down-regulation of frizzled-7 expression decreases survival,
invasion and metastatic capabilities of colon cancer cells. Br J
Cancer. 101:1374–1381. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Winn RA, Marek L, Han SY, Rodriguez K,
Rodriguez N, Hammond M, Van Scoyk M, Acosta H, Mirus J, Barry N, et
al: Restoration of WNT-7a expression reverses non-small cell lung
cancer cellular transformation through frizzled-9-mediated growth
inhibition and promotion of cell differentiation. J Biol Chem.
280:19625–19634. 2005. View Article : Google Scholar : PubMed/NCBI
|