Introduction
Breast cancer is the most common malignant cancer
among females. The highest morbidity occurs in northern America and
northern European countries (1).
Despite the fact that China is a country with low incidence, the
morbidity is increasing year by year due to changes in dietary
structures, living standards and life styles (2). According to new data, the morbidity of
breast cancer is increasing yearly, and it is becoming a tumor with
the highest death rates (3).
Meanwhile, the age at onset is becoming increasingly younger
(3).
p21-activated kinase 1 (PAK1) was first found as a
member of the Pak family (4).
Initially, it was cloned from cerebral tissues as p21 kinase
(4). Members of the Pak family play
an essential role in immune escape, motility, angiogenesis and
genetic regulation (5). Therefore,
the Pak family may constitute the critical node of signal
transduction in the process of tumor progression. During the
evolutionary process of colorectal malignant tumors, expression of
Pak1 is increased. Recent studies have found that the activation of
Pak1 is necessary for inducing lysophosphatidic acid and autotoxin
in melanoma cells (6). In addition,
Pak1 was found to be highly expressed in head and neck neoplasms
(7).
As a key transmitter factor for the Wnt signal
channel, β-catenin is expressed in many types of tumors (8). Its oncogenic potential in in
vitro culture models and in vivo animal experiments have
been extensively explored. The nuclear accumulation of β-catenin is
generally considered as the symbol of Wnt/β-catenin signal routine.
β-catenin accumulates and enters into the nucleus, which induces
the expression of target genes (9).
Waxberry is a plant of the genus Myricaceae
(10). Geographically, it is
distributed between 18 and 33° north latitude while its economic
cultivation is mainly distributed in southeast coast regions, such
as Zhejiang, Jiangsu, Fujian, Guangdong and Jiangxi Provinces.
Myricetin, found in the bark of waxberry, is bitter in taste and
warm in property with antiviral, anti-inflammatory, antioxidant,
free radical scavaging, immune adjustment, anti-androgenic and
antiallergic functions (11–13).
In the present study, we examined the anticancer effects of
myricetin. We found that myricetin suppressed the cell viability of
human breast cancer MCF-7 cells. The mechanisms involved in the
effects of myricetin were also investigated.
Materials and methods
Cell culture and cell viability
Human breast cancer MCF-7 cells were maintained in
RPMI-1640 medium supplemented with 10% US-qualified fetal bovine
serum (FBS) (both from Invitrogen, Grand Island, NY, USA) in a
humidified incubator with 5% CO2 at 37°C. MCF-7 cells
(1×104) were seeded into a 96-well plate, incubated at
37°C and then treated with different concentrations of myricetin
(0–80 µM) for 12, 24 and 48 h. The medium was removed, and
50 µl MTT (5 mg/ml) was added to each well and then
incubated at 37°C for 4 h. The supernatant was removed, and 200
µl of dimethyl sulfoxide (DMSO; Invitrogen) was dissolved
for 20 min. Absorbance was measured at 490 nm.
Flow cytometric analysis of the apoptotic
rate
MCF-7 cells (1×106) were seeded into a
6-well plate, incubated at 37°C, and then treated with different
concentrations of myricetin (0, 10, 20 and 40 µM) for 24 h.
MCF-7 cells were washed with cold phosphate-buffered saline (PBS)
twice and re-suspended in binding buffer. Subsequently, 5 µl
of FITC Annexin V and 1 µl propidium iodide (PI) were added
to the cells and incubated for 20 min at room temperature in the
dark. The apoptotic rate was determined by flow cytometry
(FACSCalibur system; BD Biosciences, San Jose, CA, USA).
Western blot analysis
MCF-7 cells (1×106) were seeded into a
6-well plate, incubated at 37°C and then treated with different
concentrations of myricetin (0, 10, 20 and 40 µM) for 24 h.
MCF-7 cells were lysed in 100 µl mammalian protein
extraction reagent (M-PER; Pierce, Rockford, IL, USA) and
centrifuged at 7,500 × g for 15 min at 4°C. Total protein levels
were determined by a BCA protein assay kit (Pierce). SDS-PAGE was
performed using equivalent protein extracts (60 µg) from
each sample, which were then blotted onto a nitrocellulose membrane
using a Mini-Protean 3 system (Bio-Rad, Hercules, CA, USA). The
blots were incubated in PBS containing 5% non-fat dry milk for 1 h.
The membranes were incubated with the primary antibodies PAK1,
MEK1/2, ERK1/2, GSK3β, β-catenin, cyclin D1, PCNA, survivin, Bax
and β-actin at 4°C overnight. The membranes were then incubated
with secondary antibody dilutions, washed with PBS containing 5%
non-fat dry milk and visualized using enhanced chemiluminescence
detection reagents (ECL Advance Western Blotting Detection kit;
Amersham, UK).
Enzyme-linked immunosorbent assay
(ELISA)
The MCF-7 cells (1×104) were seeded into
a 96-well plate, incubated at 37°C and then treated with different
concentrations of myricetin (0, 10, 20 and 40 µM) for 24 h.
The caspase-3 assay kit (Ac-DEVD-pNA, 2 mM) was used to detect
caspase-3 enzymatic activity in the MCF-7 cells. The absorbance was
measured at 405 nm.
Statistical analysis
Data are expressed as mean ± standard error of the
mean (SEM) and analyzed using ANOVA. The results were analyzed
using a post hoc test (two-sided Dunnett's test) and one-way
analysis of variance (ANOVA) to test differences between each
treatment and the control. A p-value of <0.05 was considered to
indicate a statistically significant result.
Results
Myricetin suppresses the cell viability
of human breast cancer MCF-7 cells
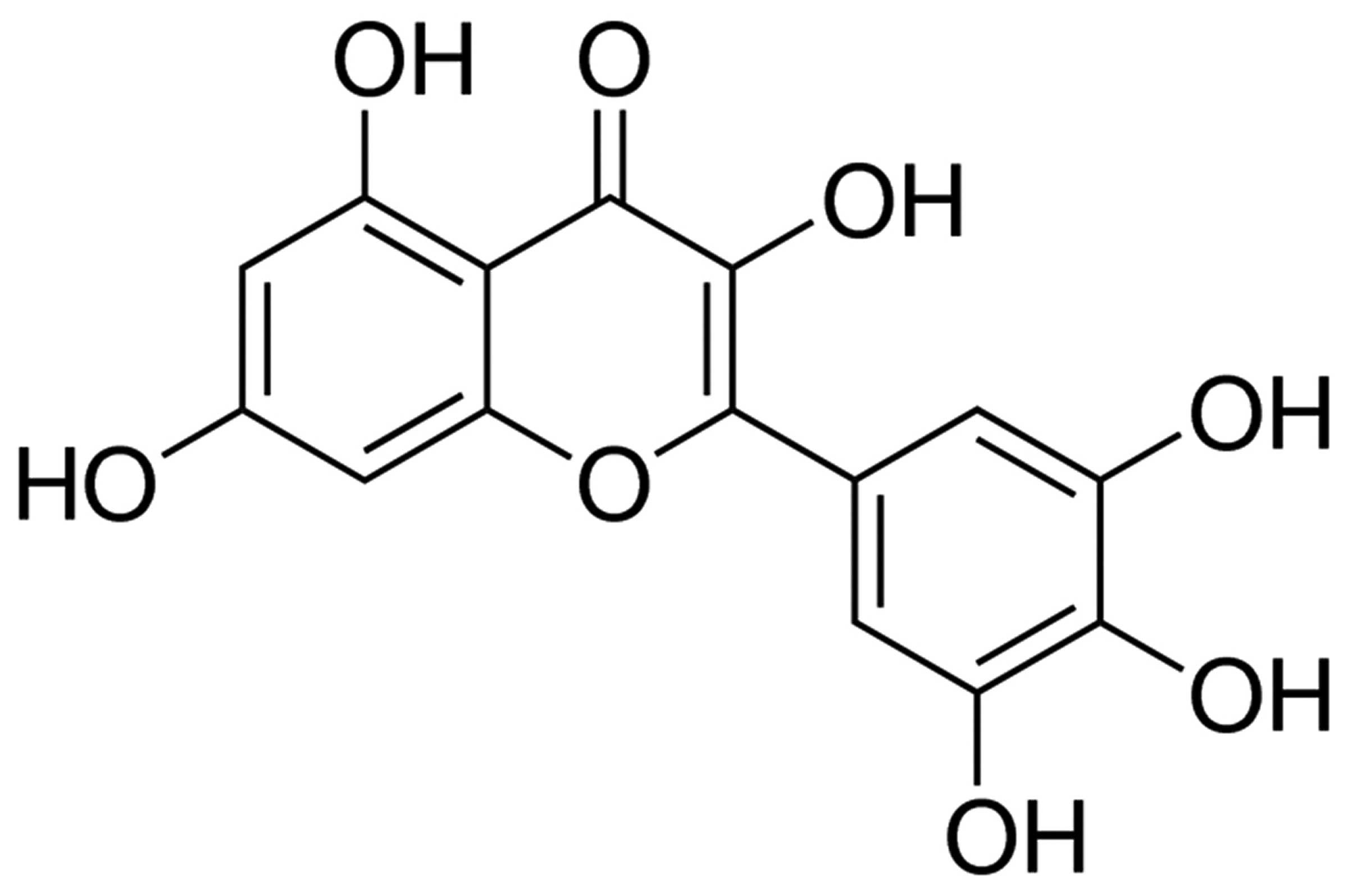
The chemical structure of myricetin is shown in
Fig. 1. MTT assay was performed to
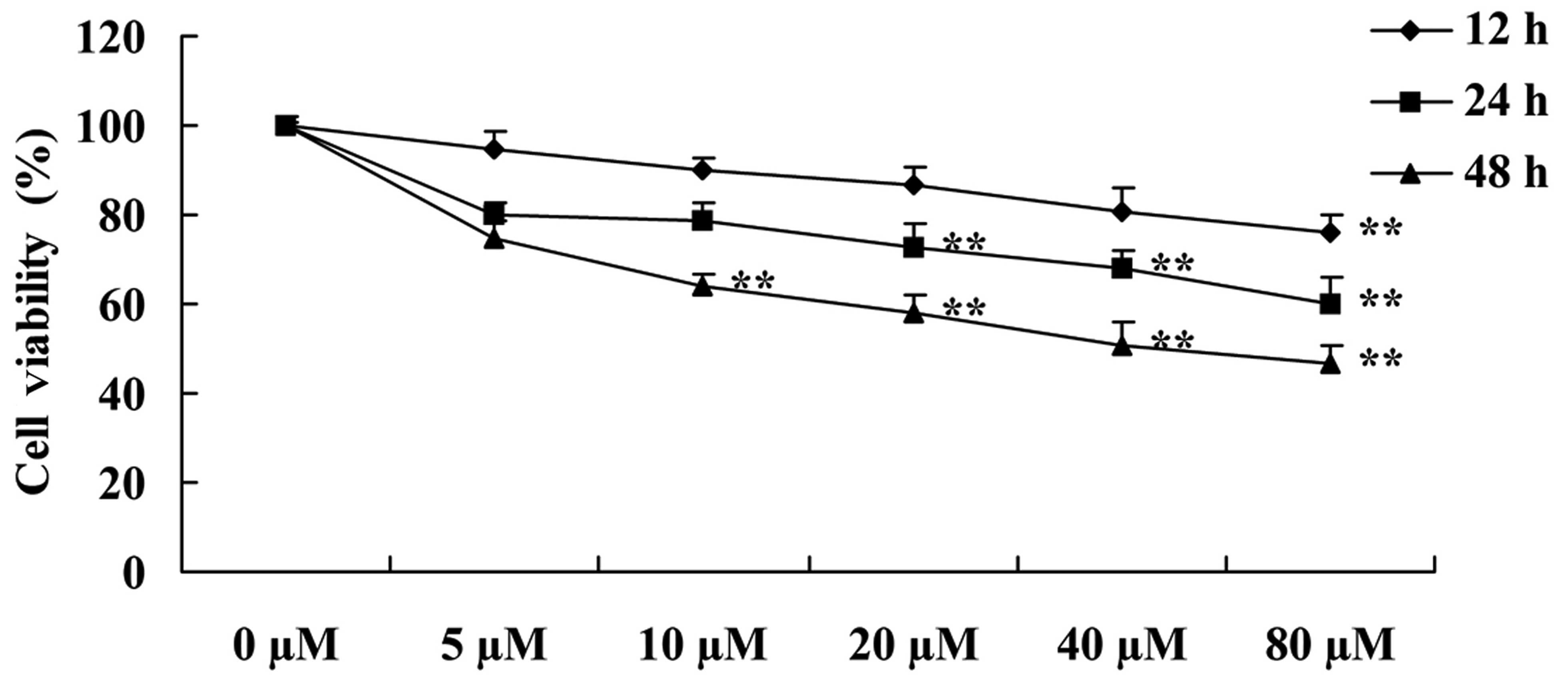
investigate the effect of myricetin on the viability of the MCF-7
cells following the treatment of myricetin. As shown in Fig. 2, myricetin suppressed the cell
viability of the MCF-7 cells in a time- and dose-dependent manner.
Particularly, the suppression of cell viability was evident after
treatment with 80 µM of myricetin for 12 h, 20–80 µM
of myricetin for 24 h and 10–80 µM of myricetin for 48 h
(Fig. 2).
Myricetin induces the cell apoptosis of
human breast cancer MCF-7 cells
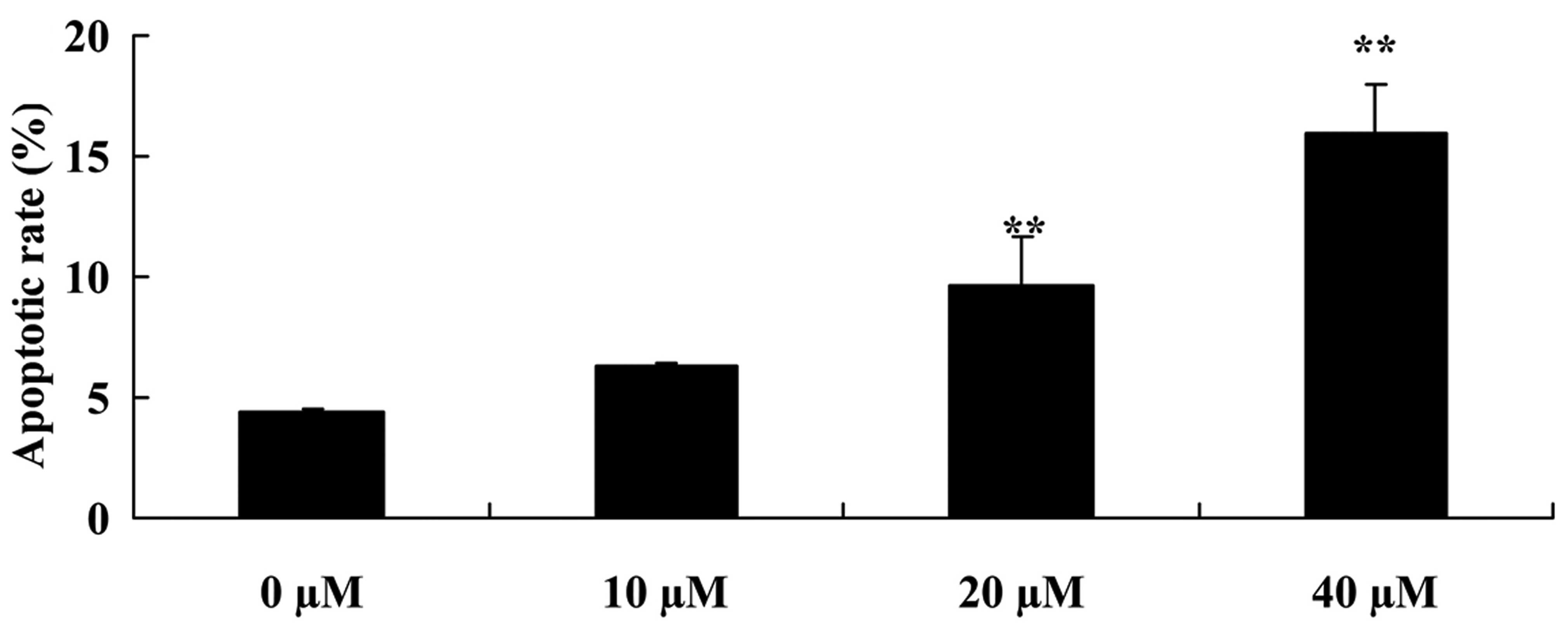
Similarly, we examined the effect of myricetin on
the cell apoptosis of MCF-7 cells using flow cytometric analysis.
We observed that compared with the controls (myricetin 0
µM), 40 or 80 µM of myricetin significantly increased
the apoptotic rate of the MCF-7 cells (Fig. 3).
Myricetin affects the PAK1 pathway in
human breast cancer MCF-7 cells
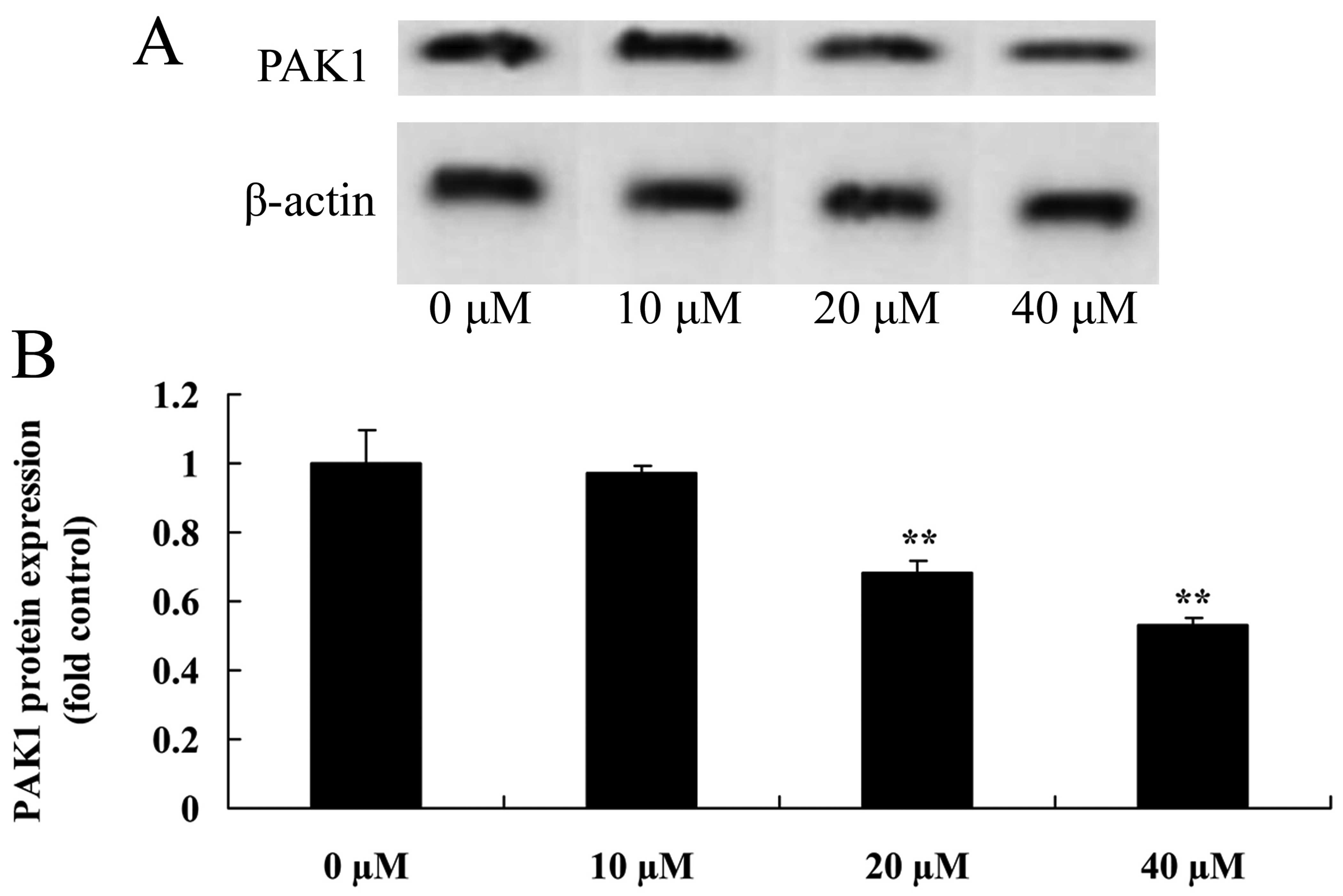
In the MCF-7 models, we also examined whether
myricetin affects the PAK1 pathway in MCF-7 cells. As shown in
Fig. 4, myricetin (40 or 80
µM) significantly inhibited the protein expression of PAK1
in the MCF-7 cells when compared with the controls (myricetin 0
µM).
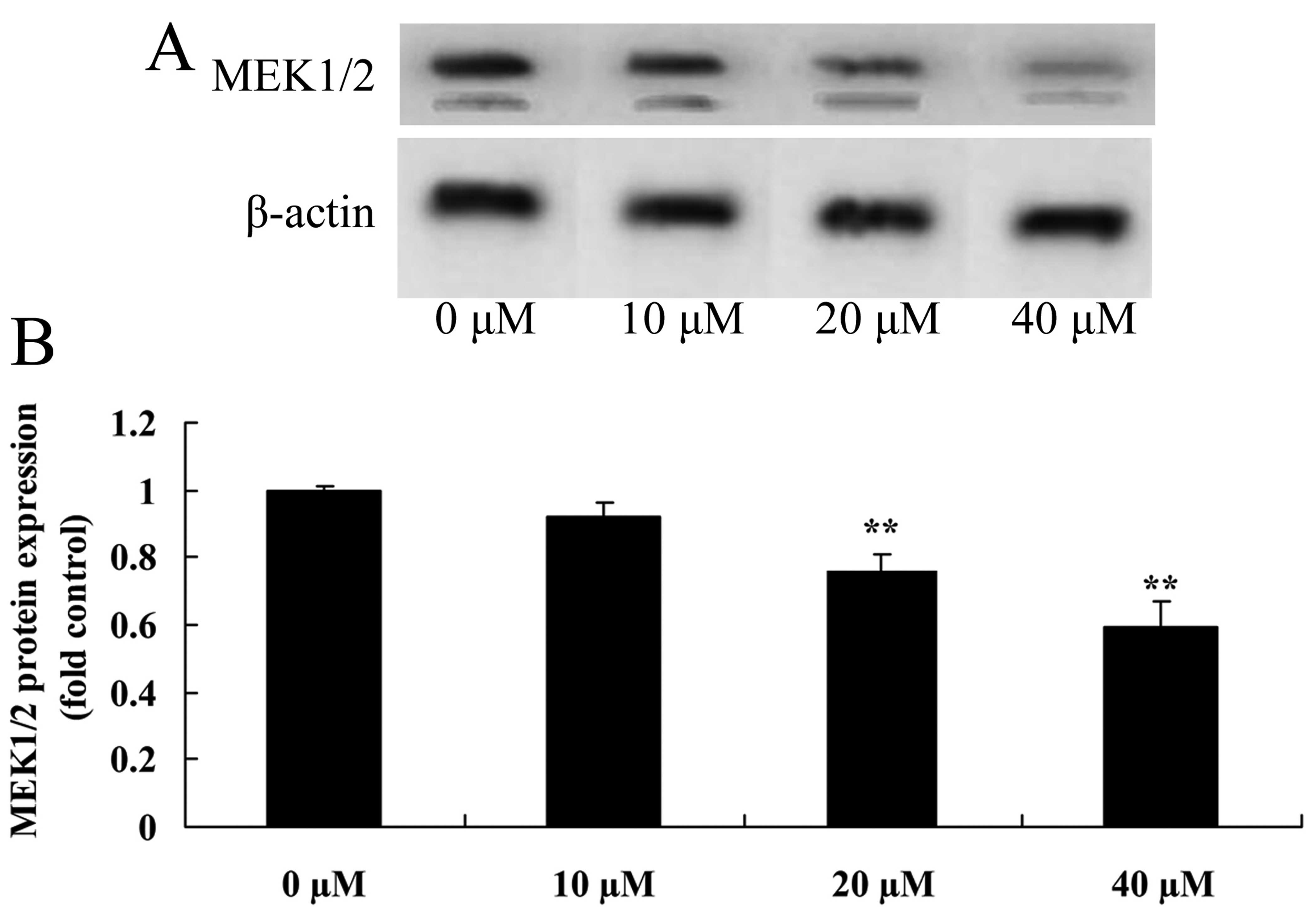
Myricetin affects the MEK1/2 pathway in
human breast cancer MCF-7 cells
To ascertain whether myricetin affects the MEK1/2
pathway in MCF-7 cells, MEK1/2 protein expression was analyzed
using western blot analysis. However, compared to the controls
(myricetin 0 µM), treatment with 40 or 80 µM of
myricetin significantly suppressed the MEK1/2 protein expression in
the MCF-7 cells (Fig. 5).
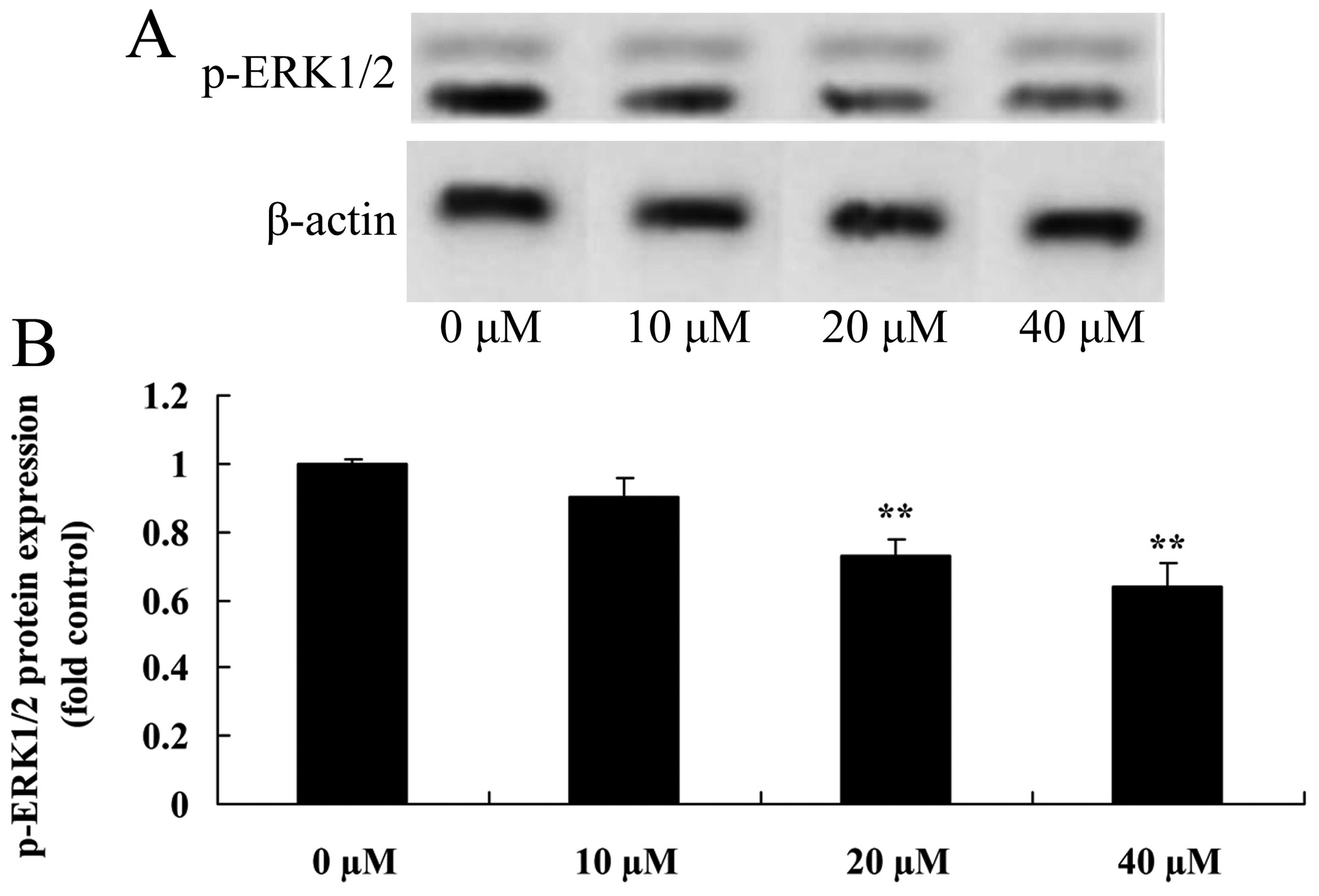
Myricetin affects the ERK1/2 pathway in
human breast cancer MCF-7 cells
Next, the role of the ERK1/2 pathway in
myricetin-induced apoptosis was determined. p-ERK1/2 protein
expression was determined in the MCF-7 cells. Treatment with 40 or
80 µM of myricetin significantly reduced the protein
expression of p-ERK1/2 in the MCF-7 cells when compared with the
controls (myricetin 0 µM) (Fig.
6).
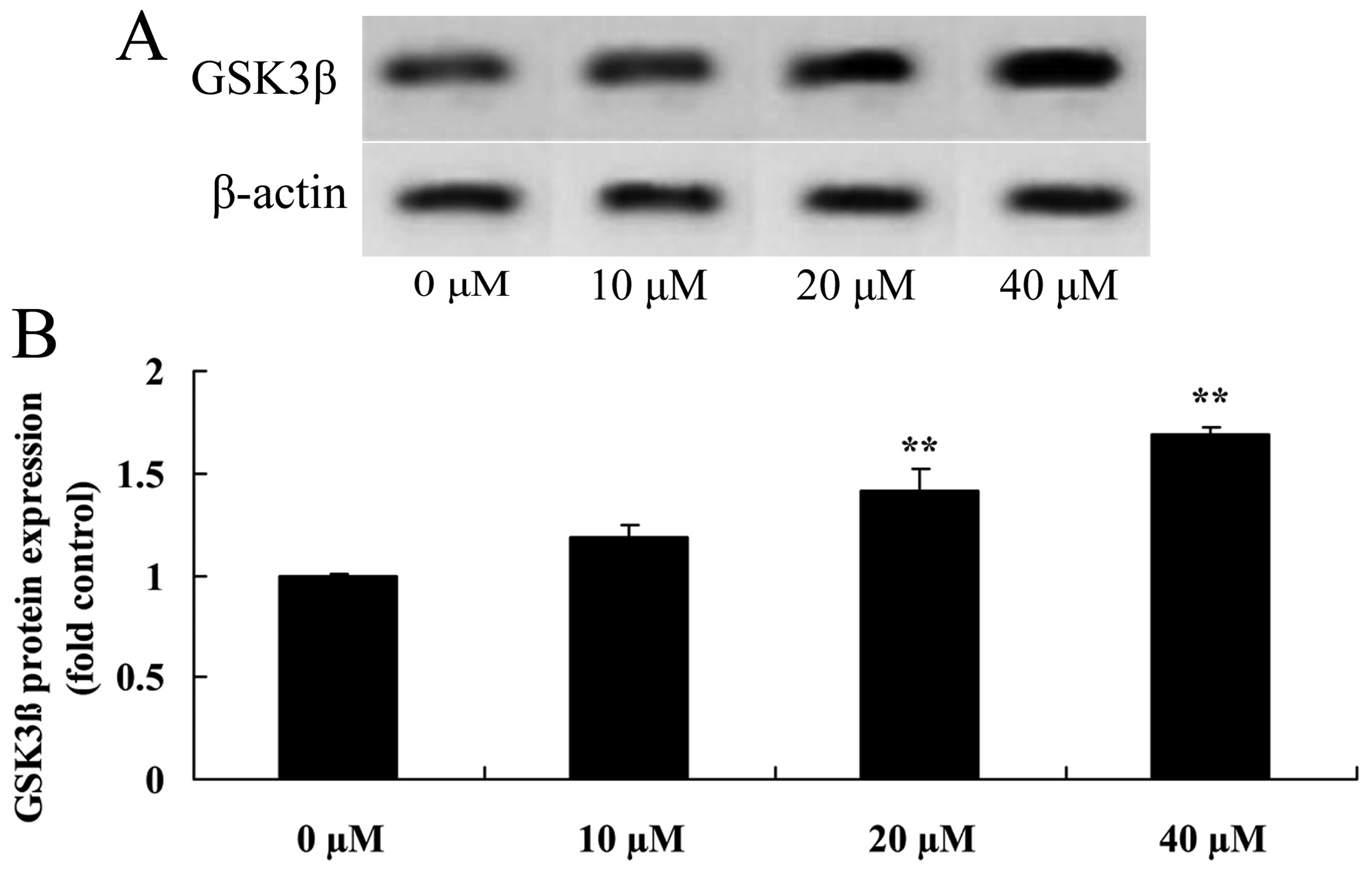
Myricetin affects the GSK3β pathway in
human breast cancer MCF-7 cells
GSK3β, a tumor-suppressor protein, was measured
using western blot analysis. Compared to the controls (myricetin 0
µM), treatment with 40 or 80 µM of myricetin
significantly activated the protein expression of GSK3β in the
MCF-7 cells (Fig. 7).
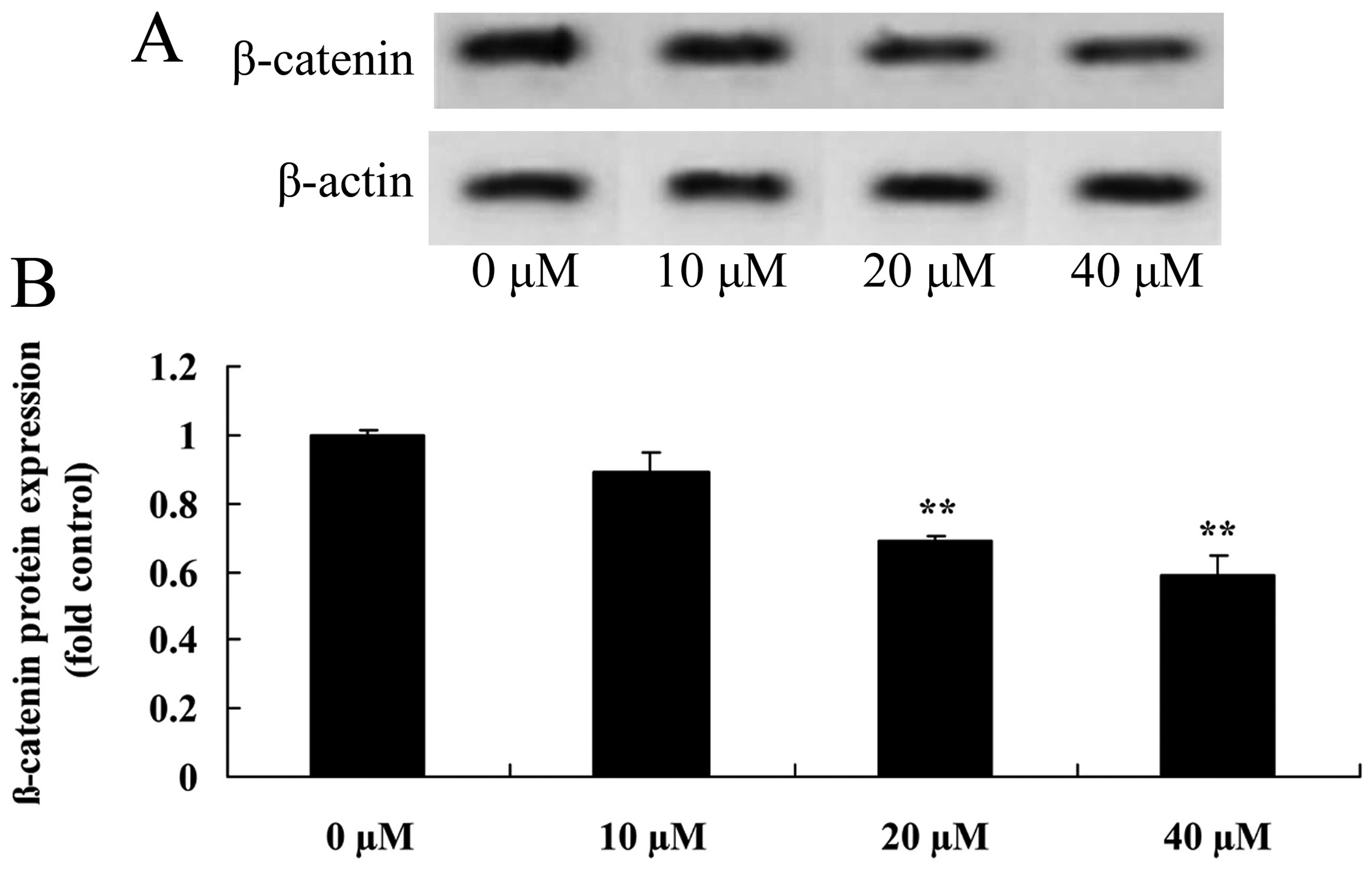
Myricetin affects the β-catenin pathway
in human breast cancer MCF-7 cells
Western blot analysis was used to investigate the
role of the β-catenin pathway on myricetin-induced apoptosis in
human breast cancer MCF-7 cells. As shown in Fig. 8, treatment with 40 or 80 µM
of myricetin significantly suppressed the β-catenin protein
expression in the MCF-7 cells when compared with the controls
(myricetin 0 µM).
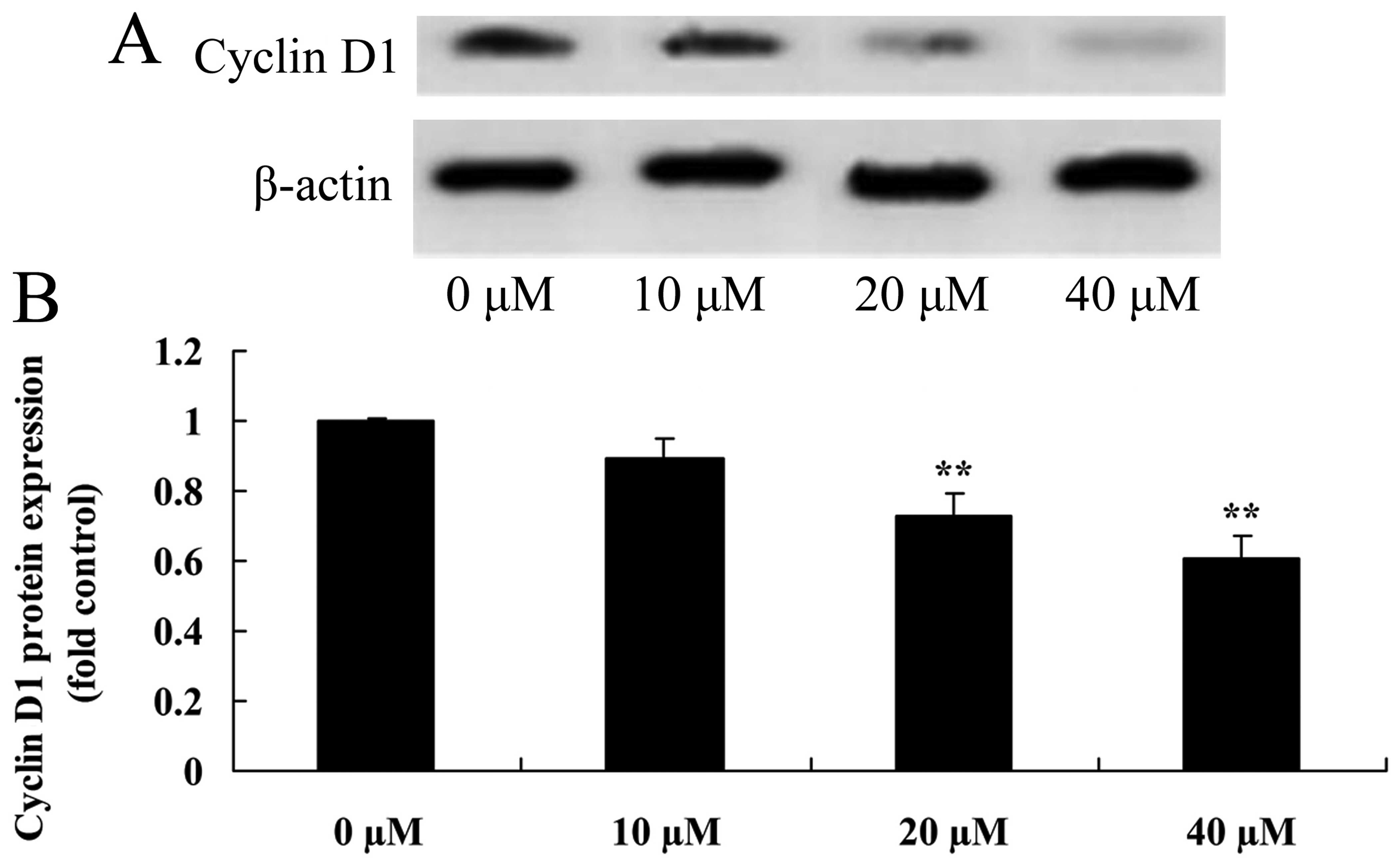
Myricetin affects the cyclin D1 pathway
in human breast cancer MCF-7 cells
Western blot analysis was used to investigate the
role of the cyclin D1 pathway in the myricetin-induced apoptosis in
human breast cancer MCF-7 cells. As shown in Fig. 9 treatment with 40 or 80 µM of
myricetin significantly inhibited the protein expression of cyclin
D1 in the MCF-7 cells when compared with the controls (myricetin 0
µM).
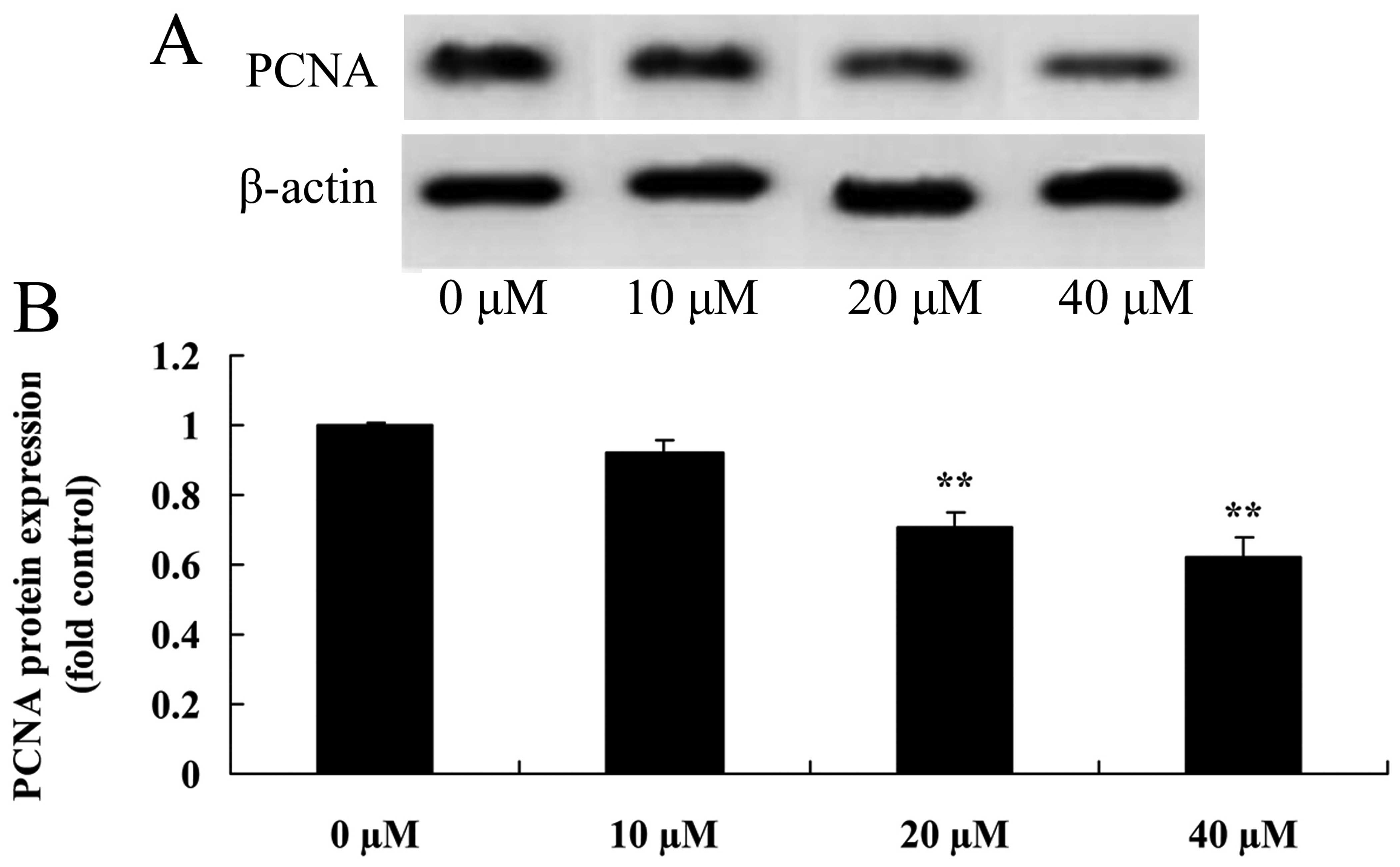
Myricetin affects the PCNA pathway in
human breast cancer MCF-7 cells
To further examine the effect of myricetin affected
on the PCNA pathway of human breast cancer MCF-7 cells, PCNA
protein expression in MCF-7 cells was detected using western blot
analysis. As shown in Fig. 10,
treatment with 40 or 80 µM of myricetin significantly
suppressed the PCNA protein expression in MCF-7 cells when compared
with the controls (myricetin 0 µM).
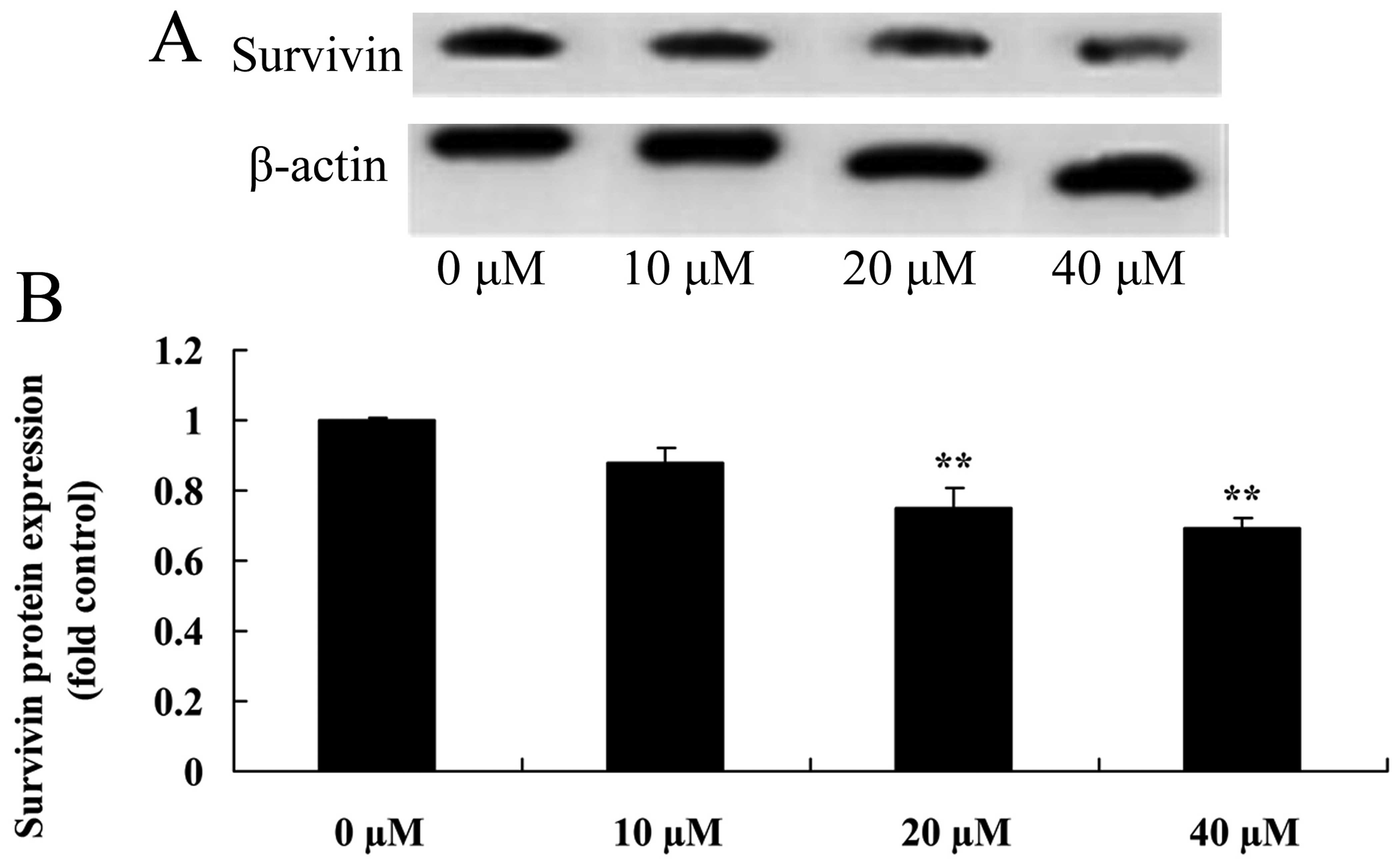
Myricetin affects the survivin pathway in
human breast cancer MCF-7 cells
The survivin pathway induces apoptosis in cancer
cells. Thus, we aimed to ascertain whether myricetin affects the
survivin pathway in human breast cancer MCF-7 cells. As shown in
Fig. 11, pretreatment with 40 or
80 µM of myricetin significantly inhibited the protein
expression of survivin in the MCF-7 cells when compared with the
controls (myricetin 0 µM).
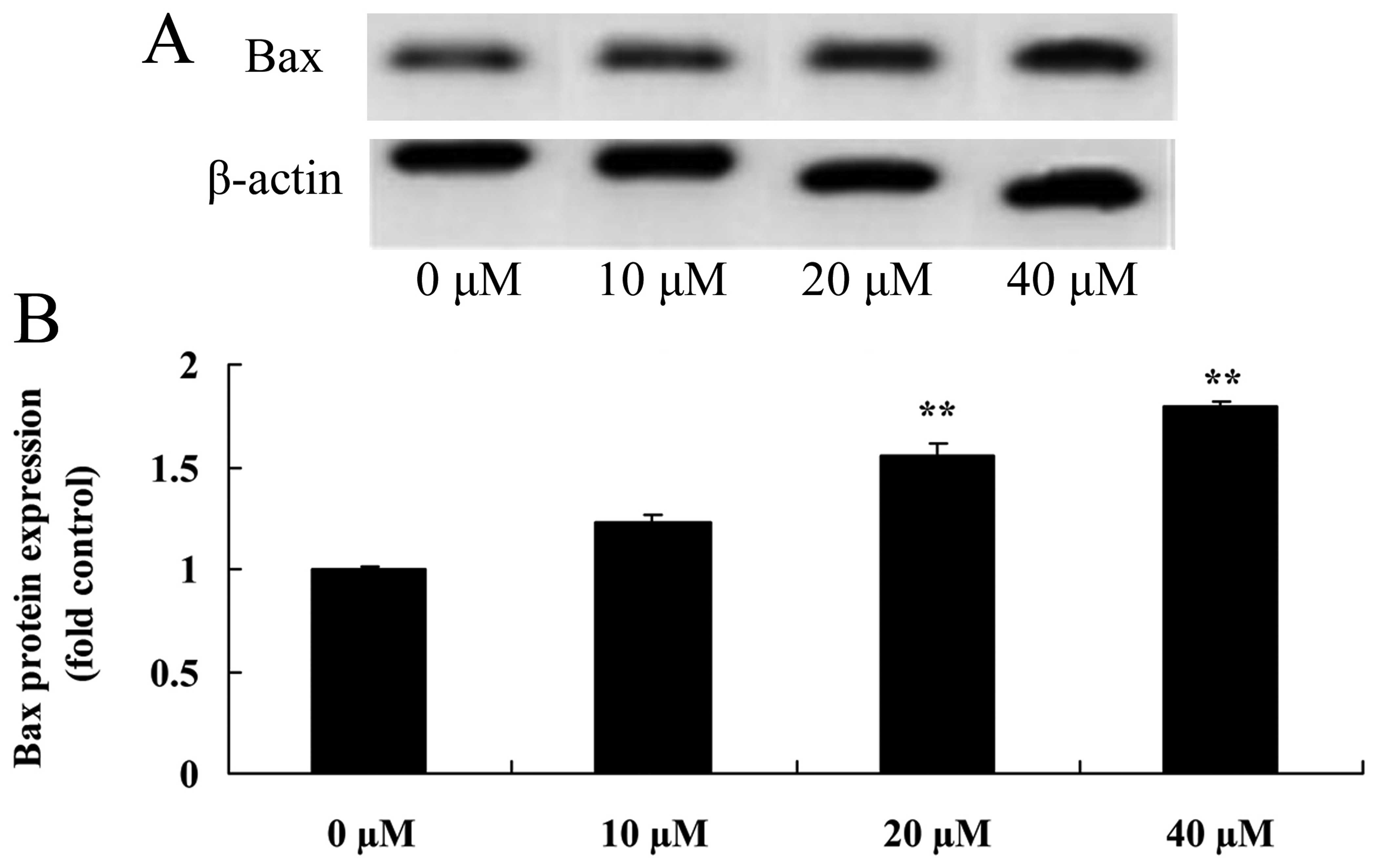
Myricetin affects the Bax pathway in
human breast cancer MCF-7 cells
To investigate whether the anticancer effect of
myricetin on human breast cancer was caused by the Bax pathway, Bax
protein expression of MCF-7 cells following treatment with
myricetin was analyzed by western blot analysis. As shown in
Fig. 12, treatment with 40 or 80
µM of myricetin significantly activated the protein
expression of Bax in the MCF-7 cells when compared with the
controls (myricetin 0 µM).
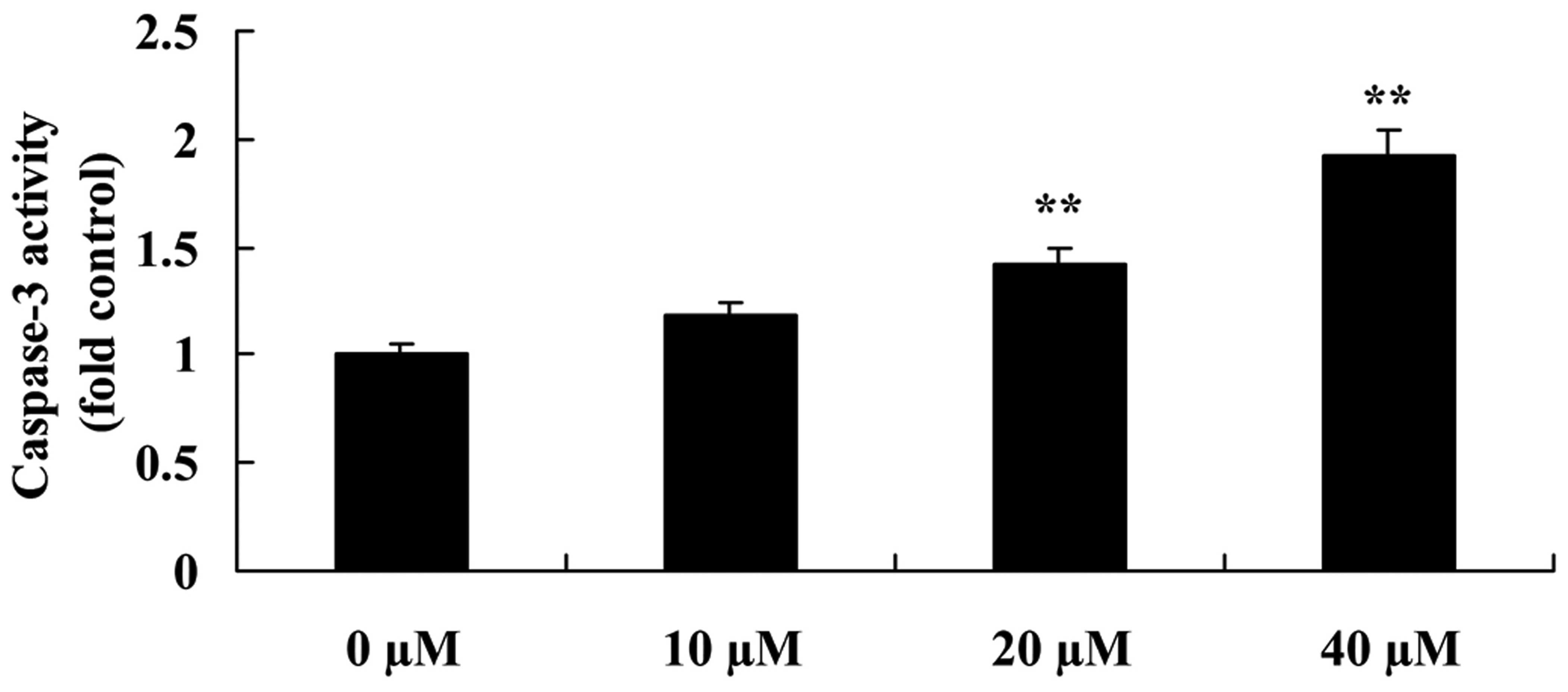
Myricetin affects the caspase-3 pathway
in human breast cancer MCF-7 cells
Caspase-3 assay kit was used to confirm the
mechanism involved in the anticancer effects of myricetin on the
apoptosis in human breast cancer MCF-7 cells. Compared to the
controls (myricetin 0 µM), myricetin (40 or 80 µM)
significantly increased the caspase-3 activity in the MCF-7 cells
(Fig. 13).
Discussion
As one of the most common malignant cancers, the
morbidity of breast cancer is increasing worldwide. In China, owing
to changes in life styles and dietary structures, the morbidity of
breast cancer is increasing rapidly and its age at onset is
becoming increasingly younger (2,14). Due
to rapid progress and the wide application of molecular biology,
research on the pathogenesis of cancer and its therapy has made
substantial achievements (15). Our
results found that myricetin suppressed the cell viability of human
breast cancer MCF-7 cells at least partly through the induction of
apoptosis.
Pak1 is pivotal to physiological processes such as
normal cell movement, mitosis, transcription and interpretation
(7). In head and neck neoplasms and
sarcoma, the activities of Pak1 have been found to be higher than
that in normal tissues (6). In the
evolutionary process of colorectal malignant tumors, expression of
Pak1 is increased (16). Studies
have confirmed that Pak1 is closely associated with the invasion
and metastasis of breast cancer, human oophoroma and prostate
cancer, indicating the Pak1 plays an important role in normal
tissue development and tumor progression (17,18).
Pak1 was found to be related to cellular orientation movement while
motility is rather important for tumor metastasis. It has been
confirmed that Pak1 has definite functions in invasion and
metastasis of breast cancers induced by HER2 (18,19).
Further studies suggest that expression of Pak1 in breast cancer
and its activities are positively related with tumor grade, and
expression levels in poorly differentiated ductal carcinoma were
higher than levels in higher differentiated ductal carcinoma
(6). These results indicate that
inhibition of the viability of MCF-7 cells following treatment with
myricetin is through the PAK1 pathway. Iyer et al provide
striking evidence that myricetin induces the apoptosis of
hepatocellular carcinoma through inhibition of PAK1 signaling
(11).
The frequency of the overexpression of MEK noted in
breast cancer is 30% and is related with the poor prognosis and
resistance to therapy (20). The
overexpression of MEK can realize autonomous activation under
conditions without extracellular ligands. This results in the
occurrence of malignant tumors through the blocking of apoptosis
induced by TNF (20). ERK can
facilitate the proliferation of tumor cells. As an important
signaling transduction pathway of MAPK, ERK can be activated by
growth factors, serum, ligands of G-protein-coupled receptors and
transcription factors (21). Growth
factors can activate ERK through the phosphorylation of the
Ras-Raf-MEK pathway. Firstly, growth factors bind with
corresponding receptors on the cell surface and induce the
phosphorylation of tyrosine residues on endochylema of receptors,
resulting in dipolymers (22).
Phosphorylated tyrosine residues can provide binding sites for
proteins with SH2 structural domain (23). Expression of ERK in pancreatic
cancer cells is significantly increased. It is known that the
MEK/ERK signaling pathway triggers the dissociation and motility of
pancreatic cancer cells and improves the invasion and metastasis of
pancreatic cancer cells (24). The
activation of the ERK signaling pathway can regulate the migratory
abilities of tumor cells and facilitate the dissemination of tumor
cells. These results indicate that myricetin induced apoptosis in
the MCF-7 cells through the MEK/ERK signaling pathway. Lim et
al found that myricetin upregulated cyclooxygenase-2 expression
in mouse epidermal cells through the MEK/ERK signaling pathway
(25).
The Wnt signaling pathway plays a pivotal role in
cell growth, progression and differentiation. Aberrant expression
of the Wnt pathway is the origin of many diseases (8). In regards to the classical
Wnt/β-catenin pathway, when the Wnt signal is lost, β-catenin in
the cytoplasm is at low levels, which can be degraded continuously
by axin compounds (26). Axin
compounds include scaffolding protein, casein kinase 1 and GSK3β.
GSK3β can continuously phosphorylate amino terminal of β-catenin,
resulting in the degradation of β-catenin by ubiquitin (27). When cells are stimulated, Wnt
signals are activated. Wnt proteins combine with FZD proteins and
low density lipoprotein receptor-related protein5/6. Dsh proteins
are activated. GSK3β is phosphorylated, which can decrease the
activity of GSK3β (24). Axin
compound cannot trigger the phosphorylation and ubiquitylation of
β-catenin, resulting in the accumulation of β-catenin. It combines
with T cell transcription factor/lymphoid enhancer binding factors
and activates the expression of cyclin D1, c-myc, MMp7, CD44,
Bcl-2, VEGF and survivin (28).
GSK3β can also activate the β-catenin signaling pathway and promote
the occurrence of hyperplasia of the mammary glands (28). These results indicate that
GSK3β/β-catenin/cyclin D1/PCNA/survivin-associated intrinsic
pathways were, at least partly, involved in the myricetin-induced
apoptosis of human breast cancer MCF-7 cells. Iyer et al
provide striking evidence that myricetin induces the apoptosis of
hepatocellular carcinoma through inhibition of
GSK3β/β-catenin/cyclin D1/PCNA/survivin signaling (11).
Bcl-2 and Bax play an essential role in cell
apoptosis. The sensitivity of cells to apoptosis-stimulating
factors largely depends on the ratio of bcl-2 proteins/bax
proteins. The proportion of bcl-2/bax in normal tissues is
constant, which creates a balance for cell division and
proliferation (29). During cell
apoptosis, many proteins in the bcl-2 family play an important role
in cell apoptosis. Therefore, the comprehensive expression levels
of bcl-2 and bax are valuable for the occurrence, progression and
prognosis of tumors (30). Our
results demonstrated that myricetin inhibited the cell growth of
MCF-7 cells through induction of Bax and caspase-3. Kim et
al reported that myricetin induced apoptosis through the
Bax/Bcl-2-dependent pathway in human colon cancer cells (10).
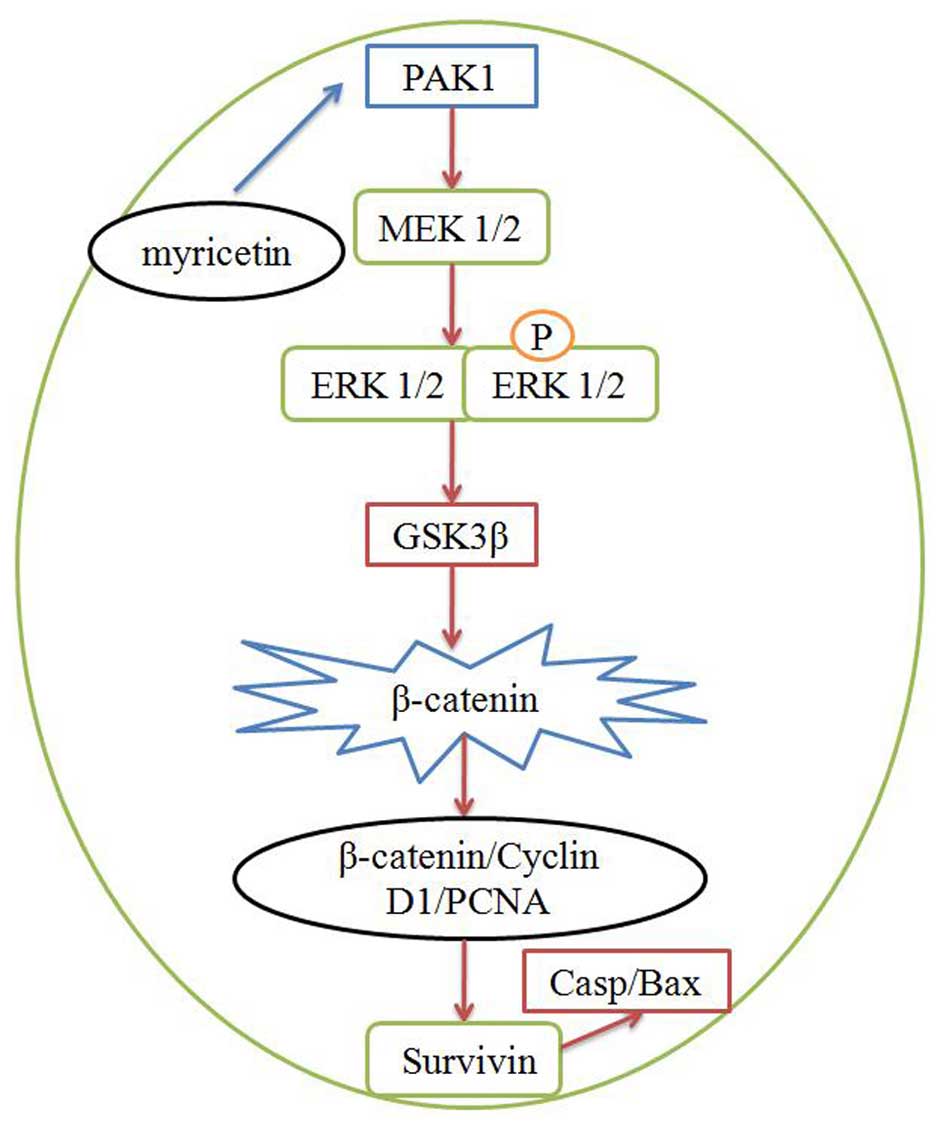
In conclusion, our data demonstrated that myricetin
suppressed the cell viability of human breast cancer MCF-7 cells at
least partly through the induction of apoptosis. Our present
results revealed that the anticancer effect of myricetin on human
breast cancer involved PAK1/MEK/ERK/GSK3β/β-catenin/cyclin
D1/PCNA/survivin/Bax-caspase-3 signaling (Fig. 14). Thus, myricetin may be a new
drug for the treatment of human breast cancer.
References
|
1
|
Kida K, Ishikawa T, Yamada A, Shimizu D,
Tanabe M, Sasaki T, Ichikawa Y and Endo I: A prospective
feasibility study of sentinel node biopsy by modified Indigocarmine
blue dye methods after neoadjuvant chemotherapy for breast cancer.
Eur J Surg Oncol. 41:566–570. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Perez EA, Dueck AC, McCullough AE, Chen B,
Geiger XJ, Jenkins RB, Lingle WL, Davidson NE, Martino S, Kaufman
PA, et al: Impact of PTEN protein expression on benefit from
adjuvant trastuzumab in early-stage human epidermal growth factor
receptor 2-positive breast cancer in the North Central Cancer
Treatment Group N9831 trial. J Clin Oncol. 31:2115–2122. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tang LC, Wang BY, Sun S, Zhang J, Jia Z,
Lu YH, Di GH, Shao ZM and Hu XC: Higher rate of skin rash in a
phase II trial with weekly nanoparticle albumin-bound paclitaxel
and cisplatin combination in Chinese breast cancer patients. BMC
Cancer. 13:2322013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yoon JH, Mo JS, Ann EJ, Ahn JS, Jo EH, Lee
HJ, Hong SH, Kim MY, Kim EG, Lee K, et al: NOTCH1 intracellular
domain negatively regulates PAK1 signaling pathway through direct
interaction. Biochim Biophys Acta. 1863:179–188. 2016. View Article : Google Scholar
|
|
5
|
Khare V, Dammann K, Asboth M, Krnjic A,
Jambrich M and Gasche C: Overexpression of PAK1 promotes cell
survival in inflammatory bowel diseases and colitis-associated
cancer. Inflamm Bowel Dis. 21:287–296. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nie J, Sun C, Faruque O, Ye G, Li J, Liang
Q, Chang Z, Yang W, Han X and Shi Y: Synapses of amphids defective
(SAD-A) kinase promotes glucose-stimulated insulin secretion
through activation of p21-activated kinase (PAK1) in pancreatic
β-cells. J Biol Chem. 287:26435–26444. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chow HY, Jubb AM, Koch JN, Jaffer ZM,
Stepanova D, Campbell DA, Duron SG, O'Farrell M, Cai KQ,
Klein-Szanto AJ, et al: p21-Activated kinase 1 is required for
efficient tumor formation and progression in a Ras-mediated skin
cancer model. Cancer Res. 72:5966–5975. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang L, Tian H, Yuan J, Wu H, Wu J and Zhu
X: CONSORT: Sam68 is directly regulated by miR-204 and promotes the
self-renewal potential of breast cancer cells by activating the
Wnt/beta-catenin signaling pathway. Medicine. 94:e22282015.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dong Y, Cao B, Zhang M, Han W, Herman JG,
Fuks F, Zhao Y and Guo M: Epigenetic silencing of NKD2, a major
component of Wnt signaling, promotes breast cancer growth.
Oncotarget. 6:22126–22138. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kim ME, Ha TK, Yoon JH and Lee JS:
Myricetin induces cell death of human colon cancer cells via
BAX/BCL2-dependent pathway. Anticancer Res. 34:701–706.
2014.PubMed/NCBI
|
|
11
|
Iyer SC, Gopal A and Halagowder D:
Myricetin induces apoptosis by inhibiting P21 activated kinase 1
(PAK1) signaling cascade in hepatocellular carcinoma. Mol Cell
Biochem. 407:223–237. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Masuda T, Miura Y, Inai M and Masuda A:
Enhancing effect of a cysteinyl thiol on the antioxidant activity
of flavonoids and identification of the antioxidative thiol adducts
of myricetin. Biosci Biotechnol Biochem. 77:1753–1758. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kang KA, Wang ZH, Zhang R, Piao MJ, Kim
KC, Kang SS, Kim YW, Lee J, Park D and Hyun JW: Myricetin protects
cells against oxidative stress-induced apoptosis via regulation of
PI3K/Akt and MAPK signaling pathways. Int J Mol Sci. 11:4348–4360.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Arving C, Brandberg Y, Feldman I,
Johansson B and Glimelius B: Cost-utility analysis of individual
psychosocial support interventions for breast cancer patients in a
randomized controlled study. Psychooncology. 23:251–258. 2014.
View Article : Google Scholar
|
|
15
|
Zhang J, Wang L, Wang Z, Hu X, Wang B, Cao
J, Lv F, Zhen C, Zhang S and Shao Z: A phase II trial of biweekly
vinorelbine and oxaliplatin in second- or third-line metastatic
triple-negative breast cancer. Cancer Biol Ther. 16:225–232. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
DeSantiago J, Bare DJ, Xiao L, Ke Y,
Solaro RJ and Banach K: p21-Activated kinase1 (Pak1) is a negative
regulator of NADPH-oxidase 2 in ventricular myocytes. J Mol Cell
Cardiol. 67:77–85. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
McDaniel AS, Allen JD, Park SJ, Jaffer ZM,
Michels EG, Burgin SJ, Chen S, Bessler WK, Hofmann C, Ingram DA, et
al: Pak1 regulates multiple c-kit mediated Ras-MAPK
gain-in-function phenotypes in Nf1+/− masT cells. Blood.
112:4646–4654. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Holm C, Rayala S, Jirström K, Stål O,
Kumar R and Landberg G: Association between Pak1 expression and
subcellular localization and tamoxifen resistance in breast cancer
patients. J Natl Cancer Inst. 98:671–680. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Smith SD, Jaffer ZM, Chernoff J and Ridley
AJ: PAK1-mediated activation of ERK1/2 regulates lamellipodial
dynamics. J Cell Sci. 121:3729–3736. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dillon LM, Bean JR, Yang W, Shee K,
Symonds LK, Balko JM, McDonald WH, Liu S, Gonzalez-Angulo AM, Mills
GB, et al: P-REX1 creates a positive feedback loop to activate
growth factor receptor, PI3K/AKT and MEK/ERK signaling in breast
cancer. Oncogene. 34:3968–3976. 2015. View Article : Google Scholar
|
|
21
|
Tarkkonen K, Ruohola J and Härkönen P:
Fibroblast growth factor 8 induced downregulation of thrombospondin
1 is mediated by the MEK/ERK and PI3K pathways in breast cancer
cells. Growth Factors. 28:256–267. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu WH, Liu HB, Gao DK, Ge GQ, Zhang P,
Sun SR, Wang HM and Liu SB: ABCG2 protects kidney side population
cells from hypoxia/reoxygenation injury through activation of the
MEK/ERK pathway. Cell Transplant. 22:1859–1868. 2013. View Article : Google Scholar
|
|
23
|
Navolanic PM, Lee JT and McCubrey JA:
Docetaxel cytotoxicity is enhanced by inhibition of the Raf/MEK/ERK
signal transduction pathway. Cancer Biol Ther. 2:677–678. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li SQ, Wang ZH, Mi XG, Liu L and Tan Y:
MiR-199a/b-3p suppresses migration and invasion of breast cancer
cells by downregulating PAK4/MEK/ERK signaling pathway. IUBMB Life.
67:768–777. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lim TG, Lee BK, Kwon JY, Jung SK and Lee
KW: Acrylamide up-regulates cyclooxygenase-2 expression through the
MEK/ERK signaling pathway in mouse epidermal cells. Food Chem
Toxicol. 49:1249–1254. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang J, Yang Z, Li P, Bledsoe G, Chao L
and Chao J: Kallistatin antagonizes Wnt/β-catenin signaling and
cancer cell motility via binding to low-density lipoprotein
receptor-related protein 6. Mol Cell Biochem. 379:295–301. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chow KH, Sun RW, Lam JB, Li CK, Xu A, Ma
DL, Abagyan R, Wang Y and Che CM: A gold(III) porphyrin complex
with antitumor properties targets the Wnt/beta-catenin pathway.
Cancer Res. 70:329–337. 2010. View Article : Google Scholar
|
|
28
|
Prasad CP, Chaurasiya SK, Axelsson L and
Andersson T: WNT-5A triggers Cdc42 activation leading to an ERK1/2
dependent decrease in MMP9 activity and invasive migration of
breast cancer cells. Mol Oncol. 7:870–883. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhu L, Zhu B, Yang L, Zhao X, Jiang H and
Ma F: RelB regulates Bcl-xl expression and the irradiation-induced
apoptosis of murine prostate cancer cells. Biomed Rep. 2:354–358.
2014.PubMed/NCBI
|
|
30
|
Guo Y, Zhang Y, Yang X, Lu P, Yan X, Xiao
F, Zhou H, Wen C, Shi M, Lu J, et al: Effects of methylglyoxal and
glyoxalase I inhibition on breast Cancer cells proliferation,
invasion, and apoptosis through modulation of MAPKs, MMP9, and
Bcl-2. Cancer Biol Ther. 1–12. 2015.
|