Introduction
Consumption of particular fruits and vegetables is
considered to prevent or even treat various diseases including
cancer. For example, the consumption of broccoli (250 g/day) and
Brussels sprouts (250 g/day) may decrease colorectal cancer risk
(1). Significant efforts have been
made to develop plant-derived dietary agents which have beneficial
effect on cancer. Quercetin (3,3′,4′,5,6-pentahydroxyflavone) is a
flavonoid that is found in many plants and foods, such as onions,
green tea, apples, berries, broccoli, red wine and others (2,3).
Quercetin exerts anti-oxidant (4),
anti-inflammatory (5),
anti-mutagenic (6), and
anti-angiogenic activities (2).
Moreover, in vitro and in vivo studies have shown
that quercetin exhibits various anticancer activities. It was
reported that quercetin-3-O-gluoside induced human DNA
topoisomerase II inhibition, cell cycle arrest and apoptosis in
hepatocelluar carcinoma cells (7).
It was also reported that quercetin derivatives demonstrated
anti-oxidant activity [monochloropivaloyl quercetin
(IC50=27 µM)] and cytotoxicity in HeLa
[chloronaphtoquinone quercetin (IC50=13.2 µM)]
and NIH-3T3 [tri(diacetylcaffeoyl) quercetin (IC50=10.6
µM)] cells (8). Quercetin
(40 mg/ml) is reported to inhibit the growth of MCF-7 breast cancer
cells and to promote apoptosis by inducing
G0/G1 phase arrest (9). Quercetin (100 µM) inhibited the
growth of colorectal cancer cells, by up-regulation of the
expression of tumor-suppressor genes and modulation of cell
cycle-related and apoptosis genes (10,11).
Moreover, quercetin (2%) inhibited carcinogen-induced rat mammary
tumor growth (12).
Apoptosis is a vital component of various processes
including normal cell turnover, proper development and functioning
of the immune system, hormone-dependent atrophy, embryonic
development and chemical-induced cell death (13). The process of apoptosis is
associated with various caspases, which are aspartate-specific
cysteine proteases and members of the interleukin-1β-converting
enzyme family (14,15). Caspases, once activated, play a key
role in the intracellular signal cascade for undergoing apoptosis.
In most tumor cells, apoptosis occurs via two different signaling
pathways: the extrinsic and intrinsic apoptosis pathways. The
extrinsic pathway is related to the activation of death receptors,
such as Fas and tumor necrosis factor receptors (TNFRs) and the
cleavage of caspase-8 and caspase-3 (16–18).
The intrinsic pathway is related to changes in the mitochondrial
membrane potential (ΔΨ m), the mitochondrial permeability
transition, and the cleavage of caspase-9 and caspase-3 (19). In both the extrinsic and intrinsic
pathways, caspase-3 is responsible for the cleavage of
poly(ADP-ribose) polymerase (PARP) during cell death (20).
Overexpression of HER2 is encountered in
approximately 25% of invasive breast cancers (21). HER2-positive breast cancers tend to
grow more quickly than HER2-negative breast cancers. HER2-positive
cancers are associated with frequent recurrence and reduced overall
survival, compared to HER2-negative tumor subtypes. The most widely
used chemotherapeutic agent is Herceptin (trastuzumab), which acts
by attaching itself to HER2 receptors on breast cancer cells and
blocking them from receiving growth signals (22,23).
Herceptin also causes arrest at the G1 phase of the cell cycle and
inhibits the phosphorylation of p27Kip1, resulting in
the suppression of cdk2 activity and reduced proliferation
(24). Herceptin suppresses
angiogenesis by both the induction of anti-angiogenic factors and
the repression of pro-angiogenic factors. However, many women do
not respond to Herceptin or develop resistance to this drug
(25). This has resulted in
significant efforts to identify other compounds that can
effectively treat HER2-overexpressing breast cancer.
Previously, we reported that phytoestrogen
suppresses cell growth and induces apoptosis by inhibiting signal
transducer and activator of transcription 3 (STAT3) and/or NF-κB
signaling in HER2-overexpressing breast cancer cells (26,27).
In the present study, we investigated whether quercetin displays
growth-suppressive activity in HER2-overexpressing BT-474 breast
cancer cells. For this purpose, we tested the effects of quercetin
on the proliferation and apoptosis of BT-474 cells. We also
investigated the mechanism by which quercetin regulates the growth
of BT-474 cells by analyzing the cell cycle and measuring the
levels of apoptotic molecules and intracellular signaling
molecules. We also aimed to ascertain whether quercetin inhibits
the STAT3 signaling pathway, leading to the growth suppression of
HER2-overexpressing breast cancer cells. Our study may advance
human health by clarifying the efficacy of quercetin for the
prevention and treatment of HER2-positive breast cancer.
Materials and methods
Compounds
Quercetin (3,3′,4′,5,6-pentahydroxyflavone) and
carbonyl cyanide 4-(trifluoromethoxy) phenylhydrazone (FCCP) were
purchased from Sigma Chemical Co. (St. Louis, MO, USA). These
compounds were dissolved in dimethyl sulfoxide (DMSO), and the
final concentration of DMSO in the controls and each sample did not
exceed 0.1%. We found that 0.1% DMSO did not affect the cell growth
rate compared to 0% DMSO (no treatment) in the breast cancer cells
(data not shown). JC-1
(5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolylcarbocyanine
iodide) was obtained from Molecular Probes (Invitrogen, Carlsbad,
CA, USA). The caspase-8 inhibitor Z-IETD-fmk and the
caspase-9 inhibitor Z-LEHD-fmk were obtained from R&D
Systems, Inc. (Minneapolis, MN, USA). The STAT3 inhibitor S3I-201
was obtained from Calbiochem (San Diego, CA, USA). An EZ-western
chemiluminescent detection kit was purchased from Daeil Lab Service
Co. (Seoul, Korea).
Cell cultures
BT474 human breast cancer cells (ATCC, American Type
Culture Collection; Manassas, VA, USA) were cultured in RPMI-1640
medium containing 50 U/ml penicillin, 50 mg/ml streptomycin and 10%
fetal bovine serum (FBS; Welgene, Daegu, Korea) at 37°C in an
atmosphere of 5% CO2.
Antibodies
Monoclonal or polyclonal antibodies (mouse or
rabbit) directed against FAS, cleaved caspase-8, caspase-3, cleaved
caspase-3 and PARP [poly(ADP-ribose) polymerase] were purchased
from Cell Signaling Technology, Inc. (Danvers, MA, USA). Monoclonal
or polyclonal antibodies (mouse or rabbit) directed against Bcl-2,
BAX, p53, phospho-p53 (Ser15), p21 and VEGF were obtained from
Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA). Monoclonal or
polyclonal antibodies (mouse or rabbit) against Bcl-XL and HIF-1α
were purchased from BD Biosciences (Franklin Lakes, NJ, USA).
Monoclonal or polyclonal antibodies (mouse or rabbit) directed
against STAT3, phospho-STAT3 (Tyr705), and phospho-JAK1
(Tyr1022/Tyr1023) were obtained from upstate-Millipore (Billerica,
MA, USA). The anti-tubulin antibody was from Sigma Chemical Co.
Horseradish peroxidase (HRP)-conjugated secondary antibodies (mouse
and rabbit) were purchased from Calbiochem and anti-goat secondary
antibody was from Jackson ImmunoResearch (West Grove, PA, USA).
Cell proliferation assay
Cells were seeded in 12-well culture plates at a
density of 5×104 cells/well. After the cells were
exposed to different concentrations of quercetin (20–60 µM)
and incubated for 3 days, they were harvested by trypsinization,
resuspended in 1–2 ml of medium, and counted using a
hemocytometer.
MTT assay
Cells were seeded in 96-multi-well culture plates at
a density of 3×103 cells/well and incubated for 24 h at
37°C. Then, they were treated with different concentrations of
quercetin (20–60 µM) for 24, 48, or 72 h. After incubation,
MTT reagents (0.5 mg/ml) were added to the each well and the plates
were incubated in the dark at 37°C for 2 h. At the end of the
incubation, the medium was removed, the resulting formazan was
dissolved in DMSO, and the optical density was measured at 570 nm
using an ELISA plate reader (fluorescence readers; Molecular
Devices, Sunnyvale, CA, USA).
Clonogenic survival assays
(anchorage-dependent and -independent)
For anchorage-dependent colony formation assay,
cells were seeded into 6-well culture plates at a density of
5×102 cells/well. After overnight incubation, they were
treated with different concentrations of quercetin (20–60
µM) or vehicle and maintained for 10 days at 37°C. Cells
were fed every 3 days by removing old medium and adding fresh
medium containing quercetin. Finally, the plates were stained with
hematoxylin and the colony number was determined. For
anchorage-independent colony formation assay, soft agar was used.
Cells (1×103) were suspended in 1 ml of 0.6% soft agar
that was layered on top of 1 ml of 1% solidified agar in each well
of 12-well plates. The plates were then incubated for 15 to 21 days
in complete RPMI medium containing quercetin (20–60
µm). The
medium was changed every 3 days during this period. At the end of
the experiment, tumor cell colonies measuring at least 30 µm
were counted using a dissection microscope.
Cell cycle analyses by flow
cytometry
Cells were harvested with 0.25% trypsin and washed
once with phosphate-buffered saline (PBS). After centrifugation,
the cells were fixed in cold 95% ethanol with 0.5% Tween-20, and
stored at −20°C for at least 30 min. The cells were incubated in 50
µg/ml of propidium iodide (PI) (including 1% of sodium
citrate and 50 µg/ml of RNase A) at room temperature in the
dark for 30 min. The analysis of apoptotic cells was performed on a
FACScan flow cytometer (Becton Dickinson, Mountain View, CA, USA)
and the data were analyzed using CellQuest software.
Analysis of mitochondrial transmembrane
potential (ΔΨm)
Cells were seeded at a density of 1×106
cells/dish in 100-mm dishes and incubated for 24 h at 37°C. After
stabilization, the cells were treated with quercetin (20–60
µm) and
vehicle for 72 h. After harvest by treatment with trypsin-EDTA, the
cells were washed with cold PBS, centrifuged at 1,500 rpm for 5 min
and stained with 4 µg/ml JC-1 for 15 min at 37°C in the
dark. The data were analyzed by FACSCalibur flow cytometry (BD
Biosciences) measuring the green fluorescence and red fluorescence
at 514/529 nm (FL-1) and 585/590 nm (FL-2), respectively.
Western blot analysis
Cells were lysed in modified RIPA buffer [150 mM
NaCl, 1% NP-40, 0.5% deoxycholate, 0.1% SDS, 50 mM Tris (pH 8.0), 1
mM EDTA, 1 mM phenylmethylsulfonyl fluoride (PMSF), 1 mM NaF, 1 mM
Na3VO4 and protease inhibitor mixture]. The
lysates were cleared by centrifugation at 10,000 × g for 15 min and
the supernatants were collected. The protein concentration was
quantified using a Bio-Rad Bradford protein assay (Bio-Rad,
Hercules, CA, USA). Equal amounts of protein lysates were used for
western blot analysis with the indicated antibodies. Immunoreactive
protein bands were detected with an EZ-Western detection kit (Daeil
Lab Service Co., Ltd., Seoul, Korea).
Immunocytochemistry
Cells (2×104 cells/well) were seeded in
8-well chamber slides, incubated for 24 h at 37°C and treated with
quercetin (60 µM) in the presence or absence of
CoCl2 for another 24 h. The cells were fixed with 4%
paraformaldehyde for 30 min and treated with 3% hydrogen peroxide
(H2O2) in methanol for 20 min to quench
endogenous peroxidase activity. The cells were washed with PBS,
blocked with 5% BSA in PBS for 1 h and incubated with the
anti-STAT3 primary antibody (1:100 dilution) overnight at 4°C.
After washing with PBS, the cells were incubated with anti-rabbit
biotin-conjugated secondary antibody for 1 h at room temperature.
Then, the cells were treated with Vectastain ABC reagent (Vector
Laboratories, Inc. Burlingame, CA, USA) for 30 min at 4°C and
stained with diaminobenzidine tetrachloride (DAB) and hematoxylin.
The cells were mounted with mounting medium and subsequently
analyzed using microscopy.
Measurement of MMP-9 secreted from BT-474
cells by ELISA
To assess the level of MMP-9 in the BT-474 cell
supernatants, the cells were treated with quercetin (20-60
µM). After 24 h, the media were collected, centrifuged to
remove the cellular debris, and stored at −70°C until assay for
MMP-9. The amount of MMP-9 secreted into the culture medium was
measured by ELISA according to the manufacturer's instructions
(R&D Systems). Briefly, 96-well plates were coated with capture
antibody in ELISA coating buffer and incubated overnight at 4°C.
The plates were then washed with PBS with 0.05% Tween-20 (PBS-T)
and subsequently blocked with 10% FBS in PBS for 1 h at 20°C.
Serial dilutions of standard antigen or sample in dilution buffer
(10% FBS in PBS) were added to the plates, and the plates were
incubated for 2 h at 20°C. After washing, biotin-conjugated
anti-mouse IgE and streptavidin-conjugated horseradish peroxidase
(SAv-HRP) were added to the plates, and the assay plates were
incubated for 1 h at 20°C. Finally, the tetramethylbenzidine (TMB)
substrate was added to the plates, and after 15 min of incubation
in the dark, 2 NH2SO4 was added to stop the
reaction. The optical density was measured at 450 nm on an
automated ELISA reader.
STAT3 luciferase reporter assay
BT-474 cells were plated and allowed to attach by
overnight incubation at 37°C. Cells were transiently transfected
with p4xM67-TK-luc plasmid (Addgene plasmid 8688; Addgene,
Cambridge, MA, USA) containing four copies of the STAT-binding site
(TTCCCGTAA). The next day, the cells were treated with different
concentrations of quercetin (20–60 µM) for 24 h and then
submitted to the luciferase assays. Luciferase assays were
performed using a dual-luciferase assay kit according to the
manufacturer's instructions (Promega, Madison, WI, USA). Finally,
luciferase activities were determined using a luminometer (BMG
Labtech, Ortenberg, Germany).
Statistical analysis
All experiments were performed in triplicate. The
data for the cell proliferation, MTT, ELISA, and STAT3 luciferase
reporter assays are expressed as the mean ± standard deviation
(SD). The standard deviations for all of the measured biological
parameters are displayed in the appropriate figures. A Student's
t-test was used for single variable comparisons, and a p-value of
<0.05 was considered to be indicative of a statistically
significant result.
Results
Quercetin suppresses the growth of BT-474
cells
The effects of quercetin on cell growth were
measured by cell proliferation and MTT assays in the BT-474 cells.
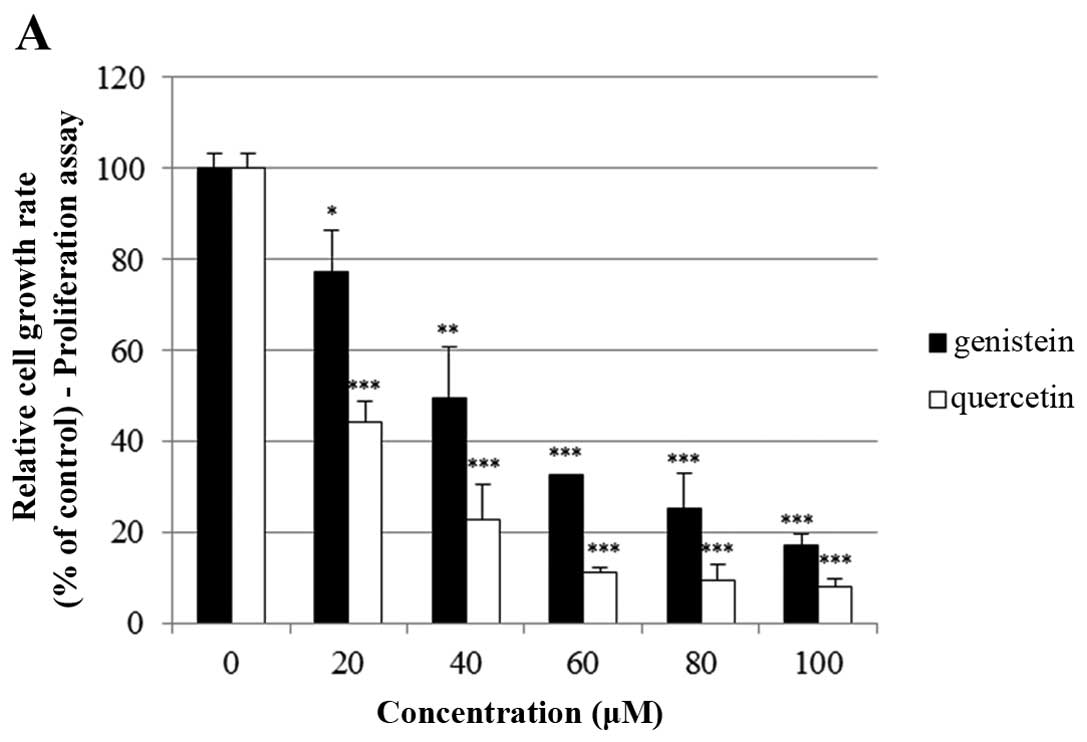
As shown in Fig. 1A, quercetin and
genistein significantly inhibited BT-474 cell proliferation in a
dose-dependent manner (20–100 µM) after 72 h of treatment
(proliferation assay). Between two phytoestrogens, quercetin had
the stronger growth suppressive activity compared to genistein in
the BT-474 cells. Therefore, we chose quercetin for our
experimental study. In addition, the time-dependent growth
suppressive activity of quercetin was measured by the MTT assay
(Fig. 1B). As shown in Fig. 1A and B, the proliferation assay
appeared to be more sensitive than the MTT assay with respect to
measuring the intensity of the cell growth inhibition. Moreover,
the growth inhibition induced by quercetin was verified by
microscopic observation. As shown in Fig. 1C, quercetin effectively inhibited
the growth rate of BT-474 monolayer cells after 72 h of treatment.
Of note, quercetin also induced morphological changes in these
cells (Fig. 1C). Since lower
concentrations of phytoestrogen could stimulate the growth of
breast cancer cells through nuclear and membrane estrogen
receptors, we performed an MTT assay to measure the growth rate of
BT-474 cells under lower concentrations of quercetin (0–20
µM). We found that lower concentrations of quercetin did not
affect the cell growth rate (Fig.
1D).
Quercetin inhibits clonogenic survival of
BT-474 cells
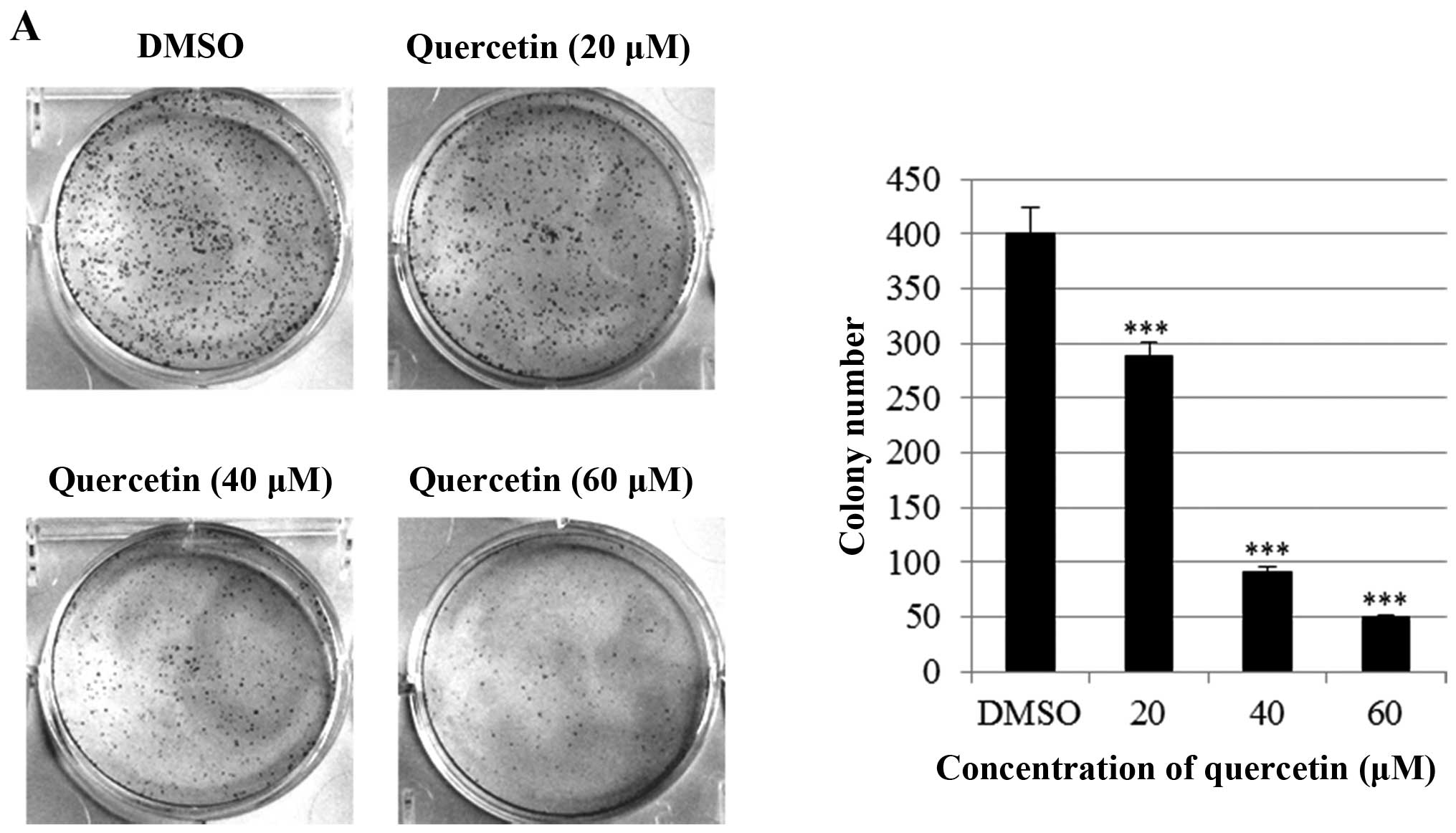
Next, we investigated the effect of quercetin on
clonogenic. survival of BT-474 cells using clonogenic survival
assays (anchorage-dependent and -independent). As shown in Fig. 2A, quercetin significantly inhibited
anchorage-dependent colony formation dose-dependently in the BT-474
cells. Consistently with this result, quercetin strongly decreased
anchorage-independent colony formation in the BT-474 cells
(Fig. 2B). These results suggest
that quercetin inhibits clonogenic survival of the BT-474
cells.
The growth-suppressive activity of
quercetin is accompanied by an increase in the
sub-G0/G1 apoptotic population in BT-474
cells
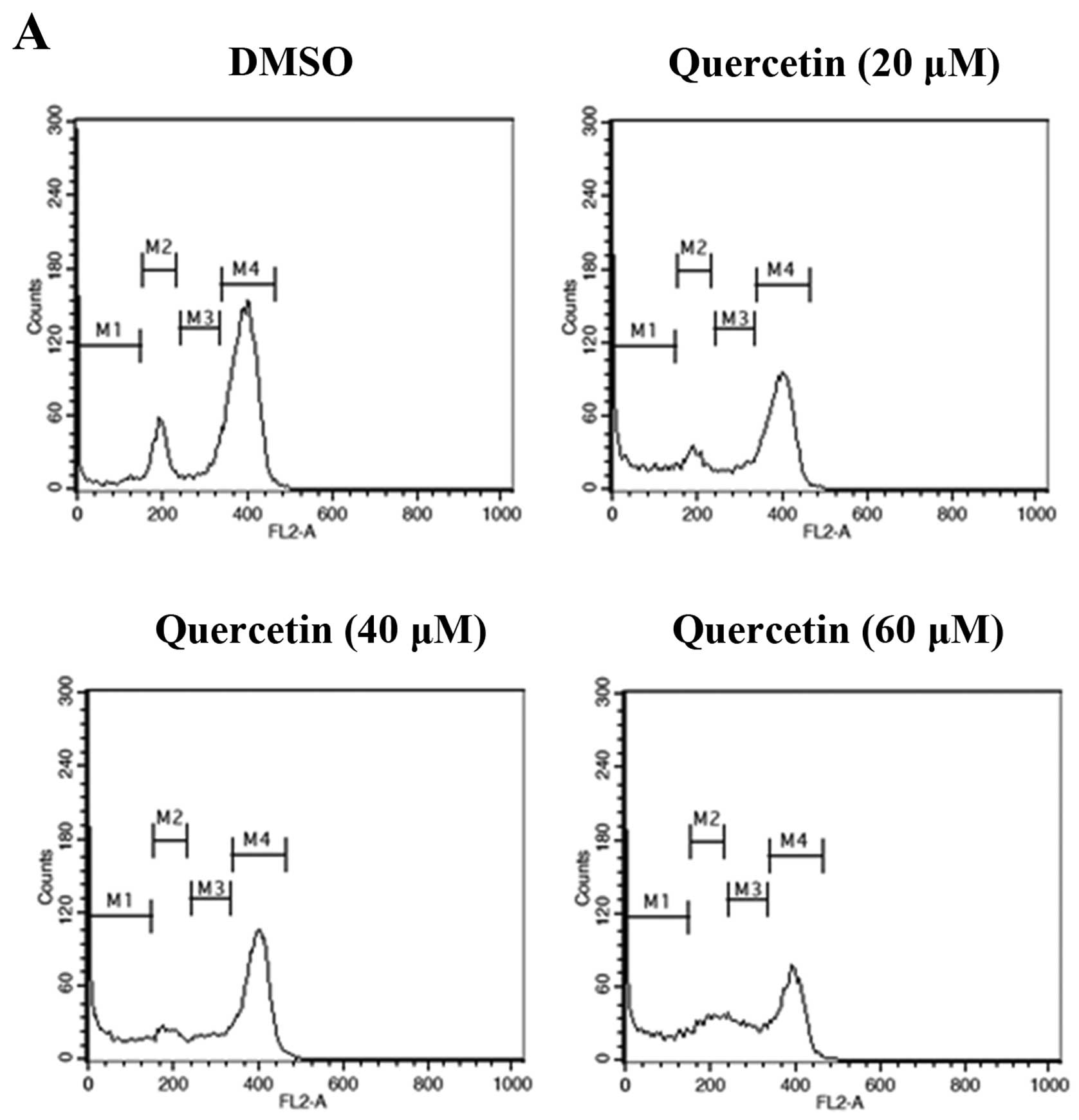
To investigate whether quercetin inhibits cell
proliferation by promoting changes in cell cycle progression, the
effect of quercetin on the cell cycle profile was assessed in the
BT-474 cells. For this purpose, the cells were treated with
quercetin (20–60 µM) for 72 h and then analyzed for cell
cycle stage by flow cytometry. The results demonstrated that
quercetin induced an increase in the
sub-G0/G1 apoptotic population in the BT-474
cells (Fig. 3).
Quercetin induces extrinsic apoptosis in
BT-474 cells
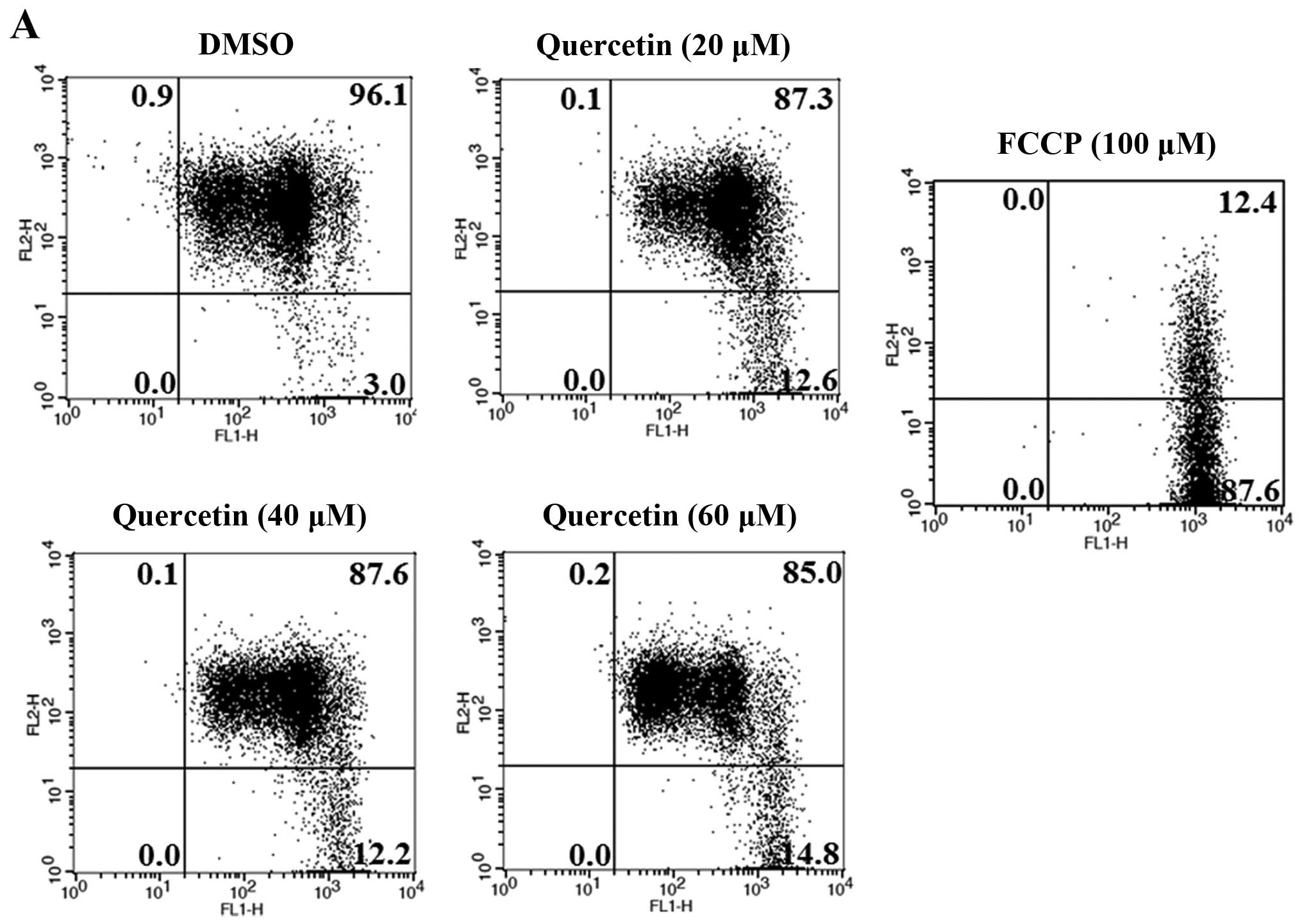
Next, we investigated whether apoptosis induced by
quercetin occurs via the extrinsic apoptosis pathway in the BT-474
cells. For this purpose, we measured the loss of mitochondrial
transmembrane potential (ΔΨm) within the cells using JC-1. JC-1 is
able to selectively enter mitochondria and reversibly transforms
the color from red to green when the membrane potential decreases.
In non-apoptotic cells with high mitochondrial ΔΨm, JC-1
spontaneously forms complexes known as J-aggregates with intense
red fluorescence. In contrast, in apoptotic cells (particularly
mitochondrial-mediated apoptotic cells) with low ΔΨm, JC-1 remains
in the monomeric form, which shows only green fluorescence. In our
study, quercetin did not induce a low mitochondrial transmembrane
potential (ΔΨm), showing relatively weak green fluorescence (DMSO,
3.0%; quer 20 µM, 12.6%; quer 40 µM, 12.2%; quer 60
µM, 14.8%) compared to FCCP (positive control, 87.6%)
(Fig. 4A). We also measured the
levels of Bcl-2 family members (BAX and Bcl-2) which are important
in the intrinsic mitochondrial apoptosis pathway. We found that
quercetin failed to decrease the level of Bcl-2 or increase the
level of BAX as shown in Fig. 4B and
C. These results demonstrate that quercetin does not induce
apoptosis via the intrinsic mitochondrial pathway but induces
apoptosis via the extrinsic pathway in BT-474 breast cancer
cells.
Quercetin induces apoptosis via the
caspase-dependent apoptosis pathway in BT-474 cells
In this step, we investigated whether quercetin
activates the caspase-dependent apoptosis pathway by measuring the
expression of caspase-8, caspase-3, and PARP. We observed that
quercetin upregulated the levels of cleaved caspase-8 and
caspase-3, and induced the cleavage of PARP in the BT-474 cells
(Fig. 5A). We also found that the
cleavage of caspase-8, caspase-3 and PARP was inhibited by the
caspase-8 inhibitor Z-IETD-fmk and the caspase-9 inhibitor
Z-LEHD-fmk (Fig. 5B), but
quercetin prevented this inhibition and was able to induce the
cleavage of caspase-8, caspase-3 and PARP in the presence of
Z-IETD-fmk and Z-LEHD-fmk (Fig. 5B). Moreover, the caspase-8 and
caspase-9 inhibitors did not suppress cell growth, while quercetin
was able to induce apoptosis even in their presence (Fig. 5C). These results confirm that
quercetin strongly promoted apoptosis via a caspase-dependent
mechanism in the BT-474 cells.
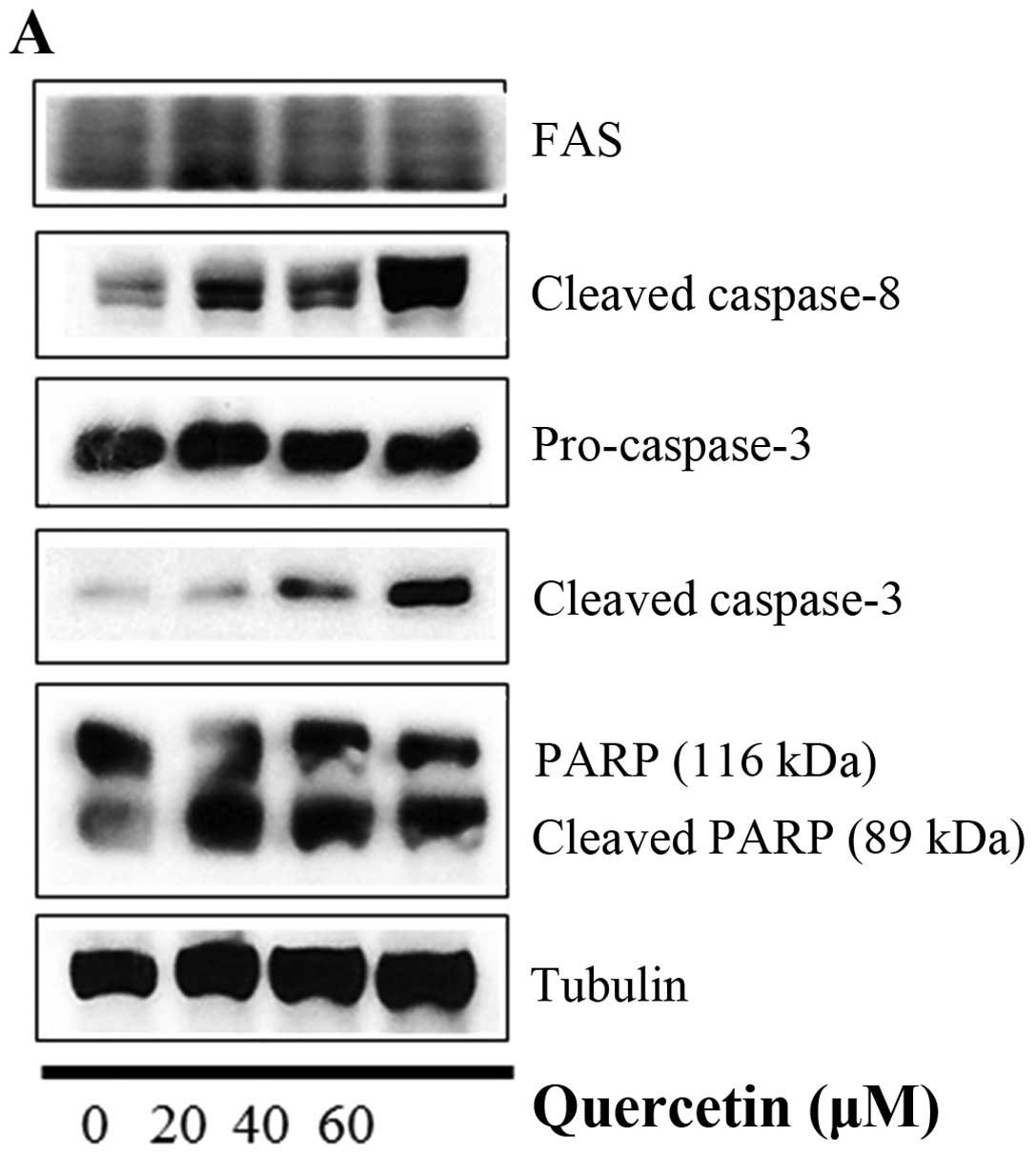 | Figure 5Quercetin induces caspase-dependent
apoptosis in BT-474 cells. (A) Quercetin induces apoptosis via a
caspase-dependent apoptosis pathway in the BT-474 cells. BT-474
cells were treated with quercetin (0–60 µM) for 24 h. Whole
cell lysates were analyzed by western blotting with anti-FAS,
anti-cleaved caspase-8, anti-caspase-3, anti-cleaved caspase-3,
anti-PARP and anti-tubulin antibodies. The data shown are
representative of three independent experiments that gave similar
results. (B) Effect of caspase-8 and caspase-9 inhibitors on
quercetin-induced apoptosis in BT-474 cells. BT-474 cells were
exposed to 60 µM quercetin with or without the caspase-8
inhibitor (40 µM) or the caspase-9 inhibitor (40 µM)
for 24 h, the cell lysates were separated by SDS-PAGE, and western
blotting with specific antibodies was performed (anti-cleaved
caspase-8, anti-cleaved caspase-3, anti-cleaved PARP, and
anti-tubulin). The data shown are representative of three
independent experiments that gave similar results. (C) Effect of
caspase-8 and caspase-9 inhibitors on BT-474 cell proliferation.
BT-474 cells were exposed to 60 µM quercetin with or without
the caspase-8 inhibitor (40 µM) or the caspase-9 inhibitor
(40 µM) for 72 h and photographed by phase contrast
microscopy (original magnification, x40). |
Effect of quercetin on STAT3 activation
in BT-474 cells
Quercetin upregulated phospho-p53 (p-p53) and p21
(p53 target gene) (Fig. 6A).
Quercetin did not affect the p53 level. As shown in Fig. 6B, we aimed to ascertain whether
quercetin affects STAT3 signaling measuring levels of p-STAT3 and
VEGF (STAT-3 target gene). We found that quercetin reduced the
expression of p-STAT3 as well as p-JAK1 (an upstream kinase of
STAT3) (Fig. 6B). Quercetin also
reduced the level of VEGF (Fig. B).
Since STAT3 is a potential modulator of HIF-1α (28), we observed the relationship between
STAT3 and HIF-1α. We found that quercetin suppressed the expression
of p-STAT3 and HIF-1α that was upregulated by CoCl2
(hypoxia mimic) (Fig. 6C).
Immunocytochemical staining indicated that quercetin decreased the
nuclear localization of STAT3 in the presence and absence of
CoCl2 (Fig. 6D).
Fig. 6E showed that quercetin
strongly decreased STAT3 transcriptional activity as revealed by
transient transfection and luciferase assays. As shown in Fig. 6F, quercetin suppressed the
production of STAT3 target gene, MMP-9, as revealed by ELISA assay.
These results suggest that quercetin decreases HER2-positive breast
cancer cell growth rate at higher concentrations (>20 µM)
by inhibiting the STAT3 signaling pathway.
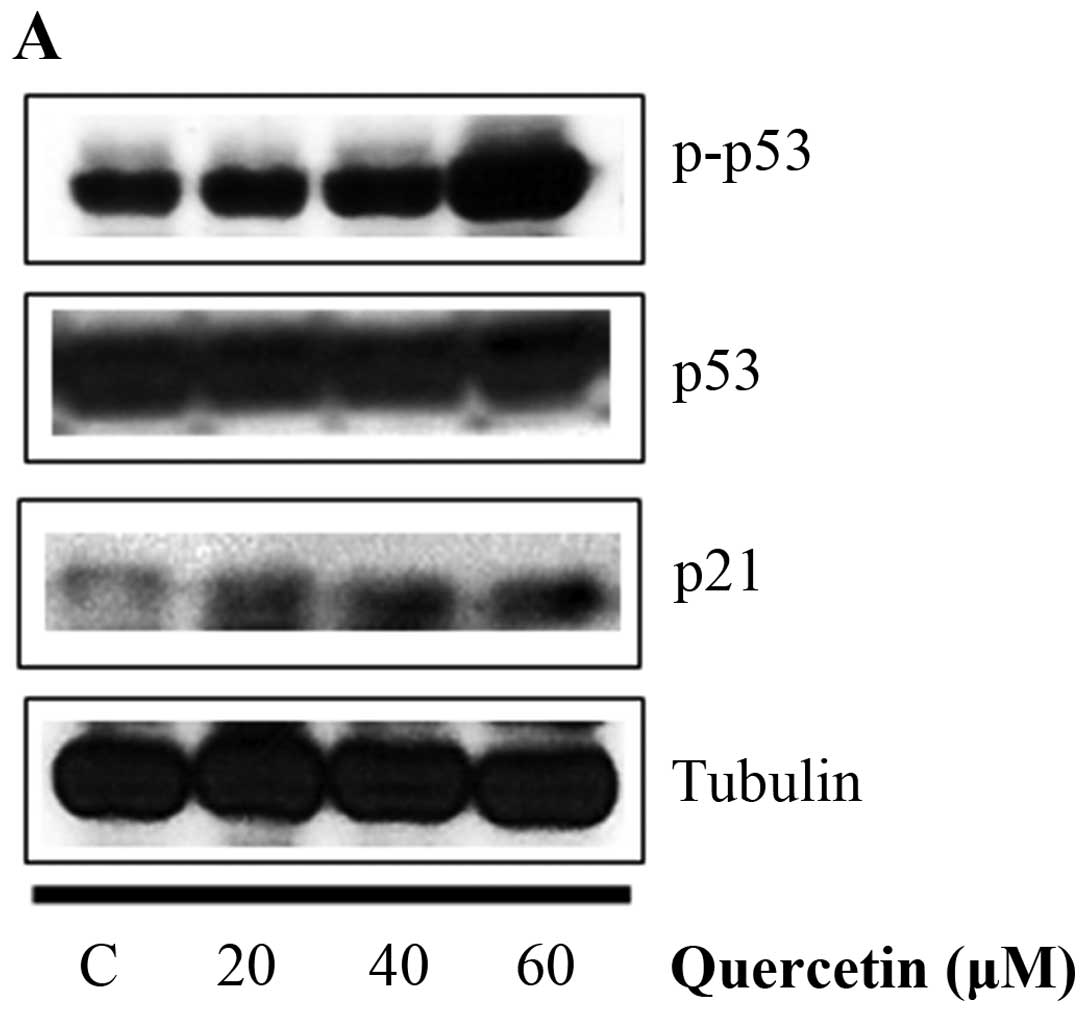 | Figure 6Effect of quercetin on STAT3
activation in BT-474 cells. (A) BT-474 cells were treated with
quercetin (0–60 µM) for 24 h. Whole cell lysates were
analyzed by western blotting with anti-p-p53, anti-p53, anti-p21
and anti-tubulin antibodies. (B) BT-474 cells were treated with
quercetin (0–60 µM) for 24 h. Whole cell lysates were
analyzed by western blotting with anti-p-JAK1, anti-p-STAT3,
anti-STAT3, anti-VEGF, and anti-tubulin antibodies. (C) BT-474
cells were treated with quercetin (60 µM) for 24 h in the
presence or absence of CoCl2 (4 h). Whole cell lysates
were analyzed by western blotting with anti-phospho-STAT3,
anti-HIF-1α, anti-STAT3, and anti-tubulin antibodies. (D) BT-474
cells were treated with quercetin (60 µM) for 24 h in the
presence or absence of CoCl2 and then submitted to
immunocytochemistry for detection of nuclear STAT3. The data shown
are representative of three independent experiments that gave
similar results. (E) BT-474 cells were transiently transfected with
p4xM67-TK-luc plasmid containing four copies of the STAT-binding
site, treated with quercetin (0–60 µM) and submitted to
dual-luciferase assay. (F) BT-474 cells were treated with quercetin
(0–60 µM) for 24 h and the intracellular MMP-9 concentration
was measured by ELISA. Data are shown as the means of three
independent experiments (error bars denote SD).
*P<0.05, **P<0.01,
***P<0.001. |
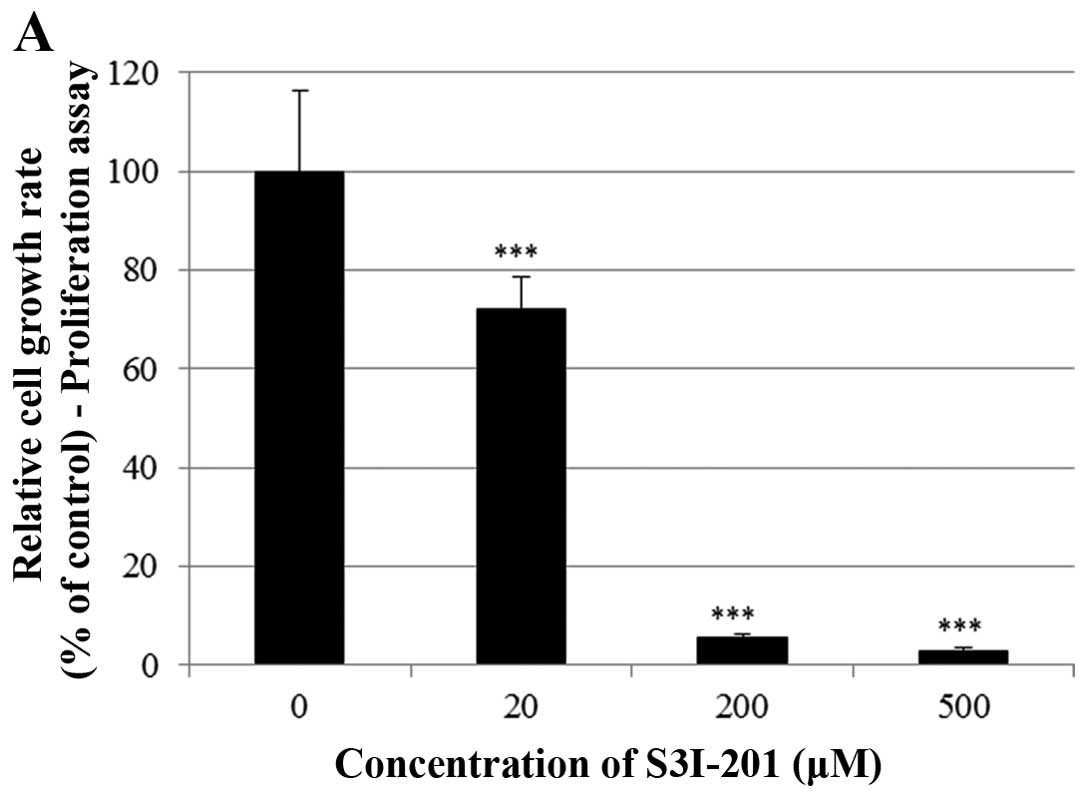
Effect of S3I-201 on STAT3 activation in
BT-474 cells
Finally, we investigated whether the STAT3 inhibitor
S3I-201 inhibits cell proliferation and STAT3 activation in BT-474
cells. As shown in Fig. 7A and B,
S3I-201 decreased cell growth in a dose-and time-dependent manner.
Furthermore, S3I-201 reduced the expression of p-STAT3, STAT-3 and
VEGF (Fig. 7C). These results
demonstrate that STAT3 inhibition induced cell growth inhibition
and repressed the expression of oncogenic molecules.
Discussion
In the present study, we investigated the mechanism
by which quercetin inhibits cell growth and induces apoptosis in
HER2-overexpressing BT-474 breast cancer cells. Quercetin
significantly inhibited BT-474 cell growth in a dose- and
time-dependent manner. Clonogenic survival assays demonstrated that
quercetin inhibited anchorage-dependent and -independent colony
formation in a dose-dependent manner. These growth inhibitions were
related with an increase in t he sub-G0/G1
apoptotic population in BT-474 cells. Quercetin increased the
number of apoptotic cells in a dose-dependent manner, as assessed
by FACS analysis. Interestingly, quercetin did not induce apoptosis
via the intrinsic mitochondrial apoptosis pathway in the BT-474
cells as revealed by JC-1 dyeing of the cells and western blot
analysis; quercetin did not reduce mitochondrial transmembrane
potential (ΔΨm) maintaining red flouorescence, and failed to
decrease the level of Bcl-2 or increase the level of BAX. Whereas,
quercetin induced apoptosis via the caspase-dependent extrinsic
apoptosis pathway since quercetin increased the cleavage of
caspase-8, caspase-3 and PARP. Moreover, quercetin reversed
inhibition of the cleavage of caspase-8, caspase-3 and PARP induced
by caspase-8 inhibitor Z-IETD-fmk and the caspase-9
inhibitor Z-LEHD-fmk. These results suggest that quercetin
contains a strong apoptotic capacity. The caspases, a family of
cysteine-dependent aspartate-directed proteases, are common death
proteases (29). Caspases are
synthesized as relatively inactive zymogens that become activated
by scaffold-mediated transactivation or by cleavage via upstream
proteases in an intracellular cascade (29). Once activated, they cleave a variety
of intracellular polypeptides, including major structural elements
of the cytoplasm and nucleus, components of the DNA repair
machinery, and a number of protein kinases (29).
Quercetin increased the expression of active p53
(p-p53) and p21 (p53 target gene), suggesting that this compound
suppresses HER2-overexpressing breast cancer cell growth via a
p53-dependent manner. In agreement with our data, quercetin has
been shown to increase the levels of p-p53 and p21 in human lung
carcinoma cells (30). The p53
tumor suppressor inhibits cellular proliferation by inducing cell
cycle arrest and apoptosis in response to cellular stresses
including DNA damage, growth factor deprivation, hypoxia and
oncogene activation (31,32). p53-dependent apoptosis is produced
by the caspase proteinases and related to pro-apoptotic proteins
such as BAX, NOXA and PUMA (33).
Interestingly, quercetin decreased the expression of
p-JAK1 (upstream kinase of STAT3), p-STAT3 and VEGF (STAT3 target
gene) suggesting its negative regulation of STAT3 pathway in BT-474
cells. Elevated p-STAT3 expression by CoCl2 was also
reduced by quercetin. Quercetin inhibited nuclear localization of
STAT3 in the presence or absence of CoCl2 as revealed by
immunocytochemistry. Quercetin inhibited the production of MMP-9 as
revealed by ELISA assay. The STAT3 inhibitor S3I-201 decreased the
cell growth and expression of p-STAT3, STAT-3 and VEGF in the
BT-474 cells. These results clearly indicate that quercetin induces
growth-suppressive activity by inhibiting the STAT3 signaling
pathway. STAT3 is a transcription factor that regulates the gene
expression in response to various cellular stimuli and plays an
important role in cell growth and apoptosis. STAT3 usually acts as
a tumor-promoter, although its role as a tumor-suppressor has been
recently reported (33,34). STAT3 accelerates cell proliferation
and angiogenesis, inhibits apoptosis, and drives invasion and
metastasis (33–35). STAT3 in melanoma tumors is
associated with poor prognosis (35–37).
Constitutive STAT3 phosphorylation is mediated by several upstream
kinases (Jak and Src) and is thought to be a key component of the
oncogenic process (38,39). Phytoestrogen (resveratrol) is known
to inhibit STAT3 signaling and induces the apoptosis of malignant
cells containing activated STAT3 (40). The VEGF promoter contains various
transcription factor binding sites, including sites for STAT3
(41) and HIF-1 (42). The physical interaction of STAT3
with HIF-1 controls VEGF transcriptional activation by their
binding to the VEGF promoter (28).
Breast cancers with HER2 gene amplification or HER2
protein overexpression are HER2-positive. Approximately, 20-25% of
invasive breast carcinomas reveal HER2 overexpression (43). A normal breast cell has 20,000 HER2
receptors, while a breast cancer cell may have up to 1.5 million.
HER2 enhances the aggressiveness of breast cancer and is associated
with recurrence when compared to HER2-negative breast cancer. HER2
is a member of the HER/ErbB2/Neu protein family, which also
includes HER1/EGFR, HER3 and HER4. HER2 crosstalks with the
estrogen receptor (ER) signal transduction pathway (44), and its expression level can be
regulated by ER. In the present study, we found that quercetin
significantly inhibited the growth and induced apoptosis in
HER2-overexpressing breast cancer cells. This indicates that
quercetin could be a useful natural therapy that inhibits
HER2-overexpressing breast cancer. Quercetin could be a promising
target for the treatment and prevention of HER2-overexpressing
breast cancer.
Acknowledgments
This research was supported by the Basic Science
Research Program through the National Research Foundation of Korea
(NRF) funded by the Ministry of Science, ICT and Future Planning
(NRF-2015R1C1A2A01051539). This research was also supported by a
grant funded by the Traditional Korean Medicine R&D Project of
the Ministry of Health and Welfare (HI12C1889 and HI11C2110).
References
|
1
|
Walters DG, Young PJ, Agus C, Knize MG,
Boobis AR, Gooderham NJ and Lake BG: Cruciferous vegetable
consumption alters the metabolism of the dietary carcinogen
2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) in humans.
Carcinogenesis. 25:1659–1669. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Igura K, Ohta T, Kuroda Y and Kaji K:
Resveratrol and quercetin inhibit angiogenesis in vitro. Cancer
Lett. 171:11–16. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hertog MG and Hollman PC: Potential health
effects of the dietary flavonol quercetin. Eur J Clin Nutr.
50:63–71. 1996.PubMed/NCBI
|
|
4
|
Coskun O, Kanter M, Korkmaz A and Oter S:
Quercetin, a flavonoid antioxidant, prevents and protects
streptozotocin-induced oxidative stress and beta-cell damage in rat
pancreas. Pharmacol Res. 51:117–123. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Boots AW, Wilms LC, Swennen EL, Kleinjans
JC, Bast A and Haenen GR: In vitro and ex vivo anti-inflammatory
activity of quercetin in healthy volunteers. Nutrition. 24:703–710.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Geetha T, Malhotra V, Chopra K and Kaur
IP: Antimutagenic and antioxidant/prooxidant activity of quercetin.
Indian J Exp Biol. 43:61–67. 2005.PubMed/NCBI
|
|
7
|
Sudan S and Rupasinghe HP:
Quercetin-3-O-glucoside induces human DNA topoisomerase II
inhibition, cell cycle arrest and apoptosis in hepatocellular
carcinoma cells. Anticancer Res. 34:1691–1699. 2014.PubMed/NCBI
|
|
8
|
Danihelová M, Veverka M, Sturdík E and
Jantová S: Antioxidant action and cytotoxicity on HeLa and NIH-3T3
cells of new quercetin derivatives. Interdiscip Toxicol. 6:209–216.
2013. View Article : Google Scholar
|
|
9
|
Deng XH, Song HY, Zhou YF, Yuan GY and
Zheng FJ: Effects of quercetin on the proliferation of breast
cancer cells and expression of survivin in vitro. Exp Ther Med.
6:1155–1158. 2013.PubMed/NCBI
|
|
10
|
van Erk MJ, Roepman P, van der Lende TR,
Stierum RH, Aarts JM, van Bladeren PJ and van Ommen B: Integrated
assessment by multiple gene expression analysis of quercetin
bioactivity on anticancer-related mechanisms in colon cancer cells
in vitro. Eur J Nutr. 44:143–156. 2005. View Article : Google Scholar
|
|
11
|
Murtaza I, Marra G, Schlapbach R,
Patrignani A, Künzli M, Wagner U, Sabates J and Dutt A: A
preliminary investigation demonstrating the effect of quercetin on
the expression of genes related to cell-cycle arrest, apoptosis and
xenobiotic metabolism in human CO115 colon-adenocarcinoma cells
using DNA microarray. Biotechnol Appl Biochem. 45:29–36. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Verma AK, Johnson JA, Gould MN and Tanner
MA: Inhibition of 7,12-dimethylbenz(a)anthracene- and
N-nitrosomethylurea-induced rat mammary cancer by dietary flavonol
quercetin. Cancer Res. 48:5754–5758. 1988.PubMed/NCBI
|
|
13
|
Elmore S: Apoptosis: A review of
programmed cell death. Toxicol Pathol. 35:495–516. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fan TJ, Han LH, Cong RS and Liang J:
Caspase family proteases and apoptosis. Acta Biochim Biophys Sin
(Shanghai). 37:719–727. 2005. View Article : Google Scholar
|
|
15
|
Bosch M, Poulter NS, Vatovec S and
Franklin-Tong VE: Initiation of programmed cell death in
self-incompatibility: Role for cytoskeleton modifications and
several caspase-like activities. Mol Plant. 1:879–887. 2008.
View Article : Google Scholar
|
|
16
|
Zhang A, Wu Y, Lai HWL and Yew DT:
Apoptosis - a brief review. Neuroembryology. 3:47–59. 2004.
View Article : Google Scholar
|
|
17
|
Waring P and Müllbacher A: Cell death
induced by the Fas/Fas ligand pathway and its role in pathology.
Immunol Cell Biol. 77:312–317. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gupta S: Molecular signaling in death
receptor and mitochondrial pathways of apoptosis (Review). Int J
Oncol. 22:15–20. 2003.
|
|
19
|
Green DR and Reed JC: Mitochondria and
apoptosis. Science. 281:1309–1312. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Boulares AH, Yakovlev AG, Ivanova V,
Stoica BA, Wang G, Iyer S and Smulson M: Role of poly(ADP-ribose)
polymerase (PARP) cleavage in apoptosis. Caspase 3-resistant PARP
mutant increases rates of apoptosis in transfected cells. J Biol
Chem. 274:22932–22940. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wilson CA, Cajulis EE, Green JL, Olsen TM,
Chung YA, Damore MA, Dering J, Calzone FJ and Slamon DJ: HER-2
overexpression differentially alters transforming growth
factor-beta responses in luminal versus mesenchymal human breast
cancer cells. Breast Cancer Res. 7:R1058–R1079. 2005. View Article : Google Scholar
|
|
22
|
Joshi JP, Brown NE, Griner SE and Nahta R:
Growth differentiation factor 15 (GDF15)-mediated HER2
phosphorylation reduces trastuzumab sensitivity of
HER2-overexpressing breast cancer cells. Biochem Pharmacol.
82:1090–1099. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Favoni RE, Daga A, Malatesta P and Florio
T: Preclinical studies identify novel targeted pharmacological
strategies for treatment of human malignant pleural mesothelioma.
Br J Pharmacol. 166:532–553. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tokunaga E, Oki E, Nishida K, Koga T,
Egashira A, Morita M, Kakeji Y and Maehara Y: Trastuzumab and
breast cancer: Developments and current status. Int J Clin Oncol.
11:199–208. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Dean-Colomb W and Esteva FJ: Her2-positive
breast cancer: Herceptin and beyond. Eur J Cancer. 44:2806–2812.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Seo HS, Choi HS, Choi HS, Choi YK, Um JY,
Choi I, Shin YC and Ko SG: Phytoestrogens induce apoptosis via
extrinsic pathway, inhibiting nuclear factor-kappaB signaling in
HER2-overexpressing breast cancer cells. Anticancer Res.
31:3301–3313. 2011.PubMed/NCBI
|
|
27
|
Seo HS, Choi HS, Kim SR, Choi YK, Woo SM,
Shin I, Woo JK, Park SY, Shin YC and Ko SG: Apigenin induces
apoptosis via extrinsic pathway, inducing p53 and inhibiting STAT3
and NFκB signaling in HER2-overexpressing breast cancer cells. Mol
Cell Biochem. 366:319–334. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jung JE, Lee HG, Cho IH, Chung DH, Yoon
SH, Yang YM, Lee JW, Choi S, Park JW, Ye SK, et al: STAT3 is a
potential modulator of HIF-1-mediated VEGF expression in human
renal carcinoma cells. FASEB J. 19:1296–1298. 2005.PubMed/NCBI
|
|
29
|
Earnshaw WC, Martins LM and Kaufmann SH:
Mammalian caspases: Structure, activation, substrates, and
functions during apoptosis. Annu Rev Biochem. 68:383–424. 1999.
View Article : Google Scholar
|
|
30
|
Kuo PC, Liu HF and Chao JI: Survivin and
p53 modulate quercetin-induced cell growth inhibition and apoptosis
in human lung carcinoma cells. J Biol Chem. 279:55875–55885. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Schuler M and Green DR: Mechanisms of
p53-dependent apoptosis. Biochem Soc Trans. 29:684–688. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Shen Y and White E: p53-dependent
apoptosis pathways. Adv Cancer Res. 82:55–84. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
de la Iglesia N, Konopka G, Puram SV, Chan
JA, Bachoo RM, You MJ, Levy DE, Depinho RA and Bonni A:
Identification of a PTEN-regulated STAT3 brain tumor suppressor
pathway. Genes Dev. 22:449–462. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lewis HD, Winter A, Murphy TF, Tripathi S,
Pandey VN and Barton BE: STAT3 inhibition in prostate and
pancreatic cancer lines by STAT3 binding sequence oligonucleotides:
Differential activity between 5′ and 3′ ends. Mol Cancer Ther.
7:1543–1550. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kortylewski M, Jove R and Yu H: Targeting
STAT3 affects melanoma on multiple fronts. Cancer Metastasis Rev.
24:315–327. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Niu G, Bowman T, Huang M, Shivers S,
Reintgen D, Daud A, Chang A, Kraker A, Jove R and Yu H: Roles of
activated Src and Stat3 signaling in melanoma tumor cell growth.
Oncogene. 21:7001–7010. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Xie TX, Huang FJ, Aldape KD, Kang SH, Liu
M, Gershenwald JE, Xie K, Sawaya R and Huang S: Activation of stat3
in human melanoma promotes brain metastasis. Cancer Res.
66:3188–3196. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Sellers LA, Feniuk W, Humphrey PP and
Lauder H: Activated G protein-coupled receptor induces tyrosine
phosphorylation of STAT3 and agonist-selective serine
phosphorylation via sustained stimulation of mitogen-activated
protein kinase. Resultant effects on cell proliferation. J Biol
Chem. 274:16423–16430. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhang Y, Turkson J, Carter-Su C, Smithgall
T, Levitzki A, Kraker A, Krolewski JJ, Medveczky P and Jove R:
Activation of Stat3 in v-Src-transformed fibroblasts requires
cooperation of Jak1 kinase activity. J Biol Chem. 275:24935–24944.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kotha A, Sekharam M, Cilenti L, Siddiquee
K, Khaled A, Zervos AS, Carter B, Turkson J and Jove R: Resveratrol
inhibits Src and Stat3 signaling and induces the apoptosis of
malignant cells containing activated Stat3 protein. Mol Cancer
Ther. 5:621–629. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Niu G, Wright KL, Huang M, Song L, Haura
E, Turkson J, Zhang S, Wang T, Sinibaldi D, Coppola D, et al:
Constitutive Stat3 activity up-regulates VEGF expression and tumor
angiogenesis. Oncogene. 21:2000–2008. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Forsythe JA, Jiang BH, Iyer NV, Agani F,
Leung SW, Koos RD and Semenza GL: Activation of vascular
endothelial growth factor gene transcription by hypoxia-inducible
factor 1. Mol Cell Biol. 16:4604–4613. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Tolaney SM and Krop IE: Mechanisms of
trastuzumab resistance in breast cancer. Anticancer Agents Med
Chem. 9:348–355. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Buzdar AU: Role of biologic therapy and
chemotherapy in hormone receptor- and HER2-positive breast cancer.
Ann Oncol. 20:993–999. 2009. View Article : Google Scholar : PubMed/NCBI
|