Introduction
Lung cancer is one of the most common malignancies
and is the leading cause of cancer-related deaths worldwide. New
cases of lung cancer diagnosed in the US exceed 2 million per year
(1). Non-small cell lung cancer
(NSCLC) accounts for 80% of lung cancer cases, including lung
adenocarcinoma, squamous cell carcinoma and large-cell carcinoma
(2). With surgical resection and
combined-modality therapy, the overall survival has improved.
However, the prognosis is still poor as most patients present with
distant metastasis before being diagnosed. Therefore, almost all
types of lung cancer have a poor 5-year survival rate (3). The most common metastatic site of
NSCLC is bone (~30 to 40% of patients with NSCLC), followed by the
brain, liver and adrenal glands (4,5). Bone
lesions caused by lung cancer metastasis are often osteolytic
lesions and have been associated with a poorer prognosis (6). However, the mechanisms of lung cancer
metastasis to the bone resulting in osteolytic lesions remain
unknown.
Paget proposed the 'seed and soil' hypothesis, which
considered that tumor cells (the 'seed') have a specific affinity
for the milieu of certain organs (the 'soil'). Metastases result
only when the seed and soil are compatible (7). Tumor progression and metastasis are
multistep processes that involve the primary tumor and distant
organ microenvironment (8). Bone
microenvironment homeostasis is maintained by various types of
cells, such as osteoblasts, osteoclasts and bone marrow-derived
mesenchymal stem cells (MSCs) (9,10). The
dynamic balance is disrupted when lung cancer cells are transferred
to the bone and osteolytic lesions occur. MSCs induce a
tumor-suppressive effect or promote tumor growth and metastasis by
secretion of a variety of cytokines, chemokines, and growth factors
through a paracrine- or autocrine-mediated pathway (11–18).
Some research has demonstrated that MSCs promote cell viability and
this is mainly attributed to decreased apoptosis (19). Other research found that MSCs
inhibited the proliferation and invasion of A549 cells in
vitro, but favored tumor formation and growth in vivo
(20). Therefore, the effect of
MSCs on lung cancer growth and metastasis remains controversial.
However, the detailed mechanisms of the interaction of MSCs with
lung cancer are largely unknown. Thus, there is an urgent need for
a better understanding, as it will lead to improvements in the
design of more effective therapy for lung cancer metastasis. The
chemokines interleukin-6 (IL-6) and IL-8 contribute to the
proliferation and metastasis of lung cancer cells or other types of
cancer cells in the microenvironment of brain metastasis which are
produced by astrocytes (21,22).
IL-6 and IL-8 are also secreted by MSCs, and they are known to
influence osteoclast formation and bone resorption (23). Whether IL-6 and IL-8 influence lung
cancer progression in the bone micro-environment remains
unclear.
BMPs are members of the TGF-β superfamily, which
participate in the development and homeostasis of diverse tissues
and organs by regulating cellular differentiation, proliferation,
apoptosis and motility. BMPs are key factors in the regulation of
bone formation (24). Furthermore,
BMPs have been recently shown to play a pivotal role in tumor
development, progression and bone metastasis (25). BMP9 has the strongest osteogenetic
effect of the BMPs (26), as it has
a promoting or inhibiting role in different tumor types (27–29).
However, whether BMP9 affects the progression of lung cancer and
osteolytic lesions of bone metastasis needs confirmation.
Based on the studies above, we aimed to investigate
the effects of BMP9 on A549 cells in an HS-5 cell-mediated
microenvironment, as well as the underlying mechanisms. We
demonstrated that BMP9 inhibited the proliferation and metastasis,
and enhanced the apoptosis of A549 cells. After the A549 cells were
co-cultured with HS-5 cells, HS-5 cells promoted the proliferation,
migration and invasion, and the mRNA and protein levels of IL-6 and
IL-8 were increased in the HS-5 and A549 cells. However BMP9
reversed the promoting effect of the HS-5 cells. The effects were
via the MAPK/ERK and NF-κB signaling pathway. These results may
offer a novel strategy to efficiently inhibit the metastasis and
invasion of lung cancer to the bone.
Materials and methods
Cell culture and adenoviral
transfection
The human lung adenocarcinoma A549 cells and bone
marrow stromal HS-5 cells were purchased from the American Type
Culture Collection (ATCC; Manassas, VA, USA) and cultured in
Dulbecco's modified Eagle's medium (DMEM; Hyclone, USA) containing
10% fetal bovine serum (FBS; Gibco, USA) and 1%
penicillin/streptomycin at 37°C in a 5% CO2 incubator
under humidified conditions. Recombinant adenovirus AdBMP9 and
negative control AdGFP were kindly provided by Dr Tongchuan He
(University of Chicago Medical Center, Chicago, IL, USA), AdBMP9 or
AdGFP was transfected into the A549 or HS-5 cells with Polybrene
(Sigma, USA). The medium was replaced with fresh medium after 8 h
of cultivation.
Co-culture system
The co-culture system was set up using 6-well
Transwell inserts (Corning, USA) with a 0.4-µm pore size. In
the Transwell chamber, A549 and HS-5 cells were plated in the upper
chambers at the density of 1×105 cells/well in 1 ml,
whereas HS-5 and A549 cells were plated in 6-well plates at the
density of 2×105 cells/well in 2 ml. Cells became
adherent after 6 h, and then were placed together in DMEM
supplemented with 2% FBS for 3 days. Cells alone were used as the
control.
Cell proliferation assay
Cell viability was detected by MTT [3-(4,
5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] assay. A
total of 4×104 A549 cells/well were cultured in 6-well
plates and 2×104 HS-5 cells were cultured in a chamber.
The A549 cells were treated with AdGFP or AdBMP9, and the cells
were cultured for 24, 48 and 72 h. At the indicated time, MTT
reagent (Sigma) was added (200 µl/well), and incubation was
carried out at 37°C for 4 h. Next, 3 ml of dimethyl sulfoxide
(DMSO) was added to the 6-well plates to dissolve the formazan
product for 10 min at room temperature, and 150 µl lysate
was added into the 96-well plate. Finally, the absorbance was
measured at 492 nm using a microplate reader. Each group was
conducted in quintuplicate, and the overall experiment was repeated
3 times.
Colony-forming assay
Log-phase A549 cells treated with AdGFP or AdBMP9
were collected and seeded in 10% FBS medium at 200 cells/well in
6-well plates for 14 days. When clones were observed, the cells
were washed twice with PBS and stained with 1% crystal violet. The
colony formation rate was calculated as: (Colony number/seeded cell
number) × 100%. Each experiment was repeated thrice.
Flow cytometry
Cell apoptosis analysis was assessed by flow
cytometry. The A549 cells were seeded into 6-well plates at a
density of 2×105 cells/well and treated with AdGFP or
AdBMP9 for 48 h. Then the cells were harvested and re-suspended in
1 ml cold PBS, and samples were added with propidium iodide (PI)
and FITC-Annexin V for cell apoptosis analysis according to the
manufacturer's protocol (Invitrogen, USA). Respectively, data were
analyzed using FACS Sorter (Becton Dickinson, San Jose, CA,
USA).
Wound-healing assay
A549 cells were seeded into 6-well plates and HS-5
cells were seeded into chambers. A549 cells were treated with AdGFP
or AdBMP9. When A549 cells had grown to 95% confluency, the
monolayer was scratched with a sterile pipette tip, and then washed
with PBS twice to remove cellular debris. Culture medium was
replaced with fresh DMEM containing 1% FBS, and then co-cultured
with the HS-5 cells. Cells that migrated into the scratched area
were compared using bright field microscopy at 48 h. Experiments
were performed in triplicate.
Transwell invasion assay
HS-5 cells are seeded into a 24-well plate
containing 10% FBS. After the cells became adherent, the A549 cells
(4×104/400 µl) in FBS-free DMEM were seeded into
the chambers (24-well Transwell chambers, 8-µm pore size;
Corning). The Transwell membrane was coated with 1:3 diluted
Matrigel (Sigma) beforehand. After 24 h, the cells that invaded to
the underside of the filter were fixed with 4% paraformaldehyde and
stained with 0.1% crystal violet, and counted by bright field
microscopy. All the experiments were repeated 3 times.
RNA interference
A549 cells were seeded into 6-well plates and
incubated at 37°C in a CO2 incubator until they were
60–80% confluent, then the cells were transfected with 50 nM IL-6
or IL-8 siRNA according to the manufacturer's instructions.
Transfected cells were incubated for 3 days. The siRNA sequences
which aimed to target IL-6 and IL-8 were designed by RiboBio and
are shown in Table I.
 | Table IThe sequences of the siRNAs. |
Table I
The sequences of the siRNAs.
| siRNAs | Sense | Antisense |
|---|
| siIL-6 | #1:
AGACAUGUAACAAGAGUAA |
UCUGUACAUUGUUCUCAUU |
| #2:
AAACAACCUGAACCUUCCA |
UUUGUUGGACUUGGAAGGU |
| #3:
GGAGACUUGCCUGGUGAAA |
CCUCUGAACGGACCACUUU |
| siIL-8 | #1:
GCCAAGGAGUGCUAAAGAA |
CGGUUCCUCACGAUUUCUU |
| #2:
GCGCCAACACAGAAAUUAU |
CGCGGUUGUGUCUUUAAUA |
| #3:
CAAAGAACUGAGAGUGAUU |
GUUUCUUGACUCUCACUAA |
RNA isolation and relative quantitative
RT-PCR
A549 cells were co-cultured with HS-5 cells or alone
or were treated with AdBMP9 in FBS-free DMEM for 3 days. Total RNA
was extracted using TRIzol reagent (Invitrogen, Carlsbad, CA, USA)
according to the manufacturer's protocol. Total RNA (2 µg)
was used for cDNA synthesis by reverse transcriptase-PCR. The mRNA
expression was quantitatively determined using a real-time
polymerase chain reaction (PCR) system (Bio-Rad, USA) using SYBR
Green PCR Master Mix. GAPDH was used as the invariant control.
Amplification was performed with the following protocol:
denaturation at 94°C for 30 sec, annealing at 55°C for 30 sec, and
extension at 72°C for 50 sec. The primer sequences used for
real-time PCR are shown in Table
II.
 | Table IIThe sequences of the primers for
RT-PCR. |
Table II
The sequences of the primers for
RT-PCR.
| Genes | Primer sequence
(5′-3′) | Product size
(bp) |
|---|
| GAPDH | F:
CAGCGACACCCACTCCTC | 120 |
| R:
TGAGGTCCACCACCCTGT | |
| BMP9 | F:
CTGCCCTTCTTTGTTGTCTT | 322 |
| R:
CCTTACACTCGTAGGCTTCATA | |
| IL-6 | F:
TAGTGAGGAACAAGCCAGAG | 234 |
| R:
TACATTTGCCGAAGAGCC | |
| IL-8 | F:
ACTCCAAACCTTTCCACC | 155 |
| R:
CTTCTCCACAACCCTCTG | |
Western blot analysis
A549 cells were cultured alone or co-cultured with
HS-5 cells, and treated with AdBMP9 for 3 days. Then the A549 cells
were washed three times with cold PBS and lysed in RIPA lysis
buffer, and the cell lysate was denatured after boiling. The
protein concentration was measured by a BCA protein assay kit.
Fifty micrograms of lysate was loaded onto 10% SDS-PAGE gels, and
subsequently transferred onto PVDF membranes. After blocking with
5% bovine serum albumin (BSA; Solarbio) in TBST for 2 h at 37°C,
the membranes were incubated with the following primary antibodies
[polyclonal rabbit anti-BMP9 (Abcam, Cambridge, MA, USA);
anti-caspase3, anti-Bax, anti-Bcl2, anti-MMP2, anti-PCNA,
anti-IL-6, anti-IL-8 (Wanleibio, Shenyang, China); monoclonal
rabbit anti-ERK1/2, anti-p-ERK1/2, anti-P38, anti-p-P38, anti-AKT,
anti-p-AKT, anti-p-P65 (Cell Signaling Technology, Danvers, MA,
USA); monoclonal mouse anti-β-actin (ZSGBBIO, Beijing, China);
rabbit anti-histone (Abmart, Shanghai, China)] at 4°C overnight,
washed with TBST for 3 times, followed by incubation with a
secondary antibody conjugating with horseradish peroxidase
(ZSGBBIO) for 1 h, and washed again. Protein levels were quantified
with the SuperSignal West Pico Chemiluminescent Substrate kit.
Animal studies
The animal experiments were performed in accordance
with the guidelines established by the Animal Care and Use
Committee of Chongqing Medical University Laboratory Animal
Research. The 4- to 6-week-old male NOD/SCID mice were randomly
divided into 4 groups (n=4/group), respectively, and were injected
subcutaneously with A549 cells (6×106); a mixture of
A549 (6×106) and HS-5 cells (3×106); A549/GFP
(6×106) and HS-5 cells (3×106); A549/BMP9
(6×106) and HS-5 cells (3×106). Tumor volume
was measured every week with Vernier calipers, and calculated in
mm3 as ab2/2, where 'a' and 'b' represent the
largest and smallest tumor diameter, respectively. The mice were
sacrificed after 7 weeks, and tumor tissues were collected,
embedded in paraffin, and cut into 4-µm sections. The
sections were stained using H&E and immunohistochemical
analyses were conducted.
Immunohistochemical staining for
Ki-67
Paraffin sections of tumor tissues were de-waxed,
rehydrated and heat-treated for antigen retrieval with citric acid
buffer. The sections were then blocked with normal goat serum for
30 min, incubated with polyclonal rabbit anti-Ki-67 (Wanleibio) at
4°C overnight, and were analyzed using an immunohistochemistry kit
(PV-9001; ZSGBBIO) following the manufacturer's protocol. Staining
procedures were performed under standardized conditions. The
sections were counterstained with hematoxylin, mounted, and
coverslipped. Staining intensity was independently assessed by the
authors.
Statistical analysis
All values are expressed as the mean ± SEM.
Statistical significance was determined using the Student's t-test
in GraphPad Prism 5. A P-value of <0.05 was considered to
indicate a statistically significant result.
Results
Overexpression of BMP9 inhibits the
proliferation, migration and invasion, and promotes the apoptosis
of A549 cells
We aimed to ascertain the effect of BMP9 on the
biological behavior of NSCLC cells AdBMP9 was used to overexpress
BMP9 in the lung adenocarcinoma A549 cells which have a low level
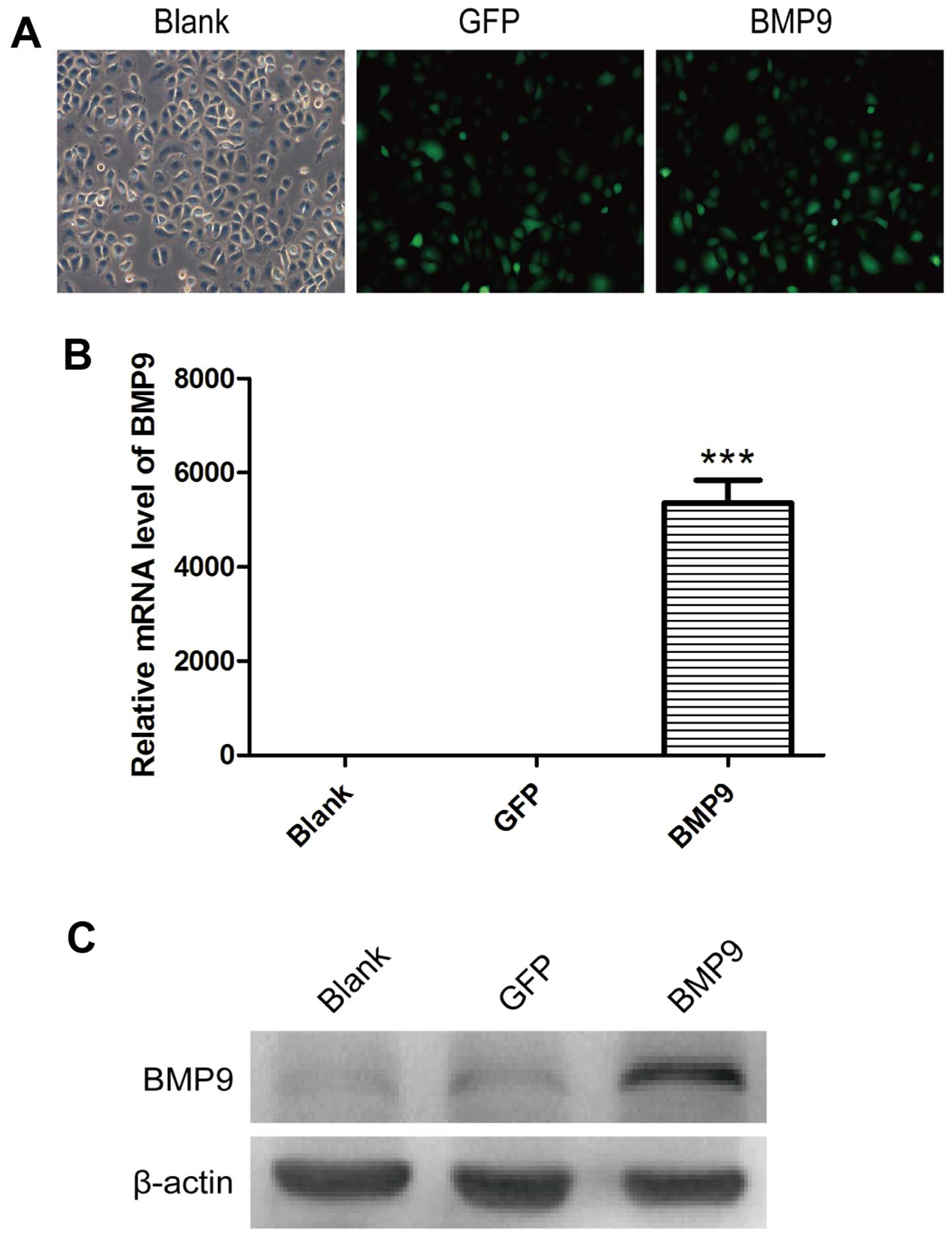
of BMP9. The infection effciency of AdBMP9 in the A549 cells was
observed under a fluorescence microscope (Fig. 1A). RT-PCR and western blot results
showed that these recombinant cells were successfully established
(P<0.001; Fig. 1B and C). Cell
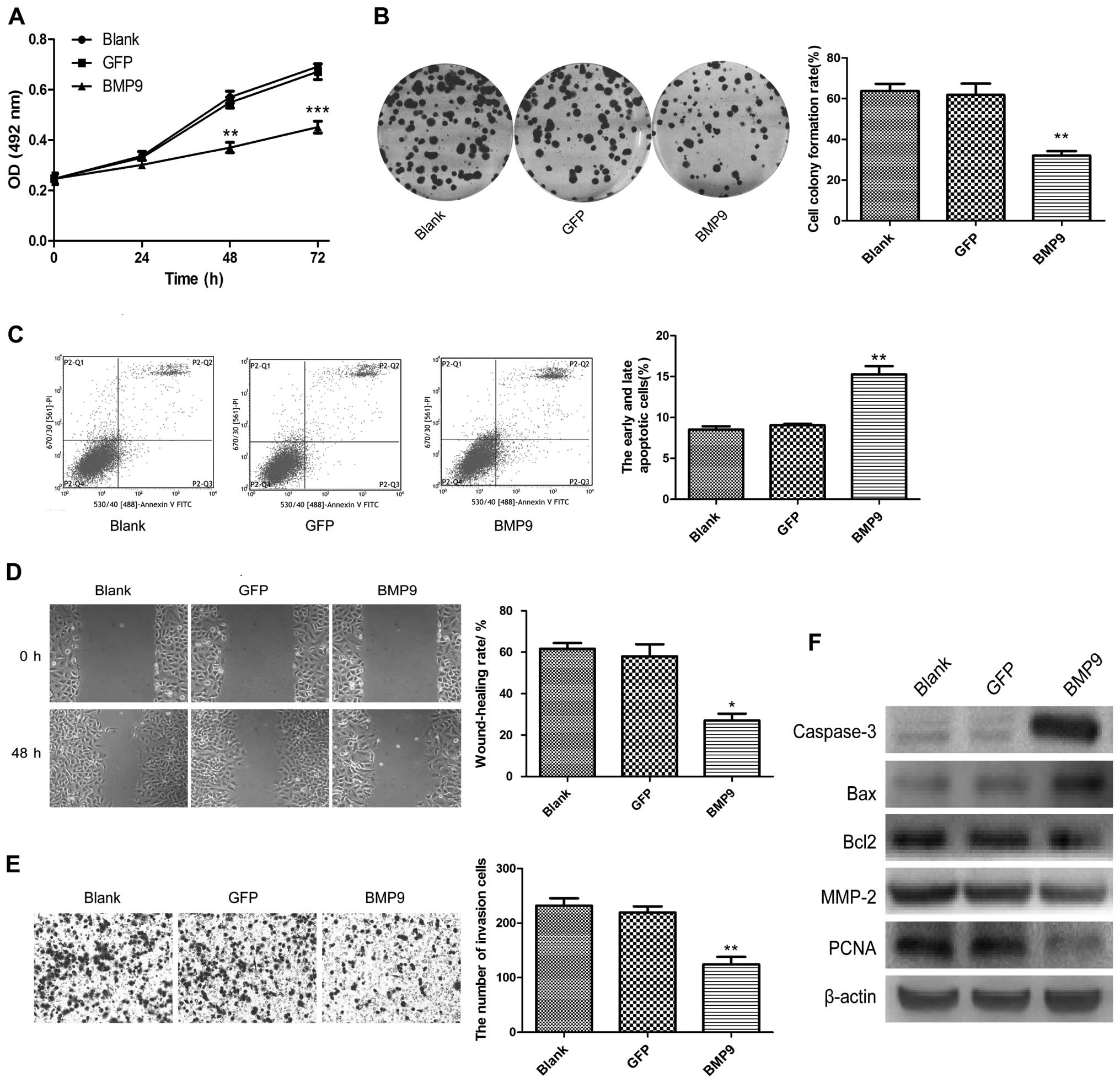
proliferation ability was assessed by MTT and colony-forming
assays. The results showed that the proliferation of the A549/BMP9
cells was decreased significantly (Fig.
2A and B). The cell apoptosis rate of the A549/BMP9 cells was
significantly increased compared with the rates noted in the blank
and GFP groups by flow cytometry (P<0.01; Fig. 2C). Cell migration and invasion
abilities were detected by wound-healing and Transwell invasion
assays. The findings demonstrated that BMP9 decreased the
wound-healing rate and the number of invasive A549 cells (Fig. 2D and E). Furthermore, we detected
the protein levels of DNA replication factor (PCNA), cell apoptosis
factors (pro-apoptosis factors caspase-3 and Bax and anti-apoptosis
factor Bcl2), and migration-related factor (MMP-2) by western
blotting. The results showed that PCNA, MMP-2 and Bcl2 were
decreased, while caspase-3 and Bax were upregulated by BMP9
overexpression in the A549 cells (Fig.
2F).
HS-5 cells stimulate the proliferation,
migration and invasion of A549 cells, and BMP9 inhibits the
malignant phenotype of A549 cells in a co-culture system
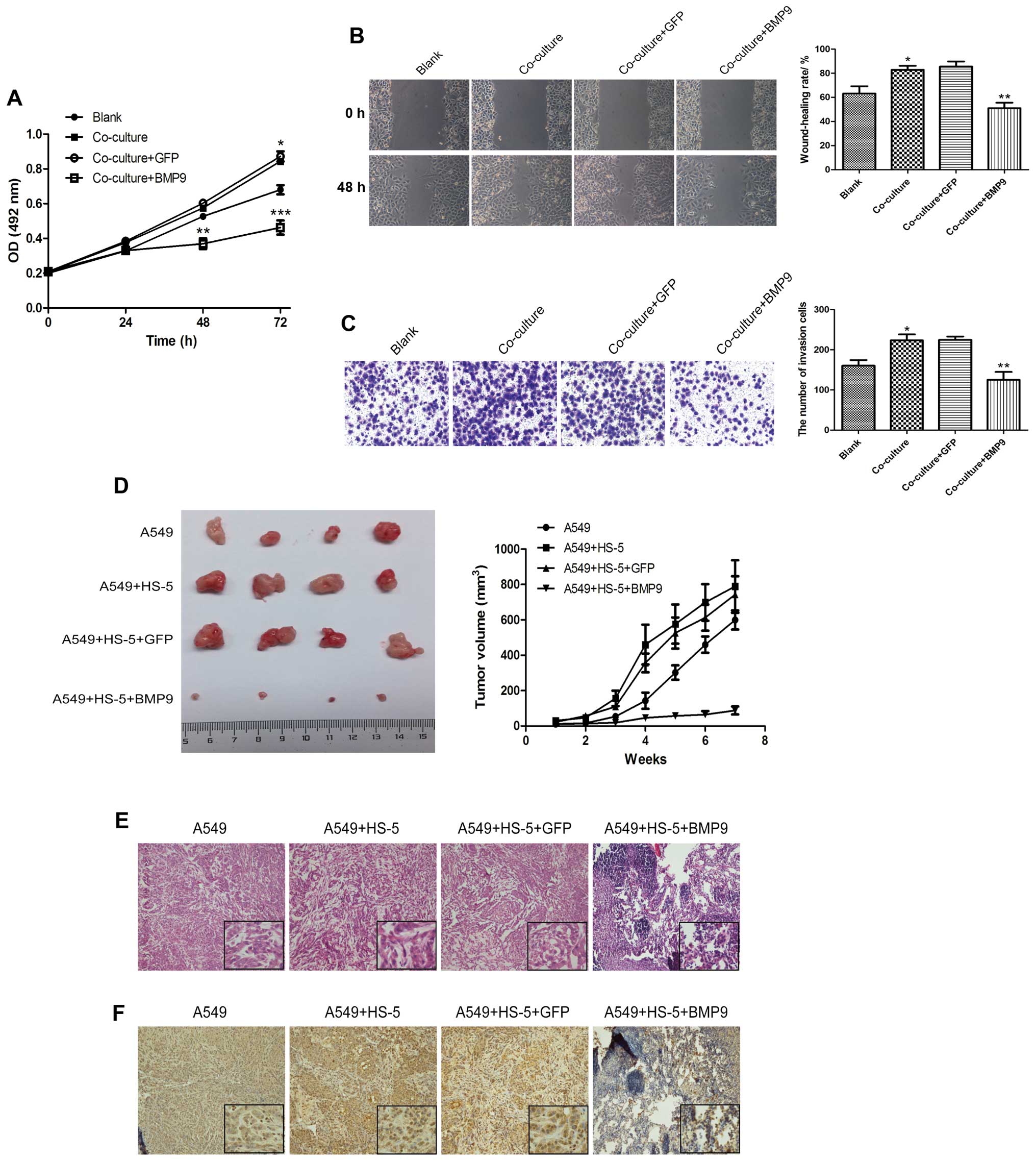
When co-cultured with HS-5 cells, the cell viability
of the A549 cells obviously increased as determined by MTT assay.
Cell migration and invasion abilities were increased as determined
by wound-healing and Transwell invasion assays (Fig. 3A–C). After AdBMP9 was added to the
co-culture system, the cell proliferation rate, the wound-healing
rate and the number of invading A549 cells in the co-culture/BMP9
group was found to be significantly decreased than these values in
the co-culture/GFP group (Fig.
3A–C). To investigate the effects of HS-5 cells and BMP9 on the
tumor growth of lung cancer cells in vivo, A549 or
adenovirus-infected A549 cells and HS-5 cells were subcutaneously
implanted into nude mice. The tumor size was monitored weekly and
the tumors were dissected after xenografting for 7 weeks. Tumor
volumes differed obviously after 4 weeks. HS-5 cells accelerated
the growth of the A549 tumors, while BMP9 significantly inhibited
the accelerative role of the HS-5 cells compared to the GFP group
(Fig. 3D). These results were
consistent with those in vitro. H&E staining showed no
variation in heterogeneity between the single A549 and A549/HS-5
group, but there were many lymphocytic invasive cells in the tumor
tissue of the BMP9 group (Fig. 3E).
Ki-67 expression was detected by immunohistochemical staining. The
results showed that the Ki-67-positive cell rate was increased in
the A549/HS-5 group when compared with the A549 group, and BMP9
inhibited the expression of Ki-67 (Fig.
3F).
IL-6 and IL-8 expression levels are
increased in the A549 and HS-5 cells in the co-culture system
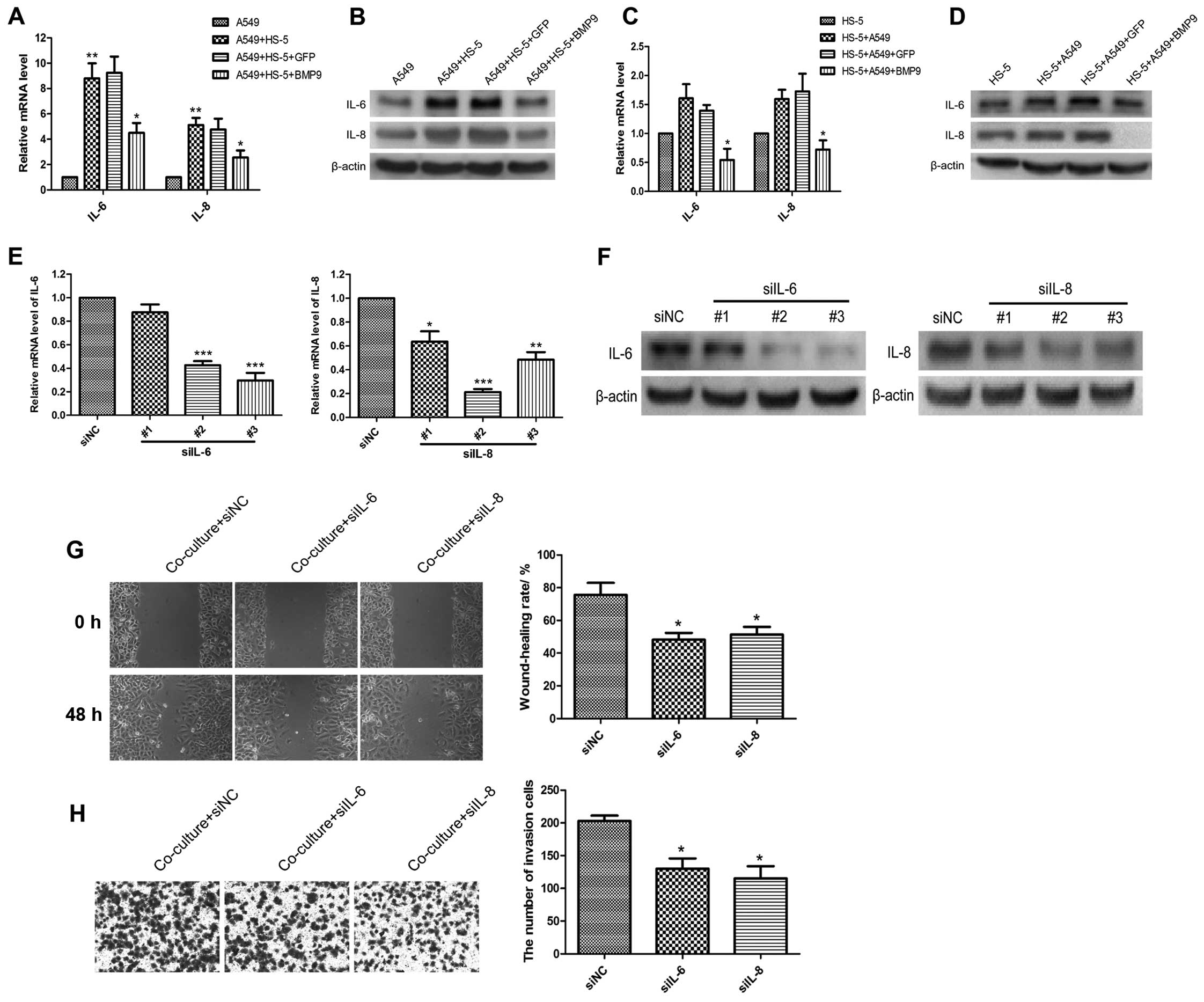
Studies have reported that expression levels of IL-6
and IL-8 are higher in lung cancer patients with metastasis than
those without metastasis. Meanwhile, IL-6 and IL-8 could be
secreted by bone marrow stromal cells, and they are known to
influence osteoclast formation and bone resorption. BMP9 is the
most effective bone formation factor of the BMPs. We investigated
whether IL-6 and IL-8 are related to the pro-metastatic effect of
A549 cells in the HS-5 cell-mediated tumor microenvironment. We
detected the mRNA and protein levels of IL-6 and IL-8 in the A549
and HS-5 cells in the co-culture system by RT-PCR and western
blotting. IL-6 and IL-8 were increased in the A549 (Fig. 4A and B) and HS-5 cells (Fig. 4C and D, but had no statistical
significance) in the co-culture system, BMP9 inhibited the
expression levels of IL-6 and IL-8 compared to the GFP group
(Fig. 4A–C). When IL-6 and IL-8
were knocked down by siRNA, the interference efficiency of the
three siRNAs targeting IL-6 and IL-8 was verified by RT-PCR and
western blotting (Fig. 4E and F).
IL-6-siRNA-3 and IL-8-siRNA-2 were the most highly functional one
and were used in the following assays. The wound-healing rate and
the number of invasive A549 cells were decreased in the co-culture
system with knockdown of IL-6 or IL-8 (Fig. 4G and H).
Effects of IL-6 and IL-8 on A549 cells
via the MAPK/ERK1/2 and NF-κB signaling pathways
NF-κB and MAPK are crucial signaling pathways
responsible for gene induction in NSCLC cell proliferation
(30,31). Activation of these pathways in lung
cancer cells initiates signaling cascades and leads to
overproduction of inflammatory cytokines and growth factors
including IL-6, IL-8 and MMPs, which promote cancer progression and
metastasis (32,33). Whether these pathways involved in
lung cancer cells thrive within the bone environment remains
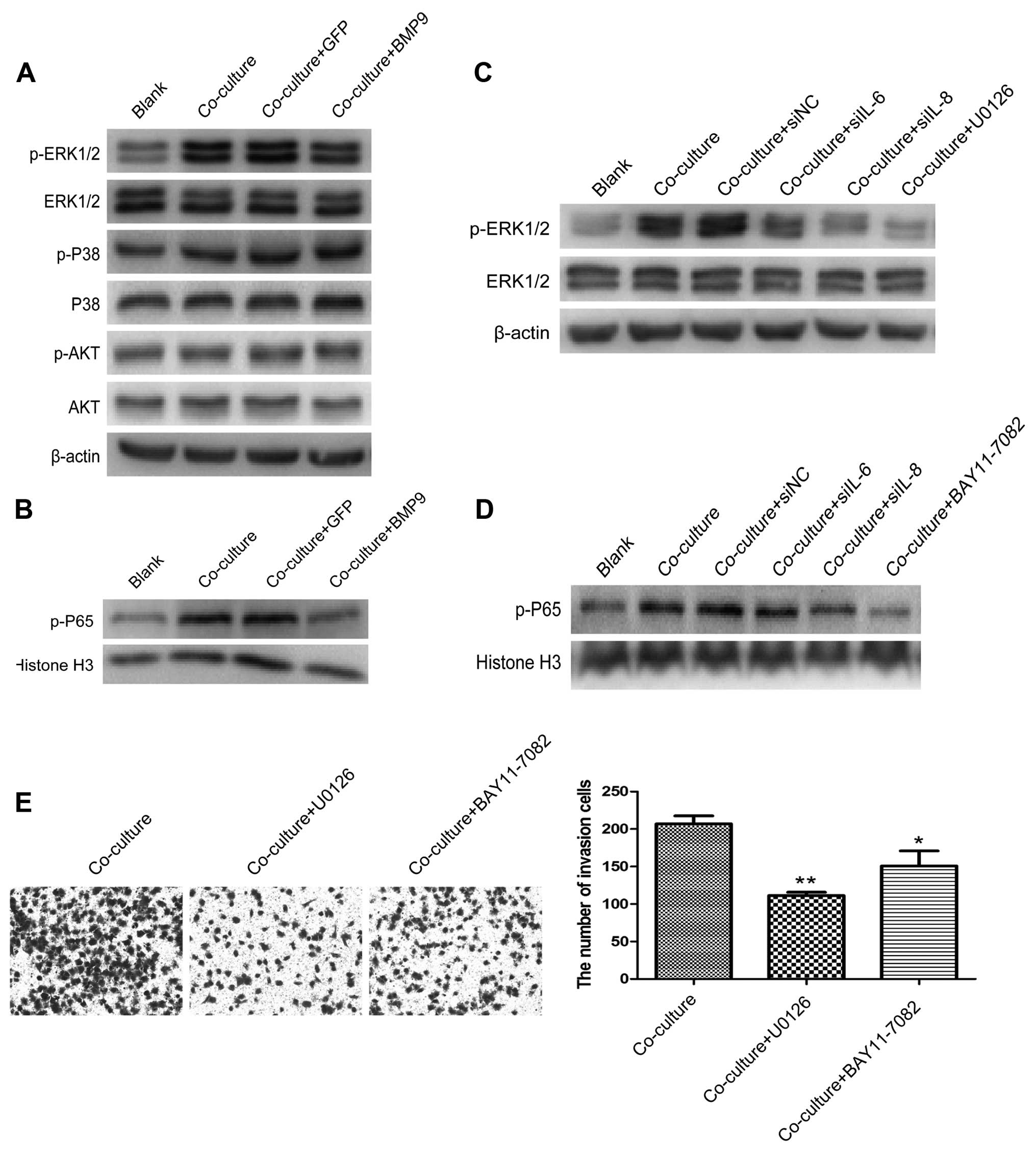
unkown. As shown in Fig. 5A and B,
the A549 cells had enhanced expression of p-ERK1/2 and p-P65, but
not p-P38 and p-AKT after co-culture with the HS-5 cells for 3
days. When IL-6 and IL-8 were silenced by small interference RNA
siIL-6 and siIL-8, or overexpression of BMP9, the expression of
p-ERK1/2 and p-P65 was decreased. The same results were found with
the MEK- and NF-κB-specific inhibitors U0126 (10 µM;
Selleckchem, USA) and BAY11-7082 (5 µM; Beyotime, Shanghai,
China), respectively (Fig. 5C and
D). Meanwhile, U0126 and BAY11-7082 decreased the invasive
ability of the A549 cells in the co-culture system (Fig. 5E).
Discussion
Bone is the most common metastatic site of
metastatic non-small cell lung cancer (4). NSCLC patients with bone metastasis
have a poor prognosis, which is due to skeletal-related events,
such as pathological fractures, spinal cord compression and
hypercalcemia of malignancy (34,35).
Bone marrow-derived cells are one of the important tumor stroma
components in the tumor microenvironment (36). Bone marrow-derived cells promote the
development of lung cancer micrometastases by releasing
extracellular matrix (ECM)-bound growth factors such as IL-6, IL-8,
PTHrp, which also promote osteolytic lesions. And this vicious
cycle is regulated by the NF-κB signaling pathway (37).
BMPs have been reported to have important effects on
many tumor types. In lung cancer, BMP2 upregulation was found to
increase the incidence and influence the prognosis of lung cancer
patients (38). The expression of
BMP5 was found to be downregulated in lung cancer tissues and may
serve as a potential prognostic biomarker or therapeutic target for
NSCLC (39). BMP9 is the most
effective BMP in bone formation. It has been reported that it can
promote the growth of ovarian cancer cells, inhibit the growth and
invasion of prostate cancer PC-3 cells, and enhance apoptosis, and
repress the invasion and migration of breast cancer cells (27,28,40).
BMP9 was also found to inhibit the invasion of breast cancer
MDA-MB-231 cells and reduce osteolytic lesions in a bone marrow
stromal cell-mediated tumor microenvironment (29). However, the role of BMP9 in lung
cancers, particularly in bone metastasis of lung cancers, requires
further investigation.
To study the role of BMP9 in lung cancer,
particularly in the bone microenvironment of lung cancer, we chose
lung adenocarcinoma A549 cells which have low expression of BMP9,
and established BMP9-overexpressing A549 cells by infection with
recombinant adenovirus AdBMP9. In vitro, we found that BMP9
inhibited the growth, migration and invasion, and promoted the
apoptosis of A549 cells. The growth, migration and invasion were
increased in A549 cells after being co-cultured with HS-5 cells.
However, BMP9 also reversed these biological activities in the
tumor microenvironment. The in vivo experiment indicated
that HS-5 cells increased tumor growth and BMP9 reversed the
promoting effect of HS-5 cells. We detected whether the chemokines
IL-6 and IL-8 are involved in regulating the progression of lung
cancer in the bone microenvironment. The data determined that the
levels of IL-6 and IL-8 were increased in the A549 and HS-5 cells
when they were co-cultured. BMP9 decreased the expression of IL-6
and IL-8. When IL-6 and IL-8 were downregulated by siRNAs in the
co-culture system, the migration and invasion of the A549 cells
were decreased. The results indicated that IL-6 and IL-8 are
associated with the migration and invasion of A549 cells in the
tumor microenvironment, and BMP9 has an inhibitory role in lung
cancer progression and osteolytic lesions. In order to further
elucidate the correlative mechanisms of IL-6, IL-8 and BMP9 with
lung cancer progression, we detected interleukin and BMP-related
MAPK, PI3K/AKT and NF-κB signaling pathways (31,41).
Our data showed that the MAPK/ERK and NF-κB signaling pathways were
activated in co-cultured A549 cells which had high expression of
IL-6 and IL-8. siIL-6 and siIL-8 inhibited the activation of the
MAPK/ERK and NF-κB signaling pathways, and had the same effect with
BMP9.
In conclusion, the study showed that MSCs can
promote the proliferation, migration and invasion of A549 cells.
This promoting role may be mediated by IL-6 and IL-8 via the
MAPK/ERK and NF-κB signaling pathways. BMP9 regulated the crosstalk
between A549 and HS-5 cells in the co-culture system, which
inhibited the proliferation and migration of A549 cells.
Acknowledgments
We thank Dr Tongchuan He (University of Chicago) for
generously providing the recombinant adenovirus BMP9 (AdBMP9). This
study was supported by the National Natural Science Foundation of
China (NSFC 31200971), Program of the Ministry of Science and
Technology of Yuzhong District, CQ, China (20150109).
References
|
1
|
Siegel R, Ma J, Zou Z and Jemal A: Cancer
statistics, 2014. CA Cancer J Clin. 64:9–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
D'Addario G: Non-small-cell lung cancer:
ESMO clinical recommendations for diagnosis, treatment and
follow-up. Ann Oncol. 20(Suppl 4): 68–70. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Siddiqui S, Ali MU, Ali MA, Shah N and
Nasreen S: Lung carcinoma: Its profile and changing trends. J Ayub
Med Coll Abbottabad. 22:116–119. 2010.PubMed/NCBI
|
|
4
|
Tamura T, Kurishima K, Nakazawa K,
Kagohashi K, Ishikawa H, Satoh H and Hizawa N: Specific organ
metastases and survival in metastatic non-small-cell lung cancer.
Mol Clin Oncol. 3:217–221. 2015.
|
|
5
|
Coleman RE: Metastatic bone disease:
Clinical features, pathophysiology and treatment strategies. Cancer
Treat Rev. 27:165–176. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tubiana-Hulin M: Incidence, prevalence and
distribution of bone metastases. Bone. 12(Suppl 1): S9–S10. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Paget S: The distribution of secondary
growths in cancer of the breast. 1889.Cancer Metastasis Rev.
8:98–101. 1989.
|
|
8
|
Langley RR and Fidler IJ: Tumor cell-organ
microenvironment interactions in the pathogenesis of cancer
metastasis. Endocr Rev. 28:297–321. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sivasubramaniyan K, Lehnen D, Ghazanfari
R, Sobiesiak M, Harichandan A, Mortha E, Petkova N, Grimm S,
Cerabona F, de Zwart P, et al: Phenotypic and functional
heterogeneity of human bone marrow- and amnion-derived MSC subsets.
Ann NY Acad Sci. 1266:94–106. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Baksh D, Song L and Tuan RS: Adult
mesenchymal stem cells: Characterization, differentiation, and
application in cell and gene therapy. J Cell Mol Med. 8:301–316.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gomes CM: The dual role of mesenchymal
stem cells in tumor progression. Stem Cell Res Ther. 4:422013.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sung SY, Hsieh CL, Law A, Zhau HE, Pathak
S, Multani AS, Lim S, Coleman IM, Wu LC, Figg WD, et al:
Coevolution of prostate cancer and bone stroma in three-dimensional
coculture: Implications for cancer growth and metastasis. Cancer
Res. 68:9996–10003. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Windus LC, Glover TT and Avery VM:
Bone-stromal cells up-regulate tumourigenic markers in a
tumour-stromal 3D model of prostate cancer. Mol Cancer. 12:1122013.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Goldstein RH, Reagan MR, Anderson K,
Kaplan DL and Rosenblatt M: Human bone marrow-derived MSCs can home
to orthotopic breast cancer tumors and promote bone metastasis.
Cancer Res. 70:10044–10050. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Karnoub AE, Dash AB, Vo AP, Sullivan A,
Brooks MW, Bell GW, Richardson AL, Polyak K, Tubo R and Weinberg
RA: Mesenchymal stem cells within tumour stroma promote breast
cancer metastasis. Nature. 449:557–563. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hernanda PY, Pedroza-Gonzalez A, van der
Laan LJ, Bröker ME, Hoogduijn MJ, Ijzermans JN, Bruno MJ, Janssen
HL, Peppelenbosch MP and Pan Q: Tumor promotion through the
mesenchymal stem cell compartment in human hepatocellular
carcinoma. Carcinogenesis. 34:2330–2340. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mizuguchi T, Hui T, Palm K, Sugiyama N,
Mitaka T, Demetriou AA and Rozga J: Enhanced proliferation and
differentiation of rat hepatocytes cultured with bone marrow
stromal cells. J Cell Physiol. 189:106–119. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Song B, Kim B, Choi SH, Song KY, Chung YG,
Lee YS and Park G: Mesenchymal stromal cells promote tumor
progression in fibrosarcoma and gastric cancer cells. Korean J
Pathol. 48:217–224. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang MH, Hu YD, Xu Y, Xiao Y, Luo Y, Song
ZC and Zhou J: Human mesenchymal stem cells enhance autophagy of
lung carcinoma cells against apoptosis during serum deprivation.
Int J Oncol. 42:1390–1398. 2013.PubMed/NCBI
|
|
20
|
Tian LL, Yue W, Zhu F, Li S and Li W:
Human mesenchymal stem cells play a dual role on tumor cell growth
in vitro and in vivo. J Cell Physiol. 226:1860–1867. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Seike T, Fujita K, Yamakawa Y, Kido MA,
Takiguchi S, Teramoto N, Iguchi H and Noda M: Interaction between
lung cancer cells and astrocytes via specific inflammatory
cytokines in the microenvironment of brain metastasis. Clin Exp
Metastasis. 28:13–25. 2011. View Article : Google Scholar :
|
|
22
|
Sierra A, Price JE, García-Ramirez M,
Méndez O, López L and Fabra A: Astrocyte-derived cytokines
contribute to the metastatic brain specificity of breast cancer
cells. Lab Invest. 77:357–368. 1997.PubMed/NCBI
|
|
23
|
Kudo O, Sabokbar A, Pocock A, Itonaga I,
Fujikawa Y and Athanasou NA: Interleukin-6 and interleukin-11
support human osteoclast formation by a RANKL-independent
mechanism. Bone. 32:1–7. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang RN, Green J, Wang Z, Deng Y, Qiao M,
Peabody M, Zhang Q, Ye J, Yan Z, Denduluri S, et al: Bone
Morphogenetic Protein (BMP) signaling in development and human
diseases. Genes Dis. 1:87–105. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ye L, Lewis-Russell JM, Kyanaston HG and
Jiang WG: Bone morphogenetic proteins and their receptor signaling
in prostate cancer. Histol Histopathol. 22:1129–1147.
2007.PubMed/NCBI
|
|
26
|
Luu HH, Song WX, Luo X, Manning D, Luo J,
Deng ZL, Sharff KA, Montag AG, Haydon RC and He TC: Distinct roles
of bone morphogenetic proteins in osteogenic differentiation of
mesenchymal stem cells. J Orthop Res. 25:665–677. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ye L, Kynaston H and Jiang WG: Bone
morphogenetic protein-9 induces apoptosis in prostate cancer cells,
the role of prostate apoptosis response-4. Mol Cancer Res.
6:1594–1606. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang K, Feng H, Ren W, Sun X, Luo J, Tang
M, Zhou L, Weng Y, He TC and Zhang Y: BMP9 inhibits the
proliferation and invasiveness of breast cancer cells MDA-MB-231. J
Cancer Res Clin Oncol. 137:1687–1696. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wan S, Liu Y, Weng Y, Wang W, Ren W, Fei
C, Chen Y, Zhang Z, Wang T, Wang J, et al: BMP9 regulates
cross-talk between breast cancer cells and bone marrow-derived
mesenchymal stem cells. Cell Oncol (Dordr). 37:363–375. 2014.
View Article : Google Scholar
|
|
30
|
Alam M, Wang JH, Coffey JC, Qadri SS,
O'Donnell A, Aherne T and Redmond HP: Characterization of the
effects of cyclooxy-genase-2 inhibition in the regulation of
apoptosis in human small and non-small cell lung cancer cell lines.
Ann Surg Oncol. 14:2678–2684. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhao J, Harper R, Barchowsky A and Di YP:
Identification of multiple MAPK-mediated transcription factors
regulated by tobacco smoke in airway epithelial cells. Am J Physiol
Lung Cell Mol Physiol. 293:L480–L490. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kausar H, Jeyabalan J, Aqil F, Chabba D,
Sidana J, Singh IP and Gupta RC: Berry anthocyanidins
synergistically suppress growth and invasive potential of human
non-small-cell lung cancer cells. Cancer Lett. 325:54–62. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lemmon CR, Woo JH, Tully E, Wilsbach K and
Gabrielson E: Nuclear factor-kappaB (NF-kappaB) mediates a
protective response in cancer cells treated with inhibitors of
fatty acid synthase. J Biol Chem. 286:31457–31465. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Bauml J, Mick R, Zhang Y, Watt CD, Vachani
A, Aggarwal C, Evans T and Langer C: Determinants of survival in
advanced non–small-cell lung cancer in the era of targeted
therapies. Clin Lung Cancer. 14:581–591. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Saad F, Lipton A, Cook R, Chen YM, Smith M
and Coleman R: Pathologic fractures correlate with reduced survival
in patients with malignant bone disease. Cancer. 110:1860–1867.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
El-Nikhely N, Larzabal L, Seeger W, Calvo
A and Savai R: Tumor-stromal interactions in lung cancer: Novel
candidate targets for therapeutic intervention. Expert Opin
Investig Drugs. 21:1107–1122. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Peng X, Guo W, Ren T, Lou Z, Lu X, Zhang
S, Lu Q and Sun Y: Differential expression of the RANKL/RANK/OPG
system is associated with bone metastasis in human non-small cell
lung cancer. PLoS One. 8:e583612013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Fei ZH, Yao CY, Yang XL, Huang XE and Ma
SL: Serum BMP-2 up-regulation as an indicator of poor survival in
advanced non-small cell lung cancer patients. Asian Pac J Cancer
Prev. 14:5293–5299. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Deng T, Lin D, Zhang M, Zhao Q, Li W,
Zhong B, Deng Y and Fu X: Differential expression of bone
morphogenetic protein 5 in human lung squamous cell carcinoma and
adenocarcinoma. Acta Biochim Biophys Sin (Shanghai). 47:557–563.
2015. View Article : Google Scholar
|
|
40
|
Herrera B, van Dinther M, Ten Dijke P and
Inman GJ: Autocrine bone morphogenetic protein-9 signals through
activin receptor-like kinase-2/Smad1/Smad4 to promote ovarian
cancer cell proliferation. Cancer Res. 69:9254–9262. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Mu Y, Gudey SK and Landström M: Non-Smad
signaling pathways. Cell Tissue Res. 347:11–20. 2012. View Article : Google Scholar
|